Abstract
Phenolic compounds in common buckwheat sprouts (CBSs) have gained research interest because of their multiple health benefits. Phenolic acids, flavanones, flavonols, flavan-3-ols, and anthocyanins are important bioactive components of CBS that exhibit biological activities, including anti-inflammatory, antioxidant, anti-proliferative, and immunomodulatory effects. The isolation and quantitative and qualitative analyses of these phenolic compounds require effective and appropriate extraction and analytical methods. The most recent analytical method developed for determining the phenolic profile is HPLC coupled with a UV–visible detector and/or MS. This review highlights the extraction, purification, analysis, and bioactive properties of phenolic compounds from CBS described in the literature.
Keywords: Common buckwheat sprouts, Phenolic compounds, Extraction, Analytical methods, Biological activity
Introduction
Sprouts, formed from germinated seeds, are among the most important vegetable foods rich in bioactive compounds, including flavonoids, phenolic acids, vitamins, and minerals (Mun et al., 2020). Additionally, sprouts are a source of functional ingredients. Because consumer perceptions of healthy foods have led to increased consumption of sprouts, various sprout-related products have emerged in the global food market. Sprouting is of interest as an effective strategy that allows rapid cultivation as well as manipulation of phenolic compounds in seeds (Jang et al., 2021). Buckwheat (Fagopyrum spp.) is a member of the Polygonaceae family mainly consumed as a pseudo cereal; the main buckwheat species are common buckwheat (F. esculentum Möench) and tartary buckwheat (F. tataricum Gaertner). Buckwheat sprouts are globally consumed as salads and fresh vegetables (Kim et al., 2004).
An increasing number of chemical, epidemiological, and pharmaceutical studies have shown that consumption of sprouts as edible plants is associated with improved health benefits (Guo et al., 2018; Huda et al., 2021). These beneficial effects, including antioxidant, antitumor, and anti-inflammatory effects, have been partially attributed to the action of bioactive compounds in buckwheat sprouts, which contain flavonoids and phenolic acids (Ishii et al., 2008; Thangaraj et al., 2019; Xiao et al., 2021). The representative phenolic compound in tartary buckwheat sprouts is quercetin-3-O-rutinoside (rutin), whereas various flavonoids including rutin and phenolic acids are detected in common buckwheat sprouts (CBSs) (Nam et al., 2015). Four C-glycosyl flavones (orientin, isoorientin, vitexin, and isovitexin) and two flavonols (rutin and quercetin-3-O-robinobioside) are the predominant flavonoids in CBSs (Horbowicz et al., 2013). CBS extracts exhibited inhibitory effects on the production of cytokines, including interleukin- (IL-) 6, IL-12, and tumor necrosis factor-α (TNF-α) in lipopolysaccharide (LPS)-induced peritoneal macrophages isolated from BALB/c mice (Nam et al., 2017). Ajaghaku et al. (2018) reported that a mixture of rutin and quercetin-3-O-robinobioside enhanced immune function through the upregulation of CD4+ T-lymphocytes. In another study, yeast-fermented CBS showed strong anti-hyperlipidemic and antioxidant activities in a mouse model (Chen et al., 2020).
Considering the numerous health benefits of phenolic compounds in CBSs, the development of effective techniques for their efficient recovery/extraction and accurate determination is essential. Buckwheat extracts, including those of CBSs, usually contain a complex mixture of phenolic compounds, which are mainly flavonoids, phenolic acids, and their derivatives (Huda et al., 2021). Phenolic compounds in plants usually occur in both free and bound forms. Free phenolics are usually extracted from plant samples with organic solvents (e.g., methanol, ethanol, and acetone), whereas bound phenolics are usually isolated using acid and alkaline hydrolysis followed by solvent extraction. The isolated phenolics are generally analyzed using LC-coupled with various detection methods to determine their specific identities and/or concentrations (Dzah et al., 2020a; Kumar, 2017).
CBSs are considered rich sources of bioactive compounds, and have received continuous research attention. Although CBSs contain many phytochemicals that can confer health benefits, including vitamins, proteins, fibers, minerals, and polysaccharides, we focused specifically on phenolic compounds. This review provides a general overview of the types of phenolic compounds present in CBSs and their potential health effects, as well as the approaches applied for analytical sample preparation (material pre-treatments, extraction, and purification methods) and phenolics determination. It thus organizes and provides relevant information to assist researchers in comparing and selecting suitable methods for the future development of an effective and efficient procedure for the analysis of phenolic compounds from CBSs.
Methodology
“Buckwheat sprouts” was used as the keyword for an extensive literature search. The published literature selected for data analysis in this review included experimental studies that analyzed phenolic compounds from CBSs or germinated buckwheat seeds. Review articles, meeting abstracts, proceedings papers, corrections, and experimental studies that characterized only total phenolics, total flavonoids, or other non-phenolic bioactive compounds were excluded. The following information was verified as a criterion for data analysis: CBS part, specific name class of the phenolic compound, analytical sample preparation technique and determination methods used for the analysis of phenolic compounds.
Phenolics in common buckwheat sprouts
CBSs are a rich source of phenolic compounds. Generally, sprouts are consumed as ready-to-eat salad vegetables and as fresh vegetables with flour-based noodles (Nam et al., 2015). CBSs play a critical role in human diets because of their bioactive compounds. Phenolic compounds, categorized as flavonoids, phenolic acids, stilbenes, and tannins, consist of at least one aromatic ring with one or more hydroxyl groups (Jung et al., 2021). Phenolic compounds are concentrated in the leaf, stem, and root portions during sprouting (Abdel-Aty et al., 2021; Lim et al., 2021). Flavonoids and phenolic acids were chosen as the representative phenolic compounds reported in this study. Details of the individual phenolic compounds identified and the contents of these compounds are summarized in Table 1.
Table 1.
Phenolic compounds reported in common buckwheat sprouts
| No | Compounds | Cultivar | Concentrations (mg/g dry weight) | References |
|---|---|---|---|---|
| Flavone | ||||
| 1 | Orientin | Kitawase | 1.5 | Nam et al. (2018a) |
| Kitawase | 6.99 | Lee et al. (2014) | ||
| – | 6.63 | Jang et al. (2019) | ||
| Hruszowska (cotyledons) | 8.18 | Wiczkowski et al. (2014) | ||
| – | 5.15 | Chen et al. (2020) | ||
| Hruszowska | 5.28 | Horbowicz et al. (2013) | ||
| 2 | Isoorientin | Kitawase | 3.2 | Nam et al. (2018a) |
| Kitawase | 8.13 | Lee et al. (2014) | ||
| – | 12.63 | Jang et al. (2019) | ||
| Hruszowska (cotyledons) | 11.82 | Wiczkowski et al. (2014) | ||
| – | 6.86 | Chen et al. (2020) | ||
| Hruszowska | 16.38 | Horbowicz et al. (2013) | ||
| 3 | Vitexin | Kitawase | 2.0 | Nam et al. (2018a) |
| Kitawase | 5.05 | Lee et al. (2014) | ||
| – | 5.90 | Jang et al. (2019) | ||
| Hruszowska (cotyledons) | 6.25 | Wiczkowski et al. (2014) | ||
| – | 1.63 | Chen et al. (2020) | ||
| Hruszowska | 4.89 | Horbowicz et al. (2013) | ||
| 4 | Isovitexin | Kitawase | 2.4 | Nam et al. (2018a) |
| Kitawase | 5.70 | Lee et al. (2014) | ||
| – | 10.48 | Jang et al. (2019) | ||
| Hruszowska (cotyledons) | 14.08 | Wiczkowski et al. (2014) | ||
| – | 5.28 | Chen et al. (2020) | ||
| Hruszowska | 10.66 | Horbowicz et al. (2013) | ||
| 5 | Apigenin | – | 0.1 (FW) | Almuhayawi et al. (2021) |
| Flavonol | ||||
| 6 | Quercetin | Hruszowska (cotyledons) | 0.15 | Wiczkowski et al. (2014) |
| – | 0.33 | Chen et al. (2020) | ||
| – | 0.05 | Park et al. (2017) | ||
| – | 0.8 (FW) | Almuhayawi et al. (2021) | ||
| 7 | Quercetin-3-O-robinobioside | Kitawase | 0.9a | Nam et al. (2018a) |
| – | 3.5a | Jang et al. (2019) | ||
| Hruszowska (cotyledons) | 2.94 | Wiczkowski et al. (2014) | ||
| Hruszowska | 1.57a | Horbowicz et al. (2013) | ||
| 8 | Rutin | Kitawase | 2.0 | Nam et al. (2018a) |
| – | 9.98 | Jang et al. (2019) | ||
| 2.94 | Wiczkowski et al. (2014) | |||
| 7.19 | Chen et al. (2020) | |||
| Hruszowska (cotyledons) | 7.60 | Ren and Sun (2014) | ||
| Hruszowska | 7.46 | Horbowicz et al. (2013) | ||
| 9 | Isoquercetin | – | 0.04 (FW) | Almuhayawi et al. (2021) |
| Flavan-3-ol | ||||
| 10 | Catechin | – | 0.97 (FW) | Almuhayawi et al. (2021) |
| – | 0.06 | Park et al. (2019) | ||
| – | 0.09 | Park et al. (2017) | ||
| 11 | Epicatechin | – | 0.04 | Park et al. (2019) |
| – | 0.05 | Park et al. (2017) | ||
| Hruszowska (cotyledons) | 0.4 | Wiczkowski et al. (2014) | ||
| Anthocyanins | ||||
| 12 | Cyanidin-3-O-galactoside | Kitawase | 0.02 | Kim et al. (2007) |
| Hruszowska (hypocotyls) | 0.11 | Wiczkowski et al. (2014) | ||
| 13 | Cyanidin 3-O-galactopyranosyl-rhamnoside | Kitawase | 0.1 | Kim et al. (2007) |
| Hruszowska (cotyledons) | 0.55 | Horbowicz et al. (2015) | ||
| Hruszowska (hypocotyls) | 0.81 | Horbowicz et al. (2015) | ||
| Hruszowska (cotyledons) | 0.49 | Wiczkowski et al. (2014) | ||
| Hruszowska (hypocotyls) | 1.57 | Wiczkowski et al. (2014) | ||
| 14 | Cyanidin-3-O-glucoside | Nepal native | 0.01 | Kim et al. (2007) |
| Hruszowska (hypocotyls) | 0.05 | Wiczkowski et al. (2014) | ||
| 15 | Cyanidin-3-O-rutinoiside | Nepal native | 0.02 | Kim et al. (2007) |
