Abstract
Diabetes mellitus (DM) type 2 is amongst the most common chronic diseases, being responsible for various problems in humans and contributing to increased mortality rates worldwide. Fructooligosaccharide, which can be produced from the roots of burdock (Arctium lappa L.), has been shown to have a wide range of pharmacological proprieties, including antiviral, anti-inflammatory, hypolipidemic, and antidiabetic effects. Moreover, burdock also contains chlorogenic acid, which has been used in traditional medicine as an antioxidant. Considering its natural origin and minimal toxicity, burdock fructooligosaccharides (BFO) has gained considerable attention from researchers owing its wide, efficient, and beneficial action against DM. Although the effectiveness of fructooligosaccharide and chlorogenic acid has been extensively discussed, limited information is available on the application of burdock for DM treatment. In this review, we discuss the beneficial contributions, and the recent in vitro and in vivo analytical findings on A. lappa extract as DM therapy.
Keywords: Burdock, Diabetes mellitus, Chlorogenic acid, Fructooligosacchaeride
Introduction
Currently, diabetes mellitus (DM) is associated with significant health and societal burdens worldwide. The number of patients with diabetes has been rapidly increasing, affecting up to 9.3% (463 million people) of the global population in 2019, a rate that is projected to continue to increase to approximately 10.2% (578 million) by 2030 and 10.9% (700 million) by 2045 (Saeedi et al., 2019). DM can be classified as type 1 and type 2 DM (T2DM), which are characterized by no secretion of insulin and insulin deficiency/resistance, respectively (ADA, 2021). All patients with T2DM have high risk of developing various complications, such as nephropathy, retinopathy, and cardiovascular disorders, as long term hyperglycemia leads to tissue and organ damage (ADA, 2021; Juster-Switlyk and Smith, 2016). Indeed, 44% of T2DM patients experience progressive impairment of their renal function, which can ultimately lead to diabetic nephropathy and renal disease (Tang and Lai, 2012).
To date, inhibition of carbohydrate, polysaccharide, and disaccharide absorption is the main strategy to control the blood glucose levels (Deng et al., 2015; Ortiz-Andrade et al., 2007; Satoh et al., 2015). As starch hydrolysis into glucose can be influenced by the enzyme α-glucosidase, inhibition of its enzymatic activity is an effective strategy to control normal blood glucose levels in diabetic patients (Kim et al., 2005; Satoh et al., 2015). Within the past three decades, some synthetic α-glucosidase inhibitors, such as acarbose, miglitol, and voglibose, have been developed (Lordan et al., 2013; Yuan et al., 2021); however, they have been associated with some serious side effects including gastrointestinal problems caused by disturbances on the microbiome Lactobacillaceae, Ruminococcaceae, and Veillonellaceae populations (Zhang et al., 2017). Therefore, identification of natural α-glucosidase inhibitors without such adverse secondary effects is warranted. Inulin-type fructans (Fig. 1) or Compositae plants have shown positive effects on preventing metabolic disorders due to the presence of bioactive compounds (Yuan et al., 2021). For example, chlorogenic acids (CGA), which is an umbrella term for a class of phenolic acids that are commonly found in coffee, including 5-O-caffeoylquinic acid (5-CQA), have been extensively studied owing its commercial availability and health benefits (Lu et al., 2020). Recent studies have also shown that fructooigosaccharides (Ding et al., 2021) and bioactive polyphenols, in particular CGA, obtained from burdock exhibit antidiabetic effects (Boonphang et al., 2021; Hussain and Hafeez, 2021; Martina et al., 2019; Saravanakumar et al., 2021; Singh et al., 2021; Yuan et al., 2021). In this review article, we systematically discuss the beneficial effects of burdock extract against T2DM, which may pave the way for the development of enhanced therapies in the future. Accordingly, in this review we provide a critical perspective of the most recent advances on the in vitro and in vivo assessment of burdock therapeutic effects.
Fig. 1.
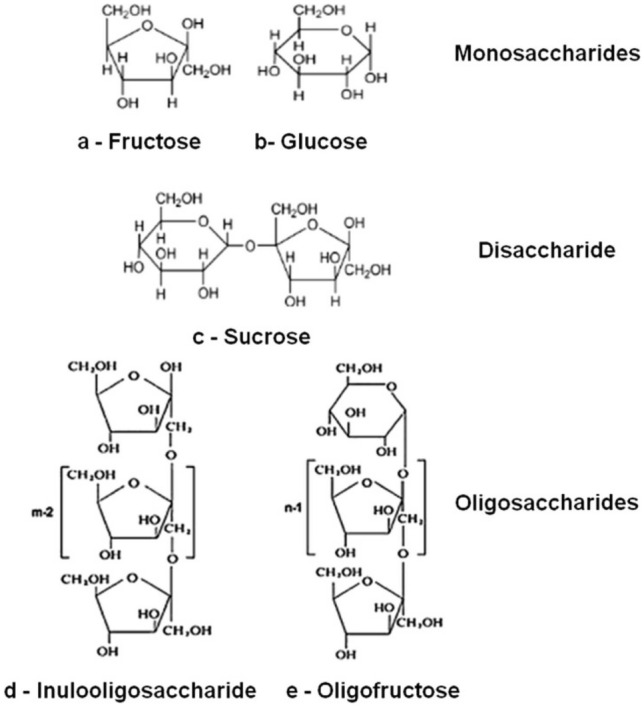
carbohydrate structure of burdock roots (Li et al., 2013)
Dietary sources of fructooligosaccharides and bioactive compounds
Fruits, vegetables, and honey contain trace amounts of fructooligosaccharides within their natural components, and many higher plants may also comprise fructooligosaccharides as reserved carbohydrates. The most common sources of fructooligosaccharides are asparagus, sugar beet, garlic, chicory, onion, Jerusalem artichoke, wheat, honey, banana, barley, tomato, rye, and burdock (Mussatto and Mancilha, 2007; Sangeetha et al., 2005), among which the most commercial fructooligosaccharide sources are chicory root (Cichorium intybus L.) (Moser et al., 2014), Jerusalem artichoke (Helianthus tuberosus L.) (Rubel et al., 2014), and burdock roots (Moro et al., 2022). Generally, fructooligosaccharide concentrations range between 0.3 and 6%; for chicory, these values are between 5 and 10%, while for Jerusalem artichoke and burdock roots, these values can reach 20% (Dominguez et al., 2014) and 0.71 g/100 g (Moro et al., 2022), respectively.
