Abstract
Lisfranc complex injuries are a spectrum of midfoot and tarsometatarsal (TMT) joint trauma, more frequent in men and in the third decade of life. Depending on the severity of the trauma can range from purely ligamentous injuries, in low-energy trauma, to bone fracture-dislocations in high-energy trauma. A quick and careful diagnosis is crucial to optimize management and treatment, reducing complications and improving functional outcomes in the middle and long-term. Up to 20% of Lisfranc fractures are unnoticed or diagnosed late, above all low-energy trauma, mistaken for simple midfoot sprains. Therefore serious complications such as post-traumatic osteoarthritis and foot deformities are not uncommon. Clinically presenting with evident swelling of the midfoot and pain, often associated with joint instability of the midfoot. Plantar region ecchymosis is highly peculiar. First level of examination is X-Ray performed in 3 projections. CT scan is useful to detect nondisplaced fractures and minimal bone sub-dislocation. MRI is the gold standard for ligament injuries. The major current controversies in literature concern the management and treatment. In stable lesions and in those without dislocation, conservative treatment with immobilization and no weight-bearing is indicated for a period of 6 weeks. Displaced injuries have worse outcomes and require surgical treatment with the two main objectives of anatomical reduction and stability of the first three cuneiform-metatarsal joints. Different surgical procedures have been proposed from closed reduction and percutaneous surgery with K-wire or external fixation (EF), to open reduction and internal fixation (ORIF) with transarticular screw (TAS), to primary arthrodesis (PA) with dorsal plate (DP), up to a combination of these last 2 techniques. There is no superiority of one technique over the other, but what determines the post-operative outcomes is rather the anatomical reduction. However, the severity of the injury and a quick diagnosis are the main determinant of the biomechanical and functional long-term outcomes.
Keywords: Lisfranc injury, Lisfranc ligament, midfoot dislocation, Lisfranc fracture-dislocations, tarsometatarsal joint, fusion
Introduction
The term “Lisfranc injuries” refers to a range of midfoot and tarsometatarsal (TMT) joint lesion that can vary from a simple single joint injury to a complex lesion that disrupts multiple different joints with multiple fractures [1], depending on the severity of the trauma. The name is attributed to a French surgeon of the Napoleonic era, which in 1825 was the first to describe injuries and amputations at this level of the foot [1].
Lisfranc injuries appear rare and account for 0.2% of all fractures, with approximately 20% of cases remaining undiagnosed or diagnosed late [2].
Lisfranc joint injuries are more frequent in the third decade of life and men are 2 to 4 times more likely than women to incur these injuries, possibly because they participate more frequently in high-speed activities [3]. High-energy injuries are more common than low-energy injuries which in most cases involve sports activities, usually occurring during football, gymnastic and running [4]. The two main mechanisms of injury are direct forces (crush injuries, fall from and height) and indirect forces (bending and torsion of the tarsus) [5].
A thorough investigation of the mechanism of trauma and a clinical examination of the foot are essential. The exact mechanism of the injury must be ascertained, including foot position during trauma, direction of the force and the amount of energy involved.
Quickly identification and management of these injuries is crucial to reduce risk of progressive midfoot instability, arch collapse, forefoot abduction, or post-traumatic osteoarthritis (OA) that results in stiffness, chronic pain, and dysfunction of the foot and ankle complex [1].
Anatomy
The Lisfranc joint complex has a specialized bony and ligamentous structure, which provides stability to this joint.
Osteology
The Lisfranc joint complex is made up by the three cuneiform bones (C1 to C3) and the cuboid bone (Cu) proximally and the five metatarsal (M1 to M5) bases distally linked together by a ligamentous capsule structure [6].
Lisfranc joint can be divided in three longitudinal columns: I) medial, composed by C1 and M1; II) central (middle), composed by C2-C3 and M2-M3; the space between the base of the second metatarsal bone and the first cuneiform bone is filled with the ligament key of the Lisfranc joint; the space between the bases of the third and fourth metatarsal bone is filled with the intermetatarsal ligament; III) lateral, composed by Cu and M4-M5.
Bone stability is determined by the trapezoidal shape of the base of the M1-M2-M3, with their respective cuneiform bones forming a stable arch known as a “transverse arch or Roman arch” with the second TMT joint as the keystone [7,8].
Ligaments
The tarsometatarsal (TMT) complex includes the TMT joints, the intermetatarsal ligaments and the intercuneiform joints.
Ligamentous structure of the Lisfranc joint can be divided schematically in: i. dorsal ligament and plantar ligament: the first the smallest and the weakest crossing each TMT joint, while the second is twice as large as the first. This explains why the dislocation is often dorsal; ii. the interosseous ligament, commonly known as the “Lisfranc ligament”, is the biggest. It is 4.5 times larger than the dorsal ligament and twice as large as the plantar ligament; iii. intermetatarsal ligaments joining the second to the fifth metatarsal, instead between M1 and M2 there isn’t an intermetatarsal ligament [9].
