Abstract
Background/Aim: A long-term effect has been confirmed in clinical practice since the introduction of nivolumab for treating various malignant tumors. A similar phenomenon is speculated to occur in head and neck cancer; however, details remain unclear due to the lack of long-term reports. We aimed to investigate the five-year outcomes in long-term responders for over two years, and evaluate the optimal duration of therapy with nivolumab.
Patients and Methods: In this retrospective observational study, we analyzed 203 cases of recurrent/metastatic head and neck squamous cell carcinoma (R/MHNSCC), including 33 long-term responders.
Results: The median overall survival (OS), 5-year OS, median progression-free survival (PFS), and 5-year PFS values in the 203 cases were 13.1 months, 19.2%, 3.1 months, and 13.2%, respectively. Of the 33 long-term responders, 14 (42.4%) continued using nivolumab for more than 2 years. The remaining 19 patients (57.6%) discontinued nivolumab. The most common reason for discontinuation was severe immune-related adverse events (irAEs) (9 cases; 27.3%); in these 9 cases, the median disease-free survival was 33.2 (range=10.7-44.3) months. Nine patients (21.2%) were considered to have progressive disease (PD) after at least 2 years of administration, and 3 patients (9.1%) requested to discontinue treatment because a complete response (CR) was achieved.
Conclusion: This study demonstrated the durable and long-term benefit of nivolumab in R/MHNSCC. In the future, we aim to accumulate real-world data for the establishment of criteria for completion of nivolumab treatment in long-term responders.
Keywords: Head and neck cancer, nivolumab, long-term responder, discontinuation
Since the CheckMate141 trial demonstrated the efficacy of nivolumab for head and neck squamous cell carcinoma (HNSCC) (1), many real-world outcome data have been reported (2-4). These data highlighted the fact that immune checkpoint inhibitors (ICIs), such as nivolumab, have properties distinctly different from those of conventional anti-cancer drugs. Immune-related adverse events (irAEs) represent one such difference. Adverse reactions to conventional anti-cancer drugs require treatment interruption or discontinuation and thus directly lead to a decreased survival rate; however, the prognosis of ICI-treated patients who developed irAEs was reported to be better than in those who did not among patients with head and neck cancer (2,4) as well as among patients with malignant melanoma (5) and various other types of cancer (6).
Another distinctive difference of ICIs from conventional anti-cancer drugs is a phenomenon called the long-term effect or durable response. Many patients receiving conventional anti-cancer drugs have to discontinue treatment due to progressive disease (PD) or intolerability to drug toxicities, and the survival rate in these patients is almost 0%. In contrast, tumor-inhibitory effects by ICIs have been reported to persist over a long period in small proportions (approximately 10-20%) of patients with lung cancer (7), malignant melanoma (8), kidney cancer (9), and various other types of cancer. However, no consensus has been established to date as to whether or not it is appropriate and when is the best time to discontinue ICI administration in cases where a long-term effect is maintained. A similar phenomenon is predicted to occur in head and neck cancer; however, details remain unclear because due to the paucity of long-term reports (10).
We herein report our investigation of the five-year outcomes in long-term responders for over two years to evaluate the optimal duration of therapy with nivolumab.
Patients and Methods
Patient cohort. Data in this retrospective observational study were derived from Kyushu University Hospital (Fukuoka, Japan) and other participating institutions (Kyushu Cancer Center and Saga University Faculty of Medicine). All patients were diagnosed with recurrent/metastatic HNSCC (R/MHNSCC) and treated with nivolumab between April 2017 to October 2021. Two hundred and three patients were eligible for inclusion. They were observed until death or until the cut-off date (March 2022). The median follow-up interval was 12.9 months (range=0.3-60.1 months).
All patients received nivolumab (240 mg/body, once every 2 weeks or 480 mg/body, once every 4 weeks) until PD or development of severe irAEs. Tumor responses were evaluated every 8 to 12 weeks by computed tomography.
Eighty-seven patients received second-line anti-cancer drugs due to PD with nivolumab treatment. Sixty-six patients received paclitaxel (80 mg/m2, once every week) plus cetuximab (250 mg/m2, once every week) chemotherapy, and the remaining 21 were treated with S-1 (80-120 mg/body/day).
