Abstract
Background/Aim: The majority of targeted therapies are focused on BRCA mutations, homologous recombination repair deficiency, and BRCA wild-type platinum-sensitive recurrent ovarian cancer. There is a growing need for platinum-resistant patients without BRCA mutations. Herein, we conducted a phase II multicenter study evaluated the efficacy and safety of bortezomib plus pegylated liposomal doxorubicin (PLD) in patients with BRCA wild-type platinum-resistant recurrent ovarian cancer (NCT03509246).
Patients and Methods: Ovarian cancer patients with wild-type BRCA who experienced platinum-resistant recurrence after three or less prior treatment cycles from three Institutions were included. All patients received bortezomib, 1.3 mg/m2 subcutaneously (days 1, 4, 8, and 11), and PLD, 40 mg/m2 intravenously (day 4), every 4 weeks. The primary endpoint was best objective response rate (ORR), and secondary endpoints included disease control rate, progression-free survival (PFS), overall survival, and safety. Targeted sequencing was performed to evaluate biomarkers and their potential association with response to treatment.
Results: The trial was terminated after 23 patients were recruited because of slow accrual. The median follow-up was 29.5 months. The overall ORR was 8.7% (2/23); partial response was observed in two patients. The median duration of response was 10.5 months, and median PFS was 2.9 months. Treatment-related adverse events (TRAEs) of grade 3/4 were reported in 43.5% of patients. One patient who exhibited TRAEs discontinued treatment. However, grade 4/5 TRAEs were not observed. Mutations in TP53 and CDK12 were detected in 67% (14/21) and 24% (12/21) of patients, respectively. Two patients with partial response harbored mutations in genes related to homologous recombination repair deficiency, including BRCA2, ATM, and CDK12.
Conclusion: The combination of bortezomib and PLD was well tolerated; however, antitumor activity was not sufficient to warrant further investigation in ovarian cancer.
Keywords: Bortezomib, pegylated liposomal doxorubicin, antitumor activity, platinum-resistant recurrent ovarian cancer, BRCA
Ovarian cancer is one of the most lethal gynecological cancers in women (1). Although the majority of patients with ovarian cancer initially respond to front-line therapy involving maximal cytoreductive surgery combined with platinum-based chemotherapy, unfortunately, most patients relapse during or after treatment with eventual drug-resistant disease (2). Platinum resistance is defined as disease relapse within six months of the last platinum-based chemotherapy dose (3). Non-platinum-based single-agent chemotherapy is the standard of care. Patients with platinum-resistant disease typically have low response rates to subsequent chemotherapy (<10%), median progression-free survival (PFS) of approximately 3 months, and median overall survival (OS) of approximately 12 months (4,5).
To achieve higher responses, novel agents or treatment regimens need to be developed. Platinum-resistant disease can be treated with non-platinum-based chemotherapy or molecularly targeted agents, such as poly-ADP-ribose polymerase inhibitors (PARPis) or immunotherapy. PARPis have emerged as novel agents for patients with recurrent ovarian cancer in various settings, including for treatment of BRCA mutation-related relapsed disease (6,7) or as maintenance therapy in patients with platinum-sensitive recurrence (8,9). However, PARPis are not very effective in patients with wild-type BRCA. The addition of bevacizumab to chemotherapy showed PFS benefit for patients with platinum-resistant disease regardless of BRCA mutation (4). The efficacy of immune checkpoint inhibitors has been disappointing in platinum-resistant settings (10,11). The introduction of targeted agents and immunotherapies have not yet shown major advances beyond the addition of bevacizumab to chemotherapy in patients with platinum-resistant disease.
The Cancer Genome Atlas suggested 22 druggable targets in 2011. Among them, CCNE1 amplification is found in approximately 20%, and BRCA mutation has been reported to be mutually exclusive (12). Preclinical data supported that CCNE1-amplified tumor cells show specific sensitivity to the proteasome inhibitor bortezomib and suggested the possibility of a unique therapeutic approach in high-grade serous ovarian cancer (HGSC) (13). Bortezomib is one of the most commonly used drugs in multiple myeloma (14). In a phase III trial, combination of bortezomib and pegylated liposomal doxorubicin (PLD) had a synergistic effect on survival compared to PLD monotherapy in patients with relapsed or refractory multiple myeloma (15). PLD is commonly used for the treatment of platinum-resistant ovarian cancer. Therefore, we hypothesized that combining bortezomib and PLD would be effective and safe in patients with BRCA wild-type platinum-resistant ovarian cancer.