Joint capsules, plantar muscles and fascia, tendons of the peroneus longus, of the tibialis anterior and of the tibialis posterior contribute to midfoot stability and support the arch of the foot. A diagram of the structure of the Lisfranc joint complex is shown in Figure 1.
Figure 1.
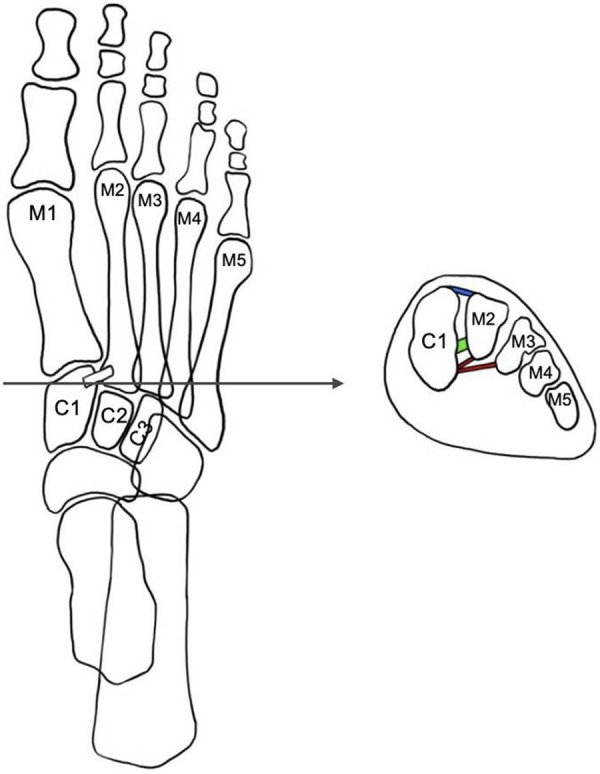
Diagram of Lisfranc joint complex. (M1: first metatarsal; M2: second metatarsal; M3: third metatarsal; M4: fourth metatarsal; M5: fifth metatarsal; C1: medial cuneiform; C2: middle cuneiform; C3: lateral cuneiform; Blue: dorsal Lisfranc ligament; Green: Interosseous Lisfranc ligament; Red: plantar Lisfranc ligament).
Diagnosis
Clinical examination
Lisfranc complex injuries subsequent to high-energy trauma, often with fractures-dislocations, present clinical and imaging evidence that make it unlikely to miss. However, low-energy Lisfranc injuries are often overlooked [2], diagnosed late or even lost due to the difficulty to identify minimal lesions; often occur during sport activities with metatarsal diastasis lesions less than 2 mm [10,11]. Patients present swelling of the midfoot region associated with pain on weight bearing activity [12].
Plantar region ecchymosis is highly peculiar. Comparison with contralateral foot can be important and help in the diagnosis; obviously except for cases with bilateral lesions, found in precipitated patients. To identify the affected TMT joint is useful the “piano key” test, consisting in moving the head of the affected metatarsal holding the midfoot and hindfoot firmly [13]. The “gap sign” indicative of separation between first and second fingers is a suggestive sign of lesion of the Lisfranc [14]. Vascular lesions are rare, in relation to which compartment syndromes or lesions of the deep peroneal nerve can occur [15].
Imaging
First level examination is X-ray, performed in 3 non-weight-bearing projections (AP, oblique, lateral) which in some cases is enough to make a diagnosis.
A craniocaudal angulation of the X-ray beam of about 30° may better show the joint [16].
On standard X-ray the signs consistent with a diagnosis of Lisfranc injuries are: 1. Loss of alignment between the medial edges of C2 and M2 (on AP view); 2. Loss of alignment between the medial edges of Cu and M4 (on oblique view); 3. Avulsion of the Lisfranc ligament represented by the “fleck” sign, a small bone fragment in the first intermetatarsal space; 4. Diastasis >2 mm between the base M1 and M2 or a greater than 1 mm difference than that of the contralateral uninjured foot (AP view); 5. Dorsal/plantar displacement of the metatarsal bases (lateral view) [17].
However, in relation to the Lisfranc joint complexity, the sensitivity of the standard X-ray is about 84.4% according to Rankine et al. [16], therefore about 20% of lesions remain undiagnosed.
The second imaging level is Computed Tomography (CT). CT is particularly useful to detect nondisplaced fractures or minimal bone sub-dislocations. A recent systematic review has shown how with the use of CT, compared to X-ray, it is possible to detect 60% more metatarsal fractures and twice the tarsal fractures and joint misalignments [18].
Three-dimensional (3-D) CT imaging provides a complete assessment of the lesion and associated with multiplanar reconstructions provides anatomical details, including neurovascular ones, that increase optimal surgical pre-operative planning [19].
In the presence of non-diagnostic X-ray and CT scans, if clinical suspicion persists, magnetic resonance imaging (MRI) is appropriate. MRI is the gold standard to detect ligament injuries. In 2009, Raikin et al. [20] observed that MRI had a sensitivity of 90% for evaluating the stability of the Lisfranc joint compared to intraoperative results [21]. MRI is also useful in the differential diagnosis with other not so infrequent pathologies [22-24].
