Abstract
Complications are increasingly recognized with SARS-CoV-2, the causative pathogen for COVID-19. Various mechanisms have been proposed to justify the cause of seizures in Covid-19 patients. To our knowledge, 13 cases of status epilepticus (SE) associated with COVID-19 have been reported so far. Here, we present a single-center case series, including the clinical, laboratory, and imaging characteristics, and the EEG and the outcome of SE in 5 Iranian patients with laboratory-confirmed SARS-CoV-2 virus. SE was para-infectious in four patients and post-infectious in one other patient. In Three patients, the causes of seizure were included severe hyponatremia, acute ischemic stroke, and meningoencephalitis. However, in two other patients, no specific reason for seizure was found, but there are possibilities for lesser-known mechanisms of Covid-19 that play roles in developing SE. Two of the patients recovered, and three patients, older and with higher comorbidities, failed to recover and died.
Keywords: Status epilepticus, COVID-19, seizures
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes different clinical characteristics, and the complications of the disease are arranged from asymptomatic infection to severe pneumonia [1]. Neurological manifestations of the disease are included hyposmia, hypogeusia, headache, seizures, stroke, sinus venous thrombosis, altered consciousness, encephalitis, demyelination, neuropathy, and polyradiculitis [2]. The prevalence and pathophysiology of seizure in SARS-CoV-2 have yet to be fully elucidated. There have been multiple reports of seizures, but rare reports of status epilepticus, in the setting of acute COVID-19 infection [3-10]. Status epilepticus (SE) is a life-threatening neurological condition in which seizure activity exceeds its duration and requires urgent medical treatment. In this case series, we reported clinical, laboratory, and imaging characteristics, EEG, and the outcome of 5 status epilepticus (SE) cases with laboratory-confirmed SARS-CoV-2 virus hospitalized during the 3rd COVID outbreak in the central hospital of COVID in Isfahan, Iran (Alzahra Hospital).
Case 1
A 19-year-old autistic and epileptic girl was admitted to the emergency room after two times generalized tonic-clonic seizures at 1-hour intervals. The patient’s witness reported a 4-minute episode of spontaneous, symmetric, tonic movements of her limbs, right gaze deviation of eye, and head turn to the right followed by clonic movement of limbs. She had no unconsciousness between the two seizures. The third and fourth seizures occurred in the emergency room. The axillary temperature was 39°C. Neurological examination revealed unconsciousness and lack of response to stimuli. The patient’s eyes were red, inflamed, and warm. No meningismus signs were detected. According to the patient history, treatment of status epilepticus began with 10 mg diazepam at the time of admission (when the patient was convulsing), followed by 3200 mg bolus Sodium ValproateIV infusion. Convulsions continued despite using the maximum dose of Sodium Valproate. Therefore, 4.5 gram IV stat Levetiracetam was infused. Convulsions still continued. Because of refractory status epilepticus, she was intubated, and Midazolam 16 mg followed by 10 mg/hr was started and increased to 30 mg/hr. Since the patient was unstable, scalp EEG was performed after 48 hours, demonstrating generalized background slowing and bilateral poly-spike and wave, suggesting generalized epilepsy, which had no evidence of status epilepticus (Figure 1A). After 24 hours of being seizure-free, midazolam tapering was started. Lung HRCT was highly suggestive of COVID-19. The patient was tested for SARS-CoV-2 reverse-transcriptase polymerase chain reaction (RT-PCR) through a nasal swab specimen, which returned positive. Infectious consultation was performed, and antibiotic agents were started. The patient’s family did not consent to Lumbar Puncture. Brain MRI revealed no pathological findings in the brain parenchyma and cortex except bilateral mastoiditis. After three days, the patient’s consciousness improved; midazolam tapering was continued, and IV Levetiracetam was changed to an oral tablet. The following week, she again had two convulsions in the morning. Levetiracetam and Sodium Valproate were changed to IV. Two seizures again occurred, and the patient’s consciousness did not improve between convulsions. 300 mg bolus of Thiopental sodium was infused and continued at 240 mg/hr. The patient’s convulsions were controlled. After 48 hours of being seizure-free, Thiopental sodium started to taper down. After obtaining consent, lumbar puncture was performed; WBC = 2, RBC = 400, Protein = 15, Glucose = 85, smear, culture, and HSV1; 2 PCR tests were all negative. The autoimmune and paraneoplastic panels in CSF were all negative (Table 1). Brain MRI was again performed and showed no new pathological findings. The patient’s consciousness started to improve gradually after seven days. Eventually, after 40 days of hospitalization, the patient’s convulsions were under control, Anti-epileptic drugs were changed to the oral form, and the patient was extubated. The Fever stopped. Inflammation and redness of the eyes were decreased. Finally, the patient was discharged after two months of hospitalization.
