Abstract
The pathogenesis of the severity of chikungunya infection is not yet fully understood. Objective: To assess the role of the cytokines/chemokines and system of complement in the evolution of chikungunya infection. Methods: In both acute and chronic phases, we measured the serum levels of 12 cytokines/chemokines and two complement mediators: mannose-binding lectin (MBL) and C3a, in 83 patients with chikungunya infection and ten healthy controls. Results: During the acute phase, 75.9% of the patients developed musculoskeletal disorders, and in 37.7% of them, these disorders persisted until the chronic phase. In general, patients had higher levels of cytokines than healthy controls, with significant differences for IFN-γ, IL-6, IL-8, IL-10, and MIP-1. Most cytokines exhibited a downward trend during the chronic phase. However, only IL-10, and MIP-1 levels were significantly lower in the chronic phase. Additionally, these levels never decreased to concentrations found in healthy controls. Moreover, MBL levels were significantly higher in the acute phase compared with the chronic phase. C3a levels were significantly higher in patients with musculoskeletal disorder compared with patients without it, in both acute-phase 118.2 (66.5-252.9), and chronic phase 68.5 (64.4-71.3), P < 0.001. Interestingly, C3a levels were significantly higher when patients had a severe disease version. Besides, in the acute phase, C3a levels were higher in patients that suffer arthritis as opposed to when they suffer arthralgia, 194.3 (69.5-282.2), and 70.9 (62.4-198.8), P = 0.013, respectively. Conclusions: Our results showed an immunological response that persisted until the chronic phase and the role of the complement system in the severity of the disease.
Keywords: Chikungunya virus, musculoskeletal disorder, disease severity, cytokines, C3a, mannose-binding lectin
Introduction
Chikungunya virus (CHIKV) is an arthritogenic virus that belongs to the Togaviridae family, genus Alphavirus, transmitted by Aedes mosquito bites. CHIKV emerged in the Americas in Saint Martin in 2013, and has currently spread to over 100 countries [1].
Most of the infected patients present with fever, headache, nausea, vomiting, myalgias, exanthema, and severe polyarthralgia/polyarthritis that are debilitants. This disease is generally self-limiting. Nevertheless, musculoskeletal manifestations can persist for months and even years, impacting the patients’ quality of life and the country’s economy [2,3].
The prevalence of patients who develop persistent musculoskeletal manifestations after three months of suffering from acute infection can range between 4.1% to 80% [4-8]. In addition to arthralgias, the musculoskeletal manifestations reported are inflammatory arthritis, synovitis, tenosynovitis/enthesitis and bursitis [9]. However, the pathophysiology of joint pain is still uncertain. One hypothesis is that the severe immune response in the post-viremia phase may be related to the severity of the infection and the development of chronic musculoskeletal manifestations [10-13]. Thus, the excessive production of cytokines driven by the activation of different cell populations in the early stages of the infection contributes to the progression of the disease.
Interleukin (IL)-1b, IL-6, IL-8, IL-12, interferon (IFN)-γ and MCP-1 (monocyte chemoattractant protein) [13], as well as IL-7 and IL-15, are involved in the acute phase of inflammation. On the other hand, cytokines IL-6 and GM-CSF (granulocyte-monocyte colony-stimulating factor) and chemokines IL-8, MCP-1 and MIP-1 (macrophage inflammatory protein-1) are associated with the persistence of arthralgia in CHIKV [11,12], though with inconclusive results. Furthermore, high levels of IL-10 have been detected in CHIKV recovered patients [14].
In addition, the role of the mannose-binding lectin (MBL) in the severe clinical evolution of the Ross River virus infection (RRV), another arthritogenic alphavirus, has been demonstrated [15]. MBL, through its binding to the microbial surface, is involved in the activation of the lectin pathway, one of three pathways by which the complement system can be activated. The activation of this cascade leads to the production of various fragments derived from component 3 (C3). One of these fragments is C3a, generated from the cleavage of C3 by C3-convertase. C3a is considered an anaphylatoxin responsible for mediating the local inflammatory process [16]. However, the role of the mannose-binding lectin and C3a have been rarely studied in CHIKV disease. To understand how the immune system is involved in this infection’s pathogenesis, especially in triggering the musculoskeletal disorder in the chronic phase, serum levels of some cytokines and immunological mediators of the complement system were measured in patients with CHIKV infection.
Materials and methods
Type and area of study
A longitudinal study was carried out between January 2016 and September 2018 in the following municipalities of Colombia: Medellín, the capital of the department of Antioquia, with 2,400,000 inhabitants; Apartadó, a municipality of the department of Antioquia, with 178,257 inhabitants; Ibagué, the capital of the department of Tolima, with 159,268 inhabitants; and Villavicencio, the capital of the department of Meta, with 551,212 inhabitants.
Study populations
The study populations were two: 1) Patients with CHIKV infection (n = 83) recruited in health institutions of the municipalities described above, and 2) Healthy controls (n = 10).
Inclusion criteria
(1) Patients with CHIKV infection
• Patients with at least 15 days of evolution of fever and arthralgia unexplained by other medical conditions.
• Patients with a laboratory diagnosis of CHIKV infection.
Exclusion criteria
• Participants with malaria confirmed by thick smear were excluded.
(2) Healthy controls
• Healthy participants in whom IgM antibodies against CHIKV were not observed.
Samples
Two serum samples were taken from each patient at different periods of infection. The first sample was taken during the acute phase of the disease, up to 15 days after the onset of symptoms, and the second sample, during the chronic phase, three months after the start of symptoms. Samples were stored at -80°C until their processing.
Diagnosis of CHIKV infection
In the acute phase sample was confirmed by detecting specific IgM antibodies against the chikungunya virus using a Novalisa® Chikungunya IgM µ-capture - ELISA (NovaTec Immunodiagnostica GMBH, Dietzenbach, Germany) commercial kit, which was processed according to the manufacturer’s instructions. Additionally, in patients between one and five days after the onset of symptoms, qRT-PCR was performed for viral genome detection, using the previously described technique [17].
