Abstract
Cancer is the leading cause of death worldwide for which effective treatments remain limited. This article aimed to critically review and discuss the potential of targeting cell cycle machineries as a vital tool for cancer treatment. Cyclin dependent kinase (CDK) 4/6 inhibitors were originally approved by the United State Food and Drug Administration (US FDA) for advanced-stage breast cancer treatment. The nearly double-prolonged survival time in patients who received CDK4/6 inhibitors are superior to the conventional chemotherapy or endocrine therapy alone and, thus, these medications have been designated a breakthrough therapy by the US FDA. The requirement of CDK4/6 in the progression of cancer cells, but probably dispensable in normal cells, makes CDK4/6 a popular target for cancer treatment. The effects of CDK4/6 inhibitors in cancer may also involve the tumor microenvironment in which the therapeutic effects are synergistically pronounced. These emerging roles, hence, prompt investigations regarding their therapeutic potential in other cancers, including gastrointestinal cancer. Many preclinical and clinical studies of CDK4/6 inhibitors in gastrointestinal cancers are underway and, as a result, several new potentials are gradually reported. Contrariwise, the primary effect of this drug group is arresting the cell cycle rather than inducing cell death. The efficacy of using CDK4/6 inhibitors as a single regimen in clinical practice is then limited. In this article, the effects of CDK4/6 inhibitors on the progression of gastrointestinal cancers, at both preclinical and clinical levels are reviewed. The future directions for research and the possibility of CDK4/6 inhibitors being “breakthrough therapy” for gastrointestinal cancers are also discussed.
Keywords: Cell cycle, cyclin, cyclin dependent kinase 4/6, gastrointestinal tract, hepatobiliary tract, review
Cancer is recognized as a leading cause of death worldwide, whereas effective treatments are underway and yet to be developed for malignancies in certain organs (1). Cancer cells possess malignant hallmarks that differentiate them from their normal counterparts in the same tissues (2). The abnormality at the genetic or epigenetic levels results in cellular transformation and gain the ability to compete and invade their surrounding tissues. Limitless replication is one of the cancer hallmarks (2). Cancer cells gain this ability through various molecular mechanisms, e.g., amplification of proliferation-associated genes, autoactivation of growth signaling pathways, and the mutation of cell cycle inhibitor genes (3). Although these mechanisms benefit cancer cells to survive, it could be a double-sided sword for their survival. The requirement of unnatural overexpression of genes or proteins makes cancer cells dependent on those oncogenes and this dependency becomes their Achilles’ heel. Targeting overexpressed and requisite proteins for cancer cell proliferation then turns into a hotspot of research to figure out a new therapeutic target to cure cancer (3,4).
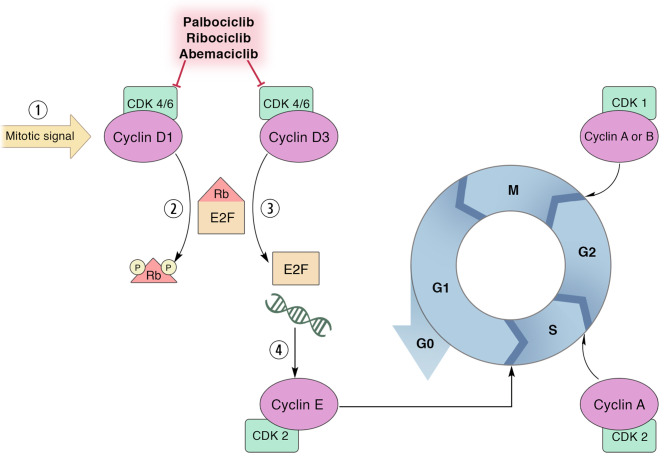
The cell cycle is a vital process and is highly regulated to maintain homeostasis. The main regulatory proteins contributing to cell cycle progression are cyclins and cyclin-dependent kinases (CDKs) (4). Different cyclins are responsible in each phase of the cell cycle so that the expression and degradation of cyclins are the dynamic processes throughout the cell cycle and are controlled like a cascade of regulation. To function as a cell cycle regulator, cyclins need to couple with CDKs; a serine/threonine kinase capable of phosphorylating other proteins that are involved with driving of the cycle. The early step in cell cycles starts when D-type cyclins (cyclin D1, cyclin D2, and cyclin D3) are expressed after cells are activated by growth signaling and those D-type cyclins in turn activate CDK4/6 which further phosphorylates the retinoblastoma protein (RB) (Figure 1). After RB is phosphorylated, the constraint E2F transcription factor releases and activates the transcription of genes that are needed for further phases, namely cyclin E and other CDKs (5). As D-type cyclins are gate keepers for the cell cycle, many cancers are frequently found with the aberrant D-type cyclin expression (3). An example is breast cancer in which CCND1 genes encoding for cyclin D1 protein are largely amplified (6). This makes a group of breast cancer cells become more dependent on cyclin D1 for their proliferation while cyclin D1 is dispensable for normal mammary epithelial cells (7,8). As aforementioned, the function of CDK4/6 is dependent on the availability of D-type cyclins for which it is required for breast cancer cells. Therefore, the development of an anticancer drug is focused on targeting the kinases which are considered druggable (9). The development of CDK4/6 inhibitors has been successful for prolonging the survival time of hormone receptor-positive breast cancer patients and pave the way for the study in other solid tumors including gastrointestinal cancers.
Figure 1. The effect of cyclin dependent kinase (CDK) 4/6 inhibitors. CDK 4/6 inhibitors, i.e., palbociclib, ribociclib, abemaciclib, inhibit the kinase function of the CDK4/6 when they are bound with their cyclin partners (D-type cyclins) and then as they prevent the phosphorylation of retinoblastoma protein (RB). Hypo-phosphorylated RB thus restrains E2F transcription factor and prevents the transcriptional activities that resulted in arresting the cell cycle at the G1 phase.
Gastrointestinal cancer is referred to as a malignancy of the gastrointestinal tract and the accessory organs, including the esophagus, stomach, pancreas, hepatobiliary tract, small intestine, large intestine, and anus (10,11). Gastrointestinal cancer accounts for approximately a quarter of global cancer incidence and causes over one-third of cancer related mortality (10). The major gastrointestinal organs with high incidences of cancers are colorectum, stomach, liver, pancreas, and esophagus (10). The curative treatment in the early stage of each cancer is surgical resection complemented with other modalities such as targeted therapy, chemotherapy, and radiotherapy. The successful rate, however, remains low in some cancers, e.g., pancreas, and in most cancers at the advanced stage. Since CDK4/6 inhibitors are appraised as breakthrough therapy in advanced-stage breast cancer which is associated with the dependency on D-type cyclins for their proliferation, the requirement of D-type cyclins for proliferation of other cancers, thereby, also holds a promise to be a potential target for therapeutic improvement. In this article, the progression of CDK4/6 investigations both at the preclinical and clinical levels in each gastrointestinal cancer are reviewed and discussed.
