Abstract
Toxic-metabolite-emitting microbes were isolated from the indoor environment of a building where the occupant was suffering serious building-related ill-health symptoms. Toxic substances soluble in methanol and inhibitory to spermatozoa at <10 μg (dry weight) ml−1 were found from six bacterial isolates and one fungus. The substances from isolates of Bacillus simplex and from isolates belonging to the actinobacterial genera Streptomyces and Nocardiopsis were mitochondriotoxic. These substances dissipated the mitochondrial membrane potential (Δψ) of boar spermatozoa. The substances from the Streptomyces isolates also swelled the mitochondria. The substances from isolates of Trichoderma harzianum Rifai and Bacillus pumilus damaged the cell membrane barrier function of sperm cells.
It is widely recognized that microbes are involved in health problems connected to water-damaged buildings (10, 11, 16, 33, 39), but no specific microbe or toxin has been identified as the dominating cause. Mycotoxins, endotoxins (lipopolysaccharide) of gram-negative bacteria, β-d-glucans, and cytotoxic and mitochondriotoxic metabolites produced by Streptomyces species have been suspected to cause health problems (4, 10, 11, 33, 34). Certain fungal genera (31) and spore-forming actinobacteria are considered indicative of health problems (6). The role of other bacteria and fungi found in large amounts in damp houses has been ignored.
In this paper we describe six toxic bacteria, identified as Bacillus pumilus, Bacillus simplex, species of Streptomyces and Nocardiopsis, and a toxic mold, Trichoderma harzianum Rifai, from a private dwelling where an occupant exhibited serious symptoms, like exacerbations of asthma, sinusitis, urticaria, blocked nose, rhinitis, otitis, hoarseness, ache in joints, myalgia, and tiredness. Toxins were detected using boar spermatozoa as indicator cells. These cells were successfully used earlier in the search for toxins in buildings with histories of water damage (2, 4, 28) and for detecting valinomycin-producing isolates of Streptomyces griseus (4) in the indoor environment and peptide toxins of Bacillus cereus and Bacillus licheniformis from food poisoning (3, 24).
The building studied was a detached house with natural ventilation, built in the 1950s. The family had lived in the house since 1986 and recently built an extension. Water damage, detected in 1993, was remediated by installing a subsurface drainage around the house. In 1996 the water damage noticed in the basement bathroom, in the roof, and in the outdoor wall of the extension was repaired.
Indoor air was sampled on 9 February 1998 with a six-stage Andersen impactor (28.3 liters min−1; Graseby Andersen, Atlanta, Ga.) on tryptic soy agar plates (Difco, Detroit, Mich.) and on corn meal agar plates (Difco) for 10 to 15 min. The plates were incubated for 7 to 14 days at 15 to 22°C, colonies were counted, and pure cultures were prepared. Cultures were also isolated from the construction materials by the dilution plating method as described by Andersson et al. (2, 5).
The pure bacterial isolates were subcultured on tryptic soy agar plates, and the fungal isolates were subcultured on 2% malt extract agar (Biokarr Diagnostics, Beauvais, France). After 10 days the biomass was harvested, extracted, evaporated, and assayed as a methanol solution for boar spermatozoon motility inhibition as described by Andersson et al. (2), judged with a phase-contrast microscope. The boar spermatozoa were commercial tradeware (AI Cooperative, Kaarina, Finland).
Damage to the spermatozoan plasma membrane permeability barrier was assessed by differential staining with propidium iodide and SYBR-14 (Live/dead sperm viability kit; Molecular Probes, Eugene, Oreg.). Propidium iodide fluoresces red when bound to DNA but needs damaged plasma membranes to penetrate the cell, where as SYBR-14 is a membrane-permeating nucleic acid stain and fluoresces bright green (12). One microliter of SYBR-14 (20 μg ml−1 of commercial semen extender BTS; IMV, L'Aigle Cedex, France) was mixed with 200 μl of extended boar semen (40 × 106 to 60 × 106 cells ml−1) and incubated for 10 min at 36°C, and 1 μl of propidium iodide (1 mg in dimethyl sulfoxide ml−1) was added. After 5 to 10 min at 36°C, the suspension was inspected with an epifluorescence microscope (390 to 490 nm for excitation; longpass emission filter, 515 nm). The mitochondrial membrane potential (Δψ) was visualized by staining 200 μl of extended boar semen with 0.7 μl of 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolocarbocyanine iodide (JC-1; 1 mg ml−1 in dimethyl sulfoxide; Molecular Probes). This mixture was shaken vigorously, incubated for 3 to 5 min at 36°C, and inspected by epifluorescence microscopy as above. JC-1 is a cationic and lipophilic dye. It permeates intact cells and accumulates in the mitochondria (7, 35). Transmission electron microscopy (TEM) was done as described earlier (1) except that 2.5% glutaraldehyde was used for fixation.