| Hruszowska (cotyledons) | 0.07 | Wiczkowski et al. (2014) | ||
| Hruszowska (hypocotyls) | 0.11 | Wiczkowski et al. (2014) | ||
| Hruszowska (cotyledons) | 0.08 | Horbowicz et al. (2015) | ||
| Hruszowska (hypocotyls) | 0.07 | Horbowicz et al. (2015) | ||
| Phenolic acids | ||||
| 16 | Chlorogenic acid | Kitawase | 2.76 | Lee et al. (2014) |
| Kitawase | 1.1 | Kim et al. (2008) | ||
| – | 0.12 | Park et al. (2017) | ||
| – | 0.13 | Zhang et al. (2015) | ||
| – | 0.06 | Park et al. (2019) | ||
| 17 | Caffeic acid | – | 0.02 | Park et al. (2017) |
| – | 0.006 | Zhang et al. (2015) | ||
| – | 0.08 | Park et al. (2019) | ||
| – | 0.8 | Almuhayawi et al. (2021) | ||
| 18 | Syringic acid | – | 0.007 | Zhang et al. (2015) |
| 19 | p-Coumaric acid |
– – |
0.006 1.2 (FW) |
Zhang et al. (2015) |
| 20 | Ferulic acid |
– – |
0.006 0.34 (FW) |
Zhang et al. (2015) |
| 21 | Sinapic acid | – | 0.003 | Zhang et al. (2015) |
| 22 | Gallic acid | – | 0.006 | Zhang et al. (2015) |
| – | 0.006 | Park et al. (2019) | ||
| – | 0.02 (FW) | Almuhayawi et al. (2021) | ||
| 23 | Ellagic acid | – | 0.08 (FW) | Almuhayawi et al. (2021) |
FW fresh weight; –: not available
aConcentration of quercetin-3-O-robinobioside is described as rutin equivalent
Flavonoids are plant secondary metabolites and an important group of phenolic compounds that are known to be strong antioxidants due to their high redox potential (Jung et al., 2021). Some flavonoids, including flavones, flavonols, flavan-3-ols, and anthocyanins, have been identified in CBSs (Table 1). Their concentrations vary depending on the cultivar, light conditions, and period of growth. Four C-glycosyl flavones, orientin, isoorientin, vitexin, and isovitexin are the predominant flavones detected in CBSs. In contrast, rutin is the most abundant flavonoid identified in tartary buckwheat sprouts (Nam et al., 2015). The concentrations of isoorientin and isovitexin in CBSs were in the range 3.2–16.38 mg/g dry weight (DW) and 2.4–14.08 mg/g DW, respectively (Chen et al., 2020; Horbowicz et al., 2013; Jang et al., 2019; Lee et al., 2014; Nam et al., 2015; Wiczkowski et al., 2014). Wiczkowski et al. (2014) reported that orientin, isoorientin, vitexin, and isovitexin are major flavones in CBSs, with their highest content observed in cotyledons. These flavones were present in traces or not detected in tartary buckwheat sprouts (Nam et al., 2015). However, although the level of rutin in CBSs was determined to be lower than that in tartary buckwheat sprouts (Kim et al., 2008; Nam et al., 2017), rutin was also an abundant flavonol in CBSs, with a concentration in the range 2.0–9.98 mg/g DW. Quercetin-3-O-robinobioside is a rutin epimer present in rooibos tea, saskatoon fruit, and CBSs (Beelders et al., 2012; Nam et al., 2015; Ozga et al., 2007). As quercetin-3-O-robinobioside elutes slightly faster than rutin in reversed-phase HPLC analysis, it is essential to achieve their optimum separation to distinguish the epimers. Quercetin-3-O-robinobioside occurs at a concentration of 0.9–2.94 mg/g DW in CBSs, whereas it was not detected in tartary buckwheat sprouts (Nam et al., 2015). Almuhayawi et al. (2021) have isolated 0.8 mg/g fresh weight (FW) of quercetin and isoquercetin (0.04 mg/g FW) from CBS extracts. Two flavan-3-ols, catechin and epicatechin, were also isolated from CBSs, although their concentrations were lower than those of other flavonoids (Almuhayawi et al., 2021; Park et al., 2017; Wiczkowski et al., 2014). Several researchers have also identified and quantified anthocyanins, which are water-soluble pigments usually present in fruits and vegetables, from CBSs. Kim et al. (2007) reported that cyanidin-3-O-galactoside, cyanidin-3-O-galactopyranosyl-rhamnoside, cyanidin-3-O-glucoside, and cyanidin-3-O-rutinoiside are the major anthocyanins in CBS. The highest contents of cyanidin-3-O-rutinoiside and cyanidin-3-O-galactopyranosyl-rhamnoside were detected in cv. Hruszowska (Wiczkowski et al., 2014). The concentrations of anthocyanins in the hypocotyls were higher than those in cotyledons (Horbowicz et al., 2013). The accumulation of anthocyanins during sprouting has been shown to strongly depend on light emission as an essential stimulus (Seo et al., 2015).
The phenolic acids isolated from CBSs were chlorogenic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, sinapic acid, gallic acid, and ellagic acid. Chlorogenic acid was the most abundant phenolic acid identified in CBSs, with a concentration in the range 0.06–2.76 mg/g DW (Kim et al., 2008; Lee et al., 2014; Park et al., 2017, 2019; Zhang et al., 2015), whereas only trace amounts of other phenolic acids were detected in CBS (Almuhayawi et al., 2021; Park et al., 2017, 2019; Zhang et al., 2015). In general, phenolic acids occur at a lower amount in CBSs than flavonoids.
Analytical sample preparation and determination of phenolic compounds in CBS
CBSs contain abundant amounts of bioactive phenolic compounds, particularly flavonoids and phenolic acids, which confer potential health benefits (Huda et al., 2021). Flavonoids, including orientin, isoorientin, vitexin, isovitexin, rutin, and quercetin-3-O-robinobioside, have been reported as the major phenolic compounds in CBSs (Table 1). For optimal utilization of the biological properties of flavonoids and other phenolic compounds from CBSs, it is essential to develop efficient methods for analytical sample preparation (extraction and purification), identification, and quantification of phenolic compounds. Studies in the past decade (Tables 2 and 3) have employed various sample preparation procedures and analytical techniques for qualitative and quantitative analysis of specific phenolic compounds in CBSs. This section discusses some of the advances made in methods to extract/recover and determine the free and bound specific phenolic compounds from CBSs.
Table 2.
Various sample preparation methods for the analysis of phenolics from common buckwheat sprouts
| Phenolicsa | Analytical sample preparationb | Purificationb | Analytical instrument c | References |
|---|---|---|---|---|
| Orientin, isoorientin, vitexin and rutin | 0.1 g sample + 1 mL 80% EtOH (3 h shaking) → centrifuged (10,000 g, 10 min) → supernatant was collected | UFLC | Lim et al. (2012) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin and Q-Gal-Rha | 0.05 g sample + 1 mL 60% MeOH containing 0.4% TFA → 0.5 min UAE and 0.5 min vortexed (2x) → centrifuged (5,000 g at 4 °C) → supernatant was collected → re-extraction of residue (5 × with same procedure) | HPLC–UV | Horbowicz et al. (2013) | |
| Orientin, isoorientin, vitexin, isovitexin and rutin |
(1) Analysis of rutin: sample + 50% acetone (2 h agitation) → suction filtration → evaporated → dried residue used (2) Analysis of other flavonoids: sample + 50% MeOH (2 h agitation) → centrifuged (10,000 rpm, 15 min) → supernatant was collected → re-extraction of residue (1 × with same procedure) → pooled supernatants were dried |
CEUV HPLC–UV HPLC–MS |
Koyama et al. (2013) | |
| Rutin | 200 mg sample + 40 mL 80% EtOH (16 h stirring, 40 °C) → centrifuged (8,000 g, 10 min) → filtered | HPLC–UV | Tsurunaga et al. (2013) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin, quercetin and chlorogenic acid | 10 mg sample + 1 mL MeOH containing 10% phosphoric acid → vortexed 5 min → incubated (3 h at 37 °C) → centrifuged (1,000 g, 5 min) and filtered | HPLC–UV | Arasu et al. (2014) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin, quercetin and chlorogenic acid | 10 mg sample + 1 mL MeOH containing 10% phosphoric acid → vortexed 5 min → incubated (3 h at 37 °C) → centrifuged (10,000 g, 5 min) and filtered | HPLC–UV | Kim et al. (2014b) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin, quercetin and chlorogenic acid | 10 mg sample + 1 mL MeOH containing 10% phosphoric acid → vortexed 5 min → incubated (3 h at 37 °C) with 0.5 min vortexing every 1 h → centrifuged (1,000 g, 5 min) and filtered | HPLC–UV | Lee et al. (2014) | |
| Rutin and quercetin | 1 g sample + 95% EtOH (1:20 w/v) → 6 h heating (70 °C) → vacuum filtered, dissolved in 95% EtOH, and stored (4 °C) | HPLC–UV | Ren and Sun (2014) | |
| Cy-Gal, Cy-Glu, Cy-Rut, Cy-Gal-Rha, orientin, isoorientin, vitexin, isovitexin, rutin, Q-Gal-Rha, Q-Glu, quercetin, epicatechin and procyanidin B2 | 0.05 g sample + 1 mL 60% MeOH containing 0.4% → TFA 0.5 min UAE and 0.5 min vortexed (2x) → centrifuged (13,200 g at 4 °C, 10 min) → supernatant was collected → re-extraction of residue (5 × with same procedure) → pooled supernatants were centrifuged (13,200 g at 4 °C, 20 min) |
HPLC–DAD HPLC–MS HPLC–CoulArray–ECD |
Wiczkowski et al. (2014) | |
| Cy-Gal, Cy-Glu, Cy-Gal-Rha, Cy-Glu-Rha, orientin, isoorientin, vitexin, isovitexin, rutin and Q-Gal-Rha | 0.05 g sample + 1 cm3 60% MeOH containing 0.4% TFA → 0.5 min UAE and 0.5 min vortexed (2x) → centrifuged (5,000 g at 4 °C, 5 min) → supernatant was collected → re-extraction of residue (5 × with same procedure) → pooled supernatants were centrifuged (5,000 g at 4 °C, 15 min) |
HPLC–DAD HPLC–MS |
Horbowicz et al. (2015) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin | 15 g sample + 150 mL 70% EtOH (48 h soaking) → filtered → re-extraction of residue (2 × with same procedure) → filtered solvent was pooled → evaporated → 840 mg obtained extract was dissolved with 40 mL water and 80 mL EtOAc/n-BuOH (3:1) → 250 mg EtOAc/n-BuOH fractionated extract was collected after separation and evaporation (2x) → suspended in 20 mL water and 40 mL EtOAc → aqueous fraction was collected and dried → 70 mg dried extract was obtained and stored (4 °C) |
HPLC–PDA HPLC–Q–TOF/MS |
