Abstract
Regenerative medicine promises a bright future where damaged body parts can be restored, rejuvenated, and replaced. The application of regenerative medicine is interdisciplinary and covers nearly all fields of medical sciences and molecular engineering. This review provides a roadmap on how regenerative medicine is applied on the levels of cell, tissue, and organ, and summarizes the advantages and limitation of human pluripotent stem cells in disease modeling and regenerative application.
Keywords: Regenerative medicine, Pluripotent stem cells, Sickle cell disease, Cartilage damage, Kidney dysfunction, Organoids, Hepatobiliary disease modeling
1. Introduction
Regenerative medicine aims to repair, rejuvenate, and replace damaged body parts that lead to disease. The idea of replacing defective and lost body parts is made possible largely by the development of stem cell technologies and tissue engineering. The field of regenerative medicine is interdisciplinary by its nature. Its applications combine the knowledge of life science, material science, applied mathematics, and various forms of engineering. From cell therapy to organ regeneration, regenerative medicine is bringing new hopes to the various untreatable diseases of the past. Aided by innovations in gene-editing techniques, a variety of regenerative interventions are being developed to treat difficult diseases of various cellular and organ bases. Additionally, stem cells have been successful in modeling a variety of diseases. These disease models provide excellent testing ground for drug design and developmental research. Human stem cell derived tissues are also being transplanted to animal models to create chimeras for more integrated studies. We are witnessing the advent of the new era, the era of regenerative medicine. In this review, we highlight the application of regenerative medicine in treating three different diseases of cellular, tissue, and organ basis, sickle cell disease, articular cartilage damage, and kidney dysfunction. Additionally, we highlight the groundbreaking advancements in human pluripotent stem cell disease modeling and discuss its current challenge and limitation.
2. Cells: Regenerative Medicine for Sickle Cell Disease
The etiology of sickle cell disease (SCD) is well understood for more than decades. Though it is the most common inherited hemoglobinopathy disease, an effective cure is still lacking. SCD, it is a monogenic disorder caused by a substitution mutation in the beta-globin gene (HBB) of chromosome 11. The mutation (A>T) at the sixth codon of HBB gives rise to the sickling hemoglobin, HbS. Structurally, the hydrophilic glutamic acid is substituted with valine that forms a hydrophobic association with alanine, phenylalanine, and leucine of the adjacent hemoglobin. Comparing to normal hemoglobin, HbS polymerizes rapidly and significantly reduced the flow rate and life span of red blood cells (Vekilov 2007).
The most common form of sickle cell disease, HbSS, constitute the homozygous mutation of rs334. HbSS patients express no normal hemoglobin and show severe signs of anemia along with other complications. It is estimated that more than 300,000 children are born with sickle cell anemia every year globally (Piel et al. 2013b). The direct pathophysiological consequence of HbS polymerization is vaso-occlusion and hemolysis. Clinically, the disease manifests itself in various forms of acute and chronic injuries. Some of the most common symptoms include swelling and painful episodes. These symptoms are often associated with other complications such as acute chest syndrome, osteonecrosis, priapism, kidney injury, and stroke (Williams and Thein 2018).
Despite the genetic simplicity of sickle cell anemia, treating this disease had been challenging. Currently, there two FDA-approved treatments for SCD, hydroxyurea (HU) and L-glutamine. Doctors are often reluctant to prescribe HU due to its associated misinformation and poor adherence (Demirci et al. 2019). On the other hand, L-glutamine treatments are extremely expensive and usually not covered by insurance. Tough these treatments can improve the patient’s quality of life, neither of them completely cures the disease. Nonetheless, with the advancement in newborn screening and vaccination technology, more than 90% of SCD patients are expected to make it to their adulthood. It is expected that by 2050, there will be over 400,000 severe SCD patients (Piel et al. 2013a).
2.1. Hematopoietic Stem Cell Transplant
In 1984, a breakthrough was made in treating sickle cell disease using the concept of regenerative medicine. It was the first time that hematopoietic stem cell transplant (HSCT) was performed on an HbSS patient. The patient was an eight-year-old girl who suffered acute myeloid leukemia at the same time (Johnson et al. 1984). The source of transplant came from her HLA-matching sister. At the time, the procedure was initially aimed to treat leukemia. However, it also improved her condition of sickle cell anemia. It was not until then, stem cell transplant has become a therapeutic option for SCD (Salinas Cisneros and Thein 2020). Currently, as of early 2021, 35 clinical trials are listed on clinicaltrials.gov investigating allogeneic stem cell transplant as a treatment for sickle cell anemia. Among these 35 trials, 12 are already completed. Meanwhile, four additional trials aim to use autologous stem cell transplant with gene correcting interventions to treat sickle cell anemia. Though each of these clinical trials features a different perspective and strategy, all of them follow the same rule of regenerative medicine: repair and replace the disease-origin cells. To date, HSCT is the only therapy that cures sickle cell disease.
Hematopoietic stem cell transplantation provides long-term benefits for SCD patients. However, the applicability is yet hindered by several obstacles. HSCT is limited by the availability of HLA-matching donors. Only 15% of patients have such matches, on top of that, only 10% undergo HSCT due to the associated risks (Chakrabarti and Bareford 2007; Walters et al. 2001). It is possible to receive transplants for an unrelated HLA-matching donor. However, it dramatically increases the risk of complications such as rejection and graft-vs-host disease (GVHD).
2.2. Gene Therapy
Gene therapy that uses autologous hematopoietic stem cells provides the unprecedented potential to permanently cure SCD without the burden of donor availability and GVHD. There are three main approaches to using gene therapy for SCD. These three approaches are parallel to each other and can be summarized by these keywords: addition, induction, and correction (Fig. 1). The addition approach adds an antisickling copy of the beta-globin gene to the patient’s hematopoietic stem cell (HSC) (Dever et al. 2016). It is usually done by in vitro viral transduction. The modified HSCs are then reinfused back to the patient. Another approach is to induce the expression of fetal hemoglobin (HbF) in patients (Paikari and Sheehan 2018). It has been observed that SCD does not affect newborn infants due to their high blood HbF levels (Watson 1948). The protective property of HbF was also confirmed in asymptomatic SCD patients with hereditary HbF persistence (Forget 1998). Thus, SCD can be rescued by procedurally elevating HbF levels. It can be done in two ways, enhancing the expression of HbF by stimulating HbF up-regulators and knocking out HbF suppressor. A clinical trial was launched in 2018 using the later approach. Lentiviral vectors carrying shRNA inhibiting BCL11A, a HbF silencer, were infused into severe SCD patients with the hope of boosting their HbF level (Demirci et al. 2019). The third approach is to correct the pathologic mutation that gives rise to SCD. It is the most straightforward yet most challenging approach. However, high hopes are given to the advancing CRISPR/Cas technology that can induce a double-strand break at the sickling mutation and initiate repair according to the provided template. Ideally, the patient’s cells would end up with homologous copies of the normal beta-globin gene. Researchers are working hard to improve the specificity and efficiency of this technique.
Fig. 1. The regenerative treatments of sickle cell disease.