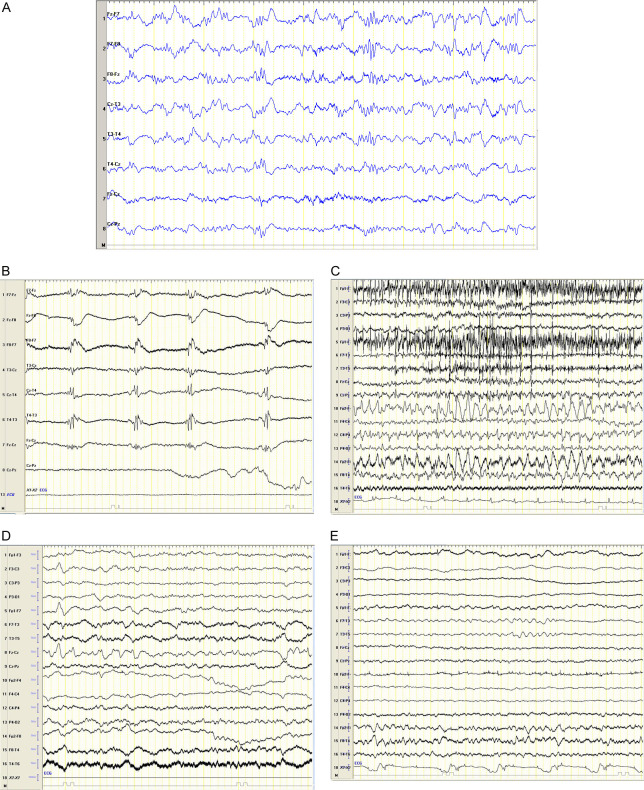
Figure 1.
Electroencephalogram (EEG) findings. A. Scalp EEG in a transverse montage from patient 1 after 48 hr, demonstrating generalized background slowing and bilateral poly-spike and wave, suggesting generalized epilepsy, no evidence in favor of status epilepticus. Sensitivity 10 uV/mm, LFF 1 Hz, HFF 70 Hz. B. Scalp EEG in a transverse montage from patient 2 after 12 hr, demonstrating continuous bilateral generalized periodic discharge, suggesting non-convulsive status epilepticus. Sensitivity 10 uV/mm, LFF 1 Hz, HFF 70 Hz. C. Scalp EEG in a bipolar montage from patient 3 after 2 hr. Demonstrating Ictal EEG was done and demonstrated continuous 4 hz spike and wave in right fronto-temporal hemisphere with evolution to right centro-parietal and left side myogenic artifact, suggested focal status epilepticus. Sensitivity 10 uV/mm, LFF 1 Hz, HFF 70 Hz. D. Scalp EEG in a bipolar montage from patient #4 after 24 hr demonstrating general background slowing and left hemisphere sharp wave, suggested epileptogenic focus but no evidence of non convulsive status epilepticus is seen at that stage. Sensitivity 10 uV/mm, LFF 1 Hz, HFF 70 Hz. E. Scalp EEG in a bipolar montage from patient 4 after 24 hr demonstrating general background slowing and left hemisphere sharp wave, suggesting epileptogenic focus but no evidence of non convulsive status epilepticus is seen at that stage. Sensitivity 10 uV/mm, LFF 1 Hz, HFF 70 Hz.
Table 1.
Clinical summary of the six patients with laboratory-confirmed SARS-CoV-2
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Demographic | 19, female | 91, Female | 81, male | 62, Female | 70, female |
| Comorbidities | Autism, Epilepsy | HTN, DM, CKD, Epilepsy | HTN, ischemic stroke | HTN | HTN, DM, PTE, CKD, opium toxicity, hypoxic-ischemic encephalopathy, status epilepticus |
| Drug history | Sodium Valproate | ASA, Losrtan, Amlodipin | ASA, Captopril | Losartan | Warfarin, sodium valproate, Levetiracetam, clonazepam, amantadin, tizanidin, trazodon |
| Sodium valproate | |||||
| COVID characteristic | Fever | Fever | Myalgia, Fever, headache cough | Fever, myalgia, headache 15 days before admission | Discharged one month ago |
| discharged 20 days ago | dyspnea nausea from 4 days ago | ||||
| Seizure characteristic | GTC | GTC | left facial twitching and left arm myoclonic jerk | The first episode of seizure | tonic movement of right arm, facial twitching, GTC |
| GTC, Meningo-encephalitis | |||||
| Brain MRI | Mastoiditis | NO | stroke suggested | Meningoencephalitis suggested | Severe atrophy, old lacunar infarct, severe small vessel, sinusitis |
| EEG | generalized slowing | continuous bilateral generalized periodic discharge | continuous four hz spike and wave in the right frontotemporal hemisphere | generalized background slowing | generalized slowing |
| bilateral poly-spike and wave | non-convulsive status epilepticus | evolution to right centro-parietal | left hemisphere sharp wave | encephalopathic state | |
| suggesting generalized epilepsy | focal status epilepticus | epileptogenic focus but no evidence of non-convulsive status epilepticus | |||
| no evidence of status epilepticus | |||||
| Lab data | CRP = 25, ESR = 23, Na = 143, K = 3.5, WBC = 7500, lym = 32%, ALT = 19, AST = 21, Hgb = 12.4, PLT = 120000, D-Dimer = 338, BUN = 10, Cr = 0.7 | Na = 149, K = 3, Hgb = 10.1, WBC = 19700, Lym = 13%, PLT = 188000, ALT = 15, AST = 38, BUN = 32, Cr = 1.1, CRP = 25, ESR = 11, D-Dimer = 3950 | Na = 139, k = 5.5, Hgb = 14.3, WBC = 8800, Lym = 13.3%, PLT = 133000, ESR = 57, CRP = 104, CPK = 734, ALT = 33, AST = 63, BUN = 21, Cr = 1.2, Ddimer = 500 | Na = 141, k = 4.4, Hgb = 12.4, WBC = 10300, Lym = 7%, PLT = 164000, ESR = 19, CRP = 14, D-Dimer = 3500, ALT = 29, AST = 43, BUN = 32, Cr = 0.8 | Na = 118, K = 2.8, Hgb = 9.9, WBC = 9800, lym = 16%, PLT = 174000, ALT = 25, AST = 35, BUN = 24, Cr = 1.78, CRP = 32, ESR = 34, D-Dimer = 297 |
| CSF | WBC = 2, RBC = 400, Pr = 15, Glu = 85, Smear: N culture: N, HSV1, 2 PCR: N,* | NO | WBC = 2, RBC = 63, Pr = 1, Glu = 63, Smear: N, culture: N, HSV-1, 2: N | WBC = 60, Net = 10% | WBC = 0, RBC = 5, Pr = 28, Glu = 86, Smear: N culture: N, HSV1, 2 PCR: N,* |
| paraneoplstic panel**: N, | Lym = 90% | paraneoplstic panel**: N, | |||
| autoimmune encephalitis panel***: N | Pr = 59, Glu = 73 | autoimmune encephalitis panel***: N | |||
| Treatment of status epilepticus | Levetiracetam, Phenytoin | Sodium valproate | Levetiracetam | Levetiracetam (40-60 mg/kg) | Levetiracetam, Phenytoin |