This retrospective study was approved by the Institutional Ethics Review Board of Kyushu University (No. 2021-138) and each participating institution and performed in accordance with the principles of the Declaration of Helsinki. Regarding informed consent, patients were able to decline to participate by opting out in response to an official announcement on the institutions’ websites.
Relevant evaluations. The response to treatment was evaluated according to the Response Evaluation Criteria in Solid Tumors version1.1 (RECIST 1.1), and the best overall response (BOR) of all patients was categorized as complete response (CR), partial response (PR), stable disease (SD), or PD.
The overall survival (OS) was defined as the time from the first cycle of Nivolumab to death, and progression-free survival (PFS) was defined the time from the first cycle of Nivolumab to first PD (according to RECIST).
Statistical analyses. The statistical analyses were performed using the SPSS statistics software program, ver. 22.0 (IBM Japan, Ltd., Tokyo, Japan). The OS and PFS were calculated using the Kaplan-Meier method. In correlation with patient background data, the categorical variables were analyzed using Fisher’s exact test, and the continuous variables were analyzed using the Mann-Whitney U-test. A p-Value of <5% was considered statistically significant.
Results
Treatment response and survival outcome. Patient clinical characteristics are shown in Table I. In all patients, the BOR was CR, PR, SD, and PD in 22 patients (10.8%), 39 patients (19.2%), 33 patients (16.3%), and 109 patients (53.7%), respectively (Figure 1).
Table I. Patient characteristics.
PFS: Progression-free survival; ECOG: Eastern Cooperative Oncology Group; PS: performance status; irAEs: immune-related adverse events. Bold p-Value indicates statistical significance.
Figure 1. Best overall responses (BORs) in all of 203 patients and the 33 long responders. In all patients, complete response (CR) and partial response (PR) rate was 10.8% and 19.2%. In contrast, in the longprogression-free survival (PFS) group, CR and PR rate was 51.5% and 39.4%, respectively.

The median OS was 13.1 months, the 5-year OS was 19.2% (Figure 2a), the median PFS was 3.1 months, and the 5-year PFS was 13.2% (Figure 2b). Of the 203 patients analyzed, 33 (16.3%) showed no tumor progression at least for 2 years and exhibited a durable response (defined as the long-PFS group). In both the long- and non-long-PFS groups, the mean age of patients was 66 years, and most patients were male (72.7% and 80.0%, respectively), had a PS 0 or 1 (97.0% and 84.1%, respectively), and were platinum-resistant (69.7% and 74.1%, respectively). At the time of the analysis, the percentages of patients who developed irAEs in the long- and non-long-PFS groups were 63.6% and 25.3%, respectively. Only the incidence of irAEs showed a significant difference between the groups (p=0.002) (Table I).
Figure 2. Survival curves. (a) Overall survival (OS) curve for 203 patients. (b) Progression-free survival (PFS) curve for 203 patients. The 5-year OS and PFS was 19.2% and 13.2%, respectively. Long-term effect was shown in recurrent/metastatic head and neck squamous cell carcinoma (R/MHNSCC) patients treated with nivolumab.
Timing of PD development. At the time of the analysis, a total of 164 patients were considered to have PD. Of these 164 patients, 147 (89.6%) developed PD within 1 year after nivolumab administration was initiated, 10 (6.1%) developed PD after 1-2 years of nivolumab administration, and 7 (4.3%) developed PD after receiving nivolumab for ≥2 years (Figure 3).
Figure 3. Frequency distribution of time to progressive disease (PD) in patients who developed PD at the time of the analysis. The 89.6% of patients developed PD within 1 year after nivolumab administration, while it was only 4.3% that developed PD after receiving nivolumab for over 2 years.

Outcomes in long-PFS patients. In the long-PFS group, the BOR was CR, PR, and SD in 17 patients (51.5%), 13 patients (39.4%), and 3 patients (9.1%), respectively (Figure 1).
Of the 33 patients, 14 (42.4%) continued nivolumab for more than 2 years (defined as the ongoing group), and 19 (57.6%) discontinued nivolumab. Nivolumab was discontinued due to severe irAEs (9 cases; 27.3%) or PD after administration for more than 2 years (7 cases; 21.2%). The remaining 3 patients (9.1%) discontinued nivolumab therapy after achieving CR (Figure 4).