The main dietary sources of bioactive compounds such as polyphenols include different herbs, foods, dicotyledonous ferns and plants species, namely berry fruits, tea, apple, cocoa, citrus fruits, roasted beans, pears, carrots, worm-wood, artichoke, potatoes, eggplant, betel, kiwi fruits, tobacco leaves, burdock, eucommia, coffee beans, tomatoes, honeysuckle, and grapes (Barreto et al., 2021; Bento-Silva et al., 2021; Moro et al., 2022; Nwafor et al., 2022; Stefanov et al., 2022). Among these sources, green coffee contains approximately 6–12% (w/w) of total CGAs (Raskar and Bhalekar, 2019), whereas fresh coffee contains approximately 8.19–23.778 mg/200 mL and instant coffee contains 9.45–41.05 mg/200 mL (Mills et al., 2015). Fruits and vegetables are also a good source of 5-CQA, with egg-plant having a high concentration of 5-CQA (1.4–28.0 mg/g) (Plazas et al., 2013), and carrot (0.3–18.8 mg/g), artichoke (1.1–1.8 mg/g), and pepper (0.7–0.9 mg/g) also making a significant contribution to 5-CQA intake in the human diet. Additionally, apples, pears, peaches, plums, cherries, tomatoes, and potatoes contain a reasonable quantity of 5-CQA (Kumar et al., 2020). Specifically, apples contain primarily 5-CQA (0.41–1.16 mg/g), 3-CQA and 5-CQA are predominant in peaches, and plums predominantly contain 3-CQA (0.54 mg/g) but also have 5-CQA (0.073 mg/g) (Upadhyay and Mohan Rao, 2013).
Burdock (Arctium lappa) is a medicinal plant that contains several bioactive polyphenols, such as chlorogenic acid and its derivatives (Herrera-Balandrano et al., 2021; Wang et al., 2001). Burdock cultivated in Japan contains 1.5–4.7 mg/g (dry weight) of chlorogenic acid along with other derivatives (Wang et al., 2001), whereas Bulgarian burdock was shown to have 5.0 ± 0.2 mg/g (dry weight) (Petkova et al., 2022) and total phenolic content was found to be approximately 150 mg/g of dry plant sample produced in France (Tousch et al., 2014). Moreover, Mondal and Eun (2021) reported that the total phenolic content of A. lappa produced in Korea was 9.28 ± 0.16 mg GAE/g of the sample dry weight. The bioactive polyphenols identified from burdock are shown in Table 1.
Table 1.
Bioactive phenols identified in burdock extracts
| Components | Burdock extract | References |
|---|---|---|
| 1-O-caffeoylquinic acid | 60% methanol | Lin and Harnly (2008) |
| 3-O-caffeoylquinic acid | 60% methanol | Lin and Harnly (2008) |
| 5-O-caffeoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008) |
| 4-O-caffeoylquinic acid | 60% methanol | Lin and Harnly (2008) |
| 1,4-Di-O-caffeoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008) |
| 1,5-Di-O-caffeoyl-3-O-maloylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| Dimaloyl-dicaffeoylquinic acid isomer 1 | 70% ethanol | Tousch et al. (2014) |
| 3,5-Di-O-caffeoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008) |
| 1,4-Di-O-caffeoyl-3-O-maloylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| Succinoyl-tricaffeoylquinic acid isomer | 70% ethanol | Tousch et al. (2014) |
| 1,5-Di-O-caffeoyl-4-O-maloylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| 1,3-Di-O-caffeoyl-4,5-di-O-maloylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| Maloyl-dicaffeoylquinic acid isomer | 70% ethanol | Tousch et al. (2014) |
| 1,5-Di-O-caffeoyl-3-O-succinoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008), Maruta et al. (1995) |
| 1,4-Di-O-maloyl-3,5-di-O-caffeoylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| 1,5-Di-O-caffeoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008), Maruta et al. (1995) |
| Dicaffeoyl-succinoyl-malonylquinic acid isomer 1 | 70% ethanol | Tousch et al. (2014) |
| Dicaffeoyl-succinoyl-malonylquinic acid isomer 2 | 70% ethanol | Tousch et al. (2014) |
| Dimaloyl-dicaffeoylquinic acid isomer 2 | 70% ethanol | Tousch et al. (2014) |
| 1,5-Di-O-caffeoyl-3-O-succinoyl-4-O-maloylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| 1,5-Di-O-caffeoyl-4-O-succinoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008), Maruta et al. (1995) |
| Dimaloyl-dicaffeoylquinic acid isomer 3 | 70% ethanol | Tousch et al. (2014) |
| Maloyl-tricaffeoylquinic isomer | 70% ethanol | Tousch et al. (2014) |
| 1,5-Di-O-caffeoyl-3,4-di-O-succinoylquinic acid | 90% methanol | Jaiswal and Kuhnert (2011) |
| 1,3,5-Tri-O-caffeoyl-4-O-maloylquinic acid | 70% ethanol | Tousch et al. (2014) |
| 1,3,5-Tri-O-caffeoyl-4-O-succinoylquinic acid | Methanol | Jaiswal and Kuhnert (2011), Lin and Harnly (2008), Maruta et al. (1995) |
| 1,3,5-Tri-O-caffeoylquinic acid | 60% methanol | Lin and Harnly (2008) |
Beneficial effects of burdock extract on health
Herbal medicines are used to treat approximately 80% diseases worldwide. Some of these herbal plants contain different bioactive polyphenols, and have attracted attention for diabetes care (Saravanakumar et al., 2021). Phenols, flavonoids, alkaloids, and saponins are among the components in A. lappa root extract (Cao et al., 2012) and evidences from in vitro experimental studies suggest that phenolic compound especially CGA inhibits α-amylase and α-glucosidase activities in a dose-dependent manner (Cui et al., 2022). Moreover, significant suppression of blood glucose, total cholesterol, triglyceride, glutamic oxaloacetic transaminase, glutamic pyruvic transaminase, γ-glutamyl transferase, alkaline phosphatase, total bilirubin, creatinine, urea, uric acid, and feed intake levels were reported in diabetic rats upon treatment with chlorogenic acid for 28 days (Singh et al., 2021). Notably, administration of CGA was also showed to delay the development of other various chronic diseases (Liang and Kitts, 2015). Hence, CGA consumption may promote a broad range of health benefits and can have several biological functions in humans, as they can target the nervous system, cardiovascular system, gastrointestinal tract, as well as the kidneys, liver, muscles, and pancreas (Lu et al., 2020). Some recent reports have also demonstrated the antioxidant (Tomac et al., 2020), anti-inflammatory (Liang and Kitts, 2015), hepato-protective (Bazool Farhood et al., 2019), anticancer (Gouthamchandra et al., 2017; Kushwaha et al., 2021; Sadeghi Ekbatan et al., 2018), and anti-obesity (He et al., 2021; Yin et al., 2021) activities of CGA. Flavonoids also are polyphenolic compounds that scavenge free radicals and reduce diabetes complications (Song et al., 2013). A number of investigators have reported flavonoids as potent antioxidants and antidiabetic agents as well as the alkaloid content of plants potentially modulating insulin secretion. In addition, saponins have been found to reduce blood glucose levels (Patel et al., 2012). Therefore, A. lappa roots extracts are capable of improving beta cell function because they contain these components.