| Sodium Valproate, Lacozamide | Midazolam | Sodium Valproate | Sodium Valproate, | ||
| Midazolam | Midazolam | ||||
| Sodium Thiopental | |||||
| Other treatment | Vancomycin, Tzocin, Ceftriaxone, Meropenem, ampicillin, acyclovir, caspofungin | - | Meropenem | vancomycin | Meropenem |
| Stroke treatment | ceftriaxone | Linezolid | |||
| acyclovir | |||||
| Symptomatic | No | No | Yes | yes | Yes |
| Outcome | SE stopped, extubated, survived | SE stopped, failed extubation, died | SE stopped, failed extubation, died | SE stopped, extubated, survived | SE continued, failed extubation, died |
N = negative;
paraneoplastic panel: Titin Ab IgG, Zic 4 Ab IgG, GAD65 Ab IgG, Tr(DNER) Ab IgG, SOX1 Ab IgG, Amphiphysin Ab IgG, CRMP-5 Ab IgG, PNMA2 Ab IgG, Ri Ab IgG, YO Ab IgG, Hu Ab IgG, Recoverine Ab IgG, SOX1 Ab IgG;
Autoimmune encephalitis panel: Anti-glutamate receptor, Anti-GABA-B, Anti LGL1, CASPR2, Anti DPPX.
Case 2
A 91-year-old woman with a known history of hypertension, diabetes mellitus, chronic kidney disease, epilepsy, and COVID-19 (1 month ago) was presented to the emergency room after three generalized tonic-colonic seizures, unconsciousness, and lack of response to stimuli. The patient’s witness reported a 1-minute episode of spontaneous, symmetric, tonic movements of her limbs and upward gaze deviation of the eye without head turn. As soon as the patient came to the emergency room, she was intubated due to low oxygen levels (80%) and unconsciousness. At the time of admission, the axillary temperature was 38.1º. According to the history taken, seizures at home were all in generalized tonic-clonic form. Treatment of status epilepticus was started with 2400 mg Sodium Valproate IV infusion. She had two seizure episodes in the emergency room. Midazolam 11 mg IV infusion followed by 10 mg/hr was started.
Another seizure occurred; thus, the maintenance dosage of Midazolam was increased to 15 mg/hr. Seizures were controlled. Because she was unstable, a brain MRI was not performed. The patient’s family did not consent to Lumbar Puncture. Lung HRCT was inconsistent for COVID-19. SARS-CoV-2 RT-PCR through a nasal swab specimen was positive. EEG was performed after 12 hours and demonstrated continuous bilateral generalized periodic discharge, which suggested non-convulsive status epilepticus (Figure 1B). After 24 hours of being seizure-free, Midazolam started to taper down. On the fifth day of hospitalization, a cardiopulmonary arrest occurred, all the medical care was withdrawn, and the patient was expired.
Case 3
An 81-year-old man with a known history of hypertension and ischemic stroke was admitted after complaining of myalgia, Fever, headache, cough, dyspnea, and nausea four days ago. At the time of admission, the axillary temperature was 38.2°C. There were no focal neurological or meningismus signs, and he was completely conscious and oriented. Lung HRCT was highly suggestive of COVID-19. The patient was tested for SARS-CoV-2 by RT-PCR through a nasal swab specimen, which returned positive. Bigeminy premature ventricular contraction (PVC) was seen on EKG. After seven days, the neurologist was called because of decreased consciousness and the sudden onset of the patient’s left facial twitching and left arm myoclonic jerk. The neurological examination revealed left hemiparesis and left Babinski sign. A stroke was diagnosed, and routine stroke management was done. Due to continuous left facial twitching and left arm myoclonic jerk that continued for more than 30 minutes, focal status epilepticus began with a 3-gram dose of Levetiracetam. After 30 minutes, twitching and left arm myoclonus were continued, a 3-gram bolus dose of Sodium Valproate was ordered, and seizures were controlled. Ictal EEG was done. It demonstrated a continuous 4 Hz spike and wave in the right frontotemporal hemisphere with evolution to the right centro-parietal and left side myogenic artifact, suggesting focal status epilepticus (Figure 1C). Brain MRI showed cortical hyper-intensity in the DWI sequence in the right temporal lobe with matched restriction ADC sequence (Figure 2). Also, a lumbar puncture was performed to rule out CNS infection due to Fever. CSF analysis was as follows: WBC = 2, RBC = 63, Protein = 15, Glucose = 80, smear, culture, and HSV-1; 2 PCR tests were all negative (note that he was receiving Meropenem from the beginning of hospitalization). For the next three weeks, multiple attempts to arouse or extubate failed after sedatives were held. Medical care was withdrawn, and the patient was expired.