Esophageal Cancer
The global incidence of esophageal cancer ranks seventh and this cancer is the sixth leading cause of death from cancer (1). Two major histological subtypes of esophageal cancer are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). Globally, 87% of all esophageal cancers are ESCC while 11% constitute EAC. The prevalence of ESCC is particularly high in East Asia, Eastern and Southern Africa, and Southern Europe, where it is associated with nutritional deficiencies, nitrosamines, spicy diets, hot beverages, heavy drinking, and smoking. On the other hand, EAC is much more common in North America and other parts of Europe, in which Barrett’s esophagus, excess body weight, gastroesophageal reflux disease, are considered key risk factors (12). Although multiple approaches are implemented in the treatment of esophageal cancer; including esophagectomy, chemotherapy, radiotherapy, and immunotherapy, the overall survival is still limited. The overall 5-year survival rate is only 20% (13). Thus, novel effective strategies are urgently needed to improve treatment outcomes.
CDK4/6 inhibitors showed anti-tumor effects in a few preclinical studies. Overexpression of CDK4 and CDK6 have been reported in esophageal cancer (14). One study demonstrated that in vitro abemaciclib treatment led to decreased proliferation and increased apoptosis in 3 EAC cell lines, OE19, OE33, and FLO1. The induction of EAC in rats by esophagojejunostomy showed that 78.9% of abemaciclib-treated animals had more than 20% decreased tumor volume (15). The study of Li et al. determined the expression of CDK4/6 in 7 ESCC and 6 EAC cell lines and found that most of the tested cell lines have the overexpressed CDK6 (16). After knocking-down CDK6, the colony formation ability decreased dramatically. In addition, another CDK4/6 inhibitor, palbociclib, was confirmed for targeting the kinase activity, delaying cell cycle progression at the G1-S boundary, and suppressing proliferation and anchorage independence of EAC cells through activation of the RB pathway (14).
A phase II trial of palbociclib in advanced esophageal cancer was also conducted (17). Thirteen patients with RB-intact esophageal cancer; 8 patients with EAC, and 5 patients with ESCC, were treated with 125-mg daily palbociclib for days 1-21 of 28-day cycles. The results showed no objective responses. In another phase I study of palbociclib in the Japanese patients, one esophageal cancer patient with palbociclib monotherapy at the 125-mg dose experienced stable disease >24 weeks (18). In a recently completed phase II trial of palbociclib in patients with advanced or metastatic esophageal and/or gastric cancer, however, neither complete nor partial responses were observed (NCT01037790) (17). Only 6 of 19 (31.6%) patients showed stable disease. In the meantime, among other CDK4/6 inhibitors, ribociclib, was also studied in another phase I clinical trial as a single agent in Japanese patients with advanced solid tumors. Among those, 9 patients with esophageal cancer were treated with 400/600 mg ribociclib on 3-weeks-on/1-week-off dosing schedules. Nonetheless, no patient in the study achieved complete or partial response, with only one patient experienced stable disease for ≥5 cycles (19). Given the limited efficacy demonstrated in these studies, CDK4/6 inhibitor monotherapy is not warranted. An ongoing trial of abemaciclib with the PD-1 inhibitor pembrolizumab in gastroesophageal cancers, however, may provide more details about the efficacy of combination strategies (NCT03997448) (20).
Gastric Cancer
Gastric cancer (GC) remains an important problem worldwide and its incidence ranks fifth and mortality ranks fourth (1). The reported incidence rates are highest in Eastern Asia and Eastern Europe. Although multiple treatments have been made in GC, the 5-year survival rate of patients with advanced GC is still less than 15%. Thus, novel drugs and new therapeutic targets are needed for GC patients. Previous studies suggested aberrant expression of Cyclin D-CDK4/6 in GC (21) as possible targets. The importance of cyclin D1 for GC cell proliferation and invasiveness was recently reported (22). Thus, the administration of CDK4/6 inhibitors might be another alternative approach that could improve the therapeutic outcome for GC patients.
The preclinical results of the anti-tumor efficacy of CDK4/6 inhibitors in GC are promising. A recent study demonstrated that palbociclib could significantly inhibit cell proliferation and induce cell senescence, cell cycle arrest, and apoptosis in human GC cell lines; AGS and HGC-27, by regulating the Notch pathway (23). The study by Wang et al. showed that human GC cell lines, namely, AGS, KATO-III, NCI-N87, and HS746T, treated with palbociclib exhibited cell cycle arrest in G1 phase and decreased number of cells in the G2/M. Similar to other studies, palbociclib inhibited cell proliferation via modulation of the cell cycle, and numerous signaling pathways, including p53, PI3K/AKT, Ras-ERK, JNK/MAPK, Wnt/β-catenin, and Smad (24). Palbociclib-induced autophagy and senescence also play crucial roles in decreasing cell proliferation in GC (25).
The clinical efficacy of palbociclib in GC, however, was very limited. A phase II clinical trial of palbociclib in 5 patients with advanced GC failed to demonstrate relevant anti-tumor effect (17). Although the efficacy of palbociclib monotherapy was not encouraging, the combination of CDK4/6 inhibitors with other agents becomes more interesting. Another phase II non-randomized, single arm, open label study of abemaciclib in combination with pembrolizumab in patients with unresectable or metastatic GC who have received at least two lines of prior therapy is ongoing (NCT03997448). The results of this trial may allow the further clinical investigation and the elucidation of relevant mechanisms of the action (20).
Liver Cancer
The most prevalent primary liver cancer is hepatocellular carcinoma (HCC) which accounted for 75-80% of cases (1). The second most prevalent type of liver cancer is the malignancy of intrahepatic bile duct epithelial cells, so called cholangiocarcinoma. HCC is the sixth most common malignancy and has been in the top five leading causes of cancer-related deaths for decades (26,27). Several studies showed that D-type cyclins and CDK4/6 are essential for the proliferation of HCC cells and associated with poor prognosis of patients (28). The aberrant regulation of the cyclin D-CDK4-RB pathway was reported in up to 73% in HCC cases (29). Thus, targeting CDK4/6 is a promising approach for the treatment of HCC and many studies showed the consistent effectiveness of CDK4/6 inhibitors.