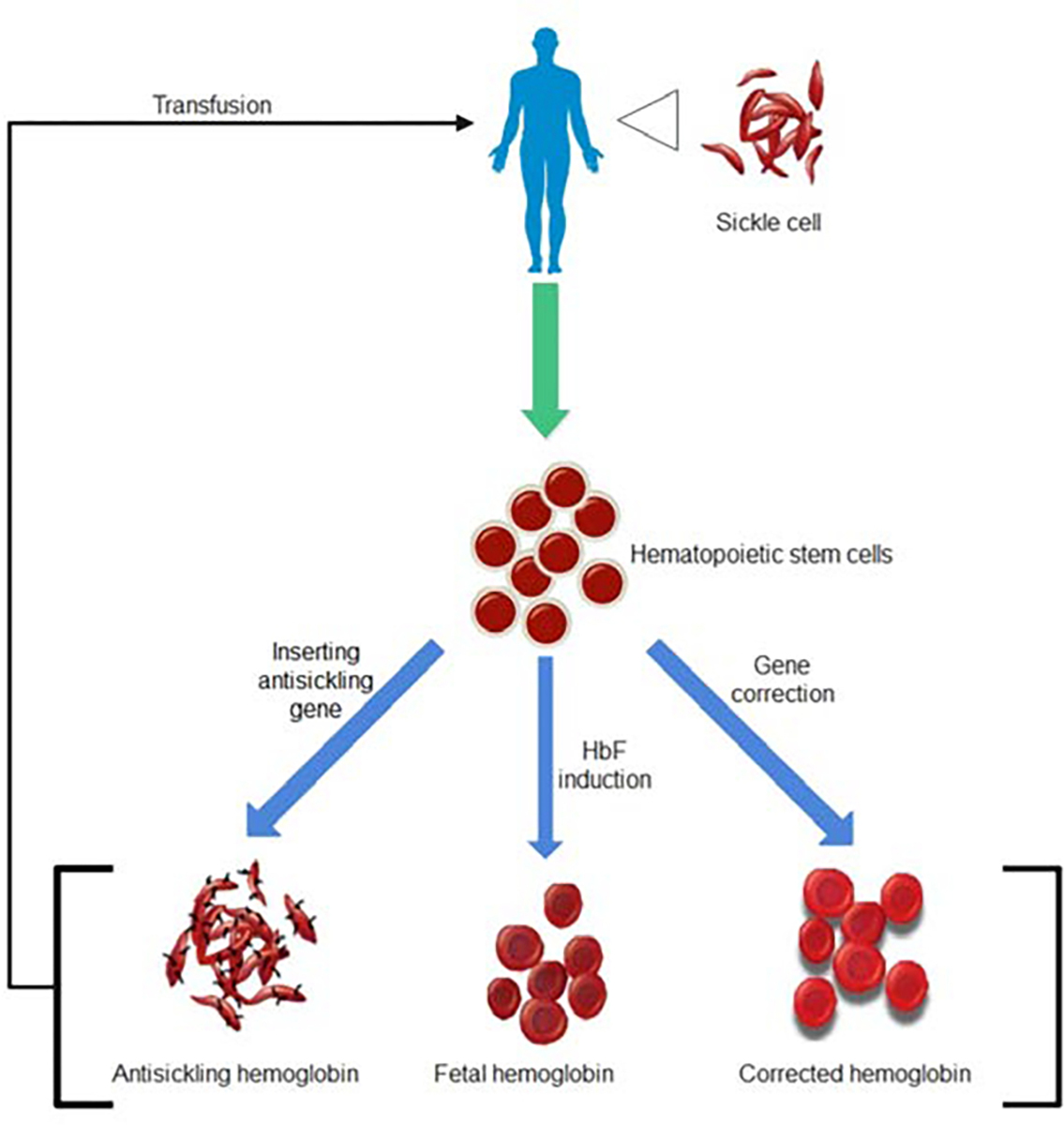
The hematopoietic stem cells derived from sickle cell patients can be differentiated into improved hemoglobin types and retransfused into the patient to replace the sickle cell.
2.3. The Challenge of Gene Therapy for SCD
Four significant challenges need to be addressed to improve the quality of gene therapy to treat SCDs. These challenges are efficiency, immunogenicity, specificity, and delivery vehicle. Genome editing techniques are generally labor-intensive, time-consuming, and costly. However, more recent techniques such as CRISPR/Cas9 are relatively easier and a lot cheaper. However, techniques have challenges surrounding the safety, delivery, and efficacy. For instance, it has been difficult to achieve a satisfactory delivery rate to a high number of mature cells with CRISPR/Cas9. The method is not always efficient in cutting and editing in some cases. Additionally, CRISPR/Cas9 editing may often lead to off-target effects and can result in severe consequences.
Future studies will be focused on improving the specificity, reducing the Off-target effect, and the delivery method of the CRISPR/Cas9. Improvements in vector technology are also in need to widen the application of gene therapy in treating sickle cell diseases. Solving the above challenges will also imply the future of germline gene therapy, potentially curing the patient’s descendants. There will be consequential social, legal, and ethical issues based on gene editing. Great hopes are given to the improvements in regenerative medicine and gene therapies.
3. Tissue: Repairing Articular Cartilage Injuries
The concept of regenerative medicine has achieved great success in treating various diseases on a cellular basis. In recent years, much attention in regenerative medicine has been drawn to develop advanced tissue engineering technologies. These developments have brought transformational changes to the treatments of articular cartilage damage.
Articular cartilage injury can occur due to normal wear and tear or trauma. It is particularly common in athletes and senior citizens. Patients who undergo arthroscopy are also likely to develop cartilage injuries (Kalson et al. 2010). The healing capability of cartilage injury is limited due to the lack of nerves and blood vessels in the cartilage. Left untreated, articular cartilage injury could lead to osteoarthritis and cause various symptoms, including swelling, pain, and compromised joint movements. Nearly half of the older citizens in the United States suffer from osteoarthritis in various degrees (Jiang et al. 2020). Economically, it has become a huge burden in medical expenses. The current treatments for articular cartilage injury are obstructed by the difficulty in binding the regenerated tissue to its surrounding environments(Muhammad et al. 2019). However, the new tissue-engineered cartilage brings unprecedented advantages to articular cartilage repair.
3.1. The Current Treatments and Limitations
The current treatment strategy for articular cartilage injury is mostly based on surgical regeneration techniques (Fig. 2). Microfracture is a common bone marrow stimulation approach for cartilage repair. First developed in the early 80s, microfracture differentiates mesenchymal stem cells from fibrous cartilage (Steadman et al. 2010). However, such technique is rather unsatisfactory in repairing larger cartilage damages that are bigger than 2.5 cm2 (Jones and Peterson 2006). Additionally, elderly patients tend to heal slowly to these procedures. Another method often used to treat cartilage injury is osteochondral transplantation (OCT) (Yamashita et al. 1985). OCT can use either autologous or allogeneic sources of osteochondral columns to fill in the defect sites of the cartilage. In the autologous case, the osteochondral columns are usually removed from the non-weight bearing sites and transplanted into the injured cartilage. This technique is often limited by the amount of transplantable osteochondral columns (Andrade et al. 2016). Thus, only suitable for small defects. On the other hand, allogeneic osteochondral tissue transplantation is more available but risks disease transmission and is very expensive. Autologous chondrocyte implantation (ACI) is an alternative technique that harvests chondrocytes from the non-weight bearing sites of the articular surface and transplants them into the damaged sites after in vitro expansion(Brittberg et al. 1994). However, this technique is still hindered by several limitations, such as the invasion surgical procedure obtaining the donor chondrocytes and the limited cell counts available. Additionally, the in vitro expansion of harvest chondrocytes is prone to dedifferentiation. Articulated articular cartilage implantation (PACI) is repairing the cartilage defect with the crushed allogenic or autologous cartilage particles (Lu et al. 2006). Comparing to OCT, this technique requires less donor cartilage. However, like the previously described techniques, PACI is only suitable for small cartilage defects no larger than 3.5 cm2 (Jiang et al. 2020).
