Abstract
Background:
Both sarcopenia and frailty are prevalent in patients with decompensated cirrhosis and associated with negative outcomes. However, few studies investigated the impact of their coexistence on mortality. We aimed to evaluate the role of sarcopenia and frailty on survival in a cohort of hospitalized cirrhotics.
Methods:
This was an observational cohort study including 221 patients hospitalized for decompensated events. The cutoff for low skeletal muscle index (SMI) at the third lumbar vertebra level on computed tomography built by our previous work (male: SMI <46.96 cm2/m2; female: SMI <32.46 cm2/m2) was used for the diagnosis of sarcopenia. Individuals with a Frailty Index >0.38 were considered frail. The sample was divided into four groups: sarcopenia and frailty (SF); sarcopenia and non-frailty (SN); non-sarcopenia and frailty (NF); and non-sarcopenia and non-frailty (NN). Follow-up for survival lasted 2 years.
Results:
Sarcopenia and frailty were present in 21.7% and 14.5% of the patients, respectively. The frequency of frailty in the group of sarcopenic patients was significantly higher than in the patients without sarcopenia (27.1% versus 11%, p = 0.009). In the survival analysis, the SF group showed a higher hazard ratio (2.604 in model 1; 4.294 in model 2) for mortality when compared with the NN group. In addition, the concurrence of those two conditions does give rise to incremental risk for mortality when compared with the group with each disturbance separately, namely, the SN/NF group.
Conclusion:
In conclusion, cirrhotic patients with sarcopenia and frailty combined showed higher mortality risk.
Keywords: frailty, Frailty Index, liver cirrhosis, mortality, sarcopenia, SMI
Graphical abstract
Introduction
Liver cirrhosis represents wide prevalence and is associated with high morbidity and mortality. 1 It is estimated that approximately 2 million deaths worldwide annually are due to liver diseases, wherein 1 million are attributed to cirrhosis and 1 million to viral hepatitis and hepatocellular carcinoma (HCC). 2 Globally, cirrhosis ranks the 11th most common cause of decease, the third leading cause of decease in subjects aged 45–64 years, and along with liver malignancies accounts for 3.5% of all deaths in the world. 3
Cirrhosis is a terminal pathology resulting from chronic hepatic inflammation that is followed by diffuse liver fibrosis; subsequently, the regular hepatic architecture is replaced by regenerative nodules and ultimately gives rise to liver failure. 4 Given the relapsing progression and multifactorial aspects of cirrhosis, a wide spectrum of complications do occur at divergent disease stages. Within the clinical setting, sarcopenia and frailty have stood out, as these entities are potentially modifiable with early identification and efficacious treatment. 5 Moreover, we and others have proved that sarcopenia or frailty in isolation is capable of predicting mortality in the ambulatory and hospitalized patients with cirrhosis.6–9 The underlying mechanism of sarcopenia and frailty is superimposed pathologically to some extent, including chronic inflammation, endotoxemia, gut dysbiosis, and endocrine disturbance. However, these two complications also exhibit conceptual discrepancy, and our previous findings demonstrated that sarcopenia is associated with multi-dimensional frailty in male patients with cirrhosis. 10 More recently, Alexopoulos et al. 11 investigated the combined impact pertaining to sarcopenia and frailty on 1-year mortality in a cohort of 115 consecutive patients with an average model for end-stage liver disease (MELD) score of 12 points. They reported that the concurrence of these complications is related to similar mortality rates compared with separate complication. However, it is not clear whether the authors used computed tomography (CT)-based skeletal muscle index (SMI) with liver-specific cutoffs to diagnose sarcopenia, whereas the Liver Frailty Index (LFI), which is specific to physical frailty, may be particularly useful for screening and follow-up in the ambulatory setting. 12 Although it is well-known that sarcopenia and frailty are associated with higher mortality risk, we herein explore the impact of two complications combined on long-term mortality by using our validated measurement and diagnostic criteria in a well-established cohort of hospitalized patients with cirrhosis.
Methods
Study protocol
This is an observational cohort study including 221 patients with decompensated cirrhosis hospitalized in Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital (TJMUGH). A detailed description of the methodology can be retained elsewhere. 13 The clinical and laboratory data were collected from our institutional database and analyzed in a retrospective manner. Specially, age, gender, body mass index (BMI), complete blood cell counts, electrolytes, cirrhosis-associated complications, indications for hospitalization, albumin, liver functional tests, renal functional tests, and etiology of cirrhosis were retrieved from electronic medical records. The traditional scoring systems [Child–Turcotte–Pugh (CTP) class and MELD score] were calculated in terms of indicative parameters. All participants were included from May 2017 to October 2019 and followed for mortality events up to 2 years.
Patients
Patients with signs of decompensation were eligible for inclusion if aged above 18 years along with confirmed diagnosis regarding cirrhosis based on liver biopsy, radiological evaluation, medical history, and laboratory information (platelet count/albumin/bilirubin). The exclusion criteria included concomitant HCC or other extrahepatic tumors, acute-on-chronic liver failure, unavailable CT scan 3 months prior to recruitment, severe hepatic encephalopathy (HE) dampening the completeness of questionnaire, liver transplantation, and refusal to scheduled follow-up (Supplemental Figure S1). The study was employed in accordance with the Declaration of Helsinki and approved by Ethics Committee of TJMUGH (IRB2021-YX-136-01). A written informed consent was obtained from all patients on admission. The present study was adherent to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement.
