Abstract
BACKGROUND
Solitary eosinophilic granuloma (EG) occurs anecdotally in the skull base region, and it has been described in only three previous publications. The authors report the first case of EG of the anterior clinoid process (ACP), which was confined to the ACP and presented with decreased vision.
OBSERVATIONS
A 38-year-old woman presented with decreased vision of the left eye of 5 months’ duration. Her visual acuity was 3/10, other neurological examinations were intact, and there were no other osseous or soft tissue lesions. The lesion was excised using a left-sided craniotomy and transdural clinoidectomy, decompressing the optic nerve both intra- and extradurally. The lesion was characteristic for EG, and no recurrence was detected after 2 years.
LESSONS
EG can be confined to the ACP and impair vision. Imaging studies are sensitive but not specific, and surgical decompression is both diagnostic and treatment oriented. Close observation and even adjuvant therapy may be indicated in similar cases.
Keywords: anterior clinoid process, clinoidectomy, eosinophilic granuloma, skull base
ABBREVIATIONS : ACP = anterior clinoid process, CT = computed tomography, EG = eosinophilic granuloma, LCH = Langerhans cell histiocytosis, LHC = Langerhans cell, MRI = magnetic resonance imaging
Solitary eosinophilic granuloma (EG) is a benign lesion growing as tumoral proliferation of antigen-presenting cells of dendritic origin coined Langerhans cells (LHCs). LHCs originate from myeloid dendritic cells, not from skin, and EG is the most common LHC histiocytosis (LCH).1 Viral infections (Epstein-Barr virus, human herpesvirus-6), immune dysfunctions, and bacteria may cause an increase in cytokines (such as interleukin-1 and -10).1,2 In the revised 2016 Histiocyte Society classification, LCH is considered an inflammatory myeloid neoplasm.1,2 LHC tumors mostly involve the flat bones, and more than 50% occur in the skull, mandible, spine, ribs, and pelvis. Skin, pituitary gland, brain, lung, liver, spleen, and gastrointestinal tract are less common tumor locations.1 EG can be differentiated from other infections and tumors by histology.1–4 To the best of our knowledge, no similar case of surgically treated, symptomatic EG confined to the anterior clinoid process (ACP) has been reported previously. We reviewed the relevant literature regarding clinical, imaging, and best treatment options for such a case.
Illustrative Case
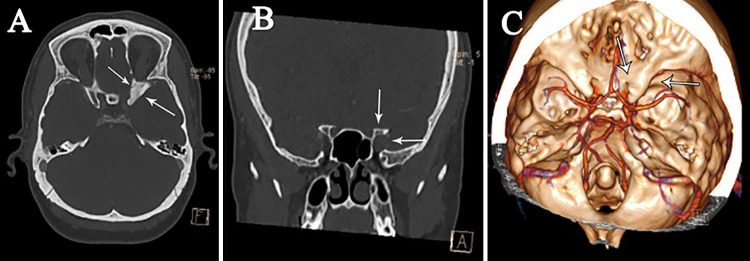
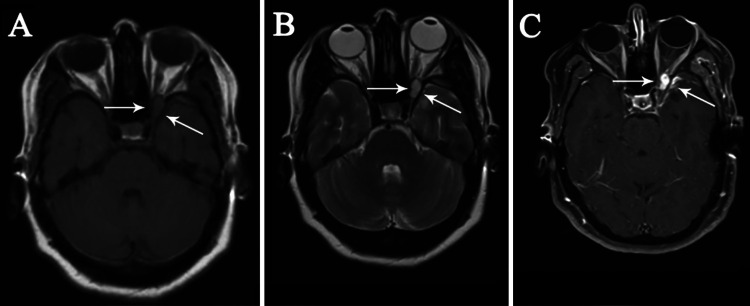
A 38-year-old woman presented with a progressive decrement of vision in her left eye of 5 months’ duration. Her visual acuity was 3/10 in the left eye; other neurological examinations were normal. Extraocular muscle movements were full, and fundoscopy was normal. She had no palpable adenopathy. Routine blood tests (such as complete blood count and erythrocyte sedimentation rate) and chest radiography results were normal. Brain computed tomography (CT) (Fig. 1) using bone window density showed a lesion of the left ACP with nonhomogeneous consistency, expanding and ballooning the left ACP in axial, coronal, and three-dimensional views. Brain magnetic resonance imaging (MRI) showed a well-defined ellipsoid lesion isointense on T1-weighted imaging and hyperintense on T2-weighted imaging, which enhanced homogeneously after contrast material injection and compressed the left optic nerve (Fig. 2).
