Abstract
Salmonella enterica serovar Typhimurium (S. Typhimurium) is one of the common causes of human colitis. In the present study, two lytic phages vB_SenS-EnJE1 and vB_SenS-EnJE6 were isolated and the therapeutic effect of the combination of phages and faecal microbiota transplantation (FMT) on S. Typhimurium-induced mouse colitis was investigated. The characteristics and genome analysis indicated that they are suitable phages for phage therapy. Results showed that vB_SenS-EnJE1 lysis 41/54 Salmonella strains of serotype O4, and vB_SenS-EnJE6 lysis 46/54 Salmonella strains of serotypes O4 and O9. Severe inflammatory symptoms and disruption of the intestinal barrier were observed in S. Typhimurium -induced colitis. Interestingly, compared with a single phage cocktail (Pc) or single FMT, the combination of Pc and FMT (PcFMT) completely removed S. Typhimurium after 72 h of treatment, and significantly improved pathological damage and restored the intestinal barrier. Furthermore, PcFMT effectively restored the intestinal microbial diversity, especially for Firmicutes/Bacteroidetes [predominantly bacterial phyla responsible for the production of short-chain fatty acids (SCFA)]. Additionally, we found that PcFMT treatment significantly increased the levels of SCFA. All these data indicated that the combination of phages and FMT possesses excellent therapeutic effects on S. Typhimurium -induced intestinal microbiota disorder diseases. Pc and FMT played roles in “eliminating pathogens” and “strengthening vital qi,” respectively. This study provides a new idea for the treatment of intestinal microbiota disorder diseases caused by specific bacterial infections.
Keywords: Salmonella enterica serovar Typhimurium, colitis, phage cocktail, FMT, SCFA
Introduction
Salmonella enterica serovar Typhimurium (S. Typhimurium) is one of most important pathogens causing enterocolitis in humans (Collaborators, 2017), which is characterised by the increased colonisation of S. Typhimurium in the intestine and the strong inflammatory response leading to colitis (Barthel et al., 2003). This foodborne pathogen causes a significant public health threat and considerable economic burden worldwide (Majowicz et al., 2010; Jajere, 2019).
Bacteriophage (phage) is a promising biocontrol strategy due to the high specificity to given bacterial species, and it thus shows minimal side effects to normal microbiota (Abedon, 2011; Alomari et al., 2021; Hassan et al., 2021). Several reports have shown the successful applications of phage therapy in animal models against different bacterial infections, such as Pseudomonas, Escherichia coli, Salmonella, and Enterococcus faecalis (Khan Mirzaei and Nilsson, 2015; Cheng et al., 2017; Waters et al., 2017; Tang et al., 2019). However, the emergence of phage-resistant variants has also frequently been reported in several animal models as well as in human pilot studies and case reports (Oechslin, 2018; McCallin et al., 2019). Phage cocktail could circumvent bacterial resistance more effectively than a single phage (McCallin et al., 2019; Pires et al., 2020). However, there are some studies in which bacteria cannot be completely removed even after the application of phage cocktail (Oliveira et al., 2010; Rivas et al., 2010). Additionally, most reports using antibiotics against Salmonella infections have led to intestinal microbiota disorders (Baumler and Sperandio, 2016) and a marked expansion of bacteria in the lumen of the murine intestine (Rivera-Chávez et al., 2016). However, phage treatment has no beneficial effect on the recovery of the intestinal microbiota. Therefore, phages combined with other therapeutic strategies that can restore the intestinal microbiota are being developed.
It has been reported that faecal microbiota transplantation (FMT) consists of the administration of faecal material into the recipient’s intestine to regulate the microbiota disorders and restore health. It has been used to treat recurrent Clostridium difficile infection for many years with a cure rate of 85–90% in patients (Van Nood et al., 2013; Lahtinen et al., 2017). Recently, it has been successfully used to alleviate mastitis caused by Staphylococcus aureus infection (Hu et al., 2020). In addition, FMT has shown potential for the intestinal decolonisation of extensively antimicrobial-resistant opportunistic pathogens, such as vancomycin-resistant Enterococci (Singh et al., 2014; Manges et al., 2016; Davido et al., 2017). However, even a balanced gut microbiota can only remove a small number of pathogens without specific bactericidal effects (Borewicz et al., 2015). Therefore, combining phage therapy and FMT may not only kills pathogens specifically, but also regulates the intestinal microbiota and removes pathogenic residues that phages fail to remove.
In this study, FMT was combined with phage cocktail to treat S. Typhimurium-induced colitis in mice. We found that the combination therapy completely removed S. Typhimurium and effectively reversed the symptoms of colitis, which was superior to single phages or single FMT treatment. Analysis of intestinal microbiota indicated that the combination of phage cocktail with FMT restored the intestinal microbial diversity and the short-chain fatty acids (SCFA) levels. In this combination therapy, phage cocktail and FMT played roles in “eliminating pathogens” and “strengthening vital qi,” respectively.
Materials and Methods
Animals
Female BALB/c mice (weights of 18–20 g) at 6–8 weeks old were purchased from Changchun Yisi Experimental Animals, Changchun, China. All of them were normally kept for a week to adapt to new surroundings. SPF grade mice breeding fodder and water were supplied ad libitum.
Bacterial Strains and Culture Conditions
The strains used in this study are listed in Supplementary Table 1. Salmonella strains were identified by universal primers (F: 5′-GTGGCGGACGGGTGAGTAA-3′, R: 3′-GTGTGACCTTGACTTCGTGCC-5′), serotyped by Salmonella antiserum (Ningbo Tianrun Biological Pharmaceutical Co., Ltd., Zhejiang, China), and cultured in Lysogeny broth (LB) (Becton, Dickinson and Company, United States) at 37°C shaker or on Salmonella-Shigella (SS) selective agar (Hopebio, China) at 37°C incubator. E. coli and Klebsiella pneumoniae strains were cultured in LB broth at 37°C shaker. S. aureus and E. faecalis were cultured in brain heart infusion (BHI) broth (Becton, Dickinson and Company, United States) at 37°C shaker.
Phage Isolation and Characteristics
Salmonella typhimurium 01E (NCBI no. SRR17717446) (Wang et al., 2019) was used as a host to isolate phages from sewage samples collected from the Changchun sewage system and yak faecal samples collected from Tibet, China (Gong et al., 2016). Yak faecal samples were soaked overnight in 10 times the volume of SM buffer before use. Briefly, 1 mL of S. typhimurium 01E was mixed with 100 mL of LB broth prepared with the cotton-filtered sewage samples and cotton-filtered supernatant of yak faeces samples. The mixture was overnight cultured at 37°C shaker and then centrifuged (10,000 × g for 10 min) and the supernatant was filtered through 0.22 μm pore size filter. Phages in the filtrate were screened by the spot assay and the double-layer agar method (Chang et al., 2005; Gu et al., 2010). Firstly, the spot assay was performed to screen the phage, 100 μL of S. typhimurium 01E was spread on 1.5% LB agar plates to prepare the lawns and 10 μL of filtrate was dropped on the lawns and incubated at 37°C for 12 h. The phage was purified by the double layer agar method, the filtrate was mixed with S. typhimurium 01E in equal volume, and the mixture was added to 0.75% LB top agar and poured onto 1.5% LB agar plates, then the plates were incubated at 37°C for 12 h. A single plaque was picked up and purified by the double-layer agar method for three times. The purified phage was stored at 4 or −80°C (mixed with 30% glycerol).
Phage morphology was observed as previously described (Kutateladze and Adamia, 2008). Briefly, the phage was amplified (800 mL) and centrifuged (8,000 × g, 10 min, 4°C) to remove bacterial debris. Ten percent polyethylene glycol 8,000 and 1 M NaCl were added to the supernatant, incubated in an ice bath overnight and then centrifuged (12,000 × g, 20 min, 4°C). The supernatant was removed, and the phage precipitate was resuspended in 2 mL of SM buffer [50 mM Tris HCl, pH 7.5, 0.1 M NaCl, 8 mM MgSO4, 0.01% (wt/vol) gelatine]. The concentrated phage was extracted with chloroform. Then, 15 μL of concentrated phage sample was dropped onto 200-mesh copper grids and negatively stained with phosphotungstic acid (2%, w/v). Transmission electron microscopy with an accelerating voltage of 80 kV (HEOL JEM-1200EXII; Japan Electronics and Optics Laboratory, Tokyo, Japan) was used to observe the phage morphology.
