Abstract
Species of Onnia are important tree pathogens and play a crucial role in forest ecosystems. The species diversity and distribution of Onnia have been studied, however, its evolutionary history is poorly understood. In this study, we reconstructed the phylogeny of Onnia using internal transcribed spacers (ITS) and large subunit (LSU) rDNA sequence data. Molecular clock analyses developed the divergence times of Onnia based on a dataset (ITS + LSU rDNA + rpb1 + rpb2 + tef1α). Reconstruct Ancestral State in Phylogenies (RASP) was used to reconstruct the historical biogeography for the genus Onnia with a Dispersal Extinction Cladogenesis (DEC) model. Here, we provide a robust phylogeny of Onnia, with a description of a new species, Onnia himalayana from Yunnan Province, China. Molecular clock analyses suggested that the common ancestor of Onnia and Porodaedalea emerged in the Paleogene period with full support and a mean stem age of 56.9 Mya (95% highest posterior density of 35.9–81.6 Mya), and most species occurred in the Neogene period. Biogeographic studies suggest that Asia, especially in the Hengduan-Himalayan region, is probably the ancestral area. Five dispersals and two vicariances indicate that species of Onnia were rapidly diversified. Speciation occurred in the Old World and New World due to geographic separation. This study is the first inference of the divergence times, biogeography, and speciation of the genus Onnia.
Keywords: Hymenochaetaceae, molecular dating, reconstruct ancestral state, new taxa, biogeographic patterns
Introduction
Onnia P. Karst. was proposed by Karsten and typified by Onnia tomentosa (Fr.) P. Karst. It is a homogeneous genus and forms a distinct clade in the Hymenochaetaceae based on phylogenetic analyses (Karsten, 1889; Wagner and Fischer, 2002; Larsson et al., 2006; Dai, 2010). Phylogenetically, Onnia is closely related to Porodaedalea Murrill, while morphologically Porodaedalea differs from Onnia by a perennial growth habit, pileate basidiocarps lacking a stipe, straight setae, and a dimitic hyphal system (Wagner and Fischer, 2002; Larsson et al., 2006; Dai, 2010; Ji et al., 2017). Almost all species of Onnia usually grow on gymnosperms, but one species, Onnia vallata (Berk.) Y.C. Dai and Niemelä, was recorded on angiosperms based on morphological features only and still without DNA data (Dai, 2010; Ryvarden and Melo, 2014; Ji et al., 2017). Some species of Onnia are well-known pathogens causing Tomentosus Root Rot on trees of Pinaceae, such as Picea and Pinus (Hunt and White, 1998; Germain et al., 2009; Ji et al., 2017).
Currently, eight species are accepted in Onnia, and their distribution is well defined. Onnia tomentosa is widespread in the Northern Hemisphere, including Canada, China, Czechia, Denmark, Finland, France, Germany, Italy, Norway, Poland, Russia, Spain, Sweden, the United Kingdom, Ukraine, and the United States (Table 1; Ryvarden and Gilbertson, 1993; Wagner and Fischer, 2001; Akata et al., 2009; Germain et al., 2009; Ji et al., 2017; Zhou and Wu, 2018; Wu et al., 2022). Onnia leporina (Fr.) H. Jahn is reported in Eurasia, such as China, Czechia, Finland, Italy, Norway, Sweden, and Ukraine (Ryvarden and Gilbertson, 1993; Wu et al., 2022). While O. himalayana Y.C. Dai, H. Zhao, and Meng Zhou, sp. nov., O. microspora Y.C. Dai and L.W. Zhou, and O. tibetica Y.C. Dai and S.H. He appear to be endemic to China, O. kesiyae M. Zhou and F. Wu, O. subtriquetra Vlasák and Y.C. Dai, and O. triquetra (Pers.) Imazeki occurred in Vietnam, the United States, and Europe (such as Czechia, Finland, France, Germany, Hungary, Poland, Russia, Spain, and Ukraine), respectively (Ryvarden and Gilbertson, 1993; Wu et al., 2022). Moreover, species of Onnia possessed host trees preferences, O. tomentosa and O. leporina mainly occurred on Picea, while other species commonly grow on Pinus (Ji et al., 2017; Wu et al., 2022). Indeed, species diversification and evolution of Onnia seem to inextricably interact with host trees and geographic separation, which provided niches for Onnia (Krah et al., 2018).
TABLE 1.
Taxa information and GenBank accession numbers used in this study.
| Species | Sample | GenBank accession nos. | Country | ||||
|
|
|||||||
| ITS | LSU rDNA | rpb1 | rpb2 | tef1α | |||
| Amylocorticium cebennense | HHB 2808 | GU187505 | GU187561 | GU187439 | GU187770 | GU187675 | United States |
| Anomoloma myceliosum | MJL 4413 | GU187500 | GU187559 | GU187441 | GU187766 | GU187677 | Canada |
| Athelia arachnoidea | CBS 418.72 | GU187504 | GU187557 | GU187436 | GU187769 | GU187672 | Netherlands |
| Auricularia heimuer | Xiaoheimao | LT716074 | KY418890 | KY418982 | KY419035 | KY419083 | China |
| Boletopsis leucomelaena | AFTOL 1527 | DQ484064 | DQ154112 | GU187494 | GU187820 | GU187763 | United States |
| Bondarzewia montana | AFTOL 452 | DQ200923 | DQ234539 | DQ256049 | AY218474 | DQ059044 | Canada |
| Coltricia perennis | Cui 10318 | KU360686 | KJ000224 | – | – | – | China |
| Cryptococcus humicola | AFTOL 1552 | DQ645516 | DQ645514 | – | DQ645517 | DQ645519 | – |
| Dacryopinax spathularia | AFTOL 454 | AY854070 | AY701525 | – | AY786054 | AY881020 | – |
| Fomitiporia hartigii | MUCL 53551 | JX093789 | JX093833 | – | JX093877 | JX093746 | Estonia |
| F. langloisii | MUCL 46375 | EF429242 | EF429225 | – | – | – | United States |
| F. mediterranea | AFTOL 688 | AY854080 | AY684157 | – | AY803748 | AY885149 | |
| Gloeophyllum sepiarium | Wilcox-3BB | HM536091 | HM536061 | – | HM536109 | HM536110 | United States |
| Gomphidius roseus | MB 95-038 | DQ534570 | DQ534669 | GU187459 | GU187818 | GU187702 | Germany |
| Grifola frondosa | AFTOL 701 | AY854084 | AY629318 | AY864876 | AY786057 | AY885153 | |
| Gymnopilus picreus | ZRL2015011 | LT716066 | KY418882 | KY418980 | KY419027 | KY419077 | China |
| Hydnoporia lamellata | Cui 7629 | JQ279603 | JQ279617 | – | – | – | China |
| Inonotus griseus | Dai 13436 | KX364802 | KX364823 | KX364871 | KX364919 | MF977775 | China |
| Jaapia argillacea | CBS 252.74 | GU187524 | GU187581 | GU187463 | GU187788 | GU187711 | Netherlands |
| Lepiota cristata | ZRL20151133 | LT716026 | KY418841 | KY418963 | KY418992 | KY419048 | China |
| Leptosporomyces raunkiaeri | HHB 7628 | GU187528 | GU187588 | GU187471 | GU187791 | GU187719 | United States |
| Neurospora crassa | OR74A | HQ271348 | AF286411 | – | AF107789 | XM959775 | – |
| Onnia kesiyae | Dai 18415 | NR_160600 | NG_068811 | – | – | OM800827 | Vietnam |