Figure 2.
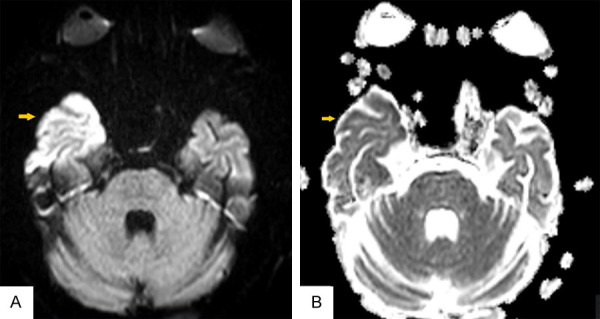
Cortical hyper-intensity in DWI sequence (A) in right temporal lobe with matched restriction ADC sequence (yellow arrow) (B).
Case 4
A 62-year-old woman with a history of HTN was admitted to the emergency room with unconsciousness and lack of response to stimuli and a history of three generalized tonic-colonic seizures, with a lack of consciousness between the convulsions. The patient’s witness reported a 2-minute episode of spontaneous, symmetric, tonic movements of her limbs and upward gaze deviation of the eye followed by brief clonic movement of limbs. At the time of admission, the fourth convulsion was seen by a neurologist. The axillary temperature was 38.8°C. Neurological examination revealed unconsciousness and lack of response to stimuli. No meningismus signs were detected. According to the patient history, treatment of status epilepticus began. Treatment was started with 10 mg diazepam when the patient was in convulsion at admission. and 4.5-gram IV Levetiracetam. The patient’s Complementary history revealed Fever, myalgia, and headache 15 days before admission. The meningoencephalitis regimen was started, including vancomycin, ceftriaxone, and acyclovir. Brain CT was normal. Lung HRCT was highly suggestive of COVID-19. The patient was tested for SARS-CoV-2 by RT-PCR through a nasal swab specimen, which returned positive. Because of heavy body weight, lumbar puncture was unsuccessful on the first day after several attempts. After 36 hours of admission, a lumbar puncture was performed by CHIBA needle. CSF analysis was as follows: WBC = 60 (Net = 10%, Lym = 90%), Protein = 59, Glucose = 73 (Table 1). According to primary CSF analysis and latency in lumbar puncture, antibiotics and antiviral drugs were continued because of the following two probable diagnoses; partially treated or viral meningoencephalitis. Scalp EEG was performed after 24 hours and demonstrated general background slowing and left hemisphere sharp wave, suggesting epileptogenic focus. Still, no evidence of non-convulsive status epilepticus was seen (Figure 1D). Brain MRI was done, and Symmetric signal change in both centrum semiovale bilateral external capsules, and caudate nuclei, was seen. Focal diffusion restriction in centrum semiovle was seen. These findings suggested meningoencephalitis (Figure 3). CSF Smear and culture were negative. HSV1, 2-PCR was reported negative after seven days; thus, acyclovir was discontinued. Vancomycin and ceftriaxone were continued for 14 days. The patient’s clinical course was significantly improved after 24 hours, and she started to become conscious. After 72 hours, she was completely conscious and oriented. Levetiracetam was changed to an oral tablet. After 14 days of hospitalization, the patient was discharged on the oral tablet of Levetiracetam and was recommended to self-quarantine.
Figure 3.
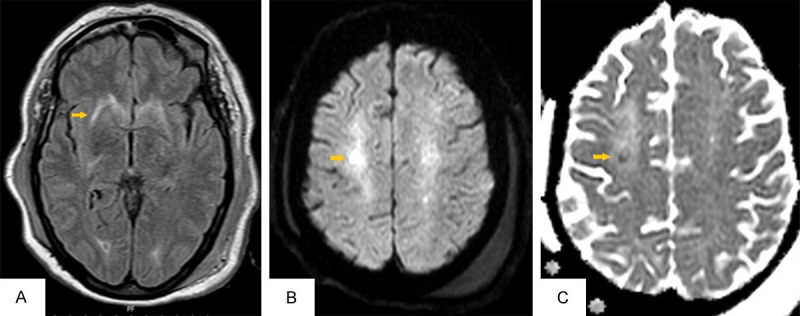
Symmetric signal change in both centrum semiovale bilateral external capsules and caudate nuclei (A). Focal diffusion restriction in centrum semiovle (B, C).