The host range of the phage was determined by the double- layer agar plate method (Gu et al., 2010), and the relative efficiency of plating (EOP) was determined, which was expressed as the ratio between the phage titre of the tested strains and the titre of the propagating host strain (Kutter, 2009). All the bacterial strains used to determine the host range of the phages are listed in Supplementary Table 1.
To analyse the thermal stability of phage, phage was incubated at different temperatures (30, 40, 50, 60, 70, and 80°C) for 40 or 80 min. The titre of the phage in the samples before and after incubation was detected by the double-layer agar plate method (Gu et al., 2010).
To analyse the pH stability of the phage, 100 μL of phage was added to 900 μL of SM buffer, which was adjusted to different pH values (2, 4, 6, 8, 10, 12, and 13) and incubated at 37°C for 1 h. The titre of the phage in the samples before and after incubation was detected by the double-layer agar plate method (Gu et al., 2010).
The phage one-step growth curve was determined as previously described (Spellberg et al., 2012). The bacteria (108 CFU/mL) were mixed with phage (107 PFU/mL) for incubating for 10 min, and the mixture was centrifuged (8,500 × g, 5 min, 4°C). Then, the precipitate was resuspended in 10 mL of LB broth and cultured at 37°C with shaking at 165 rpm. The 200 μL sample was collected at different time points, and the phage titre in the samples was determined by the double-layer agar plate method (Gu et al., 2010). Burst size was calculated as the ratio of the final number of progeny phage particles to the number of infected bacterial cells (Sritha and Bhat, 2018).
Phage Genome Sequencing and Analysis
The phage genome was extracted by a virus genome extraction kit (Omega B IO-Tek Inc., Doraville, GA, United States). The whole phage genome was sequenced using the Illumina HiSeq2500 platform at Wuhan GENEWIZ Biotechnology Co., Ltd. (Szabadosova et al., 2018). Genes and predicted open reading frames (ORFs) were assembled using Spades version 3.6.2 with 1,000 times coverage and Gene Marks version 3.6.2, respectively (Besemer et al., 2001; Bankevich et al., 2012). The presence of tRNA was detected by a transcanner.1 CLC Genomics Workbench 8.1 (CLC Bio-Qiagen, Aarhus, Denmark) was used to visualise the functional models of the genome (Kim et al., 2013). MEGA version 7.0.26 (Hall, 2013) was used to analyse the evolutionary relationship between isolated phages and reported phages based on the amino acid sequence of the terminal enzyme large subunits (Casjens et al., 2005).
Bactericidal Effect of Phages in vitro
Pc was established by mixing vB_SenS-EnJE1 (NCBI no. MN336264) and vB_SenS-EnJE6 (NCBI no. MN336265) at a ratio of 1:1, and the titre of each phage was 109 PFU/mL. The bactericidal abilities of a single phage and Pc in vitro were analysed as previously described (Pajunen et al., 2000).
The Stability of Phages in the Mouse Gastrointestinal Tract
The stability of phages in the mouse gastrointestinal tract was determined in vitro as previously described (Bosak et al., 2018). Three mice were euthanised, and the contents of the stomach, ileum, cecum and colon of each mouse were collected, homogenised and centrifuged (14,000 × g, 1 min, 4°C). The supernatant was mixed with the phage (volume 1:1), and the mixtures were incubated at 37°C. Samples were collected at 10, 30, 60, and 90 min after incubation. The phage titre in the samples was determined by the double-layer agar plate method (Gu et al., 2010).
Animal Experiment
Salmonella typhimurium 01E was used to establish the mouse colitis model (Stecher et al., 2006). Briefly, mice were orally administered 20 mg of streptomycin (Hopebio, China) after fasting for 4 h. Then, all mice were supplied with water immediately, and food was supplied ad libitum 2 h later. After streptomycin treatment for 20 h, the mice were fasted for 4 h and then orally administered 0.2 mL of S. typhimurium 01E (4 × 108 CFU/mouse).
To evaluate the therapeutic effect of the phages, 0.2 mL of 2 × 108 PFU/mouse of a single phage or Pc was orally administered to the mice at 3 h after S. Typhimurium infection. As a control, PBS was orally administered to the mouse colitis model.
FMT treatment was performed as previously described (Jacobson et al., 2018). Briefly, fresh faecal pellets were collected from healthy donor mice and homogenised in PBS (2 pellets/mL). Then, the faecal suspensions were immediately centrifuged (500 × g, 5 min, 4°C). The supernatants (200 μL/mouse) were gavaged for the first time to mice at 6 h after S. Typhimurium infection, and then, the mice continued to receive FMT at 12, 24, and 36 h after S. Typhimurium infection.
To evaluate the therapeutic effect of the combination of Pc and FMT (abbreviated as PcFMT), FMT was performed for the first time at 3 h after a single dose of Pc (0.2 mL, 2 × 108 PFU/mouse, at 3 h after infection), and then, the mice continued to receive FMT at 12, 24, and 36 h after S. Typhimurium infection. Mice in the control group were gavaged with an equal volume of PBS to replace FMT.
Disease Activity Index (DAI)
The disease activity index (DAI) of the mice was scored for seven consecutive days using three parameters, including body weight loss, stool consistency and rectal bleeding, as previously described (Zhang et al., 2015). The DAI of different treatment groups was monitored for seven consecutive days. (i) Body weight loss (0, none; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4, >15%), (ii) stool consistency (0, normal; 1, pasty and not adhering to the anus; 2, semiformed adhered to the anus; 3, pasty and adhered to the anus; 4, watery), and (iii) rectal bleeding (0, no blood (–); 1, blood (±); 2, blood (+); 3, blood (++); 4, obvious blood in stool).
Histopathological Analysis
The colonic tissues of the mice in different treatment groups were collected 48 h after S. Typhimurium infection, fixed in 4% paraformaldehyde for 48 h, and then embedded in paraffin for next-step sectioning and H&E (haematoxylin and eosin) staining. The histological score was determined by a histopathological scoring scheme as previously described (Stecher et al., 2006).
Bacterial Loads in the Colon
At indicated times after infection, mice from different groups were sacrificed. The colonic tissues of the mice were removed. The length of the colon was measured, and the colonic tissues were homogenised in 1 mL of PBS buffer. The bacterial loads of S. Typhimurium were determined by plating on SS agar plates, and the phage titres were detected by the double-layer agar plate method. The limit of detection (LOD) of bacteria was 10 CFU per organ.
Enzyme-Linked Immunosorbent Assay
The colonic tissues of mice in different treatment groups were collected 48 h after S. typhimurium infection, homogenised in 1 mL of PBS buffer, and centrifuged (10,000 × g for 10 min, 4°C). The levels of TNF-α, IL-1β, IL-6, the MPO, and EPO in the supernatant of the colon tissues and the IgA in the serum were measured using Enzyme-linked immunosorbent assay (ELISA) kits (Biolegend, United States) (Strath et al., 1985; Souza et al., 2002; Zhang et al., 2020).
RT-PCR
Total RNA of colonic tissues was extracted by TRIzol reagent (Invitrogen, United States) and reverse transcribed to cDNA using a PrimeScript RT reagent Kit (Takara, China). The RT-PCR for each gene was repeated in triplicate using SYBR Premix Ex Taq II (Takara, China). The relative expression of SCFA uptake transporters SMCT1 and MCT1, SCFA signalling genes HDAC and GPR43, intestine physical barrier-involved genes Mucin 1 and Mucin 2, and epithelial tight junction related genes (Occludin, Claudin-3 and ZO-1) were calculated by the 2–ΔΔCT method (Livak and Schmittgen, 2001) and normalised according to the expression of β-actin and GAPDH. The primer sequences used in RT-PCR experiment are listed in Supplementary Table 2.