Measurement of immunological mediators
The measurement of immunological mediators was carried out in both serum samples (acute and chronic phases of the disease). C3a and MBL levels were measured using a Human Complement C3a ELISA Kit (Novus biologicals® - Biotechne®, Canada) and a Quantikine® ELISA Human MBL Immunoassay kit (R & D Systems® - biotechne®, USA), respectively. The serum levels of the following cytokines, IFN-γ, TNF-α, IL1Ra, IL-4, IL-6, IL-8, IL-10, IL-12, MCP-1, RANTES, GM-CSF, and MIP-1, were processed using commercial enzyme-linked immunosorbent assay kits (Sigma-Aldrich®, Saint Louis, MO, USA). All tests were made following the manufacturer’s instructions.
Evaluation of the presence and severity of the musculoskeletal disorder
A physician evaluated the patients suffering from CHIKV in the acute and chronic phases. A patient was considered to have the musculoskeletal disorder (MSD) if they presented with localized pain and/or swelling in a joint group [18]; if this happened during the acute phase, it was named “presence”. If this happened during the chronic phase, it was called “persistence”. The severity of the MSD was assessed through: 1) the presence of arthritis (joint pain and swelling) or arthralgia (only joint pain); 2) pain measurement by the visual analog scale (VAS), and 3) measuring the disability index with the “Health Assessment Questionary” (HAQ). The patients were divided according to their pain into two categories: non-severe pain (scores 1 to 5) and severe pain (scores 6 to 10). They were also classified according to their functional disability in two categories: non-severe functional disability (scores 0 to 1) and severe functional disability (scores 2 to 3).
Definition of disease severity
If a patient, during the acute phase, presented at least two of the following hematological findings: thrombocytopenia < 100,000 cells/mm^3, elevated levels of alanine transaminase (ALT) or aspartate transaminase (AST) > 50 IU/L, and elevated C-reactive protein (CRP) > 3 mg/L, it was considered a severe version of the disease.
Statistical analysis
The data were analyzed using the Statistical Package for the Social Sciences, SPSS (SPSS®, version 22, Inc.01., Chicago, ILL). Quantitative data were expressed as the median (interquartile range), and the qualitative data were expressed as a proportion. The Mann-Whitney test performed a statistical comparison of the level of immunological mediators between the groups. We compared the acute and chronic phases utilizing the Wilcoxon test. The cytokines levels were correlated using Pearson’s correlation analysis. A P-value < 0.05 was considered significant.
Ethics
All subjects voluntarily signed the informed consent form, previously approved by the Ethics Committee of the Universidad CES (Medellin, Colombia), registered with number 445, which is under the ethical standards of the Helsinki Declaration of 2008 and the conditions provided by the Health Ministry of Colombia.
Results
All the 83 patients included in this study were positive for IgM antibodies to CHIKV; three were also positive for RT-PCR. The age median (IQR) of the patients was 34 (18-49) years, and 57 (68.7%) were females. The median (IQR) in days between the symptom onset and the date of evaluation during the acute phase was 6 (4-11), and the median (IQR) in months between the date of symptom onset and the date of the evaluation in the chronic phase was 3.7 (2.5-5.10). The age median (IQR) of the ten healthy controls was 32 (21.3-44.8) years, and six (60.0%) of them were men.
Clinical and laboratory findings
The most frequent clinical findings were fever, headache, myalgia/arthralgia, asthenia, anorexia, back pain, and rash. Nevertheless, other systems were affected, such as the gastrointestinal tract (diarrhea and vomiting), and the respiratory tract (cough and sore throat). Furthermore, elevated C-reactive protein (CRP) was observed in 45.3% of the patients (Table 1).
Table 1.
Clinical and laboratory findings of patients with CHIKV infection (n = 83)
| Signs and symptoms | n | % |
|---|---|---|
| General | ||
| Fever | 71 | 85.5 |
| Headache | 67 | 80.7 |
| Myalgia/arthralgia | 65 | 78.3 |
| Asthenia | 64 | 77.1 |
| Chills | 60 | 72.3 |
| Back pain | 40 | 48.2 |
| Conjunctivitis | 21 | 25.3 |
| Dermatological | ||
| Rash | 32 | 38.6 |
| Pruritus | 26 | 31.3 |
| Gastrointestinal | ||
| Anorexia | 41 | 49.4 |
| Diarrhea | 25 | 30.1 |
| Vomiting | 24 | 28.9 |
| Respiratory | ||
| Cough | 31 | 37.3 |
| Sore throat | 28 | 33.7 |
| Hemorrhagic symptom | ||
| Petechiae | 9 | 10.8 |
| Epistaxis | 4 | 4.8 |
| Bleeding gums | 4 | 4.8 |
| Biological parameters | ||
| Anemia (Hemoglobin (< 10 g/dL))* | 4 | 6.9 |
| Thrombocytopenia (< 100,000 cells/mmˆ3)* | 8 | 13.8 |
| Lymphocytopenia (< 1 × 10ˆ9/L)* | 6 | 10.3 |
| Elevated ALT (> 50 IU/L)** | 10 | 13.3 |
| Elevated AST (> 50 IU/L)** | 13 | 17.3 |
| Elevated CPR (> 3 mg/L)** | 34 | 45.3 |
n = 58 patients are with data.
n = 75 patients with data.
Frequency of musculoskeletal disorder
During the acute phase, the presence of MSD in 74.7% (62/83) of the patients was observed. MSD persisted in 33.7% (28/83) of them during the chronic phase. In this phase, the frequency of persistence was significantly lower in males compared with females (15.4% vs. 42.1, P = 0.02), and it was significantly higher in patients > 30 years compared to ≤ 30 years of age (45.7% vs. 18.9%, P = 0.012) (Table 2).
Table 2.
Frequency of musculoskeletal disorder in acute phase and chronic phase of patients with CHIKV infection according sex and age
| Variables | Acute phase | Chronic phase | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Presence of MSD | OR (95% CI) | P value* | Persistence of MSD | OR (95% CI) | P value* | |
| Sex | ||||||
| Male (n = 26) | 22 (84.6%) | 2.33 (0.69-7.81) | 0.186 | 4 (15.4%) | 0.250 (0.08-0.82) | 0.024 |
| Female (n = 57) | 40 (70.2%) | 24 (42.1%) | ||||
| Age | ||||||
| > 30 years (n = 46) | 36 (78.3%) | 1.52 (0.56-4.11) | 0.406 | 21 (45.7%) | 3.6 (1.31-9.85) | 0.012 |
| ≤ 30 years (n = 37) | 26 (70.3%) | 7 (18.9%) | ||||
| Total (n = 83) | 62 (74.7%) | 28 (33.7%) | ||||
OR: Odds Ratio; 95% CI: Confidence interval;
Chi square test.