Sample size
The calculation of sample size was carried out using http://powerandsamplesize.com/ for testing time-to-event data, with an α error of 0.05, a power (1 − β error) of 0.95, hazard ratio of 4, overall probability of event of 0.6, and proportion of sample in indicative group of 0.06, confirming that a total of 200 individuals is required.
Methods
At baseline, all participants had the multi-dimensional frail phenotype assessed by the Frailty Index which is revised from Carolina Frailty Index within 48 h of the first admission (Supplemental Table S1).8,14 Frailty Index is a self-reported questionnaire consisting of 36 items with regard to multiple aspects (see detail in our earlier publication). A valid questionnaire refers to each participant fulfilling at least 10 items. For example, a participant who obtains 11 points after completing all the 36 items has a Frailty Index of 0.31 (11/36), while another subject who gets 6 points after completing 12 items of questionnaire has a Frailty Index of 0.5 (6/12). The collection of questionnaire was performed by experienced physicians in our department. Accordingly, the frail phenotype of a patient was classified as frailty when the Frailty Index was more than 0.38 and as non-frailty when the Frailty Index was ⩽ 0.38. Transient alterations in physical and cognitive functions among hospitalized patients with cirrhosis, which may limit the utility of performance-based frailty evaluation (e.g. LFI), account for the Frailty Index of choice. 15
All CT imaging were obtained using a spectral CT scanner (Discovery 750 HD 64-row; General Electric Company, Boston, MA, USA). Skeletal muscles at the third lumbar level (L3) were evaluated using a project on the basis of Matlab (Mathworks Inc., Natick, MA, USA). 16 The area of skeletal muscles comprises the psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis muscles. Tissue-specific Hounsfield unit (HU) cutoff was adopted to identify divergent tissue types, namely, −29 to 150 HU for quantifying skeletal muscles. The SMI was retrieved by dividing the entire muscle area at L3 by stature in square meters (cm2/m2). For sarcopenia, the thresholds of low SMI with best discriminative capability to stratify high-mortality-risk patients were used: an SMI <46.96 cm2/m2 for male and <32.46 cm2/m2 for female, respectively. 6 The final results were confirmed by a radiologist (H.H.W.) who has expertise in musculoskeletal anatomy. Although handgrip strength (HGS) has been addressed as a diagnostic criterion according to the European Working Group on Sarcopenia in Older People (EWGSOP2), its utility for evaluating sarcopenia remains ambiguous among cirrhotics. 17 Moreover, HGS was weakly correlated with muscle mass and quality according to cross-sectional imaging. 18 In the field of hepatology, CT-based SMI has dominated the literature and been linked to various inferior outcomes. On the other hand, both the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend CT scans for assessing sarcopenia in patients with cirrhosis due to its routinely clinical application.19,20 Taking into consideration relatively large proportion of patients with cirrhosis and ascites, we supposed it is justified to measure dry weight. The dry weight was measured by deducting 5% for mild ascites, 10% for moderate ascites, and 15% for massive ascites for patients with ascites along with edema, and 5% of body weight was deducted for patients with peripheral edema. 21 The BMI was retrieved accordingly.
The patients were classified into four groups considering the presence of sarcopenia and frailty:
Group sarcopenia and frailty (SF, n = 13): Comprised of patients with positive criteria for sarcopenia and for frailty (Frailty Index >0.38).
Group sarcopenia and non-frailty (SN, n = 35): Comprised of patients with positive criteria for sarcopenia, but without criteria for frailty (Frailty Index ⩽0.38).
Group non-sarcopenia with frailty (NF, n = 19): Comprised of patients without criteria for sarcopenia, but with positive criteria for frailty (Frailty Index >0.38).
Group non-sarcopenia and non-frailty (NN, n = 154): Comprised of patients without criteria for sarcopenia and for frailty (Frailty Index ⩽0.38).
Statistical analysis
Categorical variables were depicted as absolute number and percentage, while continuous variables as mean ± standard deviation (SD) or as median [interquartile range (IQR)]. The comparisons of the variables among the groups of sarcopenia and frailty were performed using the Chi-square test or Fisher’s exact for categorical variables, while one-way analysis of variance (ANOVA) or Kruskal–Wallis tests with Dunn’s post hoc test for continuous variables as appropriate.
The comparisons between the survival and deceased groups were performed using univariate Cox regression analysis. The survival analysis was done by the Kaplan–Meier graphic using the log-rank test to compare the survival curves among the sarcopenia and frailty groups. Two separate Cox’s proportional risk models were used to assess the hazard ratio (HR) for mortality, using the NN group as reference. The value of p < 0.05 is used for statistical significance. All statistical analyses were carried out using SPSS 23.0 (IBM, New York, USA).