Convincing results from numerous studies have shown that fructooligosaccharide is an effective dietary protective compound that can successfully inhibit the activity of α-amylase and α-glucosidase enzyme, thereby reducing the blood glucose and bilirubin levels, while promoting creatinine excretion, and increasing blood urea nitrogen and uric acid as well as the high-density lipoprotein cholesterol levels (Singh et al., 2021). In the large profile of active compounds found in burdock's root, Sitosterol-beta-d-glucopyranoside is thought to be the most potent and effective component. It inhibits alpha glucosidase activity strongly. The enzymes involved in glycopeptide and glycogenolysis are alpha glucosidases. The inhibition of glycosidase is a potential treatment for DM and obesity (Chan et al., 2010). Also known as inulin, gamma-lucoside-fructose ester, assists in the regulation of blood glucose levels. Burdock root contains natural carbohydrates called inulin, which may be able to maintain blood glucose levels by acting on cell surface receptors. Silver and Krantz (1956) also reported an increase in short-chain fatty acid production. In a model of alloxan-induced diabetes in mice and rats, total lignan from burdock fruit has been shown to exert anti-diabetic activity. Burdock lignan has been demonstrated to be a safe and effective antidiabetic agent (Xu et al., 2008).
In vitro and in vivo protective effects of burdock
Polyphenols and fructooligosaccharides, the main components of burdock, are biologically active compounds that have several therapeutical effects and properties. Table 2 highlights the beneficial effects of burdock extract against DM, as demonstrated by in vitro and in vivo experimental studies. With regards to its health promoting attributes, the potential of burdock extract against fibrosis, cancer, and cardiovascular disorders has been clinically demonstrated. In particular, several studies conducted in the past few decades have demonstrated the positive significant effects of burdock fructooligosaccharide (BFO) against chronic diseases. In the following sections, we discuss the in vitro and in vivo antidiabetic effect of burdock.
Table 2.
In vitro and in vivo beneficial effects of chlorogenic acid from Arctium lappa
| No | Extract/compound | Experimental model | Analytical findings | References |
|---|---|---|---|---|
| 1 | Burdock leaf flavonoids | In vitro inhibition assay | α-Amilase (IC50: 92.01 μg/mL) and α-glucosidase (IC50: 29.49 μg/mL) inhibitions in a mixed-type manner | Cui et al. (2022) |
| 2 | Burdock fructooligosaccharide | NRK-52E cells | Protection against HG-induced damage by inhibiting apoptosis and oxidative stress via Nrf2/HO-1 signaling | Ding et al. (2021) |
| 3 | Hot water extract rich in fructooligosaccharide | Male, C57BL/6 J mice (n = 80) | Reduced FBG levels, body weight, and serum total triglyceride and cholesterol | Yuan et al. (2021) |
| 4 | Total lignans from Fructus arctii (250 and 125 mg/kg) for 11 weeks | KKAy type 2 diabetic and obese mice | Decreased FBG, HbA1c, and body weight; improved oral glucose tolerance; increased insulin secretion | Gao et al. (2018) |
| 5 | A. lappa ethanol and hexane extracts | In vitro inhibition assay | Inhibition of α‐glucosidase activity | Franco et al. (2018) |
| 6 | A. lappa hydro‐alcoholic extract (200 and 300 mg/kg) for 28 days | Diabetic mice | Reduced glycaemia (p < 0.001 for both 200 and 300 mg extracts); increased insulinemia (p < 0.05 for 200 mg extract); improved HOMA‐IR (p < 0.05 for 300 mg extract) | Ahangarpour et al. (2017) |
| 7 | A. lappa water extract (50 and 250 mg/kg/day) for 8 weeks | Mice | Decreased HFD‐induced weight gain and blood glucose levels | Bok et al. (2017) |
| 8 | Arctigenic acid (50 mg/kg) for 12 weeks | Goto‐Kakizaki type 2 diabetic mice | Decreased FBG and HbA1c; improved oral glucose tolerance | Xu et al. (2015) |
| 9 | Total lignans from Fructus arctii (300 mg/kg) for 12 weeks | Goto‐Kakizaki type 2 diabetic mice | Decreased blood glucose levels and HbA1c; improved glucose tolerance; stimulation of insulin and GLP‐1 release | Xu et al. (2014) |
| 10 | A. lappa root extract rich in caffeoylquinic acid derivatives | L6 myocytes | Increased glucose uptake | Tousch et al. (2014) |
| A. lappa root extract rich in caffeoylquinic acid derivatives | Hepatocytes from rats | Reduced glucose output induced by glucagon | ||
| Intraperitoneal and oral administration of dried A. lappa root extract rich in caffeoylquinic acid derivatives | Mice | Improved oral glucose tolerance | ||
| 11 | A. lappa n‐hexane extract | HepG2 cells | Activation of AMPK | Kuo et al. (2012) |
| 12 | Arctigenin | H9C2 and C2C12 cells | Promotion of AMPK phosphorylation | Tang et al. (2011) |
| 13 | Total lignans from Fructus arctii (2.0, 1.0, and 0.5 g/kg) for 10 day | Alloxan‐induced diabetic mice | Decreased blood glucose levels; increased plasma insulin levels | Xu et al. (2008) |
AMPK 5′ adenosine monophosphate‐activated protein kinase, FBG fasting blood glucose, GLP‐1 glucagon‐like peptide‐1, HbA1c glycated hemoglobin, HFD high-fat diet, HOMA‐IR homeostasis model assessment–insulin resistance
In vitro studies on diabetes mellitus
Carbohydrates are decomposed into glucose molecules due to the activity of α‐glucosidase; thus, inhibition of this process by bioactive compounds, such as phytochemicals, can help regulate the blood glucose levels in patients with DM. Sitosteol-beta-d-glycopyranoside is the main bioactive component of burdock that acts against α‐glucosidase and shows antidiabetic activity (Annunziata et al., 2019). Moreover, a recent in vitro study reported that burdock leaf extract can effectively inhibit the activity of α-amylase and α-glucosidase, and consequently impact on starch digestion (Cui et al., 2022). The study also demonstrated, by kinetic and spectroscopic experiments, that the compounds present in burdock can bind to both α-amylase and α-glucosidase, thus preventing the hydrolysis of glycogen into glucose. Notably, administration of only 4% burdock leaf extract significantly reduced the digestible starch.