Frequency of the severity of the musculoskeletal disorder
In the acute and chronic phases, the most common MSD found was arthralgia, 61.3% and 71.4%, respectively. Arthritis (pain and swelling) was observed in 38.7% of the patients in the acute phase. This finding persisted in 28.6% of the patients in the chronic phase. Severe pain was reported in 51.6% of the MSD patients in the acute phase, which decreased to 28.6% (8/28) in the chronic phase. We observed severe dysfunctionality in 21.0% (13/62). Only one female > 30 years old in the chronic phase still presented with this degree of dysfunctionality. None of the severe findings of the MSD (arthritis, severe pain, and severe dysfunctionality) showed significant differences by sex or age group in either the acute or chronic phase (Table 3).
Table 3.
Type of severity of musculoskeletal disorder in acute phase and chronic phase of patients with CHIKV infection according sex and age
| Acute phase | |||||||||
|
| |||||||||
| Type of severity | Total n = 62 | Sex | Age (years) | ||||||
|
|
|
||||||||
| Male n = 22 | Female n = 40 | OR (95% CI) | P value* | > 30 n = 36 | ≤ 30 n = 26 | OR (95% CI) | P value* | ||
|
| |||||||||
| Arthralgia | 38 (61.3%) | 15 (68.2%) | 23 (57.5%) | 0.63 (0.21-1.88) | 0.586 | 20 (55.6%) | 18 (69.2%) | 1.80 (0.62-5.20) | 0.304 |
| Arthritis | 24 (38.7%) | 7 (31.8%) | 17 (42.5%) | 0.634 (0.21-1.88) | 0.586 | 16 (44.4%) | 8 (30.8%) | 1.80 (0.62-5.20) | 0.304 |
| Severe pain | 32 (51.6%) | 8 (36.4%) | 24 (60.0%) | 0.381 (0.13-1.11) | 0.111 | 21 (58.3%) | 11 (42.3%) | 1.91 (0.68-5.30) | 0.303 |
| Severe disability | 13 (21.0%) | 3 (13.6%) | 10 (25.0%) | 0.474 (0.12-1.94) | 0.348 | 7 (19.4%) | 6 (23.1%) | 0.81 (0.21-2.75) | 0.760 |
|
| |||||||||
| Chronic phase | |||||||||
|
| |||||||||
| Type of severity | Total n = 28 | Male n = 4 | Female n = 24 | OR (95% CI) | P value* | > 30 n = 21 | ≤ 30 n = 7 | OR (95% CI) | P value* |
|
| |||||||||
| Arthralgia | 20 (71.4%) | 2 (50.0%) | 18 (75.0%) | 3.00 (0.34-26.2) | 0.555 | 15 (71.4%) | 5 (71.4%) | 1.0 (0.15-6.64) | 1.00 |
| Arthritis | 8 (28.6%) | 2 (50.0%) | 6 (25.0%) | 3.00 (0.34-26.2) | 0.555 | 6 (28.6%) | 2 (28.6%) | 1.00 (0.15-6.64) | 1.00 |
| Severe pain | 8 (28.6%) | 1 (25.0%) | 7 (29.2%) | 0.810 (0.71-9.18) | 1.00 | 5 (23.8%) | 3 (42.9%) | 0.417 (0.07-2.52) | 0.371 |
| Severe disability | 1 (3.6%) | 0 (0.0%) | 1 (4.2%) | NC** | NC** | 1 (4.8%) | 0 (0.0%) | NC** | NC** |
OR: Odds Ratio; 95% CI: Confidence interval.
Chi square test;
NC: Not calculable.
Frequency of disease severity
In 20% (15/75) of the patients, disease severity was observed because they presented at least two of the following biochemical/laboratory features: thrombocytopenia < 100,000 cells/mm^3, elevated levels of alanine transaminase (ALT), or aspartate transaminase (AST) > 50 IU/L, and C-reactive protein (CRP) level greater than 3 mg/L.
Comparison of serum levels of immunological mediators between both CHIKV patients in the acute and chronic phase and healthy controls
Most of the serum levels in immunological mediators were higher in the acute phase than the chronic phase, with significant differences for MBL, IL-10, and MIP-1. Contrastingly, C3a, MCP-1, and RANTES remained high in both phases. Serum levels of the immunological mediators were higher in patients with CHIKV infection than in healthy controls in both acute and chronic stages, with significant differences for IFN-γ, IL-6, IL-8, IL-10, and MIP-1. C3a and RANTES levels were more elevated in healthy controls (Table 4). Serum levels of IL-4 and IL1Ra were not detectable in 77.1% and 72.3% of the patients, respectively, or their levels were too low (data not shown).
Table 4.
Comparison of MBL, C3a and cytokines levels in patient with CHIKV infection and healthy controls in acute and chronic phases n = 83
| Mediator | CHIKV patients | P value* AP vs. CP | Healthy controls (HC) Median (IQR) pg/µL | P value** CHIKV patients (AP) vs. HC | |
|---|---|---|---|---|---|
|
| |||||
| Acute phase (AP) Median (IQR) pg/µL | Chronic phase (CP) Median (IQR) pg/µL | ||||
| MBL | 2.3 (1.1-3.8) | 1.7 (0.9-3.1) | 0.013 | 1.7 (1.0-2.2) | 0.281 |
| C3a | 71.1 (65.9-218.9) | 72.0 (65.5-198.6) | 0.203 | 147.0 (129.1-184.4) | 0.110 |
| IFN-γ | 65.0 (31.0-143.7) | 54.8 (24.2-123.2) | 0.091 | 9.7 (0.0-38.3) | 0.003 |
| TNF-α | 11.4 (1.9-26.5) | 13.1 (2.9-28.9) | 0.930 | 0.0 (0.0-58.8) | 0.119 |
| IL-6 | 25.8 (0.34-58.3) | 22.2 (4.9-52.9) | 0.533 | 0.0 (0.0-0.4) | < 0.001 |
| IL-12 | 1.6 (0.0-6.1) | 2.5 (0.0-14.1) | 0.096 | 0.0 (0.0-7.6) | 0.089 |
| IL-10 | 28.6 (14.7-51.4) | 17.1 (10.4-26.1) | < 0.001 | 7.7 (5.5-10.5) | < 0.001 |
| IL-8 | 74.4 (23.8-264.9) | 44.5 (14.7-317.1) | 0.882 | 7.8 (4.9-23.9) | < 0.001 |
| MIP-1 | 21.6 (5.3-50.1) | 9.5 (0.0-25.4) | 0.004 | 0.0 (0.0-1.6) | < 0.001 |
| MCP-1 | 197.9 (132.3-325.5) | 197.7 (119.9-305) | 0.172 | 135.1 (61.9-272.1) | 0.114 |
| RANTES | 5201.4 (4480.7-10478.9) | 5390.8 (4734-8451.4) | 0.499 | 6973.1 (2426.3-13174.9) | 0.926 |
| GS-CSF | 0.00 (0.00-0.901) | 0.00 (0.00-1.39) | 0.375 | 0.00 (0.00-0.48) | 0.510 |
Wilcoxon test;
Mann-Whitney test.