Intestinal Microbiota
Fresh faece from each sample was collected and frozen in liquid nitrogen immediately and then stored at −80°C. The total DNA of all samples was extracted using E.Z.N. ATM Mag-Bind Soil DNA Kit (OMEGA, United Kingdom). The 16SV3-V4 region in the 16S rRNA gene was amplified using specific primers (Nobar_341F-805R). Paired-end (PE) reads (250 bp) were generated using the Illumina Hiseq2500 platform. Then, PE reads were merged using PEAR 0.9.8. High-quality clean tags were obtained and clustered into distinct operational taxonomic units (OTUs) by Usearch software with 97% sequence identity. OTUs were further analysed using the RDP classifier against a curated database derived from the RDP database. Alpha diversity, UniFrac beta diversity and principal coordinate analysis (PCoA) were calculated using QIIME. OTUs were also used for determining the differences in taxonomic abundance with group_significance.py.
Short-Chain Fatty Acids
The supernatants of fresh faeces were collected (10,000 × g, 15 min), immediately frozen and stored at −80°C. The concentrations of SCFA, including acetate, butyrate, and propionate, were measured by a gas chromatographic system (Agilent 7890A/5975, United States).
Statistical Analysis
Statistical analyses of experimental data were performed by SPSS version 13.0 (SPSS, Inc., Chicago, IL, United States), analysed using one-way analysis of variance (ANOVA) and expressed as the means ± SEM. P < 0.05 was considered statistically significant. ∗P < 0.05; ∗∗P < 0.01; and ∗∗∗P < 0.001.
Results
Characteristics of the Phages
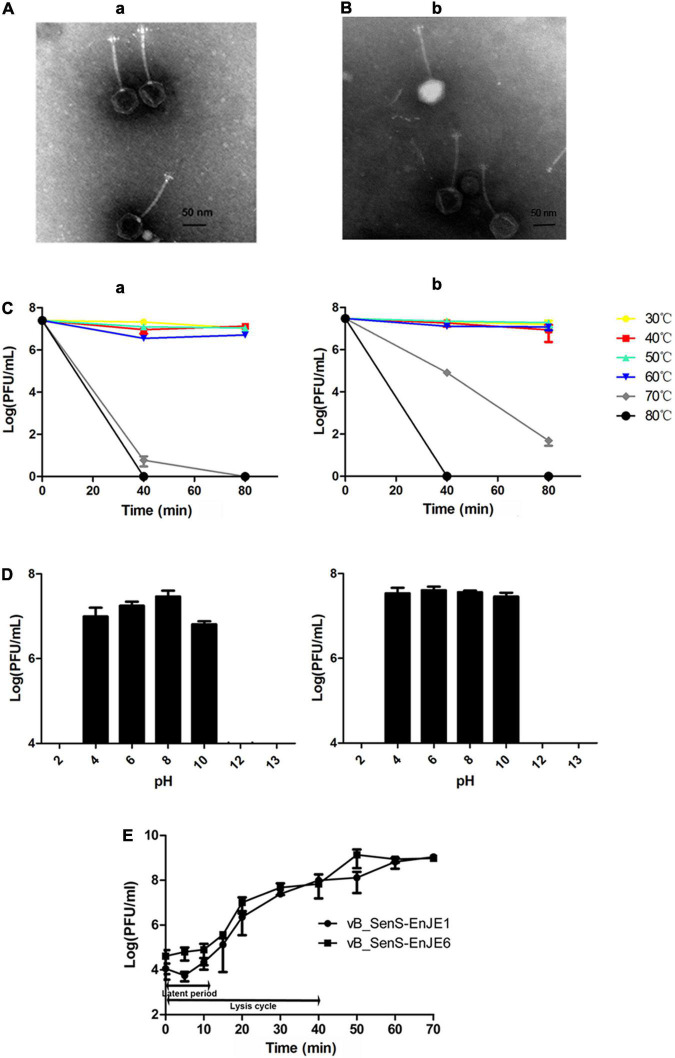
In this study, two S. Typhimurium phages were isolated and named vB_SenS-EnJE1 (NCBI no. MN336264) and vB_SenS-EnJE6 (NCBI no. MN336265). TEM showed that vB_SenS-EnJE1 and vB_SenS-EnJE6 belong to the Siphoviridae family, which consists of an icosahedral head with a diameter of approximately 60 ± 5 nm, 60.5 ± 5 and a non-contractile tail with a length of 115 ± 5 nm, 114 ± 5, respectively (Figures 1A,B). The phage titres of vB_SenS-EnJE1 and vB_SenS-EnJE6 changed little from 30 to 60°C (Figure 1C) and pH 4-10 (Figure 1D), indicating that both of them have good temperature and pH stability. vB_SenS-EnJE6 showed a better tolerance to high temperature (70°C) and extreme pH conditions (pH 10) than vB_SenS-EnJE1 (Figures 1C,D). The one-step curves of vB_SenS-EnJE1 and vB_SenS-EnJE6 showed similar latent periods (10 min) and lysis cycles (40 min). In vB_SenS-EnJE1, there was a 5-min eclipse period during which fewer phages could be detected (Figure 1E). The burst sizes of vB_SenS-EnJE1 and vB_SenS-EnJE6 were 88 PFU/cell and 97 PFU/cell, respectively (Figure 1E).
FIGURE 1.
Biological features of phages. Electron microscopy images of vB_SenS-EnJE1 (A) and vB_SenS-EnJE6 (B). The bar represents 50 nm. (C) Thermal stability of vB_SenS-EnJE1 (a) and vB_SenS-EnJE6 (b). Phage particles were incubated under different temperature conditions, and then, the samples were collected at 40 and 80 min after incubation. (D) pH stability of vB_SenS-EnJE1 (a) and vB_SenS-EnJE6 (b). Phage particles were collected after 1 h of incubation under different pH conditions. The titres of the phages at different pH values and thermal conditions were determined by the double layer agar method. (E) One-step curves of the two phages at MOI = 0.1. All experiments were repeated three times.
Host Range
The double layer agar results (Supplementary Table 1) showed vB_SenS-EnJE1 lysis 41/54 strains of Salmonella, all of which belong to the O4 serotype. vB_SenS-EnJE6 showed lytic activity against 46/54 Salmonella strains, which included the O4 and O9 serotypes (associated with most foodborne illnesses). There were 4 strains (S. typhimurium181003, 1811004, 1811007, and S. dublin SD) that only can be lysed by vB_SenS-EnJE6. Additionally, vB_SenS-EnJE1 can lyse Salmonella strains DE3, Yuch1, Yuch3 and 202035 more efficiently (EOP were higher) than vB_SenS-EnJE6. Otherwise, vB_SenS-EnJE6 showed stronger lytic activity (EOP were higher) than vB_SenS-EnJE1 in lysing strains wudi2, 0078, 01D3, 181008, 181011, and 181017. Additionally, neither vB_SenS-EnJE1 nor vB_SenS-EnJE6 has lysis activity against other species, which showed their high specificity for Salmonella species.
Genomic Characteristics
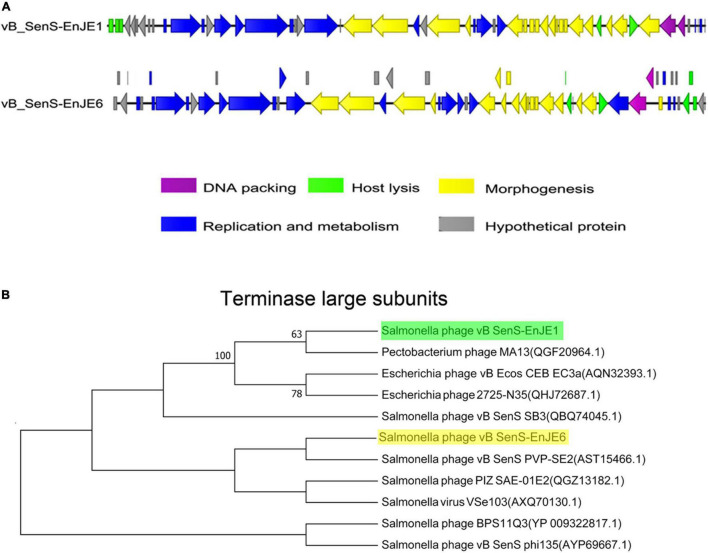
vB_SenS-EnJE1 is a linear double-stranded DNA virus composed of 42 894 bp with a GC content of 49.76% (Supplementary Table 3 and Figure 2A). A total of 54 ORFs were predicted, 25.92% were annotated as hypothetical proteins, and one of them showed no significant similarity. vB_SenS-EnJE6 is also a linear double-stranded DNA virus that is 43 129 bp in length and had an average GC content of 49.57%. Its genome carries 69 ORFs, 31.88% of which were annotated as hypothetical proteins (Supplementary Table 4 and Figure 2A). The ORFs of the two phages are shown in Supplementary Tables 3, 4. No lysogen-related genes, virulence factor genes or antibiotic resistance genes were found in vB_SenS-EnJE1 or vB_SenS-EnJE6.