FIG. 1.
Preoperative nonenhanced CT scans showing expanded left ACP in axial (A) and coronal (B) views of the optic canal, which is ballooned in the three-dimensional view (C) (small white arrows).
FIG. 2.
Preoperative axial T1-weighted (A), T2-weighted (B), and contrast-enhanced (C) MRI sequences showing an extradural, well-circumscribed round lesion as isointense on T1-weighted and hyperintense on T2-weighted images. T1-weighted images with gadolinium-DTPA demonstrate a mass with avid enhancement compressing the optic nerve. The dura overlying the ACP is also enhancing (small white arrows).
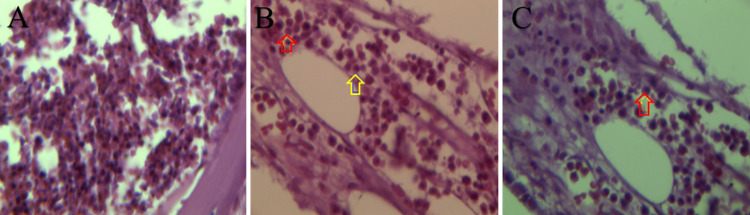
The patient underwent surgery through a left pterional craniotomy. After opening the Sylvian fissure and draining cerebrospinal fluid, the left optic nerve was identified. The nerve was squeezed by a bulging ACP. The overlying dura mater was found to be thicker and reddish colored. The falciform ligament and dura overlying the ACP were stripped to unroof the cranial side of the optic canal in the first step under microscopic dissection. ACP drilling showed porotic bone with honeycomb-like texture containing a small amount of creamy tissue and yellow liquid material. Drilling was done until reaching normal-looking bone at the bottom of ACP struts, relieving the squeezed optic nerve. Although no obvious air sinus opening was visible at this point, a small, thin piece of the fascia of the temporalis muscle was laid in the cavity and covered by a layer of SurgiCel (oxidized regenerated cellulose, Johnson & Johnson). The postoperative CT showed near-total resection of the tumor and acceptable decompression of the optic canal (Fig. 3). The postoperative course was uneventful. Histopathological examination confirmed the diagnosis of EG with typical LHCs, eosinophilic leukocyte infiltrations, coffee-bean nuclei, and eosinophilic cytoplasm (Fig. 4). Detailed staining was positive for CD1 antigen, S-100 protein, CD207 (Langerin), cyclin D1, and PNA (peanut agglutinin) and showed a paucity of nuclear atypia and atypical mitoses. Whole-body nuclear bone scan was negative. Because of the patient’s age, tumor location, and pathology, the neurooncologist started vinblastine with prednisolone for a period of 12 months.
FIG. 3.
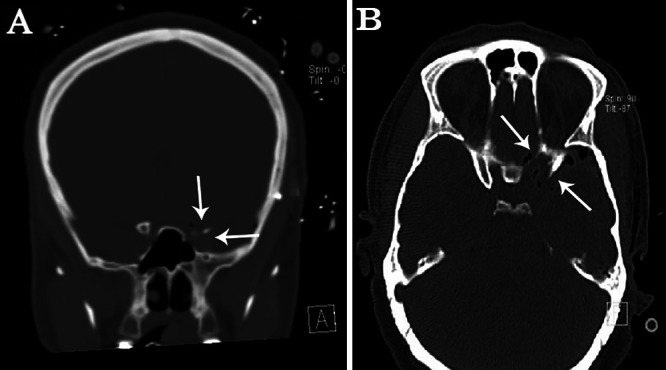
Early postoperative coronal (A) and axial (B) CT scans showing tumor removal through a right pterional approach and the surgical corridor, partially occupied by air bubbles (small white arrows).
FIG. 4.
Hematoxylin and eosin stain shows mixture of inflammatory cells (A), oval (B, yellow arrow) and coffee-bean nuclei (B, red arrow), and eosinophilic cytoplasm (C, red arrow).
The patient received clinical and radiological follow-up that showed neither residual disease nor recurrence after 2 years, and her visual acuity improved to 8/10.