Classification
To describe the Lisfranc complex injuries have been proposed numerous classification. In 1909, Quenu and Kuss [25] classified Lisfranc lesions based on the three-column concept. The lesions were classified as homolateral, isolated and divergent. Always based on concepts of Quenu and Kuss, Hardcastle et al. [26], in 1982, divided the injuries in three types: type A (with complete displacement of all the metatarsal bones), B (with displacement of one or more of the metatarsal bones), and C (divergent pattern).
In 1986 Myerson et al. [27] proposed a new modified version of Hardcastle’s classification, which included type A (injury with total incongruity, medial-lateral), type B with isolated incongruity patterns (respectively type B1 medial and type B2 lateral), and type C divided into types C2 and C1 according to whether all four or fewer metatarsals were divergently displaced respectively.
Recently, a fourth category to Myerson’s modified Hardcastle classification (type D injury) has been introduced, which corresponds to the partial injury of the Lisfranc joint [28].
Divided into D1 type in which distance between C1 bone and M2 is 2 mm and not require surgical fixation and D2 type with this distance >2 mm, with the need for surgical fixation (D2 further divided into purely ligamentous D2L and with bone avulsion D2B).
In 2002 Nunley and Vertullo [29] proposed a classification for low-energy injuries, focused on clinical features, weight-bearing foot X-ray and bone scintigraphy (BS).
In this classification, stage I is a Lisfranc ligament sprain without diastasis or loss of arch height on lateral X-ray but increased uptake on BS. Stage II sprains are lesions with M1-M2 diastasis between 1 and 5 mm on AP weight bearing X-ray due to the Lisfranc ligament injury, without loss of arch height on weight bearing lateral view.
Patients with stage III injuries have M1-M2 diastasis greater than 5 mm on AP weightbearing view and loss of midfoot arch height, showing a decreased distance M5-C1 on lateral X-ray [29].
In 2008 Coetzee [30] schematically divided the injuries into: ● incomplete ligamentous disruption, with 3 stages: stage “1” less than 2 mm diastases and no medial longitudinal arch collapse; stage “2” between 2 and 5 mm diastases and no arch collapse; stage “3” between 2 and 5 mm diastases and arch collapse; ● complete ligamentous disruption, stage “1” without significant intra-articular fractures or stage “2” with significant intra-articular comminution.
The existing classification systems have shown only a moderate grade of reliability among the interlocutors and do not add prognostic value, so much so that they are of little use in clinical practice [31,32]. The classification evolution of Lisfranc complex injuries is summarized in the flow-chart in Figure 2.
Figure 2.
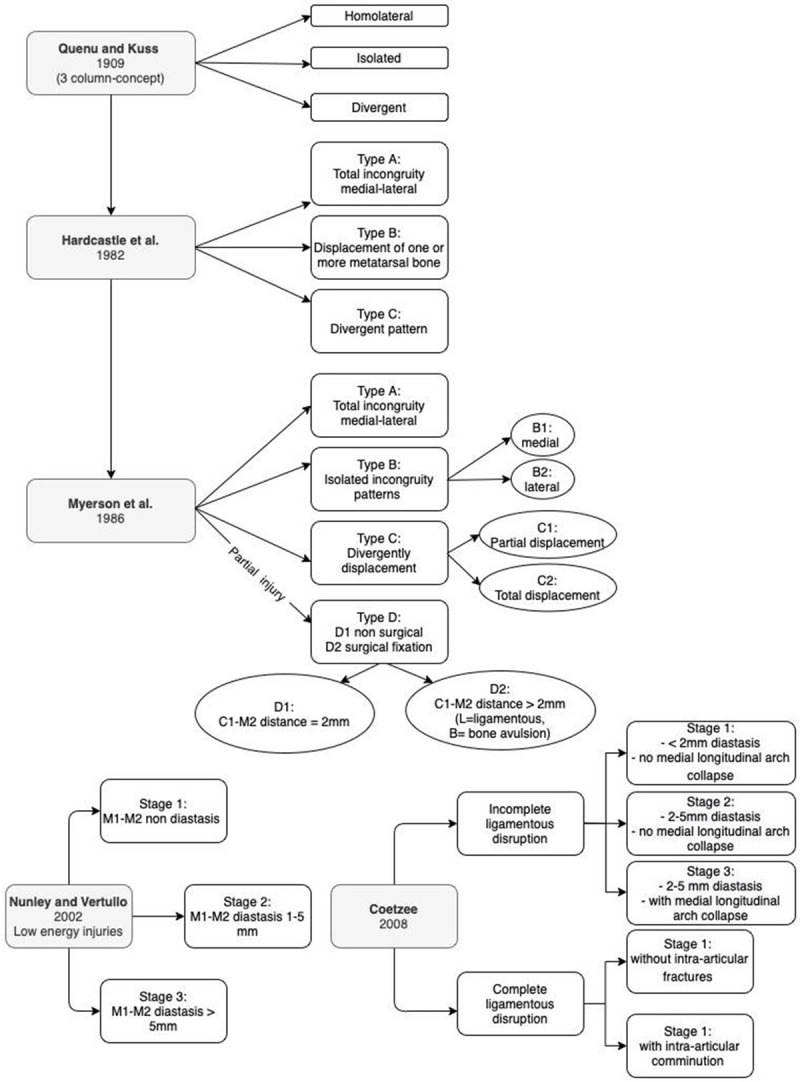
Diagram showing the evolution of the Lisfranc joint complex injuries classification. (M1: first metatarsal; M2: second metatarsal).