CDK4/6 inhibitors, namely palbociclib, show a very potent effect on HCC cell cycle arrest as similar as to that seen in estrogen receptor positive breast cancer cells. The major effect involves the cyclin D-CDK4/6-RB pathway in which a positivity of RB expression is a crucial factor for drug response (30). Given that RB loss or impairment is found in less than 30% in HCC, the possibility of using palbociclib for HCC treatment is sound. Palbociclib also exerted a synergistic effect on HCC cell inhibition when combined with sorafenib, a pan-kinase inhibitor approved for targeted therapy in HCC. The effect of palbociclib, however, was reversible after pausing the drug. Thus, the 3-week on and 1-week off regimen used in patients with breast cancer might not be effective for HCC and patients might have high risk to develop adverse effects of palbociclib. The other studies also supported that combination of palbociclib with sorafenib exerted a very potent effect probably via modulation of multiple pathways. The combination of other pathway inhibitors such as BAY-117082, an inhibitor of the nuclear factor-kB (NF-kB) pathway, showed a satisfactory effect (31). The inhibitory effects of palbociclib may also be involved in more than the cell cycle regulations via CDK4/6 inhibition (32). The study of Hsieh et al. found that palbociclib could activate AMP-activated protein kinase and inhibited protein-phosphatase 5 which resulted in the induction of autophagy and apoptosis of HCC cells in vitro and in vivo (33). These effects remained in palbociclib-treated HCC cells even if CDK4/6 were knocked down, suggesting some CDK4/6 independent effects of palbociclib. The inhibitory effects of ribociclib and abemaciclib, another two CDK4/6 inhibitors, however, were diminished in CDK4/6 depleted cells which, in contrast, suggested palbociclib specific effects. All these studies suggested that combined palbociclib with an appropriate kinase inhibitor should synergize the effect of palbociclib and was better than using either drug alone.
Apart from palbociclib, another two CDK4/6 inhibitors also exerted an effective inhibition on the growth of HCC cells. Abemaciclib showed a potent effect on cell cycle arrest similar to palbociclib (34). Inhibitory effects of ribociclib on HCC cell proliferation are also promising; nonetheless, this drug is probably highly effective in cells with a high expression of RB and low p16 proteins (28). Ribociclib also synergized the effect of infigratinib, a fibroblast growth factor receptor (FGFR) inhibitor, and reversed the resistant phenotype of HCC cells to infigratinib (26). As infigratinib is highly selective to FGFR in HCC cells, the combination of this drug with ribociclib is then anticipated as an alternative treatment for HCC with overexpression of FGFR.
Preclinical studies reported that CDK4/6 inhibitors are promising for HCC treatment, however, clinical studies are still lacking at the present time. More clinical investigation is needed to ascertain the efficacy for the practice. Since the window of toxicity is known from studies in other cancers, the results of clinical trials may be available in a near future and the direction of HCC treatment will be therefore clarified.
Biliary Tract Cancer
Biliary tract cancer includes the malignancy of gallbladder and bile duct epithelia in which the latter is also called cholangiocarcinoma (CCA). CCA is considered relatively rare, and the prevalence is approximately 3% of all gastrointestinal cancer. The prevalence of CCA, however, is particularly in high in the Southeast Asian countries where the carcinogenesis is closely associated with parasitic infections. Intrahepatic CCA is sometimes classified as primary liver cancer in which the prevalence has accounted for 30% of liver cancer. Intrahepatic CCA, however, naturally differs from hepatocellular carcinoma and even possesses heterogenous biology between the intrahepatic and extrahepatic subtypes. This results in difficulty of developing therapeutic strategies and makes this disease dismal and fatal.
The study of Sitthithumcharee et al. (35) showed that the CCA cells overexpressed cyclin D1 and thus makes the cancer cells depend on the activities of CDK4/6 for their proliferation. The treatment of cells with CDK4/6 inhibitors (palbociclib, ribociclib, and abemaciclib) thus induced cell cycle arrest and cellular senescence. The inhibitory effects were also shown in patient-derived xenografts. The major affected pathways are RB-dependent, and the pharmacological efficacy is associated with the KRAS mutation signature. The increased therapeutic efficiency of palbociclib was also shown in the combination with the pan-mTOR inhibitor, MLN0128, in another study (36). Greater effects of CDK4/6 inhibitors were shown in the combination with the inhibitors of other pro-carcinogenic pathways, namely Wnt/β-catenin and Notch 3 (37). Since the primary effect of palbociclib is to arrest the cell cycle which limits the efficacy of cancer treatments, the combined treatment with MLN0128 which promotes CCA cell apoptosis thus provides a new hope for CCA treatment. Not only pan-mTOR inhibition, the inactivation of focal adhesion kinase (FAK) and CDK4/6 by a combination of FAK inhibitor, PND1186, and palbociclib also showed a greater effect (38). In addition to a combination of CDK4/6 inhibitors and other chemical agents, palbociclib also sensitized CCA cells to radiotherapy (39). The underlying mechanism is by inhibiting the repairing of DNA damage after radiation and, hence, promote cells to apoptosis.
The preclinical studies of CDK4/6 inhibitors, especially palbociclib, showed promising roles of this drug group for CCA treatment. The clinical study, however, is limited and no data are available to date. Up to this current search, the study of CDK4/6 inhibitors for the treatment of gallbladder cancer was not available. Altogether, the investigations on effects of CDK4/6 inhibitors for biliary tract cancers are relatively limited compared with other gastrointestinal cancers and more studies are needed to guide the direction of future practice in this cancer group.
Pancreatic Cancer
Pancreatic cancer (PC), especially pancreatic ductal adenocarcinoma (PDAC), is one of the deadliest cancers with a very poor prognosis. The 5-year survival rate of patients with PC who have standard chemotherapy is less than 10% (40). Most PC harbor mutations of KRAS which is a regulating pathway of cell cycle machinery expressions and of CDKN2A, is a gene encoding p16 protein that acts as a natural cell cycle inhibitor. It is approximated that PC harboring these mutations are comprised of more than 90% of cases (41). Therefore, the study of cell cycle inhibitors, especially CDK4/6 inhibitor which has been approved for some cancers are then of the great interest. Many studies, both preclinical and clinical trials, are attempting to determine the effectiveness of using this drug group.