FIGURE 2.
Genome characterisation of phages. (A) Comparison analysis of the genomic organisation of vB_SenS-EnJE1 and vB_SenS-EnJE6. CLC workbench 8.1 was employed to visualise the putative CDSs and their transcript direction. The different colours represent different functional clusters. (B) Neighbour-joining tree of vB_SenS-EnJE1 and vB_SenS-EnJE6. The evolutionary relationship between isolated phages and reported phages was analysed based on the amino acid sequence of the terminal enzyme large subunits.
BLAST analysis of their genome against the NCBI on the redundant DNA database was performed. The genome of vB_SenS-EnJE1 was about 94% homogenous to three Salmonella phages BPS11Q3 (Accession number: KX405002.1), BPS11T2 (Accession number: MG646668.1), and vB_SpuS_Sp4 (Accession number: MH358359.1) and a Xanthomonas phage f29-Xaj (Accession number: KU595434). The vB_SenS-EnJE6 genome showed 94.60% similarity with Salmonella SE2 (Accession number: NC_016763.1), about 93% similarity to vB_SenS-Ent1, 2 (Accession number: HE775250.1 and HG934469.1), Salmonella phage wksl3 (Accession number: JX202565.1), BPS11Q3 and BPS11T2. Additionally, the nucleotide sequences of vB_SenS-EnJE1 and vB_SenS-EnJE6 showed 95% identity (coverage of 92%) with each other.
Phylogenetic tree analysis showed that vB_SenS-EnJE1 and vB_SenS-EnJE6 belong to the sister clade of reported Pectobacterium phage M13 and Salmonella phage vB_SenS PVP-SE2, respectively. However, they are at different levels of development (Figure 2B).
The in vitro Bactericidal Effect of the Single Phage and Pc
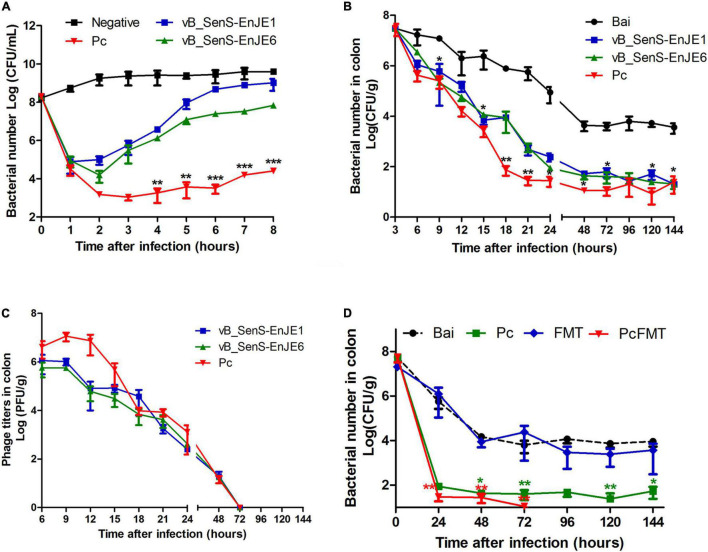
Before the application of the single phage and Pc in vivo, their antibacterial activity against S. Typhimurium in vitro was determined. From Figure 3A, the number of bacteria in the negative control group increased slowly with time from 108 CFU/mL to 1010 CFU/mL. The number of bacteria in the single phage treatment group decreased rapidly in the first 1h (vB_SenS-EnJE1) or 2 h (vB_SenS-EnJE6) and then increased rapidly (reaching ≥ 108 CFU/mL at 8 h after treatment). The number of bacteria in the Pc treatment group decreased rapidly (approximately as low as 103 CFU/mL) in the first 2 h and then increased slowly (reaching approximately 104 CFU/mL at 8 h). This shows that Pc can kill Salmonella more effectively and delay the emergence of resistant bacteria.
FIGURE 3.
Pc combined with FMT completely removed S. Typhimurium in vivo. (A) The antibacterial effect of single phage and Pc on S. Typhimurium in vitro at MOI = 0.1. Each independent experiment was repeated three times. The bacterial loads (B) and phage titres (C) in the colonic tissues of healthy, bacterially infected, single phage-treated and Pc-treated mice. (D) The bacterial loads in the colonic tissues of the bacterial-infected, single Pc-treated, and PcFMT-treated mice. Data are expressed as the means ± SEM, n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Therapeutic Effect of the Pc in vivo
First, the stability of the single phage and Pc under gastrointestinal conditions was detected. We found that the phage titres were not significantly changed after the single phage or Pc was incubated with the contents of the stomach, ileum, caecum and colon at 37°C for 90 min (Supplementary Figure 1), which showed good stability under gastrointestinal conditions.
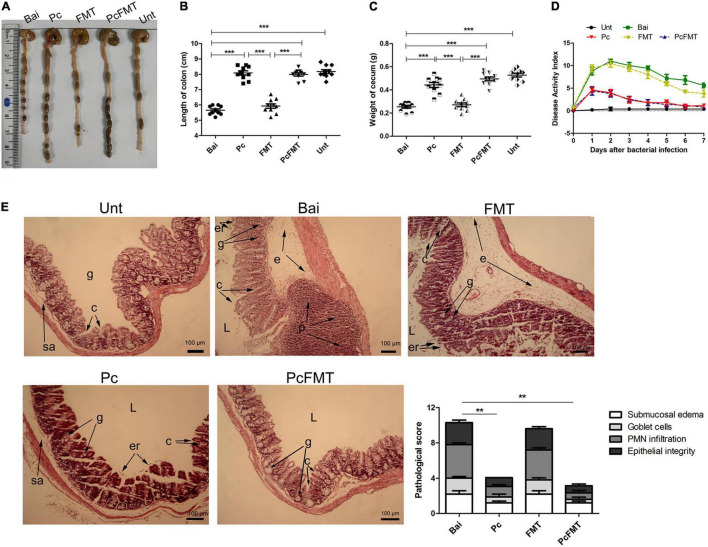
The mouse colitis model was successfully obtained when S. typhimurium 01E (0.2 mL, 4 × 108 CFU/mouse) was administered by gavage to the mice. In this model, the length of the colon was significantly shortened (Figures 4A,B). The weight of the caecum was significantly decreased (Figure 4C). The DAI score was increased (Figure 4D). At 24 h after S. Typhimurium infection, the bacterial load in the colonic tissues of non-treated mice was approximately 104 CFU/g. The Pc treatment group more effectively reduced the Salmonella load (<102 CFU/g) of the mouse colon than a single phage (P < 0.05). However, the Salmonella remained detectable in colonic tissues of single phage- or Pc-treated mice for 144 h (Figure 3B). In addition, the phage titre in the colonic tissues of the Pc treatment group showed transient proliferation in the first 3 h after gavage and then decreased gradually (Figure 3C). In the colon tissues of mice in the single phage treatment group, there was no significant proliferation of phages, and the titre of phages began to decrease after 3 h. No phage was detected in either the single phage or Pc treatment group at 72 h after gavage. However, Pc treatment can alleviate the severe pathological damage observed in the colon tissue (Figures 4A,E), and recover the transcription levels of the epithelial tight junction related genes (Claudin-3, Occludin and ZO-1) and epithelial mucus genes (Mucin 1 and Mucin 2) (Figures 5A,B), and restore the contents of inflammatory cytokines (IL-6, IL-1β and TNF-α), MPO and EPO in colonic tissues, and the IgA in blood (P < 0.01) (Supplementary Figure 2). All these results indicated that although Pc showed better bactericidal activity and anti-inflammatory activity in vivo, Pc could not completely remove Salmonella from the colonic tissues of infected mice.