Management and treatment
Management of Lisfranc injuries depends on the severity of the trauma, with the primary goals of treatment being pain relief and foot stability preventing later OA and disability. First of all it is necessary to distinguish between high and low energy injuries, in order to approach them with the correct imaging examinations up to the appropriate treatment (non-surgical or surgical), as shown in Figure 3 in the flow-chart for management and treatment of Lisfranc complex injuries.
Figure 3.
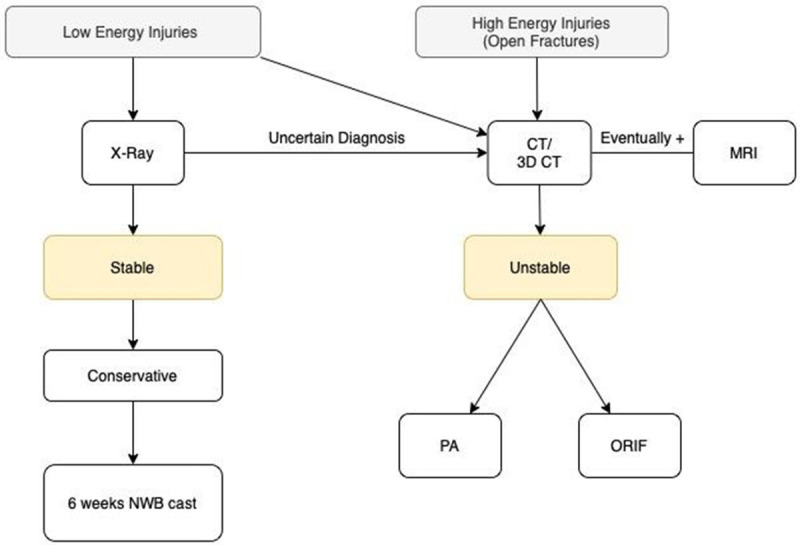
Lisfranc complex injuries management and treatment flowchart. (CT: Computed Tomography; MRI: Magnetic Resonance Imaging; PA: Primary Arthrodesis; ORIF: Open Reduction and Internal Fixation).
Non-surgical treatment
Currently, there aren’t in literature randomized controlled trials that compare nonoperative and surgical treatment for Lisfranc injuries. Hence the results on conservative treatment of nonoperative treatment are based on a few retrospective case series, without widely extended consent on the indications [10].
However, based on our clinical experience and above all on the available literature, conservative treatment is indicated only for stable and non-displaced injuries with pure ligament sprains (stage I according to Nunley and Vertullo) [29].
The conservative treatment involves immobilization in a non-weight-bearing short leg for 6 weeks; after this period, if the midfoot pain disappears, physical activity can be gradually resumed using an orthopedic insole to discharge the medial longitudinal arch. If at six weeks the pain persists, an orthopedic boot with weight-bearing is used for four more weeks and is undertaken a course of physical therapy including taping, modalities (ice, ultrasound, and iontophoresis), and sports-specific exercises [33].
In 1994, Shapiro et al. [34] reported successful conservative treatment for stable injuries even in 9 athletes, showing a return to competition in an average time of 4 months.
In a recent case series on 55 patients [10] (22 avulsion fractures and 33 simple un-displaced intra-articular fractures) the authors reported good results for pain and function with conservative treatment at a follow-up of 2 to 6 years.
Surgical treatment
Nowadays, there is strong consensus that high-energy trauma with displaced or unstable lesions require surgical treatment in order to obtain anatomic reduction and stable internal fixation [35]. However, also after open reduction and internal fixation (ORIF), about 40 to 94% of patients will suffer post-traumatic OA, demanding conversion to a joint fusion to solve the pain, to such an extent that some authors recommend primary arthrodesis (PA) in some cases to avoid the need for re-surgery [36].
High-energy injuries also require careful soft tissue examination (to select appropriate treatment, timing for surgery and minimize postoperative skin and wound complications), and if the evidence leads to a high suspicion of compartment syndrome a surgical fasciotomy is required [4,37].
Percutaneous surgery
In case of open Lisfranc injuries or severe soft tissue compromise and major metatarsal diastasis, temporary stabilization with multiple Kirschner wires (K-wire) or external fixator (EF) should be considered especially in case of comminution and soft-tissue loss. Subsequent conversion should be scheduled 10-15 days later, once soft tissues are back in good condition [38], and the “wrinkles sign” can be helpful [39].