Figure 4. Treatment status in the 33 long responders.

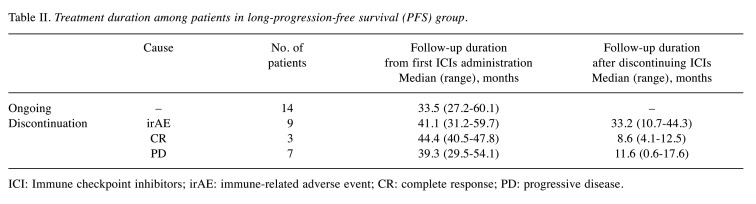
In the ongoing group, the median duration of nivolumab administration was 33.5 (range=27.2-60.1) months, and one patient continued to receive nivolumab for five years. In patients who discontinued nivolumab for severe irAEs, the median disease-free survival after discontinuation was 33.2 (range=10.7-44.3) months. In patients who discontinued nivolumab upon their request after achieving CR, the median disease-free survival after discontinuation was 8.6 (range=4.1-12.5) months. The treatment duration and withdrawal period among patients in the long-PFS group are shown in Table II.
Table II. Treatment duration among patients in long-progression-free survival (PFS) group.
ICI: Immune checkpoint inhibitors; irAE: immune-related adverse event; CR: complete response; PD: progressive disease.
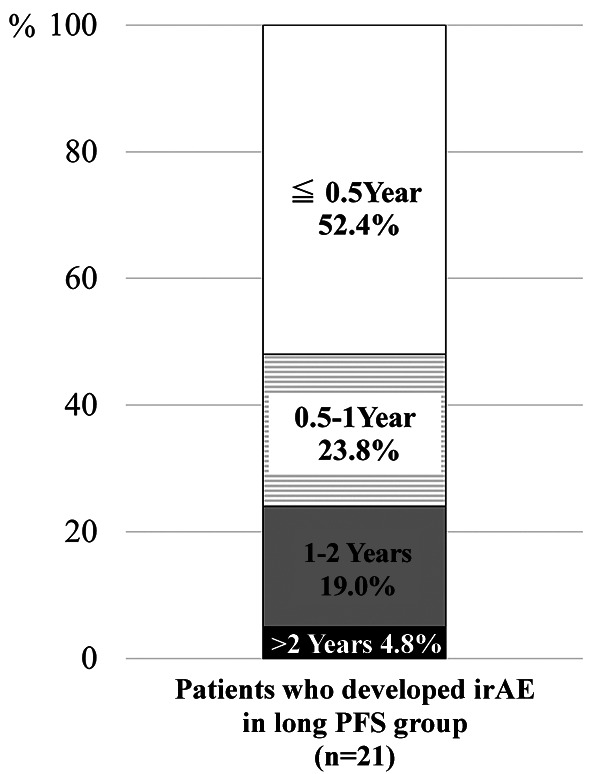
At the time of the analysis, a total of 21 patients in the long-PFS group had developed irAEs. Of these 21 patients, 11 (52.4%) developed irAEs within 6 months after starting nivolumab administration, 5 (23.8%) developed irAEs from 6 months to 1 year, 4 (19.0%) developed irAEs from 1-2 years, and 1 (4.8%) developed irAEs after receiving nivolumab for more than 2 years (Figure 5).
Figure 5. Timing of immune-related adverse events (irAE) developing in the long- progression-free survival (PFS) group (n=21). In the longPFS group, 52.4% of the patients developed irAEs within 6 months after starting nivolumab administration. The 4.8% of the patients developed irAEs even if more than two years passed after receiving nivolumab.
Discussion
The long-tail phenomenon has been confirmed in survival curves for cancer patients in whom ICIs were introduced. This phenomenon reflects the durable and long-term benefits of ICIs, which have not been seen with conventional anti-cancer drugs. The reported 5-year survival rates in lung cancer, malignant melanoma, and kidney cancer patients treated with ICIs were 16% (11), 34% (12), and 26% (9), respectively, showing that long-term benefits are commonly seen with various malignant tumors despite these patients having incurable, advanced diseases. Long-term benefits have also been noted in esophageal cancer and head and neck cancer patients, with 2-year survival rates of 17.2% (13) and 16.9% (10), respectively. The 5-year survival rate shown in our study in R/MHNSCC patients who underwent nivolumab treatment was 19.2%, confirming that some patients can achieve a durable long-term benefit even 5 years after treatment.