FIGURE 4.
PcFMT treatment effectively improved mouse colitis caused by S. typhimurium. The colonic tissues were collected from healthy, bacterial-infected, single-dose Pc-treated, FMT-treated, and PcFMT-treated mice 48 h after S. Typhimurium (0.2 mL, 4 × 108 CFU/mouse) infection. “Unt” represents untreated healthy mice group; “Bai” represents the bacterial-infected mice group; “Pc” means phage cocktail-treated mice group; “PcFMT” represents the combined phage cocktail and FMT mice group. (A) Macroscopic pathological changes in the colon and caecum. The length of the colon (B) and the weight of the caecum (C) (n = 6) from untreated, bacterially infected, single-dose Pc-treated, FMT-treated, and PcFMT-treated mice were measured at 48 h post-infection. (D) Disease activity index (DAI). (E) H&E staining. Colonic tissue was stained with H&E (100×). L, intestinal lumen; e, edema; p, PMN; er, erosion of the epithelial layer; c, crypt; g, goblet cell; sa, submucosa. Magnifications are indicated by the black bars. Data are expressed as the means ± SEM, n = 6. **P < 0.01; ***P < 0.001.
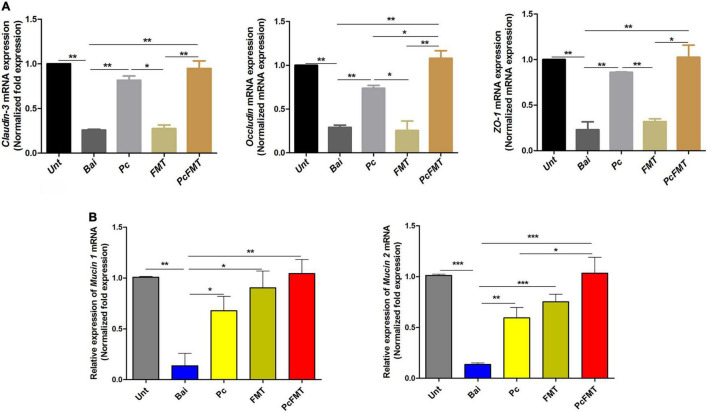
FIGURE 5.
Therapeutic effect of PcFMT on mouse colitis caused by S. Typhimurium. The colonic tissues were collected from healthy, bacterial-infected, single-dose Pc-treated, FMT-treated, and PcFMT-treated mice 48 h after S. Typhimurium (0.2 mL, 4 × 108 CFU/mouse) infection. “Unt” represents untreated healthy mice group; “Bai” represents the bacterial-infected mice group; “Pc” means phage cocktail-treated mice group; “PcFMT” represents the combined phage cocktail and FMT mice group. (A) The transcription levels of the tight junction proteins Occludin, Claudin-3 and ZO-1. (B) The transcription levels of epithelial mucus Mucin 1 and Mucin 2 in colonic tissues. Data are the means ± SEM, n = 6. *P < 0.05; **P < 0.01; ***P < 0.001 indicate a significant difference.
Therapeutic Effect of PcFMT on Mouse Colitis Caused by S. Typhimurium
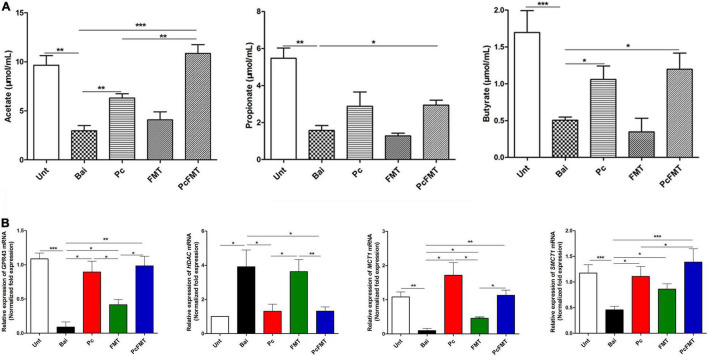
The therapeutic effect of PcFMT on colitis mice caused by S. Typhimurium was further studied. PcFMT completely removed Salmonella on the 3rd day (Figure 3D). The length of the colon, the weight of the caecum and DAI score were restored (Figures 4A–D). The pathological damage to the colonic tissues (Figure 4E) and intestinal physical barrier (Figures 5A,B) was repaired. The levels of inflammatory cytokines (IL-6, IL-1β, and TNF-α), MPO and EPO in colonic tissue were significantly reduced. The levels of IgA in blood were also significantly reduced (P < 0.05) (Supplementary Figure 2). In addition, compared with the non-treatment group after bacterial infection, the SCFA levels in the faeces of mice in the PcFMT treatment groups were significantly increased, especially the acetate content (P < 0.001) (Figure 6A). the transcription level of SCFA metabolism-related genes (GPR43, MCT1, and MSCT1) was also increased (P < 0.05). At the same time, the transcription level of the SCFA metabolism gene HDAC was significantly decreased (P < 0.05) after PcFMT treatment (Figure 6B). Compared with Pc treatment, PcFMT treatment significantly increased the transcription levels of the intestinal barrier-related genes Occludin and Mucin 2 and the SCFA metabolism-related gene MSCT1 (P < 0.05) (Figures 5A,B, 6B), and the acetate content also increased (P < 0.01) (Figure 6A). Compared with Pc treatment, PcFMT treatment is more conducive to the recovery of the intestinal barrier and the metabolism of SCFA.
FIGURE 6.
SCFA concentrations and the relative expression of SCFA metabolism related genes. The colonic tissues and faeces were collected from healthy, bacterial-infected, single-dose Pc-treated, FMT-treated, and PcFMT-treated mice 48 h after S. Typhimurium (0.2 mL, 4 × 108 CFU/mouse) infection. (A) SCFA contents (acetate, propionate, butyrate) in the faeces of healthy, bacteria-infected, single-dose Pc-treated, FMT-treated, and PcFMT-treated mice. (B) The transcription levels of the SCFA transporters SMCT1 and MCT1 and the SCFA receptors HDAC and GPR43 in the colonic tissues of different treatment groups. The relative expression was calculated as the ratio of the target gene to the internal reference genes (β-actin and GAPDH). Healthy mice were used as controls. Data are expressed as the means ± SEM, n = 6. *P < 0.05; **P < 0.01; ***P < 0.001.
Effect of PcFMT Treatment on Intestinal Microbiota
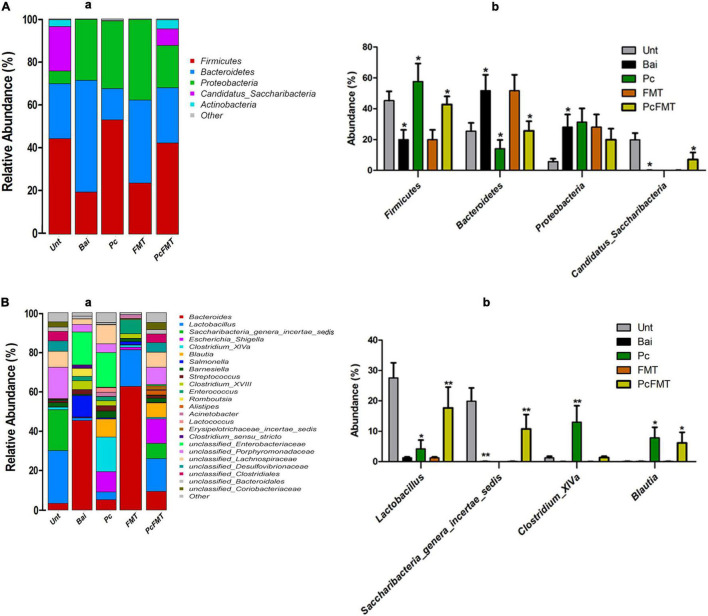
To investigate the change of intestinal microbiota after Pc and PcFMT treatment, the species richness and diversity of the intestinal bacterial community of each sample were analysed. Pc or FMT alone had no significant effect on the intestinal microbiota of healthy mice (Supplementary Figure 3). Compared with healthy mice, the intestinal microbiota structure of the mice in the non-treatment group after bacterial infection was significantly destroyed (Supplementary Figures 4A,B). Compared with the non-treatment bacterial infection group, the structure of the intestinal microbiota in the Pc treatment group was partially restored, while that in the PcFMT treatment group was restored to be close to that in the healthy mouse group (Supplementary Figures 4A,B). The results of bacterial phylum classification showed that compared with the intestinal microbiota of healthy mice, the relative abundance of Bacteroidetes and Proteobacteria in the intestinal microbiota of untreated mice after bacterial infection increased significantly (P < 0.05), while the relative abundance of Firmicutes and Candidatus_Saccharibacteria decreased significantly (P < 0.05) (Figure 7A). The proportion of Firmicutes and Bacteroidetes in the intestinal tracts of infected mice was partially restored in the Pc treatment group (Figure 7A), while the microbial community structure in the intestinal microbiota of mice in the PcFMT treatment group was restored close to that of healthy mice. At the genus level, the relative abundance of Lactobacillus and Saccharibacteria_genera_incertae_sedis in the intestinal microbiota of bacterial infection mice without treatment was significantly lower than that in the intestinal microbiota of healthy mice (P < 0.01), while PcFMT treatment significantly increased the abundance of Lactobacillus (P < 0.01) (Figure 7B).