Fig. 2. The regenerative approaches to repair cartilage injury.
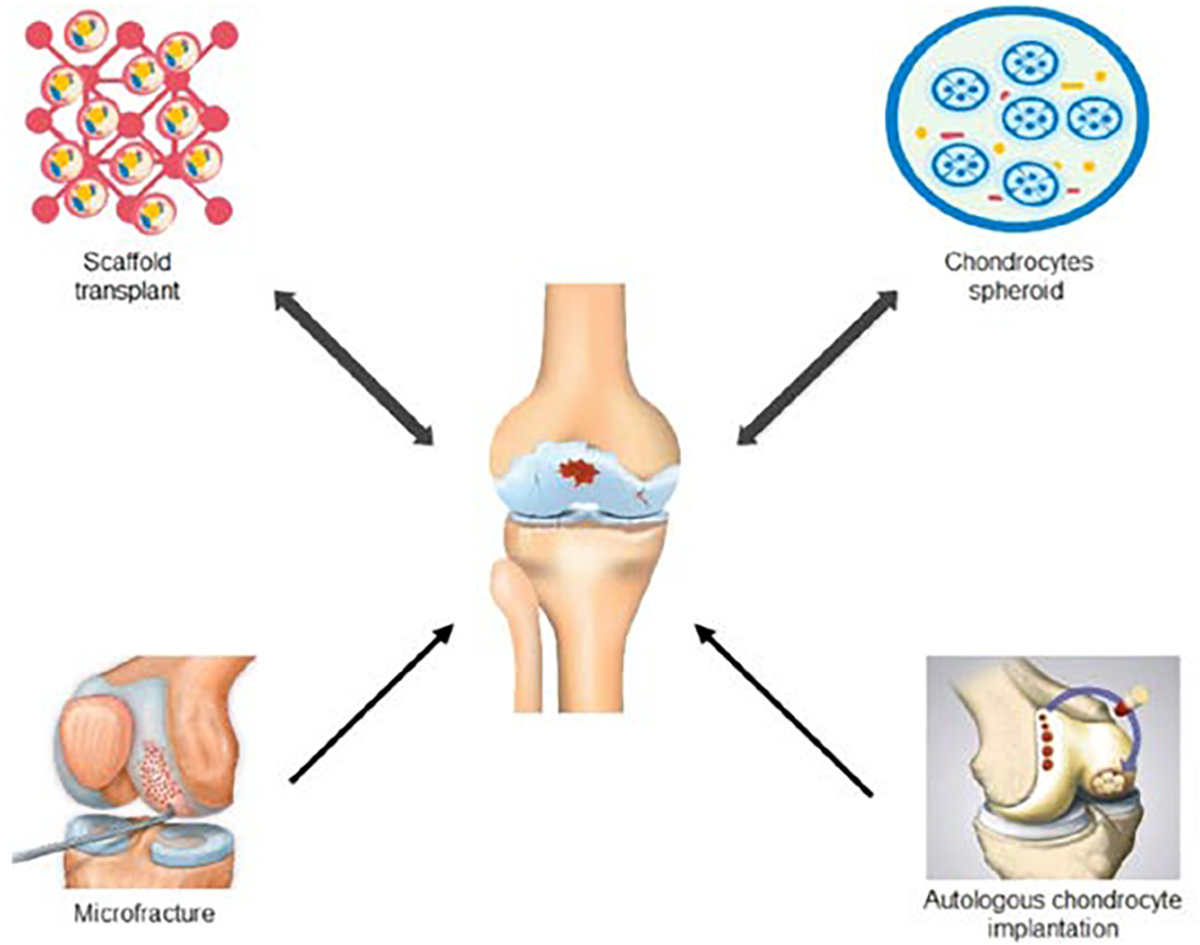
Various regenerative approaches have been developed to elaborately repair the articular cartilage injury.
3.1. Tissue Engineering for Cartilage Injuries
Cartilage tissue engineering combines advancements in material science, biomechanics, biochemistry, and cell biology. It has shown great promise in regenerating hyaline cartilage and repair the entire cartilage defect. Engineered tissue based on mesenchymal stem cells displays excellent proliferation potential, differentiation ability, and low immunogenicity (Harrell et al. 2019). Three main strategies are currently in use to generate tissue-engineered cartilage (Fig. 2). The first strategy is by establishing a cell-scaffold construct. The scaffold is often made of biocompatible materials such as collagen matrix and hydrogel. Chondrocytes and various types of stem cells are planted into these scaffolds before transplanting into the patients. The scaffolds offer support for the seed cells to differentiate and expand. Once completely integrated with the patient, these scaffolds slow degrade. Another approach to generating tissue-engineered cartilage is the cell-free strategy. It is important to note that the cell-free strategy is, in fact, not free of cells. It indirectly uses stem cells, such as mesenchymal stem cells, to regenerate cartilage without directly transplanting these mesenchymal stem cells into the patients. Two subcategories fall under this technique. The first induces cartilage regeneration by stimulating bone marrow stem cells in situ combined with a transplanted biocompatible scaffold. The second approach is to integrate mesenchymal stem cell derivatives, including cytokines, various RNAs, etc., to the transplanted scaffold to stimulate the regeneration of cartilage. These “cell-free” strategies are still in the animal testing stage (Jiang et al. 2020). The last strategy commonly used to generate tissue-engineered cartilage is the scaffold-free strategy. This strategy uses chondrocytes spheroids for cartilage transplantation. It is similar to ACI. However, the difference is that ACI uses cell suspensions. The advantage of the scaffold-free approach is that it avoids the problem of developing complex scaffolds that are often unavailable. It has been challenging to develop ideal scaffolds that meet the various criteria of transplantation. These criteria include but not limited to promoting cell growth, biodegradable at an appropriate rate, and adhesive. Chondrosphere® is an approved scaffold-free product that uses chondrocytes spheroid to repair cartilage damage. The phase III clinical trial has demonstrated that Chondrosphere® is at least as effective as microfracture in patients with small cartilage effects (Armoiry et al. 2019). In patients with cartilage defects bigger than cm2, Chondrosphere® is proven to be a more effective treatment.
To make tissue-engineered cartilages more available and practical, we must overcome several obstacles. These obstacles include producing an ideal scaffold that promotes adhesion and growth of seed cells, optimizing the differentiation and expansion of cartilage-related cells, and minimizing the tumorigenicity and heterogenicity of stem cells used. Also, the possibility of disease transmission and immune rejection of transplanted allogeneic cell sources must be addressed and prevented. Fundamentally, we need to grab a more detailed understanding of how cartilage developments are regulated in vivo. Many interactions of immune responses between secreted factors, synovial fluids, and exosomes are yet to be determined. These interactions are the key factors to consider when optimizing the physical property of tissue scaffold and maintaining a local environment ideal for recovery.
