Abstract
Background:
We aimed to compare the efficacy of local injection therapies for lateral epicondylitis in a Bayesian framework.
Methods:
We searched the Embase, PubMed, Cochrane Central Register of Controlled Trials, Web of Science, Scopus, and ProQuest, for randomized controlled trials published from inception to February 2021 in any languages. The injection therapies included corticosteroids (CSs), autologous blood (AB), botulinum toxin (BT), and platelet-rich plasma (PRP). Placebo was the reference group for comparison. The study outcomes were pain, function, and strength, at 1, 3 and 6 months after injection.
Results:
Thirty-one trials were finally included in this network meta-analysis, comprising 1,948 patients. In the first month of treatment, CS and BT were more efficacious than placebo in terms of pain reduction, and CS was superior to BT. In the same follow-up time, CS was also superior to placebo in terms of functional improvement. In the third month of treatment, BT was the only intervention that was more efficient than placebo in pain relief. With regard to functional improvement, none of the treatments significantly had a higher effectiveness than placebo in the same period. Moreover, no therapies were found to be more efficient than placebo in the sixth month of treatment in terms of any study outcomes. In addition, we did not identify an intervention superior to placebo regarding strength improvement outcome in any times of follow-up.
Conclusion:
CSs and BT are efficient in improving clinical outcomes of lateral epicondylitis in the short term. Also, the efficacy of CSs seems to be greater than BT. On the other hand, AB and PRP were not significantly more efficient than placebo in any times of follow-up.
Key Words: Lateral epicondylitis, Tennis elbow, Injection therapies, Systematic review, Network meta-analysis
Lateral epicondylitis, or tennis elbow, is a common cause of lateral elbow pain and can be seen in 1-3% of general population (1). It is stated that the problem is caused by a degenerative process due to repetitive microtrauma and strain along with vascular deprivation at the extensor tendon, typically extensor carpi radialis brevis tendon, which sometimes results in severe local pain that can impede proper upper limb function (2). There are various surgical and non-surgical treatments for lateral epicondylitis. However, it is recommended to select non-surgical treatments for the patients as much priority as possible, which include nonsteroidal anti-inflammatory drugs, electro physiotherapy, physical therapy, and local injection therapies. The usual injection therapies include corticosteroids (CSs), autologous blood (AB), botulinum toxin (BT), and platelet-rich plasma (PRP) (3, 4).
Until now, different studies have evaluated the effectiveness of injection therapies for lateral epicondylitis, but with contradictory findings (5, 6). In a meta-analysis study by Li et al. (7), it was shown that CSs were more effective than PRP in improving pain and function in a short-term (up to 2 months) follow-up, while in a long-term follow-up (6 months), the results were in favor of PRP. On the other hand, a network meta-analysis reported that CSs had small effect on improving pain compared to other injection therapies, and were not recommended (8). These conflicting results may be due to differences in the study objectives and methods. Previous meta-analyses had also some limitations, such as limited databases searched, or only English papers included. In the present study, we tried to comprehensively assessed the available evidence on the efficacy of local injection therapies for lateral epicondylitis with more precise objectives and overcoming the limitations of previous meta-analyses. These data will hopefully help clinicians to better manage the patients.
Methods
Study protocol: The protocol of the present systematic review has previously been documented online in the PROSPERO registry (CRD42021244239). This study has been presented according to the guidelines of the Preferred Reporting Items for Systematic Review and Meta‐Analysis (PRISMA) extension statement for network meta-analyses (table S1) (9).
Information sources and search strategy: We performed a search on the literature published from inception to February 2021 in the bibliographic databases of the Embase, PubMed, Cochrane Central Register of Controlled Trials, Web of Science, Scopus, and ProQuest, without language restrictions. The relevant terms were searched in the Medical Subject Headings (MeSH) database, and finally, the keywords included “lateral epicondylitis” OR “tennis elbow” OR “lateral epicondylalgia” OR “elbow epicondylitis” AND “corticosteroid” OR “corticosteroids” OR “glucocorticoid” OR “glucocorticoids” OR “steroid” OR “steroids” OR “autologous blood” OR “botulinum toxin” OR “platelet-rich plasma” OR “PRP”. The search was limited to title or abstract. In addition, we conducted a hand search of the reference lists of relevant review articles and the retrieved papers for additional sources.
Inclusion and exclusion criteria: We included all randomized controlled trial (RCT) studies with the following criteria:
Including adult patients (aged ≥18 years).
Comparing clinical outcomes between at least two of the following treatments: CS, AB, BT, PRP, and placebo.
Investigating at least one of the following outcomes: visual analog score (VAS), disabilities of arm, shoulder and hand (DASH) score, modified Nirschl score (MNS), patient-related tennis elbow evaluation (PRTEE) score, grip strength (GS), at 1, 3, and/or 6 months.
The exclusion criteria were as follows:
Reviews, case reports, editorials, and letter to the editors.
Duplicate papers or evaluating the same sample.
Trials without clear methodology or results.
Full‐texts not being available
Study selection and data extraction: Two independent reviewers (MT, MZ) screened the titles and abstracts of all the articles obtained from the initial search for potential eligibility. Then, full-text of the potential papers were retrieved for final evaluation. Any disagreements related to the inclusion of articles were resolved by discussion between the investigators. The following data were extracted from each trial and finally entered into a Microsoft Excel spreadsheet (Microsoft Corporation, Redmond, Washington): first author’s name, study location (country), publication year, final follow-up (month), treatment names, total sample size, number of patients by gender (if available), patient’s age (if available), outcome measure. Non‐English papers were translated by Google Translate, where required. We contacted the corresponding authors by email if missing or unclear information existed. In case of duplicates, we selected those with the most comprehensive details.