Palbociclib, the first CDK4/6 inhibitor approved by the US FDA, has been studied in PC for a decade. In 2012, Liu et al. (42) found that PD-0332991 (which was later approved in a generic name of palbociclib) exerted a potent anti-proliferative effect on PC cells. The treatment using palbociclib, however, could induce epithelial-mesenchymal transition (EMT) and probably increase metastatic ability of PC by activating the transcriptional activities of the SMAD4 pathway. This resulted in the concern for using palbociclib for PC treatment. The same group of authors then applied SB505124 which is the inhibitor of type-I transforming growth factor-β receptor to block the EMT. The better inhibitory effect on PC cells after combination with SB505124 was observed compared with using palbociclib alone. The later studies of CDK4/6 inhibitors were carried forward and they found what might provide only a modest effect if they were used as single agents (43). In addition, CDK4 expression itself could not be a good predictor for the response to CDK4/6 inhibitor (40). The anti-proliferative effects were more pronounced when CDK4/6 inhibitors were combined with a certain chemotherapy (44). The importance of selecting the right chemotherapeutic drug was reported by the study of Kumaraswamy et al. (43). The combination of palbociclib and taxane resulted in a satisfactory anti-tumor effect. The administration of palbociclib at the same time with gemcitabine conversely ablated the effects of both drugs compared with the single agent treatment (43). These effects were suspected to be the mechanistically result of G1 arrest by palbociclib which protected PC cells form undergoing the S phase which is the point of gemcitabine’s action. The in-depth mechanisms were then recently discovered by Savador-Babero et al. (45,46) in that the sequence of applying chemotherapeutic drugs and CDK4/6 inhibitors mattered. CDK4/6 inhibitors should be given after the treatment of chemotherapy as it then allowed the cells to undergo the primary effects of each chemotherapeutic drug, e.g., breaking of DNA strands and inhibiting the microtubule polymerization and de-polymerization. In addition to the cell cycle arrest of chemotherapeutic-treated cells re-entering the cell cycle, CDK4/6 inhibitors also prevented the repairing of DNA that had been bombarded by the chemotherapeutic drug resulting in cell apoptosis. The sequence of administration of CDK4/6 inhibitor for patients, hence, needs to be taken into the account, otherwise the results of combination could be worsened and distort the clinical outcome.
The results of combining palbociclib with the other agents rather than those chemotherapeutic drugs are also promising. The co-treatment of PC cells with an mTOR inhibitor demonstrated a great effect in vitro and in vivo. The administration of the mTOR inhibitor also prevented the resistance to CDK4/6 inhibitors which is likely an adaptive ability for PC cells carrying KRAS mutation (47). The combination of palbociclib with trametinib, a MEK inhibitor, also showed a potent inhibitory effect on PC growth in vivo (48). This combination also increased the infiltration of antigen presenting cells and cytotoxic T cells into the PC tumor microenvironment. Therefore, adding the immunotherapeutic agents such as anti-PDL1 to the group of the combination of palbociclib and trametinib resulted in a super-synergistic effect. The phase I clinical trial of palbociclib and trametinib combination also showed a suggestive result. Among 9 patients with solid cancers, 2 patients with PC receiving palbociclib-trametinib had a partial remission (49). As this study only recruited a limited number of patients, more investigation is needed to clarify the actual benefit. Studies of the inhibitory effects of palbociclib are ongoing as well as the discovery of new mechanisms (50) and the development of drug delivery studies are also reported (51).
Apart from palbociclib, another two CDK4/6 inhibitors; abemaciclib and ribociclib, are also reported for their therapeutic aims in PC. Abemaciclib exerted a potent effect on the inhibition of the PDAC xenograft model and the effects were greater when combined with the inhibitor of HuR (ELAVL1), a positive regulator of cyclin D1, and Yes-associated protein 1 (YAP1) (51). The combination of ribociclib with MEK162, a MEK inhibitor (53), or sorafenib, a pan-kinase inhibitor (41), also showed an effective inhibition of PC growth and increased apoptosis of PC cells. In a phase I clinical trial of an add-on of ribociclib and everolimus, an inhibitor of mTOR pathway, compared to the standard chemotherapy of gemcitabine and 5-fluorouracil, the results did not show any satisfactory effect (54). Among 21 patients receiving add-on combination of ribociclib and everolimus, only 2 of them had stable disease whereas the rest of them did not respond well. Therefore, more clinical studies are extremely needed to clarify how benefit the patients can get compared with the risk of developing the adverse effects of multiple drug administration.
Colorectal Cancer
Colorectal cancer (CRC) is one of the most problematic cancers for public health worldwide. In 2020, the incidence of CRC ranked third whereas the mortality ranked second in both males and females (1). Curative-intent surgery and a standard chemotherapy regimen provided a satisfactory result, however, the recurrence and the resistance to chemotherapy remained. One of the oncogenic driver genes frequently found mutated in colorectal cancer is KRAS. The frequency of KRAS mutation in colorectal cases was reported up to 50% (55). The development of targeted therapy, hence, aims to target Ras associated pathways and its downstreams, e.g., Raf, MEK, ERK. Although, CRC with the KRAS mutation responds well to the inhibitors of the extracellular signal-regulated kinases (ERK) pathway, a subfamily of mitogen-activated protein kinase (MAPK), when modest effects were observed in some models of preclinical studies which may not suffice for clinical translation. Because a major group of patients with CRC are likely to benefit from targeting ERK downstream, the therapeutic agent synergizing the effects of ERK pathways inhibitors are then of interest to develop a novel treatment regimen.
Several studies have shown that KRAS mutation signatures of CRC respond well to palbociclib treatment as the ERK pathway influenced by Ras mutation directly involves the expression of cell cycle regulatory proteins. The expression of D-type cyclins and the transcription factors that regulate cell mitosis such as forkhead box M1 (FOXM1) are under the regulation of ERK (56). Reciprocally, CDK4/6 is also reported to control FOXM1 stability by their kinase functions (57). The combination of MEK inhibitors; namely trametinib (58), PD0325901 (56), and palbociclib, thereby, exerted the synergistic effects on growth inhibition of CRC in vitro and in vivo. The super-synergistic effects on inhibiting cell proliferation were observed when MEK inhibitor and palbociclib were given in combination with cetuximab, a monoclonal antibody targeting epidermal growth factor receptor (EGFR) (59). In this particular circumstance, palbociclib can also induce apoptosis of CRC cells. For instance, in hypoxic conditions, CRC cells developed hypoxia-resistance by upregulation of the transcription factor hypoxia-inducible factor 1α (HIF1α) (60). The treatment of palbociclib downregulated HIF1α expression and also affected other pro-proliferative and pro-survival pathways such as ERK and glycogen synthase kinase 3β (GSK3β). Moreover, palbociclib also synergized the effect of the chemotherapeutic drug, irinotecan, and increased apoptotic cell death of CRC. The synergistic effects are not only reported with chemotherapeutic or targeted therapeutic drugs. Palbociclib also exerted synergistic effects with radiotherapy in CRC cells with wild type p53 (61). A few studies, however, showed that not all KRAS mutated CRC responded well to palbociclib. A specific mutation of KRAS at p.G12D made CRC cells less sensitive to palbociclib (62). The other factors, such as high p27 expression, could compromise the inhibitory activity of palbociclib in CRC (63). Although promising data suggests palbociclib as a good candidate for CRC treatment, more investigation is needed to clarify who will benefit from the treatment. The discovery of the molecular signature of palbociclib response may assist in clinical judgement to determine candidates who would have maximal benefit over the risk of adverse drug reaction.