Patients and Methods
Study design and participants. A phase II, single-arm, multicenter study was conducted at three cancer centers across the Korean Gynecologic Oncology Group. Eligible patients were women aged 19 years or older, who had platinum-resistant (defined as progression within 6 months after the last platinum-based chemotherapy) or refractory (defined as progression within 1 month after the last platinum-based chemotherapy) recurrent ovarian cancer with BRCA wild-type and a histologically confirmed diagnosis of high-grade serous, endometrioid, carcinosarcoma, mixed Müllerian with high-grade serous component, clear-cell, or low-grade serous, primary peritoneal cancer, or fallopian tube cancer. Other inclusion criteria were an Eastern Cooperative Oncology Group performance status of 0-2, adequate bone marrow function (absolute neutrophil count ≥1,500 cells/ml, hemoglobin level ≥9.0 g/dl, and platelet count ≥100,000/ml), adequate renal function [creatinine level ≤1.5× the upper limit of normal (ULN)], and hepatic function (alanine and aspartate aminotransferase concentrations ≤3× ULN or ≤3× ULN in case of liver metastases and total bilirubin level ≤1.5× ULN).
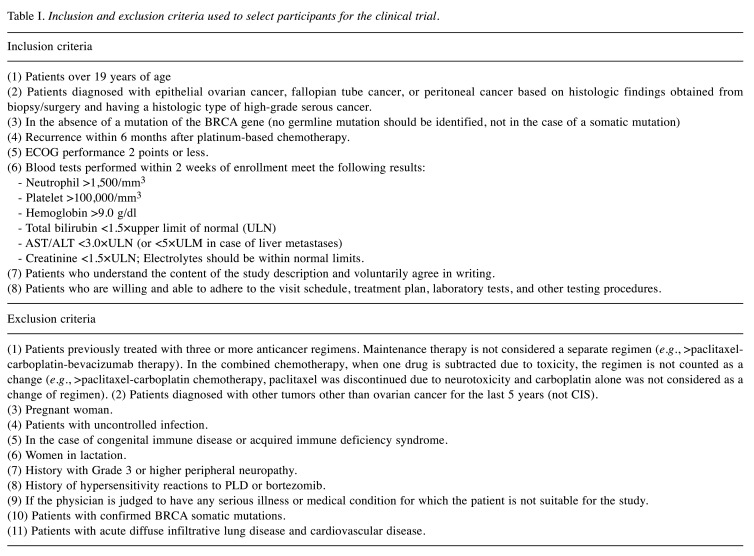
Key exclusion criteria included prior use of four or more lines of anticancer therapies, suspected deleterious mutation (germline or somatic) in BRCA, known additional malignancy that is progressing or has required active treatment within the last 3 years, and active infection, including tuberculosis, hepatitis B, hepatitis C, or human immunodeficiency virus. All inclusion and exclusion criteria are listed in Table I.
Table I. Inclusion and exclusion criteria used to select participants for the clinical trial.
This study was approved by the institutional review boards of the participating centers (IRB No., Yonsei University Severence Hospital 4-2017-1189; Seoul National University Bundang Hospital 1712-034-905; Seoul National University College of Medicine B-1709-420-004). All participants provided informed consent, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines.
Procedures. Patients received bortezomib, 1.3 mg/m2, as a subcutaneous injection (days 1, 4, 8, and 11), and PLD, 40 mg/m2, intravenous infusion, for more than 60 min on day 4 of a 4-week cycle. Patients could receive up to six cycles and continued treatment until disease progression or unacceptable side-effects.
Dose modifications (interruptions and reductions) were allowed for the management of adverse events, and dose re-escalation was not allowed. Two dose reductions were allowed for both bortezomib (first reduction to 1.1 mg/m2, second reduction to 0.9 mg/m2) and PLD (first reduction to 30 mg/m2, second reduction to 20 mg/m2) in the event of toxicity. Bortezomib and PLD were interrupted in patients with grade 4 toxicities. For grade 3 toxicities, patients underwent dose reduction, and for grade 2 or below toxicities, patients were maintained on the same dose. At first occurrence of grade 3/4 toxicities, bortezomib and PLD were delayed up to 3 weeks until recovery to grade 1 or improvement. When non-hematologic toxicities were recovered, but the hematologic toxicities were grade 2, patients underwent one dose reduction of PLD, and when hematological toxicities were grade 3, patients underwent permanent discontinuation. When non-hematologic toxicity except for hair loss and neurotoxicity did not recover to grade 1 or lower by 3 weeks from the scheduled administration time, patients underwent permanent discontinuation. When non-hematologic toxicities and hematologic toxicities were recovered, but neurotoxicity was grade 2, patients underwent one dose reduction of bortezomib, and for grade 3/4, patients underwent permanent discontinuation. When neutropenic fever occurred in the previous cycle, patients underwent one dose reduction of PLD.
Measurable disease and tumor response were assessed using RECIST version 1.1. Tumor response was assessed using contrast CT scans after three and six cycles of chemotherapy. Additional chest CT, pelvic MRI, and whole-body PET-CT were recommended if investigators considered it necessary for the evaluation of tumor response. Safety and adverse events were assessed on the day of each cycle and were graded according to the NCI Common Terminology Criteria for Adverse Events version 4.0.