Nam et al. (2015) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin, K3R, quercitrin, myricetin, luteolin, quercetin, kaempferol, gallic acid, DHBA, THBA, p-HBA, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, ferulic acid, sinapic acid and THCA | 3 g sample + 50 mL 70% MeOH (1 h UAE) → filtered and concentrated to 10 mL → filtered |
HPLC–UV HPLC–MS |
Zhang et al. (2015) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin, Q-Gal-Rha, Cy-Gal-Rha and Cy-Glu-Rha | Sample + 60% MeOH containing 0.4% TFA (1:4) | HPLC–UV | Dębski et al. (2016) | |
| Rutin | Sample + 50% MeOH → lyophilized → dissolved in 1 mL 10% aqueous acetonitrile with 0.1% formic acid | HPLC–UV | Nakamura et al. (2016) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin, apigenin, quercetin, luteolin, kaempferol, naringenin, gallic acid and caffeic acid | 10 g sample + 90 mL absolute MeOH → HAE (22,000 rpm; 2 × 30 s extraction, 10 s pause each round) → 3 min incubation → solvent/supernatant was filtered |
● Sorbent (100 mg Strata-X RP cartridges) ● 1st conditioning (3 mL absolute MeOH); 2nd conditioning (3 mL 10% MeOH) ● 30 mL diluted methanol extract (1:9 in water v/v) was loaded → washed with 3 mL 10% MeOH → dried with vacuum pump → elution (3 mL absolute MeOH) ● Eluted extract was evaporated, dissolved in 1 mL 50% MeOH (ultrasound and vortexing), and filtered |
HPLC–MS | Terpinc et al. (2016) |
| Derivatives of benzoic acid and cinnamic acid | 800 mg sample + 20 mL water (adjusted to pH 2; 6 M HCl) → 5 × extraction with 20 mL DEE → evaporated under vacuum → neutralized and lyophilized → residue was hydrolyzed in 20 mL 2 M NaOH (4 h, nitrogen atmosphere) → acidified to pH 2 (6 M HCl) → 5 × extraction with 30 mL DEE → 15 mL 6 M HCl was added, placed under nitrogen atmosphere → hydrolyzed in a boiling water bath (1 h) → 45 mL DEE was added → ether extracts were evaporated → dissolved in 10 mL methanol and filtered | HPLC–DAD | Wiczkowski et al. (2016) | |
| Orientin, isoorientin, vitexin and rutin | Sample + absolute MeOH (1:20 w/v) → extract was dissolved in EtOH | HPLC–UV | Yang et al. (2016) | |
| 4-HBA, catechin, chlorogenic acid, caffeic acid, epicatechin, rutin and quercetin | 0.1 g sample + 5 mL 80% MeOH containing 10% acetic acid → UAE (10 min at 28 °C) → water bath (1 h at 37 °C) → centrifugation (3,000 rpm, 10 min) → re-extraction of residue (1 × with same procedure) → pooled supernatant was dried using nitrogen gas → dissolved with 5 mL absolute MeOH → diluted, filtered and stored |
HPLC–UV HPLC–MS |
Park et al. (2017) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin and quercetin | Sample + 1 mL MeOH containing 10% phosphoric acid → vortexed 5 min → incubation (3 h at 37 °C) with vortexing every 1 h → centrifuged (1,000 g, 5 min) and filtered | HPLC | Jeong et al. (2018) | |
| Orientin, isoorientin, vitexin, isovitexin and rutin | 100 mg sample + 1 mL MeOH (overnight at −20 °C) → centrifuged (17,000 g, 10 min) and filtered | UPLC–MS | Koja et al. (2018) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin | 15 g sample + 150 mL absolute MeOH (48 h soaking) → 10 min HAE → solvent containing extract was filtered → re-extraction of residue (2 × with same procedure) → filtered solvent was pooled → evaporated and stored at −20 °C | HPLC–PDA | Nam et al. (2018a) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin | 15 g sample + 150 mL absolute MeOH (24 h soaking) → solvent containing extract was filtered → re-extraction of residue (2 × with same procedure) → filtered solvent was pooled → evaporated | HPLC–PDA | Nam et al. (2018b) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin | Sample + 90% MeOH (30 min UAE) → centrifuged (2,232 g, 10 min) and filtered → re-extraction of residue (1 × with same procedure) → filtered supernatant was pooled → filtrate/supernatant was evaporated and stored at −50 °C | HPLC–PDA | Jang et al. (2019) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin |
● Ultrasound-assisted DES-based extraction technique ● Optimum: 80% CCTG, extraction temperature of 56 °C, extraction time of 40 min ● Sample:solvent (100 mg:1 mL) ● Extract was centrifuged (10,000 rpm, 30 min) → supernatant was filtered |
● Column containing sorbent (Sep-Pak C18 cartridges) ● 1st conditioning (2 × 5 mL absolute MeOH); 2nd conditioning (2 × 5 mL 0.01 N aqueous HCl) → evaporation ● Extract (0.5 mL) was loaded to column ● 1st elution with 6 mL 0.01 N aqueous HCl ● 2nd elution with 2 × 4 mL absolute MeOH ● Evaporated extract was dissolved (10 mL) with absolute MeOH |
HPLC–PDA HPLC–Q–TOF/MS |
Mansur et al. (2019) |
| Benzoic acid, caffeic acid, catechin, chlorogenic acid, epicatechin, gallic acid and rutin | 0.2 g sample + 2 mL 80% MeOH → vortexed 0.5 min → UAE (1 h at 37 °C) → centrifuged (16,000 g, 15 min) → re-extraction of residue (1 × with same procedure) → pooled supernatant was evaporated → dissolved with 2 mL absolute MeOH and filtered | HPLC–UV | Park et al. (2019) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin and quercetin | 1 g sample + 15 mL 70% EtOH (1 h UAE) → filtered | UPLC–MS | Chen et al. (2020) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin |
● MSPD-based extraction ● Optimum: C18 sorbent/sample (2:1; 100 mg) and 10 min static extraction with 5 mL 80% EtOH ● Extract was filtered |
HPLC–PDA | Mansur et al. (2020) | |
| Orientin, isoorientin, vitexin, isovitexin, rutin and quercetin | Sample + 1 mL MeOH containing 10% phosphoric acid → vortexed 5 min → incubated (3 h at 37 °C) with vortexing every 1 h → centrifuged (1,000 g, 5 min) and filtered | HPLC–UV | Sim et al. (2020) | |
| Rutin | 1 g sample + 95% EtOH (2 h) → filtered and stored (−20 °C) | HPLC–PDA | Witkowicz et al. (2020) | |
| Gallic acid, caffeic acid, ferulic acid, protocatechuic acid, p-coumaric acid, catechin, OHD, luteolin, apigenin, quercetin, isoquercetin, ellagic acid, velutin orientin, isoorientin, vitexin, isovitexin and rutin | 50 mg sample + acetone:water (4:1 v/v) (24 h shaking) → filtered and centrifuged (4,000 g, 10 min) → filtrate evaporation → dissolved with absolute MeOH | HPLC–DAD | Almuhayawi et al. (2021) | |
| Epicatechin, luteolin, orientin, vitexin, apigenin, naringenin, kaempferol, iso-rhamnetin, quercetin, 4-HBA, caffeic acid, sinapic acid, syringic acid, p-coumaric acid, ferulic acid and chlorogenic acid | Sample + MeOH, water, and formic acid (5 × extraction) → extracts were acidified (pH 2) with 6 M HCl → extraction with DEE → residues were hydrolyzed with 4 M NaOH and 6 M HCl → extraction with DEE → DEE extracts were pooled, evaporated, and diluted in 80% MeOH | HPLC–MS/MS | Dębski et al. (2021) | |
| Rutin and quercetin |
1) Free phenolics (ethanol extract): 1 g sample + 10 mL 80% EtOH (20 min) → centrifuged (6,500 g, 10 min) → re-extraction of residue (2 × with same procedure) → 3 supernatants were pooled and evaporated → diluted (10 mL) with MeOH and stored 2) Bound phenolics (hydrolysis): solid residue (after 3 × EtOH extraction) + 20 mL 2 M NaOH (24 h shaking) → acidified (pH 2) with 6 M HCl → centrifuged → supernatant was extracted with DEE/EtOAc (1:1) → evaporated, diluted (10 mL) with MeOH and stored |
HPLC–UV | Hung et al. (2021) | |
| Orientin, isoorientin, vitexin, isovitexin, Q3R and rutin | Sample + 90% MeOH (30 min UAE) → centrifuged (2,232 g, 10 min) and filtered → re-extraction of residue (1 × with same procedure) → filtered supernatant was pooled → filtrate/supernatant was evaporated |
● Column containing sorbent (100 g Diaion HP-20 resin) ● Extract was loaded to column ● 1st elution (1 L water); 2nd elution (1 L absolute MeOH) → evaporation ● Evaporated extract was freeze-dried and stored (−50 °C) |
HPLC–PDA HPLC–MS |
Jang et al. (2021) |
| Vitexin and isovitexin | 0.25 g sample + 3 mL 70% MeOH (10 min UAE) → centrifuged (1,800 g, 15 min) → supernatant was stored (−18 °C) | HPLC–DAD | Kalinová et al. (2021) | |
| Orientin, isoorientin, vitexin, rutin, catechin, epicatechin, hyperin and p-coumaric acid |
1) Free phenolics (methanol extract): 0.5 g sample + 3 mL 70% MeOH (40 min shaking at 200 rpm in the dark) → centrifuged (8,709 g, 8 min, 10 °C) → re-extraction of residue (2 × with same procedure) → 3 supernatants were pooled → diluted (10 mL) with 70% MeOH, filtered, stored (2 °C) 2) Bound phenolics (hydrolysis): solid residue (after 3 × MeOH extraction) + 20 mL 2 M NaOH (4 h shaking at 200 rpm in the dark) → acidified (pH 3.2–3.4) with 3.5 mL formic acid centrifuged (8,709 g, 8 min, 10 °C) → filtered and stored (2 °C) |
● Sorbent (100 mg Strata-X RP cartridges) ● 1st conditioning (3 mL absolute MeOH); 2nd conditioning (3 mL water) ● 30 mL diluted methanol extract (1:9 in water v/v) or 3 mL hydrolyzed extract was loaded → washed with 4 mL water → dried with vacuum pump → elution (2 mL 70% MeOH) ● Eluted extract was filtered and stored (−80 °C) |
HPLC–MS | Živković et al. (2021) |
aQ-Gal-Rha quercetin-3-O-galactorhamnoside, Cy-Gal cyanidin-3-O-galactoside, Cy-Glu cyanidin-3-O-glucoside, Cy-Rut cyanidin-3-O-rutinoside, Cy-Gal-Rha cyanidin-3-O-galactorhamnoside, Q-Glu quercetin-3-O-glucoside, Cy-Glu-Rha cyanidin-3-O-glucorhamnoside, Q3R quercetin 3-O-robinobioside, K3R kaempferol-3-O-rutinoside, DHBA 3,4-dihydroybenzoic acid, THBA 2,3,4-trihydroxybenzoic acid, p-HBA p-hydroxybenzoic, THCA trans-3-hydroxycinnamic acid, 4-HBA 4-hydroxybenzoic acid, OHD ortho-hydroxydaidzein
bThe extractions were performed under lab/room temperature unless otherwise mentioned. EtOH ethanol, MeOH methanol, TFA trifluoroacetic acid, UAE ultrasound-assisted extraction, EtOAc ethyl acetate, n-BuOH n-butanol, HAE homogenizer-assisted extraction, DEE diethyl ether, DES deep eutectic solvent, CCTG a DES composed of choline chloride and triethylene glycol, MSPD matrix solid-phase dispersion extraction
cThe more detailed information is available in Table 3
Table 3.