Serum levels of immunological mediators according to the presence/persistence of musculoskeletal disorder (MSD) in the acute phase and chronic phase
In the acute phase, C3a, IL-8, MIP-1, and MCP-1 were more elevated in patients with MSD than patients without it, with significant differences for C3a and MCP-1. In contrast, IL-6 serum levels were significantly higher in patients without musculoskeletal disorders. In the chronic phase, serum levels of C3a and RANTES were significantly higher in patients with persistence of MSD. On the other hand, IFN-γ, IL-6, IL-8, IL-10, and MIP-1 were considerably higher in patients without persistence of MSD than in patients with persistence of MSD (Table 5).
Table 5.
Comparison of MBL, C3a and cytokines levels in patient with CHIKV infection according to presence/persistence of musculoskeletal disorder in acute and chronic phases
| Mediator | Acute phase (AP) Musculoskeletal disorder Median (IQR) pg/µL | Chronic phase (CP) Musculoskeletal disorder Median (IQR) pg/µL | P value** Presence (AP) vs. Persistence (CP) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Presence n = 62 | No presence n = 21 | P value* Presence/No presence | Persistence n = 28 | No persistence n = 55 | P value* Persistence/No persistence | ||
| MBL | 2.3 (1.2-3.8) | 2.5 (1.0-3.7) | 0.853 | 1.4 (1.0-2.9) | 1.9 (0.9-3.5) | 0.398 | 0.425 |
| C3a | 122.7 (66.6-253.3) | 68.5 (64.5-71.2) | 0.022 | 190.9 (72.5-248.2) | 68.4 (63.2-164.1) | < 0.001 | 0.501 |
| IFN-γ | 59.4 (18.3-139.7) | 72.1 (47.8-183.7) | 0.166 | 31.0 (1.2-72.6) | 61.8 (40.6-150.3) | 0.005 | 0.101 |
| TNF-α | 11.0 (0.0-25.8) | 12.0 (3.7-28.1) | 0.467 | 5.3 (0.0-44.7) | 14.6 (4.7-27.5) | 0.198 | 0.502 |
| IL-6 | 12.8 (0.0-45.0) | 47.5 (23.5-94.9) | 0.002 | 7.9 (2.4-32.8) | 23.0 (8.6-77.6) | 0.004 | 0.510 |
| IL-12 | 1.8 (0.0-9.7) | 1.4 (0.1-2.7) | 0.496 | 0.3 (0.0-14.1) | 4.6 (0.5-14.1) | 0.090 | 0.398 |
| IL-10 | 26.4 (13.5-51.4) | 34.3 (19.6-60.4) | 0.341 | 11.6 (7.9-19.5) | 20.8 (14.4-30.0) | 0.001 | 0.002 |
| IL-8 | 82.1 (23.2-243.8) | 67.9 (34.9-781.6) | 0.516 | 20.4 (10.5-41.3) | 104.7 (20.0-742.0) | 0.002 | 0.112 |
| MIP-1 | 23.9 (3.1-50.2) | 17.8 (8.7-49.2) | 0.963 | 3.4 (0.0-13.7) | 16.1 (0.0-36.6) | 0.008 | 0.002 |
| MCP-1 | 229.0 (148.8-342.5) | 144.7 (106.9-193.3) | 0.008 | 223.7 (124.8-352.8) | 176.1 (119.9-268.4) | 0.149 | 0.349 |
| RANTES | 5153.0 (4495.8-10923.1) | 5201.4 (4401.9-9539.6) | 0.554 | 7721.1 (4759.8-14824.1) | 5226.1 (4676.9-6965.7) | 0.037 | 0.918 |
Mann-Whitney test;
Wilcoxon test.