Studies have begun to explore whether or not ICIs can be discontinued without disease progression during the long-term durable benefit, and if they can, when is the best time to discontinue. A study in lung cancer patients who received pembrolizumab for 2 years before it was discontinued reported good PFS rates of 72.5% and 57.7% at 1 and 2 years after discontinuation, respectively, suggesting that pembrolizumab can be discontinued after 2 years (14). A different study in lung cancer patients who received pembrolizumab for 1 year before it was discontinued reported that the median PFS was better in the continuation group than in the discontinuation group (24.7 vs. 9.4 months), suggesting that treatment discontinuation after 1 year requires careful consideration (15). Yet another study in lung cancer patients who underwent ICI treatment for palliation for at least 24 consecutive months reported that ICI treatment could be discontinued without issue after positron emission tomography-based confirmation of completely lost tumor growth activity and CR (16).
In the present study, we found that, among R/MHNSCC patients, those who developed PD after more than 2 years of nivolumab treatment accounted for about 4.3% of all patients who were diagnosed with PD. Based on this finding, we feel that treatment discontinuation may be considered after maintenance administration of nivolumab for two years. However, in our study, only 3 patients who achieved CR discontinued nivolumab after 2 years of treatment, and the median follow-up duration in these patients was short (8.5 months). Thus, a longer observation in a larger number of patients will be necessary.
There is no settled theory as to what cases are long-term responders to nivolumab. However, the possibility that PDL-1 expression may be involved in the efficacy of nivolumab has been previously pointed out (1), it has also recently been suggested that tumor proportion score (TPS) may be a predictor of nivolumab efficacy (17). We intend to add a further study of the relationship between PDL-1 expression and long-term responders to nivolumab. In this study, the incidence of irAEs was significantly higher in long-term responders. Although a factor after treatment, the development of irAEs is considered a predictor of long-term nivolumab efficacy.
Regarding irAEs, approximately 10% of patients present with grade ≥3 irAEs (1). However, approximately 60% of long-term responders in this study developed irAEs, and approximately 25% of them had to discontinue nivolumab due to severe irAEs. The patients who discontinued nivolumab due to severe irAEs remained tumor-free for a median of 33 months (up to 44 months) after discontinuation. In fact, previous studies have reported that tumors remained suppressed even after patients had to discontinue ICI treatment for a long-time due to severe irAEs in head and neck cancer patients (18) as well as in patients with malignant melanoma (19) and various carcinomas (20). Therefore, we should consider the development of irAEs to be a good sign suggesting a favorable prognosis, and we should observe the progress with appropriate management for irAEs. We believe that the above follow-up will lead to prognostic improvement for R/MHNSCC patients receiving nivolumab.
Conclusion
Nivolumab has been used to treat R/MHNSCC in clinical practice for only five years, and the evaluation of its long-term benefits has just begun. We are pleased that the durable and long-term benefits of nivolumab in R/MHNSCC patients were confirmed in this study. We hope to accumulate further real-world data for the establishment of criteria concerning the completion of nivolumab treatment in long-term responders.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
Ryuji Yasumatsu, the corresponding author, designed the work. Mioko Matsuo had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of data analysis, and wrote the manuscript. Muneyuki Masuda and Moriyasu Yamauchi revised and edited the work critically. Takahiro Wakasaki and Kazuki Hashimoto statistically interpreted the data. Rina Jiromaru and Tomomi Manako performed collection of data. Takashi Nakagawa drafted the work. All Authors approved the final version of the manuscript.