| Onnia leporina | Dai 13501 | KT281958 | – | – | – | – | China |
| Dai 20866 | OM677245 | OM677252 | – | – | OM800829 | China | |
| JV0609/15 | KT281959 | – | – | – | – | Czechia | |
| JV1207/2 | KT281960 | KT281972 | – | – | – | Czechia | |
| Phaeo1 | KF996514 | – | – | – | – | Italy | |
| Onnia microspora | Dai 11886 | KT281956 | KT281970 | – | – | – | China |
| Dai 11897 | KT281957 | KT281971 | – | – | – | China | |
| Onnia himalayana | Dai 22620 | OM677247 | OM677254 | – | – | – | China |
| Onnia subtriquetra | Dai 23686 | OM677244 | OM677251 | ON007276 | OM937018 | OM800828 | United States |
| Dai 23687 | OM967274 | OM967335 | – | – | – | United States | |
| MB2 | KT281955 | KT281969 | – | – | – | United States | |
| JV0410/12J | KT281954 | KT281968 | – | – | – | United States | |
| JV0109/D6J | KT281953 | KT281967 | – | – | – | United States | |
| Onnia tibetica | Cui 12254 | KT281961 | KT281973 | – | – | – | China |
| Dai 23621 | OM967275 | OM967336 | – | – | – | China | |
| Dai 23622 | – | OM967337 | – | – | – | China | |
| Dai 23642 | OM677246 | OM677253 | ON007277 | OM937019 | OM800830 | China | |
| Dai 23643 | OM967276 | OM967338 | – | – | – | China | |
| Yuan 1964 | KT281962 | KT281974 | – | – | – | China | |
| Onnia triquetra | CBS 278.55 | MH857481 | MH869023 | – | – | – | Germany |
| JV1410/3 | KT281963 | KT281975 | – | – | – | Czechia | |
| Onnia tomentosa | Dai 14806B | KT281965 | KT281976 | – | – | – | China |
| Dai 18900 | OM677241 | OM677248 | – | OM937015 | OM800824 | China | |
| Dai 22935 | OM677242 | OM677249 | ON007278 | OM937016 | OM800825 | China | |
| Dai 23682 | OM967277 | OM967339 | – | – | – | United States | |
| Dai 23683 | OM677243 | OM677250 | OM007279 | OM937017 | OM80082 | United States | |
| Dai 23685 | OM967279 | OM967341 | – | – | – | United States | |
| Vampola 2010 | KT281966 | KT281977 | – | – | – | Czechia | |
| FP-100585-5p | KF996516 | – | – | – | – | Canada | |
| OT-Slu | KF996518 | – | – | – | – | Sweden | |
| T. Niemela 9079 | MF319075 | MF319006 | – | – | – | Finland | |
| SFC20170810-01 | MT044403 | – | – | – | – | Russia | |
| Cui 9986 | KT281964 | – | – | – | – | China | |
| HHB-18573 | KT955001 | – | – | – | – | United States | |
| LOO-13789-Q | KF996517 | – | – | – | – | United States | |
| TW 445 | AF311023 | – | – | – | Germany | ||
| Phellinopsis conchata | L7601 | KU139188 | KU139257 | – | – | – | United States |
| Phellinopsis andina | MR 1203 | KP347542 | KP347528 | – | – | – | Argentina |
| Phellinus igniarius | 85-917 | AY340048 | AF311027 | – | – | – | Germany |
| Porodaedalea chinensis | Cui 10252 | KX673606 | MH152358 | – | MH101479 | MG585301 | China |
| P. pini | No-6170-T | JX110037 | JX110081 | – | – | JX109993 | United States |
| FP102111T | JX110036 | JX110080 | – | – | – | United States | |
| P. yunnanensis | Dai 3072 | MG585282 | MH152380 | – | – | MG585292 | China |
| Ramaria rubella | AFTOL 724 | AY854078 | AY645057 | – | AY786064 | AY883435 | United States |
| Sanghuangporus sanghuang | Cui 14419 | MF772789 | MF772810 | MF972246 | MF973483 | MF977790 | China |
| Suillus pictus | AFTOL 717 | AY854069 | AY684154 | AY858965 | AY883429 | AY883429 | – |
| Thelephora ganbajun | ZRL20151295 | LT716082 | KY418908 | KY418987 | KY419043 | KY419093 | China |
| Trametes versicolor | ZRL20151477 | LT716079 | KY418903 | KY418984 | KY419041 | KY419091 | China |
| Trechispora alnicola | AFTOL 665 | DQ411529 | AY635768 | – | – | DQ059052 | United States |
| Ustilago maydis | AFTOL 505 | AY854090 | AF453938 | – | AY485636 | AY885160 | – |
New sequences are in bold; “–” represents missing data.
Recently, important research advances have been made in the studies of species diversity and divergence times of fungi (He et al., 2019; Varga et al., 2019; Wu et al., 2020; Dai et al., 2021; Wang K. et al., 2021; Zong et al., 2021). At present, more than 140,000 species of fungi were described, accounting for 3.50%–6.04% of an estimate of 2,200,000–3,800,000 (Hawksworth and Lücking, 2017; Wang et al., 2020). Hymenochaetaceae, the core family of wood-inhabiting fungi, recognizes 672 poroid species in the world (Wu et al., 2022). In addition, the determination of the divergence times within Basidiomycota based on fossil evidence has provided a robust set of age estimates for higher taxa (Zhao et al., 2017; He et al., 2019; Wang X. W. et al., 2021), with fossil species such as Quatsinoporites cranhamii S.Y. Smith et al. (2004) and Berbee and Taylor (2010) representing a minimum age of 125 Mya for Hymenochaetaceae. Meanwhile, the molecular dating studies of macrofungi widely pay attention to ectomycorrhizal fungi, saprotrophic fungi, and pathogenic fungi (Hibbett and Matheny, 2009; Chen et al., 2015; Song et al., 2016; Truong et al., 2017; Li et al., 2020; Liu et al., 2022; Wang X. W. et al., 2022). A series of studies related to the divergence time of pathogenic fungi, such as Coniferiporia L.W. Zhou and Y.C. Dai, Heterobasidion Bref., and Phytophthora ramorum Werres et al., have been published (Chen et al., 2015; Jung et al., 2021; Wang X. W. et al., 2022). However, divergence times of important coniferous pathogenic fungal Onnia have not been well resolved.
In the study of biogeography, the evolution of species is an important issue requiring reconstructing the origin, speciation, and distribution patterns of organisms (Page, 2003; Seehausen et al., 2014). To date, macrofungi, especially wood-inhabiting fungi, being closely interacted with host plants, are an interesting subject in biogeographic research (Chen et al., 2015; Song et al., 2016; Varga et al., 2019; Li et al., 2020; Wang X. W. et al., 2022). For example, the ancestral geographic origin analyses suggested that coniferous pathogenic fungal Coniferiporia originated in Asia and then extend to Europe and North America (Wang X. W. et al., 2022). Regrettably, Onnia, a crucial member of wood-inhabiting fungi, is very much understudied in this regard.
In this article, a new species from Yunnan Province, China, Onnia himalayana, is phylogenetically and morphologically described. Meanwhile, a hypothesis for species diversification and origin of Onnia is proposed, namely, species of this genus seem to originate in the coniferous forests of southwest China.
Materials and Methods
Sample Collection
Species, voucher specimens, and GenBank accession numbers of Onnia used in the present study were obtained from Asia, Europe, and North America. They are listed in Table 1.