Outcomes. The primary endpoint was the objective response rate (ORR) according to RECIST version 1.1, which included the patients with measurable disease who had a complete or partial response. Secondary endpoints were PFS, OS, duration of response, proportion of disease control, and safety. PFS was defined as the time from the start of treatment until the first documented sign of disease progression or death from any cause, and OS was defined as the time from the first treatment until death from any cause. Duration of response was assessed in patients who achieved a response and was defined as the time from the first documented response until the time of documented progression or death from any cause. Disease control was defined as the proportion of patients who achieved a complete or partial response or stable disease.
Statistical analysis. We used the A’Hern single-stage design to calculate sample size. Previously reported data showed that the ORR of single-agent chemotherapy was 11.8% and that of single-agent chemotherapy combined with bevacizumab was 27.3% in platinum-resistant recurrent ovarian cancer (4). We expected that the objective response for bortezomib plus PLD would be 15%. If a total of 8 or more responses were observed, the treatment regimen was considered a success. In a single-stage phase II design with a one-sided 5% level of significance and 80% power, a single arm requires approximately 40 patients. Allowing for a follow-up loss rate of 10%, the total sample size was expected to be 44 patients.
We analyzed efficacy and safety in the intention-to-treat patients; all patients received at least one treatment dose. We calculated the proportions of patients achieving responses and associated 95% CIs using the Clopper-Pearson method as well as the median duration of response and PFS and associated 95% CIs using the Kaplan-Meier method. Survival analysis was performed using the Kaplan-Meier method with a log-rank test. Based on a decision of the Data Safety Monitoring Board, this study was terminated before completion of the planned patient recruitment because of poor accrual, and we analyzed data that were collected by the cutoff date of May 31, 2021. Statistical analyses were performed using the SPSS statistical software (version 21.0; IBM, Armonk, NY, USA).
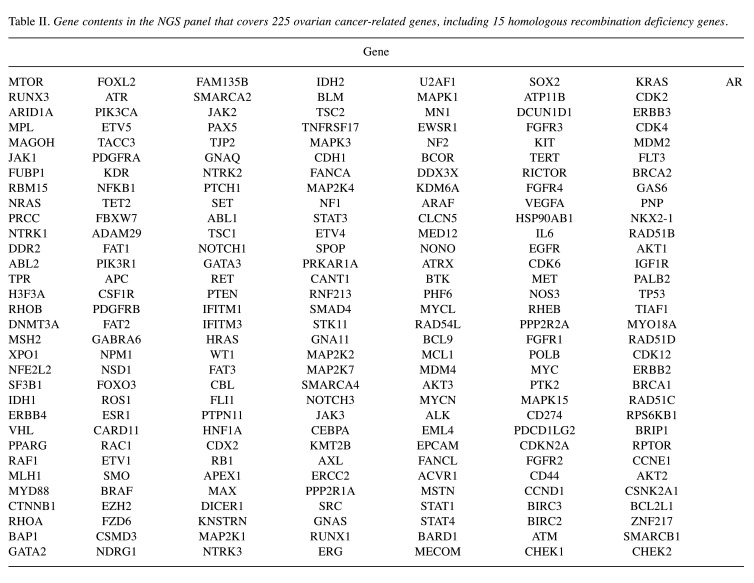
Library preparation and next-generation sequencing (NGS). An NGS panel was designed for the detection of mutations in 225 ovarian cancer-related genes, including 15 homologous recombination deficiency genes (Table II). Custom RNA probes were designed for target-enrichment sequencing (Celemics, Seoul, Republic of Korea) and covered all unions of reported exons of the 225 genes (total count of regions was 3,690). The panel covered 637,421 bases of the human genome (hg19). All transcripts of genes reported in the UCSC database were included as targets to comprehensively detect SNVs, small insertion-deletion mutations, and structural variants.
Table II. Gene contents in the NGS panel that covers 225 ovarian cancer-related genes, including 15 homologous recombination deficiency genes.
For exploratory genomic analyses, tumor samples were prepared from formalin-fixed, paraffin-embedded tissues before treatment. Eighteen specimens were included in this study. Genomic DNA was sheared and processed by end-repair, dA-tailing, adapter ligation, and pre-PCR for indexation of the NGS library for Illumina sequencing. Genomic DNA and capture probes were hybridized to the capture target regions using a Celemics Target Enrichment Kit. Captured regions were amplified by post-PCR for enrichment. The captured library was then sequenced on an Illumina NextSeq550 instrument (Illumina, San Diego, CA, USA), generating 2×150-bp reads.