Flavonoid compounds have been pointed out as being responsible for the antioxidant and other biological activities of burdock extract. Ferreres et al. (2012) suggested that kaempferol, myricetin, and quercetin derivatives are the main biologically active components in hydro-methanolic extracts of burdock leaves. In particular, these components were associated with the inhibition of α-amylase and lipase activities, along with antioxidant proprieties (Tan et al., 2017). Franco et al. (2018) showed that ethanol and hexane extracts of burdock have α‐glucosidase inhibitory activity (25.2 ± 1.1% and 20.8 ± 0.4%, respectively).
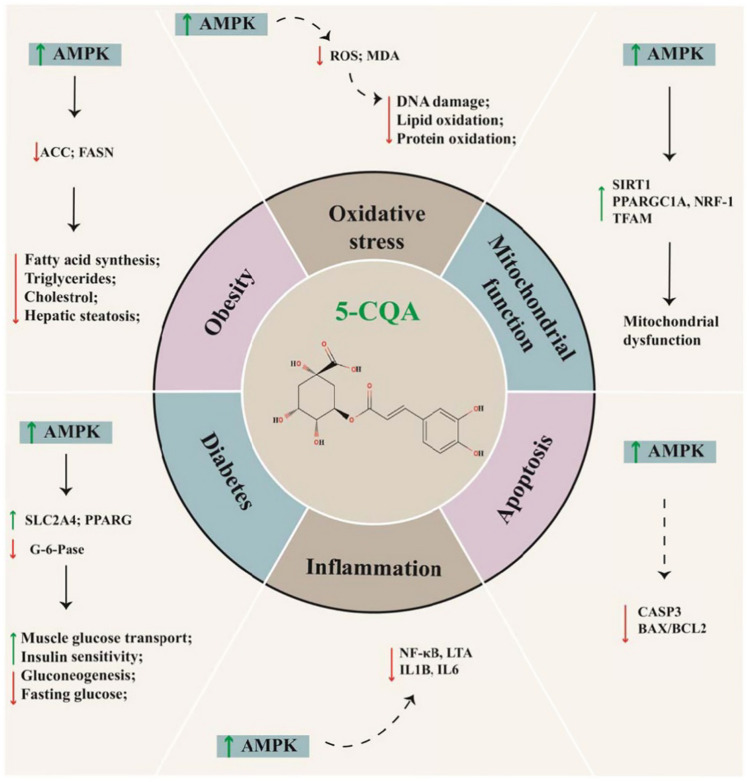
In addition to α‐glucosidase, regulation of the activity of 5′ adenosine monophosphate‐activated protein kinase (AMPK) has also been suggested as an important strategy for T2DM management (Mihaylova and Shaw, 2011). AMPK can inhibit the activity of several enzymes involved in anabolic processes, such as the glycerol‐3‐phosphate acyltransferase, which participates in triacylglycerols synthesis; 3‐hydroxy‐3‐methylglutaryl‐CoA reductase, involved in sterols synthesis; and acetyl‐CoA carboxylase 1, which contributes to fatty acid synthesis. Thus, AMPK inhibition results in enhanced activity of the glucose transporter (GLUT)‐1 and GLUT-4 translocation on cell membranes, which in turn promotes glucose uptake in the muscles (Mihaylova and Shaw, 2011). The putative mechanisms and effects of 5-CQA via activating AMPK signaling pathway has been shown in Fig. 2. According to reports, CGA affects only insulin-sensitive tissues such as skeletal muscles, livers and adipocytes. However, AMPK has been linked to the calcium-dependent protein kinase kinase-β (CaMKKβ), in addition to the calcium-dependent protein kinase and LKB-1 (Ong et al., 2012). In addition, arctigenin present in burdock extract can promote the activation of AMPK via Ca2+/calmodulin-mediated protein kinase- and liver kinase B1‐dependent pathways in in vitro rodent models of cardiomyocytes and muscle cells (Tang et al., 2011). Kuo et al. (2012) reported similar results in a human liver cancer cell line treated with A. lappa n‐hexane fraction. Interestingly, A. lappa root extract showed a significant impact on the glucose uptake in rat myocytes, reducing the glucose levels in rat hepatocytes (Tousch et al., 2014).
Fig. 2.
Putative mechanisms and effects of 5-CQA via activating AMPK signaling pathway. The phenolic compound 5-CQA activates AMPK, which contributes to main the energy and metabolic homeostasis through modulating the mechanisms above. AMPK, 5′-Adenosine monophosphate-activated protein kinase; ACC acetyl-CoA carboxylase, FASN fatty acid synthase, SLC2A4 solute carrier family 2, facilitated glucose transporter member 4, PPARG peroxisome proliferator-activated receptor gamma, G-6-Pase glucose-6-phosphatase, ROS reactive oxygen species, MDA malondialdehyde, NF-κB nuclear factor kappa, LTA lymphotoxin-alpha, also known as TNF-beta, IL1B interleukin-1 beta, IL6 interleukin-6, SIRT1 NAD-dependent protein deacetylase sirtuin 1, PPARGC1A peroxisome proliferator-activated receptor gamma coactivator 1-alpha, NRF-1 nuclear respiratory factor 1, TFAM transcription factor A, mitochondrial, CASP3 caspase-3; BAX, BCL2-associated X protein, BCL2 apoptosis regulator Bcl-2/B-cell lymphoma 2 (Lu et al., 2020)
In vivo studies on diabetes mellitus
Persistent hyperglycemia can damage the islet β cells due to continuous and excessive insulin secretion to reduce blood glucose, which in turn aggravates the hyperglycemic status and triggers DM development. Hence, chronic complications may occur under persistent hyperglycemia. 5-CQA has been shown to have beneficial effects against DM by promoting the uptake of glucose in the skeletal muscles (Yan et al., 2020). Moreover, fructooligosaccharide and high amounts of polyphenols, especially chlorogenic acid, present in burdock (Cui et al., 2022; Moro et al., 2022) were shown to significantly reduce fasting blood glucose (FBG) levels in male, diabetic C57BL/6 J mice upon 6 weeks of treatment (Yuan et al., 2021). Indeed, the FBG levels were significantly reduced from 23.97 ± 5.65 mmol/L before treatment to 19.77 ± 2.56 mmol/L after treatment in mice treated with a high concentration of burdock oligosaccharides (Yuan et al., 2021).
High glucose levels in the blood can significantly reduce cell viability; however, BFO was shown to protect kidney cells from apoptosis (Ding et al., 2021). Indeed, cell viability increased (63.16%, 72.97%, and 77.98% of the control value at high glucose) in the presence of increasing BFO concentrations (62.5, 125, and 250 μg/mL, respectively). Importantly, BFO protected the kidney cells from the oxidative damage induced by the high glucose levels by significantly inhibiting the production and stabilization of reactive oxygen species (Tsikas, 2017). Moreover, BFO can enhance the mitochondrial membrane potential, increase the levels of the antioxidant enzymes catalase and superoxide dismutase, and adjust the Bcl-2/Bax ratio, which are critical for the regulation of the antioxidant pathway and cell death.