Morphology
The studied Onnia specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Morphological descriptions are based on field notes and herbarium specimens. Sections were studied at a magnification of up to 1,000 × using a Nikon Eclipse 80i microscope and phase contrast illumination. Microscopic features and measurements were made from slide preparations stained with Cotton Blue and Melzer’s reagent. Basidiospores were measured from sections cut from the tubes. To represent variation in the size of basidiospores, 5% of measurements were excluded from each end of the range and are given in parentheses. In the description: KOH = 5% potassium hydroxide, IKI = Melzer’s reagent, IKI– = neither amyloid nor dextrinoid, CB = Cotton Blue, CB + = cyanophilous in Cotton Blue, CB– = acyanophilous in Cotton Blue, L = arithmetic average of basidiospore length, W = arithmetic average of basidiospore width, Q = L/W ratios, and n = number of basidiospores/measured from given number of specimens. Color terms are from Anonymous (1969) and Petersen (1996).
DNA Extraction, Polymerase Chain Reaction, and Sequencing
Total DNA was extracted from dried specimens with a rapid plant genome extraction kit (Aidlab Biotechnologies Co., Ltd, Beijing, China), modified following Cao et al. (2012) and Zhao and Cui (2013). The internal transcribed spacers (ITS), large subunit of nuclear ribosomal RNA gene (LSU rDNA), partial DNA-directed RNA polymerase II subunit one gene (rpb1) and subunit two gene (rpb2), and partial translation elongation factor 1-alpha gene (tef1α) were amplified with primer pairs ITS 4 (5′-TCC TCC GCT TAT TGATAT GC-3′) and ITS 5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′; White et al., 1990), LR0R (5′-ACC CGC TGA ACT TAA GC-3′) and LR7 (5′-TAC TAC CAC CAA GAT CT-3′), RPB1-Af (5′-GAR TGY CCD GGD CAY TTY GG-3′) and RPB1-Cf (5′-CCN GCD ATN TCR TTR TCC ATR TA-3′; Matheny et al., 2002), fRPB2-5F (5′-GAY GAY MGW GAT CAY TTY GG-3′) and fRPB2-7cR (5′-CCC ATR GCT TGY TTR CCC AT-3′; Liu et al., 1999; Matheny, 2005), and EF1-1567R (5′-ACH GTR CCR ATA CCA CCS ATC TT-3′) and EF1-983F (5′-GCY CCY GGH CAY CGT CAY TTY AT-3′; Rehner and Buckley, 2005; Matheny et al., 2007), respectively. The polymerase chain reaction (PCR) procedures were as follows: for ITS sequences, an initial denaturation at 95°C for 3 min, followed by 34 cycles at 94°C for 40 s, 54°C for 45 s, 72°C for 1 min, and a final extension of 72°C for 10 min (Zhou et al., 2021a,b); for LSU rDNA region, an initial denaturation at 94°C for 1 min, followed by 34 cycles at 94°C for 30 s, 50°C for 1 min, 72°C for 1.5 min, and a final extension of 72°C for 10 min (Shen et al., 2016); for rpb1, rpb2, and tef1α regions, an initial denaturation at 94°C for 2 min, followed by 10 cycles at 94°C for 40 s, 60°C for 40 s, and 72°C for 2 min, then followed by 37 cycles at 94°C for 45 s, 55°C for 1.5 min, 72°C for 2 min, and a final extension at 72°C for 10 min (Chen et al., 2015; Wang X. W. et al., 2021). Sequencing for PCR products was conducted by BGI Tech Solutions Beijing Liuhe Co., Ltd., Beijing, China. Sequences were assembled and proofread with Geneious (version 9.0.21, accessed 1 May 2021) and then submitted to GenBank under the accession numbers in Table 1.
Phylogenetic Analyses
All sequences were aligned with AliView (version 3.0; Larsson, 2014) and MAFFT (version 7; Katoh and Standley, 2013), and then manually adjusted. A dataset of 30 specimens composed of ITS + LSU rDNA sequences was subjected to maximum likelihood (ML), maximum parsimony (MP), and Bayesian inference (BI) phylogenetic analyses using RAxML (version 8, Stamatakis, 2014), PAUP (version 4.0b10; Swofford, 2002), and MrBayes (version 3.2.7a; Ronquist et al., 2012), respectively, following Zhao et al. (2021, 2022a,b). The GTRGAMMA model was chosen as the substitution model for ML analysis. Obtained phylograms were viewed with FigTree (version 1.4.4).
Divergence Time Estimation
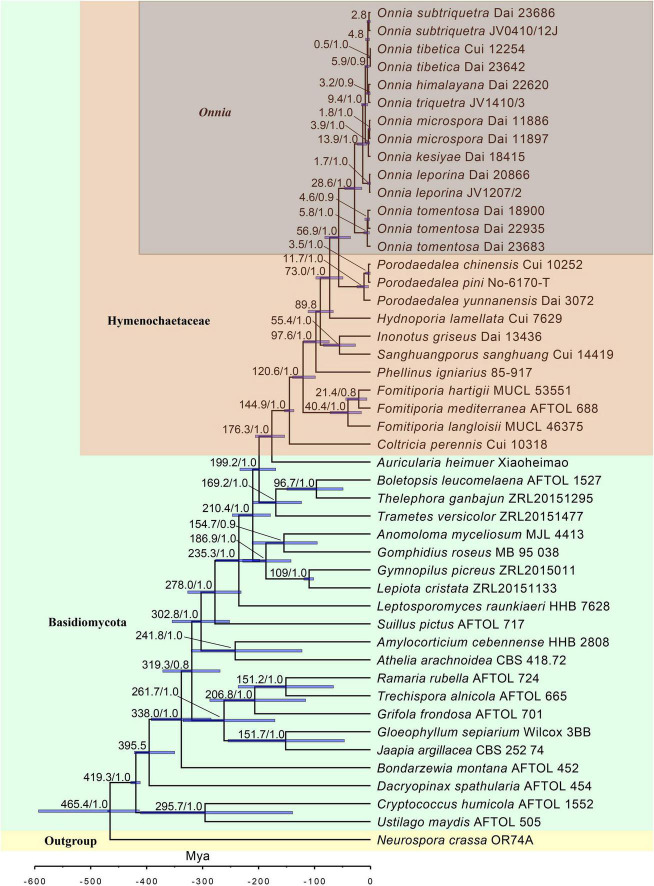
In this study, a dataset with 47 specimens (Figure 2) was used to infer the divergences times of species in the genus Onnia based on a dataset composed of ITS + LSU rDNA + rpb1 + rpb2 + tef1α sequences. The divergence times were estimated with BEAST (version 2.6.5; Bouckaert et al., 2014), using two ribosomal RNA genes (ITS and LSU rDNA) and three protein-coding genes (rpb1, rpb2, and tef1α). An XML (Extensible Markup Language) file was generated with BEAUti (version 2). The rates of evolutionary changes at nuclear acids were estimated using ModelTest (version 3.7) with the GTR substitution model (Posada and Crandall, 1998). Divergence time and corresponding CIs were conducted with a log-normal relaxed molecular clock and the Yule speciation prior. Three fossil time points, i.e., Archaeomarasmius leggettii Hibbett et al. (1995, 1997), Quatsinoporites cranhamii S.Y. Smith et al. (2004) and Berbee and Taylor (2010), and Paleopyrenomycites devonicus Taylor et al. (1999, 2005), representing the divergence time at Agaricales, Hymenochaetaceae, and between Ascomycota and Basidiomycota, respectively, were selected for calibration. The offset age with a gamma distributed prior (scale = 20 and shape = 1) was set as 90, 125, and 400 Mya for Agaricales, Hymenochaetaceae, and Basidiomycota, respectively. After 10,000,000 generations, the first 10% were removed as burn-in. The log file was checked for convergence with Tracer (version 1.52). Consequently, a maximum clade credibility (MCC) tree was summarized with TreeAnnotator (version 2.6.5), annotating clades with more than 0.8 posterior probability (PP).