4. Organ: Kidney Regeneration
Whole organ regeneration remains challenging at the current stage of regenerative medicine. However, significant improvements towards kidney generation have been made in recent years. The need for modern organ regeneration and transplantation is on the global rise. Kidney is the most frequently transplanted organ in the United States (Alachkar et al. 2011). Around 69,000 kidneys are transplanted every year globally, according to WTO (Organization 2008). In 2014, US alone had nearly 16,000 kidney transplants (Wragg et al. 2019). These large numbers do not nearly meet the needs of organ shortage. The average waiting time for an available kidney in the US is almost two and a half years (Wragg et al. 2019). This wait time is much longer in other places of the world (Lee et al. 2019). More than 16% of patients die or get too sick receive their kidney transplant by the end of their wait time (Cassuto et al. 2010). The extreme scarcity of kidneys has led to problems beyond the field of medicine, such as trafficking, compensated donation, and the expansion in black markets. Researchers around the world are seeking ways to grow transplantable kidneys and devices that can mimic the function of kidneys.
4.1. Organoids
Kidney organoids derived from human pluripotent stem cells (hPSCs) are given hopes to bring the future of renal replacement therapy in regenerative medicine. Numerous protocols are established to differentiate hPSCs into kidney organoids (Freedman et al. 2015; Morizane et al. 2015; Taguchi et al. 2014; Taguchi and Nishinakamura 2017; Takasato et al. 2015). These protocols use a combination of small molecules and growth factors to direct a stage-specific differentiation similar to the development of embryonic kidneys. Renal structures including nephron, glomeruli, interstitium, and collecting ducts are self-organized in these organoids. However, the functional capability of these cultures is often no match to their natural counterparts. Advanced understanding of how kidney develops in vivo are in need to optimize these differentiation protocols. Additionally, three major obstacles block the reality of renal organoid replacement therapy. These obstacles are off-target cells, vascularization, and reproducibility (Geuens et al. 2020). Kidney organoids derived from established protocol may contain up to 20% of the nonrenal cell population (Combes et al. 2019; Wu et al. 2018). These off-target cells increase in prevalence as the organoid gets larger and eventually disrupts the integrity (Geuens et al. 2020). It remains unclear how these off-target cells come to place. The cells in this off-target population resemble many cell types, including neuronal, muscle cells, cartilage, and anywhere in between (Bantounas et al. 2018; Morizane et al. 2015). The lack of vascular structure is another major difficulty in renal organoids. To date, there is no kidney organoid that shows patent vasculature. These organoids simply do not have enough endothelial cells nor the proper guiding cues from the vasculature. As organoids grow larger, cell death in the center mass becomes inevitable due to the lack of vascular nutrients and waste exchange. The third major problem is scaling. Current kidney organoids are roughly 1/10,000 of a single human kidney by nephron counts (Geuens et al. 2020). Automated systems for generating these organoids are being constructed and improved to scale up the liver organoids production (Czerniecki et al. 2018). However, without vasculature, these organoids cannot be assembled into a functional liver.
4.2. Wearable Artificial Kidney
As the regeneration of a whole kidney remains a tremendous challenge, dialysis is the mainstream of current kidney replacement therapy. Though dialysis provides the means to survive live failure, it requires significant changes in the patient’s daily routine. Beyond its physical inconvenience, long sessions of dialysis are becoming a burden that affects the mental health of patients (Pereira et al. 2017). In recent years, efforts are made into the development of wearable artificial kidneys. These wearable kidney devices are currently under clinical trials (Davenport et al. 2007; Gura et al. 2005; Lee and Roberts 2008). The portability of these devices allows the patient to continue to work and travel. Typically, these devices are a few kilograms in weight and operated by battery (Salani et al. 2018). Inside the wearable artificial kidney, blood is anticoagulated and pumped through a polysulfide hollow-fiber dialyzer (Gura et al. 2009). The dialysates are pushed in a rhythm that compensates for the peak and trough of the blood flow as the dialyzer transmembrane oscillates. A blood flow of 100 mL/min is achieved with this system. To avoid the risk of accidental disconnection, wearable artificial kidneys are advised to use catheters instead of needles in a fistula. However, the consequences of continuous catheter use remain unclear (Salani et al. 2018). Some technological improvements are still needed before the general application of wearable artificial kidneys.
5. Disease Modeling with human Pluripotent Stem Cells
Advancements in regenerative medicine bring new hope to treating diseases of various mechanisms. However, further innovations in regenerative medicine still largely rely on the development of accurate disease models. The human pluripotent stem cell (hPSC) is an excellent tool in disease modeling. The advantages of pluripotent stem cells include availability, flexibility, and genomic integrity. Once the pluripotent stem cell population is established, these cells can expand indefinitely, providing large numbers of cells for differentiation and testing. Additionally, induced pluripotent stem cells (iPSCs) can be generated from various adult cells and tissues. The abundance of iPSC and its easy maintenance offers an unparalleled advantage for developing disease models. At the same time, hPSCs are extremely flexible as they can theoretically differentiate into any cell type of the human body. The differentiation protocol of hPSCs to many cell types are well established and publicly available. The most common cell types differentiated from iPSCs include dopaminergic neurons, motor neurons, astrocytes, oligodendrocytes, cardiomyocytes, hepatocytes, pancreatic β-cell, and lung epithelial cells (Fig. 3) (Abo et al. 2020; Bianchi et al. 2018; Corbett and Duncan 2019; Ehrlich et al. 2017; Hallett et al. 2015; Karakikes et al. 2015; Ma et al. 2018; Soubannier et al. 2020). Patient-derived iPSCs are often developed into the diseased cell types to identify the linkages between the patient genotype and disease phenotype as the iPSC reprogramming preserves the genomic integrity of the patient. In the past decade, iPSCs have been successful in modeling countless diseases of genetic defects. However, it remains a challenge to use iPSCs to accurately model more complex diseases, such as cancer. Nevertheless, human iPSC offers an unprecedented alternative to disease modeling. Most importantly, these cell and organoid-based disease models are compatible with high-throughput screening, providing a faster and more efficient solution to drug discovery and pathology research.
Fig. 3. Human iPSC disease models of various organ and tissue types.