References
- 1.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hori R, Shinohara S, Kojima T, Kagoshima H, Kitamura M, Tateya I, Tamaki H, Kumabe Y, Asato R, Harada H, Kitani Y, Tsujimura T, Honda K, Ichimaru K, Omori K. Real-world outcomes and prognostic factors in patients receiving nivolumab therapy for recurrent or metastatic head and neck carcinoma. Cancers (Basel) 2019;11(9):1317. doi: 10.3390/cancers11091317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okamoto I, Sato H, Kondo T, Koyama N, Fushimi C, Okada T, Miura K, Matsuki T, Yamashita T, Omura G, Tsukahara K. Efficacy and safety of nivolumab in 100 patients with recurrent or metastatic head and neck cancer - a retrospective multicentre study. Acta Otolaryngol. 2019;139(10):918–925. doi: 10.1080/00016489.2019.1648867. [DOI] [PubMed] [Google Scholar]
- 4.Matsuo M, Yasumatsu R, Masuda M, Toh S, Wakasaki T, Hashimoto K, Taura M, Uchi R, Nakagawa T. Relationship between immune-related adverse events and the long-term outcomes in recurrent/metastatic head and neck squamous cell carcinoma treated with nivolumab. Oral Oncol. 2020;101:104525. doi: 10.1016/j.oraloncology.2019.104525. [DOI] [PubMed] [Google Scholar]
- 5.Cybulska-Stopa B, Ziętek M, Czarnecka AM, Piejko K, Dziura R, Galus Ł, Ziółkowska B, Kieszko S, Kempa-Kamińska N, Calik J, Seredyńska J, Gądek K, Zemełka T, Teterycz P, Kubiatowski T, Suwiński R, Mackiewicz J, Rutkowski P. Development of immunity-related adverse events correlates with baseline clinical factors, survival and response to anti-PD-1 treatment in patients with inoperable or metastatic melanoma. J Dermatolog. 2021;Treat:1–7. doi: 10.1080/09546634.2021.1937477. [DOI] [PubMed] [Google Scholar]
- 6.Hussaini S, Chehade R, Boldt RG, Raphael J, Blanchette P, Maleki Vareki S, Fernandes R. Association between immune-related side effects and efficacy and benefit of immune checkpoint inhibitors - A systematic review and meta-analysis. Cancer Treat Rev. 2021;92:102134. doi: 10.1016/j.ctrv.2020.102134. [DOI] [PubMed] [Google Scholar]
- 7.Ito S, Asahina H, Honjo O, Tanaka H, Honda R, Oizumi S, Nakamura K, Takamura K, Hommura F, Kawai Y, Ito K, Sukoh N, Yokoo K, Morita R, Harada T, Takashina T, Goda T, Dosaka-Akita H, Isobe H, Hokkaido Lung Cancer Clinical Study Group Trial Prognostic factors in patients with advanced non-small cell lung cancer after long-term Anti-PD-1 therapy (HOT1902) Lung Cancer. 2021;156:12–19. doi: 10.1016/j.lungcan.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Hwu WJ, Hamid O, Ribas A, Weber JS, Daud AI, Hodi FS, Wolchok JD, Mitchell TC, Hersey P, Dronca R, Joseph RW, Boutros C, Min L, Long GV, Schachter J, Puzanov I, Dummer R, Lin J, Ibrahim N, Diede SJ, Carlino MS, Joshua AM. Long-term safety of pembrolizumab monotherapy and relationship with clinical outcome: A landmark analysis in patients with advanced melanoma. Eur J Cancer. 2021;144:182–191. doi: 10.1016/j.ejca.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer RJ, Escudier B, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Plimack ER, Procopio G, McDermott DF, Castellano D, Choueiri TK, Donskov F, Gurney H, Oudard S, Richardet M, Peltola K, Alva AS, Carducci M, Wagstaff J, Chevreau C, Fukasawa S, Tomita Y, Gauler TC, Kollmannsberger CK, Schutz FA, Larkin J, Cella D, McHenry MB, Saggi SS, Tannir NM. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156–4167. doi: 10.1002/cncr.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington KJ, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Docampo LCI, Haddad R, Rordorf T, Kiyota N, Tahara M, Lynch M, Jayaprakash V, Li L, Gillison ML. Nivolumab vs. investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51. doi: 10.1016/j.oraloncology.