Various analytical methods for the determination of phenolics from common buckwheat sprouts
| Instrument typea | Stationary phaseb | Mobile phasec | Flow rate; injection | Identification and/or quantificationd | Phenolics analytee | References |
|---|---|---|---|---|---|---|
| UFLC | C18 RP (5 μm, 250 × 4.6 mm) |
A: 2% acetic acid in water B: 2% acetic acid in 45% acetonitrile |
1 mL/min; 10 μL |
355 nm External standard (RT) External standard calibration |
Flavonoid | Lim et al. (2012) |
| HPLC–UV | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
350 nm External standard (RT) External standard calibration |
Flavonoid | Horbowicz et al. (2013) |
| CE–UV | Silica NP (75 μm); 25 °C | 50 mM boric acid buffer containing 0.1 M SDS (pH 9.0) | Pressure injection (20 psi × 10 s) |
280 nm External standard (RT) External standard calibration |
Flavonoid | Koyama et al. (2013) |
| HPLC–UV | C18 RP (150 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 10 μL |
280 nm External standard (RT) External standard calibration |
||
| HPLC–MS | C18 RP (150 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 10 μL |
ESI positive mode Scanning (100–1000 m/z) External standard (RT) + MS spectra |
||
| HPLC–UV | C18 RP (150 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 10 μL |
280 nm External standard (RT) Rutin standard calibration |
Flavonoid | Nakamura et al. (2013) |
| HPLC–UV | C18 RP (250 × 4.6 mm) | Acetonitrile/0.1% formic acid (16:84) | 1 mL/min |
370 nm External standard (RT) External standard calibration |
Flavonoid | Tsurunaga et al. (2013) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: methanol/water/acetic acid (5:92.5:2.5) B: methanol/water/acetic acid (95:2.5:2.5) |
1 mL/min; 10 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid and phenolic acid | Arasu et al. (2014) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: methanol/water/acetic acid (5:92.5:2.5) B: methanol/water/acetic acid (95:2.5:2.5) |
1 mL/min; 10 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid and phenolic acid | Kim et al. (2014b) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: methanol/water/acetic acid (5:92.5:2.5) B: methanol/water/acetic acid (95:2.5:2.5) |
1 mL/min; 10 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid and phenolic acid | Lee et al. (2014) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 50 °C |
A: 0.2% acetic acid in water B: 0.2% acetic acid in methanol |
5 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid | Ren and Sun (2014) |
| HPLC–DAD | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
350 nm (flavonoid); 520 nm (anthocyanin) External standard (RT) External standard calibration |
Flavonoid | Wiczkowski et al. (2014) |
| HPLC–MS | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
ESI positive mode External standard (RT) + MS spectra |
||
| HPLC–CoulArray–ECD | C18 RP (3 μm, 250 × 2 mm); 45 °C | Acetonitrile/water/phosphoric acid (9.99:89.99:0.02) | 0.2 mL/min |
+ 800–950 mV External standard (RT) External standard calibration |
||
| HPLC–DAD | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
350 nm (flavonoid); 520 nm (anthocyanin) External standard (RT) External standard calibration |
Flavonoid | Horbowicz et al. (2015) |
| HPLC–MS | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
ESI positive mode External standard (RT) + MS spectra |
||
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 25 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 20 μL |
350 nm External standard (RT) |
Flavonoid | Nam et al. (2015) |
| HPLC–Q–TOF/MS | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 20 μL |
ESI positive and negative mode Scanning (50 – 1700 m/z) External standard (RT) + MS spectra |
||
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 30 °C |
1) C-glycosyl flavones analysis: A: 0.1% formic acid in water B: methanol 2) Other flavonoid analysis: A: 0.15% phosphoric acid in water B: acetonitrile 3) Phenolic acid analysis: A: 0.15% phosphoric acid in water B: methanol |
1 mL/min (flavonoid), 0.7 mL/min (phenolic acid); 20 μL |
355 nm (flavonoid); 270 nm (phenolic acid) External standard (RT) |
Flavonoid and phenolic acid | Zhang et al. (2015) |
| HPLC–MS | C18 RP (5 μm, 250 × 4.6 mm); 30 °C |
1) C-glycosyl flavones analysis: A: 0.1% formic acid in water B: methanol 2) Other flavonoid analysis: A: 0.15% phosphoric acid in water B: acetonitrile 3) Phenolic acid analysis: A: 0.15% phosphoric acid in water B: methanol |
1 mL/min (flavonoid), 0.7 mL/min (phenolic acid); 20 μL |
ESI negative mode Scanning (50 – 1000 m/z) External standard (RT) + MS spectra External standard calibration |
||
| HPLC–UV | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
350 nm (flavonoid); 520 nm (anthocyanin) External standard (RT) External standard calibration |
Flavonoid | Dębski et al. (2016) |
| HPLC–UV | C18 RP (150 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 10 μL |
280 nm External standard (RT) Rutin standard calibration |
Flavonoid | Nakamura et al. (2016) |
| HPLC–MS | C18 RP (2.6 μm, 100 × 2 mm); 25 °C |
A: 0.1% formic acid in water B: acetonitrile |
0.3 mL/min; 10 μL |
ESI negative mode External standard (RT) + MS spectra External standard calibration |
Flavonoid and phenolic acid | Terpinc et al. (2016) |
| HPLC–DAD | C18 RP (3 μm, 250 × 2 mm); 45 °C |
A: acetonitrile/water/formic acid (6:89:5) B: acetonitrile/water/formic acid (80:15:5) |
0.2 mL/min |
280 and 320 nm External standard (RT) External standard calibration |
Phenolic acid | Wiczkowski et al. (2016) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 35 °C |
A: acetic acid/water (2:98) B: acetic acid/ acetonitrile/water (2:45:53) |
1 mL/min; 10 μL |
355 nm External standard (RT) External standard calibration |
Flavonoid | Yang et al. (2016) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 30 °C |
A: acetic acid/water (0.15:99.85) B: methanol |
1 mL/min; 20 μL |
280 nm External standard (RT) External standard calibration |
Flavonoid and phenolic acid | Park et al. (2017) |
| HPLC–MS | C18 RP (5 μm, 250 × 4.6 mm); 30 °C |
A: acetic acid/water (0.15:99.85) B: methanol |
1 mL/min; 20 μL |
ESI negative mode Scanning (100–1300 m/z) External standard (RT) + MS spectra External standard calibration |
||
| HPLC | C18 RP (5 μm, 250 × 4.6 mm) |
A: water B: acetic acid/methanol (5:95) |
1 mL/min; 5 μL |
254 nm External standard (RT) External standard calibration |
Flavonoid | Jeong et al. (2018) |
| UPLC-MS | C18 RP (1.7 μm, 50 × 2.1 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in methanol |
0.25 mL/min |
ESI negative mode External standard (RT) + MS spectra External standard calibration Internal standard (chrysin) |
Flavonoid | Koja et al. (2018) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 20 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid | Nam et al. (2018a) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 20 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid | Nam et al. (2018b) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
1 mL/min; 5 μL |
360 nm External standard (RT) External standard calibration |
Flavonoid | Jang et al. (2019) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 10 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid | Mansur et al. (2019) |
| HPLC–Q–TOF/MS | C18 RP (1.8 μm, 150 × 2.1 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.3 mL/min; 2 μL |
ESI negative mode Scanning (100–1000 m/z) External standard (RT) + MS spectra |
||
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm); 30 °C |
A: acetic acid/water (0.15:99.85) B: methanol |
1 mL/min; 20 μL |
280 nm External standard (RT) External standard calibration |
Flavonoid and phenolic acid | Park et al. (2019) |
| UPLC-MS | C18 RP (1.7 μm, 150 × 2.1 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.3 mL/min; 1 μL |
ESI positive mode External standard (RT) + MS spectra External standard calibration |
Flavonoid | Chen et al. (2020) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
0.8 mL/min; 10 μL |
350 nm External standard (RT) External standard calibration |
Flavonoid | Mansur et al. (2020) |
| HPLC–UV | C18 RP (5 μm, 250 × 4.6 mm) |
A: water B: 5% acetic acid in methanol |
1 mL/min; 20 μL |
254 nm External standard (RT) External standard calibration |
Flavonoid | Sim et al. (2020) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 25 °C |
A: 1% formic acid in water B: 1% formic acid in acetonitrile |
1 mL/min |
370 nm External standard (RT) External standard calibration |
Flavonoid | Witkowicz et al. (2020) |
| HPLC–DAD | Silica NP (7 μm, 150 × 3 mm) |
A: water-formic acid (90:10) B: acetonitrile/water/formic acid (85:10:5) |
0.8 mL/min; 20 μL |
External standard (RT) External standard calibration Internal standard (3,5-dichloro-4-hydroxybenzoic) |
Flavonoid and phenolic acid | Almuhayawi et al. (2021) |
| HPLC–MS/MS | C18 RP (2.7 μm, 50 × 0.5 mm); 45 °C |
A: water/formic acid (99.05:0.95) B: acetonitrile/formic acid (99.05:0.95) |
0.015 mL/min |
ESI negative mode (MRM) External standard (RT) + MS spectra External standard calibration |
Flavonoid and phenolic acid | Dębski et al. (2021) |
| HPLC–UV | C18 RP (5 μm, 150 × 4.6 mm); 35 °C |
A: 1% phosphoric acid in water B: methanol |
1 mL/min |
280 nm External standard (RT) External standard calibration |
Flavonoid | Hung et al. (2021) |
| HPLC–PDA | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
1 mL/min; 5 µL |
360 nm External standard (RT) External standard calibration |
Flavonoid | Jang et al. (2021) |
| HPLC–MS | C18 RP (5 μm, 250 × 4.6 mm); 40 °C |
A: 0.1% formic acid in water B: 0.1% formic acid in acetonitrile |
1 mL/min; 5 µL |
ESI negative and positive mode Scanning (200–1200 m/z) External standard (RT) + MS spectra |
||
| HPLC–DAD | C18 RP (3 μm, 150 × 2 mm); 25 °C |
A: 5% acetonitrile + 0.1% o-phosphoric acid B: 80% acetonitrile + 0.1% o-phosphoric acid |
0.25 mL/min |
350 nm External standard (RT) External standard calibration |
Flavonoid | Kalinová et al. (2021) |
| HPLC–MS | C18 RP (2.7 μm, 150 × 2.1 mm); 30 °C |
A: 0.1% aqueous formic acid B: acetonitrile |
0.25 mL/min; 20 µL |
ESI negative mode External standard (RT) + MS spectra External standard calibration |
Flavonoid and phenolic acid | Živković et al. (2021) |
aUFLC ultra-fast liquid chromatography, CE capillary electrophoresis, UPLC ultra-high performance liquid chromatography, DAD diode array detector, ECD electrochemical array detector, PDA photodiode array detector, Q-TOF/MS quadrupole time-of-flight/MS
bRP reversed-phase, NP normal phase
cSDS: sodium dodecyl sulfate; C-glycosyl flavones: orientin, isoorientin, vitexin and isovitexin
dRT: retention time comparison; ESI: electron spray ionization; MS spectra: data from MS analysis
eThe specific name for polyphenol analyte is described in Table 2
The preparation of analytical samples usually involves several steps, including sample dehydration and size reduction, extraction, hydrolysis, purification, and storage (Dzah et al., 2020a). Table 2 summarizes the various techniques used to prepare analytical CBS samples for the determination of their phenolic compounds. In most studies, CBS samples were first subjected to dehydration using oven drying or freeze-drying (lyophilization), followed by particle size reduction by milling or grinding combined with sieving as required. Reduced moisture after dehydration minimizes the rate of lipid oxidation (Kim et al., 2014a) and enzyme activity in the sample (George et al., 2014). Moreover, reduced particle size leads to an increase in surface area, resulting in increased interaction with solvent and mass transfer of phenolic analytes, while sieving enhances the homogeneity and extraction efficiency (Dzah et al., 2020a).