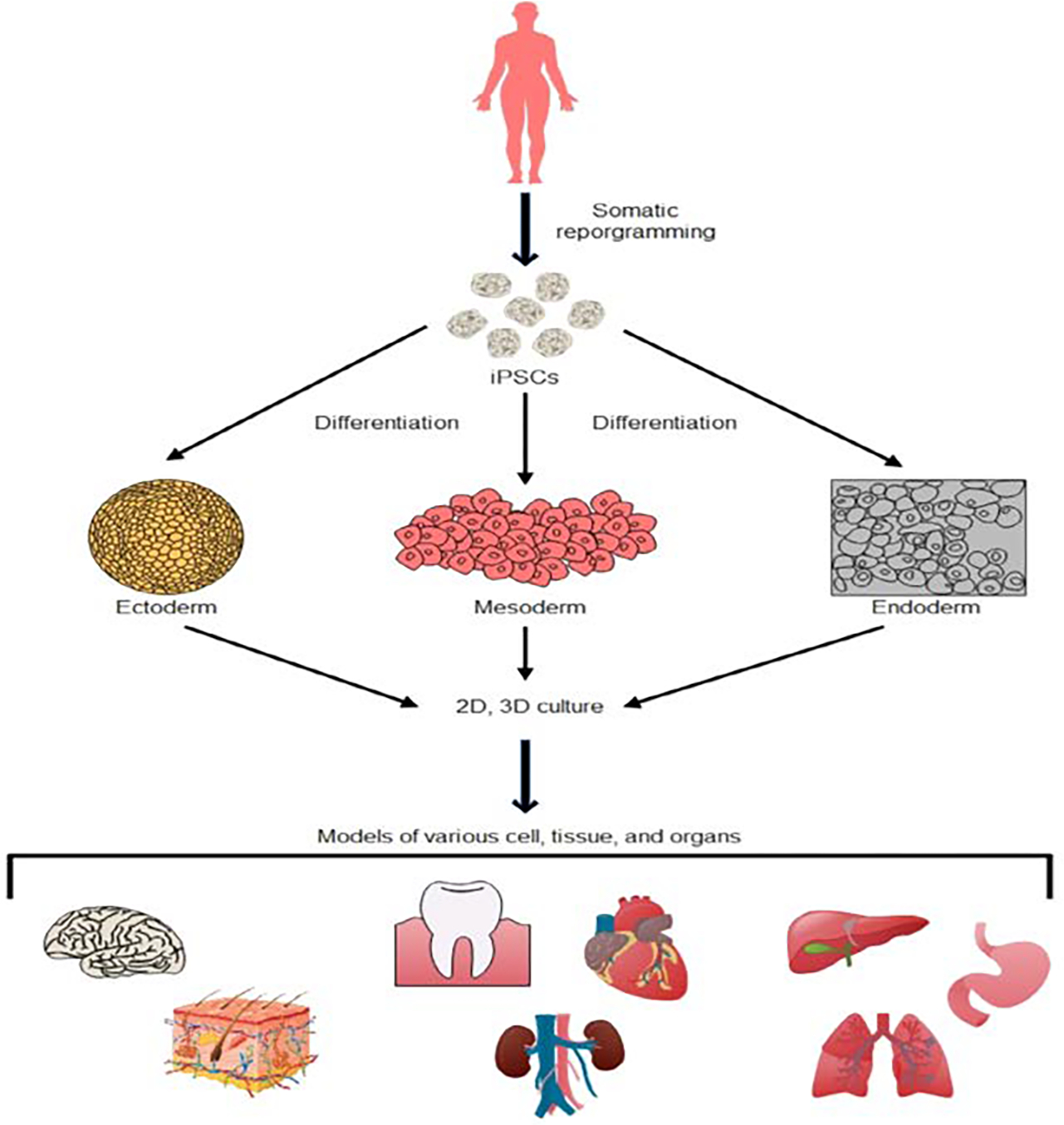
Patient-specific cells can be derived into iPSCs that are capable of differentiating into all three germ layers. These iPSCs preserves the genome of the patient and can be further developed into 2D and 3D disease models of various origins.
5.1. iPSC-based Modeling of Hepatobiliary Diseases
The complexity and vital roles of hepatobiliary organs them a detrimental target of diseases. Despite the recent developments, the treatment of many complex hepatobiliary disease remains challenging. Some of these diseases include biliary atresia, primary sclerosing cholangitis, biliary fibrosis, liver cirrhosis, and hepatobiliary cancers. There is a desperate need for further understanding of the molecular and pathodevelopmental mechanisms of liver diseases. For the past decades, many advancements in the understanding of such diseases are supported by primary cells, tissues, as well as various animal models. However, major drawbacks of primary culture and animal models include high cost and low availability. Alternatively, immortalized disease lines are often too altered to accurately reflect their supposed physiology. Though useful in many ways, animal models of hepatology are neither close to recapitulating hepatobiliary responses in humans.
In recent years, much focus has been dedicated to creating hepatobiliary “disease in a dish.” In a nutshell, this concept is enabled by the in vitro differentiation of patient-derived stem cells. Normal stem cells can also be genetically altered to express enhanced disease phenotypes, depending on the configurable culture conditions. The major advantage of this approach is that these in vitro disease models can be abundantly available and high-throughput compatible. It holds the potential to dramatically reduce the time span of fundamental research and drug discovery processes.
The differentiation protocols of human iPSC to hepatobiliary cell types, including hepatocyte-like cells (HLC) and cholangiocyte-like cells (CLC), have been reported from various sources (Corbett and Duncan 2019; Sampaziotis et al. 2017). These protocols follow a multistep and stage-specific procedure involving a symphony of key cytokines and growth factors. When iPSCs are derived from patients of genetic diseases, the genetic linkages of disease progressions are retained in the differentiation of these iPSCs, allowing in vitro observation and drug testing. Additionally, by altering the chemical composition of iPSC culture media, researchers are also able to recapitulate the development of hepatobiliary conditions caused by environmental factors, for example, drug-induced hepatotoxicity and alcohol liver disease (Sirenko and Cromwell 2018; Tian et al. 2016). Altogether, genetic and environmental, iPSC models are an excellent tool for studying hepatobiliary diseases.
In the past decades, a list of hepatobiliary diseases was successfully modeled by iPSC technology, many of which have led to the discovery of promising therapy candidates. A list of these diseases and their phenotypes is included in Table 1.
Table 1.
iPSC-based hepatobiliary disease models and their phenotypes.
| Type | iPSC-derived disease models | Model phenotypes | References |
|---|---|---|---|
| Hepatocytic | Alpha-1-antitrypsin deficiency | ZAAT polymer accumulation | (Choi et al. 2013; Kaserman and Wilson 2018; Tafaleng et al. 2015) |
| Alpers syndrome | Reduced optic atrophy 1 protein | (Li et al. 2015) | |
| Citrin deficiency | Impaired ureagenesis | (Kim et al. 2016) | |
| Hemophilia A | FVm deficiency | (Jia et al. 2014; Olgasi et al. 2018) | |
| Infantile-onset Pompe disease | Lysosomal glycogen accumulation | (Yoshida et al. 2019) | |
| Liver fibrosis (iPSC-HSC) | Retinyl esters accumulation | (Coll et al. 2018) | |
| Niemann-pick disease type C | Cholesterol accumulation | (Soga et al. 2015) | |
| Tangier disease | Impaired cholesterol efflux | (Bi et al. 2017) | |
| Wilson’s disease | Rapid ATP7B degradation | (Parisi et al. 2018; Yi et al. 2012) | |
| Biliary | Alagille syndrome | Organoid malformation | (Sampaziotis et al. 2015) |
| Biliary atresia | Increased fibrosis Reduced biliary differentiation | (Tian et al. 2019) | |
| Cystic fibrosis | Impaired Cl− channel activity | (Simsek et al. 2016) | |
| Polycystic liver disease | Cholangiocytic cysts | (Kamiya et al. 2018) |
5.2. The Current Limitation of iPSCs in Regenerative Applications
Three major challenges hinder the downstream applications of human iPSCs. These challenges are potential tumorigenicity, immunogenicity, and heterogenicity (Yamanaka 2020). The tumorigenicity of iPSC is mainly caused by three different reasons, incorrect patterning, reprogramming factors, and genetic abnormalities. The fate of stem cells is strongly influenced by their patterning along with other cell types. In the occurrence of incorrect or incomplete patterning, niche-specific stem cells within the transplant often end up forming tumors. One example is the emergence of neural rosettes, which will maintain normal development when it is patterned towards the cortex. However, simple in vivo injection of the same cells leads to cancerous growth (Malchenko et al. 2014). The tumorigenicity of iPSCs can also derive from the intrinsic property of reprogramming factors. The common factors used to generate iPSCs, including ct3/4, Sox2, Klf4, c-Myc, all have reported roles in cancer development. C-Myc is one of the most frequently discussed proto-oncogenes. iPSC-associated chimeric mice often develop tumors due to the reactivation of c-Myc and other reprogramming factors (Yamanaka 2020).