Results
Table 1 shows the main characteristics of the studied sample comprised of hospitalized patients with decompensated cirrhosis. Generally, the median age was around 63 years (IQR: 57–68), and the majority of sample was comprised of females (53.8%). The major etiology of cirrhosis was attributed to autoimmune/cholestatic liver diseases, and 41 patients received a maintenance dose of corticosteroid therapy (e.g. 10 mg prednisolone daily). The cirrhosis-associated complications comprised gastroesophageal varices (GEV) in 155, ascites in 133, variceal bleeding in 75, infection in 29, and HE in 14 subjects, respectively. The median MELD score was 9 points (IQR: 6–12) and the majority of the sample was stratified as CTP class B/C (70.1%). The indications for hospitalization included ascites in 120, variceal bleeding in 75, hyponatremia in 21, portal vein thrombosis in 17, and HE in 14 patients (subjects may have concomitant symptoms). The comorbidities included hypertension in 60, diabetes mellitus in 53, and cardiovascular disease in 32 patients. When evaluating the presence of sarcopenia, 21.7% of the sample had low skeletal muscle mass, which is highly compatible to findings derived from a large-scale multicenter in China. 22 When assessing the presence of frail phenotype, 14.5% of the entire cohort was classified as frail.
Table 1.
Main demographic and clinical characteristics of patients with decompensated cirrhosis.
| Variables | SF (n = 13) | SN (n = 35) | NF (n = 19) | NN (n = 154) | Total (n = 221) | p value |
|---|---|---|---|---|---|---|
| Age (years) | 63 (55.5–65.5) | 59 (57–69) | 66 (57–74) | 63 (57–68) | 63 (57–68) | 0.1052 |
| Gender (%) | <0.0001 | |||||
| Male | 11 (84.6) | 30 (85.7) | 7 (36.8) | 54 (35.1) | 102 (46.2) | |
| Female | 2 (15.4) | 5 (14.3) | 12 (63.2) | 100 (64.9) | 119 (53.8) | |
| Hemoglobin (g/l) | 70 (61–107) | 86 (66–99) | 86 (65–119) | 88.5 (74–113) | 88 (71–110) | 0.1887 |
| Outcome (%) | <0.0001 | |||||
| Dead | 11 (84.6) | 8 (22.9) | 5 (26.3) | 21 (13.6) | 45 (20.4) | |
| Alive | 2 (15.4) | 27 (77.1) | 14 (73.7) | 133 (86.4) | 176 (79.6) | |
| BMI (kg/m2) | 19.5 (18.7–21.8) | 21.4 (19.5–24.7) | 22.9 (20.2–27.1) | 25.4 (22.5–28.3) | 24.4 (20.9–27.3) | <0.0001 |
| Bilirubin (μmol/l) | 32.7 (22.4–51.4) | 23.9 (16.2–49.5) | 23.1 (19.6–53.2) | 19.5 (13.95–36.4) | 21.7 (14.6–38.5) | 0.0684 |
| WBC (×109/l) | 4.11 (3.38–4.63) | 3.47 (1.96–6.19) | 3.84 (2.20–5.41) | 3.40 (2.37–4.78) | 3.45 (2.37–4.88) | 0.8100 |
| Sodium (mmol/l) | 133 (126–139) | 141 (138–143) | 140 (136–142) | 140 (138–142) | 140 (138–142) | 0.0019 |
| Potassium (mmol/l) | 3.5 (2.7–3.85) | 3.7 (3.4–4.0) | 3.7 (3.3–4.1) | 3.9 (3.5–4.1) | 3.8 (3.5–4.1) | 0.1094 |
| Platelets (×109/l) | 94 (44–126) | 83 (50–144) | 76 (42–134) | 81(51–112) | 81 (49–115.5) | 0.9244 |
| Albumin (g/l) | 22 (19.5–28) | 27 (24–32) | 27 (21–31) | 28.5 (25–33) | 28 (24.5–32) | 0.0032 |
| Urea (mmol/l) | 6.6 (4.05–11.5) | 4.9 (3.2–8) | 5.9 (3.1–9.3) | 5.0 (3.7–5.9) | 5.0 (3.7–6.6) | 0.1076 |
| Creatinine (mmol/l) | 61 (57–90.5) | 58 (54–74) | 64 (58–82) | 56.5 (46.5–67.5) | 58 (49–72) | 0.0174 |
| NLR | 7.46 (3.5–9.38) | 3.52 (1.98–6.21) | 4.91 (2.62–6.06) | 2.68 (1.66–3.81) | 3.05 (1.93–4.80) | <0.0001 |
| MELD score | 12 (9.5–16) | 8 (5.75–11) | 11 (8–13) | 9 (6–11) | 9 (6–12) | 0.0124 |
| MELD score (%) | 0.6108 | |||||
| >15 | 3 (23.1) | 5 (14.3) | 2 (10.5) | 17 (11) | 27 (12.2) | |
| ⩽15 | 10 (76.9) | 30 (85.7) | 17 (89.5) | 137 (89) | 194 (87.8) | |
| CTP score | 9 (8–10.5) | 8 (7–9) | 9 (7–10) | 7 (6–9) | 9 (8–13) | <0.0001 |
| CTP class (%) | 0.0072 | |||||