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gettinger S, Horn L, Jackman D, Spigel D, Antonia S, Hellmann M, Powderly J, Heist R, Sequist LV, Smith DC, Leming P, Geese WJ, Yoon D, Li A, Brahmer J. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36(17):1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 12.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph R, Weber JS, Dronca R, Mitchell TC, Patnaik A, Zarour HM, Joshua AM, Zhao Q, Jensen E, Ahsan S, Ibrahim N, Ribas A. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann Oncol. 2019;30(4):582–588. doi: 10.1093/annonc/mdz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato K, Doki Y, Ura T, Hamamoto Y, Kojima T, Tsushima T, Hironaka S, Hara H, Kudo T, Iwasa S, Muro K, Yasui H, Minashi K, Yamaguchi K, Ohtsu A, Kitagawa Y. Long-term efficacy and predictive correlates of response to nivolumab in Japanese patients with esophageal cancer. Cancer Sci. 2020;111(5):1676–1684. doi: 10.1111/cas.14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Garon EB, Kim DW, Cho BC, Perez-Gracia JL, Han JY, Arvis CD, Majem M, Forster MD, Monnet I, Novello S, Szalai Z, Gubens MA, Su WC, Ceresoli GL, Samkari A, Jensen EH, Lubiniecki GM, Baas P. Long-term outcomes and retreatment among patients with previously treated, programmed death-ligand 1-positive, advanced non-small-cell lung cancer in the KEYNOTE-010 study. J Clin Oncol. 2020;38(14):1580–1590. doi: 10.1200/JCO.19.02446. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse DM, Garon EB, Chandler J, McCleod M, Hussein M, Jotte R, Horn L, Daniel DB, Keogh G, Creelan B, Einhorn LH, Baker J, Kasbari S, Nikolinakos P, Babu S, Couture F, Leighl NB, Reynolds C, Blumenschein G Jr, Gunuganti V, Li A, Aanur N, Spigel DR. Continuous versus 1-year fixed-duration nivolumab in previously treated advanced non-small-cell lung cancer: CheckMate 153. J Clin Oncol. 2020;38(33):3863–3873. doi: 10.1200/JCO.20.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferdinandus J, Metzenmacher M, Kessler L, Umutlu L, Aigner C, Karl KO, Grünwald V, Eberhardt W, Fendler WP, Herrmann K, Faehling M, Christoph DC. Complete metabolic response in patients with advanced nonsmall cell lung cancer with prolonged response to immune checkpoint inhibitor therapy. J Immunother Cancer. 2021;9(3):e002262. doi: 10.1136/jitc-2020-002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito T, Okamoto I, Tokashiki K, Sato H, Okada T, Yamashita G, Nagao T, Hirai H, Saigusa N, Tsukahara K. PD-L1 expression and survival rates using TPS and CPS for nivolumab-treated head-and-neck cancer. Anticancer Res. 2022;42(3):1547–1554. doi: 10.21873/anticanres.15628. [DOI] [PubMed] [Google Scholar]
- 18.Yasumatsu R, Matsuo M, Wakasaki T, Masuda M, Takeuchi T, Manako T, Jiromaru R, Uchi R, Hashimoto K, Nakagawa T. Clinical outcome in recurrent and/or metastatic head and neck cancer patients after discontinuation of nivolumab monotherapy due to immune-related adverse events. Acta Otolaryngol. 2020;140(12):1043–1048. doi: 10.1080/00016489.2020.1807601. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Rodrigues AJ, Bhambhvani HP, Fatemi P, Pollom EL, Gibbs IC, Thomas RP, Soltys SG, Hancock SL, Chang SD, Reddy SA, Gephart MH, Li G. Intracranial tumor control after immune-related adverse events and discontinuation of immunotherapy for melanoma. World Neurosurg. 2020;144:e316–e325. doi: 10.1016/j.wneu.2020.08.124. [DOI] [PubMed] [Google Scholar]
- 20.Albandar HJ, Fuqua J, Albandar JM, Safi S, Merrill SA, Ma PC. Immune-related adverse events (irAE) in cancer immune checkpoint inhibitors (ICI) and survival outcomes correlation: To rechallenge or not. Cancers (Basel) 2021;13(5):989. doi: 10.3390/cancers13050989. [DOI] [PMC free article] [PubMed] [Google Scholar]