Results
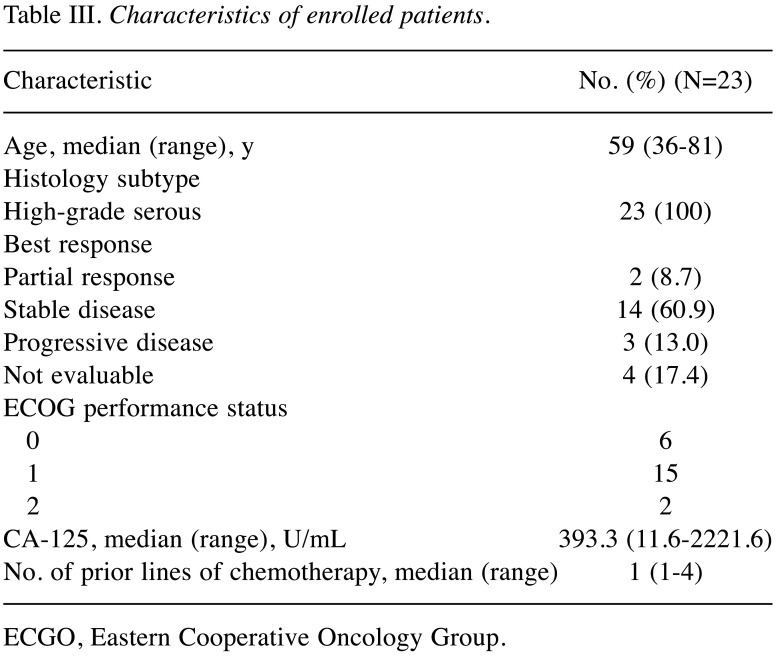
Patient characteristics. Between May 2018 and Jan 2020, 23 patients were enrolled in the study (Figure 1) and included in the intention-to-treat analysis. The median duration of follow-up at the time of data analysis (data cutoff point was Jan 31, 2021) was 29.5 months. At the data cutoff point, all 23 (100%) patients had discontinued the study; 19 (82.6%) patients discontinued of disease progression, 1 (4.4%) patient discontinued because of adverse events, and 3 (13%) patients were lost to follow-up. The clinical characteristics of the patients are shown in Table III.
Figure 1. Trial design.
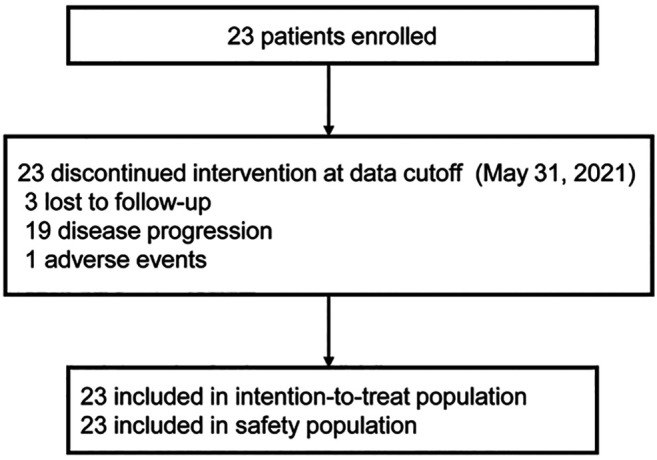
Table III. Characteristics of enrolled patients.
ECGO, Eastern Cooperative Oncology Group.
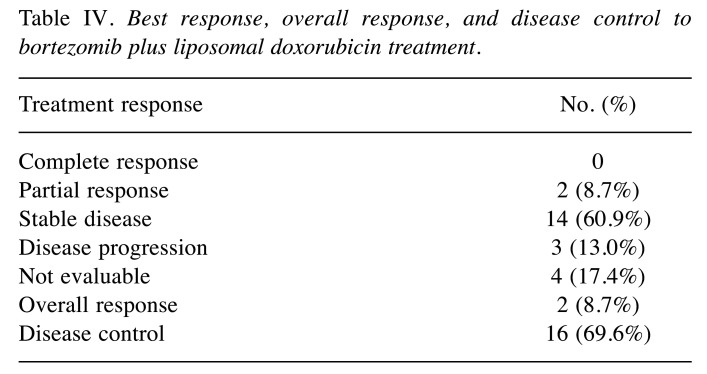
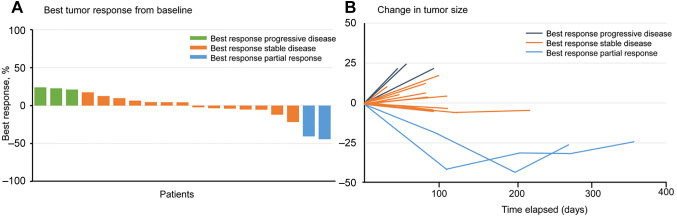
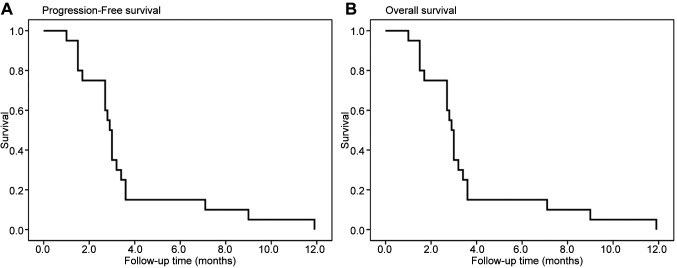
Outcomes. Of the 23 patients, 19 patients were evaluable for objective response using the RECIST criteria. The overall confirmed ORR was 8.7% (2/23); partial response was observed in two patients. Disease control was achieved in 69.6% (16/23) patients (Table IV). Tumor shrinkage was noted in 9 (39.1%) of 23 patients who had at least one post-baseline efficacy assessment (Figure 2). The mean best percentage change of the target lesion size from the baseline was –0.6% (SD 19.1). The median duration of response was 10.5 months. At the data cutoff point, all 23 (100%) patients had disease progression and 9 (39.1%) patients had died. Furthermore, the median PFS was 2.9 months, and median OS was 19.0 months (Figure 3).