Nithyananth et al. [38] on 22 patients, with open Lisfranc injuries (all type IIIa and IIIb according to Gustilo-Anderson classification [40]) and a mean age of 36 years, treated with multiple K-wire fixation, found at a 56 months of follow-up a mean American Orthopaedic Foot & Ankle (AOFAS) score of 82 (range 59-100) and mean wound healing time of 16 days (range 10-30). Chandran et al. [39] in a group of 10 patients (11 feet) with midfoot open injuries treated with uniplanar EF, maintained for a mean duration of 9 weeks (range 6-15 weeks), experienced a high rate of complications including residual pain and foot and ankle function, ability to stand on tiptoe, presence of a limp, deformity of plantar arch, range of motion of the ankle, subtalar and metatarsophalangeal joints. These results demonstrated that crush injuries with severe trauma and soft tissue injury of the midfoot often result in persistent morbidity despite even early management with external fixation.
Closed reduction and K-wire percutaneous fixation can also be used for definitive fixation, but screw fixation has been shown to provide better biomechanical stability of the medial and middle columns. Therefore, screw fixation is the preferred method of internal fixation with the exception of the lateral column which can be stabilized with K-wire. Open reduction is also to avoid inaccurate reduction and possible interposition of soft tissues [39].
ORIF and primary arthrodesis (PA)
Some controversies persist regarding surgical treatment of Lisfranc injuries: the most appropriate surgical approach and the choice between ORIF with transarticular screws (TAS) versus PA of the first TMT joint with dorsal plates (DP) or a combination of both.
Surgery is usually performed with patient in supine position and knee at 90° of flexion. An incision in the first intermetatarsal space that allows access to the first and second TMT joints and, if necessary, a second longitudinal incision in line with the fourth metatarsal bone are used.
Philpott et al. [41] described a modified approach consisting of a single longitudinal incision on the second metatarsal, from the TMT joint to the metatarsophalangeal (MTP) joint. Through this incision it is achieved access to all the individual TMT joints. The approach was a viable option with complication rates similar to previous approaches.
Controversies remain as to which internal fixation implants are most appropriate.
According to most authors, PA has better results in terms of function and clinical outcomes compared to ORIF treatment with TAS and with a combination of the two techniques [11,42,43].
TAS have been questioned because of joint damage (from 2% to 6% depending on the cases) [44]. For this reason, PA with DP and screws that do not cross the joint have been used in recent years.
In a study of Alberta et al. [44] TAS and DP showed similar ability to resist TMT joint displacement with weight-bearing load.
In a comparison study [42] patients treated with DP showed better functional and radiological outcomes than those treated with TAS or a combination technique. The DP group had a mean AOFAS score of 82.5 points, compared with 71.0 for the TAS group and 63.3 for the combination group (P<0.001).
In a study involving 25 patients [43] that compared PA to ORIF, PA showed better results in terms of reduced foot deformity, biomechanical and function of the foot, complications and surgical duration of the procedure.
In two recent studies [45,46] three different types of surgery were compared: ORIF with TAS, PA with DP and a combination of the two techniques, concluding that functional outcomes mainly depend on the quality of the anatomical reduction and not on the choice of the fixation implant used, significant differences was reported only for the reoperation rate for the removal of the implant.
However, decision making may be influenced by the reoperation rate. In a study on 217 patients with 12 months follow up the authors concluded that, excluding surgery for implant removal, patients treated with ORIF or PA didn’t have a different reoperation rate [47].
The most common causes of re-surgeries are post-traumatic OA in patients treated with ORIF and non-union in those treated with PA.
In a prospective randomized study analyzing 101 patients with purely ligamentous injuries, 92% of the patients treated with PA achieved previous level of activity in the postoperative period. In cases treated with ORIF only 65% achieved pre-injury activity levels in the post-operative period [48].
In a recent systematic review Van Den Boom et al. [49] found a statistically significant difference in patient-reported outcomes scores (PROMs), as measured by the AOFAS score, in favor of PA for the treatment of Lisfranc injuries.
The use of suture endobutton fixation has also been described, placed along the path of the Lisfranc ligament (C1 to M2 base). In this report 3 patients were treated with this relatively fast and minimally invasive technique, achieving satisfactory short-term results. Furthermore, with this device there is no need for subsequent implant removal [50].
Complications
According to the literature, the most common and feared complication after ORIF is post-traumatic OA, reaching in some cases 45% and more. According to Lau et al. [2], treatment with combinations of plates and trans-articular screws have a high risk of OA with respect to only bridging plates and the risk is strictly related to the quality of anatomic reduction. According to Dubois-Ferrière et al. [51], the radiographic evidence of OA was observed in 72.1% of patients and symptomatic OA in 51.1%, the latter with worse outcomes.
Other complications can be screws problems (intended as pull-out or breakage) 16.0%; spontaneous arthrodesis of adjacent joints 7.8%; soft tissue and wound complications 3.6%; compartment syndrome 2.6%; implants infection 1.5%; reflex sympathetic dystrophy 1% and deep vein thrombosis 0.5% [3,45].