FIGURE 2.
Estimated divergence of Onnia generated from molecular clock analyses using a combined dataset of ITS, LSU rDNA, rpb1, rpb2, and tef1α sequences. Estimated mean divergence time (Mya) and posterior probabilities (PP) > 0.8 are annotated at the internodes. The 95% highest posterior density (HPD) interval of divergence time estimates is marked by horizontal blue bars.
Inferring Historical Biogeography
Reconstruct Ancestral State in Phylogenies (RASP) (version 4.2) was used to reconstruct historical biogeography for the genus Onnia with a dispersal-extinction-cladogenesis (DEC) model (Yu et al., 2015, 2020). For historical biogeographic analyses, the posterior distributions of the dataset (Table 1), including two ribosomal RNA genes (ITS and LSU rDNA) and three protein-coding genes (rpb1, rpb2, and tef1α), were estimated with BEAST. The geographic distributions for Onnia were identified in three areas: (A) Asia, (B) Europe, and (C) North America.
Results
Phylogeny of Onnia
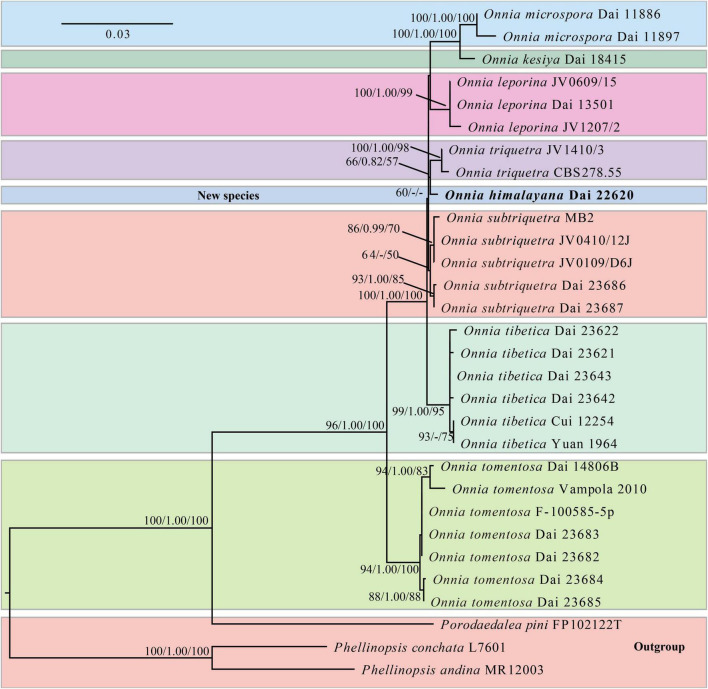
The ITS and LSU rDNA sequences are provided in Tables 1, 30 voucher specimens represent eight species of Onnia, one species of Porodaedalea Murrill, and two species of Phellinopsis Y.C. Dai. The dataset had an aligned length of 2,127 characters, including 1,750 constant, 155 parsimony-uninformative, and 222 parsimony-informative characters. MP analysis yielded a tree (tree length = 495, consistency index = 0.8889, homoplasy index = 0.1111, retention index = 0.9073, and rescaled consistency index = 0.8064). The best model of BI for the ITS and LSU rDNA dataset was GTR + I + G, and the average SD of split frequencies was less than 0.01. The topology of the ML tree was chosen to represent the phylogenetic relationship with Porodaedalea pini (Brot.) Murrill, Phellinopsis conchata (Pers.) Y.C. Dai, and P. andina (Plank and Ryvarden) Rajchenb. and Pildain as outgroups, since ML, MP, and BI resulted in similar topologies. The result suggests that O. himalayana is closely related to O. triquetra (Figure 1).
FIGURE 1.
Maximum likelihood (ML) phylogenetic tree of Onnia based on ITS and LSU rDNA sequences, with Porodaedalea pini, Phellinopsis conchata, and P. andina as outgroups. ML bootstrap values (≥ 50%)/maximum parsimony (MP) bootstrap values (≥ 50%)/Bayesian inference (BI) posterior probabilities (≥ 0.8) of each clade are indicated along branches. A scale bar in the upper left indicates substitutions per site.
Divergence Time Estimation for Onnia
The results of divergence time estimation show (Figure 2) that Hymenochaetaceae emerged earlier with a mean stem age of 176.3 Mya [95% highest posterior density (HPD) of 153.5–205.7 Mya] and a mean crown age of 144.9 Mya (95% HPD of 136.8–153.8 Mya), which is consistent with previous studies (Wang X. W. et al., 2021; Ji et al., 2022). In Hymenochaetaceae, Onnia is closely related to the genus Porodaedalea, which is most deeply diversified during the Paleogene, with a mean stem age of 56.9 Mya (95% HPD of 35.9–81.6 Mya) and full support (1.0 PP, Figure 2 and Table 2). The majority of species of Onnia emerged in the Neogene, especially in the Pliocene. Onnia tomentosa is the oldest species with a mean stem age of 28.6 Mya (95% HPD of 15.5–46.2 Mya), while O. triquetra and O. himalayana are younger than the other species with a stem age of 3.2 Mya (95% HPD of 0.7–7.1 Mya).
TABLE 2.
Inferred divergence time of species in the genus Onnia.
| Genus/Species | Means of stem age (Mya)/95% HPD (Mya)/Posterior probabilities | Means of crown age (Mya)/95% HPD (Mya)/Posterior probabilities |
| Onnia | 56.9/35.9–81.6/1.0 | 28.6/15.5–46.2/1.0 |
| O. tomentosa | 28.6/15.5–46.2/1.0 | 5.8/2.0–12.0/1.0 |
| O. leporina | 13.9/7.3–23.6/1.0 | 1.7/0.1–5.6/1.0 |
| O. kesiyae | 3.9/1.4–7.6/1.0 | 3.9/1.4–7.6/1.0 |
| O. microspora | 3.9/1.4–7.6/1.0 | 1.8/0.4–4.1/1.0 |
| O. triquetra | 3.2/0.7–7.1/0.9 | 3.2/0.7–7.1/0.9 |
| O. himalayana | 3.2/0.7–7.1/0.9 | 3.2/0.7–7.1/0.9 |
| O. tibetica | 4.8/1.9–9.0/– | 0.5/0–1.8/1.0 |
| O. subtriquetra | 4.8/1.9–9.0/– | 2.8/0.5–6.4/– |
Hyphen “–” represents a posterior probability (PP) < 0.8.