Apart from palbociclib, another CDK4/6 inhibitor, abemaciclib, is also effective for CRC treatment both in KRAS and BRAF mutations. The synergistic effect was pronounced when abemaciclib was combined with LY3009120, a pan-raf inhibitor (64). Using machine learning to identify the prognostic signature of colon adenocarcinoma, Linares-Blanco et al. found that several genes are potential candidates for therapeutic targeting including fatty acid binding protein 6 (FABP6) (65). The in silico molecular docking revealed that FABP6 strongly interacted with abemaciclib. This suggested the novel potential strategies to improve the efficacy of abemaciclib. Biological validation is still lacking and needs further study. Altogether, the CDK4/6 inhibitors are promising for therapeutic aims in a large proportion of patients with KRAS mutated CRC but findings of biomarkers for drug responses are needed with more clinical studies and are required to make appropriate decisions.
Prospective and Future Direction of Research
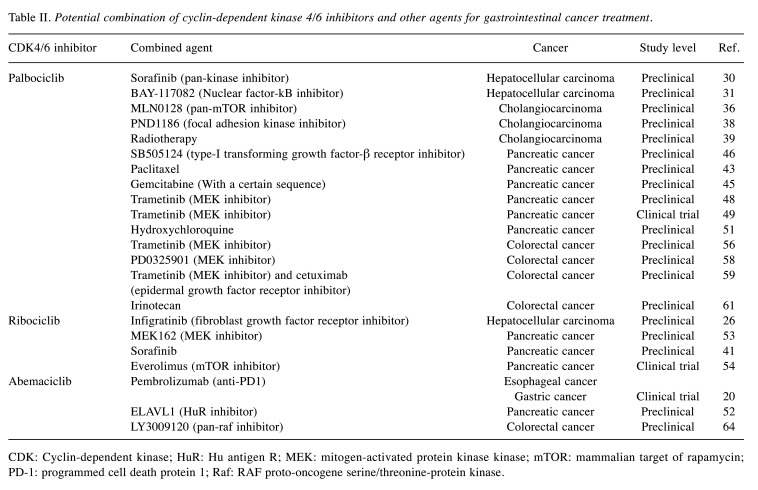
Although CDK4/6 inhibitors are approved for use in some cancers and hold a very high promise for use in the GI cancers, information at present is still limited. Studies at the preclinical level showed great inhibitory effects on GI cancer cell proliferation, but in clinical studies, only a modest effect was observed. The key clinical trials that may suggest the future direction of the study of CDK4/6 inhibitors on GI cancers are summarized in Table I. Some CDK4/6 inhibitors are likely affecting more than inhibiting CDK4/6 which could increase their efficacy. On the other hand, these off-target effects can cause unexpected adverse reactions in patients. The inhibition of the G1 phase of the cell cycle may also disturb the effects of other drugs targeting the downstream process in cell proliferation, such as DNA replication or mitosis. This could antagonize the effect of other therapeutic aims. The potential combinations that may result in synergistic effects for each drug are summarized in Table II. In summary, studies to fully understand the comprehensive mechanisms of these inhibitors are needed for an appropriate selection and the safety of administration to the patients. More clinical trials, especially a randomized controlled trial, are urgently needed to guide clinical practice of these potentially breakthrough-therapy medications.
Table I. Published clinical trials of cyclin-dependent kinase 4/6 inhibitors in gastrointestinal cancer.
MEK: Mitogen-activated protein kinase kinase; mTOR: mammalian target of rapamycin.
Table II. Potential combination of cyclin-dependent kinase 4/6 inhibitors and other agents for gastrointestinal cancer treatment.
CDK: Cyclin-dependent kinase; HuR: Hu antigen R; MEK: mitogen-activated protein kinase kinase; mTOR: mammalian target of rapamycin; PD-1: programmed cell death protein 1; Raf: RAF proto-oncogene serine/threonine-protein kinase.
Conflicts of Interest
The Authors declare that they do not have conflicts of interest. We did not receive any specific funding for this work. CS received research grants from the National Research Council of Thailand (N41A640108), The Medical Council of Thailand, Faculty of Medicine (IN65126), and Fundamental Fund of Khon Kaen University.
Authors’ Contributions
FZ, YZ, CS conceptualized the paper, reviewed literature, and wrote first draft of manuscript. CS supervised and revised the manuscript. TK summarized the literature and created the table and figure. All Authors critically reviewed and approved the final manuscript.
Acknowledgements
CS is supported by the Fundamental Fund of Khon Kaen University and The Research Grant from The Medical Council of Thailand (Pol. Gen. Dr. Jongjate Aojanepong Fund). We did not receive any specific funding for this work. We would like to thank Professor James A Will for editing the English presentation of this manuscript via KKU publication Clinic, Khon Kaen University, Thailand.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Suski JM, Braun M, Strmiska V, Sicinski P. Targeting cell-cycle machinery in cancer. Cancer Cell. 2021;39(6):759–778. doi: 10.1016/j.ccell.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Alonso D, Malumbres M. Mammalian cell cycle cyclins. Semin Cell Dev Biol. 2020;107:28–35. doi: 10.1016/j.semcdb.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Elsheikh S, Green AR, Aleskandarany MA, Grainge M, Paish CE, Lambros MB, Reis-Filho JS, Ellis IO. CCND1 amplification and cyclin D1 expression in breast cancer and their relation with proteomic subgroups and patient outcome. Breast Cancer Res Treat. 2008;109(2):325–335. doi: 10.1007/s10549-007-9659-8. [DOI] [PubMed] [Google Scholar]
- 7.Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von Boehmer H, Sicinski P. The requirement for cyclin D function in tumor maintenance. Cancer Cell. 2012;22(4):438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411(6841):1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- 9.Fassl A, Geng Y, Sicinski P. CDK4 and CDK6 kinases: From basic science to cancer therapy. Science. 2022;375(6577):eabc1495. doi: 10.1126/science.abc1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020;159(1):335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shao Y, Hua Z, Zhao L, Shen Y, Guo X, Niu C, Wei W, Liu F. Time trends of gastrointestinal cancers incidence and mortality in Yangzhong from 1991 to 2015: An updated age-period-cohort analysis. Front Oncol. 2018;8:638. doi: 10.3389/fonc.2018.00638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021. doi: 10.1007/s12328-020-01237-x. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 14.Ismail A, Bandla S, Reveiller M, Toia L, Zhou Z, Gooding WE, Kalatskaya I, Stein L, D’Souza M, Litle VR, Peters JH, Pennathur A, Luketich JD, Godfrey TE. Early G1 cyclin-dependent kinases as prognostic markers and potential therapeutic targets in esophageal adenocarcinoma. Clin Cancer Res. 2011;17(13):4513–4522. doi: 10.1158/1078-0432.CCR-11-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosovec JE, Zaidi AH, Omstead AN, Matsui D, Biedka MJ, Cox EJ, Campbell PT, Biederman RWW, Kelly RJ, Jobe BA. CDK4/6 dual inhibitor abemaciclib demonstrates compelling preclinical activity against esophageal adenocarcinoma: a novel therapeutic option for a deadly disease. Oncotarget. 2017;8(59):100421–100432. doi: 10.18632/oncotarget.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li F, Xu Y, Liu B, Singh PK, Zhao W, Jin J, Han G, Scott AW, Dong X, Huo L, Ma L, Pizzi MP, Wang Y, Li Y, Harada K, Xie M, Skinner HD, Ding S, Wang L, Krishnan S, Johnson RL, Song S, Ajani JA. YAP1-mediated CDK6 activation confers radiation resistance in esophageal cancer - rationale for the combination of YAP1 and CDK4/6 inhibitors in esophageal cancer. Clin Cancer Res. 2019;25(7):2264–2277. doi: 10.1158/1078-0432.CCR-18-1029. [DOI] [PubMed] [Google Scholar]
- 17.Karasic TB, O’Hara MH, Teitelbaum UR, Damjanov N, Giantonio BJ, d’Entremont TS, Gallagher M, Zhang PJ, O’Dwyer PJ. Phase II trial of palbociclib in patients with advanced esophageal or gastric cancer. Oncologist. 2020;25(12):e1864–e1868. doi: 10.1634/theoncologist.2020-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tamura K, Mukai H, Naito Y, Yonemori K, Kodaira M, Tanabe Y, Yamamoto N, Osera S, Sasaki M, Mori Y, Hashigaki S, Nagasawa T, Umeyama Y, Yoshino T. Phase I study of palbociclib, a cyclin-dependent kinase 4/6 inhibitor, in Japanese patients. Cancer Sci. 2016;107(6):755–763. doi: 10.1111/cas.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doi T, Hewes B, Kakizume T, Tajima T, Ishikawa N, Yamada Y. Phase I study of single-agent ribociclib in Japanese patients with advanced solid tumors. Cancer Sci. 2018;109(1):193–198. doi: 10.1111/cas.13428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uboha N, Eickhoff J, Chandrasekharan C, Jalal S, Benson A, Deming D, Lindemann S, Hochster H. Phase II study of the combination of abemaciclib and pembrolizumab in locally advanced unresectable or metastatic gastroesophageal adenocarcinoma: Big Ten Cancer Research Consortium BTCRC-GI18-149. Journal of Clinical Oncology. 2022;38(4_suppl):TPS461–TPS461. doi: 10.1200/JCO.2020.38.4_suppl.TPS461. [DOI] [Google Scholar]
- 21.Lee SR, Shin JW, Kim HO, Son BH, Yoo CH, Shin JH. Determining the effect of transforming growth factor-β1 on cdk4 and p27 in gastric cancer and cholangiocarcinoma. Oncol Lett. 2013;5(2):694–698. doi: 10.3892/ol.2012.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takao K, Konishi H, Matsubara D, Shoda K, Arita T, Shimizu H, Komatsu S, Shiozaki A, Yamamoto Y, Kubota T, Fujiwara H, Okamoto K, Otsuji E. MiR-3663-3p inhibits the progression of gastric cancer through the CCND1 pathway. Anticancer Res. 2021;41(5):2441–2449. doi: 10.21873/anticanres.15019. [DOI] [PubMed] [Google Scholar]
- 23.Bi H, Shang J, Zou X, Xu J, Han Y. Palbociclib induces cell senescence and apoptosis of gastric cancer cells by inhibiting the Notch pathway. Oncol Lett. 2021;22(2):603. doi: 10.3892/ol.2021.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Sun Y, Li W, Ye F, Zhang Y, Guo Y, Zhang DY, Suo J. Antiproliferative effects of the CDK6 inhibitor PD0332991 and its effect on signaling networks in gastric cancer cells. Int J Mol Med. 2018;41(5):2473–2484. doi: 10.3892/ijmm.2018.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenzuela CA, Vargas L, Martinez V, Bravo S, Brown NE. Palbociclib-induced autophagy and senescence in gastric cancer cells. Exp Cell Res. 2017;360(2):390–396. doi: 10.1016/j.yexcr.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Prawira A, Le TBU, Vu TC, Huynh H. Ribociclib enhances infigratinib-induced cancer cell differentiation and delays resistance in FGFR-driven hepatocellular carcinoma. Liver Int. 2021;41(3):608–620. doi: 10.1111/liv.14728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Chen K, Shang R, Chen X, Zhang Y, Song X, Evert M, Zhong S, Li B, Calvisi DF, Chen X. Alpelisib combination treatment as novel targeted therapy against hepatocellular carcinoma. Cell Death Dis. 2021;12(10):920. doi: 10.1038/s41419-021-04206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiter FP, Denk G, Ziesch A, Ofner A, Wimmer R, Hohenester S, Schiergens TS, Spampatti M, Ye L, Itzel T, Munker S, Teufel A, Gerbes AL, Mayerle J, De Toni EN. Predictors of ribociclib-mediated antitumour effects in native and sorafenib-resistant human hepatocellular carcinoma cells. Cell Oncol (Dordr) 2019;42(5):705–715. doi: 10.1007/s13402-019-00458-8. [DOI] [PubMed] [Google Scholar]
- 29.Lu JW, Lin YM, Chang JG, Yeh KT, Chen RM, Tsai JJ, Su WW, Hu RM. Clinical implications of deregulated CDK4 and Cyclin D1 expression in patients with human hepatocellular carcinoma. Med Oncol. 2013;30(1):379. doi: 10.1007/s12032-012-0379-5. [DOI] [PubMed] [Google Scholar]
- 30.Bollard J, Miguela V, Ruiz de Galarreta M, Venkatesh A, Bian CB, Roberto MP, Tovar V, Sia D, Molina-Sánchez P, Nguyen CB, Nakagawa S, Llovet JM, Hoshida Y, Lujambio A. Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma. Gut. 2017;66(7):1286–1296. doi: 10.1136/gutjnl-2016-312268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng J, Kohno S, Okada N, Okahashi N, Teranishi K, Matsuda F, Shimizu H, Linn P, Nagatani N, Yamamura M, Harada K, Horike SI, Inoue H, Yano S, Kumar S, Kitajima S, Ajioka I, Takahashi C. Treatment of retinoblastoma 1-intact hepatocellular carcinoma with cyclin-dependent kinase 4/6 inhibitor combination therapy. Hepatology. 2021;74(4):1971–1993. doi: 10.1002/hep.31872. [DOI] [PubMed] [Google Scholar]