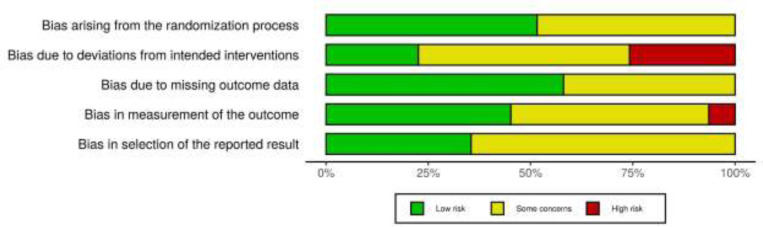
Risk of bias assessment: Two authors (MT and MZ) independently contributed to the assessment of the quality of the included studies, using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2) (10). This tool examines a study bias in five distinct domains, including randomization process, deviations from intended interventions, missing outcome data, outcome measurement, and selection of the reported result. Each domain has three quality levels, including ‘low risk’, ‘some concerns’, and ‘high risk’.
Study outcomes and statistical analysis: The outcomes for the present network meta-analysis included:
“Pain intensity”, measuring by VAS (ranging from 0 [no pain] to 100 [worst pain] score, and MNS (ranging from 0 [no pain with exercise] to 4 [severe pain with normal activities] score).
“Function”, measuring by DASH (ranging from 0 [no disability] to 100 [most severe disability] score), and PRTEE (ranging from 0 [no disability] to 100 [significant disability] score).
“Strength”, measuring by GS (higher values mean more strength).
We did a network meta-analysis combining direct and indirect comparisons in a Bayesian framework using the R package ‘gemtc’ (https://cran.r-project.org/package=gemtc). With regard to the study outcomes, the pooled estimates were presented as the standardized mean difference (SMD) and 95% credible interval (CrI). SMD was selected as the effect size measure when the studies used different outcome scales, otherwise we used unstandardized mean difference (UMD). Data were combined using a random-effects model (11) to give more conservative estimates. Also, we used the Markov chains Monte Carlo method for all analyses. Node splitting models were used to obtain indirect estimates and to evaluate local inconsistency (12). Treatments were ranked for study outcomes using the surface under the cumulative ranking curve (SUCRA). We also presented the summary results of all pairwise comparisons and network meta-analysis in the league tables. For the present network meta-analysis, “placebo” was considered as the reference group for comparison. We assessed the publication bias with a comparison-adjusted funnel plot and Egger’s test. A p‐value <0.05 was considered significant for all relevant analyses.
Results
Search results, study selection, and characteristics: Searching the databases initially generated 2,151 records, of which 2,107 were excluded due to duplication or meeting exclusion criteria through screening titles or abstracts. Full-texts of 44 articles were evaluated, and finally, 31 eligible papers comprising 1948 patients with lateral epicondylitis were included in this systematic review (fig. 1). The pain intensity was investigated in 23 studies (13-35), the functional status was investigated in 16 studies (13, 14, 18, 20, 21, 23, 26, 28, 33, 36-42), and the strength was investigated in 8 studies (15, 17, 20, 21, 23, 25, 26, 43). Out of 23 trials assessing the pain reduction outcome, 20 studies used VAS pain score only, and 3 studies used both of VAS and MNS. Out of 16 RCTs investigating the functional improvement outcome, 8 studies used DASH only, 7 studies used PRTEE only, and one study used both. Baseline characteristics of the included RCTs were summarized in table 1. Moreover, the results of risk of bias assessment were reported for all of the included studies in figs. 2 and 3.
Fig 1.
PRISMA flowdiagram
Table 1.
Characteristics of the studies included in the systematic review and network meta-analysis
| First author | Publication year | Country | Final follow-up (m) | Outcomes | Treatment | Total patients (n) | Male (n) | Female (n) | Mean age (years) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Arik (13) | 2014 | Turkey | 6 | VAS, PRTEE | AB | 40 | 11 | 29 | 43.7 | |
| CS | 40 | 10 | 30 | 46.7 | ||||||
| Branson (36) | 2017 | Australia | 6 | PRTEE | AB | 14 | 10 | 4 | 47.9 | |
| CS | 14 | 8 | 6 | 48.1 | ||||||
| Coombes (14) | 2013 | Australia | 12 | VAS, PRTEE | CS | 43 | 27 | 16 | 49.3 | |
| PL | 41 | 14 | 17 | 49.9 | ||||||