The antidiabetic activity of A. lappa root extract was also evaluated in nicotinamide/streptozotocin (NA/STZ)-induced type 2 diabetes mice (Ahangarpour et al., 2017). Briefly, adult male, NMRI diabetic mice were pretreated with 200 and 300 mg/kg A. lappa root extract. Upon continuous treatment for 28 days, the blood glucose levels were significantly reduced regardless of the BFO dose administrated. In addition, BFO were shown to reduce the levels of triglycerides, very low density lipoproteins, and alkaline phosphatases in these diabetic mice. Furthermore, 200 mg/kg of BFO increased the insulin levels, whereas the levels of high-density lipoproteins and leptin increased upon administration of 300 mg/kg of the extract. The main mechanism is the ability of STZ to generate ROS and impair insulin production by beta cells (Patel et al., 2012). Inhibiting glucose absorption from the intestine is another anti-hyperglycemic mechanisms. However, increasing insulin production and pancreatic tissue function, A. lappa root extract may decrease the intestinal absorption of glucose (Ahangarpour et al., 2017).
Similar results were also reported by Bok et al. (2017). In this study, blood glucose levels were found to be significantly reduced in high-fat diet-induced diabetic rats after 8 weeks of treatment with 50 or 250 mg/kg/day of A. lappa water extract. In addition, due to high content of caffeoylquinic acid derivatives, A. lappa extract exerts anti‐hyperglycemic effects, both after acute (intraperitoneal) and subchronic (oral) administration (Tousch et al., 2014).
Among various complications, obesity due to DM is also a serious problem. Some investigations have suggested that A. lappa extract has the potential to treat both diabetes and obesity (Gao et al., 2018). Treatment with 125 and 250 mg/kg of A. lappa extract for 11 weeks was shown to promote significant reduction in fasting glycemia, HbA1c levels, and body weight, and improved oral glucose tolerance in KK-Ay rodent models of diabetes and obesity. In addition, the extract also activated several important cellular signals, such as the phosphatidylinositol 3-kinase/protein kinase B and AMPK signaling pathways (Gao et al., 2018). Arctigenin, which is also the main component present in A. lappa extract, has also been described as having antidiabetic proprieties. In particular, oral administration of arctigenic acid (50 mg/kg, twice daily for 12 weeks) in Goto‐Kakizaki rats was shown to stimulate insulin secretion and significantly reduce the blood glucose levels (Xu et al., 2015). It is known that arctigenin (2(3H)-furanone, 4-[(3,4-dimethoxyphenyl) methyl] dihydro-3-[(4-hydroxy-3-methoxyphenyl)methyl]-, (3R, 4R)-), the main component of lignan, could be hydrolyzed in vivo, resulting in arctigenic acid (AA). As with nateglinide, AA has an asymmetric carbon and a carboxyl group. Accordingly, AA is speculated to be the principal active metabolite of lignan with similar hypoglycemic properties to nateglinide. Possibly it is the key substance responsible for hypoglycemia (Xu et al., 2015).
In line with these findings, Cao et al. (2012) demonstrated that the administration of burdock root extract (200–400 mg/kg body weight) to STZ-induced diabetic rats induced antidiabetic effects in dose-dependent manner. The effectiveness of the burdock root extract was clearly demonstrated by reduced fasting blood glucose levels, along with beneficial effects on serum insulin, lipid profile, urea and creatinine, liver and skeletal muscle glycogen content, lipid peroxidation of the liver and kidney tissues, as well as by lowering the body weight. In addition, administration of 400 mg/kg of body weight of burdock root extract promoted an overall health outcome, similar to that observed in rats treated with a commercially available antidiabetic drug (glibenclamide, 2.5 mg/kg of body weight). Taken together, these results demonstrate that oral administration of burdock root extract holds potential for managing the underlying features of DM.
As a result of administering A. lappa root extract and glibenclamide, serum leptin might be elevated, but may decrease in the diabetic group. There are reports of reduced leptin production in diabetic animals, likely as a result of impaired glucose uptake and adipose tissue metabolism. In addition, insulin increases the serum level of leptin in adipocytes and facilitates glucose uptake and oxidation. According to some studies, A. lappa root extract increased serum insulin levels in diabetic mice, so perhaps its beneficial effects on insulin release from beta cells are related to its effect on leptin levels (Benhaddou-Andaloussi et al., 2011).
In conclusion, the active ingredients (isolated from different parts of the plant) found in burdock have been shown to be effective in treating a wide variety of conditions and numerous in vitro and in vivo evidences have suggested that A. lappa extract has the potential to impart therapeutic effect in diabetes due to the phytochemical content. Evidences also showed that burdock (A. lappa) extract contains inulin-type fructooligosaccharides, bioactive compounds such as chlorogenic acid, caffeic acid and its derivatives, tannins, saponins etc. which may exert positively on muscle glucose transport and insulin sensitivity rather than only inhibit the enzymatic activities. In addition, studying the acute toxicity of burdock root also revealed no toxic effects, which means the consumption of phenolic and flavone containing burdock root in foods and beverages or extract would be beneficial in alleviating diabetic complications. However, future investigations are still necessary to better understand the exact underlying antidiabetic mechanisms and activity pathways of the compounds present in the extracts. Moreover, as the activity of the bioactive compounds can be influenced by the solvent used during the extraction process, an optimal extraction method should be established to investigate the enhanced therapeutic activity of the antidiabetic compounds.
Acknowledgements
We would like to thanks Food Science and Biotechnology for its special issue “Polyphenols and Health” and giving us this opportunity to write this review. We did not receive any fund for this review.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shakti Chandra Mondal, Email: shakti.c.mondal@hstu.ac.bd.
Jong-Bang Eun, Email: jbeun@jnu.ac.kr.