The Historical Biogeography of Onnia
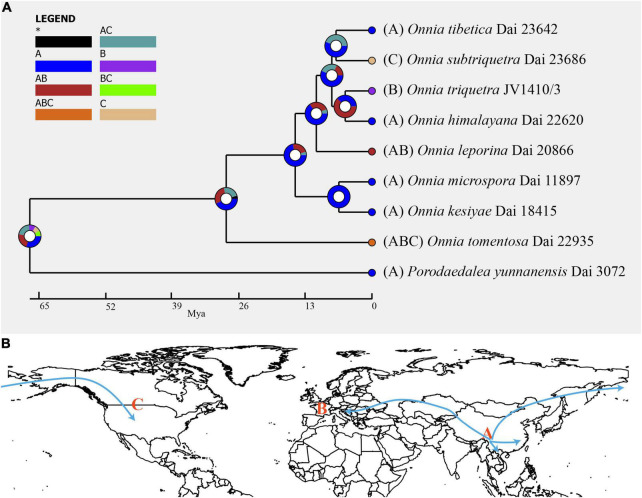
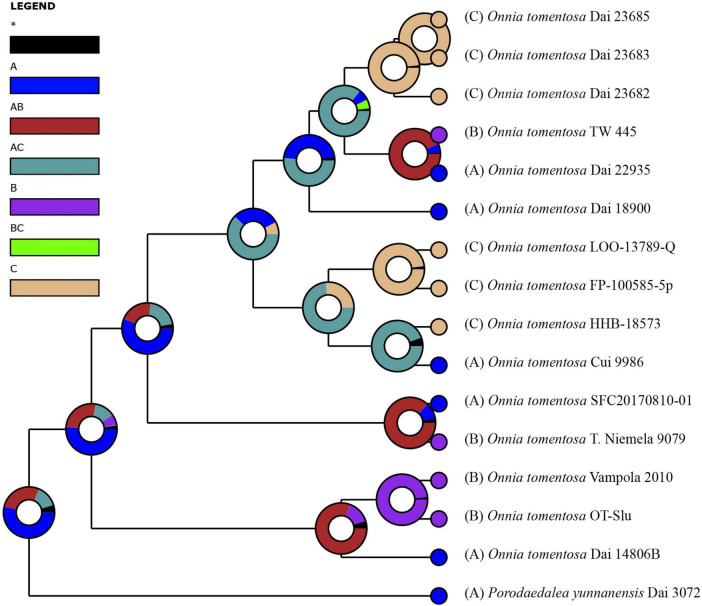
Inferred historical biogeography scenarios using RASP are shown in Figure 3. The RASP analysis suggests that Asia is the center of origin of the genus Onnia, and suggests that five dispersal events (three from Asia to Europe, and two from Asia to North America) and two vicariance (Eurasia and North America) events occurred during the distribution of this genus. Six species are found in Asia, three in Europe, and two in North America, suggesting that Asia is still the center of Onnia species. Moreover, there are three species, O. tomentosa, O. tibetica, and O. himalayana, distributed in southwest China, which implies that this region may be a more precise center of origin within Asia. Indeed, a total of 15 specimens of O. tomentosa, namely, six in North America, five in Asia, and four in Europe, have been collected (Figure 4 and Table 1). The dataset of ancestral state reconstruction suggested that Asia is the ancestral area (Figure 4). Meanwhile, possible concealed dispersal routes were inferred (Figure 3B): (1) Asia to North America and (2) Asia to Europe.
FIGURE 3.
(A) Ancestral state reconstruction and divergence time estimation of Onnia using a dataset containing ITS, LSU rDNA, rpb1, rpb2, and tef1α sequences. A pie chart at each node indicates the possible ancestral distributions inferred from dispersal-extinction-cladogenesis (DEC) analysis implemented in RASP. A black asterisk represents other ancestral ranges. (B) Possible dispersal routes of Onnia in the Northern hemisphere. Regions are labeled as follows: (A) Asia, (B) Europe, (C) North America, (AB) Asia and Europe, (AC) Asia and North America, (BC) Europe and North America, and (ABC) Asia, Europe, and North America.
FIGURE 4.
Ancestral state reconstruction and divergence time estimation of Onnia tomentosa using a dataset containing ITS and LSU rDNA sequences. A pie chart at each node indicates the possible ancestral distributions inferred from dispersal-extinction-cladogenesis (DEC) analysis implemented in RASP. A black asterisk represents other ancestral ranges. Regions are labeled as follows: (A) Asia, (B) Europe, (C) North America, (AB) Asia and Europe, (AC) Asia and North America, and (BC) Europe and North America.
Taxonomy
Onnia himalayana Y.C. Dai, H. Zhao and Meng Zhou, sp. nov. (Figure 5).
FIGURE 5.

Basidiocarps and microscopic structures of Onnia himalayana (Holotype, Dai 22620). (a) Basidiocarps of Onnia himalayana; (b) Basidiospores; (c) Basidia and basidioles; (d) Hymenial setae; (e) Hyphae from upper tomentum; and (f) Hyphae from trama.
MycoBank: MB: 844317.
Type: CHINA. Yunnan Province, Dali, Cangshan Geopark, on root of Pinus yunnanensis, 30 VIII 2021, Dai 22620 (Holotype, BJFC037194).
Etymology: Himalayana (Lat.), refers to the species being found in the eastern Himalayan area.
Basidiocarps annual, laterally to centrally stipitate, solitary, without odor or taste and corky when fresh, becoming hard corky upon drying. Pilei dimidiate to circular, projecting up to 3 cm, 4 cm wide, and 8 mm thick at the center. Pileal surface clay buff with cream to buff margin, velutinate, and azonate when fresh, becoming cinnamon, homogeneous, distinctly velutinate, and azonate when dry; margin sharp, curving downward when dry. Pore surface clay pink when fresh, becoming fulvous when dry, sterile margin distinct, up to 2 mm wide; pores angular, 3–4 per mm; and dissepiments thin, strongly lacerate to dentate. Context duplex, upper layer fulvous, more or less spongy, up to 4 mm thick, lower layer umber, hard corky, up to 2 mm thick, no demarcation zone between the two layers. Tubes are concolorous with pores, hard corky, and up to 2 mm long. Stipe clay buff, hard corky when dry, velutinate, up to 1 cm long, 8 mm diam; pores decurrent on the stipe.
Hyphal system monomitic, generative hyphae simple septate, IKI–, CB–; tissues darkening but otherwise unchanged in KOH. Context: hyphae in the upper layer are pale yellowish to golden yellow, slightly thick-walled, occasionally branched, frequently simple septate, straight, regularly arranged, and 5–7 μm diam; hyphae in the lower layer are yellowish to golden brown, slightly thick- to thick-walled, occasionally branched, with frequent simple septa, straight, regularly arranged, not agglutinated, and 4–5.5 μm diam; hyphae in stipe similar to those in context. Tubes: Tramal hyphae hyaline to yellowish, thin- to slightly thick-walled, rarely branched, frequently septate, more or less flexuous, subparallel along the tubes, not agglutinated, and 2.5–4.5 μm diam.
Hymenium: Setae hooked, sharply pointed at apex, dark brown, thick-walled, deep-rooting, embedded in trama and projecting from hymenium, and 40–78 × 14–20 μm; cystidia and cystidioles absent; basidia clavate, with four sterigmata and a simple septum at the base, 12–15 × 5–6 μm; and basidioles dominant, in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid to oblong-ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, 5-6 × 3.2-4 (-4.1) μm, L = 5.62 μm, W = 3.63 μm, and Q = 1.55 (n = 30/1).
Discussion
The discovery of new fungal species has rapidly increased with the development of molecular techniques, drawing attention to the huge fungal diversity that exists on earth (Cui et al., 2019; He et al., 2019; Wu et al., 2020, 2022; Dai et al., 2021; Wang K. et al., 2021; Zhang and Dai, 2021; Ji et al., 2022). Hymenochaetaceae is a core family of macrofungi that consists of approximately 670 poroid species (Wu et al., 2022) and is an interesting subject for species diversity studies (Dai, 2010; Wu et al., 2020, 2022; Dai et al., 2021; Wang X. W. et al., 2022). Although Onnia is a small genus in this family, some species of Onnia are important pathogenic fungi that cause Tomentosus Root Rot on trees of Picea and Pinus (Hunt and White, 1998; Germain et al., 2009; Dai, 2010; Ji et al., 2017). As species distribution of Onnia is usually closely related to host trees (Ji et al., 2017; Wu et al., 2022), the genus is ideal for studying species diversity, divergence times, and biogeography.