| Creaney (37) | 2011 | UK | 6 | PRTEE | PRP | 63 | 36 | 27 | 53.0 | |
| AB | 48 | 27 | 21 | 48.0 | ||||||
| Creuzé (15) | 2018 | France | 3 | VAS, GS | BT | 30 | 17 | 13 | 47.3 | |
| PL | 30 | 16 | 14 | 46.7 | ||||||
| Dojode (16) | 2012 | India | 6 | VAS, MNS | AB | 30 | 13 | 17 | 42.9 | |
| CS | 30 | 12 | 18 | 42.2 | ||||||
| Espandar (17) | 2010 | Iran | 4 | VAS, GS | BT | 24 | 2 | 22 | 43.3 | |
| PL | 24 | 2 | 22 | 44.2 | ||||||
| Gautam (18) | 2015 | India | 6 | VAS, DASH, GS | PRP | 15 | NA | NA | NA | |
| CS | 15 | NA | NA | NA | ||||||
| Gosens (19) | 2011 | Netherlands | 24 | VAS | PRP | 51 | 23 | 28 | 46.8 | |
| CS | 49 | 23 | 26 | 47.3 | ||||||
| Guo (20) | 2017 | Taiwan | 4 | VAS, PRTEE, GS | BT | 15 | 6 | 9 | 49.9 | |
| CS | 11 | 5 | 6 | 53.4 | ||||||
| Gupta (21) | 2020 | India | 12 | VAS, DASH, GS | PRP | 40 | NA | NA | 42.4 | |
| CS | 40 | NA | NA | 39.4 | ||||||
| Jindal (22) | 2013 | India | 1 | VAS, MNS | AB | 25 | 14 | 11 | 39.0 | |
| CS | 25 | 17 | 8 | 37.3 | ||||||
| Kazemi (23) | 2010 | Iran | 2 | VAS, MNS, DASH, GS | AB | 30 | 7 | 23 | 47.2 | |
| CS | 30 | 4 | 26 | 47.0 | ||||||
| Khaliq (24) | 2015 | Pakistan | 1 | VAS | PRP | 51 | 21 | 30 | 34.0 | |
| CS | 51 | 24 | 27 | 34.0 | ||||||
| Krogh (38) | 2013 | Denmark | 3 | PRTEE | PRP | 20 | 9 | 11 | 47.6 | |
| CS | 20 | 11 | 9 | 44.7 | ||||||
| PL | 20 | 9 | 11 | 43.9 | ||||||
| Lebiedziński (39) | 2015 | Poland | 12 | DASH | AB | 53 | 28 | 25 | 47.0 | |
| CS | 46 | 12 | 34 | 54.0 | ||||||
| Lin (25) | 2010 | Taiwan | 3 | VAS, GS | BT | 8 | 3 | 5 | 45.9 | |
| CS | 9 | 6 | 3 | 44.6 | ||||||
| Linnanmaki (26) | 2020 | Finland | 12 | VAS, DASH, GS | PRP | 40 | 18 | 22 | 46.0 | |
| AB | 40 | 20 | 20 | 46.0 | ||||||
| PL | 39 | 17 | 22 | 49.0 | ||||||
| Montalvan (27) | 2016 | France | 12 | VAS | PRP | 25 | 17 | 8 | 47.0 | |
| PL | 25 | 17 | 8 | 46.4 | ||||||
| Omar (28) | 2012 | Egypt | 1.5 | VAS, DASH | PRP | 15 | 6 | 9 | 40.5 | |
| CS | 15 | 5 | 10 | 37.5 | ||||||
| Ozturan (43) | 2010 | Turkey | 12 | GS | AB | 18 | 7 | 11 | 44.0 | |
| CS | 20 | 10 | 10 | 45.8 | ||||||
| Palacio (40) | 2016 | Brazil | 6 | PRTEE, DASH | PRP | 20 | NA | NA | 46.6 | |
| CS | 20 | NA | NA | 46.2 | ||||||
| Placzek (29) | 2007 | Germany | 4 | VAS | BT | 68 | 31 | 37 | 47.4 | |
| PL | 62 | 30 | 32 | 46.9 | ||||||
| Raeissadat (30) | 2014 | Iran | 12 | VAS | PRP | 31 | 8 | 23 | 43.0 | |
| AB | 30 | 6 | 24 | 44.0 | ||||||
| Schöffl (41) | 2017 | Germany | 6 | DASH | PRP | 18 | 9 | 9 | 52.6 | |
| PL | 18 | 9 | 9 | 52.6 | ||||||
| Singh (42) | 2013 | India | 3 | PRTEE | AB | 30 | 12 | 18 | 35.2 | |
| CS | 30 | 16 | 14 | 33.0 | ||||||
| Thanasas (31) | 2011 | Greece | 6 | VAS | PRP | 14 | NA | NA | 35.9 | |
| AB | 14 | NA | NA | 36.6 | ||||||
| Varshney (32) | 2017 | India | 6 | VAS | PRP | 33 | NA | NA | NA | |
| CS | 50 | NA | NA | NA | ||||||
| Wolf (33) | 2011 | USA | 6 | VAS, DASH | AB | 9 | NA | NA | NA | |
| CS | 9 | NA | NA | NA | ||||||
| PL | 10 | NA | NA | NA | ||||||
| Wong (34) | 2005 | China | 3 | VAS | BT | 30 | 5 | 25 | 45.0 | |
| PL | 30 | 6 | 24 | 44.2 | ||||||
| Yerlikaya (35) | 2018 | Turkey | 2 | VAS | PRP | 60 | 15 | 45 | 45.8 | |
| PL | 30 | 11 | 19 | 47.6 | ||||||
Abbreviations: VAS, visual analog score; DASH, Disabilities of Arm Shoulder and Hand score; MNS, modified Nirschl score; PRTEE, Patient-Related Tennis Elbow Evaluation score; GS, grip strength; CS, corticosteroid; AB, autologous blood; BT, botulinum toxin; PRP, platelet-rich plasma; PL, placebo
Fig 2.
Risk of bias assessment for the individual domains
Fig 3.
Risk of bias assessment for the individual studies
Pain relief: The results of pairwise and network meta-analyses about the pain relief outcome are represented in table 2. Due to different assessment tools, we used SMD to represent the pooled effect size. The network plots for the pain relief in different follow-ups have been provided in figs. 4A-C. The findings of inconsistency assessment and publication bias are also shown in figs. S1 and S2, respectively. Funnel plot and Egger’s test showed no publication bias in the network meta-analyses at any follow-ups.
Table 2.