References
- ADA (American Diabetes Association). 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2021. Diabetes Care. 44: S15-S33 (2021) [DOI] [PubMed]
- Ahangarpour A, Heidari H, Akbar Oroojan A, Mirzavandi F, Nasr Esfehani K, Dehghan Mohammadi Z, Kh E, Mohammadi DZ. Antidiabetic, hypolipidemic and hepatoprotective effects of arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna Journal of Phytomedicine. 2017;7:169. [PMC free article] [PubMed] [Google Scholar]
- Annunziata G, Barrea L, Ciampaglia R, Cicala C, Arnone A, Savastano S, Nabavi SM, Tenore GC, Novellino E. Arctium lappa contributes to the management of type 2 diabetes mellitus by regulating glucose homeostasis and improving oxidative stress: A critical review of in vitro and in vivo animal-based studies. Phytotherapy Research. 2019;33:2213–2220. doi: 10.1002/ptr.6416. [DOI] [PubMed] [Google Scholar]
- Barreto NMB, Pimenta NG, Braz BF, Freire AS, Santelli RE, Oliveira AC, Bastos LHP, Cardoso MHWM, Monteiro M, Diogenes MEL, Perrone D. Organic black beans (Phaseolus vulgaris L.) from Rio de Janeiro state, Brazil, present more phenolic compounds and better nutritional profile than nonorganic. Foods. 2021;10:900. doi: 10.3390/foods10040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazool Farhood H, Balas M, Gradinaru D, Margină D, Dinischiotu A. Hepatoprotective effects of chlorogenic acid under hyperglycemic conditions. Romanian Biotechnological Letters. 2019;24:301–307. doi: 10.25083/rbl/24.2/301.307. [DOI] [Google Scholar]
- Benhaddou-Andaloussi A, Martineau L, Vuong T, Meddah B, Madiraju P, Settaf A, Haddad PS. The in vivo antidiabetic activity of Nigella sativa is mediated through activation of the AMPK pathway and increased muscle Glut4 content. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9. doi: 10.1155/2011/538671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bento-Silva A, Duarte N, Mecha E, Belo M, Serra AT, Vaz Patto MC, Bronze MR. Broa, an ethnic maize bread, as a source of phenolic compounds. Antioxidants. 2021;10:672. doi: 10.3390/antiox10050672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok SH, Cho SS, Bae CS, Park DH, Park KM. Laboratory Animal Research Safety of 8-weeks oral administration of Arctium lappa L. Laboratory Animal Research. 2017;33:251–255. doi: 10.5625/lar.2017.33.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonphang O, Ontawong A, Pasachan T, Phatsara M, Duangjai A, Amornlerdpison D, Jinakote M, Srimaroeng C. Antidiabetic and renoprotective effects of Coffea arabica pulp aqueous extract through preserving organic cation transport system mediated oxidative stress pathway in experimental type 2 diabetic rats. Molecules. 2021;26:1907. doi: 10.3390/molecules26071907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Li C, Zhang P, Cao X, Huang T, Bai Y, Chen K. Antidiabetic effect of burdock (Arctium lappa L.) root ethanolic extract on streptozotocin-induced diabetic rats. African Journal of Biotechnology. 2012;11:9079–9085. [Google Scholar]
- Chan YS, Cheng LN, Wu JH, Chan E, Kwan YW, Lee SMY, Leung GPH, Yu PHF, Chan SW. A review of the pharmacological effects of Arctium lappa (burdock) Inflammopharmacology. 2010;19:245–254. doi: 10.1007/s10787-010-0062-4. [DOI] [PubMed] [Google Scholar]
- Cui J, Zeng S, Zhang C. Anti-hyperglycaemic effects of burdock (Arctium lappa L.) leaf flavonoids through inhibiting α-amylase and α-glucosidase. International Journal of Food Science & Technology. 2022;57:541–551. doi: 10.1111/ijfs.15026. [DOI] [Google Scholar]
- Deng YT, Lin-Shiau SY, Shyur LF, Lin JK. Pu-erh tea polysaccharides decrease blood sugar by inhibition of α-glucosidase activity in vitro and in mice. Food and Function. 2015;6:1539–1546. doi: 10.1039/C4FO01025F. [DOI] [PubMed] [Google Scholar]
- Ding M, Tang Z, Liu W, Shao T, Yuan P, Chen K, Zhou Y, Han J, Zhang J, Wang G. Burdock fructooligosaccharide attenuates high glucose-induced apoptosis and oxidative stress injury in renal tubular epithelial cells. Frontiers in Pharmacology. 2021;12:784187–784187. doi: 10.3389/fphar.2021.784187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez AL, Rodrigues LR, Lima NM, Teixeira JA. An overview of the recent developments on fructooligosaccharide production and applications. Food and Bioprocess Technology. 2014;7:324–337. doi: 10.1007/s11947-013-1221-6. [DOI] [Google Scholar]
- Ercan P, El SN. Inhibitory effects of chickpea and Tribulus terrestris on lipase, α-amylase and α-glucosidase. Food Chemistry. 2016;205:163–169. doi: 10.1016/j.foodchem.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Ferreres F, Gil-Izquierdo A, Vinholes J, Silva ST, Valentão P, Andrade PB. Bauhinia forficata link authenticity using flavonoids profile: relation with their biological properties. Food Chemistry. 2012;134:894–904. doi: 10.1016/j.foodchem.2012.02.201. [DOI] [PubMed] [Google Scholar]
- Franco RR, da Silva Carvalho D, de Moura FBR, Justino AB, Silva HCG, Peixoto LG, Espindola FS. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. Journal of Ethnopharmacology. 2018;215:140–146. doi: 10.1016/j.jep.2017.12.032. [DOI] [PubMed] [Google Scholar]
- Gao Y, Gu C, Wang K, Wang H, Ruan K, Xu Z, Feng Y. The effects of hypoglycemia and weight loss of total lignans from Fructus Arctii in KKAy mice and its mechanisms of the activity. Phytotherapy Research. 2018;32:631–642. doi: 10.1002/ptr.6003. [DOI] [PubMed] [Google Scholar]
- Gouthamchandra K, Sudeep HV, Venkatesh BJ, Shyam Prasad K. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Science and Human Wellness. 2017;6:147–153. doi: 10.1016/j.fshw.2017.06.001. [DOI] [Google Scholar]
- He X, Zheng S, Sheng Y, Miao T, Xu J, Xu W, Huang K, Zhao C. Chlorogenic acid ameliorates obesity by preventing energy balance shift in high-fat diet induced obese mice. Journal of the Science of Food and Agriculture. 2021;101:631–637. doi: 10.1002/jsfa.10675. [DOI] [PubMed] [Google Scholar]
- Herrera-Balandrano DD, Beta T, Cai Z, Zhang X, Li Y, Huang W. Effect of in vitro gastro-intestinal digestion on the phenolic composition and antioxidant capacity of burdock roots at different harvest time. Food Chemistry. 2021;358:129897. doi: 10.1016/j.foodchem.2021.129897. [DOI] [PubMed] [Google Scholar]