- 32.Jo H, Park Y, Kim T, Kim J, Lee JS, Kim SY, Chung JI, Ko HY, Pyun JC, Kim KS, Lee M, Yun M. Modulation of SIRT3 expression through CDK4/6 enhances the anti-cancer effect of sorafenib in hepatocellular carcinoma cells. BMC Cancer. 2020;20(1):332. doi: 10.1186/s12885-020-06822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh FS, Chen YL, Hung MH, Chu PY, Tsai MH, Chen LJ, Hsiao YJ, Shih CT, Chang MJ, Chao TI, Shiau CW, Chen KF. Palbociclib induces activation of AMPK and inhibits hepatocellular carcinoma in a CDK4/6-independent manner. Mol Oncol. 2017;11(8):1035–1049. doi: 10.1002/1878-0261.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen J, Hu J, Wu J, Luo X, Li Y, Li J. Molecular characterization of long-term survivors of hepatocellular carcinoma. Aging (Albany NY) 2021;13(5):7517–7537. doi: 10.18632/aging.202615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sittithumcharee G, Suppramote O, Vaeteewoottacharn K, Sirisuksakun C, Jamnongsong S, Laphanuwat P, Suntiparpluacha M, Matha A, Chusorn P, Buraphat P, Kakanaporn C, Charngkaew K, Silsirivanit A, Korphaisarn K, Limsrichamrern S, Tripatara P, Pairojkul C, Wongkham S, Sampattavanich S, Okada S, Jirawatnotai S. Dependency of cholangiocarcinoma on cyclin D-dependent kinase activity. Hepatology. 2019;70(5):1614–1630. doi: 10.1002/hep.30704. [DOI] [PubMed] [Google Scholar]
- 36.Song X, Liu X, Wang H, Wang J, Qiao Y, Cigliano A, Utpatel K, Ribback S, Pilo MG, Serra M, Gordan JD, Che L, Zhang S, Cossu A, Porcu A, Pascale RM, Dombrowski F, Hu H, Calvisi DF, Evert M, Chen X. Combined CDK4/6 and Pan-mTOR inhibition is synergistic against intrahepatic cholangiocarcinoma. Clin Cancer Res. 2019;25(1):403–413. doi: 10.1158/1078-0432.CCR-18-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitchen P, Lee KY, Clark D, Lau N, Lertsuwan J, Sawasdichai A, Satayavivad J, Oltean S, Afford S, Gaston K, Jayaraman PS. A runaway PRH/HHEX-Notch3-positive feedback loop drives cholangiocarcinoma and determines response to CDK4/6 inhibition. Cancer Res. 2020;80(4):757–770. doi: 10.1158/0008-5472.CAN-19-0942. [DOI] [PubMed] [Google Scholar]
- 38.Song X, Xu H, Wang P, Wang J, Affo S, Wang H, Xu M, Liang B, Che L, Qiu W, Schwabe RF, Chang TT, Vogl M, Pes GM, Ribback S, Evert M, Chen X, Calvisi DF. Focal adhesion kinase (FAK) promotes cholangiocarcinoma development and progression via YAP activation. J Hepatol. 2021;75(4):888–899. doi: 10.1016/j.jhep.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang CY, Hsieh FS, Wang CY, Chen LJ, Chang SS, Tsai MH, Hung MH, Kuo CW, Shih CT, Chao TI, Chen KF. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia-mutated kinase-mediated DNA damage response. Eur J Cancer. 2018;102:10–22. doi: 10.1016/j.ejca.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 40.Jiggens E, Mortoglou M, Grant GH, Uysal-Onganer P. The role of CDK4 in the pathogenesis of pancreatic cancer. Healthcare (Basel) 2021;9(11):1478. doi: 10.3390/healthcare9111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee T, Kim K, Lee J, Park SH, Park YS, Lim HY, Kang WK, Park JO, Kim ST. Antitumor activity of sorafenib plus CDK4/6 inhibitor in pancreatic patient derived cell with KRAS mutation. J Cancer. 2018;9(18):3394–3399. doi: 10.7150/jca.26068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Korc M. Cdk4/6 inhibition induces epithelial-mesenchymal transition and enhances invasiveness in pancreatic cancer cells. Mol Cancer Ther. 2012;11(10):2138–2148. doi: 10.1158/1535-7163.MCT-12-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumarasamy V, Ruiz A, Nambiar R, Witkiewicz AK, Knudsen ES. Chemotherapy impacts on the cellular response to CDK4/6 inhibition: distinct mechanisms of interaction and efficacy in models of pancreatic cancer. Oncogene. 2020;39(9):1831–1845. doi: 10.1038/s41388-019-1102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chou A, Froio D, Nagrial AM, Parkin A, Murphy KJ, Chin VT, Wohl D, Steinmann A, Stark R, Drury A, Walters SN, Vennin C, Burgess A, Pinese M, Chantrill LA, Cowley MJ, Molloy TJ, Australian Pancreatic Cancer Genome Initiative (APGI) , Waddell N, Johns A, Grimmond SM, Chang DK, Biankin AV, Sansom OJ, Morton JP, Grey ST, Cox TR, Turchini J, Samra J, Clarke SJ, Timpson P, Gill AJ, Pajic M. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut. 2018;67(12):2142–2155. doi: 10.1136/gutjnl-2017-315144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salvador-Barbero B, Álvarez-Fernández M, Zapatero-Solana E, El Bakkali A, Menéndez MDC, López-Casas PP, Di Domenico T, Xie T, VanArsdale T, Shields DJ, Hidalgo M, Malumbres M. CDK4/6 inhibitors impair recovery from cytotoxic chemotherapy in pancreatic adenocarcinoma. Cancer Cell. 2020;37(3):340–353.e6. doi: 10.1016/j.ccell.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Salvador-Barbero B, Alvarez-Fernández M, Zapatero-Solana E, El Bakkali A, Menéndez MDC, López-Casas PP, Di Domenico T, Xie T, VanArsdale T, Shields DJ, Hidalgo M, Malumbres M. CDK4/6 inhibitors impair recovery from cytotoxic chemotherapy in pancreatic adenocarcinoma. Cancer Cell. 2020;38(4):584. doi: 10.1016/j.ccell.2020.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Knudsen ES, Kumarasamy V, Ruiz A, Sivinski J, Chung S, Grant A, Vail P, Chauhan SS, Jie T, Riall TS, Witkiewicz AK. Cell cycle plasticity driven by MTOR signaling: integral resistance to CDK4/6 inhibition in patient-derived models of pancreatic cancer. Oncogene. 