Table IV. Best response, overall response, and disease control to bortezomib plus liposomal doxorubicin treatment.
Figure 2. Best percentage change from the baseline in target lesion size in patients. (A) Waterfall plot showing the percentage change in tumor size from baseline constituting the best response for each patient. (B) Spider plot showing responses for all patients.
Figure 3. Kaplan-Meier curves of progression-free survival (A) and overall survival (B).
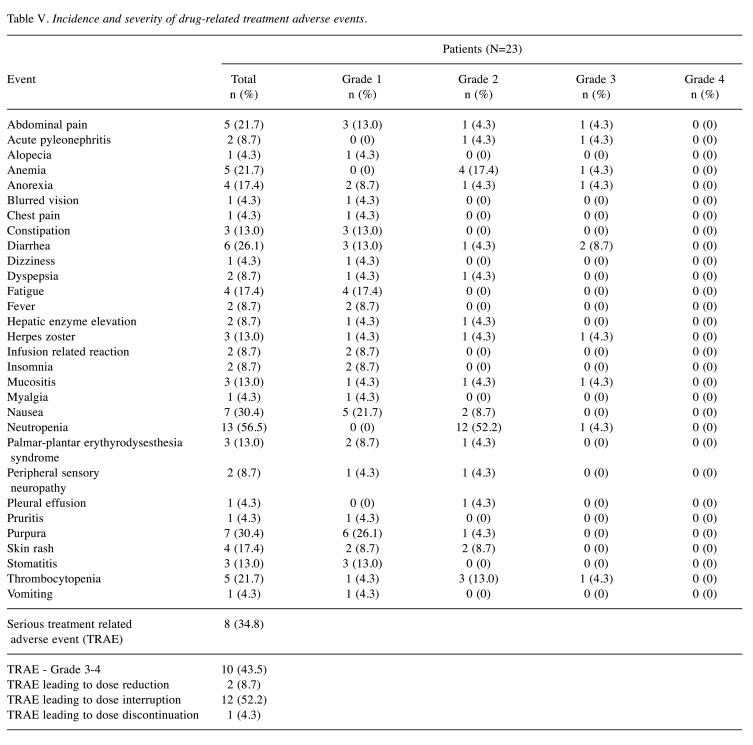
Treatment-related adverse events (TRAEs) (any grade) occurred in 21 (91.3%) patients (Table V). One patient (4.3%) discontinued the study because of an adverse event due to diarrhea. Grade 3/4 TRAEs were reported in 43.5% of patients. The most common grade 3/4 adverse event was diarrhea [two (8.7%)], followed by abdominal pain [one (4.3%)], acute pyelonephritis [one (4.3%)], anemia [one (4.3%)], anorexia [one (4.3%)], herpes zoster infection [one (4.3%)], mucositis [one (4.3%)], neutropenia [one (4.3%)], and thrombocytopenia [one (4.3%)]. Grade 4/5 TRAEs were not observed. Serious TRAEs, including abdominal pain, acute pyelonephritis, diarrhea, dyspepsia, hepatic enzyme level elevation, herpes zoster infection, stomatitis, thrombocytopenia, were reported in eight patients. Dose reduction occurred in two (8.7%) patients, and all patients were subjected to a PLD dose reduction. Dose interruption occurred in 12 (52.2%) patients.
Table V. Incidence and severity of drug-related treatment adverse events.