Various factors, including the chemical nature of the isolated analytes, methods employed for extraction and purification, storage conditions, and interfering analytes, may influence the extraction and recovery yields of specific phenolic analytes from the extracts (Naczk and Shahidi, 2004). As shown in Table 2, methods used for preparation of analytical CBS samples vary widely among studies. For instance, most studies used low sample weights between 0.01 and 1 g, with only a few studies using high sample weights between 3 and 15 g (Nam et al., 2015, 2018a, b; Terpinc et al., 2016; Zhang et al., 2015). The lowest sample-to-solvent ratio was approximately 0.05:10 (w/v) (Tsurunaga et al., 2013) and the highest was approximately 1.67:10 (w/v) (Živković et al., 2021). Conventional organic solvents, such as methanol (50–100%), ethanol (70–100%), and aqueous acetone (50–80%) have been frequently used for the solid–liquid extractions of free phenolic compounds and their derivatives from CBS, in the order methanol > ethanol > aqueous acetone. In some cases, the extraction solvents were acidified with aqueous phosphoric acid (Arasu et al., 2014; Jeong et al., 2018; Kim et al., 2014b; Lee et al., 2014; Sim et al., 2020), acetic acid (Park et al., 2017), trifluoroacetic acid (Dębski et al., 2016; Horbowicz et al., 2015; Wiczkowski et al., 2014) or formic acid (Dębski et al., 2021). In addition, the use of deep eutectic solvents (DESs), a new group of organic solvents developed as promising environmentally friendly alternatives to conventional solvents, has been investigated for the extraction of major flavonoids from CBS. A DES composed of choline chloride (hydrogen bond acceptor) and triethylene glycol (hydrogen bond donor) (80% in water v/v) was found to be more effective than methanol for the extraction of vitexin and quercetin-3-O-robinobioside (Mansur et al., 2019).
Solid–liquid extractions of phenolics from CBSs (Table 2) were performed either under static conditions (conventional soaking with solvent, with or without heating) or under dynamic conditions using classical techniques such as vortexing, agitation, shaking, and stirring. In some studies, more advanced techniques such as ultrasound-assisted extraction (UAE) and homogenizer-assisted extraction (HAE) have also been used to increase the extraction yield and efficiency, with UAE being employed more often than HAE. A comparative assessment based on literature by Dzah et al. (2020b) revealed that the UAE method not only contributes to increased extraction yield of phenolic compounds, but also better preserves and increases their biological activity. The solid–liquid extractions of phenolics from CBSs (Table 2) were performed mostly at room temperature. Heating treatments ranging from 28 to 70 °C were applied in some studies; Ren and Sun (2014) performed heat treatment at 70 °C for 6 h using a water boiler. Alternatively, Koja et al. (2018) applied overnight extraction at a freezing temperature of −20 °C.
Phenolic compounds extracted from CBSs in most of the studies summarized in Table 2 contained the only free phenolics and not the bound fraction, which could be partly attributed to the residues being discarded after extraction with organic solvents. Some studies used the residues to perform re-extraction up to one to five times using the same procedure as that for the first extraction, to enhance the extraction yields of free phenolic compounds. Moreover, after re-extraction, simultaneous fractionation of the residue using water, ethyl acetate/n-butanol (EtOAc/n-BuOH, 3:1 v/v), water, and EtOAc was performed by Nam et al. (2015). To extract bound phenolic compounds from the residues of CBS extracts, some studies combined alkaline (NaOH) and acid (HCl or formic acid) hydrolysis with diethyl ether (DEE) (Debski et al., 2021; Wiczkowski et al., 2016; Živković et al., 2021) or DEE/EtOAc (1:1 v/v) (Hung et al., 2021). The combination of acid and alkaline hydrolysis allows the extracts to be neutralized and prevents further hydrolytic degradation, thereby allowing successful separation and purification of the phenolic compounds (Dzah et al., 2020a).
The unwanted compounds or fractions that may affect the final extraction/recovery yield and interfere in the determination of the target compounds can be removed by purifying the extract samples (Kumar, 2017). In some studies, the solid-phase extraction (SPE)-based purification technique was employed (Table 2). The CBS extracts containing phenolic compounds were retained in a specific sorbent (C18 or resin) with or without column preconditioning using organic solvents (methanol, aqueous methanol, aqueous HCl, or water). The unwanted fractions in the extracts were washed with aqueous methanol, aqueous HCl, or water, and the retained phenolic compounds were eluted with methanol. An alternative to traditional SPE is the matrix solid-phase dispersion extraction technique, which has the advantage of simultaneously extracting and purifying major flavonoids (Mansur et al., 2020). The CBS extracts were obtained using centrifugation (1,000–17,000 × g; 3,000–22,000 rpm), filtration (using filter paper, vacuum filter or membrane filter), and drying (via vacuum evaporation, nitrogen gas, or freeze-drying) during and/or after the extraction and/or purification steps. The obtained extracts were stored either in liquid form dissolved in organic solvents (methanol, ethanol, and acetonitrile) or in dried form at 4 or −50 °C. The preparation times for the CBS analytical sample varied widely among studies, from a few minutes up to days to complete the procedure. Sample preparation procedures that required the longest time used conventional extraction techniques such as soaking or shaking (Nam et al., 2015, 2018a, b; Almuhayawi et al., 2021).
Various analytical methods have been used for the qualitative and quantitative determination of multiple phenolic compounds in plant extracts. Among them, LC-based techniques have been the most widely applied (Dzah et al., 2020a; Kumar, 2017), especially for the analysis of buckwheat extracts (Huda et al., 2021). Regarding the analysis of CBS extracts, LC [HPLC, ultra-fast liquid chromatography (UFLC), and ultra-high performance liquid chromatography (UPLC)] systems equipped with an UV detector, diode array detector (DAD), or photodiode array detector (PDA) have been frequently applied for the separation and determination of phenolic compounds (Table 3). To improve the sensitivity of the analytical method, some studies employed LC coupled with MS techniques, such as MS, tandem MS/MS, and quadrupole time-of-flight/MS (Q–TOF/MS).
The stationary phase (column) is a major component of LC, and is used mainly for the separation of analytes. The non-polar C18 reverse-phase (RP) column was most frequently used for the analysis of phenolic compounds in CBSs, although its specifications varied across studies, including particle size (1.7–5 µm), length (50–250 mm), and inner diameter (ID; 0.5–4.6 mm). The UPLC and MS-based techniques used columns with lower particle sizes, lengths, and IDs. According to Žuvela et al. (2019), although hundreds of RP-LC column types are available in the market, none of them are almost identical. The differences between columns are mainly in the medium type (monolithic, porous, or non-porous), geometry (particle size and shape, diameter and pore volume, and area of the bed), bed chemical properties (type of attached ligands and their density), and stationary-phase carrier composition (carbon, polymers, or silica). Therefore, there is no universal RP-LC column that can cover a variety of analytes.
The polarity and pH of mobile phase solvents, as well as column temperature, are also important LC parameters for the separation of analytes (Dzah et al., 2020a). Various mobile phase systems (Table 3) composed of polar solvents, such as water and methanol or acetonitrile (either in absolute or aqueous forms), with or without the addition of formic acid (0.1–10% v/v), acetic acid (0.15–5% v/v), or phosphoric acid (0.02–1% v/v), have been used for the elution and separation of phenolic compounds from CBS extracts by LC with binary gradient elution (elution flow rates of 0.015–1 mL/min) and column temperatures of 25–50 °C. According to Bligh et al. (2013), binary gradient elution systems help separate compounds from different classes of flavonoids using RP-LC columns. The addition of acids to mobile phase solvents serves to avoid a rise in pH and ionization, as well as a reduced retention time during RP-LC separation, thereby enhancing the resolution and reproducibility of each separation.
The detector in the LC system is responsible for turning a physical or chemical attribute into a measurable signal corresponding to the identity and/or concentration of the analyte. The UV, DAD, and PDA detectors are the most common LC detectors in use today because many analytes of interest absorb in the UV and visible regions between 190 and 600 nm (Swartz, 2010). Table 3 shows that phenolic acids, anthocyanins, and flavonoids other than anthocyanin were identified at 270–320 nm, 520 nm, and 254–370 nm, respectively. The phenolic analytes were identified based on the consistency in retention time of detected analytes in the sample with that of the corresponding reference standards, and quantified using an external calibration curve derived from the reference standards analyzed at different concentration levels. The LC method provides only limited structural information (UV spectrum) and tentative identification of the eluted analyte peaks in the samples by comparison with reference standards (Kumar, 2017). Thus, complete peak separation is necessary for both qualitative and quantitative determination using LC analysis to enhance its reliability.
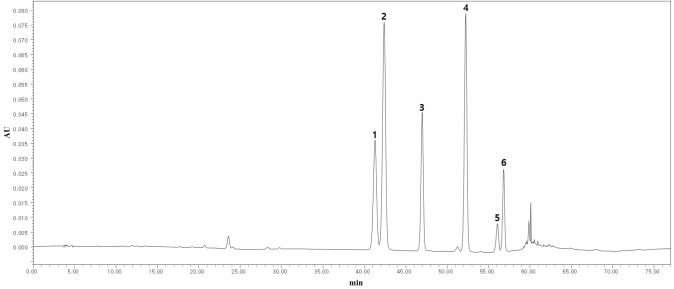
More advanced detectors, including MS, MS/MS, and Q–TOF/MS, have also been applied for the identification and quantification of phenolic compounds in CBS extracts. Electron spray ionization (ESI) modes, either negative, positive, or both positive and negative, are usually applied to determine the fragmentation patterns of the ions. After ionization of the sample, the molecular masses of the phenolic compounds were mostly calculated based on the m/z in the range 50–1700. The analytes were identified based on their retention times and MS spectra, and their concentrations were calculated relative to the corresponding external standards from the calibration curves (Table 3). Several MS-based analytical techniques have become popular choices in the analysis of buckwheat extracts (Huda et al., 2021) because their sensitivity and selectivity facilitate the analysis of phenolic compounds present at low concentrations in plant extracts (Dzah et al., 2020a; Kumar, 2017). The ESI–MS analysis is also highly compatible with the polar solvents used as mobile phase in RP-LC because they readily undergo electrochemical reactions in the spraying nozzle, thus enabling the combination of the LC system with MS detectors (Banerjee and Mazumdar, 2012; Lim and Lord, 2002). The use of LC–MS techniques also allows simultaneous monitoring of the retention time of the isolated compound peak and determination of their MS data during the analysis (Santi and Dipjyoti, 2013). In addition, LC–MS techniques are widely considered more powerful than conventional LC because they can be used for qualitative analysis of phenolic compounds even when baseline separation is not achieved. However, flavonoid isomers (e.g., orientin, isoorientin, vitexin, isovitexin, rutin, and quercetin-3-O-robinobioside) need to be fully separated (Fig. 1) to enable their accurate quantitative analysis in food, as they exhibit identical molecular masses and similar fragment patterns, and cannot be distinguished based on only MS information (Nam et al., 2015; Jang et al., 2019; Mansur et al., 2019).
Fig. 1.