Serum levels of immunological mediators according to the severity of the MSD
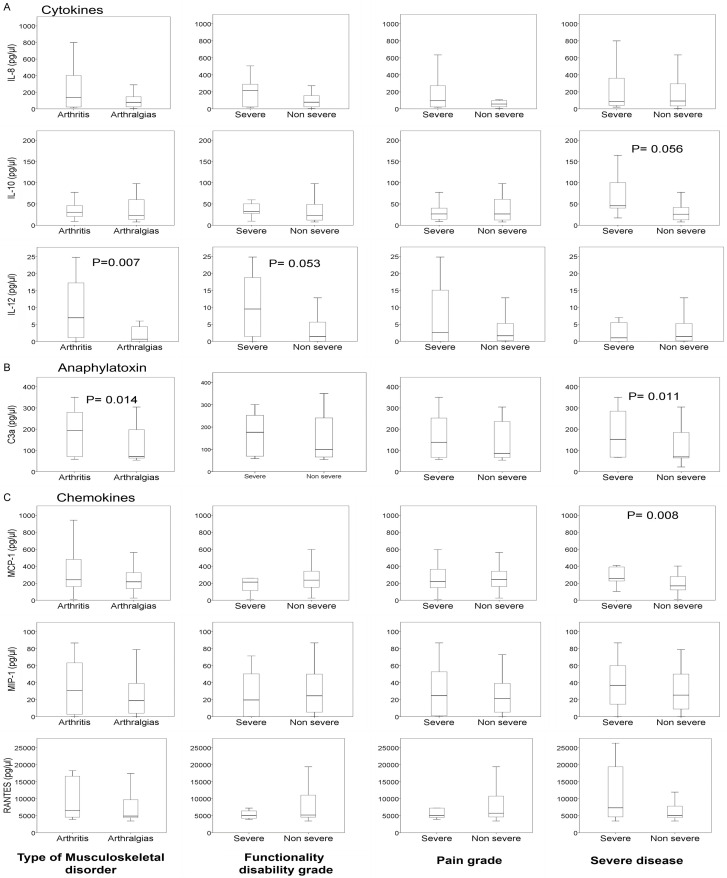
C3a, IL-8, IL-10, IL-12, MIP-1, MCP-1, and RANTES levels were higher in patients with arthritis than patients with arthralgias, with significant differences in C3a and IL-12. In this same phase, C3a, IL-8, IL-10, IL-12, and IFN-γ were more elevated in patients with a severe functional disability than in patients with non-severe functional disability. Concerning the pain level, the levels of C3a, IL-8, and L-12, although higher in patients with severe pain, were found to have no significant differences (Figure 1). TNF-α levels were significantly lower in patients with severe pain than in non-severe pain, 15.2 (6.4-32.9) vs. 4.5 (0.0-20.7), P = 0.033. During the chronic phase, in the eight patients with arthritis, some of the cytokines in the acute phase (IL-12, MIP-1, MCP-1, and RANTES) presented higher levels, which were not significant. Moreover, in this same phase, serum levels of TNF-α were significantly lower in patients with arthritis compared with patients with arthralgias, 0.0 (0.0-2.2) vs. 15.0 (3.2-57.3), P = 0.004, and in patients with severe pain compared to those who did not present with severe pain, 12.3 (0.7-57.3) vs. 0.0 (0.0-4.7), P = 0.038.
Figure 1.
Serum levels of (A) Cytokines, (B) Anaphylatoxin and (C) Chemokines, in the acute phase of CHIKV infection, according to type of arthritis n = 24, arthralgia n = 38; degree of functional disability (severe n = 13, non-severe n = 49) and pain grade (severe = 32, non-severe = 30) and severity of the disease (severe n = 15, non-severe n = 60).
Serum levels of immunological mediators according to the severity of the disease in acute phase
In patients with the severe version of the disease, C3a, IFN-γ, IL-8, IL-10, MIP-1, MCP-1, and RANTES were more elevated than in non-severe cases, with C3a and MCP-1 being significantly different.
Correlations
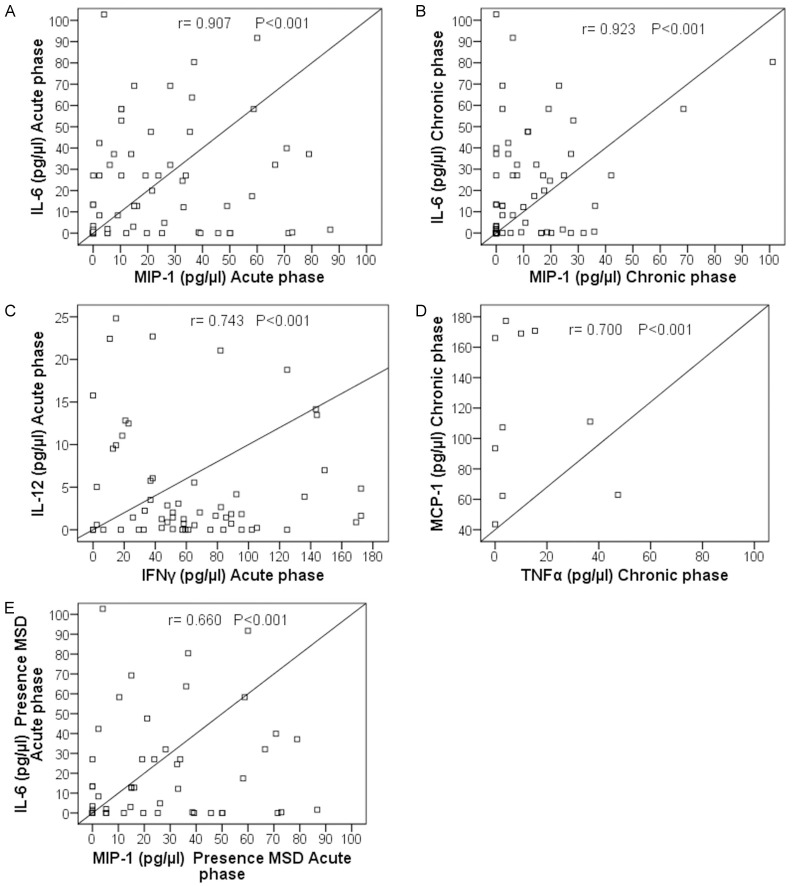
In this study, during the acute phase of the infection, a robust positive correlation between IL-6 and MIP-1 (r = 0.907; P < 0.001) and a moderate positive correlation between IL-12 and IFN-γ (r = 0.743; P < 0.001) levels were observed. During the chronic phase, a robust positive correlation was observed between IL-6 and MIP-1 (r = 0.923; P < 0.001). In patients with MSD presence in the acute phase, we observed a moderated positive correlation between IL-6 and MIP-1 (r = 0.660; P < 0.001). In patients with MSD persistence in the chronic phase, a moderated positive correlation between MCP-1 and TNF-α (r = 0.700; P < 0.001) was found (Figure 2).
Figure 2.
Correlations of cytokines/chemokines in patients with chikungunya infection. A. Correlation between IL-6 and MIP-1 in the acute phase. B. Correlation between IL-6 and MIP-1 in the chronic phase. C. Correlation between IL-12 and IFN-γ in the acute phase. D. Correlation between MCP-1 and TNF-α in the chronic phase. E. Correlation between IL-6 and MIP-1 in patients with presence of musculoskeletal disorder in the acute phase.
Discussion
The clinical signs of severe joint pain from CHIKV infection may last long after the infection. However, there is still no clarity on the causes. Therefore, in this study, we assessed serum levels of some cytokines and two complement system mediators in individuals with CHIKV infection according to the evolution of the disease. The clinical manifestations found were consistent with the results of other studies [19-23].