Currently, dating analyses have provided a deep insight into the evolution of macrofungi using multigene analyses (Zhao et al., 2017; He et al., 2019; Varga et al., 2019). Our analysis of divergence times using a dataset of two ribosomal RNA genes (ITS and LSU rDNA) and three protein-coding genes (rpb1, rpb2, and tef1α) suggests that Onnia and Porodaedalea possibly emerged in the Paleogene with a mean stem age of 56.9 Mya (95% HPD of 35.9–81.6 Mya) and full support (1.0 PP; Figure 2 and Table 2). Considering the divergence estimation of Pinaceae (206 Mya) and the fossil record of Hymenochaetaceae (125 Mya), this estimation of Onnia and Porodaedalea seems reasonable (Smith et al., 2004; Berbee and Taylor, 2010; Magallón et al., 2015; Ran et al., 2018). Moreover, the basal modern species, O. tomentosa, occurred in 28.6 Mya, which is consistent with the timing of the second pulse of rapid uplift of the Qinghai-Tibet Plateau (between 20 and 30 Mya; Wang et al., 2012, 2018). Most species of Onnia emerged about 5 Mya (Figure 2 and Table 2), i.e., late Miocene to Pliocene, and adapted to a low temperature, facilitating survival in the Quaternary Ice Age.
Biogeographic studies of macrofungi have been very successful for ectomycorrhizal fungi, such as Amanita (see Sánchez-Ramírez et al., 2015; Truong et al., 2017), saprotrophic Lentinula (see Hibbett et al., 1998), and pathogenic fungi, e.g., Heterobasidion (Chen et al., 2015) based on molecular analyses. Our results suggest that the species distribution of Onnia has a distinct biogeographical pattern, similar to other wood-decaying fungi (Sato et al., 2017; Han et al., 2018; Li et al., 2020). Species of Onnia appear to have originated in Asia, especially in the Hengduan-Himalayan region which is a global biodiversity hotspot, and this conclusion supports previous studies on the origination of wood-decaying fungi (Song et al., 2016; Li et al., 2020; Wang X. W. et al., 2022). Three species, O. himalayana, O. tibetica, and O. tomentosa, occur in the Hengduan-Himalayan region. The basal species, O. tomentosa, emerged at 28.6 Mya (Figure 2 and Table 2), and maybe dispersal occurred between East Asia and North America via the Beringia (Bering Land Bridge). However, a vicariance event, such as the opening of the Bering Strait, could limit gene flow and species dispersal in the Old World and the New World (Hibbett, 2001; Cai et al., 2014; Li et al., 2020).
Conclusion
In this study, our dataset of divergence times suggests that Onnia and Porodaedalea possibly emerged in the Paleogene. Most species of Onnia emerged in the late Miocene to Pliocene and adapted to a low temperature, and therefore survived in the Quaternary Ice Age. Species appear to have originated in the coniferous forests of southwest China, then spread across the Northern Hemisphere with host plants. Geographic separation led to a diversification of new species in the Old World and New World. A total of nine species are recognized, namely, eight species that grow on gymnosperms and one species that grows on angiosperms. Furthermore, a new species, Onnia himalayana, is proposed and illustrated based on phylogenetic and morphological evidence.
Data Availability Statement
All the sequences have been deposited in GenBank; the accession numbers are listed in Table 1.
Author Contributions
HZ: data analyses, formal analyses, conceived the ideas, and original draft and review. MZ: data curation and the draft of new species. X-YL: review and editing. FW: project administration and review and editing. Y-CD: funding acquisition, investigation, description of new species, and review and editing. All authors have read the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Hong-Min Zhou and Ablat Tohtirjep (Institute of Microbiology, School of Ecology and Nature Conservation, Beijing Forestry University) for DNA extraction, PCR reaction, and sequencing.
Footnotes
Funding
This work was supported by the National Natural Science Foundation of China (Project Nos. 32161143013 and U1802231) and by the Second Tibetan Plateau Scientific Expedition and Research Program (STEP), Grant No. 2019QZKK0503.
References
- Akata I., Dogan H. H., Cetin B., Isiloglu M. (2009). Onnia tomentosa Fr. P. Karst, a new genus record for Turkey. Biol. Divers. Conserv. 2 78–81. [Google Scholar]
- Anonymous (1969). Flora of British Fungi. Colour Identification Chart. London, UK: Her Majesty’s Stationery Office, 1–3. [Google Scholar]
- Berbee M. L., Taylor J. W. (2010). Dating the molecular clock in fungi – how close are we? Fungal Biol. Rev. 24 1–16. 10.1016/j.fbr.2010.03.001 [DOI] [Google Scholar]
- Bouckaert R., Heled J., Kühnert D., Vaughan T., Wu C. H., Xie D., et al. (2014). BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 10:e1003537. 10.1371/journal.pcbi.1003537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Tulloss R. E., Tang L. P., Tolgor B., Zhang P., Chen Z. H., et al. (2014). Multi-locus phylogeny of lethal amanitas: implications for species diversity and historical biogeography. BMC Evol. Biol. 14:143. 10.1186/1471-2148-14-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Wu S. H., Dai Y. C. (2012). Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 56 49–62. 10.1007/s13225-012-0178-5 [DOI] [Google Scholar]
- Chen J. J., Cui B. K., Zhou L. W., Korhonen K., Dai Y. C. (2015). Phylogeny, divergence time estimation, and biogeography of the genus Heterobasidion (Basidiomycota, Russulales). Fungal Divers. 71 185–200. 10.1007/s13225-014-0317-2 [DOI] [Google Scholar]
- Cui B. K., Li H. J., Ji X., Zhou J. L., Song J., Si J., et al. (2019). Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers. 97 137–392. 10.1007/s13225-019-00427-4 [DOI] [Google Scholar]
- Dai Y. C. (2010). Hymenochaetaceae (Basidiomycota) in China. Fungal Divers. 45 131–343. 10.1007/s13225-010-0066-9 [DOI] [Google Scholar]
- Dai Y. C., Yang Z. L., Cui B. K., Wu G., Yuan H. S., Zhou L. W., et al. (2021). Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 40 770–805. 10.13346/j.mycosystema.210036 [DOI] [Google Scholar]
- Germain H., Bergeron M. J., Bernier L., Laflamme G., Hamelin R. (2009). Patterns of colonization and spread in the fungal spruce pathogen Onnia tomentosa. Mol. Ecol. 18 4422–4433. 10.1111/j.1365-294X.2009.04370.x [DOI] [PubMed] [Google Scholar]
- Han L. H., Feng B., Wu G., Halling R. E., Buyck B., Yorou N. S., et al. (2018). African origin and global distribution patterns: evidence inferred from phylogenetic and biogeographical analyses of ectomycorrhizal fungal genus Strobilomyces. J. Biogeogr. 45 201–212. 10.1111/jbi.13094 [DOI] [Google Scholar]
- Hawksworth D. L., Lücking R. (2017). Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 5 1–17. 10.1128/microbiolspec.FUNK-0052-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M. Q., Zhao R. L., Hyde K. D., Begerow D., Kemler M., Yurkov A. (2019). Notes, outline and divergence times of Basidiomycota. Fungal Divers. 99 105–367. 10.1007/s13225-019-00435-4 [DOI] [Google Scholar]