HPLC chromatogram of major flavonoids in common buckwheat sprouts at 350 nm. Peak 1: orientin; 2: isoorientin; 3: vitexin; 4: isovitexin; 5: quercetin-3-O-robinobioside; 6: rutin
The determination of phenolic compounds in CBS is a complex process that requires a combination of multiple analytical approaches, such as analytical sample preparation, separation, identification, and quantification of analytes. It is difficult to recommend a universally suitable combined method from the available studies because none of the methods are comprehensive enough to cover all the analytes (e.g., flavonoids, phenolic acids, anthocyanin, free or bound forms, isomers), and each method has its own advantages and disadvantages. Nevertheless, by considering the nature of the target analytes and the characteristics, advantages, and disadvantages of the analytical techniques, a combination of effective and efficient approaches can be utilized for better outcomes.
Health benefits of CBS and its phenolic compounds
Antioxidant activity
Sprouts contain a wide range of antioxidants (Guo et al., 2018), including flavonoids, which possess many biological properties, such as anti-cancer, anti-aging, and anti-inflammatory effects (Lee et al., 2018; Nam et al., 2017; Rha et al., 2020). Regular consumption of natural antioxidants has been shown to significantly reduce and delay reactive oxygen species (ROS)-related diseases (Ravula et al., 2021). The in vitro antioxidant activity of CBSs was evaluated using the commonly used DPPH and ABTS radical scavenging assays. The results are expressed as the percentage of radical scavenging activity, Trolox equivalents, or vitamin C equivalents. CBS ethanol extracts showed approximately 88% DPPH scavenging activity at a concentration of 5 mg/mL (Liu et al., 2008). Jeong et al. (2018) and Witkowicz et al. (2020) reported DPPH scavenging activity in CBS extracts to be 8000 mg Trolox equivalents/100 g DW and 46.88 μM Trolox equivalents/g DW, respectively. The antioxidant activity of common buckwheat seeds against DPPH was 1138 mg Trolox equivalents/100 g (Kim et al., 2019), indicating that the sprouts had significantly higher antioxidant activity than the seeds. The DPPH scavenging activity of CBSs grown in the dark was 25 mg vitamin C equivalents/g DW (Nam et al., 2018a, b). With the ABTS radical scavenging assay, CBS ethanol extracts exhibited approximately 2 μM Trolox equivalents/g FW (Almuhayawi et al., 2021). Sim et al. (2020) reported that the CBS methanol extract produced approximately 5 mg Trolox equivalents/g FW. Against the ABTS radical, the flavonoid isomers exhibited scavenging activity in the order isoorientin > rutin > orientin > isovitexin > vitexin (Nam et al., 2018a, b). Accumulation of intracellular ROS is a significant cause of numerous metabolic diseases. Jeong et al. (2018) found that CBS extracts decreased ROS generation in a dose-dependent manner in a tert-butyl hydroperoxide-induced HepG2 cell model. CBSs also significantly reduced intracellular oxidative stress, including peroxides and superoxide anions, in HepG2 cells (Liu et al., 2008). Although the intracellular antioxidant activities of CBSs are not well described, CBSs and their flavonoids are potential sources of antioxidants that can play a critical role in the prevention of ROS-related diseases.
Anti-inflammatory activity
Significant amounts of inflammatory mediators are produced during inflammation, and excessive production of mediators is a major cause of chronic inflammatory diseases (Lee et al., 2018). Biomedicine research has focused on finding natural therapeutic agents for inflammation-associated diseases with minimal toxicity and high potency. The anti-inflammatory activities of CBSs and their flavonoids are listed in Table 4. CBS extracts have been reported to show strong inhibitory effects on the inflammatory mediators [nitric oxide (NO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2)] and cytokines including tumor TNF-α, IL-6, and IL-12 in LPS-induced RAW 264.7 and peritoneal macrophages (Nam et al., 2017). Almuhayawi et al. (2021) reported that CBS extracts directly inhibited COX-2 and lipoxygenase activities. Ishii et al. (2008) demonstrated the suppressive effects of CBS extracts on human colon cancer cells and mice via the inhibition of cytokine upregulation. The activation of nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (MAPKs) is closely linked to cytokine production (Kim et al., 2020). Isoorientin attenuated LPS-induced pro-inflammatory responses by downregulating the MAPKs and NF-κB signaling pathways (Yuan et al., 2014). Yoo et al. (2014) found that rutin downregulated high-mobility group box 1 protein-dependent inflammatory responses in human umbilical vein endothelial cells. Orientin significantly reduced the inflammatory response in LPS-stimulated acute lung injury in mice by suppressing the activation of NF-κB and the nucleotide-binding domain-like receptor protein 3 inflammasome (Xiao et al., 2021). Thus, CBSs and their flavonoids are potential natural therapeutic agents against inflammation.
Table 4.
Health benefits of common buckwheat sprout extracts and their phenolics
| Extract/phenolicsa | Beneficial effects | Mechanismb | References |
|---|---|---|---|
| 80% (v/v) ME | Anti-inflammatory effect | Inhibition of cytokines, COX-2, NO production, NF-κB activation, and MAPK in LPS-induced RAW 264.7 and peritoneal macrophages | Nam et al. (2017) |
| 80% (v/v) EE | Anti-inflammatory effect | Suppression of NO and cytokines production in LPS-induced RAW 264.7 cells | Kim et al. (2019) |
| 80% (v/v) EE | Anti-inflammatory effect | Inhibition of COX-2 and lipoxygenase activities | Almuhayawi et al. (2021) |
| 70% (v/v) EE | Anti-inflammatory effect | Inhibition of upregulation of inflammation cytokines in LPS-stimulated CoLoTC cells and mice model | Ishii et al. (2008) |
| Isoorientin | Anti-inflammatory effect | Down-regulation of MAPK and NF-κB signaling pathway in LPS-induced mouse microglial cells | Yuan et al. (2014) |
| Rutin | Anti-inflammatory effect | Down-regulation of high mobility group box 1 protein-dependent inflammatory responses | Yoo et al. (2014) |
| Orientin | Anti-inflammatory effect | Suppression of NF-κB and nucleotide-binding domain-like receptor protein 3 inflammasome; Upregulation of nuclear factor erythroid 2-related factor 2 | Xiao et al. (2021) |
| Rutin and quercetin-3-O-robinobioside | Immune-enhancing properties | Upregulation of CD4+ T-lymphocytes | Ajaghaku et al. (2018) |
| Orientin | Anti-proliferation of cancer cells | Anti-proliferative effects against colorectal carcinoma cells | Thangaraj et al. (2019) |
| Orientin | Anti-proliferation of cancer cells | Suppression of colonic cell proliferation via down-regulation of proliferative antigens | Thangaraj and Vaiyapuri (2017) |
| Vitexin and isovitexin | Neuroprotective effect | Neuroprotective effects on amyloid β-induced PC-12 cells | Guimarães et al. (2015) |
| Isoorientin | Neuroprotective effect | Protective effect against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells by increasing Nrf2-antioxidant signaling pathway | Ma et al. (2020) |
| Vitexin | Antidiabetic effect | Protective effect on pancreatic β-cells against high-glucose induced damages | Ganesan et al. (2020) |
aME methanol extract, EE ethanol extract
bCOX-2 cyclooxygenase-2, NF-κB nuclear factor-kappa B, MAPK mitogen-activated protein kinases, LPS lipopolysaccharide, NO nitric oxide, Nrf2: nuclear factor erythroid 2 related factor 2
Other biological activities
The biological activities of CBS extracts have not been extensively described. Orientin exhibited anti-proliferative effects against colorectal carcinoma cells by arresting the cell cycle in a dose-dependent manner and regulating cyclin and cyclin-dependent protein kinases (Thangaraj et al., 2019). It downregulated proliferative markers, including proliferative cell nuclear antigen and Ki67, which are associated with tumor initiation (Thangaraj and Vaiyapuri, 2017). Neuroprotective effects of isoorientin were observed against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells by activation of the nuclear factor erythroid 2-related factor 2 antioxidant signalling pathway (Ma et al., 2020). Vitexin exhibited a protective effect on pancreatic β-cells against high-glucose-induced damage and enhanced insulin release (Ganesan et al., 2020). Isovitexin attenuated amyloid β-induced neuronal death in PC-12 cells (Guimarães et al., 2015). Vitexin and isovitexin were also detected in the brain using LC–MS/MS, indicating their ability to penetrate the blood–brain barrier (Bai et al., 2017).
Summary and future research approaches
CBS extracts and their phenolic compounds have been shown to exhibit potent antioxidant and anti-inflammatory activities both in vitro and in vivo, as detailed in this review. However, other biological activities of CBS extracts are not as well described as those of tartary buckwheat sprouts. The biological activity of CBSs is closely linked to the amount and class of phenolic compounds. Studies have focused on the bioactivities of major flavonoids (C-glycosyl flavones and rutin) in CBS, while the health benefits of quercetin-3-O-robinobioside have been rarely reported. Greater attention must be paid to the further discovery of bioactivities of CBS extracts and phenolics that have been less studied. Effective sample preparation and analytical techniques are needed to determine the health benefits of CBS phenolics. In the past decade, various sample preparation procedures and analytical techniques have been employed for qualitative and/or quantitative analysis of specific phenolic compounds, both free and bound, in CBS. Solid–liquid extractions using methanol (50–100%), performed at room temperature and under static or dynamic conditions, were the most frequently applied for the isolation of phenolics from CBS. The phenolics isolated from the CBS extracts were further purified using SPE-based approaches. The phenolics in the CBS extracts were mostly determined by LC. Thus, there does not exist any consolidated method that can cover all phenolic analytes in CBS. However, from available studies, an effective and efficient combined analytical approach can be developed by considering the nature of the target phenolics and the characteristics of the analytical methods, as well as their advantages and disadvantages.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2021R1G1A1010510).
Declarations
Conflict of interest
The author declares that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ahmad Rois Mansur and Sang Gil Lee are contributed equally to this work.
Contributor Information
Ahmad Rois Mansur, Email: ahmadroismansur@gmail.com.
Sang Gil Lee, Email: sglee1125@pknu.ac.kr.
Bong-Han Lee, Email: green-food@naver.com.
Sang Gyu Han, Email: hn5459@kyonggi.ac.kr.
Sung-Won Choi, Email: csw0365@osan.ac.kr.
Won-Jae Song, Email: wjsong@kyonggi.ac.kr.
Tae Gyu Nam, Email: tgzoo0706@kyonggi.ac.kr.