The bacterial isolates were identified based on morphology, whole-cell fatty acid composition phenotypic properties, and full or partial 16S rRNA gene sequencing. Whole-cell fatty acids were analyzed as described earlier (4, 5, 41) using MIDI Aerobic Library version 3.90 (Microbial ID, Newark, Del.). The extraction of genomic DNA, PCR amplification of the 16S rRNA gene, and sequencing of the purified PCR products were carried out as described previously (30). Sequence reaction products were purified by ethanol precipitation and electrophoresed with a model 310 genetic analyzer (Applied Biosystems, Foster City, Calif.). The 16S rRNA gene sequences obtained were aligned against previously determined actinobacterial sequences using the ae2 editor (21).
Indoor air from the basement and the living room of the studied residence contained approximately 103 CFU of cultivatable bacteria m−3 harvested with the Andersen sampler. Colony counts of air-borne spore-forming actinobacteria and fungi in the basement air were higher than those in the living room air (Table 1). Over 50% of the colonies which propagated from the air were from stages four to six of the Andersen sampler, indicating particle sizes of 3.3 to 0.65 μm. This is the fraction respirable via the bronchi into alveoli.
TABLE 1.
Culturable heterotrophic aerobic microorganisms collected from the indoor air and construction materials of a water-damaged residential buildinga
| Sample | CFU
|
||
|---|---|---|---|
| Total bacteria | Spore-forming actinobacteriab | Fungi | |
| Basement air (CFU m−3) | 1,030 | 64 | 540 |
| Living room air (CFU m−3) (ground floor) | 1,100 | 7 | 130 |
| Basement materials (CFU g−1) | |||
| Wood from the doorstep | 5.0 × 104 | 1.8 × 104 | 5.2 × 104 |
| Thermal insulation material (made of rag-based fiber) | 4.5 × 104 | <103 | 9.3 × 104 |
| Filter from vacuum cleaner | 1.9 × 105 | 5.5 × 103 | 9.4 × 104 |
| Upstairs materials (CFU g−1) | |||
| Fiberboard ceiling (water-damaged) | 1.3 × 105 | 5.3 × 104 | 6.3 × 106 |
| Fiberboard ceiling (not water-damaged) | 7.2 × 103 | 1.5 × 103 | 3.8 × 104 |
| Woody ceiling board | 6.7 × 103 | <103 | 2.0 × 103 |
Indoor air was sampled using a six-stage Andersen sampler on tryptic soy agar (bacteria) and corn meal agar plates (fungi).
Based on colony morphology.
Construction materials from the basement contained high numbers (104 to 105 CFU g−1) of cultivatable bacteria and fungi (Table 1). Actinobacteria grew from a woody doorstep and also from a filter of the vacuum cleaner. The fungal plate count in the fiber board of the upstairs ceiling with a known history of water damage was up to 6.3 × 106 CFU g−1 and that of bacteria was 105 CFU g−1, of which 41% were identified as spore-forming actinobacteria (Table 1). The results indicated that there were sources of spore-forming actinobacteria and of fungi in the water-damaged ceiling and in the basement. The recent renovation and drainage operation may have halted further water damage but did not eliminate the microbes resulting from the past history of the building.