Another challenge iPSCs are facing is immune rejection. It has been controversial whether autologous iPSCs are immunogenic. Zhao et al. has suggested that the immunogenicity of autologous iPSC is caused by its abnormal gene expression (Zhao et al. 2011). Their research provides an example of immune rejection of autologous iPSC transplants. The teratomas formed in the iPSC-transplanted mice showed obvious signs of rejection, such as T cell infiltration. A more recent study suggested that neoepitopes of autologous iPSCs can also originate from de novo mutations in the mitochondria (Deuse et al. 2019). However, in most cases, autologous iPSCs grafts do not trigger an immunogenic response. On the other hand, allografts of iPSCs are much more immunogenic. Nonetheless, allogenic approaches are much preferred due to their low cost and high production. Immunogenicity caused by allogeneic transplants is often mitigated by immunosuppressants. One limitation of immunosuppressants is that it is often a lifelong treatment, especially in organ transplants. However, it is possible to avoid excessive immunosuppressing medication when the graft sites are immune-privileged, for example, the central nervous system, spinal cord, and the eye.
Heterogenicity originates from the intrinsic differences between the iPSC lines. These differences include their morphology, growth curve, gene expression, and propensity to differentiate into various cell lineages. The downstream applications such as modeling, drug testing, and therapies are hugely hurdled by heterogenicity. This problem was first addressed in mouse ESCs. It was later revealed that heterogenic mouse ESCs could be converted into a neutral “ground” state by two defined kinases, MEK and GSK3 inhibitors (Ying et al. 2008). The “ground state” of these lines characterize an undifferentiated morphology, lower DNA methylation content, and greater potential to produce chimeric mice and germline competent ESCs. Heterogenicity is not unique to mice PSCs. Human ESC and iPSCs also have troubles with heterogenicity. The example of heterogenicity in humans can be demonstrated by the comparison of hESC and hiPSC lines. Such comparisons revealed significant differences in their gene expression, epigenetic status, and differentiation potentials (Yamanaka 2012). On the other hand, when twenty or more hESC and hiPSC lines are compared, it was shown that overlapping variations do exist. In the attempts to overcome heterogeneity, researchers attempted to convert the “primed” state of hPSCs into a “naive” state. Multiple approaches have been taken to induce the naïve or ground state pluripotency in hPSCs. The concerning points of naïve hPSCs hinder the studies on heterogenicity. These concerns are about hPSCs genetic integrity and the loss of imprinting (Yamanaka 2020).
6. Concluding Remarks
Regenerative medicine is a translational field that requires the constant exchange of knowledge between many related fields. Regenerative medicine is also a powerful concept that can potentially cure nearly all diseases. Instead of treating the symptoms of a disease, regenerative medicine is designed to repair the cause of disease and to replace the defect with functional tissues. The application of regenerative medicine goes way beyond what this review can capture. The purpose of this review is to demonstrate how the concept of regenerative medicine is being applied to different magnitudes, from cells to tissues to organs. The advantages and challenges of regenerative medicine mentioned above are often similar in other disease settings. The future of regenerative medicine relies on the advancements of associated fields, including iPSC differentiation technology, disease modeling, genetic engineering, material science, and most importantly, fundamental science.
Financial support:
This work was supported by NIH grant R01 DK122982 and PSC Partners Research Grant, Johns Hopkins Pediatric Liver Center.
Abbreviations
- SCD
sickle cell disease
- HU
hydroxyurea
- HSCT
hematopoietic stem cell transplant
- HSC
hematopoietic stem cell
- GVHD
graft-vs-host disease
- HbF
fetal hemoglobin
- HbS
hemoglobin S
- hPSC
human pluripotent stem cell
- ACI
autologous chondrocyte implantation
- PACI
articulated articular cartilage implantation
- OCT
osteochondral transplantation
- iPSC
induced pluripotent stem cell
- hiPSC
human induced pluripotent stem cell
- ESC
embryonic stem cell
- hESC
human embryonic stem cell
- HLC
hepatocyte-like cell
- CLC
cholangiocyte-like cell
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
References:
- Abo KM et al. (2020) Human iPSC-derived alveolar and airway epithelial cells can be cultured at air-liquid interface and express SARS-CoV-2 host factors bioRxiv doi: 10.1101/2020.06.03.132639 [DOI] [Google Scholar]
- Alachkar N, Rabb H, Jaar BG (2011) Urinary biomarkers in acute kidney transplant dysfunction Nephron Clin Pract 118:c173–181; discussion c181 doi: 10.1159/000321381 [DOI] [PubMed] [Google Scholar]
- Andrade R et al. (2016) Knee donor-site morbidity after mosaicplasty - a systematic review J Exp Orthop 3:31 doi: 10.1186/s40634-016-0066-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armoiry X et al. (2019) Autologous Chondrocyte Implantation with Chondrosphere for Treating Articular Cartilage Defects in the Knee: An Evidence Review Group Perspective of a NICE Single Technology Appraisal Pharmacoeconomics 37:879–886 doi: 10.1007/s40273-018-0737-z [DOI] [PubMed] [Google Scholar]
- Bantounas I et al. (2018) Generation of Functioning Nephrons by Implanting Human Pluripotent Stem Cell-Derived Kidney Progenitors Stem Cell Reports 10:766–779 doi: 10.1016/j.stemcr.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi X et al. (2017) ATP-Binding Cassette Transporter A1 Deficiency in Human Induced Pluripotent Stem Cell-Derived Hepatocytes Abrogates HDL Biogenesis and Enhances Triglyceride Secretion EBioMedicine 18:139–145 doi: 10.1016/j.ebiom.2017.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi F et al. (2018) Rapid and efficient differentiation of functional motor neurons from human iPSC for neural injury modelling Stem Cell Res 32:126–134 doi: 10.1016/j.scr.2018.09.006 [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L (1994) Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation N Engl J Med 331:889–895 doi: 10.1056/NEJM199410063311401 [DOI] [PubMed] [Google Scholar]
- Cassuto JR et al. (2010) Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients Am J Transplant 10:2502–2511 doi: 10.1111/j.1600-6143.2010.03292.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Bareford D (2007) A survey on patient perception of reduced-intensity transplantation in adults with sickle cell disease Bone Marrow Transplant 39:447–451 doi: 10.1038/sj.bmt.1705622 [DOI] [PubMed] [Google Scholar]
- Choi SM et al. (2013) Efficient drug screening and gene correction for treating liver disease using patient-specific stem cells Hepatology 57:2458–2468 doi: 10.1002/hep.26237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll M et al. (2018) Generation of Hepatic Stellate Cells from Human Pluripotent Stem Cells Enables In Vitro Modeling of Liver Fibrosis Cell Stem Cell 23:101–113 e107 doi: 10.1016/j.stem.2018.05.027 [DOI] [PubMed] [Google Scholar]