References
- Abdel-Aty AM, Elsayed AM, Salah HA, Bassuiny RI, Mohamed SA. Egyptian chia seeds (Salvia hispanica L.) during germination: Upgrading of phenolic profile, antioxidant, antibacterial properties and relevant enzymes activities. Food Science and Biotechnology. 30: 723-734 (2021) [DOI] [PMC free article] [PubMed]
- Ajaghaku DL, Akah PA, Ilodigwe EE, Nduka SO, Osonwa UE, Okoye FBC. Upregulation of CD4+ T-lymphocytes by isomeric mixture of quercetin-3-O-rutinoside and quercetin-3-O-robinobioside isolated from Millettia aboensis. Immunological Investigations. 2018;47(4):372–388. doi: 10.1080/08820139.2018.1433201. [DOI] [PubMed] [Google Scholar]
- Almuhayawi MS, Hassan AH, Abdel-Mawgoud M, Khamis G, Selim S, Al Jaouni, SK, AbdElgawad H. Laser light as a promising approach to improve the nutritional value, antioxidant capacity and anti-inflammatory activity of flavonoid-rich buckwheat sprouts. Food Chemistry. 345: 128788 (2021) [DOI] [PubMed]
- Arasu MV, Kim SJ, Al-Dhabi NA, Suzuki T, Yamauchi H, Lee SW. Comparison of flavonoid contents between common and tartary buckwheat (Fagopyrum) Sprouts cultured with/without soil. Asian Journal of Chemistry. 2014;26:5985–5990. doi: 10.14233/ajchem.2014.16355. [DOI] [Google Scholar]
- Bai Y, Zhang Q, Wang B, Zhang M, Xu Y, Li S, Zhao Y, Yu Z. Plasma pharmacokinetics, bioavailability, and tissue distribution of four C-glycosyl flavones from mung bean (Vigna radiata L.) seed extracts in rat by ultrahigh-performance liquid chromatography–tandem mass spectrometry. Journal of Agricultural and Food Chemistry. 65: 5570-5580 (2017) [DOI] [PubMed]
- Banerjee S, Mazumdar S. Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte. International journal of Analytical Chemistry. 2012: 282574 (2012) [DOI] [PMC free article] [PubMed]
- Beelders T, Sigge GO, Joubert E, de Beer D, de Villiers A. Kinetic optimisation of the reversed phase liquid chromatographic separation of rooibos tea (Aspalathus linearis) phenolics on conventional high performance liquid chromatographic instrumentation. Journal of Chromatography a. 2012;1219:128–139. doi: 10.1016/j.chroma.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Bligh SWA, Ogegbo O, Wang, Z. Flavonoids by HPLC. pp. 2107–2144. In: Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Ramawat KG, Mérillon JM (eds). Spronger-Verlag: Berlin, Heidelberg (2013)
- Chen T, Piao M, Rahman SME, Zhang L, Deng Y. Influence of fermentation on antioxidant and hypolipidemic properties of maifanite mineral water-cultured common buckwheat sprouts. Food Chemistry. 321: 126741 (2020) [DOI] [PubMed]
- Dębski H, Szwed M, Wiczkowski W, Szawara-Nowak D, Bączek N, Horbowicz M. UV-B radiation increases anthocyanin levels in cotyledons and inhibits the growth of common buckwheat seedlings. Acta Biologica Hungarica. 2016;67:403–411. doi: 10.1556/018.67.2016.4.6. [DOI] [PubMed] [Google Scholar]
- Debski H, Wiczkowski W, Szawara-Nowak D, Horbowicz M. Elicitation with sodium silicate and iron chelate affects the contents of phenolic compounds and minerals in buckwheat sprouts. Polish Journal of Food and Nutrition Sciences. 2021;71:21–28. doi: 10.31883/pjfns/131061. [DOI] [Google Scholar]
- Dzah CS, Duan Y, Zhang H, Boateng NAS, Ma H. Latest developments in polyphenol recovery and purification from plant by-products: A review. Trends in Food Science and Technology. 2020;99:375–388. doi: 10.1016/j.tifs.2020.03.003. [DOI] [Google Scholar]
- Dzah CS, Duan Y, Zhang H, Wen C, Zhang J, Chen G, Ma H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Bioscience. 35: 100547 (2020b).
- Ganesan K, Ramkumar KM, Xu B. Vitexin restores pancreatic β-cell function and insulin signaling through Nrf2 and NF-κB signaling pathways. European Journal of Pharmacology. 888: 173606 (2020) [DOI] [PubMed]
- George E, Emami S, Tabil LG, Campbell L. Effect of initial moisture and temperature on the enzyme activity of pelleted high protein/fiber biomass. Canadian Biosystems Engineering. 2014;56:17–24. [Google Scholar]
- Guimarães CC, Oliveira DD, Valdevite M, Saltoratto ALF, Pereira SIV, de Castro França S, Pereira AMS, Pereira PS. The glycosylated flavonoids vitexin, isovitexin, and quercetrin isolated from Serjania erecta Radlk (Sapindaceae) leaves protect PC12 cells against amyloid-β25-35 peptide-induced toxicity. Food and Chemical Toxicology. 2015;86:88–94. doi: 10.1016/j.fct.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Guo L, Zhu Y, Wang F. Calcium sulfate treatment enhances bioactive compounds and antioxidant capacity in broccoli sprouts during growth and storage. Postharvest Biology and Technology. 2018;139:12–19. doi: 10.1016/j.postharvbio.2018.01.010. [DOI] [Google Scholar]
- Horbowicz M, Dębski H, Wiczkowski W, Szawara-Nowak D, Koczkodaj D, Mitrus J, Sytykiewicz H. The impact of short-term exposure to Pb and Cd on flavonoid composition and seedling growth of common buckwheat cultivars. Polish Journal of Environmental Studies. 2013;22:1723–1730. [Google Scholar]
- Horbowicz M, Wiczkowski W, Szawara-Nowak D, Sawicki T, Kosson R, Sytykiewicz H. The level of flavonoids and amines in de-etiolated and methyl jasmonate treated seedling of common buckwheat. Phytochemistry Letters. 2015;13:15–19. doi: 10.1016/j.phytol.2015.05.011. [DOI] [Google Scholar]
- Huda MN, Lu S, Jahan T, Ding M, Jha R, Zhang K, Zhang W, Georgiev MI, Park SU, Zhou M. Treasure from garden: Bioactive compounds of buckwheat. Food Chemistry. 335: 127653 (2021) [DOI] [PMC free article] [PubMed]
- Hung PV, Trinh LND, Thuy NTX, Morita N. Changes in nutritional composition, enzyme activities and bioactive compounds of germinated buckwheat (Fagopyrum esculantum M.) under unchanged air and humidity conditions. International Journal of Food Science and Technology. 56: 3209-3217 (2021)
- Ishii S, Katsumura T, Shiozuka C, Ooyauchi K, Kawasaki K, Takigawa S, Fukushima T, Tokuji Y, Kinoshita M, Ohnishi M, Kawahara M, Ohba K. Anti-inflammatory effect of buckwheat sprouts in lipopolysaccharide-activated human colon cancer cells and mice. Bioscience, Biotechnology, and Biochemistry. 2008;72:3148–3157. doi: 10.1271/bbb.80324. [DOI] [PubMed] [Google Scholar]
- Jang D, Jung YS, Kim MS, Oh SE, Nam TG, Kim DO. Developing and validating a method for separating flavonoid isomers in common buckwheat sprouts using HPLC-PDA. Foods. 2019;8:549. doi: 10.3390/foods8110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang D, Jung YS, Seong H, Kim MS, Rha CS, Nam TG, Han NS, Kim DO. Stability of enzyme-modified flavonoid C- and O-glycosides from common buckwheat sprout extracts during in vitro digestion and colonic fermentation. Journal of Agricultural and Food Chemistry. 2021;69:5764–5773. doi: 10.1021/acs.jafc.1c00542. [DOI] [PubMed] [Google Scholar]
- Jeong H, Sung J, Yang J, Kim Y, Jeong HS, Lee J. Effect of sucrose on the functional composition and antioxidant capacity of buckwheat (Fagopyrum esculentum M.) sprouts. Journal of Functional Foods. 43: 70-76 (2018)
- Jung YS, Kwak IA, Lee SG, Cho HS, Cho YS, Kim DO. Influence of production systems on phenolic characteristics and antioxidant capacity of highbush blueberry cultivars. Journal of Food Science. 2021;86:2949–2961. doi: 10.1111/1750-3841.15784. [DOI] [PubMed] [Google Scholar]
- Kalinová JP, Vrchotová N, Tříska J. Vitexin and isovitexin levels in sprouts of selected plants. Journal of Food Composition and Analysis. 100: 103895 (2021)
- Kim SL, Kim SK, Park CH. Introduction and nutritional evaluation of buckwheat sprouts as a new vegetable. Food Research International. 2004;37:319–327. doi: 10.1016/j.foodres.2003.12.008. [DOI] [Google Scholar]
- Kim SJ, Maeda T, Sarker MZI, Takigawa S, Matsuura-Endo C, Yamauchi H, Mukasa Y, Saito K, Hashimoto N, Noda T, Saito T, Suzuki T. Identification of anthocyanins in the sprouts of buckwheat. Journal of Agricultural and Food Chemistry. 2007;55:6314–6318. doi: 10.1021/jf0704716. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Zaidul ISM, Suzuki T, Mukasa Y, Hashimoto N, Takigawa S, Noda T, Matsuura-Endo C, Yamauchi H. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chemistry. 2008;110:814–820. doi: 10.1016/j.foodchem.2008.02.050. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim MJ, Lee J. Role of moisture on the lipid oxidation determined by D2O in a linoleic acid model system. Food Chemistry. 2014;146:134–140. doi: 10.1016/j.foodchem.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Rahman MM, Lee MK, Seo JM, Arasu MV, Suzuki T, Al-Dhabi NA, Yoon YH, Shim JH. Identification and quantification of volatile and phenolic compounds composition in buckwheat sprouts by GC/MS and HPLC. Asian Journal of Chemistry. 2014;26:777–782. doi: 10.14233/ajchem.2014.15538. [DOI] [Google Scholar]
- Kim SJ, Sohn HB, Lee KT, Shin JS, Kim S, Nam JH, Hong SY, Suh JT, Chang DC, Kim YH. Anti-inflammatory effects of seed ethanolic extracts of the common buckwheat and tartary buckwheat are mediated through the suppression of inducible nitric oxide synthase and pro-inflammatory cytokines in LPS-induced RAW 264.7 macrophage cells. Korean Journal of Food Science and Technology. 51: 565-575 (2019)
- Kim HY, Bae WY, Yu HS, Chang KH, Hong YH, Lee NK, Paik HD. Inula britannica fermented with probiotic Weissella cibaria D30 exhibited anti-inflammatory effect and increased viability in RAW 264.7 cells. Food Science and Biotechnology. 29: 569-578 (2020) [DOI] [PMC free article] [PubMed]
- Koja E, Ohata S, Maruyama Y, Suzuki H, Shimosaka M, Taguchi G. Identification and characterization of a rhamnosyltransferase involved in rutin biosynthesis in Fagopyrum esculentum (common buckwheat) Bioscience, Biotechnology, and Biochemistry. 2018;82:1790–1802. doi: 10.1080/09168451.2018.1491286. [DOI] [PubMed] [Google Scholar]
- Koyama M, Nakamura C, Nakamura K. Changes in phenols contents from buckwheat sprouts during growth stage. Journal of Food Science and Technology. 2013;50:86–93. doi: 10.1007/s13197-011-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BR. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs) Journal of Pharmaceutical Analysis. 2017;7:349–364. doi: 10.1016/j.jpha.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Seo JM, Lee MK, Chun JH, Antonisamy P, Arasu MV, Suzuki T, Al-Dhabi NA, Kim SJ. Influence of different LED lamps on the production of phenolic compounds in common and tartary buckwheat sprout. Industrial Crops and Products. 2014;54:320–326. doi: 10.1016/j.indcrop.2014.01.024. [DOI] [Google Scholar]