Results of pairwise and network meta-analysis for pain relief in different follow-ups
| At 1 st month | CS | -1.17 (-2.20 to -0.01) | -0.36 (-0.97 to 0.23) | -0.62 (-1.39 to 0.10) | -0.92 (-2.24 to 0.44) |
| -0.22 (-1.06 to 0.59) | BT | NA | NA | -1.40 (-2.18 to -0.60) | |
| -0.60 (-1.14 to -0.04) | -0.37 (-1.32 to 0.58) | AB | 0.27 (-0.75 to 1.34) | 0.18 (-1.13 to 1.51) | |
| -0.70 (-0.13 to -1.28) | -0.48 (-1.38 to 0.42) | -0.11 (-0.79 to 0.56) | PRP | 0.09 (-0.80 to 0.93) | |
| -1.04 (-1.78 to -0.34) | -0.83 (-1.54 to -0.05) | -0.44 (-1.24 to 0.33) | 0.32 (-1.05 to 0.39) | PL | |
| At 3 rd month | BT | NA | NA | 0.27 (-0.65 to 1.17) | -1.30 (-1.97 to -0.63) |
| -0.11 (0.99 to 0.82) | AB | 0.31 (-0.65 to 1.29) | -0.73 (-1.37 to -0.08) | -0.04 (-1.22 to 1.19) | |
| -0.10 (-1.02 to 0.84) | 0.01 (-0.68 to 0.69) | PRP | -0.52 (-1.34 to 0.33) | -0.03 (-1.12 to 1.13) | |
| -0.59 (-1.38 to 0.27) | -0.46 (-1.05 to 0.11) | -0.47 (-1.11 to 0.15) | CS | 0.04 (-1.76 to 1.80) | |
| -0.79 (-1.47 to -0.05) | -0.67 (-1.49 to 0.16) | -0.68 (-1.52 to 0.16) | -0.20 (-1.02 to 0.58) | PL | |
| At 6 th month | PRP | 0.18 (-1.14 to 1.47) | -0.27 (-1.41 to 0.82) | -1.91 (-2.91 to -0.91) | |
| -0.20 (-1.29 to 0.83) | PL | -0.13 (-1.45 to 1.07) | -0.48 (-2.26 to 1.34) | ||
| -0.54 (-1.36 to 0.26) | -0.35 (-1.44 to 0.78) | AB | -0.67 (-1.60 to 0.28) | ||
| -1.50 (-2.33 to -0.73) | -1.30 (-2.49 to -0.17) | -0.96 (-1.76 to -0.22) | CS |
Note: Treatments are ranked according to their SUCRA. The comparative therapeutic efficacies are reported as standardized mean difference with 95% credible intervals (in parentheses). The comparison between the treatments should be read from left to right. The network meta-analysis results are presented in the left lower (by comparing columns with rows), and the pairwise meta-analysis results are presented in the right upper (by comparing rows with columns). Abbreviations: CS, corticosteroid; AB, autologous blood; BT, botulinum toxin; PRP, platelet-rich plasma; PL, placebo
Fig 4.
Network plot of comparisons for pain relief in the first (A), third (B) and sixth (C) month of treatment. Each node (circle) exhibits an injection therapy. The line width corresponds to the number of trials comparing the individual treatments
Abbreviations: CS, corticosteroid; AB, autologous blood; BT, botulinum toxin; PRP, platelet-rich plasma; PL, placebo
Within the first month of follow-up, based on the network meta-analysis, CS had the most efficacy on the pain reduction versus placebo (SMD=-1.04, 95% CrI: -1.78 to -0.34), followed by BT (SMD=-0.83, 95% CrI: -1.54 to -0.05) (Fig 5A). Also, CS was ranked first and non-significantly more efficient than BT in pain relief. In the pairwise meta-analysis, BT was significantly associated with lower pain scores compared with placebo (SMD = -1.40, 95% CrI: -2.18 to -0.60).
Fig 5.
Forest plot of network meta-analysis results for pain relief in the first (A), third (B) and sixth (C) month of treatment. Treatments are ranked according to their SUCRA. Treatments crossing zero are not significantly different from placebo
Within the third month of follow-up, according to the network meta-analysis, BT was the only intervention that significantly decreased pain scores versus placebo (SMD = -0.79, 95% CrI: -1.47 to -0.05) (fig. 5B). Also, in the pairwise meta-analysis, BT was found to be superior to placebo (SMD = -1.30, CrI: -1.97 to -0.63).
Within sixth months follow-up, none of the treatments were significantly more efficient than placebo in pain reduction, either based on network meta-analysis or pairwise meta-analysis (fig. 5C).
Functional improvement: The results of pairwise and network meta-analyses about the functional improvement outcome are represented in table 3. Due to different assessment tools, we used SMD to represent the pooled effect size. The network plots for the pain relief in different follow-ups have been provided in figs. 6A-C. The findings of inconsistency assessment and publication bias are also indicated in figs. S3 and S4, respectively. Funnel plot and Egger’s test showed no publication bias in the network meta-analyses at any follow-ups.
Table 3.