- Combes AN, Zappia L, Er PX, Oshlack A, Little MH (2019) Single-cell analysis reveals congruence between kidney organoids and human fetal kidney Genome Medicine 11:3 doi: 10.1186/s13073-019-0615-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett JL, Duncan SA (2019) iPSC-Derived Hepatocytes as a Platform for Disease Modeling and Drug Discovery Front Med (Lausanne) 6:265 doi: 10.3389/fmed.2019.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniecki SM et al. (2018) High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping Cell Stem Cell 22:929–940 e924 doi: 10.1016/j.stem.2018.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport A, Gura V, Ronco C, Beizai M, Ezon C, Rambod E (2007) A wearable haemodialysis device for patients with end-stage renal failure: a pilot study Lancet 370:2005–2010 doi: 10.1016/S0140-6736(07)61864-9 [DOI] [PubMed] [Google Scholar]
- Demirci S, Leonard A, Haro-Mora JJ, Uchida N, Tisdale JF (2019) CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges Adv Exp Med Biol 1144:37–52 doi: 10.1007/5584_2018_331 [DOI] [PubMed] [Google Scholar]
- Deuse T et al. (2019) De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans Nat Biotechnol 37:1137–1144 doi: 10.1038/s41587-019-0227-7 [DOI] [PubMed] [Google Scholar]
- Dever DP et al. (2016) CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells Nature 539:384–389 doi: 10.1038/nature20134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M et al. (2017) Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors Proc Natl Acad Sci U S A 114:E2243–E2252 doi: 10.1073/pnas.1614412114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget BG (1998) Molecular basis of hereditary persistence of fetal hemoglobin Ann N Y Acad Sci 850:38–44 doi: 10.1111/j.1749-6632.1998.tb10460.x [DOI] [PubMed] [Google Scholar]
- Freedman BS et al. (2015) Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids Nat Commun 6:8715 doi: 10.1038/ncomms9715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuens T, van Blitterswijk CA, LaPointe VLS (2020) Overcoming kidney organoid challenges for regenerative medicine NPJ Regen Med 5:8 doi: 10.1038/s41536-020-0093-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gura V, Beizai M, Ezon C, Polaschegg HD (2005) Continuous renal replacement therapy for end-stage renal disease. The wearable artificial kidney (WAK) Contrib Nephrol 149:325–333 doi: 10.1159/000085694 [DOI] [PubMed] [Google Scholar]
- Gura V, Macy AS, Beizai M, Ezon C, Golper TA (2009) Technical breakthroughs in the wearable artificial kidney (WAK) Clin J Am Soc Nephrol 4:1441–1448 doi: 10.2215/CJN.02790409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett PJ et al. (2015) Successful function of autologous iPSC-derived dopamine neurons following transplantation in a non-human primate model of Parkinson’s disease Cell Stem Cell 16:269–274 doi: 10.1016/j.stem.2015.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V (2019) Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives Biomed Pharmacother 109:2318–2326 doi: 10.1016/j.biopha.2018.11.099 [DOI] [PubMed] [Google Scholar]
- Jia B et al. (2014) Modeling of hemophilia A using patient-specific induced pluripotent stem cells derived from urine cells Life Sci 108:22–29 doi: 10.1016/j.lfs.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Jiang S et al. (2020) Clinical Application Status of Articular Cartilage Regeneration Techniques: Tissue-Engineered Cartilage Brings New Hope Stem Cells Int 2020:5690252 doi: 10.1155/2020/5690252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson FL, Look AT, Gockerman J, Ruggiero MR, Dalla-Pozza L, Billings FT, 3rd (1984) Bone-marrow transplantation in a patient with sickle-cell anemia N Engl J Med 311:780–783 doi: 10.1056/NEJM198409203111207 [DOI] [PubMed] [Google Scholar]
- Jones DG, Peterson L (2006) Autologous chondrocyte implantation J Bone Joint Surg Am 88:2502–2520 doi: 10.2106/00004623-200611000-00025 [DOI] [PubMed] [Google Scholar]
- Kalson NS, Gikas PD, Briggs TW (2010) Current strategies for knee cartilage repair Int J Clin Pract 64:1444–1452 doi: 10.1111/j.1742-1241.2010.02420.x [DOI] [PubMed] [Google Scholar]
- Kamiya A, Chikada H, Ida K, Ando E, Tsuruya K, Kagawa T, Inagaki Y (2018) An in vitro model of polycystic liver disease using genome-edited human inducible pluripotent stem cells Stem Cell Res 32:17–24 doi: 10.1016/j.scr.2018.08.018 [DOI] [PubMed] [Google Scholar]
- Karakikes I, Ameen M, Termglinchan V, Wu JC (2015) Human induced pluripotent stem cell-derived cardiomyocytes: insights into molecular, cellular, and functional phenotypes Circ Res 117:80–88 doi: 10.1161/CIRCRESAHA.117.305365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaserman JE, Wilson AA (2018) Patient-Derived Induced Pluripotent Stem Cells for Alpha-1 Antitrypsin Deficiency Disease Modeling and Therapeutic Discovery Chronic Obstr Pulm Dis 5:258–266 doi: 10.15326/jcopdf.5.4.2017.0179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Choi JY, Lee SH, Lee BH, Yoo HW, Han YM (2016) Malfunction in Mitochondrial beta-Oxidation Contributes to Lipid Accumulation in Hepatocyte-Like Cells Derived from Citrin Deficiency-Induced Pluripotent Stem Cells Stem Cells Dev 25:636–647 doi: 10.1089/scd.2015.0342 [DOI] [PubMed] [Google Scholar]
- Lee DB, Roberts M (2008) A peritoneal-based automated wearable artificial kidney Clin Exp Nephrol 12:171–180 doi: 10.1007/s10157-008-0050-9 [DOI] [PubMed] [Google Scholar]
- Lee S, Yoo KD, An JN, Oh YK, Lim CS, Kim YS, Lee JP (2019) Factors affecting mortality during the waiting time for kidney transplantation: A nationwide population-based cohort study using the Korean Network for Organ Sharing (KONOS) database PLoS One 14:e0212748 doi: 10.1371/journal.pone.0212748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S et al. (2015) Valproic acid-induced hepatotoxicity in Alpers syndrome is associated with mitochondrial permeability transition pore opening-dependent apoptotic sensitivity in an induced pluripotent stem cell model Hepatology 61:1730–1739 doi: 10.1002/hep.27712 [DOI] [PubMed] [Google Scholar]
- Lu Y, Dhanaraj S, Wang Z, Bradley DM, Bowman SM, Cole BJ, Binette F (2006) Minced cartilage without cell culture serves as an effective intraoperative cell source for cartilage repair J Orthop Res 24:1261–1270 doi: 10.1002/jor.20135 [DOI] [PubMed] [Google Scholar]
- Ma H, Wert KJ, Shvartsman D, Melton DA, Jaenisch R (2018) Establishment of human pluripotent stem cell-derived pancreatic beta-like cells in the mouse pancreas Proc Natl Acad Sci U S A 115:3924–3929 doi: 10.1073/pnas.1702059115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchenko S et al. (2014) Onset of rosette formation during spontaneous neural differentiation of hESC and hiPSC colonies Gene 534:400–407 doi: 10.1016/j.gene.2013.07.101 [DOI] [PubMed] [Google Scholar]
- Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV (2015) Nephron organoids derived from human pluripotent stem cells model kidney development and injury Nat Biotechnol 33:1193–1200 doi: 10.1038/nbt.3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad SA, Nordin N, Mehat MZ, Fakurazi S (2019) Comparative efficacy of stem cells and secretome in articular cartilage regeneration: a systematic review and meta-analysis Cell Tissue Res 375:329–344 doi: 10.1007/s00441-018-2884-0 [DOI] [PubMed] [Google Scholar]
- Olgasi C et al. (2018) Patient-Specific iPSC-Derived Endothelial Cells Provide Long-Term Phenotypic Correction of Hemophilia A Stem Cell Reports 11:1391–1406 doi: 10.1016/j.stemcr.2018.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH (2008) Transplantation. https://www.who.int/transplantation/gkt/statistics/en/#:~:text=Based%20on%20activity%20data%20analysed,%25%20from%20living%20donors)%2C%205.