The cytokine profile in our patients during CHIKV infection in the acute phase coincided with an innate immune response, with high production of proinflammatory cytokines, in agreement with the previous reports [10,13,14,24-27]. These cytokines are dependent on the early activation of Th1 and Th2, which has also been observed in other studies [13]. Consistent with Suhrbier et al., we observed that the serum levels of cytokines IFN-γ, IL-6, IL-8, IL-10, and MIP-1, were elevated in patients in the acute phase. They also remained high in the chronic phase than in healthy controls [28]. These findings could be explained by the CHIKV persistence in inflammatory cells infiltrating the joint with a high viral load in the acute phase [9,10,14,19]. Interestingly, IL-10 (Th2 cytokine) levels were significantly higher in the acute phase of CHIKV patients compared with the chronic phase, and healthy controls, indicating an anti-inflammatory response, in line with previous studies [13,14].
It is well known that the musculoskeletal disorder, a consequence of CHIKV infection, has an immunological origin [28-30], which could be related to the presence of CHIKV antigens and CHIKV RNA in joint tissue [19,25]. In this study, presence of musculoskeletal disorder was observed in 74.7% of patients in the acute phase, and it persisted for at least three months in 37.7%, as reported by other authors [5,7,8,31,32]. When comparing the presence of the musculoskeletal disorder in patients in the acute phase, C3a, IL-8, MIP-1, and MCP-1 levels, were more elevated. In the chronic phase, C3a, MCP-1, and RANTES/CCL5 were increased in patients with persistence of MSD. These results showed the predominant role of the chemokines in the development of MSD which is consistent with other studies [12,14]. In the chronic phase, IFN-γ, IL-6, IL-12, IL-10, IL-8, and MIP-1 were more elevated in patients without the persistence of MSD, contrary to what was found by other authors [9,10,25]; nevertheless, in a meta-analysis, in spite of these last biomarkers and other having elevated levels in non-recovered patients, the differences were not significant [13]. This means that this topic might not be fully clarified.
According to severity of the MSD, we observed arthritis in 38.7% and 28.6% of the patients in acute and chronic phases, respectively. In line with the previously reported data, some authors reported arthritis in 20.5% of the patients in the acute phase, and around 7.4% to 25.2% in patients in the chronic phases [4,18,33], as opposed to another study that reported 53.7% of arthritis in the chronic phase [34]. This difference could be explained because, in this previous study, the population was constituted mostly of postmenopausal women. Alternatively, we found that severe joint pain occurred in 51.6% and 28.6% of the patients in the acute and chronic phases, respectively, and severe functional disability in 21.0% and 3.6%, respectively, in both phases. Other authors had reported disability in 10.5% to 16.6% in the chronic phase [20,35].
We observed higher C3a, IL-8, IL-10, and IL-12 levels in patients with arthritis and severe disability. On the other hand, elevated levels of MCP-1 were associated with arthritis and with severe disease. In contrast, RANTES levels were lower in patients with severe pain and severe disability. Other authors have associated the severity with increased levels of IFN-γ, IL-6, IL-12, interferon γ-induced protein 10 kDa (IP-10), IL-1b, monokine induced by interferon-gamma (MIG), MCP-1, IL-17A, IL-27 and reduced levels of RANTES, and IL-8 [13,14,36,37]. The decrease in RANTES levels is a consequence of reducing platelets during the disease, which are its main reservoir [13,29]. It is essential to highlight the role of MCP-1 in the induction of monocytes/macrophages, which are involved in the pathophysiology of arthritis [13].
In our study, MBL levels were significantly higher in the acute phase in comparison to the chronic stage; this could be explained because the role of MBL pathway in the complement activation in CHIKV infection. Previous studies have evidenced that activating the complement mediated by MBL pathway is essential in the severity progression of the infection RRV, another endemic arthritogenic alphavirus [15,38].
As described above, we observed that elevated C3a levels are associated with chronicity and CHIKV infection severity. Studies have evidenced the role of C3a in enhancing the severity of RRV-induced disease in a murine model. Additionally, in patients with RRV polyarthritis, the levels of C3a in synovial fluid were significantly higher compared with patients with non-inflammatory osteoarthritis [39,40].
In summary, our study showed the role of the complement system in CHIKV infection and an evident immunological response that persisted until the chronic phase. Chemokines such as C3a, MCP-1, and RANTES were related to the persistence of MSD. C3a, IL-12, and MCP-1 levels were significantly involved with the severity of chikungunya virus infection, suggesting they could be considered biomarkers of severity.
The recent findings about the mechanisms that CHIKV has developed to neutralize the complement system [41,42] and our results highlight the importance of delving into the study of the role of the complement in this disease, as well as the need to identify biomarkers for patient follow-up and the development of possible treatments.
The limitation of our study was the low frequency of severe manifestations of MSD in the chronic phase, with the consequent decrease in the sample size in this patient group.
Finally, the differences in the frequency of persistence and severity of the CHIKV infection and the production of cytokines of the results between studies could be explained by the definition of variables, study population, and the measurement methods of immunology mediators.
Acknowledgements
This study was supported by the “Instituto Colombiano para el desarrollo de la Ciencia y la Tecnología Francisco José de Caldas”, COLCIENCIAS, Colombia, grant: 32567.
Disclosure of conflict of interest
None.