FIGURE 7.
PcFMT recovered the microbiota community structures of S. Typhimurium-induced colitis mice. The microbiota of fresh faeces collected from healthy, bacteria-infected, single-dose Pc-treated, FMT-treated, and PcFMT-treated mice were detected by amplifying the V3–V4 region of the 16S rRNA gene using specific primers (Nobar_341F-805R). Relative abundance of predominant bacteria at the phylum (A) level and genus (B). “Unt” represents the untreated healthy mice group; “Bai” represents the bacterial-infected mice group; “Pc” means the phage cocktail-treated mice group; “PcFMT” represents the combined phage cocktail and FMT mice group. Data are expressed as the means ± SEM, n = 6. *P < 0.05; **P < 0.01.
Discussion
Salmonella enterica serovar Typhimurium is a common pathogen that causes invasive intestinal bacterial diseases, such as colitis (Herp et al., 2019). Initially, in this study, we intended to treat the mouse colitis model caused by S. Typhimurium using phage therapy. Usually, phage show strong specificity in infecting and killing bacteria. On the one hand, this feature can ensure the safety of phage application without damaging the normal intestinal microbiota of the body. However, at the same time, this feature also limits the application of phage when the antibacterial spectrum is too narrow. Moreover, when only one phage is used, the treated bacteria can quickly produce resistant strains against this phage. Fortunately, Pc can not only maintain specificity for specific pathogens but also broaden the antibacterial spectrum and delay the production of resistant strains (Tanji et al., 2004; Gu et al., 2012; Pires et al., 2020). Therefore, a Pc composed of two phages was used for treatment. In the early stage of treatment, the loads of S. Typhimurium in the colon of the colitis model were effectively reduced by Pc, but they could not be completely removed in the later stage. In addition, the analysis of intestinal microbiota showed that Pc treatment had a positive effect on the recovery of intestinal microbiota and reduced the ratio of Bacteroides and Firmicutes, indicating that colitis was alleviated (Wang et al., 2017; Zhang et al., 2018). This may be because most S. Typhimurium were killed, which indirectly restored the intestinal microbiota to a certain extent (Hsu et al., 2019). However, the diversity of the intestinal microbiota cannot be completely restored, so S. Typhimurium can be colonised for a long time. At the same time, the persistence of S. Typhimurium further hinders the recovery of the microbiota (Borewicz et al., 2015).
Antibiotics are the most mainstream treatment therapy for intestinal pathogen infection. However, antibiotics easily cause intestinal microbial disorders (Rivera-Chávez et al., 2016). Moreover, the prevalence of drug-resistant pathogens also greatly reduces the effect of antibiotic therapy (Dodds, 2017). Many studies have shown that FMT is effective in preventing intestinal inflammation caused by chemical substances but not pathogenic bacteria (Wang et al., 2014; Kang et al., 2019). In addition, some case studies have shown that FMT has a good effect in the treatment of human intestinal infections caused by pathogenic bacteria on the premise that antibiotics and other therapies are ineffective (Cohen and Maharshak, 2017). The pathogens in these clinical cases are mainly antibiotic-resistant bacteria, including ESBL-producing and carbapenemase-producing Enterobacteriaceae, vancomycin-resistant Enterococci, and methicillin-resistant S. aureus (Singh et al., 2014; Manges et al., 2016; Davido et al., 2017). Moreover, these bacteria exist in the intestine at a low level, which might be the main reason for the remarkable therapeutic effect (Lahtinen et al., 2017). We used the established mouse colitis model caused by S. Typhimurium to evaluate the therapeutic effect of FMT. Unfortunately, the therapeutic effect of FMT on this model was not significant. The loads of S. Typhimurium in the colon tissues did not decrease significantly, inflammatory injury was not improved, and the intestinal microbiota did not recover significantly. This may be because FMT was used for treatment rather than prevention in this study, and our treatment time (6, 12, 24, 36 h after infection, observed until 48 h) was shorter than the reported preventive application of FMT (1 time/24 h before infection, at least 1 week) (Jacobson et al., 2018; Hu et al., 2020). In addition, FMT was not able to exert a strong antibacterial effect on S. Typhimurium and was not able to effectively reduce the level of inflammation. Inflammation is the main driver of intestinal microbial disorders. Single FMT treatment might only have a transient effect during severe intestinal inflammation and might even produce potential side effects, such as microbial translocation (Winter and Bäumler, 2014; Pigneur and Sokol, 2016).
Could the combination of Pc and FMT have an ideal therapeutic effect? In vivo results showed that PcFMT treatment completely eliminated S. Typhimurium, significantly alleviated colitis symptoms, restored the intestinal physical barrier, and increased the expression of Mucin 1 and Mucin 2. Increased expression of Mucin 2 has been reported to promote mucus secretion, which contributes to rapid repair of the intestinal mucosa and elimination of foreign pathogens (Birchenough et al., 2015; Mccauley and Guasch, 2015). Additionally, the content of SCFAs, especially acetate after PcFMT treatment were increased significantly. It has been reported that FMT can improve inflammatory diseases caused by bacterial infection by releasing SCFAs (Hu et al., 2020). So, the good treatment effect of PcFMT in vivo may be related to SCFAs. Additionally, compared with the bacterial infection group, PcFMT restored the microbial microbiota diversity. It has been reported the balance intestinal microbiota can clear pathogens better (Borewicz et al., 2015). In addition, PcFMT significantly increased the abundance of Lactobacillus, Saccharibateria_genera_incertae_sedis, and Blautia. Especially for the increase in Lactobacillus. It has been reported that Lactobacillus can effectively reduce the inflammatory level of colitis in mice (Mao et al., 1996). Therefore, the good treatment effect of PcFMT in vivo may also be related to the prebiotic effect of increased Lactobacillus (Mu et al., 2018).
In conclusion, we found that the combination of Pc and FMT can effectively treat colitis in severe inflammation caused by S. Typhimurium. In this combination therapy, phage plays the role of “eliminating pathogen,” that is, specifically killing specific pathogens; FMT mainly plays the role of “strengthening vital qi,” that is, helping to restore the intestinal barrier and microbiota structure and assisting phages in removing S. Typhimurium. Because the components of FMT are complex and may even have adverse components (Tan and Johnson, 2019), SCFA or Lactobacillus may be directly used to replace FMT and combined with phages of specific pathogens to treat intestinal inflammation caused by corresponding pathogens, and this speculation will be further explored in our follow-up research. This study provides a new idea for the treatment of intestinal inflammatory diseases caused by specific bacterial infections.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Jilin University.