Case 5
The patient was a 70-year-old woman, bedridden three years ago, with a history of suicide with opium five years ago, followed by hypoxic-ischemic encephalopathy. The patient also had status epilepticus five months ago and was diagnosed with COVID 1 month ago. She was admitted to the emergency room after several abnormal movements beginning with tonic movement of the right arm, facial twitching, and then generalized colonic movement. She was intubated before admission. Vital signs were as follows: axillary temperature: 37.2°C, respiratory rate: 18, heart rate: 72, blood pressure: 175/100 mmHg, and oxygen saturation: 100%. Neurological examination revealed unconsciousness and lack of response to stimuli. Drug history included Sodium Valproate, Levetiracetam, Clonazepam, Warfarin, and other sedative drugs (Table 1). Treatment of status epilepticus was started; Sodium Valproate 2400 mg IV infusion and Levetiracetam 3.5 gram IV infusion. Midazolam was initially performed because of continuous facial twitching, initially 12 mg followed by 10 mg/hr. Facial twitch was continued; thus, Midazolam was increased to 20 mg/hr. During hospitalization, the patient had a fever up to 39.8°C. Infectious consultation was done, and Meropenem and Linazolide were started. Lung HRCT showed late COVID stages, and COVID-PCR was negative. Lumbar puncture was done, and then the results of CSF analysis were included WBC = 0, RBC = 5, Protein = 28, Glucose = 86, negative smear and culture for HSV-PCR, and negative autoimmune encephalitis, and paraneoplastic panel. EEG was performed after 72 hours. At the electroencephalography, convulsions were controlled, and EEG demonstrated generalized slowing, suggesting an encephalopathy (Figure 1E). Brain MRI showed severe brain atrophy, sinusitis, old lacunar infarctions, and multiple confluent bilateral centrum semiovale and subcortical hyperintense foci, which is compatible with ischemic change (Fazekas 3). For the next four weeks, numerous attempts to arouse or extubate failed. Medical care was withdrawn, and the patient expired.
Discussion
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is rapidly becoming a global concern [11]. COVID-19 causes different clinical characteristics and complications ranging from asymptomatic to severe pneumonia, leading to respiratory failure and death [1]. Many neurological manifestations, including encephalitis, stroke, headache, vertigo, and Guillain-barre syndrome, have been highlighted in numerous studies [12,13]. To our knowledge, 13 cases of status epilepticus (SE) associated with COVID-19 have been reported so far (Table 2).
Table 2.
Clinical summary of the six patients with laboratory-confirmed SARS-CoV-2
| Article | demographic | Past medical history | Covid-19 Symptoms | Seizure characteristic | Treatment of status epilepticus | CSF analysis | imaging | EEG findings | outcome | Symptomatic |
|---|---|---|---|---|---|---|---|---|---|---|
| Saeed A. etc. [29] | 3y, M | Neg | Fever + | GTC | Phenobarbital, levetiracetam, midazolam | Normal | Brain MRI: intracerebral hemorrhage in the right occipital lobe * | Note done | discharged | Not provoked |
| Asymptomatic | ||||||||||
| Somani S. etc. [3] | 49, F | RA, Schizoaffective disorder | Fever: + | Focal to Generalized | lorazepam, | Not done | Brain MRI: Normal | Background-delta slowing | discharged | Not provoked |
| case 1 | Asymptomatic | levetiracetam | Interictal-none | |||||||
| Ictal-frequent (4-6/hour) cyclical | ||||||||||
| seizures | ||||||||||
| Somani S. etc. [3] case 2 | 73, F | ESRD.DM | Fever: - | Myoclonic status epilepticus with coma (MSE) | lorazepam, levetiracetam, lacosamide, | Not done | CT brain and perfusion study: Normal | Background-very low voltage, 1-2 Hz activity | expired | Not Provoked |
| Skull base encephalocele, VPS | Respiratory distress | phenytoin, Midazolam | Interictal- 0.5-0.75 Hz bilateral independent | |||||||
| periodic discharges | ||||||||||
| Ictal-frequent cyclical seizures | ||||||||||
| emanating from the left and right frontocentral | ||||||||||
| regions | ||||||||||
| Carroll E. etc. [4] | 69, F | DM. renal transplant | Neg (post infectious) | Focal | levetiracetam. Clonazepam lacosamide, midazolam | Protein: 91 mg/dL | Brain MRI: mild hippocampal atrophy | continuous | discharged | Not Provoked |
| Seizure | WBC: 1 | lateralized periodic polyspike and wave discharges, often with | ||||||||
| Csf culture: negative | sporadic superimposed fast activity in the right posterior quadrant | |||||||||
| SARS-CoV-2 PCR, negative | and left occipital pole | |||||||||
| Autoimmune encephalitis panel: negative | ||||||||||
| IgG: 16.8 mg/d | ||||||||||
| IgG synthesis rate: 23.3 mg/dL; | ||||||||||
| CSF IgG index: 0.64 | ||||||||||
| CSF albumin: 52 mg/dL | ||||||||||
| albumin index: 25.6; | ||||||||||
| Repeat lumbar puncture | ||||||||||
| revealed protein of 384 mg/dL. | ||||||||||
| Farley M. etc [22] | 8, M | PANDAS | Fever: + | Focal with impaired consciousness | levetiracetam | Note done | Brain CT: Normal | diffuse cerebral dysfunction of nonspecific etiology | discharged | Provoked (use of Seizure threshold lowering drugs) |
| Asymptomatic | ||||||||||
| Abdulsalam M [7], etc | 32, M | Neg | Fever: - | GTC | midazolam. | Protein: 2212 mg/L | Ct: normal | EEG not done | discharged | unknown |
| Asymptomatic | Levetiracetam | Glucose (3.9 mmol/L). | ||||||||
| CSF culture - | ||||||||||
| herpes simplex virus polymerase chain reaction - | ||||||||||
| Sokolov E, [23] | 57, F | schizoaffective disorder, depression, COPD, PTE, traumatic fall, DM | Febrile: + | NCSE | Levetiracetam | Note done | Brain MRI: Normal | Frequent | discharged | Provoked (use of Seizure threshold lowering drugs) |