Extracts prepared from 47 microbial isolates were tested for toxicity in the boar sperm cell motility assay. Extracts of three isolates of Streptomyces (ES9, ES14, and ES16), one Nocardiopsis (ES10.1), two Bacillus (ES20 and ES21), and one fungal isolate (ES39) inhibited the motility of boar spermatozoa with low 50% effective concentration (EC50) values, <10 μg of methanol-soluble substance ml−1. The toxic bacteria originated from air inside the building. The toxic air-borne Nocardiopsis and Streptomyces isolates were from stages five and six of the Andersen sampler (particle sizes of 2.1 to 0.65 μm), indicating that they may penetrate the alveoli in the lung. The toxic fungal isolate ES39, identified as Trichoderma harziamum Rifai, was found in thermal insulation material in the basement.
The 16S rRNA gene sequences were determined for the toxic spore-forming actinobacteria. The sequence obtained from Nocardiopsis sp. strain ES10.1 was 99.4% similar to that of Nocardiopsis prasina DSM 43845T. The partial 16S rRNA gene sequences and the phenotypic properties showed that isolates ES9, ES14, and ES16 were species of Streptomyces. Isolates ES9 and ES14 showed 100% 16S rRNA gene sequence similarity to S. griseus, and phenotypic properties were also similar to those of S. griseus. Isolate ES16 was 99.1% similar to Streptomyces abikoensis and to Streptomyces ehimensis, but differed from these strains in phenotypic properties. The partial 16S rRNA gene sequence obtained from toxic Bacillus isolate ES21 was 100% similar to that of Bacillus macroides, but the closest (99.6%) validity described species was Bacillus simplex. Another toxic Bacillus isolate, ES20, was identified as Bacillus pumilus based on the whole-cell fatty acid composition and phenotypic properties (40).
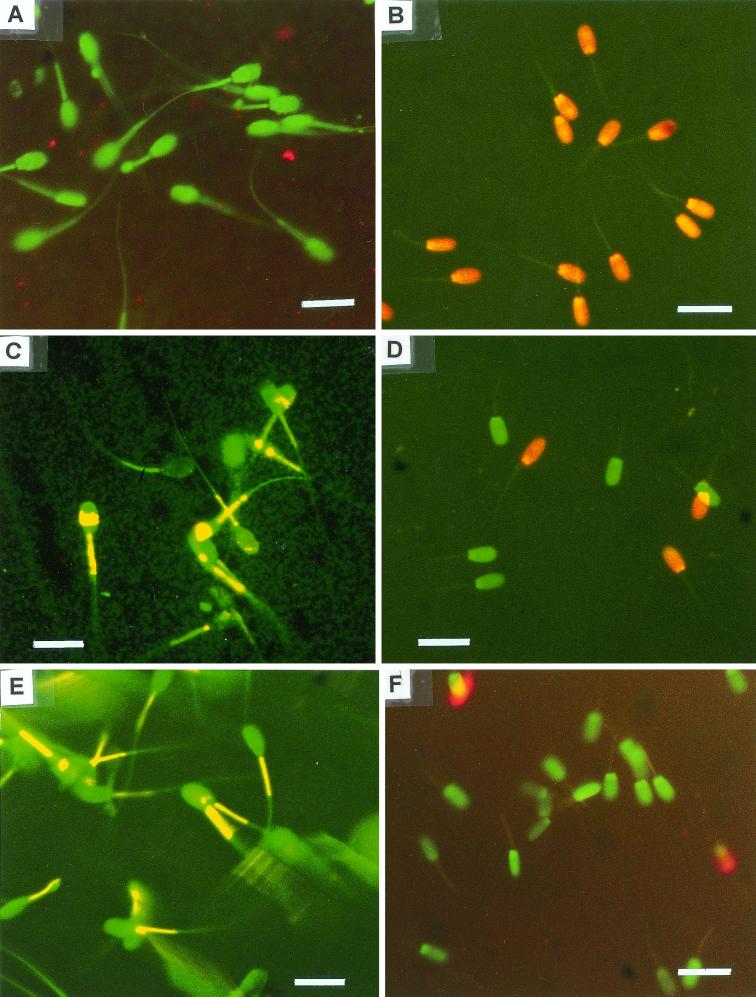
Exposure of boar spermatozoa to an extract from T. harzianum ES39 relaxed the cell membrane permeability barrier to allow propidium iodide to enter, visible as red fluorescence of the cells (Fig. 1B). Exposure also resulted in quenching of the yellow fluorescence of the midpiece of boar spermatozoa stained with JC-1, indicating dissipation of the mitochondrial membrane potential, Δψ (Fig. 1A). The agent in the extract of T. harzianum ES39 responsible for paralyzing the sperm cell motility was heat stable (100°C for 20 min). T. harzianum species have earlier been reported to produce various secondary metabolites (13, 36), for example, hydrophobic antibiotics interacting with phospholipid-containing membranes (13, 37). A strongly membrane-damaging substance was also detected in an extract prepared from B. pumilus ES20. Several species of the genus Bacillus are known producers of low-molecular-weight nonproteinaceous toxins. For instance, B. pumilus produces the bioactive cyclic peptides surfactin and pumilacidin (25, 26). Toxin-producing B. pumilus has been suspected to have a role in the etiology of byssinosis (18).