- Hibbett D. S. (2001). Shiitake mushrooms and molecular clocks: historical biogeography of Lentinula. J. Biogeogr. 28 231–241. 10.1046/j.1365-2699.2001.00528.x [DOI] [Google Scholar]
- Hibbett D. S., Matheny P. B. (2009). The relative ages of ectomycorrhizal mushrooms and their plant hosts estimated using Bayesian relaxed molecular clock analyses. BMC Biol. 7:13. 10.1186/1741-7007-7-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbett D. S., Grimaldi D., Donoghue M. J. (1995). Cretaceous mushrooms in amber. Nature 377 487–487. 10.1038/377487a0 [DOI] [Google Scholar]
- Hibbett D. S., Grimaldi D., Donoghue M. J. (1997). Fossil mushrooms from Miocene and Cretaceous ambers and the evolution of Homobasidiomycetes. Am. J. Bot. 84 981–991. 10.2307/2446289 [DOI] [PubMed] [Google Scholar]
- Hibbett D. S., Hansen K., Donoghue M. J. (1998). Phylogeny and biogeography of Lentinula inferred from an expanded rDNA dataset. Mycol. Res. 102 1041–1049. 10.1017/S0953756297005996 [DOI] [Google Scholar]
- Hunt R., White T. (1998). First report of Inonotus tomentosus, the cause of tomentosus root disease, from the Yukon Territory. Plant Dis. 82 264–264. 10.1094/PDIS.1998.82.2.264C [DOI] [PubMed] [Google Scholar]
- Ji X. H., He S. H., Chen J. J., Si J., Wu F., Zhou L. W., et al. (2017). Global diversity and phylogeny of Onnia (Hymenochaetaceae) species on gymnosperms. Mycologia 109 27–34. 10.1080/00275514.2016.1274619 [DOI] [PubMed] [Google Scholar]
- Ji X., Zhou J. L., Song C. G., Xu T. M., Wu D. M., Cui B. K. (2022). Taxonomy, phylogeny and divergence times of Polyporus (Basidiomycota) and related genera. Mycosphere 13 1–52. 10.5943/mycosphere/13/1/1 [DOI] [Google Scholar]
- Jung T., Horta Jung M., Webber J. F., Kageyama K., Hieno A., Masuya H., et al. (2021). The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. J. Fungi 7:226. 10.3390/jof7030226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsten P. (1889). Kritisk öfversigt af Finlands Basidsvampar (Basidiomycetes; Gastero- & Hymenomycetes). Bidrag till Kännedom af Finlands Natur och Folk 48 1–470. [Google Scholar]
- Katoh K., Standley D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krah F. S., Bässler C., Heibl C., Soghigian J., Schaefer H., Hibbett D. S. (2018). Evolutionary dynamics of host specialization in wood-decay fungi. BMC Evol. Biol. 18:119. 10.1186/s12862-018-1229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson A. (2014). AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30 3276–3278. 10.1093/bioinformatics/btu531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K. H., Parmasto E., Fischer M., Langer E., Nakasone K. K., Redhead S. A. (2006). Hymenochaetales: a molecular phylogeny for the hymenochaetoid clade. Mycologia 98 926–936. 10.1080/15572536.2006.11832622 [DOI] [PubMed] [Google Scholar]
- Li J., Han L. H., Liu X. B., Zhao Z. W., Yang Z. L. (2020). The saprotrophic Pleurotus ostreatus species complex: late Eocene origin in East Asia, multiple dispersal, and complex speciation. IMA Fungus 11 1–21. 10.1186/s43008-020-00031-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. J., Whelen S., Hall B. D. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 16 1799–1808. 10.1093/oxfordjournals.molbev.a026092 [DOI] [PubMed] [Google Scholar]
- Liu Z. B., Wu Y. D., Zhao H., Lian Y. P., Wang Y. R., Wang C. G., et al. (2022). Outline, divergence Times, and phylogenetic analyses of Trechisporales (Agaricomycetes. Basidiomycota). Front. Microbiol. 13:818358. 10.3389/fmicb.2022.818358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magallón S., Gómez-Acevedo S., Sánchez-Reyes L. L., Hernández-Hernández T. (2015). A metacalibrated time-tree documents the early rise of flowering plant phylogenetic diversity. New Phytol. 207 437–453. 10.1111/nph.13264 [DOI] [PubMed] [Google Scholar]
- Matheny P. B. (2005). Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 35 1–20. 10.1016/j.ympev.2004.11.014 [DOI] [PubMed] [Google Scholar]
- Matheny P. B., Liu Y. J., Ammirati J. F., Hall B. D. (2002). Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am. J. Bot. 89 688–698. 10.3732/ajb.89.4.688 [DOI] [PubMed] [Google Scholar]
- Matheny P. B., Wang Z., Binder M., Curtis J. M., Lim Y. W., Henrik N. R. (2007). Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 43 430–451. 10.1016/j.ympev.2006.08.024 [DOI] [PubMed] [Google Scholar]
- Page R. D. (2003). Tangled Trees: Phylogeny, Cospeciation, and Coevolution. Chicago: University of Chicago Press. [Google Scholar]
- Petersen J. H. (1996). The Danish Mycological Society’s Colour-Chart. Greve: Foreningen til Svampekundskabens Fremme, 1–6. [Google Scholar]
- Posada D., Crandall K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 14 817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- Ran J. H., Shen T. T., Wu H., Gong X., Wang X. Q. (2018). Phylogeny and evolutionary history of Pinaceae updated by transcriptomic analysis. Mol. Phylogenet. Evol. 129 106–116. 10.1016/j.ympev.2018.08.011 [DOI] [PubMed] [Google Scholar]
- Rehner S. A., Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97 84–98. 10.1080/15572536.2006.11832842 [DOI] [PubMed] [Google Scholar]
- Ronquist F., Teslenko M., Van Der Mark P., Ayres D. L., Darling A., Höhna S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61 539–542. 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvarden L., Gilbertson R. L. (1993). European polypores 1. Synopsis Fungorum 6 1–387. [Google Scholar]
- Ryvarden L., Melo I. (2014). Poroid fungi of Europe. Synopsis Fungorum 31 1–455. [Google Scholar]
- Sánchez-Ramírez S., Tulloss R. E., Amalfi M., Moncalvo J. M. (2015). Palaeotropical origins, boreotropical distribution and increased rates of diversification in a clade of edible ectomycorrhizal mushrooms (Amanita section Caesareae). J. Biogeogr. 42 351–363. 10.1111/jbi.12402 [DOI] [Google Scholar]
- Sato H., Tanabe A. S., Toju H. (2017). Host shifts enhance diversification of ectomycorrhizal fungi: diversification rate analysis of the ectomycorrhizal fungal genera Strobilomyces and Afroboletus with an 80-gene phylogeny. New Phytol. 214 443–454. 10.1111/nph.14368 [DOI] [PubMed] [Google Scholar]
- Seehausen O., Butlin R. K., Keller I., Wagner C. E., Boughman J. W., Hohenlohe P. A., et al. (2014). Genomics and the origin of species. Nat. Rev. Genet. 15 176–192. 10.1038/nrg3644 [DOI] [PubMed] [Google Scholar]
- Shen L. L., Chen J. J., Wang M., Cui B. K. (2016). Taxonomy and multi-gene phylogeny of Haploporus (Polyporales, Basidiomycota). Mycol. Prog. 15 731–742. 10.1007/s11557-016-1203-y [DOI] [Google Scholar]