| A | 0 (0) | 7 (20) | 3 (15.8) | 56 (36.4) | 66 (29.9) | |
| B/C | 13 (100) | 28 (80) | 16 (84.2) | 98 (63.6) | 155 (70.1) | |
| Comorbidity (%) | ||||||
| Hypertension | 2 (15.4) | 10 (28.6) | 5 (26.3) | 43 (27.9) | 60 (27.1) | 0.1274 |
| Diabetes mellitus | 3 (23.1) | 10 (28.6) | 6 (31.6) | 34 (22.1) | 53 (24.0) | 0.2201 |
| Cardiovascular disease | 3 (23.1) | 3 (8.6) | 7 (36.8) | 19 (12.3) | 32 (14.5) | 0.4082 |
| Complication (%) | ||||||
| GEV | 8 (61.5) | 25 (71.4) | 11 (57.8) | 111 (72.1) | 155 (70.1) | 0.5472 |
| Variceal bleeding | 5 (38.5) | 14 (40.0) | 4 (21.1) | 52 (33.8) | 75 (33.9) | 0.0499 |
| HE | 3 (23.1) | 2 (5.7) | 2 (10.5) | 7 (4.5) | 14 (6.3) | 0.0561 |
| Infection | 6 (46.2) | 4 (11.4) | 6 (31.6) | 13 (8.4) | 29 (13.1) | <0.0001 |
| Ascites | 13 (100) | 26 (74.3) | 17 (89.5) | 77 (50) | 133 (60.1) | <0.0001 |
| Etiology (%) | 0.0030 | |||||
| Alcohol | 6 (46.1) | 17 (48.6) | 6 (31.5) | 29 (18.8) | 58 (26.2) | |
| Viral | 4 (30.8) | 10 (28.6) | 1 (5.3) | 42 (27.3) | 57 (25.8) | |
| Autoimmune/cholestasis | 2 (15.4) | 4 (11.4) | 6 (31.6) | 52 (33.8) | 64 (29) | |
| Others | 1 (7.7) | 4 (11.4) | 6 (31.6) | 31 (20.1) | 42 (19) |
BMI, body mass index; CTP, Child–Turcotte–Pugh; GEV, gastroesophageal varices; HE, hepatic encephalopathy; MELD, model for end-stage liver disease; NF, non-sarcopenia and frailty; NLR, neutrophil-to-lymphocyte ratio; NN, non-sarcopenia and non-frailty; SF, sarcopenia and frailty; SN, sarcopenia and non-frailty; WBC, white blood cell count.
Considering that the presence of two inter-related conditions, sarcopenia and frailty, was investigated, we evaluated whether the frequency of frailty differs between the sarcopenia groups. Figure 1 demonstrates the frequency of patients with frailty (assessed as Frailty Index >0.38) in the groups stratified as sarcopenia and non-sarcopenia. As can be observed, the prevalence of patients with frailty significantly differed among the sarcopenia groups, indicating that frailty was more common in the group of sarcopenia (27.1% versus 11%, p = 0.009). Notably, 11% of the patients in the group of non-sarcopenia had frailty. We then expanded our analyses by investigating the role that these two conditions combined (sarcopenia and frailty) have on other laboratory exams and clinical features.
Figure 1.
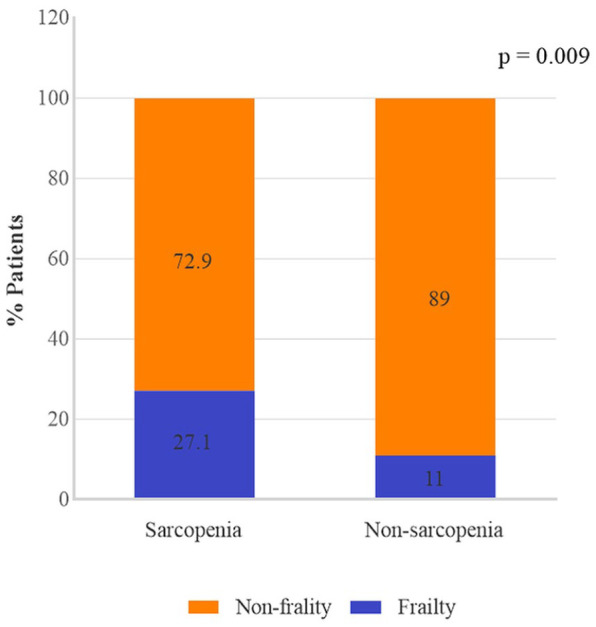
Prevalence of frailty (assessed by Frailty Index) in groups classified as sarcopenia and non-sarcopenia.
Comparisons of demographics, clinical characteristics, and laboratory data among the groups classified by the presence of sarcopenia and frailty are shown in Table 1. BMI and the percentage of males differed significantly among the groups, with BMI lower in the SF group and male gender more prevalent in the SN group. Regarding laboratory data, most of them varied significantly among the groups, as evidenced by lower sodium, lower albumin, higher neutrophil-to-lymphocyte ratio (NLR), higher MELD score, and higher prevalence of CTP class B/C in the SF group. Cirrhosis-associated complications including variceal bleeding, HE, infection, and ascites differed markedly among the groups, which indicated worse disease severity in the SF group compared with other groups. After 2 years of follow-up, there were 45 deaths. The causes of death were due to liver failure in 18, severe infection in 14, HE in 7, and variceal bleeding in 6 subjects. The group of deceased patients was with higher MELD score and prevalence of CTP class B/C and prevalence of infection/HE compared with the patients who survived (Supplemental Table S2).