References
- 1.Vairo F, Haider N, Kock R, Ntoumi F, Ippolito G, Zumla A. Chikungunya: epidemiology, pathogenesis, clinical features, management, and prevention. Infect Dis Clin North Am. 2019;33:1003–1025. doi: 10.1016/j.idc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Brito CA, Sohsten AK, Leitão CC, Brito RC, Valadares LD, Fonte CA, Mesquita ZB, Cunha RV, Luz K, Leão HM, Brito CM, Frutuoso LC. Pharmacologic management of pain in patients with Chikungunya: a guideline. Rev Soc Bras Med Trop. 2016;49:668–679. doi: 10.1590/0037-8682-0279-2016. [DOI] [PubMed] [Google Scholar]
- 3.Wimalasiri-Yapa BMCR, Stassen L, Huang X, Hafner LM, Hu W, Devine GJ, Yakob L, Jansen CC, Faddy HM, Viennet E, Frentiu FD. Chikungunya virus in Asia - Pacific: a systematic review. Emerg Microbes Infect. 2019;8:70–79. doi: 10.1080/22221751.2018.1559708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertolotti A, Thioune M, Abel S, Belrose G, Calmont I, Césaire R, Cervantes M, Fagour L, Javelle É, Lebris C, Najioullah F, Pierre-François S, Rozé B, Vigan M, Laouénan C, Cabié A Chronic Chikungunya working group of University Medical Center of Martinique. Prevalence of chronic chikungunya and associated risks factors in the French West Indies (La Martinique): a prospective cohort study. PLoS Negl Trop Dis. 2020;14:e0007327. doi: 10.1371/journal.pntd.0007327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Consuegra-Rodríguez MP, Hidalgo-Zambrano DM, Vásquez-Serna H, Jimenez-Canizales CE, Parra-Valencia E, Rodriguez-Morales AJ. Post-chikungunya chronic inflammatory rheumatism: follow-up of cases after 1 year of infection in Tolima, Colombia. Travel Med Infect Dis. 2018;21:62–68. doi: 10.1016/j.tmaid.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 6.Chopra A, Anuradha V, Ghorpade R, Saluja M. Acute chikungunya and persistent musculoskeletal pain following the 2006 Indian epidemic: a 2-year prospective rural community study. Epidemiol Infect. 2012;140:842–850. doi: 10.1017/S0950268811001300. [DOI] [PubMed] [Google Scholar]
- 7.Pathak H, Mohan MC, Ravindran V. Chikungunya arthritis. Clin Med (Lond) 2019;19:381–385. doi: 10.7861/clinmed.2019-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Morales AJ, Villamil-Gomez W, Merlano-Espinosa M, Simone-Kleber L. Post-chikungunya chronic arthralgia: a first retrospective follow-up study of 39 cases in Colombia. Clin Rheumatol. 2016;35:831–832. doi: 10.1007/s10067-015-3041-8. [DOI] [PubMed] [Google Scholar]
- 9.Benjamanukul S, Osiri M, Chansaenroj J, Chirathaworn C, Poovorawan Y. Rheumatic manifestations of chikungunya virus infection: prevalence, patterns, and enthesitis. PLoS One. 2021;16:e0249867. doi: 10.1371/journal.pone.0249867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoarau JJ, Jaffar Bandjee MC, Krejbich Trotot P, Das T, Li-Pat-Yuen G, Dassa B, Denizot M, Guichard E, Ribera A, Henni T, Tallet F, Moiton MP, Gauzère BA, Bruniquet S, Jaffar Bandjee Z, Morbidelli P, Martigny G, Jolivet M, Gay F, Grandadam M, Tolou H, Vieillard V, Debré P, Autran B, Gasque P. Persistent chronic inflammation and infection by Chikungunya arthritogenic alphavirus in spite of a robust host immune response. J Immunol. 2010;184:5914–5927. doi: 10.4049/jimmunol.0900255. [DOI] [PubMed] [Google Scholar]
- 11.Chow A, Her Z, Ong EK, Chen JM, Dimatatac F, Kwek DJ, Barkham T, Yang H, Rénia L, Leo YS, Ng LF. Persistent arthralgia induced by chikungunya virus infection is associated with interleukin-6 and granulocyte macrophage colony-stimulating factor. J Infect Dis. 2011;203:149–157. doi: 10.1093/infdis/jiq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaaitanya IK, Muruganandam N, Sundaram SG, Kawalekar O, Sugunan AP, Manimunda SP, Ghosal SR, Muthumani K, Vijayachari P. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011;24:265–271. doi: 10.1089/vim.2010.0123. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira AS, Baldoni NR, Cardoso CS, Oliveira CDL. Biomarkers of severity and chronification in chikungunya fever: a systematic review and meta-analysis. Rev Inst Med Trop Sao Paulo. 2021;63:e16. doi: 10.1590/S1678-9946202163016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirathaworn C, Chansaenroj J, Poovorawan Y. Cytokines and chemokines in chikungunya virus infection: protection or induction of pathology. Pathogens. 2020;9:415. doi: 10.3390/pathogens9060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gunn BM, Morrison TE, Whitmore AC, Blevins LK, Hueston L, Fraser RJ, Herrero LJ, Ramirez R, Smith PN, Mahalingam S, Heise MT. Mannose binding lectin is required for alphavirus-induced arthritis/myositis. PLoS Pathog. 2012;8:e1002586. doi: 10.1371/journal.ppat.1002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison TE, Simmons JD, Heise MT. Complement receptor 3 promotes severe ross river virus-induced disease. J Virol. 2008;82:11263–11272. doi: 10.1128/JVI.01352-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, Campbell GL. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaseen HM, Simon F, Deparis X, Marimoutou C. Identification of initial severity determinants to predict arthritis after chikungunya infection in a cohort of French gendarmes. BMC Musculoskelet Disord. 2014;15:249. doi: 10.1186/1471-2474-15-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar R, Ahmed S, Parray HA, Das S. Chikungunya and arthritis: an overview. Travel Med Infect Dis. 2021;44:102168. doi: 10.1016/j.tmaid.2021.102168. [DOI] [PubMed] [Google Scholar]
- 20.Hossain S, Choudhury MR, Islam MA, Hassan MM, Yeasmin S, Hossain F, Zaman MM. Post-chikungunya arthritis: a longitudinal study in a tertiary care hospital in Bangladesh. Trop Med Health. 2022;50:21. doi: 10.1186/s41182-022-00412-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto JR, Silva Junior GBD, Mota RMS, Martins P, Santos AKT, Moura DCN, Pires Neto RDJ, Daher EF. Clinical profile and factors associated with hospitalization during a chikungunya epidemic in Ceará, Brazil. Rev Soc Bras Med Trop. 2019;52:e20190167. doi: 10.1590/0037-8682-0167-2019. [DOI] [PubMed] [Google Scholar]