Author Contributions
JG and WH conceived the idea and designed the experiment. XW, YX, and YJ carried out the animal experiments. HX and XL performed the bacteriological experiments. XW and YJ analysed the data. LL carried out the bioinformatics analysis. XW and YX wrote the manuscript. JG and WH supervised the study and reviewed the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Jianbao Dong (Liaocheng University, Liaocheng, China), and Yuqing Liu (Shandong Academy of Agricultural Sciences, Jinan, China) for generously providing the Salmonella strains.
Footnotes
Funding
This work was financially supported through grants from the National Natural Science Foundation of China (grant nos. U19A2038, 31872505, and 32072824), the Natural Science Foundation of Jilin Province (grant no. 20200201120JC), the Fundamental Research Funds for the Central Universities, the Shandong Provincial Modern Agricultural Industry Technology System (SDAIT-27), and the Key Research and Development Plan of Shandong Province (2021TZXD012).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.944495/full#supplementary-material
References
- Abedon S. T. (2011). Lysis from without. Bacteriophage 1 46–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alomari M. M. M., Dec M., Urban-Chmiel R. (2021). Bacteriophages as an Alternative Method for Control of Zoonotic and Foodborne Pathogens. Viruses 2021:13. 10.3390/v13122348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A. A., Dvorkin M., Kulikov A. S., et al. (2012). SPAdes: a New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 19 455–477. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel M., Hapfelmeier S., Quintanilla-Martinez L., Kremer M., Rohde M., Hogardt M., et al. (2003). Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71 2839–2858. 10.1128/IAI.71.5.2839-2858.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumler A. J., Sperandio V. (2016). Interactions between the microbiota and pathogenic bacteria in the gut. Nature 535 85–93. 10.1038/nature18849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besemer J., Lomsadze A., Borodovsky M. (2001). GeneMarkS: a self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 29 2607–2618. 10.1093/nar/29.12.2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchenough G. M. H., Johansson M. E., Gustafsson J. K., Bergström J. H., Hansson G. C. (2015). New developments in goblet cell mucus secretion and function. Mucosal Immunol. 8 712–719. 10.1038/mi.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borewicz K. A., Kim H. B., Singer R. S., Gebhart C. J., Sreevatsan S., Johnson T., et al. (2015). Changes in the Porcine Intestinal Microbiome in Response to Infection with Salmonella enterica and Lawsonia intracellularis. PLoS One 10:e0139106. 10.1371/journal.pone.0139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosak J., Micenkova L., Hrala M., Pomorska K., Kunova Bosakova M., Krejci P., et al. (2018). Colicin FY inhibits pathogenic Yersinia enterocolitica in mice. Sci. Rep. 8:12242. 10.1038/s41598-018-30729-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S. R., Gilcrease E. B., Winn-Stapley D. A., Schicklmaier P., Schmieger H., Pedulla M. L., et al. (2005). The Generalized Transducing Salmonella Bacteriophage ES18: complete Genome Sequence and DNA Packaging Strategy. J. Bacteriol. 187 1091–1104. 10.1128/JB.187.3.1091-1104.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-C., Chen C.-R., Lin J.-W., Shen G.-H., Chang K.-M., Tseng Y.-H., et al. (2005). Isolation and Characterization of Novel Giant Stenotrophomonas maltophilia Phage φSMA5. Appl. Env. Microbiol. 71 1387–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M., Liang J., Zhang Y., Hu L., Gong P., Cai R., et al. (2017). The Bacteriophage EF-P29 Efficiently Protects against Lethal Vancomycin-Resistant Enterococcus faecalis and Alleviates Gut Microbiota Imbalance in a Murine Bacteremia Model. Front. Microbiol. 8:837. 10.3389/fmicb.2017.00837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen N. A., Maharshak N. (2017). Novel Indications for Fecal Microbial Transplantation: update and Review of the Literature. Digest. Dis. Sci. 62 1131–1145. 10.1007/s10620-017-4535-9 [DOI] [PubMed] [Google Scholar]
- Collaborators G. B. D. D. D. (2017). Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 17 909–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davido B., Batista R., Michelon H., Lepainteur M., Bouchand F., Lepeule R., et al. (2017). Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J. Hosp. Infect. 95 433–437. 10.1016/j.jhin.2017.02.001 [DOI] [PubMed] [Google Scholar]
- Dodds D. R. (2017). Antibiotic resistance: a current epilogue. Biochem. Pharmacol. 134 139–146. 10.1016/j.bcp.2016.12.005 [DOI] [PubMed] [Google Scholar]
- Gong P., Cheng M., Li X., Jiang H., Yu C., Kahaer N., et al. (2016). Characterization of Enterococcus faecium bacteriophage IME-EFm5 and its endolysin LysEFm5. Virology 492 11–20. 10.1016/j.virol.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Gu J., Liu X., Li Y., Han W., Lei L., Yang Y., et al. (2012). A method for generation phage cocktail with great therapeutic potential. PLoS One 7:e31698. 10.1371/journal.pone.0031698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Xu W., Lei L., Huang J., Feng X., Sun C., et al. (2010). LysGH15, a Novel Bacteriophage Lysin, Protects a Murine Bacteremia Model Efficiently against Lethal Methicillin-Resistant Staphylococcus aureus Infection. J. Clin. Microbiol. 49 111–117. 10.1128/JCM.01144-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G. (2013). Building Phylogenetic Trees from Molecular Data with MEGA. Mole. Biol. Evol. 30 1229–1235. 10.1093/molbev/mst012 [DOI] [PubMed] [Google Scholar]
- Hassan A. Y., Lin J. T., Ricker N., Anany H. (2021). The Age of Phage: Friend or Foe in the New Dawn of Therapeutic and Biocontrol Applications? Pharmaceuticals 2021:14. 10.3390/ph14030199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herp S., Brugiroux S., Garzetti D., Ring D., Jochum L. M., Beutler M., et al. (2019). Mucispirillum schaedleri Antagonizes Salmonella Virulence to Protect Mice against Colitis. Cell Host Microbe 25 681.e–694.e. 10.1016/j.chom.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Hsu B. B., Gibson T. E., Yeliseyev V., Liu Q., Lyon L., Bry L., et al. (2019). Dynamic Modulation of the Gut Microbiota and Metabolome by Bacteriophages in a Mouse Model. Cell Host Microbe 25 803–814e5. 10.1016/j.chom.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Guo J., Zhao C., Jiang P., Maimai T., Yanyi L., et al. (2020). The gut microbiota contributes to the development of Staphylococcus aureus-induced mastitis in mice. ISME J. 14 1897–1910. 10.1038/s41396-020-0651-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Lam L., Rajendram M., Tamburini F., Honeycutt J., Pham T., et al. (2018). A Gut Commensal-Produced Metabolite Mediates Colonization Resistance to Salmonella Infection. Cell Host Microbe 24 296.e–307.e. 10.1016/j.chom.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajere S. M. (2019). A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet. World 12 504–521. 10.14202/vetworld.2019.504-521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.-W., Adams J. B., Coleman D. M., Pollard E. L., Maldonado J., Mcdonough-Means S., et al. (2019). Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Scient. Rep. 2019:9. 10.1038/s41598-019-42183-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Mirzaei M., Nilsson A. S. (2015). Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS One 10:e0118557. 10.1371/journal.pone.0127606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. U., Park S. K., Kang S. A., Park M. K., Cho M. K., Jung H. J., et al. (2013). Comparison of functional gene annotation of Toxascaris leonina and Toxocara canis using CLC genomics workbench. Korean J. Parasitol. 51 525–530. 10.3347/kjp.2013.51.5.525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutateladze M., Adamia R. (2008). Phage therapy experience at the Eliava Institute. Médecine et Maladies Infectieuses 38 426–430. 10.1016/j.medmal.2008.06.023 [DOI] [PubMed] [Google Scholar]