2019;38(18):3355–3370. doi: 10.1038/s41388-018-0650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knudsen ES, Kumarasamy V, Chung S, Ruiz A, Vail P, Tzetzo S, Wu J, Nambiar R, Sivinski J, Chauhan SS, Seshadri M, Abrams SI, Wang J, Witkiewicz AK. Targeting dual signalling pathways in concert with immune checkpoints for the treatment of pancreatic cancer. Gut. 2021;70(1):127–138. doi: 10.1136/gutjnl-2020-321000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato S, Adashek JJ, Shaya J, Okamura R, Jimenez RE, Lee S, Sicklick JK, Kurzrock R. Concomitant MEK and Cyclin Gene Alterations: Implications for response to targeted therapeutics. Clin Cancer Res. 2021;27(10):2792–2797. doi: 10.1158/1078-0432.CCR-20-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang B, Li D, Jin X, Zhang K. The CDK4/6 inhibitor PD0332991 stabilizes FBP1 by repressing MAGED1 expression in pancreatic ductal adenocarcinoma. Int J Biochem Cell Biol. 2020;128:105859. doi: 10.1016/j.biocel.2020.105859. [DOI] [PubMed] [Google Scholar]
- 51.Ji Y, Liu X, Li J, Xie X, Huang M, Jiang J, Liao YP, Donahue T, Meng H. Use of ratiometrically designed nanocarrier targeting CDK4/6 and autophagy pathways for effective pancreatic cancer treatment. Nat Commun. 2020;11(1):4249. doi: 10.1038/s41467-020-17996-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhir T, Schultz CW, Jain A, Brown SZ, Haber A, Goetz A, Xi C, Su GH, Xu L, Posey J 3rd, Jiang W, Yeo CJ, Golan T, Pishvaian MJ, Brody JR. Abemaciclib is effective against pancreatic cancer cells and synergizes with HuR and YAP1 inhibition. Mol Cancer Res. 2019;17(10):2029–2041. doi: 10.1158/1541-7786.MCR-19-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinberg BA, Wang H, Witkiewicz AK, Marshall JL, He AR, Vail P, Knudsen ES, Pishvaian MJ. A Phase I study of ribociclib plus everolimus in patients with metastatic pancreatic adenocarcinoma refractory to chemotherapy. J Pancreat Cancer. 2020;6(1):45–54. doi: 10.1089/pancan.2020.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Willobee BA, Gaidarski AA, Dosch AR, Castellanos JA, Dai X, Mehra S, Messaggio F, Srinivasan S, VanSaun MN, Nagathihalli NS, Merchant NB. Combined blockade of MEK and CDK4/6 pathways induces senescence to improve survival in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2021;20(7):1246–1256. doi: 10.1158/1535-7163.MCT-19-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–2467. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pek M, Yatim SMJM, Chen Y, Li J, Gong M, Jiang X, Zhang F, Zheng J, Wu X, Yu Q. Oncogenic KRAS-associated gene signature defines co-targeting of CDK4/6 and MEK as a viable therapeutic strategy in colorectal cancer. Oncogene. 2017;36(35):4975–4986. doi: 10.1038/onc.2017.120. [DOI] [PubMed] [Google Scholar]
- 57.Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM, Zhai H, Vidal M, Gygi SP, Braun P, Sicinski P. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20(5):620–634. doi: 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee MS, Helms TL, Feng N, Gay J, Chang QE, Tian F, Wu JY, Toniatti C, Heffernan TP, Powis G, Kwong LN, Kopetz S. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget. 2016;7(26):39595–39608. doi: 10.18632/oncotarget.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, Liao H, Zheng T, Wang J, Guo D, Lu Z, Li Z, Chen Y, Shen L, Zhang Y, Gao J. Conditionally reprogrammed colorectal cancer cells combined with mouse avatars identify synergy between EGFR and MEK or CDK4/6 inhibitors. Am J Cancer Res. 2020;10(1):249–262. [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang J, Zhou L, Zhao S, Dicker DT, El-Deiry WS. The CDK4/6 inhibitor palbociclib synergizes with irinotecan to promote colorectal cancer cell death under hypoxia. Cell Cycle. 2017;16(12):1193–1200. doi: 10.1080/15384101.2017.1320005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández-Aroca DM, Roche O, Sabater S, Pascual-Serra R, Ortega-Muelas M, Sánchez Pérez I, Belandia B, Ruiz-Hidalgo MJ, Sánchez-Prieto R. P53 pathway is a major determinant in the radiosensitizing effect of Palbociclib: Implication in cancer therapy. Cancer Lett. 2019;451:23–33. doi: 10.1016/j.canlet.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 62.Menon M, Elliott R, Bowers L, Balan N, Rafiq R, Costa-Cabral S, Munkonge F, Trinidade I, Porter R, Campbell AD, Johnson ER, Esdar C, Buchstaller HP, Leuthner B, Rohdich F, Schneider R, Sansom O, Wienke D, Ashworth A, Lord CJ. A novel tankyrase inhibitor, MSC2504877, enhances the effects of clinical CDK4/6 inhibitors. Sci Rep. 2019;9(1):201. doi: 10.1038/s41598-018-36447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rampioni Vinciguerra GL, Dall’Acqua A, Segatto I, Mattevi MC, Russo F, Favero A, Cirombella R, Mungo G, Viotto D, Karimbayli J, Pesce M, Vecchione A, Belletti B, Baldassarre G. p27kip1 expression and phosphorylation dictate Palbociclib sensitivity in KRAS-mutated colorectal cancer. Cell Death Dis. 2021;12(10):951. doi: 10.1038/s41419-021-04241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen SH, Gong X, Zhang Y, Van Horn RD, Yin T, Huber L, Burke TF, Manro J, Iversen PW, Wu W, Bhagwat SV, Beckmann RP, Tiu RV, Buchanan SG, Peng SB. RAF inhibitor LY3009120 sensitizes RAS or BRAF mutant cancer to CDK4/6 inhibition by abemaciclib via superior inhibition of phospho-RB and suppression of cyclin D1. Oncogene. 2018;37(6):821–832. doi: 10.1038/onc.2017.384. [DOI] [PubMed] [Google Scholar]
- 65.Liñares-Blanco J, Munteanu CR, Pazos A, Fernandez-Lozano C. Molecular docking and machine learning analysis of Abemaciclib in colon cancer. BMC Mol Cell Biol. 2020;21(1):52. doi: 10.1186/s12860-020-00295-w. [DOI] [PMC free article] [PubMed] [Google Scholar]