FIG. 1.
Epifluorescence micrographs of boar spermatozoa stained with the dye JC-1 to visualize the mitochondrial Δψ (A, C, and E) and with a live/dead staining with SYBR-14 and propidium iodide (B, D, and F) to demonstrate effects on the cell membrane. Boar spermatozoa were exposed to 9.6 μg of methanol-soluble metabolites from Trichoderma harzianum ES39 ml−1 for 3 days (A and B) or to 9.0 μg of methanol-soluble metabolites from Streptomyces sp. strain ES16 ml−1 for 3 days (C and D) or to the reagents only (E and F). Excitation light, 390 to 490 nm. Bars, 10 μm.
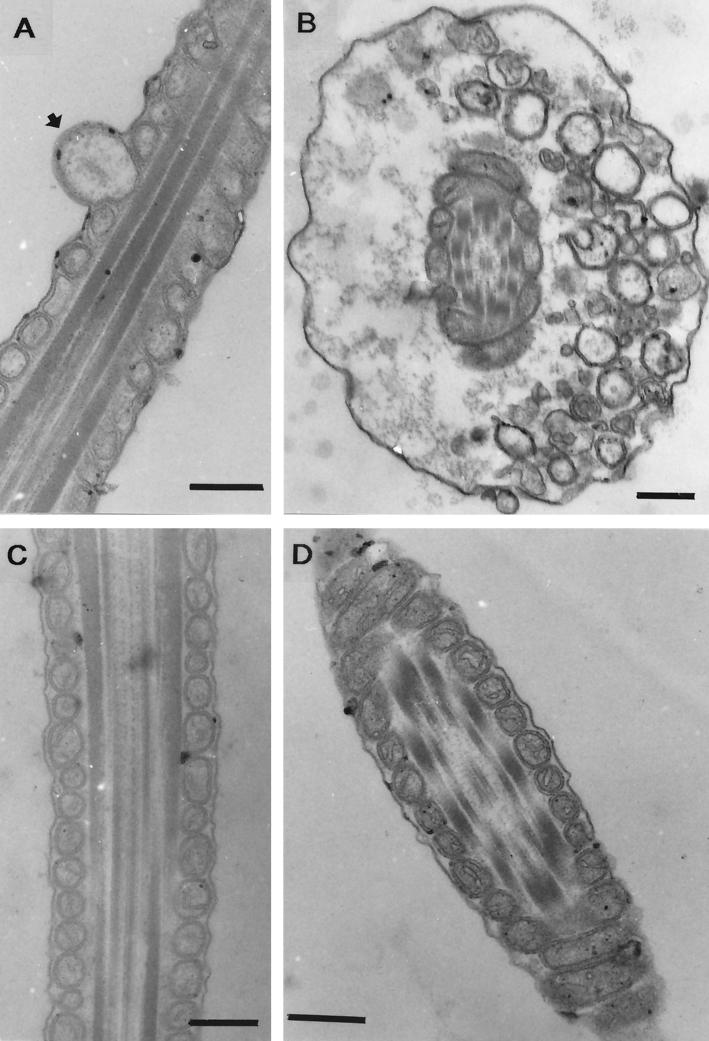
The agents in extracts from Streptomyces griseus ES9 and ES14 and Streptomyces sp. strain ES16 responsible for paralyzing sperm cell motility were heat stable (100°C for 20 min). Microscopic inspection of spermatozoa differentially stained with the dye JC-1 showed that exposure to these extracts quenched the yellow fluorescence in the midpiece of the sperm cell, as shown in Fig. 1C for ES16. The mitochondria of the sperm cells are located in the midpiece. The result thus indicates dissipation of the mitochondrial membrane potential, Δψ. Staining of the exposed sperm cells with SYBR-14 and propidium iodide showed that propidium iodide was excluded by the cells, indicating that the plasma membrane was intact in spite of the observed mitochondrial damage (Fig. 1D). Extracts from Streptomyces isolates ES9, ES14, and ES16 caused swelling of mitochondria, as shown for ES16 in Fig. 2A. Specific mitochondrial toxicity, i.e., dissipation of Δψ and swelling, has earlier been reported for spermatozoa exposed to cereulide from Bacillus cereus (3, 23) and valinomycin (23) produced by indoor-air isolates of S. griseus (4). The effects observed in this study for S. griseus ES9 and ES14 and Streptomyces sp. strain ES16 were thus similar to the effects caused by two known potassium ionophores, valinomycin and cereulide. Valinomycin produced by indoor isolates of S. griseus was earlier shown to cause mitochondrial swelling, activity loss, and apoptosis in human natural killer cells (27).