| Respiratory distress | (2.5-4 Hz), florid bilateral, non-synchronous | |||||||||
| epileptiform | ||||||||||
| discharges were seen over both hemispheres | ||||||||||
| Monti G, etc [19] | 50, M | HTN | Febrile: + | focal motor seizures with impaired awareness | valproic acid lacosamide. Midazolam Ketamine perampanel | Total cells count: 76/uL | Brain MRI: Normal | anterior sub-continuous periodic theta activity | discharged | Symptomatic (autoimmune encephalitis) |
| Asymptomatic | Proteins: slightly | |||||||||
| Elevated | ||||||||||
| Glucose: normal | ||||||||||
| Neurotropic viruses, bacteria | ||||||||||
| and yeast PCR: neg | ||||||||||
| SARS-Cov-2 PCR: neg | ||||||||||
| Autoimmune encephalitis and onconeurals Ab: NMDA-R Ab + | ||||||||||
| Rodrigo-Armenteros P [20] | 62, M | Neg | Fever: + | NCSE | Levetiracetam valproic | slightly increased protein (49 mg/dL), no cells and normal glucose. | MRI: focal leptomeningeal enhancement in the right parietal peri-Rolandic sulci | continuous, abrupt and rhythmic sharply-contoured delta waves at 2-2.5 Hz., bilaterally, more evident in the frontotemporal regions. There was a temporal-spatial evolution towards central and posterior electrodes | discharged | Symptomatic (hypoxemia) |
| severe respiratory distress syndrome | RT-PCR for SARS-Cov-2 in the CSF was negative | |||||||||
| Chen W etc. [5] case 1 | 37, F | ESRD | Diarrhea | Generalized myoclonic | Levetiracetam phenytoin | Normal | Brain CT: Normal | continuous spike and slow-wave discharges that appeared bifrontal predominant at up to 3 Hz associated with myoclonic movements | discharged | Provoked (hypoxia) |
| Chen W etc [5] case 2 | 60, F | HTN | Respiratory distress Myalgia, diarrhea, chest and abdominal pain | NCSE | Levetiracetam | Note done | Brain CT: normal | abundant 1 Hz GPDs with occasional bursts at 2-3 Hz | discharged | Symptomatic (hypernatremia, hypoxia) |
| Chen W etc [5] case 3 | 50, M | Neg | Respiratory distress | NCSE | Levetiracetam | Normal | Brain ct: nr | moderate to severe diffuse slowing and intermittent GRDA | Not mentioned | Not provoked |
| Myalgia | ||||||||||
| Chen W etc. [5] case 4 | 38, F | heart transplant, kidney transplant, pulmonary | Fever: + | NCSE | levetiracetam | Normal | Note done | Moderate to severe diffuse slowing with intermittent GRDA | NM | Note provoked |
| HTN, DM, chronic congestive hepatopathy |
RA: Rheumatoid arthritis; ESRD: End-Stage Renal Disease; DM: diabetes mellitus; VPS: Ventriculoperitoneal shunt; PANDAS: pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections; COPD: Chronic obstructive pulmonary disease; PTE: Pulmonary thromboembolism; HTN: Hypertension; PCR: Polymerase chain reaction; CSF: Cerebrospinal fluid; NCSE: Non-convulsive status epilepticus; GRDA: generalized rhythmic delta activity.
Brain MRI was done a few days after SE.
In this article, we reported five patients with SE associated with COVID-19. This association could be both para-infectious (case 1, 2, 3, 4) and post-infectious (case 5). Due to persistently high levels of inflammatory factors in patient 2, we consider it a para-infectious case. To our knowledge, only one post-infection status epilepticus has been reported so far [4].
The relationship between seizures in patients with Covid-19 can be justified in several ways. This could be due to specific mechanisms associated with SARS-CoV-2 (as an idiopathic seizure), nonspecific mechanisms related to hypoxia, sepsis, metabolic disturbance, other (as an acute symptomatic seizure), or it could be an utterly co-accidental finding.
The acute symptomatic seizure occurs at the time of a systemic insult or in close temporal association with a documented brain insult [14]. According to the international league against epilepsy (ILAE) [14], cases 3, 4, and 5 include symptomatic seizures.
SARS-CoV-2 could predispose patients to cerebrovascular events by inducing coagulopathy, disrupting endothelial function, and promoting a hypercoagulative state collectively [15], which happened in case 3. In case 5, SE occurred due to hypernatremia, which can be caused by multi-organ damage and metabolic disturbance potential of SARS-CoV-2 [16]. CSF (Cerebrospinal fluid) analysis of case 4 indicated an acute viral menigio-encephalitis as the cause of symptomatic SE. We could not detect the viral pathogens of these patients due to the lack of performance of the covid-19 PCR (Polymerase chain reaction) test in the CSF sample.
Reports show that the genomic analysis of SARS-CoV-2 is similar to SARS-coV-1 [17]. Previously, SARS-CoV-1 has been extracted in CNS autopsies of infected patients [17]. Human coronaviruses such as SARS-CoV can accumulate in infected leukocytes, thereby disseminating to the CNS. Another route of coronavirus transmission is through the olfactory bulb; it is believed that the virus can move retrogradely from the olfactory nerve or other cranial nerves into the CNS [18]. We did not find any evidence for the presence of the virus in the CNS. One reason could be the lack of inflammation signs in the CSF analysis sample of patients. Due to technical problems, we could not perform PCR on CSF samples of patients. However, this same issue was investigated in 3 previous studies [4,19,20], and the PCRs were negative in all the studies. In studies with abnormal CSF [4], there was other valid reason for CSF abnormality, such as autoimmune encephalitis [19] and post-infection inflammatory syndrome [4]. Abdulsalam M [7] reported a patient with a high protein level of CSF and negative HSV PCR; however, it is impossible to determine the exact cause of the abnormality of CSF due to lack of details such as cell counts.