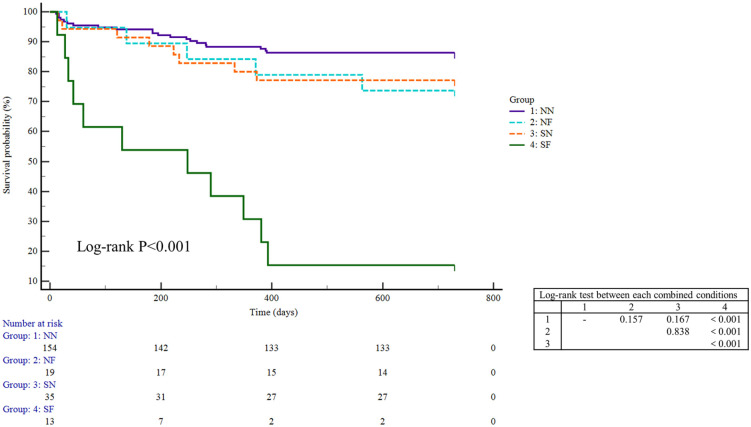
The survival analysis showed that there was a significant difference in the survival curves among the groups, with the group combining both conditions (sarcopenia and frailty) being the one with lower survival rate (Figure 2 and Figure S2). To further confirm this finding, we set out to build two multivariate Cox regression models on the basis of univariate analysis (Tables 2 and 3). In model 1 adjusted for ascites, albumin, MELD score, sodium, WBC, NLR, alcoholism, and BMI, the SF group had an HR of 2.604 [95% confidence interval (CI): 1.023–6.630]. In model 2 adjusted for CTP score, creatinine, sodium, infection, NLR, alcoholism, and BMI, the SF group had an HR of 4.294 (95% CI: 1.766–10.439). Intriguingly, the HRs for the integral SN/NF group were lower than those for the SF group in both models (0.922 versus 2.604 in model 1 and 1.038 versus 4.294 in model 2).
Figure 2.
Survival curves according to the groups with sarcopenia and frailty in patients with decompensated cirrhosis.
NF, non-sarcopenia and frailty; NN, non-sarcopenia and non-frailty; SF, sarcopenia and frailty; SN, sarcopenia and non-frailty.
Table 2.
Factors associated with mortality by univariate Cox analysis in cirrhosis.
| Variables | HR | 95% CI | p value |
|---|---|---|---|
| Ages (years) | 0.992 | 0.964–1.022 | 0.617 |
| Gender (%) | |||
| Male | 1.503 | 0.835–2.706 | 0.174 |
| Female | |||
| Hemoglobin (g/l) | 0.989 | 0.978–1.001 | 0.065 |
| BMI (kg/m2) | 0.913 | 0.846–0.986 | 0.021 |
| Bilirubin (μmol/l) | 1.005 | 1.003–1.008 | <0.001 |
| WBC (×109/l) | 1.082 | 1.023–1.144 | 0.006 |
| Sodium (mmol/l) | 0.904 | 0.872–0.937 | <0.001 |
| Potassium (mmol/l) | 1.144 | 0.753–1.738 | 0.529 |
| Platelets (×109/l) | 1.003 | 0.999–1.007 | 0.183 |
| Albumin (g/l) | 0.862 | 0.815–0.911 | 0.001 |
| Urea (mmol/l) | 1.007 | 0.994–1.021 | 0.281 |
| Creatinine (mmol/l) | 1.009 | 1.005–1.013 | <0.001 |
| NLR | 1.019 | 1.004–1.035 | 0.016 |
| MELD score | 1.142 | 1.061–1.230 | <0.001 |
| CTP score | 1.662 | 1.293–2.134 | <0.001 |
| CTP class (%) | |||
| A | |||
| B/C | 7.113 | 2.202–22.977 | 0.001 |
| Complication (%) | |||
| GEV | 0.560 | 0.307–1.021 | 0.058 |
| Variceal bleeding | 1.503 | 0.832–2.717 | 0.177 |
| HE | 1.624 | 0.706–3.736 | 0.254 |
| Infection | 2.262 | 1.102–4.643 | 0.026 |
| Ascites | 3.145 | 1.461–6.767 | 0.003 |
| Etiology (%) | |||
| Alcohol | 1.985 | 1.093–3.606 | 0.024 |
| SF (versus NN group) | 10.216 | 4.885–21.365 | <0.001 |
| SN (versus NN group) | 1.774 | 0 .786–4.004 | 0.168 |
| NF (versus NN group) | 1.998 | 0.754–5.300 | 0.164 |
| SN/NF (versus NN group) | 1.854 | 0.928–3.703 | 0.080 |
BMI, body mass index; CI, confidence interval; CTP, Child–Turcotte–Pugh; GEV, gastroesophageal varices; HE, hepatic encephalopathy; HR, hazard ratio; MELD, model for end-stage liver disease; NF, non-sarcopenia and frailty; NLR, neutrophil-to-lymphocyte ratio; NN, non-sarcopenia and non-frailty; SF, sarcopenia and frailty; SN, sarcopenia and non-frailty; WBC, white blood cell count.