Conclusions
Lisfranc complex injuries are a spectrum of injuries of the TMT joints, ranging from purely ligamentous sprains, usually occurring in athletes, to fracture dislocations, commonly a consequence of high-energy trauma.
In accordance with the recent literature the current trend is non-surgical treatment for undisplaced injuries, whilst all injuries that show load instability or diastasis of the TMT joints required surgical treatment with anatomical reduction and internal fixation. Based on biomechanical studies, there are no differences between the use of TAS and DP.
All authors agree that the severity of the injury, a quick diagnosis and anatomical reduction are the main determinants of the biomechanical and functional long-term outcomes.
Acknowledgements
The authors certify that they have NOT affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Welck MJ, Zinchenko R, Rudge B. Lisfranc injuries. Injury. 2015;46:536–541. doi: 10.1016/j.injury.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Lau S, Bozin M, Thillainadesan T. Lisfranc fracture dislocation: a review of a commonly missed injury of the midfoot. Emerg Med J. 2017;34:52–56. doi: 10.1136/emermed-2015-205317. [DOI] [PubMed] [Google Scholar]
- 3.Moracia-Ochagavia I, Rodriguez-Merchan EC. Lisfranc fracture-dislocations: current management. EFORT Open Rev. 2019;4:430–444. doi: 10.1302/2058-5241.4.180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeOrio M, Erickson M, Usuelli FG, Easley M. Lisfranc injuries in sport. Foot Ankle Clin. 2009;14:169–186. doi: 10.1016/j.fcl.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Wiley JJ. The mechanism of tarso-metatarsal joint injuries. J Bone Joint Surg Br. 1971;53:474–482. [PubMed] [Google Scholar]
- 6.Tafur M, Rosenberg ZS, Bencardino JT. MR Imaging of the midfoot including chopart and Lisfranc joint complexes. Magn Reson Imaging Clin N Am. 2017;25:95–125. doi: 10.1016/j.mric.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Peicha G, Labovitz J, Seibert FJ, Grechenig W, Weiglein A, Preidler KW, Quehenberger F. The anatomy of the joint as a risk factor for Lisfranc dislocation and fracture-dislocation. An anatomical and radiological case control study. J Bone Joint Surg Br. 2002;84:981–985. doi: 10.1302/0301-620x.84b7.12587. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher SM, Rodriguez NA, Andersen CR, Granberry WM, Panchbhavi VK. Anatomic predisposition to ligamentous Lisfranc injury: a matched case-control study. J Bone Joint Surg Am. 2013;95:2043–2047. doi: 10.2106/JBJS.K.01142. [DOI] [PubMed] [Google Scholar]
- 9.Johnson A, Hill K, Ward J, Ficke J. Anatomy of the lisfranc ligament. Foot Ankle Spec. 2008;1:19–23. doi: 10.1177/1938640007312300.. [DOI] [PubMed] [Google Scholar]
- 10.Ponkilainen VT, Partio N, Salonen EE, Laine HJ, Maenpaa HM, Mattila VM, Haapasalo HH. Outcomes after nonoperatively treated non-displaced Lisfranc injury: a retrospective case series of 55 patients. Arch Orthop Trauma Surg. 2021;141:1311–1317. doi: 10.1007/s00402-020-03599-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith N, Stone C, Furey A. Does open reduction and internal fixation versus primary arthrodesis improve patient outcomes for Lisfranc trauma? A systematic review and meta-analysis. Clin Orthop Relat Res. 2016;474:1445–1452. doi: 10.1007/s11999-015-4366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grewal US, Onubogu K, Southgate C, Dhinsa BS. Lisfranc injury: a review and simplified treatment algorithm. Foot (Edinb) 2020;45:101719. doi: 10.1016/j.foot.2020.101719. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes D, Leather M, Parker R. Case study: the conservative management of a complex mid foot injury in an elite professional footballer. Res Sports Med. 2021:1–10. doi: 10.1080/15438627.2021.1895785. [DOI] [PubMed] [Google Scholar]
- 14.Davies MS, Saxby TS. Intercuneiform instability and the “gap” sign. Foot Ankle Int. 1999;20:606–609. doi: 10.1177/107110079902000912. [DOI] [PubMed] [Google Scholar]
- 15.Eleftheriou KI, Rosenfeld PF, Calder JD. Lisfranc injuries: an update. Knee Surg Sports Traumatol Arthrosc. 2013;21:1434–1446. doi: 10.1007/s00167-013-2491-2. [DOI] [PubMed] [Google Scholar]
- 16.Rankine JJ, Nicholas CM, Wells G, Barron DA. The diagnostic accuracy of radiographs in Lisfranc injury and the potential value of a craniocaudal projection. AJR Am J Roentgenol. 2012;198:W365–369. doi: 10.2214/AJR.11.7222. [DOI] [PubMed] [Google Scholar]