- Hussain F, Hafeez J. Therapeutic attributes of Stevia rebaudiana leaves in diabetic animal model plant sample collection and extract preparation. RADA Journal of Biological Research & Applied Sciences. 2021;12:1–7. [Google Scholar]
- Jaiswal R, Kuhnert N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food & Function. 2011;2:63–71. doi: 10.1039/C0FO00125B. [DOI] [PubMed] [Google Scholar]
- Juster-Switlyk K, Smith AG. Updates in diabetic peripheral neuropathy. F1000Research. 2016;5:738. doi: 10.12688/f1000research.7898.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YM, Jeong YK, Wang MH, Lee WY, Rhee HI. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition. 2005;21:756–761. doi: 10.1016/j.nut.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Kumar R, Sharma A, Iqbal MS, Srivastava JK. Therapeutic promises of chlorogenic acid with special emphasis on its anti-obesity property. Current Molecular Pharmacology. 2020;13:7–16. doi: 10.2174/1874467212666190716145210. [DOI] [PubMed] [Google Scholar]
- Kuo DH, Hung MC, Hung CM, Liu LM, Chen FA, Shieh PC, Ho CT, Way TD. Body weight management effect of burdock (Arctium lappa L.) root is associated with the activation of AMP-activated protein kinase in human HepG2 cells. Food Chemistry. 2012;134:1320–1326. doi: 10.1016/j.foodchem.2012.03.023. [DOI] [PubMed] [Google Scholar]
- Kushwaha PP, Kumar R, Neog PR, Behara MR, Singh P, Kumar A, Prajapati KS, Singh AK, Shuaib M, Sharma AK, Pandey AK, Kumar S. Characterization of phytochemicals and validation of antioxidant and anticancer activity in some Indian polyherbal ayurvedic products. Vegetos. 2021;34:286–299. doi: 10.1007/s42535-021-00205-1. [DOI] [Google Scholar]
- Li J, Cheong KL, Zhao J, Hu DJ, Chen XQ, Qiao CF, Zhang QW, Chen YW, Li SP. Preparation of inulin-type fructooligosaccharides using fast protein liquid chromatography coupled with refractive index detection. Journal of Chromatography A. 2013;1308:52–57. doi: 10.1016/j.chroma.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8:16. doi: 10.3390/nu8010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LZ, Harnly JM. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. Journal of Agricultural and Food Chemistry. 2008;56:10105–10114. doi: 10.1021/jf802412m. [DOI] [PubMed] [Google Scholar]
- Lordan S, Smyth TJ, Soler-Vila A, Stanton C, Paul Ross R. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chemistry. 2013;141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Lu H, Tian Z, Cui Y, Liu Z, Ma X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Comprehensive Reviews in Food Science and Food Safety. 2020;19:3130–3158. doi: 10.1111/1541-4337.12620. [DOI] [PubMed] [Google Scholar]
- Martina SJ, Govindan PAP, Wahyuni AS. The difference in effect of Arabica coffee gayo beans and leaf (Coffea Arabica Gayo) extract on decreasing blood sugar levels in healthy mice. Open Access Macedonian Journal of Medical Sciences. 2019;7:3363. doi: 10.3889/oamjms.2019.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruta Y, Kawabata J, Niki R. Antioxidative caffeoylquinic acid derivatives in the roots of burdock (Arctium lappa L.) Journal of Agricultural and Food Chemistry. 1995;43:2592–2595. doi: 10.1021/jf00058a007. [DOI] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biology. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CE, Tzounis X, Oruna-Concha MJ, Mottram DS, Gibson GR, Spencer JPE. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. British Journal of Nutrition. 2015;113:1220–1227. doi: 10.1017/S0007114514003948. [DOI] [PubMed] [Google Scholar]
- Mondal, S. C. and Eun, J. B. Extraction of phenolic compounds from burdock root waste using ultrasonication as a green technology. In: Abstract: The Korean Society of Food Science and Nutrition (KFN). Oct. 27-29, BEXCO, Busan, Korea (2021)
- Moro, T. de M. A., Pereira, A. P. A., Lopes, A. S., Pastore, G. M., & Clerici, M. T. P. S. Retention of bioactive compounds and bifidogenic activity of burdock roots subjected to different processes. International Journal of Gastronomy and Food Science. 27: 100448 (2022)
- Moser, M., Agemans, A., & Caers, W. Production and bioactivity of oligosaccharides from chicory roots. Food oligosaccharides: production, Analysis and Bioactivity. 55-75 (2014)
- Mussatto SI, Mancilha IM. Non-digestible oligosaccharides: A review. Carbohydrate Polymers. 2007;68:587–597. doi: 10.1016/j.carbpol.2006.12.011. [DOI] [Google Scholar]
- Nwafor EO, Lu P, Zhang Y, Liu R, Peng H, Xing B, Liu Y, Li Z, Zhang K, Zhang Y, Liu Z. Chlorogenic acid: potential source of natural drugs for the therapeutics of fibrosis and cancer. Translational Oncology. 2022;15:101294. doi: 10.1016/j.tranon.2021.101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KW, Hsu A, Tan BKH. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: a contributor to the beneficial effects of coffee on diabetes. PLOS ONE. 2012;7:e32718. doi: 10.1371/journal.pone.0032718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Andrade RR, García-Jiménez S, Castillo-España P, Ramírez-Ávila G, Villalobos-Molina R, Estrada-Soto S. α-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. Journal of Ethnopharmacology. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pacific Journal of Tropical Biomedicine. 2012;2:320–330. doi: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkova N, Hambarlyiska I, Tumbarski Y, Vrancheva R, Raeva M, Ivanov I. Phytochemical composition and antimicrobial properties of burdock (Arctium lappa L.) roots extracts. Biointerface Research in Applied Chemistry. 2022;12:2826–2842. [Google Scholar]
- Plazas M, Andujar I, Vilanova S, Hurtado M, Gramazio P, Herraiza FJ, Prohens J. Breeding for chlorogenic acid content in eggplant: interest and prospects. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2013;41:26–35. doi: 10.15835/nbha4119036. [DOI] [Google Scholar]
- Raskar V, Bhalekar MR. Formulation of coffee bean extract (Chlorogenic Acid) solid lipid nanoparticles for lymphatic uptake on oral administration. Journal of Drug Delivery and Therapeutics. 2019;9:477–484. doi: 10.22270/jddt.v9i4-A.3444. [DOI] [Google Scholar]
- Rubel IA, Pérez EE, Genovese DB, Manrique GD. In vitro prebiotic activity of inulin-rich carbohydrates extracted from Jerusalem artichoke (Helianthus tuberosus L.) tubers at different storage times by Lactobacillus paracasei. Food Research International. 2014;62:59–65. doi: 10.1016/j.foodres.2014.02.024. [DOI] [Google Scholar]