FIG. 2.
TEM of thin sections of middle piece of boar spermatozoa exposed to methanol-soluble metabolites from toxic bacterial isolates of indoor-environment origin. Boar spermatozoa were exposed to 9.0 μg of methanol-soluble substances of Streptomyces sp. strain ES16 ml−1 for 3 days (A) or to 40.0 μg of B. simplex ES21 ml−1 for 3 days (B). Longitudinal (C) and transverse (D) thin sections of spermatozoon exposed to reagents only. A swollen mitochondrion (arrow) is visible in panel A. Swelling of the midpiece of the spermatozoan tail is visible in panel B. Thin sections of panels C and D show unaffected cell membranes and normal-sized mitochondria. Bars, 0.2 μm.
The amount of valinomycin in S. griseus may be high, contents up to 1% of dry weight have been noted for isolates from the indoor environment (4) and from animal feces (29). Taking into account that usually only 1% of environmental bacteria show up in the colony counts and that nonculturable cells may exhibit the same toxicity as the viable ones, the building materials in the residence studied in this work likely contained mitochondriotoxic substances in amounts that may be immunotoxic to humans. Streptomyces species are known to be productive sources of secondary metabolites (14). Therefore, other metabolites may also have been present in the building studied.
Exposure to extracts of the indoor isolate B. simplex (“B. macroides”) ES21 caused proximal droplet-like swellings in the spermatozoan midpiece (Fig. 2B) at a frequency higher than was observed in spermatozoa exposed to methanol only (Fig. 2C and D). There was no visible damage to the plasma membrane of the boar sperm cell (Fig. 2B). Extracts prepared from the indoor Nocardiopsis isolate ES10.1 inhibited boar spermatozoon motility in the absence of any ultrastructural change visible by TEM. Extracts prepared from this isolate and from B. simplex ES21 dissipated the mitochondrial Δψ similarly to the extracts from the Streptomyces isolates. But since no swelling of mitochondria was observed in the exposed spermatozoa, the mitochondriotoxin extracted from these isolates may not have been a potassium ionophore. Mitochondrial membrane potential (Δψ) energizes the motility of boar spermatozoa (23), thus explaining the motility loss by mitochondrial damage. Dissipation of mitochondrial ion gradient initiates programmed cell death in eukaryotic cells (reviewed in references 7 and 15). Thus, the exposure of humans to mitochondriotoxin-emitting microbes present in the water-damaged building is of concern.
Further bacterial isolates from the indoor air were identified as members of the genera Bacillus, Nocardia, Gordonia, Rhodococcus, Dietzia, Micrococcus, Methylobacterium, and Flavobacterium. The air-borne species in the basement were mainly the same genera as those in the living room except for Micrococcus, which was dominant in the basement, and Bacillus spp., which were dominant in the living room. The Dietzia (identified by partial 16S rRNA gene sequence), Rhodococcus, Nocardia, and Gordonia species contain tuberculostearic acid (8), which is a component of lipoarabinomannan in the cell membrane (19). Lipoarabinomannan is a powerful stimulator of tumor necrosis factor alpha in human macrophages (9, 32). The genera Rhodococcus, Nocardia, and Gordonia also contain pathogenic species (22).