Results of pairwise and network meta-analysis for functional improvement in different follow-ups
| At 1 st month | CS | -0.52 (-1.18 to 0.14) | -0.94 (-2.02 to 0.30) | -1.11 (-2.04 to -0.24) |
| -0.64 (-1.25 to -0.03) | AB | 0.24 (-0.99 to 1.55) | -0.18 (-1.93 to 1.67) | |
| -0.87 (-1.66 to -0.12) | -0.24 (-1.06 to 0.62) | PL | -0.09 (-1.24 to 1.01) | |
| -1.04 (-1.74 to -0.37) | -0.41 (-1.21 to 0.41) | -0.17 (-0.97 to 0.63) | PRP | |
| At 3 rd month | PRP | 0.19 (-1.12 to 1.46) | -0.23 (-1.58 to 1.12) | 0.84 (-1.71 to -0.02) |
| -0.06 (-0.84 to 0.66) | AB | 0.11 (-1.24 to 1.52) | -0.67 (-1.56 to 0.15) | |
| -0.29 (-1.27 to 0.65) | -0.23 (-1.20 to 0.76) | PL | -0.04 (-1.39 to 1.29) | |
| -0.77 (-1.44 to -0.16) | -0.71 (-1.36 to -0.08) | -0.48 (-1.45 to 0.44) | CS | |
| At 6 th month | PRP | 0.12 (-1.24 to 1.48) | 0.38 (-0.04 to 0.91) | -1.13 (-1.76 to -0.63) |
| 0.02 (-1.04 to 1.03) | PL | -0.29 (-1.86 to 1.27) | -0.34 (-2.37 to 1.74) | |
| -0.27 (-1.23 to 0.63) | -0.29 (-1.44 to 0.78) | AB | 0.42 (0.02 to 0.73) | |
| -0.44 (-1.39 to 0.48) | -0.46 (-1.70 to 0.69) | -0.16 (-1.02 to 0.68) | CS |
Note: Treatments are ranked according to their SUCRA. The comparative therapeutic efficacies are reported as standardized mean difference with 95% credible intervals (in parentheses). The comparison between the treatments should be read from left to right. The network meta-analysis results are presented in the left lower (by comparing columns with rows), and the pairwise meta-analysis results are presented in the right upper (by comparing rows with columns).
Abbreviations: CS, corticosteroid; AB, autologous blood; PRP, platelet-rich plasma; PL, placebo
Fig 6.
Network plot of comparisons for functional improvement in the first (A), third (B) and sixth (C) month of treatment. Each node (circle) exhibits an injection therapy. The line width corresponds to the number of trials comparing the individual treatments. Abbreviations: CS, corticosteroid; AB, autologous blood; PRP, platelet-rich plasma; PL, placebo
Within the first month of follow-up, network meta-analysis showed that CS was the only treatment that was significantly more effective than placebo in functional improvement (SMD = -0.87, 95% CrI: -1.66 to -0.12) (fig. 7A). However, no significant differences were seen between any treatments and placebo in functional improvement according to the pairwise meta-analysis.
Fig 7.
Forest plot of network meta-analysis results for functional improvement in the first (A), third (B) and sixth (C) month of treatment. Treatments are ranked according to their SUCRA. Treatments crossing zero are not significantly different from placebo
Within the third and sixth months of follow-up, none of the injection therapies were significantly more efficient than placebo in functional improvement, either based on network meta-analysis or based on pairwise meta-analysis (figs. 7B-C).
Strength improvement: The results of pairwise and network meta-analyses about the strength improvement outcome are represented in table 4. Due to different assessment tools, we used SMD to represent the pooled effect size. The network plots for the pain relief in different follow-ups have been provided in figs. 8A-B. The findings of inconsistency assessment and publication bias are also shown in figs. S5 and S6, respectively. Funnel plot and Egger’s test showed no publication bias in the network meta-analyses at any follow-ups.
Table 4.
Results of pairwise and network meta-analysis for strength improvement in different follow-ups
| At 1 st month | CS | 0.15 (-18.93 to 19.50) | NA | 12.04 (-6.84 to 32.17) | 9.37 (-18.44 to 38.14) |
| 3.67 (-12.57 to 19.27) | AB | 0.07 (-30.72 to 31.67) | -2.39 (-33.30 to 28.09) | NA | |
| 6.49 (-14.06 to 26.42) | 2.85 (-17.27 to 23.45) | PL | -2.72 (-33.63 to 28.51) | 3.50 (-16.99 to 23.32) | |
| 7.81 (-7.10 to 24.68) | 4.13 (-13.10 to 23.66) | 1.47 (-18.44 to 23.02) | PRP | NA | |
| 10.02 (-10.55 to 29.33) | 6.70 (-15.79 to 26.87) | 3.49 (-13.23 to 19.86) | 2.21 (-21.30 to 22.57) | BT | |
| At 3 rd month | AB | 0.45 (-22.29 to 23.25) | 2.86 (-20.62 to 26.96) | 6.80 (-8.10 to 21.81) | NA |
| 1.46 (-12.01 to 15.06) | PRP | 2.25 (-19.86 to 25.35) | 4.28 (-10.58 to 19.90) | NA | |
| 4.89 (-10.91 to 20.68) | 3.36 (-12.75 to 19.00) | PL | NA | 2.89 (-11.24 to 18.63) | |
| 5.47 (-5.46 to 17.04) | 4.06 (-6.89 to 15.86) | 0.93 (-13.20 to 16.31) | CS | 4.15 (-16.96 to 25.30) | |
| 8.00 (-8.23 to 26.36) | 6.62 (-9.30 to 23.54) | 3.49 (-7.92 to 15.96) | 2.79 (-12.60 to 18.02) | BT |
Note: Treatments are ranked according to their SUCRA. The comparative therapeutic efficacies are reported as unstandardized mean difference with 95% credible intervals (in parentheses). The comparison between the treatments should be read from left to right. The network meta-analysis results are presented in the left lower (by comparing columns with rows), and the pairwise meta-analysis results are presented in the right upper (by comparing rows with columns).
Abbreviations: CS, corticosteroid; AB, autologous blood; BT, botulinum toxin; PRP, platelet-rich plasma; PL, placebo
Fig 8.