- Paikari A, Sheehan VA (2018) Fetal haemoglobin induction in sickle cell disease Br J Haematol 180:189–200 doi: 10.1111/bjh.15021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi S et al. (2018) Characterization of the most frequent ATP7B mutation causing Wilson disease in hepatocytes from patient induced pluripotent stem cells Sci Rep 8:6247 doi: 10.1038/s41598-018-24717-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira BDS, Fernandes NDS, de Melo NP, Abrita R, Grincenkov F, Fernandes N (2017) Beyond quality of life: a cross sectional study on the mental health of patients with chronic kidney disease undergoing dialysis and their caregivers Health Qual Life Outcomes 15:74 doi: 10.1186/s12955-017-0646-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN (2013a) Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions PLoS Med 10:e1001484 doi: 10.1371/journal.pmed.1001484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB et al. (2013b) Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates Lancet 381:142–151 doi: 10.1016/S0140-6736(12)61229-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salani M, Roy S, Fissell WHt (2018) Innovations in Wearable and Implantable Artificial Kidneys Am J Kidney Dis 72:745–751 doi: 10.1053/j.ajkd.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Salinas Cisneros G, Thein SL (2020) Recent Advances in the Treatment of Sickle Cell Disease Front Physiol 11:435 doi: 10.3389/fphys.2020.00435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaziotis F, de Brito MC, Geti I, Bertero A, Hannan NR, Vallier L (2017) Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells Nat Protoc 12:814–827 doi: 10.1038/nprot.2017.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaziotis F et al. (2015) Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation Nat Biotechnol 33:845–852 doi: 10.1038/nbt.3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simsek S et al. (2016) Modeling Cystic Fibrosis Using Pluripotent Stem Cell-Derived Human Pancreatic Ductal Epithelial Cells Stem Cells Transl Med 5:572–579 doi: 10.5966/sctm.2015-0276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko O, Cromwell EF (2018) Determination of Hepatotoxicity in iPSC-Derived Hepatocytes by Multiplexed High Content Assays Methods Mol Biol 1683:339–354 doi: 10.1007/978-1-4939-7357-6_19 [DOI] [PubMed] [Google Scholar]
- Soga M et al. (2015) HPGCD outperforms HPBCD as a potential treatment for Niemann-Pick disease type C during disease modeling with iPS cells Stem Cells 33:1075–1088 doi: 10.1002/stem.1917 [DOI] [PubMed] [Google Scholar]
- Soubannier V, Maussion G, Chaineau M, Sigutova V, Rouleau G, Durcan TM, Stifani S (2020) Characterization of human iPSC-derived astrocytes with potential for disease modeling and drug discovery Neurosci Lett 731:135028 doi: 10.1016/j.neulet.2020.135028 [DOI] [PubMed] [Google Scholar]
- Steadman JR, Rodkey WG, Briggs KK (2010) Microfracture: Its History and Experience of the Developing Surgeon Cartilage 1:78–86 doi: 10.1177/1947603510365533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafaleng EN et al. (2015) Induced pluripotent stem cells model personalized variations in liver disease resulting from alpha1-antitrypsin deficiency Hepatology 62:147–157 doi: 10.1002/hep.27753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells Cell Stem Cell 14:53–67 doi: 10.1016/j.stem.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Taguchi A, Nishinakamura R (2017) Higher-Order Kidney Organogenesis from Pluripotent Stem Cells Cell Stem Cell 21:730–746 e736 doi: 10.1016/j.stem.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Takasato M et al. (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis Nature 526:564–568 doi: 10.1038/nature15695 [DOI] [PubMed] [Google Scholar]
- Tian L, Prasad N, Jang YY (2016) In Vitro Modeling of Alcohol-Induced Liver Injury Using Human-Induced Pluripotent Stem Cells Methods Mol Biol 1353:271–283 doi: 10.1007/7651_2014_168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Ye Z, Kafka K, Stewart D, Anders R, Schwarz KB, Jang YY (2019) Biliary Atresia Relevant Human Induced Pluripotent Stem Cells Recapitulate Key Disease Features in a Dish J Pediatr Gastroenterol Nutr 68:56–63 doi: 10.1097/MPG.0000000000002187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vekilov PG (2007) Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia? Br J Haematol 139:173–184 doi: 10.1111/j.1365-2141.2007.06794.x [DOI] [PubMed] [Google Scholar]
- Walters MC et al. (2001) Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia Biol Blood Marrow Transplant 7:665–673 doi: 10.1053/bbmt.2001.v7.pm11787529 [DOI] [PubMed] [Google Scholar]
- Watson J (1948) The significance of the paucity of sickle cells in newborn Negro infants Am J Med Sci 215:419–423 doi: 10.1097/00000441-194804000-00008 [DOI] [PubMed] [Google Scholar]
- Williams TN, Thein SL (2018) Sickle Cell Anemia and Its Phenotypes Annu Rev Genomics Hum Genet 19:113–147 doi: 10.1146/annurev-genom-083117-021320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wragg NM, Burke L, Wilson SL (2019) A critical review of current progress in 3D kidney biomanufacturing: advances, challenges, and recommendations Renal Replacement Therapy 5:18 doi: 10.1186/s41100-019-0218-7 [DOI] [Google Scholar]
- Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD (2018) Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics Cell Stem Cell 23:869–881 e868 doi: 10.1016/j.stem.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S (2012) Induced pluripotent stem cells: past, present, and future Cell Stem Cell 10:678–684 doi: 10.1016/j.stem.2012.05.005 [DOI] [PubMed] [Google Scholar]
- Yamanaka S (2020) Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges Cell Stem Cell 27:523–531 doi: 10.1016/j.stem.2020.09.014 [DOI] [PubMed] [Google Scholar]
- Yamashita F, Sakakida K, Suzu F, Takai S (1985) The transplantation of an autogeneic osteochondral fragment for osteochondritis dissecans of the knee Clin Orthop Relat Res:43–50 [PubMed] [Google Scholar]
- Yi F, Qu J, Li M, Suzuki K, Kim NY, Liu GH, Belmonte JC (2012) Establishment of hepatic and neural differentiation platforms of Wilson’s disease specific induced pluripotent stem cells Protein Cell 3:855–863 doi: 10.1007/s13238-012-2064-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL et al. (2008) The ground state of embryonic stem cell self-renewal Nature 453:519–523 doi: 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Jonouchi T, Osafune K, Takita J, Sakurai H (2019) A Liver Model of Infantile-Onset Pompe Disease Using Patient-Specific Induced Pluripotent Stem Cells Front Cell Dev Biol 7:316 doi: 10.3389/fcell.2019.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y (2011) Immunogenicity of induced pluripotent stem cells Nature 474:212–215 doi: 10.1038/nature10135 [DOI] [PubMed] [Google Scholar]