Discussion
Genesis and Prevalence
The LCH group of diseases includes Letter-Siewe disease, Hand-Schuller-Christian disease, and EG. Whereas the former two diseases are systemic, the latter disease is a localized form of histiocytosis. It is a nonneoplastic chronic disease of granulomatous nature with unknown cause.1,2 EG occurs mostly in children up to young adolescence (i.e., 4 to 5 cases/million/year in children younger than 15 years versus 1 to 2 cases/million/year in adults similar to our case).1
Paraclinical Studies
Paraclinical test results are mostly normal in EG patients except for mild eosinophilia with punched-out lytic lesions on skull radiographs. These lytic lesions show a double contour and beveled edge due to greater erosion of the inner table than the outer and no sclerosis. On CT scans, the cortical erosion and soft tissue involvement can be better detected, as in our case (Fig. 1). On MRI, T1-weighted images typically show low signal intensity; on T2-weighted images, it can be iso- or hyperintense. Contrast enhancement can also be present.3,4
Natural History
The natural history and prognosis of LCH are variable. There is involvement of the hematopoietic system. Lung, liver, and spleen can change the natural course and prognosis of patients with LCH, but those with solitary bone lesions may even regress gradually and have a good prognosis. Involvement of multiple organs and more than one bone, with wide skull base orbit, ethmoid, sphenoid, and temporal bone being affected in patients with a mature skeleton, all have higher risk complications and recurrences with worse prognosis.3,4
Short Review of the Literature
To the best of our knowledge, only one case has been reported of primary skull base EG invading and enlarging the left optic canal with an enhancing mass showing on CT of the left ACP extending to the left orbital apex and to the medial face of the cavernous sinus presenting with left exophthalmos.3 Another primary EG extending from the cavernous sinus to the ACP and posterior ethmoidal region4 was a similar case we found in our review.
We think that timely referral of our patient may be another reason that prevented the lesion from progressing on the same course and presentation as other reported cases in the literature.
Treatment and Techniques
Excision of the lesion with curettage with normal margins is the treatment of choice as in our case, but biopsy and follow-up with adjuvant low-dose local irradiation, steroids, and cytotoxic agents are other treatment modalities for unresectable or recurrent LCH lesions.1–4 If a lesion in this location were not surgically curable,3,4 subtotal excision and adjuvant therapies would be indicated.
EG of ACP is rare, and approaches to this location can be extradural or intradural or a combination of both approaches.3,4 We used the intradural approach to extirpate the enhancing dura of the ACP also.
Observations
The patient was a 38-year-old woman who was referred with decreased vision in her left eye due to EG ballooning the left ACP, which could be extirpated via left transdural clinoidectomy. Histopathological features of the mass were characteristic of EG with low-grade histological invasion. No recurrence of the lesion was demonstrated 2 years after surgery.
Lessons
This is the first case of EG confined to the ACP and compromising the optic canal that presented with visual impairment. For symptomatic ACP lesions, surgical excision remains the main diagnostic and treatment option. It was treated successfully with decompression of the optic nerve and clinoidectomy and chemotherapy. The invasive nature of EG confined to ACP, as in our patient, mandated close follow-up and adjuvant therapy even after apparently gross total tumor excision. EG should be included in the differential diagnosis of the lesions of ACP.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Amirjamshidi. Acquisition of data: Amirjamshidi, Pour-Rashidi, Asem. Analysis and interpretation of data: Amirjamshidi. Drafting the article: Amirjamshidi, Pour-Rashidi, Abbasioun. Critically revising the article: Amirjamshidi, Abbasioun. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Amirjamshidi. Administrative/technical/material support: Amirjamshidi. Study supervision: Amirjamshidi.
References
- 1. Cohen Aubart F, Idbaih A, Emile JF, et al. Histiocytosis and the nervous system: from diagnosis to targeted therapies. Neuro Oncol. 2021;23(9):1433–1446. doi: 10.1093/neuonc/noab107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127(22):2672–2681. doi: 10.1182/blood-2016-01-690636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lunardi P, Farah JO, Qhaso R, Puzzilli F, Siciliano P, Di Stefano D. Primary eosinophilic granuloma invading the skull base: case report and critical review of the literature. Tumori. 1996;82(4):397–400. doi: 10.1177/030089169608200421. [DOI] [PubMed] [Google Scholar]
- 4. Harris NL, Mcneely WF, Jo-Anne O, et al. Case records of Massachusetts General Hospital. N Engl J Med. 2002;346(7) [Google Scholar]