- Lee MH, Nam TG, Lee I, Shin EJ, Han AR, Lee P, Lee SY, Lim TG. Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food Science and Nutrition. 2018;6:2560–2567. doi: 10.1002/fsn3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Park KJ, Kim BK, Jeong JW, Kim HJ. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chemistry. 135: 1065-1070 (2012) [DOI] [PubMed]
- Lim YJ, Lyu IL, Kwon SJ, Eom SH. Effects of UV-A radiation on organ-specific accumulation and gene expression of isoflavones and flavonols in soybean sprout. Food Chemistry. 339: 128080 (2021) [DOI] [PubMed]
- Lim CK, Lord G. Current developments in LC-MS for pharmaceutical analysis. Biological and Pharmaceutical Bulletin. 2002;25:547–557. doi: 10.1248/bpb.25.547. [DOI] [PubMed] [Google Scholar]
- Liu CL, Chen YS, Yang JH, Chiang BH. Antioxidant activity of tartary (Fagopyrum tataricum (L.) Gaertn.) and common (Fagopyrum esculentum Moench) buckwheat sprouts. Journal of Agricultural and Food Chemistry. 56: 173-178 (2008) [DOI] [PubMed]
- Ma L, Zhang B, Liu J, Qiao C, Liu Y, Li S, Lv H. Isoorientin exerts a protective effect against 6-OHDA-induced neurotoxicity by activating the AMPK/AKT/Nrf2 signalling pathway. Food and Function. 2020;11:10774–10785. doi: 10.1039/D0FO02165B. [DOI] [PubMed] [Google Scholar]
- Mansur AR, Song NE, Jang HW, Lim TG, Yoo M, Nam TG. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chemistry. 2019;293:438–445. doi: 10.1016/j.foodchem.2019.05.003. [DOI] [PubMed] [Google Scholar]
- Mansur AR, Kim KJ, Kim DB, Yoo M, Jang HW, Kim DO, Nam TG. Matrix solid-phase dispersion extraction method for HPLC determination of flavonoids from buckwheat sprouts. LWT. 133: 110121 (2020)
- Mun JH, Kim ID, Dhungana SK, Park YS, Kim JH, Shin DH. Yield and quality characteristics of Korean red bean sprouts produced with different time of seed soaking. Food Science and Biotechnology. 2020;29:197–206. doi: 10.1007/s10068-019-00657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naczk M, Shahidi F. Extraction and analysis of phenolics in food. Journal of Chromatography a. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Naramoto K, Koyama M. Blood-pressure-lowering effect of fermented buckwheat sprouts in spontaneously hypertensive rats. Journal of Functional Foods. 2013;5:406–415. doi: 10.1016/j.jff.2012.11.013. [DOI] [Google Scholar]
- Nakamura K, Koyama M, Ishida R, Kitahara T, Nakajima T, Aoyama T. Characterization of bioactive agents in five types of marketed sprouts and comparison of their antihypertensive, antihyperlipidemic, and antidiabetic effects in fructose-loaded SHRs. Journal of Food Science and Technology. 2016;53:581–590. doi: 10.1007/s13197-015-2048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TG, Lee SM, Park JH, Kim DO, Baek NI, Eom SH. Flavonoid analysis in buckwheat sprouts. Food Chemistry. 2015;170:97–101. doi: 10.1016/j.foodchem.2014.08.067. [DOI] [PubMed] [Google Scholar]
- Nam TG, Kim DO, Eom SH. Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Science and Biotechnology. 2018;27:169–176. doi: 10.1007/s10068-017-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TG, Lim YJ, Eom SH. Flavonoid accumulation in common buckwheat (Fagopyrum esculentum) sprout tissues in response to light. Horticulture, Environment, and Biotechnology. 2018;59:19–27. doi: 10.1007/s13580-018-0003-5. [DOI] [Google Scholar]
- Nam TG, Lim TG, Lee BH, Lim S, Kang H, Eom SH, Yoo M, Jang HW, Kim DO. Comparison of anti-inflammatory effects of flavonoid-rich common and tartary buckwheat sprout extracts in lipopolysaccharide-stimulated RAW 264.7 and peritoneal macrophages. Oxidative Medicine and Cellular Longevity. 2017: 96658030 (2017) [DOI] [PMC free article] [PubMed]
- Ozga JA, Saeed A, Wismer W, Reinecke DM. Characterization of cyanidin-and quercetin-derived flavonoids and other phenolics in mature saskatoon fruits (Amelanchier alnifolia Nutt.). Journal of Agricultural and Food Chemistry. 55: 10414-10424 (2007) [DOI] [PubMed]
- Park CH, Yeo HJ, Park YJ, Morgan A, Valan Arasu M, Al-Dhabi NA, Park SU. Influence of indole-3-acetic acid and gibberellic acid on phenylpropanoid accumulation in common buckwheat (Fagopyrum esculentum Moench) sprouts. Molecules. 2017;22:374. doi: 10.3390/molecules22030374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Yeo HJ, Park YE, Chun SW, Chung YS, Lee SY, Park SU. Influence of chitosan, salicylic acid and jasmonic acid on phenylpropanoid accumulation in germinated buckwheat (Fagopyrum esculentum Moench) Foods. 2019;8:153. doi: 10.3390/foods8050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravula AR, Teegala SB, Kalakotla S, Pasangulapati JP, Perumal V, Boyina HK. Fisetin, potential flavonoid with multifarious targets for treating neurological disorders: An updated review. European Journal of Pharmacology. 910: 174492 (2021) [DOI] [PubMed]
- Ren SC, Sun JT. Changes in phenolic content, phenylalanine ammonia-lyase (PAL) activity, and antioxidant capacity of two buckwheat sprouts in relation to germination. Journal of Functional Foods. 2014;7:298–304. doi: 10.1016/j.jff.2014.01.031. [DOI] [Google Scholar]
- Rha CS, Jung YS, Lee JD, Jang D, Kim MS, Lee MS, Hong YD, Kim DO. Chemometric analysis of extracts and fractions from green, oxidized, and microbial fermented teas and their correlation to potential antioxidant and anticancer effects. Antioxidants. 2020;9:1015. doi: 10.3390/antiox9101015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi MM, Dipjyoti C. Mass spectrometric detection of phenolic acids. pp. 2047–2059 In: Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes. Ramawat KG, Mérillon JM (eds). Spronger-Verlag: Berlin, Heidelberg (2013)
- Seo JM, Aras MV, Kim YB, Park SU, Kim SJ. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chemistry. 2015;177:204–213. doi: 10.1016/j.foodchem.2014.12.094. [DOI] [PubMed] [Google Scholar]
- Sim U, Sung J, Lee H, Heo H, Jeong HS, Lee J. Effect of calcium chloride and sucrose on the composition of bioactive compounds and antioxidant activities in buckwheat sprouts. Food Chemistry. 312: 126075 (2020) [DOI] [PubMed]
- Swartz M. HPLC detectors: a brief review. Journal of Liquid Chromatography and Related Technologies. 2010;33:1130–1150. doi: 10.1080/10826076.2010.484356. [DOI] [Google Scholar]
- Terpinc P, Cigić B, Polak T, Hribar J, Požrl. LC–MS analysis of phenolic compounds and antioxidant activity of buckwheat at different stages of malting. Food Chemistry. 210: 9-17 (2016) [DOI] [PubMed]
- Thangaraj K, Vaiyapuri M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1, 2-dimethylhydrazine induced colorectal carcinogenesis. Biomedicine and Pharmacotherapy. 2017;96:1253–1266. doi: 10.1016/j.biopha.2017.11.088. [DOI] [PubMed] [Google Scholar]
- Thangaraj K, Balasubramanian B, Park S, Natesan K, Liu W, Manju V. Orientin induces G0/G1 cell cycle arrest and mitochondria mediated intrinsic apoptosis in human colorectal carcinoma HT29 cells. Biomolecules. 2019;9(9):418. doi: 10.3390/biom9090418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurunaga Y, Takahashi T, Katsube T, Kudo A, Kuramitsu O, Ishiwata M, Matsumoto S. Effects of UV-B irradiation on the levels of anthocyanin, rutin and radical scavenging activity of buckwheat sprouts. Food Chemistry. 2013;141:552–556. doi: 10.1016/j.foodchem.2013.03.032. [DOI] [PubMed] [Google Scholar]
- Wiczkowski W, Szawara-Nowak D, Dębski H, Mitrus J, Horbowicz M. Comparison of flavonoids profile in sprouts of common buckwheat cultivars and wild tartary buckwheat. International Journal of Food Science and Technology. 2014;49:1977–1984. doi: 10.1111/ijfs.12484. [DOI] [Google Scholar]
- Wiczkowski W, Szawara-Nowak D, Sawicki T, Mitrus J, Kasprzykowski Z, Horbowicz M. Profile of phenolic acids and antioxidant capacity in organs of common buckwheat sprout. Acta Alimentaria. 2016;45:250–257. doi: 10.1556/066.2016.45.2.12. [DOI] [Google Scholar]
- Witkowicz R, Biel W, Skrzypek E, Chłopicka J, Gleń-Karolczyk K, Krupa M, Prochownik E, Galanty A. Microorganisms and biostimulants impact on the antioxidant activity of buckwheat (Fagopyrum esculentum Moench) sprouts. Antioxidants. 2020;9:584. doi: 10.3390/antiox9070584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Cui Y, Zhao Y, Liu L, Wang H, Yang L. Orientin relieves lipopolysaccharide-induced acute lung injury in mice: The involvement of its anti-inflammatory and anti-oxidant properties. International Immunopharmacology. 90: 107189 (2021) [DOI] [PubMed]
- Yang HJ, Lim JH, Park KJ, Kang S, Kim DS, Park S. Methyl jasmolate treated buckwheat sprout powder enhances glucose metabolism by potentiating hepatic insulin signaling in estrogen-deficient rats. Nutrition. 2016;32:129–137. doi: 10.1016/j.nut.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Yoo H, Ku SK, Baek YD, Bae JS. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflammation Research. 2014;63:197–206. doi: 10.1007/s00011-013-0689-x. [DOI] [PubMed] [Google Scholar]
- Yuan L, Wu Y, Ren X, Liu Q, Wang J, Liu X. Isoorientin attenuates lipopolysaccharide-induced pro-inflammatory responses through down-regulation of ROS-related MAPK/NF-κB signaling pathway in BV-2 microglia. Molecular and Cellular Biochemistry. 2014;386:153–165. doi: 10.1007/s11010-013-1854-9. [DOI] [PubMed] [Google Scholar]
- Zhang G, Xu Z, Gao Y, Huang X, Zou Y, Yang T. Effects of germination on the nutritional properties, phenolic profiles, and antioxidant activities of buckwheat. Journal of Food Science. 2015;80:H1111–H1119. doi: 10.1111/1750-3841.12830. [DOI] [PubMed] [Google Scholar]
- Živković A, Polak T, Cigić B, Požrl T. Germinated Buckwheat: Effects of dehulling on phenolics profile and antioxidant activity of buckwheat seeds. Foods. 2021;10:740. doi: 10.3390/foods10040740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žuvela P, Skoczylas M, Jay Liu J, Bączek T, Kaliszan R, Wong MW, Buszewski B. Column characterization and selection systems in reversed-phase high-performance liquid chromatography. Chemical Reviews. 119: 3674-3729 (2019) [DOI] [PubMed]