The most prevalent symptoms reported in water-damaged houses are irritation of the respiratory tract and eyes (20). In this paper we show the presence of mitochondriotoxic actinobacteria (Streptomyces and Nocardiopsis) and B. simplex as well as the presence of eukaryotic membrane damage-inducing B. pumilus and T. harzianum Rifai in indoor air and construction material. Surface-active compounds may adversely affect the tear film in the eyes and thus account for the eye irritation (38). It has also been suggested that species of Streptomyces may be involved in respiratory disorders observed in individuals living in moldy houses (17).
The evidence in this paper indicates that the occupant of the moisture-problem house was exposed to multiple, differently acting toxins of microbial origin as well as to potential pathogens.
Nucleotide sequence accession numbers.
Accession numbers for the 16S rRNA gene sequences of the bacterial isolates are Nocardiopsis sp. ES10.1, DSM 44407, AY028325; Streptomyces griseus ES9 (DSM 41772), AY028322; Streptomyces griseus ES14 (DSM 41773), AY028324; Streptomyces sp. strain ES16 (DSM 41774), AY028323; and Dietzia sp. strain ES18, AY028326. B. pumilus ES20 and B. simplex ES21 are available in the DSMZ culture collection under the numbers DSM 13835 and DSM 13997, respectively.
Acknowledgments
This work was supported by a scholarship from the ABS Graduate School to J.P., the Academy of Finland (grant 50733), and the Yrjö Jahnsson Fund.
We thank Magnus C. Andersson for advice on analyzing boar spermatozoa, Tuire Koro for preparing the thin sections, and Viikki Science Library for expert information service. We also acknowledge access to the facilities of the Laboratory of Electron Microscopy of Helsinki University.
REFERENCES
- 1.Andersson M A, Laukkanen M, Nurmiaho-Lassila E-L, Rainey F A, Niemelä S, Salkinoja-Salonen M S. Bacillus thermosphaericus sp. nov.: a new thermophilic ureolytic Bacillus isolated from air. Syst Appl Microbiol. 1995;18:203–220. [Google Scholar]
- 2.Andersson M A, Nikulin M, Köljalg U, Andersson M C, Rainey F A, Reijula K, Hintikka E-L, Salkinoja-Salonen M S. Bacteria, molds, and toxins in water-damaged building materials. Appl Environ Microbiol. 1997;63:387–393. doi: 10.1128/aem.63.2.387-393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersson M A, Mikkola R, Helin J, Andersson M C, Salkinoja-Salonen M S. A novel sensitive bioassay for the detection of Bacillus cereus emetic toxin and related depsipeptide ionophores. Appl Environ Microbiol. 1998a;64:1338–1343. doi: 10.1128/aem.64.4.1338-1343.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson M A, Mikkola R, Kroppenstedt R, Rainey F A, Peltola J, Helin J, Sivonen K, Salkinoja-Salonen M S. Mitochondrial toxin produced by Streptomyces griseus strains isolated from indoor environment is valinomycin. Appl Environ Microbiol. 1998b;64:4767–4773. doi: 10.1128/aem.64.12.4767-4773.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson M A, Weiss N, Rainey F A, Salkinoja-Salonen M S. Dust-borne bacteria in animal sheds, schools and children's day care centers. J Appl Microbiol. 1999;86:622–634. doi: 10.1046/j.1365-2672.1999.00706.x. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. Indoor air guideline. Department of Social Affairs and Health. Helsinki, Finland: Edita; 1997. . (In Finnish.) [Google Scholar]
- 7.Bernardi P, Scorrano L, Colonna R, Petronilli V, Di Lisa F. Mitochondria and cell death (a review): mechanistic aspects and methodological issues. Eur J Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 8.Chun J, Kang S-O, Goodfellow M. Phylogeny of mycolic acid-containing actinomycetes. J Ind Microbiol. 1996;17:205–213. [Google Scholar]