- Smith S. Y., Currah R. S., Stockey R. A. (2004). Cretaceous and Eocene poroid hymenophores from Vancouver Island, British Columbia. Mycologia 96 180–186. 10.1080/15572536.2005.11833010 [DOI] [PubMed] [Google Scholar]
- Song J., Chen J. J., Wang M., Chen Y. Y., Cui B. K. (2016). Phylogeny and biogeography of the remarkable genus Bondarzewia (Basidiomycota, Russulales). Sci. Rep. 6 1–10. 10.1038/srep34568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30 1312–1313. 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swofford D. L. (2002). PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods); Version 4.0b10. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Taylor T. N., Hass H., Kerp H. (1999). The oldest fossil ascomycetes. Nature 399 648–648. 10.1038/21349 [DOI] [PubMed] [Google Scholar]
- Taylor T. N., Hass H., Kerp H., Krings M., Hanlin R. T. (2005). Perithecial ascomycetes from the 400 million year old Rhynie chert: an example of ancestral polymorphism. Mycologia 97 269–285. 10.1080/15572536.2006.11832862 [DOI] [PubMed] [Google Scholar]
- Truong C., Sánchez-Ramírez S., Kuhar F., Kaplan Z., Smith M. E. (2017). The Gondwanan connection – Southern temperate Amanita lineages and the description of the first sequestrate species from the Americas. Fungal Biol. 121 638–651. 10.1016/j.funbio.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Varga T., Krizsán K., Földi C., Dima B., Sánchez-García M., Sánchez-Ramírez S., et al. (2019). Megaphylogeny resolves global patterns of mushroom evolution. Nat. Ecol. Evol. 3 668–678. 10.1038/s41559-019-0834-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner T., Fischer M. (2001). Natural groups and a revised system for the European poroid Hymenochaetales (Basidiomycota) supported by nLSU rDNA sequence data. Mycol. Res. 105 773–782. 10.1017/S0953756201004257 [DOI] [Google Scholar]
- Wagner T., Fischer M. (2002). Proceedings towards a natural classification of the worldwide taxa. Phellinus sl and Inonotus sl, and phylogenetic relationships of allied genera. Mycologia 94 998–1016. 10.1080/15572536.2003.11833156 [DOI] [PubMed] [Google Scholar]
- Wang E., Kirby E., Furlong K. P., van Soest M., Xu G., Shi X., et al. (2012). Two-phase growth of high topography in eastern Tibet during the Cenozoic. Nat. Geosci. 5 640–645. 10.1038/ngeo1538 [DOI] [Google Scholar]
- Wang K., Chen S. L., Dai Y. C., Jia Z. F., Li T. H., Liu T. Z., et al. (2021). Overview of China’s nomenclature novelties of fungi in the new century (2000-2020). Mycosystema 40 822–833. [Google Scholar]
- Wang K., Kirk P. M., Yao Y. J. (2020). Development trends in taxonomy, with special reference to fungi. J. Syst. Evol. 58 406–412. 10.1111/jse.12538 [DOI] [Google Scholar]
- Wang X. W., Jiang J. H., Liu S. L., Gafforov Y., Zhou L. W. (2022). Species diversification of the coniferous pathogenic fungal genus Coniferiporia (Hymenochaetales, Basidiomycota) in association with its biogeography and host plants. Phytopathology 112 404–413. 10.1094/PHYTO-05-21-0181-R [DOI] [PubMed] [Google Scholar]
- Wang X. W., May T. W., Liu S. L., Zhou L. W. (2021). Towards a natural classification of Hyphodontia sensu lato and the trait evolution of basidiocarps within Hymenochaetales (Basidiomycota). J. Fungi 7:478. 10.3390/jof706048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. X., Shen Y. J., Pang Z. B. (2018). Three main stages in the uplift of the Tibetan Plateau during the Cenozoic period and its possible effects on Asian aridification: a review. Clim. Past Discuss. [Preprint]. 10.5194/cp-2018-64 [DOI] [Google Scholar]
- White T. J., Bruns T., Lee S., Taylor J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR Protocols: a Guide to Methods and Applications eds Innis M. A., Gelfand D. H., Sninsky J. J., White T. J. (New York, NY: Academic Press; ), 315–322. [Google Scholar]
- Wu F., Yuan H. S., Zhou L. W., Yuan Y., Cui B. K., Dai Y. C. (2020). Polypore diversity in South China. Mycosystema 39 653–681. 10.13346/j.mycosystema.200087 [DOI] [Google Scholar]
- Wu F., Zhou L. W., Josef V., Dai Y. C. (2022). Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers. 113 1–192. [Google Scholar]
- Yu Y., Blair C., He X. (2020). RASP 4: ancestral state reconstruction tool for multiple genes and characters. Mol. Biol. Evol. 37 604–606. 10.1093/molbev/msz257 [DOI] [PubMed] [Google Scholar]
- Yu Y., Harris A. J., Blair C., He X. (2015). RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Mol. Phylogenet. Evol. 87 46–49. 10.1016/j.ympev.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Zhang Q. Y., Dai Y. C. (2021). Taxonomy and Phylogeny of the Favolaschia calocera complex (Mycenaceae) with descriptions of four new species. Forests 12:1397. 10.3390/f12101397 [DOI] [Google Scholar]
- Zhao C. L., Cui B. K. (2013). Morphological and molecular identification of four new resupinate species of Perenniporia (Polyporales) from southern China. Mycologia 105 945–958. 10.3852/12-201 [DOI] [PubMed] [Google Scholar]
- Zhao H., Nie Y., Zong T. K., Dai Y. C., Liu X. Y. (2022a). Three new species of Absidia (Mucoromycota) from China based on phylogeny, morphology and physiology. Diversity 14:132. 10.3390/d14020132 [DOI] [Google Scholar]
- Zhao H., Nie Y., Zong T. K., Wang Y. J., Wang M., Dai Y. C., et al. (2022b). Species diversity and ecological habitat of Absidia (Cunninghamellaceae, Mucorales) with emphasis on five new species from forest and grassland soil in China. J. Fungi 8:471. 10.3390/jof8050471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhu J., Zong T. K., Liu X. L., Ren L. Y., Lin Q., et al. (2021). Two new species in the family Cunninghamellaceae from China. Mycobiology 49 142–150. 10.1080/12298093.2021.1904555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R. L., Li G. J., Sánchez-Ramírez S., Stata M., Yang Z. L., Wu G., et al. (2017). A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Divers. 84 43–74. [Google Scholar]
- Zhou M., Wu F. (2018). A new species of Onnia (Hymenochaetales, Basidiomycota) from Vietnam. Phytotaxa 349 73–78. 10.11646/phytotaxa.349.1.9 [DOI] [Google Scholar]
- Zhou M., Dai Y. C., Vlasák J., Yuan Y. (2021a). Molecular phylogeny and global diversity of the genus Haploporus (Polyporales, Basidiomycota). J. Fungi 7:96. 10.3390/jof7020096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Wang C. G., Wu Y. D., Liu S., Yuan Y. (2021b). Two new brown rot polypores from tropical China. MycoKeys 82 173–197. 10.3897/mycokeys.82.68299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong T. K., Zhao H., Liu X. L., Ren L. Y., Zhao C. L., Liu X. Y. (2021). Taxonomy and. phylogeny of four new species in Absidia (Cunninghamellaceae, Mucorales) from China. Front. Microbiol. 12:677836. 10.3389/fmicb.2021.677836 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the sequences have been deposited in GenBank; the accession numbers are listed in Table 1.