- 17.Burroughs KE, Reimer CD, Fields KB. Lisfranc injury of the foot: a commonly missed diagnosis. Am Fam Physician. 1998;58:118–124. [PubMed] [Google Scholar]
- 18.Sripanich Y, Weinberg MW, Krahenbuhl N, Rungprai C, Haller J, Saltzman CL, Barg A. Surgical outcome of chronic Lisfranc injury without secondary degenerative arthritis: a systematic literature review☆. Injury. 2020;51:1258–1265. doi: 10.1016/j.injury.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Kalia V, Fishman EK, Carrino JA, Fayad LM. Epidemiology, imaging, and treatment of Lisfranc fracture-dislocations revisited. Skeletal Radiol. 2012;41:129–136. doi: 10.1007/s00256-011-1131-5. [DOI] [PubMed] [Google Scholar]
- 20.Raikin SM, Elias I, Dheer S, Besser MP, Morrison WB, Zoga AC. Prediction of midfoot instability in the subtle Lisfranc injury. Comparison of magnetic resonance imaging with intraoperative findings. J Bone Joint Surg Am. 2009;91:892–899. doi: 10.2106/JBJS.H.01075. [DOI] [PubMed] [Google Scholar]
- 21.Llopis E, Carrascoso J, Iriarte I, Serrano Mde P, Cerezal L. Lisfranc injury imaging and surgical management. Semin Musculoskelet Radiol. 2016;20:139–153. doi: 10.1055/s-0036-1581119. [DOI] [PubMed] [Google Scholar]
- 22.Greco T, Cianni L, De Mauro D, Dughiero G, Bocchi MB, Cazzato G, Ragonesi G, Liuzza F, Maccauro G, Perisano C. Foot metastasis: current knowledge. Orthop Rev (Pavia) 2020;12:8671. doi: 10.4081/or.2020.8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perisano C, Greco T, Vitiello R, Maccauro G, Liuzza F, Tamburelli FC, Forconi F. Mueller-Weiss disease: review of the literature. J Biol Regul Homeost Agents. 2018;32:157–162. [PubMed] [Google Scholar]
- 24.Cianni L, Bocchi MB, Vitiello R, Greco T, De Marco D, Masci G, Maccauro G, Pitocco D, Perisano C. Arthrodesis in the Charcot foot: a systematic review. Orthop Rev (Pavia) 2020;12:8670. doi: 10.4081/or.2020.8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quenu E, Kuss G. Etude sur les luxations du metatarse (luxations métatarso-tarsiennes) du diastasis entre le 1er et le 2e métatarsien. Rev Chir Paris. 1909;39:1–72. [Google Scholar]
- 26.Hardcastle PH, Reschauer R, Kutscha-Lissberg E, Schoffmann W. Injuries to the tarsometatarsal joint. Incidence, classification and treatment. J Bone Joint Surg Br. 1982;64:349–356. doi: 10.1302/0301-620X.64B3.7096403. [DOI] [PubMed] [Google Scholar]
- 27.Myerson MS, Fisher RT, Burgess AR, Kenzora JE. Fracture dislocations of the tarsometatarsal joints: end results correlated with pathology and treatment. Foot Ankle. 1986;6:225–242. doi: 10.1177/107110078600600504. [DOI] [PubMed] [Google Scholar]
- 28.Sivakumar BS, An VVG, Oitment C, Myerson M. Subtle Lisfranc injuries: a topical review and modification of the classification system. Orthopedics. 2018;41:e168–e175. doi: 10.3928/01477447-20180213-07. [DOI] [PubMed] [Google Scholar]
- 29.Nunley JA, Vertullo CJ. Classification, investigation, and management of midfoot sprains: Lisfranc injuries in the athlete. Am J Sports Med. 2002;30:871–878. doi: 10.1177/03635465020300061901. [DOI] [PubMed] [Google Scholar]
- 30.Coetzee JC. Making sense of Lisfranc injuries. Foot Ankle Clin. 2008;13:695–704. ix. doi: 10.1016/j.fcl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Weatherford BM, Bohay DR, Anderson JG. Open reduction and internal fixation versus primary arthrodesis for Lisfranc injuries. Foot Ankle Clin. 2017;22:1–14. doi: 10.1016/j.fcl.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Talarico RH, Hamilton GA, Ford LA, Rush SM. Fracture dislocations of the tarsometatarsal joints: analysis of interrater reliability in using the modified hardcastle classification system. J Foot Ankle Surg. 2006;45:300–303. doi: 10.1053/j.jfas.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Crates JM, Barber FA, Sanders EJ. Subtle lisfranc subluxation: results of operative and nonoperative treatment. J Foot Ankle Surg. 2015;54:350–355. doi: 10.1053/j.jfas.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro MS, Wascher DC, Finerman GA. Rupture of Lisfranc’s ligament in athletes. Am J Sports Med. 1994;22:687–691. doi: 10.1177/036354659402200518. [DOI] [PubMed] [Google Scholar]