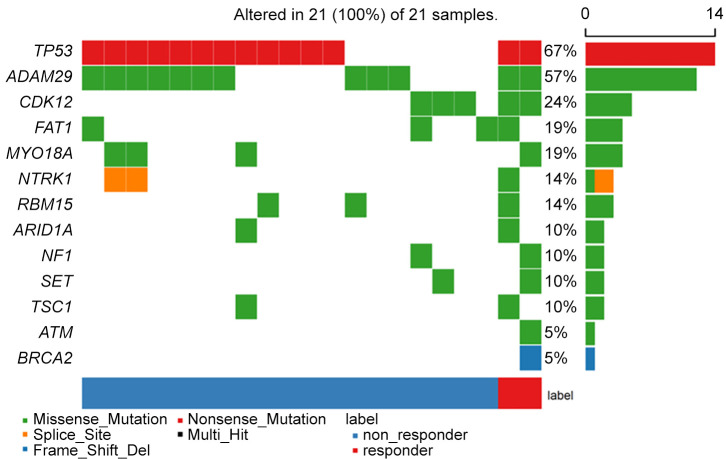
Exploratory gene expression profiles obtained from 21 samples from the patients enrolled in the study are shown in Figure 4. Missense mutations accounted for the majority (60.5%) of identified variants, followed by nonsense mutation (32.6%), splice-site mutation (4.7%), and frameshift deletion (2.3%). TP53 and cyclin-dependent kinase 12 (CDK12) mutations were detected in 67% (14/21) and 24% (12/21) of patients, respectively. Other genes, including FAT1, MYO18A, and NTRK1 were also identified. However, mutation in CCNE-1 was not detected.
Figure 4. Mutation profiles.
Cases of interest. There were two cases with partial remission with a durable response in this study. As a representative case, patient #6, with BRCA2, ATM, and CDK12 mutations, demonstrated the best overall radiologic response of PR with a durable response to bortezomib and Caelyx of 5.8 months and received six cycles of PLD plus bortezomib. Patient #8, with CDK12 mutation, demonstrated the best overall radiologic response of PR with a durable response to bortezomib and Caelyx of 8.2 months and received six cycles of PLD plus bortezomib.
Discussion
In this study, bortezomib plus PLD did not seem to provide substantial benefit in the treatment of patients with BRCA wild-type platinum-resistant recurrent ovarian cancer. The median PFS was 2.9 months, and the median duration of response was 3.0 months. Furthermore, bortezomib plus and PLD was found to have a manageable toxicity profile.
The incorporation of targeted therapies based on genetic testing has significantly altered the treatment landscape for patients with ovarian cancer. These targeted therapies are mainly focused on first-line therapy or platinum-sensitive recurrent ovarian cancer. However, effective treatments for platinum-resistant or platinum-refractory ovarian cancer remain limited. Non-platinum single-agent chemotherapies, including PLD, gemcitabine, topotecan, oral etoposide and paclitaxel, are used for platinum-resistant recurrent ovarian cancer patients. However, response rates to these agents are approximately 10%, and the PFS of these regimens is only approximately 3 months (16). PLD is the most commonly used chemotherapeutic agent in platinum-resistant ovarian cancer patients. Hence, optimal treatment strategies are warranted to enhance the effectiveness of PLD. Recently, addition of bevacizumab to these non-platinum single agents improved the PFS and increased the response rate (14); however, the effect of bevacizumab in patients with platinum-resistant ovarian cancer is still unsatisfactory.
PARPis are approved for the treatment of patients with ovarian cancer harboring BRCA mutations and olaparib plus bevacizumab for first-line maintenance treatment of homologous recombination repair deficiency, as well as in BRCA wild-type platinum-sensitive recurrent ovarian cancer. However, studies on targeted therapy and optimal therapeutic strategies in platinum-resistant patients without BRCA mutations are scarce. Recently, it has been reported that ovarian cancer patients with CCNE-1 amplification express mutually exclusive BRCA mutations and exhibit poor prognosis (13). Furthermore, the results showed that these patients were sensitive to bortezomib and suggested the possibility of unique therapeutic strategies in HGSC (13). PLD can activate anti-apoptotic pathways via the activation of nuclear factor-ĸB (NF-ĸB), which potentially limited the effectiveness of the drug (17). Agents that inhibit the proteasome, such as bortezomib, are particularly effective in blocking the NF-ĸB pathway and provide a rationale for the combination of bortezomib plus PLD (18). Based on the aforementioned results, we designed this study with a combination of bortezomib plus PLD in platinum-resistant recurrent ovarian cancer patients without BRCA mutations.
In the AURELIA trial (4), a combination of bevacizumab and chemotherapy yielded an ORR of 30.0%, and the median PFS was 6.7 months in patients with platinum-resistant recurrent ovarian cancer. In the MITO 11 study (19), the addition of pazopanib to weekly paclitaxel for patients with platinum-resistant or platinum-refractory ovarian cancer yielded an ORR of 56% and a median PFS of 6.3 months. In a previous phase II trial (20), the addition of bortezomib to PLD did not seem to provide substantial benefit in recurrent ovarian cancer. The ORR was 16% in 56 recurrent ovarian cancer patients, and the platinum-resistant group was closed at the interim analysis for lack of efficacy. The median duration of response was 4.8 months. In our study, the PFS was 2.9 months, and the median duration of response was 3.0 months.