- Sadeghi Ekbatan S, Li X-Q, Ghorbani M, Azadi B, Kubow S. Molecular sciences chlorogenic acid and its microbial metabolites exert anti-proliferative effects, S-Phase cell-cycle arrest and apoptosis in human colon cancer caco-2 cells. International Journal of Molecular Sciences. 2018;19:723. doi: 10.3390/ijms19030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., Colagiuri, S., Guariguata, L., Motala, A. A., Ogurtsova, K., Shaw, J. E., Bright, D., & Williams, R. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice. 157: 107843 (2019) [DOI] [PubMed]
- Sangeetha PT, Ramesh MN, Prapulla SG. Fructooligosaccharide production using fructosyl transferase obtained from recycling culture of Aspergillus oryzae CFR 202. Process Biochemistry. 2005;40:1085–1088. doi: 10.1016/j.procbio.2004.03.009. [DOI] [Google Scholar]
- Saravanakumar K, Park S, Sathiyaseelan A, Kim KN, Cho SH, Vijaya A, Mariadoss A, Wang MH, Sathiyaseelan S, Kim A, Cho KN, Mariadoss SH, Wang AVA, Oszmianski J. Metabolite profiling of methanolic extract of Gardenia jaminoides by LC-MS/MS and GC-MS and its anti-diabetic, and anti-oxidant activities. Pharmaceuticals. 2021;14:102. doi: 10.3390/ph14020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Igarashi M, Yamada S, Takahashi N, Watanabe K. Inhibitory effect of black tea and its combination with acarbose on small intestinal α-glucosidase activity. Journal of Ethnopharmacology. 2015;161:147–155. doi: 10.1016/j.jep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Silver AA, Krantz JC. The effect of the ingestion of burdock root on normal and diabetic individuals. Southern Medical Journal. 1956;49:1086. doi: 10.1097/00007611-195609000-00026. [DOI] [Google Scholar]
- Singh AK, Rana HK, Singh V, Chand Yadav T, Varadwaj P, Pandey AK. Evaluation of antidiabetic activity of dietary phenolic compound chlorogenic acid in streptozotocin induced diabetic rats: molecular docking, molecular dynamics, in silico toxicity, in vitro and in vivo studies. Computers in Biology and Medicine. 2021;134:104462. doi: 10.1016/j.compbiomed.2021.104462. [DOI] [PubMed] [Google Scholar]
- Song Y, Manson JE, Buring JE, Sesso HD, Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. Journal of the American College of Nutrition. 2013;24:376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- Stefanov, S. M., Fetzer, D. E. L., de Souza, A. R. C., Corazza, M. L., Hamerski, F., Yankov, D. S., & Stateva, R. P. Valorization by compressed fluids of arctium lappa seeds and roots as a sustainable source of valuable compounds. Journal of CO2 Utilization. 101821 (2022)
- Tan Y, Chang SKC, Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry. 2017;214:259–268. doi: 10.1016/j.foodchem.2016.06.100. [DOI] [PubMed] [Google Scholar]
- Tang SCW, Lai KN. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrology Dialysis Transplantation. 2012;27:3049–3056. doi: 10.1093/ndt/gfs260. [DOI] [PubMed] [Google Scholar]
- Tang X, Zhuang J, Chen J, Yu L, Hu L, Jiang H, Shen X. Arctigenin efficiently enhanced sedentary mice treadmill endurance. PLOS ONE. 2011;6:e24224. doi: 10.1371/journal.pone.0024224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomac I, Šeruga M, Labuda J. Evaluation of antioxidant activity of chlorogenic acids and coffee extracts by an electrochemical DNA-based biosensor. Food Chemistry. 2020;325:126787. doi: 10.1016/j.foodchem.2020.126787. [DOI] [PubMed] [Google Scholar]
- Tousch D, Bidel LPR, Cazals G, Ferrare K, Leroy J, Faucanié MF, Chevassus H, Tournier M, Lajoix A-D, Azay-Milhau J. Chemical analysis and antihyperglycemic activity of an original extract from burdock root (Arctium lappa) Journal of Agricultural and Food Chemistry. 2014;62:7738–7745. doi: 10.1021/jf500926v. [DOI] [PubMed] [Google Scholar]
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Analytical Biochemistry. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- Upadhyay R, Mohan Rao LJ. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Critical Reviews in Food Science and Nutrition. 2013;53:968–984. doi: 10.1080/10408398.2011.576319. [DOI] [PubMed] [Google Scholar]
- Wang, R., Ayano, H., Furumoto, T., Kondo, A., & Fukui, H. Variation of the content of chlorogenic acid derivatives among cultivars and market items of burdock (Arctium lappa L.). In Nippon Shokuhin Kagaku Kogaku Kaishi. 48: 857-862 (2001)
- Xu Z, Gu C, Wang K, Ju J, Wang H, Ruan K, Feng Y. Arctigenic acid, the key substance responsible for the hypoglycemic activity of Fructus Arctii. Phytomedicine. 2015;22:128–137. doi: 10.1016/j.phymed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- Xu Z, Ju J, Wang K, Gu C, Feng Y. Evaluation of hypoglycemic activity of total lignans from Fructus Arctii in the spontaneously diabetic Goto-Kakizaki rats. Journal of Ethnopharmacology. 2014;151:548–555. doi: 10.1016/j.jep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang X, Zhou M, Ma L, Deng Y, Zhang H, Zhao A, Zhang Y, Jia W. The antidiabetic activity of total lignan from Fructus Arctii against alloxan-induced diabetes in mice and rats. Phytotherapy Research. 2008;22:544–549. doi: 10.1002/ptr.2406. [DOI] [PubMed] [Google Scholar]
- Yan, Y., Zhou, X., Guo, K., Zhou, F., & Yang, H. Use of chlorogenic acid against diabetes mellitus and its complications. Journal of Immunology Research. 2020 (2020) [DOI] [PMC free article] [PubMed]
- Yin P, Xie S, Zhuang Z, Fang H, Tian L, Liu Y, Niu J. Chlorogenic acid improves health in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets: Involvement of lipid metabolism, antioxidant ability, inflammatory response, and intestinal integrity. Aquaculture. 2021;545:737169. doi: 10.1016/j.aquaculture.2021.737169. [DOI] [Google Scholar]
- Yuan, P. chuan, Shao, T. li, Han, J., Liu, C. yan, Wang, G. dong, He, S. guang, Xu, S. xia, Nian, S. hui, & Chen, K. shan. Burdock fructooligosaccharide as an α-glucosidase inhibitor and its antidiabetic effect on high-fat diet and streptozotocin-induced diabetic mice. Journal of Functional Foods. 86: 104703 (2021)
- Zhang, X., Fang, Z., Zhang, C., Xia, H., Jie, Z., Han, X., Chen, Y., & Ji, L. Effects of acarbose on the gut microbiota of prediabetic patients: a randomized, double-blind, controlled crossover trial. Diabetes Therapy. 8: 293-307 (2017) [DOI] [PMC free article] [PubMed]