- 9.Colston M I. The cellular and molecular basis of immunity against mycobacterial diseases. J Appl Bacteriol Symp Suppl. 1996;81:33S–39S. [PubMed] [Google Scholar]
- 10.Etzel R, Montana E, Sorenson W, Kullman G, Allan T, Dearborn D. Acute pulmonary hemorrhage in infants associated with exposure to Stachybotrys atra and other fungi. Arch Pediatr Adolesc Med. 1998;152:757–762. doi: 10.1001/archpedi.152.8.757. [DOI] [PubMed] [Google Scholar]
- 11.Flannigan B, Miller J D. Health implications of fungi in indoor environments — an overview. In: Samson R A, Flannigan B, Flannigan M E, Verhoeff A P, editors. Health implications of fungi in indoor environments. Amsterdam, The Netherlands: Elsevier; 1994. pp. 3–26. [Google Scholar]
- 12.Garner D L, Thomas C A, Gravance C G. The effect of glycerol on the viability, mitochondrial function and acrosomal integrity of bovine spermatozoa. Reprod Domest Anim. 1999;34:399–404. [Google Scholar]
- 13.Ghisalberti E L, Sivasithamparam K. Antifungal antibiotics produced by Trichoderma spp. Soil Biol Biochem. 1991;23:1011–1020. [Google Scholar]
- 14.Gräfe U. Biochemie der Antibiotika: Struktur-Biosynthese-Wirkmechanismus. Heidelberg, Germany: Spektrum Akademischer-Verlag GmbH; 1992. Biosynthesen ausgewandten Strukturklassen; pp. 219–318. [Google Scholar]
- 15.Green D, Reed J. Mitochondria and apoptosis. Science. 1998;281:309–312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 16.Hendry K, Cole E. A review of mycotoxins in indoor air. J Toxicol Environ Health. 1993;38:183–198. doi: 10.1080/15287399309531711. [DOI] [PubMed] [Google Scholar]
- 17.Hirvonen M-R, Nevalainen A, Makkonen N, Mönkkönen J, Savolainen K. Streptomycetes spores from moldy houses induce nitric oxide, TNFα and IL-6 secretion from RAW 264.7 macrophage cell line without causing subsequent cell death. Environ Toxicol Pharmacol. 1997;3:57–63. doi: 10.1016/s1382-6689(96)00140-8. [DOI] [PubMed] [Google Scholar]
- 18.Hoult B, Tuxford A F. Toxin production of Bacillus pumilus. J Clin Pathol. 1991;44:455–458. doi: 10.1136/jcp.44.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter S W, Gaylord H, Brennan P J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 20.Husman T. Health effects of indoor-air microorganisms: a review. Scand J Work Environ Health. 1996;22:5–13. doi: 10.5271/sjweh.103. [DOI] [PubMed] [Google Scholar]
- 21.Maidak B L, Cole J R, Parker C T, Jr, Garrity G M, Larsen N, Bing L, Lilburn T G, McCaughey M J, Olsen G J, Overbeek R, Pramanik S, Schmidt T M, Tiedje J M, Woese C R. A new version of the RDP (Ribosomal Database Project) Nucleic Acids Res. 1999;27:171–173. doi: 10.1093/nar/27.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNeil M M, Brown J M. The medically important aerobic actinomycetes. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikkola R, Saris N-E, Grigoriev P A, Andersson M A, Salkinoja-Salonen M S. Ionophoretic properties and mitochondrial effects of cereulide, the emetic toxin of B. cereus. Eur J Biochem. 1999;263:112–117. doi: 10.1046/j.1432-1327.1999.00476.x. [DOI] [PubMed] [Google Scholar]
- 24.Mikkola R, Kolari M, Andersson M A, Helin J, Salkinoja-Salonen M S. Toxic lactonic lipopeptide from food poisoning isolates of Bacillus licheniformis Eur. J Biochem. 2000;267:4068–4074. doi: 10.1046/j.1432-1033.2000.01467.x. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa M, Ito M, Imanaka T. Isolation of a new surfactin producer Bacillus pumilus A-1, and cloning and nucleotide sequence of the regulator gene, psf-1. J Ferment Bioeng. 1992;74:255–261. [Google Scholar]
- 26.Naruse N, Tenmyo O, Kobaru S, Kamei H, Miyaki T, Konishi M, Oki T. Pumilacidin, a complex of new antiviral antibiotics—production, isolation, chemical properties, structure and biological activity. J Antibiot. 1990;43:267–280. doi: 10.7164/antibiotics.43.267. [DOI] [PubMed] [Google Scholar]