Myelosuppression, gastrointestinal complaints, and dermatologic toxicities are considered to be the most common adverse events related to the combination of bortezomib and PLD (15). In our trial, the most frequent TRAEs were neutropenia, nausea, and dermatologic toxicities, which were predominantly of low grade. Grade 3 or higher-grade hematological toxicities (4.3% neutropenia and 4.3% thrombocytopenia) were generally consistent with those reported in previous studies in ovarian cancer (7% neutropenia and 10.3% thrombocytopenia). In our study, gastrointestinal complaints were the most frequently reported grade 3/4 TRAEs, and no treatment-related death was recorded. Peripheral neuropathy, an established toxicity (of low grade) of bortezomib, was reported in 8.7% of patients. PLD-related toxicities were less frequent than in previous reports. No cardiac toxicities occurred, grade 3 mucositis was experienced only by one patient, and no grade 3 palmar-plantar erythyrodysesthesia syndrome was reported.
We performed NGS of tumor samples to identify gene expression profiles related to the bortezomib-PLD response. We observed partial response in two patients with somatic mutations in BRCA2, ATM, and CDK12. Ovarian cancers with somatic mutations in BRCA1/2, ATM and CDK12 genes could cause a defect in double-strand break repair by homologous recombination repair, which can be defined as a BRCAness (21). In addition, the incorporation of boltezomib may have affected BRCAness state. Proteasome is involved in homologous recombination repair, and inhibition of proteasome blocks the recruitment of DNA repair components BRCA1, FANCD2, and RAD51 (22). Neri et al. (23) showed that bortezomib induces a functional state of BRCAness in multiple myeloma cells. PLD is known to act as single-stranded and double-stranded DNA breaks by interfering with topoisomerase II-mediated repair. Previous study showed that HGSC patients with BRCA1/2 mutations experience superior sensitivity to PLD (24). In our study, sensitivity to PLD may have increased by inducing increased BRCAness with the somatic mutation in BRCA1/2, ATM and CDK12 genes. The NGS results in our study are only hypothesis generating and should be validated in studies of bortezomib with or without the addition of PLD. Further study is needed to investigate the predictive biomarker of therapeutic response of PLD with bortezomib.
Our study had several limitations. First, patient recruitment was closed earlier than the predesigned protocol. Second, the sample size was small, which limits the conclusions. Third, we observed no CCNE-1 amplification in the sequencing data; therefore, we could not determine whether bortezomib plus PLD is effective in BRCA wild-type patients with CCNE-1 amplification.
In conclusion, we observed no new toxicity profile for bortezomib plus PLD in patients with BRCA wild-type platinum-resistant recurrent ovarian cancer; however, insufficient antitumor activity of this combination does not warrant further phase III investigations.
Conflicts of Interest
The Authors have no competing interests to disclose in relation to this study.
Authors’ Contributions
KDK, ML, JYL, YJL and ARS designed the study and collected the data. YJL and JYL analyzed and interpreted the data. YJL, ARS, and JYL wrote the manuscript. JWK, HSK, KDK, DHS, SHK, SWK revised the manuscript. All Authors contributed to the article and approved the submitted version.
Acknowledgements
This study was funded by Samyang Biopharmaceuticals Corporation.
References
- 1.Sant M, Chirlaque Lopez MD, Agresti R, Sánchez Pérez MJ, Holleczek B, Bielska-Lasota M, Dimitrova N, Innos K, Katalinic A, Langseth H, Larrañaga N, Rossi S, Siesling S, Minicozzi P, EUROCARE-5 Working Group Survival of women with cancers of breast and genital organs in Europe 1999-2007: Results of the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2191–2205. doi: 10.1016/j.ejca.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 2.Davis A, Tinker AV, Friedlander M. “Platinum resistant” ovarian cancer: what is it, who to treat and how to measure benefit. Gynecol Oncol. 2014;133(3):624–631. doi: 10.1016/j.ygyno.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 3.Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S, Pujade-Lauraine E, Sehouli J, Vergote I, Beale P, Bekkers R, Calvert P, Copeland L, Glasspool R, Gonzalez-Martin A, Katsaros D, Kim JW, Miller B, Provencher D, Rubinstein L, Atri M, Zeimet A, Bacon M, Kitchener H, Stuart GC, Gynecologic Cancer InterGroup Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21(4):771–775. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 4.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P, Bamias A, Pereira D, Wimberger P, Oaknin A, Mirza MR, Follana P, Bollag D, Ray-Coquard I. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 5.Lee JY, Park JY, Park SY, Lee JW, Kim JW, Kim YB, Jeong DH, Lee KB, Kim TH, Lee IH, Choi MC, Kim KH, Kim YM, Lee YJ, Kang S, KGOG Investigators , Pujade-Lauraine E. Real-world effectiveness of bevacizumab based on AURELIA in platinum-resistant recurrent ovarian cancer (REBECA): A Korean Gynecologic Oncology Group study (KGOG 3041) Gynecol Oncol. 