Network plot of comparisons for strength improvement in the first (A) and third (B) month of treatment. Each node (circle) exhibits an injection therapy. The line width corresponds to the number of trials comparing the individual treatments
Abbreviations: CS, corticosteroid; AB, autologous blood; BT, botulinum toxin; PRP, platelet-rich plasma; PL, placebo
Within the first and third months of follow-up, none of the treatments were significantly more efficient than placebo in strength improvement, either based on network meta-analysis or based on pairwise meta-analysis (figs. 9A-B). Due to lack of enough data, performing network meta-analysis for strength improvement was not appliable in a sixth-month follow-up.
Fig 9.
Forest plot of network meta-analysis results for functional improvement in the first (A) and third (B) month of treatment. Treatments are ranked according to their SUCRA. Treatments crossing zero are not significantly different from placebo
Discussion
In the present systematic review and network meta-analysis, we investigated the clinical efficacy of different injection therapies for lateral epicondylitis in different courses of follow-up. We assessed three different outcomes for this study, including pain reduction, functional improvement, and strength improvement. In the short-term follow-up (first month of treatment), it was found that CS and BT were more efficacious than placebo in terms of pain reduction, and CS was ranked first and was superior to BT. CS was also superior to placebo in terms of functional improvement in the short-term follow-up; However, we could not assess the efficacy of BT in this course due to insufficient data. In the mid-term follow-up (third month of treatment), BT was the only intervention that was more efficient than placebo in pain relief. Regarding functional improvement, none of the treatments significantly had a higher effectiveness than placebo in this period. Moreover, no therapies were found to be more efficient than placebo in the long-term follow-up (sixth month of treatment) in terms of any study outcomes. In addition, we did not identify an intervention superior to placebo regarding strength improvement outcome in any times of follow-up.
The anti-inflammatory mechanism of CSs can be explained by reduction of immune function and inflammatory cells and mediators, such as macrophages, mast cells, lymphocytes, prostaglandin and leukotrienes, leading to decrease in pain (44) Concerning BT, one mechanism can relate to temporary paralysis of the proximal extensors of the forearm, allowing a period of rest and aiding tissue recovery (45). In addition, releasing some mediators, such as calcitonin gene-related peptide, substance P, bradykinin, and glutamate, have been suggested as the other mechanism of analgesic properties of BT (45, 46).
So far, different meta-analyses have been conducted to explore the clinical effective of injection treatments for lateral epicondylitis. In the network meta-analysis by Dong et al. (8), hyaluronic acid and prolotherapy had significantly a higher efficacy than placebo in terms of pain reduction, and other treatments such as CS, PRP, AB and BT were not more efficacious compared with placebo. The present study has superiorities over Dong et al.’s study. First, we assessed the therapeutic efficacy of the injections at different times of follow-up, while Dong et al. did not categorize the follow-up times. Second, we evaluated three different outcomes in our study, whereas they investigated pain score only in their study. The other difference between the above-mentioned study and our network meta-analysis was the studied injection therapies, that is, we considered treatments that are more practical and have sufficient data for the analyses. There has been another network meta-analysis recently published related to the topic of our study (47); However, one of the major limitations of that study was lack of a placebo group for comparison. Also, we categorized the follow-up duration into three times, while that study grouped it into two periods. In the meta-analysis by Simental-Mendia et al. (48), the authors stated that PRP was not significantly more efficient than placebo in relieving pain and joint functionality, which was similar to our results. Other meta-analysis by Lin et al. (49) demonstrated that BT significantly reduced pain versus placebo within the first and third months of follow-up. They also declared that CS was more effective than placebo in the first month of treatment, but not in the next follow-ups. Additionally, they mentioned that CS is superior to BT in the first month of treatment. These results are similar to our findings obtained by pairwise or network meta-analyses.
In the present study, we attempted to overcome some of the limitations of the previous systematic review and meta-analyses by extending searched databases without language restriction, including placebo as the reference group for comparison, assessing three different clinical outcomes, and categorizing the follow-up duration into three different times (short-term, mid-term, and long-term).
This study has also some limitations. First, not enough data existed on follow-ups longer than six months to perform the network meta-analysis; however, considering that we did not find an efficient injection therapy in the sixth month, the presence of an efficient treatment in longer follow-ups might be improbable. Second, we witnessed wide and overlapped CrIs in some of the results, which were mainly due to limited number of studies. Therefore, it is needed to conduct more relevant RCTs. Third, the treatment substances and dosages sometimes differ between various trials and these factors need to be considered in further reviews when sufficient data are available for analysis.
In conclusion showed that corticosteroids and botulinum toxin are efficient in improving clinical outcomes of lateral epicondylitis. The effects of corticosteroids remained for one month. This time for botulinum toxin was three months. Also, the efficacy of corticosteroids seems to be greater than botulinum toxin within the first month of treatment. After three months, no significant therapeutic effects were found for corticosteroids or botulinum toxin. Regarding autologous blood and platelet-rich plasma, they were not significantly more efficient than placebo in any times of follow-up.
Funding:
None.
Conflict of interests:
The authors declare no competing interests.
Author contribution:
MT, RJ, SK and SME contributed to the study design. MT and MZ contributed to data collection. MZ contributed to data analysis. MT, RJ, SK and MZ contributed to drafting the manuscript. SME contributed to intellectual input and manuscript revision. All authors have read the manuscript and approved its final version.
Data availability:
No additional data available.
Ethics approval:
Not applicable.
Consent to participate and for publication:
Not applicable.