Table 3.
Factors associated with mortality by multivariate Cox analysis in cirrhosis.
| Variables | Multivariate analysis: model 1 | Multivariate analysis: model 2 | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| BMI (kg/m2) | 0.917 (0.842–0.998) | 0.044 | ||
| Bilirubin (μmol/l) | ||||
| WBC (×109/l) | ||||
| Sodium (mmol/L) | ||||
| Albumin (g/L) | 0.908 (0.843–0.977) | 0.010 | ||
| Creatinine (mmol/l) | 1.008 (1.004–1.013) | <0.001 | ||
| NLR | ||||
| MELD score | 1.081 (1.028–1.138) | 0.003 | ||
| CTP score | 1.438 (1.216–1.700) | <0.001 | ||
| Infection | ||||
| Ascites | ||||
| Alcohol | 2.291 (1.156–4.539) | 0.017 | ||
| SF (versus NN group) | 2.604 (1.023–6.630) | 0.045 | 4.294 (1.766–10.439) | 0.001 |
| SN/NF (versus NN group) | 0.922 (0.413–2.055) | 0.842 | 1.038 (0.471–2.286) | 0.927 |
BMI, body mass index; CI, confidence interval; CTP, Child–Turcotte–Pugh; HR, hazard ratio; MELD, model for end-stage liver disease; NF, non-sarcopenia and frailty; NLR, neutrophil-to-lymphocyte ratio; NN, non-sarcopenia and non-frailty; SF, sarcopenia and frailty; SN, sarcopenia and non-frailty; WBC, white blood cell count.
Multivariate regression analysis model 1: ascites, albumin, MELD, MF, sodium, WBC, NLR, alcohol, and BMI.
Multivariate regression analysis model 2: CTP score, creatinine, MF, sodium, infection, NLR, alcohol, and BMI.
Discussion
In this study, we aimed to evaluate the role of sarcopenia and frailty on survival in a cohort of hospitalized cirrhotics. Frailty (diagnosed by Frailty Index) was present in 14.5%, which was similar to a report by Deng et al. 23 that 19% of 233 patients undergoing outpatient liver transplantation evaluation exhibited frail assessed by LFI. Furthermore, in a comprehensive review to promote recognition of both conditions, namely, frailty and sarcopenia, it was shown that the prevalence of frailty ranges from 18% to 43% in the context of cirrhosis. 5 Therefore, our findings on the presence of frailty are somehow lower than those from previous studies, most likely due to the application of varying measured metrics as well as discrete conceptual construct pertaining to global frailty versus physical frailty.
Markers of abnormal skeletal muscle quantity, such as low SMI, designated as sarcopenia in the current study were present in 21.7% of the patients, a percentage closely similar to the findings by Zeng et al. in a multicenter study on the basis of 911 cirrhotic Chinese patients (22.5% of the patients diagnosed with sarcopenia). In that paper, the L3-SMI cutoff value for sarcopenia was 44.77 cm2/m2 in male patients and 32.50 cm2/m2 in female patients, which is in line with our thresholds. Moreover, we acknowledge that the currently estimated prevalence of sarcopenia is lower to those from previous studies in the context of cirrhosis (30–70%). 19 This discrepancy is most likely due to the cutoffs for application, anatomical landmark for measurement, study design, and outcome of interest. 24 Actually, Tandon et al. 5 have addressed that it is unlikely to use a universal prognostic cutoff or single optimal site of measurement for all populations due to a higher incidence of sarcopenia in Asian populations if Western cutoffs were arbitrarily used.
In addition, we identified that among the groups stratified by sarcopenia status, 27.1% of the sarcopenic patients also had frailty. In another study recruiting adult patients with cirrhosis who were actively listed for transplantation, 25% of the patients with sarcopenia using L3-SMI on CT were also diagnosed with frailty. 25 The similar frequency of sarcopenia and frailty combined in our study and in the aforesaid one indicates that these pathological disturbances can coexist, and a careful evaluation of both conditions should be carried out in patients with cirrhosis. Of note, a fraction of patients (11%) without sarcopenia also exhibited frail. In other words, the absence of sarcopenia dose not exclude the existence of frailty. This finding highlights a fact that sarcopenia and frailty share some common drivers; however, these are different abnormalities and the investigation of both is crucial.
In the current study, frailty was diagnosed by Frailty Index, which evaluates several domains of frail components [instrumental activities of daily living (IADL) score, physical function score, falls, exhausted, depressed, medications, comorbidities, visual/hearing impairment, loss of weight, and low levels social activity]. Therefore, it provides a global and multi-dimensional assessment of frail status, including facets not reflected by sarcopenia or physical frailty. This likely interprets the reason why individuals in the group of non-sarcopenia had frailty when diagnosed by Frailty Index. Adding to these findings, we also showed, as expected, that when sarcopenia and frailty occur concomitantly (SF group), most parameters regarding liver function were worse in comparison with the NN group (discussed below).