- 27.Paananen A, Mikkola R, Sareneva T, Matikainen S, Andersson M A, Julkunen I, Salkinoja-Salonen M S, Timonen T. Inhibition of human NK cell function by valinomycin, a toxin from Streptomyces griseus in indoor air. Infect Immun. 2000;68:165–169. doi: 10.1128/iai.68.1.165-169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltola J, Andersson M A, Mikkola R, Mussalo-Rauhamaa H, Salkinoja-Salonen M S. Membrane toxic substances in water-damaged construction materials and fungal pure cultures. In: Johanning E, editor. Bioaerosols, fungi and mycotoxins: health effects, assessment, prevention and control. Eastern New York Occupational and Environmental Health Center. N.Y: Albany; 1999. pp. 432–443. [Google Scholar]
- 29.Pettit G, Tan R, Melody N, Kielty J, Pettit R, Herald D, Tucker B, Mallavia L, Doubek D, Schmidt J. Antineoplastic agents. 409. Isolation and structure of montanastatin from a terrestrial actinomycete. Bioorg Med Chem. 1999;7:895–899. doi: 10.1016/s0968-0896(99)00024-3. [DOI] [PubMed] [Google Scholar]
- 30.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 31.Rao C, Burge H, Chang J. Review of quantitative standards and guidelines for fungi in indoor air. J Air Waste Management Assoc. 1996;46:899–908. doi: 10.1080/10473289.1996.10467526. [DOI] [PubMed] [Google Scholar]
- 32.Rhoades E, Ullrich H J. How to establish a lasting relationship with your host: lessons learned from Mycobacterium spp. Immunol Cell Biol. 2000;78:301–311. doi: 10.1046/j.1440-1711.2000.00938.x. [DOI] [PubMed] [Google Scholar]
- 33.Rylander R. Microbial cell wall constituents in indoor air and their relation to disease. Indoor Air. 1998;4(Suppl.):59–65. [Google Scholar]
- 34.Rylander R. Indoor air-related effects and airborne (1→3)-β-d-glucan. Environ Health Perspect. 1999;107(Suppl. 3):501–503. doi: 10.1289/ehp.99107s3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6 or rhodamine 123, is a reliable fluorescent probe to assess Δψ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 36.Samson R A, Hoekstra E, Frisvad J, Filtenborg O, editors. Introduction to food-borne fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcultures; 1995. [Google Scholar]
- 37.Segalas I, Prigent Y, Davoust D, Bodo B, Rebuffat S. Characterization of a type of β-bend ribbon spiral generated by the repeating (Xaa-Yaa-Aib-Pro) motif: the solution structure of harzianin HC IX, a 14-residue peptaibol forming voltage-dependent ion channels. Biopolymers. 1999;50:71–85. [Google Scholar]
- 38.Skov P, Petersen L N, Wolkoff P. Indoor Air 99, Proceedings of the 8th Conference on Indoor Air Quality and Climate. Vol. 1. London, England: Construction Research Communications Ltd.; 1999. Eye irritation in the office environment, part II: possible mechanisms; pp. 123–128. [Google Scholar]
- 39.Sorenson W, Frazer D, Jarvis B B, Simpson J, Robinson V. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl Environ Microbiol. 1987;53:1370–1375. doi: 10.1128/aem.53.6.1370-1375.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suominen, I., M. A. Andersson, M. C. Andersson, A.-L. Hallaksela, P. Kämpfer, F. A. Rainey, and M. S. Salkinoja-Salonen. Toxic Bacillus pumilus from indoor air, recycled paper pulp and Norway spruce, food poisoning outbreaks and clinical samples. Syst. Appl. Microbiol., in press. [DOI] [PubMed]
- 41.Väisänen O, Nurmiaho-Lassila E-L, Marmo S, Salkinoja-Salonen M S. Structure and composition of biological slimes on paper and board machines. Appl Environ Microbiol. 1994;60:641–653. doi: 10.1128/aem.60.2.641-653.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]