References
- 1.Sanders Jr TL, Maradit Kremers H, Bryan AJ, et al. The epidemiology and health care burden of tennis elbow: a population-based study. Am J Sports Med. 2015;43:1066–71. doi: 10.1177/0363546514568087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai WC, Erickson BJ, Mlynarek RA, Wang D. Chronic lateral epicondylitis: challenges and solutions. Open Access J Sports Med. 2018;9:243. doi: 10.2147/OAJSM.S160974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaquero-Picado A, Barco R, Antuña SA. Lateral epicondylitis of the elbow. EFORT Open Rev. 2016;1:391–7. doi: 10.1302/2058-5241.1.000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amroodi MN, Mahmuudi A, Salariyeh M, Amiri A. Surgical treatment of tennis elbow; minimal incision technique. Arch Bone Jt Surg. 2016;4:366. [PMC free article] [PubMed] [Google Scholar]
- 5.Girgis B, Duarte JA. Efficacy of physical therapy interventions for chronic lateral elbow tendinopathy: a systematic review. Phys Ther Rev. 2020;25:42–59. [Google Scholar]
- 6.Ma K-L, Wang H-Q. Management of lateral epicondylitis: a narrative literature review. Pain Res Manag. 2020;2020:6965381. doi: 10.1155/2020/6965381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li A, Wang H, Yu Z, et al. Platelet-rich plasma vs corticosteroids for elbow epicondylitis: a systematic review and meta-analysis. Medicine. 2019;98:e18358. doi: 10.1097/MD.0000000000018358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong W, Goost H, Lin X-B, et al. Injection therapies for lateral epicondylalgia: a systematic review and Bayesian network meta-analysis. Br J Sports Med. 2016;50:900–8. doi: 10.1136/bjsports-2014-094387. [DOI] [PubMed] [Google Scholar]
- 9.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–84. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 10.Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.van Valkenhoef G, Dias S, Ades A, Welton NJ. Automated generation of node‐splitting models for assessment of inconsistency in network meta‐analysis. Res Synth Methods. 2016;7:80–93. doi: 10.1002/jrsm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arik HO, Kose O, Guler F, et al. Injection of autologous blood versus corticosteroid for lateral epicondylitis: a randomised controlled study. J Orthop Surg. 2014;22:333–7. doi: 10.1177/230949901402200313. [DOI] [PubMed] [Google Scholar]
- 14.Coombes BK, Bisset L, Brooks P, Khan A, Vicenzino B. Effect of corticosteroid injection, physiotherapy, or both on clinical outcomes in patients with unilateral lateral epicondylalgia: a randomized controlled trial. JAMA. 2013;309:461–9. doi: 10.1001/jama.2013.129. [DOI] [PubMed] [Google Scholar]
- 15.Creuzé A, Petit H, de Sèze M. Short-term effect of low-dose, electromyography-guided botulinum toxin A injection in the treatment of chronic lateral epicondylar tendinopathy: a randomized, double-blinded study. J Bone Joint Surg Am. 2018;100:818–26. doi: 10.2106/JBJS.17.00777. [DOI] [PubMed] [Google Scholar]
- 16.Dojode C. A randomised control trial to evaluate the efficacy of autologous blood injection versus local corticosteroid injection for treatment of lateral epicondylitis. Bone Joint Res. 2012;1:192–7. doi: 10.1302/2046-3758.18.2000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espandar R, Heidari P, Rasouli MR, et al. Use of anatomic measurement to guide injection of botulinum toxin for the management of chronic lateral epicondylitis: a randomized controlled trial. CMAJ. 2010;182:768–73. doi: 10.1503/cmaj.090906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gautam V, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong) 2015;23:1–5. doi: 10.1177/230949901502300101. [DOI] [PubMed] [Google Scholar]
- 19.Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–8. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 20.Guo YH, Kuan TS, Chen KL, et al. Comparison between steroid and 2 different sites of botulinum toxin injection in the treatment of lateral epicondylalgia: A randomized, double-blind, active drug-controlled pilot study. Arch Phys Med Rehabil. 2017;98:36–42. doi: 10.1016/j.apmr.2016.08.475. [DOI] [PubMed] [Google Scholar]
- 21.Gupta PK, Acharya A, Khanna V, et al. PRP versus steroids in a deadlock for efficacy: long-term stability versus short-term intensity—results from a randomised trial. Musculoskelet Surg. 2020;104:285–94. doi: 10.1007/s12306-019-00619-w. [DOI] [PubMed] [Google Scholar]
- 22.Jindal N, Gaury Y, Banshiwal RC, Lamoria R, Bachhal V. Comparison of short term results of single injection of autologous blood and steroid injection in tennis elbow: a prospective study. J Orthop Surg Res. 2013;8:10. doi: 10.1186/1749-799X-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazemi M, Azma K, Tavana B, Moghaddam FR, Panahi A. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy: a randomized clinical trial of efficacy. Am J Phys Med Rehabil. 2010;89:660–7. doi: 10.1097/PHM.0b013e3181ddcb31. [DOI] [PubMed] [Google Scholar]
- 24.Khaliq A, Khan I, Inam M, et al. Effectiveness of platelets rich plasma versus corticosteroids in lateral epicondylitis. J Pak Med Assoc. 2015;65:S100–S4. [PubMed] [Google Scholar]
- 25.Lin YC, Tu YK, Chen SS, et al. Comparison between botulinum toxin and corticosteroid injection in the treatment of acute and subacute tennis elbow: a prospective, randomized, double-blind, active drug-controlled pilot study. Am J Phys Med Rehabil. 2010;89:653–9. doi: 10.1097/PHM.0b013e3181cf564d. [DOI] [PubMed] [Google Scholar]