It is interesting to note that various laboratory data and clinical features, such as BMI, sodium, albumin, creatinine, and NLR, differed among the groups stratified by frailty and sarcopenia status, this difference being more remarkable between the SF group and the NN group. Among those, we also observed that patients in the SF group have lower BMI, lower sodium, lower albumin, and higher NLR relative to subjects in the SN or NF group. Furthermore, the MELD score and CTP class were more advanced in the coexistence of sarcopenia and frailty relative to sarcopenia or frailty in isolation (Table 1). Similar results can be found in relation to complications, namely, HE, infection, and ascites, which is partially expected since the group combining both conditions (sarcopenia and frailty) exhibits the severest grading of liver disease compared with either condition separately. Taken together, we suppose the collective information underlies the reason why either condition separately dropped out the Cox regression model, and a therapeutic strategy should be developed for the SF group patients (Table 3).
Finally, when evaluating survival, we demonstrated that the mortality risk of the SF group is around three to four times (HR: 2.604 in model 1 and HR: 4.294 in model 2) higher than the group without any of these abnormalities. As far as we are concerned, this is the first study reporting synergistically inferior impact of sarcopenia and frailty combined on mortality in the context of cirrhosis. In contrast, a preliminary investigation implemented by Alexopoulos et al. 11 addressed no incremental mortality risk of concurrence of these two conditions compared with the respective condition. Therefore, further studies are warranted to validate our findings.
Several limitations and strengths of the current study can be listed. As a limitation, the design of observational study can impair the establishment of a causality–effect association. Second, the relatively small sample size may underpower the comparison among the sarcopenia and frailty groups, although statistical significance was already observed with this sample size. Third, the lack of measuring muscle strength, namely, HGS, is in contrast to the sarcopenia definition recently proposed by EWGSOP2, although the vast majority of literature on cirrhosis has operationalized sarcopenia as loss of muscle mass. 26 As positive issues, we consider the originality of assessing the concomitance of sarcopenia and frailty in cirrhosis, and the relationship of these conditions with survival. In addition, the performance-based metrics used to identify frail phenotype in the ambulatory patients necessitate active participation of subjects, which can limit their use in severely decompensated or acutely ill populations. Thus, we have adopted a self-reported questionnaire to diagnose frailty. Furthermore, we selected cross-sectional imaging by CT to accurately and objectively evaluate skeletal muscle mass, whose utility is not affected by fluid retention. Although CT carries potential for radiation exposure, renal injury, and high cost, this modality has been regarded as a routine examination for screening HCC risk and disease progression among cirrhotics.
Conclusion
In conclusion, hospitalized patients with decompensated cirrhosis who have sarcopenia and frailty showed higher mortality risk. In addition, the concurrence of those two conditions does give rise to incremental mortality when compared with the group with each disturbance separately. Moreover, we reported that frailty can occur in patients without sarcopenia. Altogether, these findings highlight the importance of developing therapeutic strategy and palliative management for these specific subset in the context of cirrhosis.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221109651 for Sarcopenia and frailty combined increases the risk of mortality in patients with decompensated cirrhosis by Gaoyue Guo, Chaoqun Li, Yangyang Hui, Lihong Mao, Mingyu Sun, Yifan Li, Wanting Yang, Xiaoyu Wang, Zihan Yu, Xiaofei Fan, Kui Jiang and Chao Sun in Therapeutic Advances in Chronic Disease
Acknowledgments
We thank Dr Huanhuan Wu for her technical support.
Footnotes
ORCID iD: Chao Sun  https://orcid.org/0000-0002-0380-7999
https://orcid.org/0000-0002-0380-7999
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Gaoyue Guo, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Chaoqun Li, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Department of Internal Medicine, Tianjin Hexi Hospital, Tianjin, China.
Yangyang Hui, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Lihong Mao, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Mingyu Sun, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Yifan Li, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Wanting Yang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Xiaoyu Wang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Zihan Yu, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Xiaofei Fan, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Kui Jiang, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Tianjin, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China.
Chao Sun, Department of Gastroenterology and Hepatology, Tianjin Medical University General Hospital, Anshan Road 154, Heping District, Tianjin 300052, China; Tianjin Institute of Digestive Disease, Tianjin Medical University General Hospital, Tianjin, China; Department of Gastroenterology, Tianjin Medical University General Hospital Airport Hospital, Tianjin, China.
Declarations
Ethics approval and consent to participate: The study was employed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of TJMUGH (IRB2021-YX-136-01). A written informed consent was obtained from all patients on admission.
Consent for publication: Not applicable.
Author contributions: Gaoyue Guo: Conceptualization; Data curation.
Chaoqun Li: Conceptualization; Data curation.
Yangyang Hui: Conceptualization; Data curation.
Lihong Mao: Formal analysis.
Mingyu Sun: Methodology.
Yifan Li: Investigation.
Wanting Yang: Resources.
Xiaoyu Wang: Investigation.
Zihan Yu: Resources.
Xiaofei Fan: Software.
Kui Jiang: Supervision.
Chao Sun: Conceptualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly supported by the Science and Technology Program of Tianjin (Grant 19ZXDBSY00020 to K.J.).