Lu et al. [21] reported risk factors in covid-19 that cause new-onset acute symptomatic seizures, including acute cerebrovascular disease, history of traumatic brain injury, CNS infection, hypoxia, shock, sepsis, Imipenem use, multiple organ dysfunction syndromes, metabolic disturbance, and exposure to drugs or toxic substances. Hypoxemia was the most prevalent factor, especially in prolonged conditions [21]. Farley M [22] and Sokolov E [23] reported two patients with SE due to drugs that decreased the threshold of seizures. Although there is no precise definition of hypoxia as a cause of seizures, SE seems to be the consequence of hypoxemia in some studies [5,20].
According to the international league against epilepsy (ILAE) case, 1 and 2 could not categorize as symptomatic SE. Theoretically, SARS-CoV-2 can cause seizures by several specific mechanisms, leading to idiopathic seizures. Some evidence suggests the neurotropic mechanism of Sars-Cov-2 in developing SE in covid-19 patients. An indirect mechanism may be due to the ability of SARS-Cov-2 to bind to the ACE2 receptor in brain cells. The interaction with ACE2 can result in its downregulation. This may cause an imbalance between the protective function of ACE2 versus the detrimental effects mediated by ACE. An individual imbalance results in differences in blood supply to different parts of the brain, hence an increased risk for seizures [24].
Another mechanism by which cytokine storm mechanisms in COVID-19 could mediate SARS-CoV-2 causes seizures. These polypeptides can cross the blood-brain barrier and cause nerve inflammation, leading to brain damage [25]. Evidence for a cytokine storm has also been reported in patients with severe COVID-19 [1,26,27]. Thus, cytokine storm must be considered another possible cause of neuronal damage in patients with a severe COVID-19 disease course. The cytokines released during a COVID-19-associated cytokine storm include interleukins such as IL-6 (standard marker in COVID-19 with the severe condition) [1,27]. We were unable to measure interleukin levels; however, increased inflammatory factors, such as D-dimer, leukocytosis, ESR, and CRP, in inpatient 2 raises the possibility that this mechanism might be involved in the development of status seizures in this patient. Carroll et al. [4] reported a SE patient with an increased albumin index and IgG synthesis rate of CSF. They suspected that the patient’s RSE resulted from a post-infectious inflammatory response.
Another significant issue is occurring SE in patients without a history of epilepsy. Previously, patients with NORSE (new-onset refractory status epilepticus) criteria associated with COVID-19 have been reported [3,19]. None of our cases fulfill NORSE or febrile infection-related epilepsy syndrome (FIRES) criteria. SE without epilepsy history is a rare condition reported in autoimmune, inflammatory, and infective encephalitis [28]. IL-6 and IL-8 are known pro-inflammatory cytokines/chemokines that are increased in CSF of patients with NORSE FIRES [28], suggesting that their rise may be related to SE.
Regardless of the etiology, the relationship between Covid-19 and SE can also be evaluated epidemiologically. Studies in this area are minimal. Leitinger et al. [8] showed that the frequency of the first SE in Salzburg did not change in the two months of the first peak of COVID-19 compared with previous months. They found a non-significant increase in focal motor SE and relatively more minor NCSE Non-convulsive status epilepticus). It may be due to the infrequent use of EEG during the COVID-19 outbreak. Also, none of our cases were subjected to long-term monitoring (LTM) due to the limitations in the corona center.
Critically ill patients are prone to SE and carry a high risk for SE. The critical point is that SE could be a primary manifestation of COVID-19 in asymptomatic and critically ill patients (patient 1). Reports confirm this [3,7,19,22,29].
Patients 4 to 6 expired while hospitalized. Status epilepticus has significant mortality, reaching up to 30% in adults [30]. Also, mortality in refractory status epilepticus may reach 16% to 39% [31].
Conclusion
Status epilepticus can occur during covid-19 and also in asymptomatic patients. Five patients with COVID-19 and status epilepticus were evaluated in the current study. The reason for complication in three cases was due to COVID-19. No specific cause for the seizure was found in the other two cases. Since this manuscript reports 5 cases of status epileptics after COVID-19, we cannot generalize our results to the population. For this purpose, case-control studies and systematic reviews which compare seizure and status epilepticus between patients with and without COVID-19 are required. The highlight of this study was the development of non-symptomatic status seizures in 2 of our patients, which can indicate that the SARS-CoV-2 virus can cause status epilepsy by unknown etiology. Further disquisition to discuss the correlation between these two is necessary.
Acknowledgements
Informed consent has been obtained from the ppatient that this information may be published. This study was approved by the Institutional Review Boarde Institutional Review Board approved this study of the Isfahan University of Medical Sciences (IR.MUI.MED.REC.1401.055).