- 35.Desmond EA, Chou LB. Current concepts review: Lisfranc injuries. Foot Ankle Int. 2006;27:653–660. doi: 10.1177/107110070602700819. [DOI] [PubMed] [Google Scholar]
- 36.Ponkilainen VT, Mattila VM, Laine HJ, Paakkala A, Maenpaa HM, Haapasalo HH. Nonoperative, open reduction and internal fixation or primary arthrodesis in the treatment of Lisfranc injuries: a prospective, randomized, multicenter trial - study protocol. BMC Musculoskelet Disord. 2018;19:301. doi: 10.1186/s12891-018-2222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benirschke SK, Meinberg E, Anderson SA, Jones CB, Cole PA. Fractures and dislocations of the midfoot: lisfranc and Chopart injuries. J Bone Joint Surg Am. 2012;94:1325–1337. doi: 10.2106/JBJS.L00413. [DOI] [PubMed] [Google Scholar]
- 38.Nithyananth M, Boopalan PR, Titus VT, Sundararaj GD, Lee VN. Long-term outcome of high-energy open Lisfranc injuries: a retrospective study. J Trauma. 2011;70:710–716. doi: 10.1097/TA.0b013e3181f02ab9. [DOI] [PubMed] [Google Scholar]
- 39.Chandran P, Puttaswamaiah R, Dhillon MS, Gill SS. Management of complex open fracture injuries of the midfoot with external fixation. J Foot Ankle Surg. 2006;45:308–315. doi: 10.1053/j.jfas.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am. 1976;58:453–458. [PubMed] [Google Scholar]
- 41.Philpott A, Lawford C, Lau SC, Chambers S, Bozin M, Oppy A. Modified dorsal approach in the management of Lisfranc injuries. Foot Ankle Int. 2018;39:573–584. doi: 10.1177/1071100717750837. [DOI] [PubMed] [Google Scholar]
- 42.Kirzner N, Zotov P, Goldbloom D, Curry H, Bedi H. Dorsal bridge plating or transarticular screws for Lisfranc fracture dislocations: a retrospective study comparing functional and radiological outcomes. Bone Joint J. 2018;100-B:468–474. doi: 10.1302/0301-620X.100B4.BJJ-2017-0899.R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiao YS, Li JK, Shen H, Bao HY, Jiang M, Liu Y, Kapadia W, Zhang HT, Yang HL. Comparison of arthrodesis and non-fusion to treat Lisfranc injuries. Orthop Surg. 2017;9:62–68. doi: 10.1111/os.12316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberta FG, Aronow MS, Barrero M, Diaz-Doran V, Sullivan RJ, Adams DJ. Ligamentous Lisfranc joint injuries: a biomechanical comparison of dorsal plate and transarticular screw fixation. Foot Ankle Int. 2005;26:462–473. doi: 10.1177/107110070502600607. [DOI] [PubMed] [Google Scholar]
- 45.Lau S, Howells N, Millar M, De Villiers D, Joseph S, Oppy A. Plates, screws, or combination? Radiologic outcomes after Lisfranc fracture dislocation. J Foot Ankle Surg. 2016;55:799–802. doi: 10.1053/j.jfas.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Sheibani-Rad S, Coetzee JC, Giveans MR, DiGiovanni C. Arthrodesis versus ORIF for Lisfranc fractures. Orthopedics. 2012;35:e868–873. doi: 10.3928/01477447-20120525-26. [DOI] [PubMed] [Google Scholar]
- 47.Buda M, Kink S, Stavenuiter R, Hagemeijer CN, Chien B, Hosseini A, Johnson AH, Guss D, DiGiovanni CW. Reoperation rate differences between open reduction internal fixation and primary arthrodesis of Lisfranc injuries. Foot Ankle Int. 2018;39:1089–1096. doi: 10.1177/1071100718774005. [DOI] [PubMed] [Google Scholar]
- 48.Ly TV, Coetzee JC. Treatment of primarily ligamentous Lisfranc joint injuries: primary arthrodesis compared with open reduction and internal fixation. A prospective, randomized study. J Bone Joint Surg Am. 2006;88:514–520. doi: 10.2106/JBJS.E.00228. [DOI] [PubMed] [Google Scholar]
- 49.van den Boom NAC, Stollenwerck G, Lodewijks L, Bransen J, Evers S, Poeze M. Lisfranc injuries: fix or fuse?: a systematic review and meta-analysis of current literature presenting outcome after surgical treatment for Lisfranc injuries. Bone Jt Open. 2021;2:842–849. doi: 10.1302/2633-1462.210.BJO-2021-0127.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cottom JM, Hyer CF, Berlet GC. Case report: treatment of Lisfranc fracture dislocations with an interosseous suture buttontechnique: a review of 3 cases. J Foot Ankle Surg. 2008;47:250–258. doi: 10.1053/j.jfas.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 51.Dubois-Ferriere V, Lubbeke A, Chowdhary A, Stern R, Dominguez D, Assal M. Clinical outcomes and development of symptomatic osteoarthritis 2 to 24 years after surgical treatment of tarsometatarsal joint complex Injuries. J Bone Joint Surg Am. 2016;98:713–720. doi: 10.2106/JBJS.15.00623. [DOI] [PubMed] [Google Scholar]