- Kutter E. (2009). Phage Host Range and Efficiency of Plating. Bacteriophages 501 141–149. [DOI] [PubMed] [Google Scholar]
- Lahtinen P., Mattila E., Anttila V.-J., Tillonen J., Teittinen M., Nevalainen P., et al. (2017). Faecal microbiota transplantation in patients with Clostridium difficile and significant comorbidities as well as in patients with new indications: a case series. World J. Gastroenterol. 23 7174–7184. 10.3748/wjg.v23.i39.7174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Majowicz S. E., Musto J., Scallan E., Angulo F. J., Kirk M., O’brien S. J., et al. (2010). The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect Dis. 50 882–889. 10.1086/650733 [DOI] [PubMed] [Google Scholar]
- Manges A. R., Steiner T. S., Wright A. J. (2016). Fecal microbiota transplantation for the intestinal decolonization of extensively antimicrobial-resistant opportunistic pathogens: a review. Infect. Dis. 48 587–592. 10.1080/23744235.2016.1177199 [DOI] [PubMed] [Google Scholar]
- Mao Y., Nobaek S., Kasravi B., Adawi D., Stenram U., Molin G., et al. (1996). The effects of Lactobacillus strains and oat fiber on methotrexate- induced enterocolitis in rats. Gastroenterology 111 334–344. 10.1053/gast.1996.v111.pm8690198 [DOI] [PubMed] [Google Scholar]
- McCallin S., Sacher J. C., Zheng J., Chan B. K. (2019). Current State of Compassionate Phage Therapy. Viruses 2019:11. 10.3390/v11040343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccauley H. A., Guasch G. (2015). Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mole. Med. 21 492–503. 10.1016/j.molmed.2015.06.003 [DOI] [PubMed] [Google Scholar]
- Mu Q., Tavella V. J., Luo X. M. (2018). Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018:9. 10.3389/fmicb.2018.00757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oechslin F. (2018). Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018:10. 10.3390/v10070351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A., Sereno R., Azeredo J. (2010). In vivo efficiency evaluation of a phage cocktail in controlling severe colibacillosis in confined conditions and experimental poultry houses. Veterin. Microb. 146 303–308. 10.1016/j.vetmic.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Pajunen M., Kiljunen S., Skurnik M. (2000). Bacteriophage φYeO3-12, Specific for Yersinia enterocolitica Serotype O:3, Is Related to Coliphages T3 and T7. J. Bacteriol. 182 5114–5120. 10.1128/JB.182.18.5114-5120.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigneur B., Sokol H. (2016). Fecal microbiota transplantation in inflammatory bowel disease: the quest for the holy grail. Mucos. Immunol. 9 1360–1365. 10.1038/mi.2016.67 [DOI] [PubMed] [Google Scholar]
- Pires D. P., Costa A. R., Pinto G., Meneses L., Azeredo J. (2020). Current challenges and future opportunities of phage therapy. FEMS Microbiol. Rev. 44 684–700. 10.1093/femsre/fuaa017 [DOI] [PubMed] [Google Scholar]
- Rivas L., Coffey B., Mcauliffe O., Mcdonnell M. J., Burgess C. M., Coffey A., et al. (2010). In Vivo and Ex Vivo Evaluations of Bacteriophages e11/2 and e4/1c for Use in the Control of Escherichia coli O157:H7. Appl. Env. Microb. 76 7210–7216. 10.1128/AEM.01530-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chávez F., Lillian F., Faber F., Christopher A., Mariana X., Erin E., et al. (2016). Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 19 443–454. 10.1016/j.chom.2016.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Van Nood E., Nieuwdorp M., Van Dam B., Ten Berge I. J. M., Geerlings S. E., et al. (2014). Donor feces infusion for eradication of Extended Spectrum beta-Lactamase producing Escherichia coli in a patient with end stage renal disease. Clin. Microbiol. Infect. 20 O977–O978. 10.1111/1469-0691.12683 [DOI] [PubMed] [Google Scholar]
- Souza D. G., Soares A. C., Pinho V., Torloni H., Reis L. F. L., Martins M. T., et al. (2002). Increased Mortality and Inflammation in Tumor Necrosis Factor-Stimulated Gene-14 Transgenic Mice after Ischemia and Reperfusion Injury. Am. J. Pathol. 160 1755–1765. 10.1016/s0002-9440(10)61122-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B., Gu J., Liu X., Li Y., Han W., Lei L., et al. (2012). A Method for Generation Phage Cocktail with Great Therapeutic Potential. PLoS ONE 2012:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritha K. S., Bhat S. G. (2018). Genomics of Salmonella phage ΦStp1: candidate bacteriophage for biocontrol. Virus Genes 54 311–318. [DOI] [PubMed] [Google Scholar]
- Stecher B. R., Paesold G. N., Barthel M., Kremer M., Jantsch J., Stallmach T., et al. (2006). Chronic Salmonella enterica Serovar Typhimurium-Induced Colitis and Cholangitis in Streptomycin-Pretreated Nramp1 +/+ Mice. Infect. Immun. 74 5047–5057. 10.1128/IAI.00072-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strath M., Warren D. J., Sanderson C. J. (1985). Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J. Immunol. Methods 83 209–215. 10.1016/0022-1759(85)90242-x [DOI] [PubMed] [Google Scholar]
- Szabadosova V., Boronova I., Ferenc P., Tothova I., Bernasovska J., Zigova M., et al. (2018). Analysis of selected genes associated with cardiomyopathy by next-generation sequencing. J. Clin. Lab. Anal. 2018:32. 10.1002/jcla.22254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X., Johnson S. (2019). Fecal microbiota transplantation (FMT) for C. difficile infection, just say ‘No’. Anaerobe 2019:60. 10.1016/j.anaerobe.2019.102092 [DOI] [PubMed] [Google Scholar]
- Tang F., Zhang P., Zhang Q., Xue F., Ren J., Sun J., et al. (2019). Isolation and characterization of a broad-spectrum phage of multiple drug resistant Salmonella and its therapeutic utility in mice. Microb. Pathog. 126 193–198. 10.1016/j.micpath.2018.10.042 [DOI] [PubMed] [Google Scholar]
- Tanji Y., Shimada T., Yoichi M., Miyanaga K., Hori K., Unno H. (2004). Toward rational control of Escherichia coli O157:H7 by a phage cocktail. Appl. Microbiol. Biotechnol. 64 270–274. 10.1007/s00253-003-1438-9 [DOI] [PubMed] [Google Scholar]
- Van Nood E., Vrieze A., Nieuwdorp M., Fuentes S., Zoetendal E. G., De Vos W. M., et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368 407–415. [DOI] [PubMed] [Google Scholar]
- Wang H., Liu K. J., Sun Y. H., Cui L. Y., Meng X., Jiang G. M., et al. (2019). Abortion in donkeys associated with Salmonella abortus equi infection. Equine Vet. J. 51 756–759. 10.1111/evj.13100 [DOI] [PubMed] [Google Scholar]
- Wang K., Jin X., You M., Tian W., Le Leu R. K., Topping D. L., et al. (2017). Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet. Nutrients 2017:9. 10.3390/nu9080875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. K., Yang Y. S., Chen Y., Yuan J., Sun G., Peng L. H. (2014). Intestinal microbiota pathogenesis and fecal microbiota transplantation for inflammatory bowel disease. World J. Gastroenterol. 20 14805–14820. 10.3748/wjg.v20.i40.14805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters E. M., Neill D. R., Kaman B., Sahota J. S., Clokie M. R. J., Winstanley C., et al. (2017). Phage therapy is highly effective against chronic lung infections with Pseudomonas aeruginosa. Thorax 72 666–667. 10.1136/thoraxjnl-2016-209265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter S. E., Bäumler A. J. (2014). Dysbiosis in the inflamed intestine. Gut. Microbes 5 71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Hua R., Zhang B., Zhang X., Yang H., Zhou X. (2018). Serine Alleviates Dextran Sulfate Sodium-Induced Colitis and Regulates the Gut Microbiota in Mice. Front. Microbiol. 9:3062. 10.3389/fmicb.2018.03062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Xia X., Han F., Jiang Q., Rong Y., Song D., et al. (2015). Cathelicidin-BF, a Novel Antimicrobial Peptide from Bungarus fasciatus, Attenuates Disease in a Dextran Sulfate Sodium Model of Colitis. Mole. Pharm. 12 1648–1661. 10.1021/acs.molpharmaceut.5b00069 [DOI] [PubMed] [Google Scholar]
- Zhang W., Zou G., Li B., Du X., Sun Z., Sun Y., et al. (2020). Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation. J. Microbiol. Biotechnol. 30 1132–1141. 10.4014/jmb.2002.02044 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.