Disclosure of conflict of interest
None.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leonardi M, Padovani A, McArthur JC. Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol. 2020;267:1573–1576. doi: 10.1007/s00415-020-09896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De Novo status epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020;7:1240–1244. doi: 10.1002/acn3.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll E, Neumann H, Aguero-Rosenfeld ME, Lighter J, Czeisler BM, Melmed K, Lewis A. Post-COVID-19 inflammatory syndrome manifesting as refractory status epilepticus. Epilepsia. 2020;61:e135–e139. doi: 10.1111/epi.16683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Toprani S, Werbaneth K, Falco-Walter J. Status epilepticus and other EEG findings in patients with COVID-19: a case series. Seizure. 2020;81:198–200. doi: 10.1016/j.seizure.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peña-Salazar C, López Cuiña M, Chavarría V, Robles Olmo B. Convulsive status epilepticus as a possible symptom of COVID-19 in a patient with intellectual disability and autistic spectrum disorder. Neurología (English Edition) 2020;35:703–705. doi: 10.1016/j.nrleng.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdulsalam MA, Abdulsalam AJ, Shehab D. Generalized status epilepticus as a possible manifestation of COVID-19. Acta Neurol Scand. 2020;142:297–298. doi: 10.1111/ane.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leitinger M, Poppert KN, Mauritz M, Rossini F, Zimmermann G, Rohracher A, Kalss G, Kuchukhidze G, Höfler J, Bosque Varela P, Kreidenhuber R, Volna K, Neuray C, Kobulashvili T, Granbichler CA, Siebert U, Trinka E. Status epilepticus admissions during the COVID-19 pandemic in Salzburg-A population-based study. Epilepsia. 2020;61:e198–e203. doi: 10.1111/epi.16737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swarz JA, Daily S, Niemi E, Hilbert SG, Ibrahim HA, Gaitanis JN. COVID-19 infection presenting as acute-onset focal status epilepticus. Pediatr Neurol. 2020;112:7. doi: 10.1016/j.pediatrneurol.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vollono C, Rollo E, Romozzi M, Frisullo G, Servidei S, Borghetti A, Calabresi P. Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure. 2020;78:109–112. doi: 10.1016/j.seizure.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hopkins J. Coronavirus resource center. https://coronavirus.jhu.edu/
- 12.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, Collange O, Boulay C, Fafi-Kremer S, Ohana M. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beghi E, Carpio A, Forsgren L, Hesdorffer DC, Malmgren K, Sander JW, Tomson T, Hauser WA. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51:671–675. doi: 10.1111/j.1528-1167.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- 15.Qi X, Keith KA, Huang JH. COVID-19 and stroke: a review. Brain Hemorrhages. 2021;2:76–83. doi: 10.1016/j.hest.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmer MA, Zink AK, Weißer CW, Vogt U, Michelsen A, Priebe HJ, Mols G. Hypernatremia-A manifestation of COVID-19: a case series. A A Pract. 2020;14:e01295. doi: 10.1213/XAA.0000000000001295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monti G, Giovannini G, Marudi A, Bedin R, Melegari A, Simone AM, Santangelo M, Pignatti A, Bertellini E, Trenti T, Meletti S. Anti-NMDA receptor encephalitis presenting as new onset refractory status epilepticus in COVID-19. Seizure. 2020;81:18–20. doi: 10.1016/j.seizure.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodrigo-Armenteros P, Uterga-Valiente JM, Zabala-Del-Arco J, Taramundi-Argüeso S, Erburu-Iriarte M, Antón-Méndez L, Gómez-Muga JJ, Garcia-Monco JC. Non-convulsive status epilepticus in a patient with COVID-19 infection. Clin Neurophysiol. 2020;131:2588–2590. doi: 10.1016/j.clinph.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, Mu J, Guo J, Li W, Wang G, Gao H, Zhang Y, Lin M, Chen L, Shen S, Zhang H, Sander JW, Luo J, Chen S, Zhou D. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61:e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farley M, Zuberi J. COVID-19 precipitating status epilepticus in a pediatric patient. Am J Case Rep. 2020;21:e925776. doi: 10.12659/AJCR.925776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sokolov E, Hadavi S, Mantoan Ritter L, Brunnhuber F. Non-convulsive status epilepticus: COVID-19 or clozapine induced? BMJ Case Rep. 2020;13:e239015. doi: 10.1136/bcr-2020-239015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glowacka I, Bertram S, Herzog P, Pfefferle S, Steffen I, Muench MO, Simmons G, Hofmann H, Kuri T, Weber F, Eichler J, Drosten C, Pöhlmann S. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark IA, Vissel B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin Immunopathol. 2017;39:505–516. doi: 10.1007/s00281-017-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie S, Zhao X, Zhao K, Zhang Z, Zhang Z, Zhang Z. Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. medRxiv. 2020 [Google Scholar]
- 28.Gaspard N, Foreman BP, Alvarez V, Kang CC, Probasco JC, Jongeling AC, Meyers E, Espinera A, Haas KF, Schmitt SE. New-onset refractory status epilepticus: etiology, clinical features, and outcome. Neurology. 2015;85:1604–1613. doi: 10.1212/WNL.0000000000001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saeed A, Shorafa E. Status epilepticus as a first presentation of COVID-19 infection in a 3 years old boy; case report and review the literature. IDCases. 2020;22:e00942. doi: 10.1016/j.idcr.2020.e00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, Bare M, Bleck T, Dodson WE, Garrity L, Jagoda A, Lowenstein D, Pellock J, Riviello J, Sloan E, Treiman DM. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American epilepsy society. Epilepsy Curr. 2016;16:48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rossetti AO, Lowenstein DH. Management of refractory status epilepticus in adults: still more questions than answers. Lancet Neurol. 2011;10:922–930. doi: 10.1016/S1474-4422(11)70187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]