- 22.Anwar S, Taslem Mourosi J, Khan MF, Ullah MO, Vanakker OM, Hosen MJ. Chikungunya outbreak in Bangladesh (2017): clinical and hematological findings. PLoS Negl Trop Dis. 2020;14:e0007466. doi: 10.1371/journal.pntd.0007466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rueda JC, Santos AM, Angarita JI, Giraldo RB, Saldarriaga EL, Ballesteros Muñoz JG, Forero E, Valencia H, Somoza F, Martin-Arsanios D, Quintero EJ, Reyes-Martinez V, Padilla D, Cuervo FM, Peláez-Ballestas I, Cardiel MH, Pavía PX, Londono J. Demographic and clinical characteristics of chikungunya patients from six Colombian cities, 2014-2015. Emerg Microbes Infect. 2019;8:1490–1500. doi: 10.1080/22221751.2019.1678366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venugopalan A, Ghorpade RP, Chopra A. Cytokines in acute chikungunya. PLoS One. 2014;9:e111305. doi: 10.1371/journal.pone.0111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy MK, Davenport BJJ, Morrison TE. Chronic chikungunya virus disease. Curr Top Microbiol Immunol. 2022;435:55–80. doi: 10.1007/82_2018_147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanabe ISB, Santos EC, Tanabe ELL, Souza SJM, Santos FEF, Taniele-Silva J, Ferro JFG, Lima MC, Moura AA, Anderson L, Bassi ÊJ. Cytokines and chemokines triggered by Chikungunya virus infection in human patients during the very early acute phase. Trans R Soc Trop Med Hyg. 2019;113:730–733. doi: 10.1093/trstmh/trz065. [DOI] [PubMed] [Google Scholar]
- 27.de Sousa Palmeira PH, Gois BM, Guerra-Gomes IC, Peixoto RF, de Sousa Dias CN, Araújo JMG, Amaral IPG, Keesen TSL. Downregulation of CD73 on CD4+ T cells from patients with chronic chikungunya infection. Hum Immunol. 2022;83:306–318. doi: 10.1016/j.humimm.2022.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Suhrbier A. Rheumatic manifestations of chikungunya: emerging concepts and interventions. Nat Rev Rheumatol. 2019;15:597–611. doi: 10.1038/s41584-019-0276-9. [DOI] [PubMed] [Google Scholar]
- 29.Ng LF, Chow A, Sun YJ, Kwek DJ, Lim PL, Dimatatac F, Ng LC, Ooi EE, Choo KH, Her Z, Kourilsky P, Leo YS. IL-1beta, IL-6, and RANTES as biomarkers of Chikungunya severity. PLoS One. 2009;4:e4261. doi: 10.1371/journal.pone.0004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Locke MC, Fox LE, Dunlap BF, Young AR, Monte K, Lenschow DJ. Interferon alpha, but not interferon beta, acts early to control chronic chikungunya virus pathogenesis. J Virol. 2022;96:e0114321. doi: 10.1128/JVI.01143-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaller Raad J, Segura Rosero A, Vidal Martínez J, Parody A, Jaller Raad R, Caballero Tovar D, Camargo López P, Giraldo Ramírez M, Blanco Magdaniel J, Andrade Celedón L. Immunological response of a population from the Caribbean region of Colombia infected with the chikungunya virus. Rev Colomb Reumatol (English Ed) 2016;23:85–91. [Google Scholar]
- 32.O’Driscoll M, Salje H, Chang AY, Watson H. Arthralgia resolution rate following chikungunya virus infection. Int J Infect Dis. 2021;112:1–7. doi: 10.1016/j.ijid.2021.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramachandran V, Kaur P, Kanagasabai K, Vadivoo S, Murhekar MV. Persistent arthralgia among chikungunya patients and associated risk factors in Chennai, South India. J Postgrad Med. 2014;60:3–6. doi: 10.4103/0022-3859.128795. [DOI] [PubMed] [Google Scholar]
- 34.Segura-Charry JS, Parada-Martinez MA, Segura-Puello HR, Muñoz-Forero DM, Nieto-Mosquera DL, Villamil-Ballesteros AC, Cortés-Muñoz AJ. Musculoskeletal disorders due to chikungunya virus: a real experience in a rheumatology department in Neiva, Huila. Reumatol Clin (Engl Ed) 2021;17:456–460. doi: 10.1016/j.reumae.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Rahim AA, Thekkekara RJ, Bina T, Paul BJ. Disability with persistent pain following an epidemic of chikungunya in rural South India. J Rheumatol. 2016;43:440–444. doi: 10.3899/jrheum.141609. [DOI] [PubMed] [Google Scholar]
- 36.Ninla-Aesong P, Mitarnun W, Noipha K. Proinflammatory cytokines and chemokines as biomarkers of persistent arthralgia and severe disease after chikungunya virus infection: a 5-year follow-up study in Southern Thailand. Viral Immunol. 2019;32:442–452. doi: 10.1089/vim.2019.0064. [DOI] [PubMed] [Google Scholar]
- 37.Kelvin AA, Banner D, Silvi G, Moro ML, Spataro N, Gaibani P, Cavrini F, Pierro A, Rossini G, Cameron MJ, Bermejo-Martin JF, Paquette SG, Xu L, Danesh A, Farooqui A, Borghetto I, Kelvin DJ, Sambri V, Rubino S. Inflammatory cytokine expression is associated with chikungunya virus resolution and symptom severity. PLoS Negl Trop Dis. 2011;5:e1279. doi: 10.1371/journal.pntd.0001279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunn BM, Jones JE, Shabman RS, Whitmore AC, Sarkar S, Blevins LK, Morrison TE, Heise MT. Ross River virus envelope glycans contribute to disease through activation of the host complement system. Virology. 2018;515:250–260. doi: 10.1016/j.virol.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison TE, Whitmore AC, Shabman RS, Lidbury BA, Mahalingam S, Heise MT. Characterization of Ross River virus tropism and virus-induced inflammation in a mouse model of viral arthritis and myositis. J Virol. 2006;80:737–749. doi: 10.1128/JVI.80.2.737-749.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison TE, Fraser RJ, Smith PN, Mahalingam S, Heise MT. Complement contributes to inflammatory tissue destruction in a mouse model of Ross River virus-induced disease. J Virol. 2007;81:5132–5143. doi: 10.1128/JVI.02799-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kril V, Aïqui-Reboul-Paviet O, Briant L, Amara A. New insights into chikungunya virus infection and pathogenesis. Annu Rev Virol. 2021;8:327–347. doi: 10.1146/annurev-virology-091919-102021. [DOI] [PubMed] [Google Scholar]
- 42.Nag J, Mukesh RK, Suma SM, Kunnakkadan U, Kumar NA, Johnson JB. A factor I-like activity associated with chikungunya virus contributes to its resistance to the human complement system. J Virol. 2020;94:e02062–19. doi: 10.1128/JVI.02062-19. [DOI] [PMC free article] [PubMed] [Google Scholar]