2019;152(1):61–67. doi: 10.1016/j.ygyno.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, Matei D, Fielding A, Spencer S, Dougherty B, Orr M, Hodgson D, Barrett JC, Matulonis U. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 7.Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, Korach J, Huzarski T, Poveda A, Pignata S, Friedlander M, Colombo N, Harter P, Fujiwara K, Ray-Coquard I, Banerjee S, Liu J, Lowe ES, Bloomfield R, Pautier P, SOLO2/ENGOT-Ov21 investigators Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 8.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, Matei D, Macpherson E, Watkins C, Carmichael J, Matulonis U. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 9.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I, Ben-Baruch NE, Marth C, Mądry R, Christensen RD, Berek JS, Dørum A, Tinker AV, du Bois A, González-Martín A, Follana P, Benigno B, Rosenberg P, Gilbert L, Rimel BJ, Buscema J, Balser JP, Agarwal S, Matulonis UA, ENGOT-OV16/NOVA Investigators Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med. 2016;375(22):2154–2164. doi: 10.1056/NEJMoa1611310. [DOI] [PubMed] [Google Scholar]
- 10.Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, Patel MR, Chaves J, Park H, Mita AC, Hamilton EP, Annunziata CM, Grote HJ, von Heydebreck A, Grewal J, Chand V, Gulley JL. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: Phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS, Birrer M, Provencher DM, Sehouli J, Colombo N, González-Martín A, Oaknin A, Ottevanger PB, Rudaitis V, Katchar K, Wu H, Keefe S, Ruman J, Ledermann JA. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30(7):1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etemadmoghadam D, Weir BA, Au-Yeung G, Alsop K, Mitchell G, George J, Australian Ovarian Cancer Study Group , Davis S, D’Andrea AD, Simpson K, Hahn WC, Bowtell DD. Synthetic lethality between CCNE1 amplification and loss of BRCA1. Proc Natl Acad Sci U.S.A. 2013;110(48):19489–19494. doi: 10.1073/pnas.1314302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Terpos E. Multiple myeloma. Ann Oncol. 2010;21 Suppl 7:vii143–vii150. doi: 10.1093/annonc/mdq370. [DOI] [PubMed] [Google Scholar]
- 15.Orlowski RZ, Nagler A, Sonneveld P, Bladé J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan Z, Rackoff W, Harousseau JL. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 16.Hanker LC, Loibl S, Burchardi N, Pfisterer J, Meier W, Pujade-Lauraine E, Ray-Coquard I, Sehouli J, Harter P, du Bois A, AGO and GINECO study group The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann Oncol. 2012;23(10):2605–2612. doi: 10.1093/annonc/mds203. [DOI] [PubMed] [Google Scholar]
- 17.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009;9(5):338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlowski RZ. The role of the ubiquitin-proteasome pathway in apoptosis. Cell Death Differ. 1999;6(4):303–313. doi: 10.1038/sj.cdd.4400505. [DOI] [PubMed] [Google Scholar]
- 19.Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S, Mosconi AM, Orditura M, Brandes AA, Arcangeli V, Panici PB, Pisano C, Cecere SC, Di Napoli M, Raspagliesi F, Maltese G, Salutari V, Ricci C, Daniele G, Piccirillo MC, Di Maio M, Gallo C, Perrone F, MITO 11 investigators Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16(5):561–568. doi: 10.1016/S1470-2045(15)70115-4. [DOI] [PubMed] [Google Scholar]
- 20.Parma G, Mancari R, Del Conte G, Scambia G, Gadducci A, Hess D, Katsaros D, Sessa C, Rinaldi A, Bertoni F, Vitali A, Catapano CV, Marsoni S, van de Velde H, Colombo N. An open-label phase 2 study of twice-weekly bortezomib and intermittent pegylated liposomal doxorubicin in patients with ovarian cancer failing platinum-containing regimens. Int J Gynecol Cancer. 2012;22(5):792–800. doi: 10.1097/IGC.0b013e318251051a. [DOI] [PubMed] [Google Scholar]
- 21.Lord CJ, Ashworth A. BRCAness revisited. Nat Rev Cancer. 2016;16(2):110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 22.Krajewska M, Fehrmann RS, de Vries EG, van Vugt MA. Regulators of homologous recombination repair as novel targets for cancer treatment. Front Genet. 2015;6:96. doi: 10.3389/fgene.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neri P, Ren L, Gratton K, Stebner E, Johnson J, Klimowicz A, Duggan P, Tassone P, Mansoor A, Stewart DA, Lonial S, Boise LH, Bahlis NJ. Bortezomib-induced “BRCAness” sensitizes multiple myeloma cells to PARP inhibitors. Blood. 2011;118(24):6368–6379. doi: 10.1182/blood-2011-06-363911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollis RL, Meynert AM, Churchman M, Rye T, Mackean M, Nussey F, Arends MJ, Sims AH, Semple CA, Herrington CS, Gourley C. Enhanced response rate to pegylated liposomal doxorubicin in high grade serous ovarian carcinomas harbouring BRCA1 and BRCA2 aberrations. BMC Cancer. 2018;18(1):16. doi: 10.1186/s12885-017-3981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]