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: All data are available from the corresponding author upon reasonable request.
References
- 1. Gines P, Krag A, Abraldes JG, et al. Liver cirrhosis. Lancet 2021; 398: 1359–1376. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020; 5: 245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol 2019; 70: 151–171. [DOI] [PubMed] [Google Scholar]
- 4. Pellicoro A, Ramachandran P, Iredale JP, et al. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol 2014; 14: 181–194. [DOI] [PubMed] [Google Scholar]
- 5. Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol 2021; 75: S147–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hou L, Deng Y, Fan X, et al. A sex-stratified prognostic nomogram incorporating body compositions for long-term mortality in cirrhosis. J Parenter Enteral Nutr 2021; 45: 403–413. [DOI] [PubMed] [Google Scholar]
- 7. Paternostro R, Bardach C, Hofer BS, et al. Prognostic impact of sarcopenia in cirrhotic patients stratified by different severity of portal hypertension. Liver Int 2021; 41: 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng Y, Lin L, Hou L, et al. A self-reported Frailty Index predicts long-term mortality in hospitalized patients with cirrhosis. Ann Transl Med 2020; 8: 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai JC, Covinsky KE, McCulloch CE, et al. The Liver Frailty Index improves mortality prediction of the subjective clinician assessment in patients with cirrhosis. Am J Gastroenterol 2018; 113: 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feng H, Wang X, Mao L, et al. Relationship between sarcopenia/myosteatosis and frailty in hospitalized patients with cirrhosis: a sex-stratified analysis. Ther Adv Chronic Dis 2021; 12: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexopoulos T, Vasilieva L, Kontogianni MD, et al. Letter: patients who have both sarcopenia and frailty have similar prognosis to those with either condition separately. Aliment Pharmacol Ther 2021; 54: 981–982. [DOI] [PubMed] [Google Scholar]
- 12. Bunchorntavakul C, Reddy KR. Letter: patients who have both sarcopenia and frailty have similar prognosis to those with either condition separately-authors’ reply. Aliment Pharmacol Ther 2021; 54: 983–984. [DOI] [PubMed] [Google Scholar]
- 13. Feng H, Wang X, Zhao T, et al. Myopenic obesity determined by visceral fat area strongly predicts long-term mortality in cirrhosis. Clin Nutr 2021; 40: 1983–1989. [DOI] [PubMed] [Google Scholar]
- 14. Guerard EJ, Deal AM, Chang Y, et al. Frailty Index developed from a cancer-specific geriatric assessment and the association with mortality among older adults with cancer. J Natl Compr Canc Netw 2017; 15: 894–902. [DOI] [PubMed] [Google Scholar]
- 15. Puchades Renau L, Herreras Lopez J, Cebria I Iranzo MA, et al. Frailty and sarcopenia in acute-on-chronic liver failure. Hepatol Commun 2021; 5: 1333–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang SH, Jeong WK, Baik SK, et al. Impact of sarcopenia on prognostic value of cirrhosis: going beyond the hepatic venous pressure gradient and MELD score. J Cachexia Sarcopenia Muscle 2018; 9: 860–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buchard B, Boirie Y, Cassagnes L, et al. Assessment of malnutrition, sarcopenia and frailty in patients with cirrhosis: which tools should we use in clinical practice? Nutrients 2020; 12: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang CW, Feng S, Covinsky KE, et al. A comparison of muscle function, mass, and quality in liver transplant candidates: results from the functional assessment in liver transplantation study. Transplantation 2016; 100: 1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. European Association for the Study of the Liver. Electronic address EEE and European Association for the Study of the L. EASL clinical practice guidelines on nutrition in chronic liver disease. J Hepatol 2019; 70: 172–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 2021; 74: 1611–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tandon P, Low G, Mourtzakis M, et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 2016; 14: 1473–1480. [DOI] [PubMed] [Google Scholar]
- 22. Zeng X, Shi ZW, Yu JJ, et al. Sarcopenia as a prognostic predictor of liver cirrhosis: a multicentre study in China. J Cachexia Sarcopenia Muscle 2021; 12: 1948–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deng LX, Bischoff KE, O’Kent DS, et al. Frailty is strongly associated with self-reported symptom burden among patients with cirrhosis. Eur J Gastroenterol Hepatol 2021; 33: e395–e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ebadi M, Bhanji RA, Tandon P, et al. Review article: prognostic significance of body composition abnormalities in patients with cirrhosis. Aliment Pharmacol Ther 2020; 52: 600–618. [DOI] [PubMed] [Google Scholar]
- 25. Fozouni L, Wang CW, Lai JC. Sex differences in the association between frailty and sarcopenia in patients with cirrhosis. Clin Transl Gastroenterol 2019; 10: e00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221109651 for Sarcopenia and frailty combined increases the risk of mortality in patients with decompensated cirrhosis by Gaoyue Guo, Chaoqun Li, Yangyang Hui, Lihong Mao, Mingyu Sun, Yifan Li, Wanting Yang, Xiaoyu Wang, Zihan Yu, Xiaofei Fan, Kui Jiang and Chao Sun in Therapeutic Advances in Chronic Disease