- 26.Linnanmäki L, Kanto K, Karjalainen T, Leppänen OV, Lehtinen J. Platelet-rich plasma or autologous blood do not reduce pain or improve function in patients with lateral epicondylitis: a randomized controlled trial. Clin Orthop Relat Res. 2020;478:1892–900. doi: 10.1097/CORR.0000000000001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montalvan B, Le Goux P, Klouche S, et al. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology (Oxford) 2016;55:279–85. doi: 10.1093/rheumatology/kev326. [DOI] [PubMed] [Google Scholar]
- 28.Omar AS, Ibrahim ME, Ahmed AS, Said M. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: randomized clinical trial. Egypt Rheumatol. 2012;34:43–9. [Google Scholar]
- 29.Placzek R, Drescher W, Deuretzbacher G, Hempfing A, Meiss AL. Treatment of chronic radial epicondylitis with botulinum toxin A: a double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg Am. 2007;89:255–60. doi: 10.2106/JBJS.F.00401. [DOI] [PubMed] [Google Scholar]
- 30.Raeissadat SA, Rayegani SM, Hassanabadi H, et al. Is platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Sci Med Rehabil. 2014;6:12. doi: 10.1186/2052-1847-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med. 2011;39:2130–4. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 32.Varshney A, Maheshwari R, Juyal A, Agrawal A, Hayer P. Autologous platelet-rich plasma versus corticosteroid in the management of elbow epicondylitis: a randomized study. Int J Appl Basic Med Res. 2017;7:125–8. doi: 10.4103/2229-516X.205808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf JM, Ozer K, Scott F, Gordon MJ, Williams AE. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg Am. 2011;36:1269–72. doi: 10.1016/j.jhsa.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Wong SM, Hui AC, Tong PY, et al. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2005;143:793–7. doi: 10.7326/0003-4819-143-11-200512060-00007. [DOI] [PubMed] [Google Scholar]
- 35.Yerlikaya M, Çaliş HT, Sütbeyaz ST, et al. Comparison of effects of leukocyte-rich and leukocyte-poor platelet-rich plasma on pain and functionality in patients with lateral epicondylitis. Arch Rheumatol. 2018;33:73–9. doi: 10.5606/ArchRheumatol.2018.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Branson R, Naidu K, du Toit C, et al. Comparison of corticosteroid, autologous blood or sclerosant injections for chronic tennis elbow. J Sci Med Sport. 2017;20:528–33. doi: 10.1016/j.jsams.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med. 2011;45:966–71. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 38.Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med. 2013;41:625–35. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 39.Lebiedziński R, Synder M, Buchcic P, et al. A randomized study of autologous conditioned plasma and steroid injections in the treatment of lateral epicondylitis. Int Orthop. 2015;39:2199–203. doi: 10.1007/s00264-015-2861-0. [DOI] [PubMed] [Google Scholar]
- 40.Palacio EP, Schiavetti RR, Kanematsu M, et al. Effects of platelet-rich plasma on lateral epicondylitis of the elbow: prospective randomized controlled trial. Rev Bras Ortop. 2016;51:90–5. doi: 10.1016/j.rboe.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schöffl V, Willauschus W, Sauer F, et al. Autologous conditioned plasma versus placebo injection therapy in lateral epicondylitis of the elbow: a double blind, randomized study. Sportverletz Sportschaden. 2017;31:31–6. doi: 10.1055/s-0043-101042. [DOI] [PubMed] [Google Scholar]
- 42.Singh A, Gangwar D. Autologous blood versus corticosteroid local injection for treatment of lateral epicondylosis: a randomized clinical trial. Online J Health Allied Scs. 2013;12:11. [Google Scholar]
- 43.Ozturan KE, Yucel I, Cakici H, Guven M, Sungur I. Autologous blood and corticosteroid injection and extracoporeal shock wave therapy in the treatment of lateral epicondylitis. Orthopedics. 2010;33:84–91. doi: 10.3928/01477447-20100104-09. [DOI] [PubMed] [Google Scholar]
- 44.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nature Rev Immunol. 2017;17:233–47. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim GM, Yoo SJ, Choi S, Park YG. Current trends for treating lateral epicondylitis. Clin Shoulder Elb. 2019;22:227–34. doi: 10.5397/cise.2019.22.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matak I, Bölcskei K, Bach-Rojecky L, Helyes Z. Mechanisms of botulinum toxin type A action on pain. Toxins (Basel) 2019;11:459. doi: 10.3390/toxins11080459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang S, Wang X, Wu P, et al. Platelet‐Rich plasma vs autologous blood vs corticosteroid injections in the treatment of lateral epicondylitis: a systematic review, pairwise and network meta‐analysis of randomized controlled trials. PM R. 2020;12:397–409. doi: 10.1002/pmrj.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simental-Mendia M, Vilchez-Cavazos F, Alvarez-Villalobos N, et al. Clinical efficacy of platelet-rich plasma in the treatment of lateral epicondylitis: a systematic review and meta-analysis of randomized placebo-controlled clinical trials. Clin Rheumatol. 2020;39:2255–65. doi: 10.1007/s10067-020-05000-y. [DOI] [PubMed] [Google Scholar]
- 49.Lin YC, Wu WT, Hsu YC, Han DS, Chang KV. Comparative effectiveness of botulinum toxin versus non-surgical treatments for treating lateral epicondylitis: a systematic review and meta-analysis. Clin Rehabil. 2018;32:131–45. doi: 10.1177/0269215517702517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data available.