Abstract
Background/Aim: Esophagectomy and gastrectomy are procedures with considerable physical burden and intense post-operative care of which the patient’s physical condition seems to be a relevant predictor. The gold standard of the cardiorespiratory fitness is the peak oxygen consumption (VO2peak). This pilot study examined the prognostic value of VO2peak on post-surgery outcomes in esophageal and gastric cancer patients.
Patients and Methods: In this prospective cross-sectional study, patients scheduled for esophagectomy or gastrectomy were examined 24 h before the surgery regarding their VO2peak. The post-operative complications according to Clavien-Dindo grade IIIb/IV/V, Intensive-Care-Unit days, and overall hospital stay were documented following surgery. In a subset, body weight changes from surgery until hospital discharge and first aftercare visit were recorded.
Results: The functional capacity was significantly reduced in 34/35 of the included patients compared to matched norm-values (p<0.01). The only significant correlation was found between VO2peak values and body weight change from surgery to the first aftercare visit. A subgroup comparison of patients with a VO2peak <17 ml/min/kg and ≥17 ml/min/kg suggested small, non-significant differences in post-surgery outcomes and significant differences in the body weight change from surgery to hospital discharge, favoring the higher-VO2peak subgroup.
Conclusion: The impaired functional capacity following esophagectomy or gastrectomy may strengthen the rational for exercise programs during neoadjuvant and pre-surgery phases. The prognostic value of VO2peak on post-operative outcomes remains uncertain due to noticeable descriptive differences, but no significant correlations, potentially limited by the small-sized population. Nonetheless, a correlation between VO2peak and body weight change post-surgery was observed and indicates a potential prognostic value of VO2peak.
Keywords: Esophagectomy, VO2peak, post-surgery outcomes, cardiopulmonary fitness, tumor surgery
Esophageal and gastric cancer are the fifth and eight most common cancer types worldwide. Both are associated with high mortality rates and poor prognoses (1). Esophagectomy and gastrectomy are essential treatment procedures in localized esophageal and gastric cancer, respectively. Both procedures are impactful interventions with high risks of postoperative complications, such as pneumonia, sepsis, anastomotic leaks or respiratory complications (2). These complications may lengthen the patients’ hospital stay and days in the intensive care unit (ICU). The patient’s physical health condition seems to be a prominent prognostic factor for surgery outcomes (3). The question that arises is whether a better physical performance would influence the prevalence of postoperative complications, ICU-time, and length of hospital stay in patients following esophagectomy and gastrectomy. The gold standard for assessment of the cardiorespiratory fitness is provided by the peak oxygen consumption (VO2peak) (4). Therefore, this study investigated the VO2peak values of esophageal and gastric cancer patients with planned surgery and documented the medical recovery post-surgery.
Patients and Methods
The present study was approved by the Institutional Review Board of the Medical Faculty of the University of Cologne (No. 13-050). The trial was registered in the ICMJE-conform German Clinical Trials Register (DRKS00005037). Patients with esophageal and gastric cancer were screened between April 2013 and April 2014 in the Clinic and Policlinic for General-, Visceral-, and Tumor-surgery of the University Hospital Cologne, Germany for inclusion. Patients were eligible if they were (I) scheduled for esophagectomy or gastrectomy (II) adults aged ≥18 y (III) and provided informed consent. Patients were excluded if they (a) were not suited for surgery (b) had instable bone metastases (c) or had severe psychological or physiological diseases that contraindicated exercise. Anthropometric data, diagnosis, and medical history including the Charlson-Comorbidity-Index (CCI) (5) of eligible patients were documented.
Physical performance. Included patients performed a heart rate monitored spiroergometry (device: cortex medical) 24 h before surgery. The spiroergometry was realized on a bicycle-ergometer (Ergoline), starting with 30 watts, and increasing 15 watts every 2nd minute until physical exertion. The maximum aerobic capacity VO2peak (in ml/min/kg) were documented and further accounted to rate the patient’s physical performance. Also, the patients’ attained VO2peak data were compared to the predicted sex-, age-, and body weight-specific norm values (6). The norm values were predicted as followed:
Male: VO2 (l/min)=Body weight×(50.72-(0.372×age))/1.000
Female: VO2 (l/min)=(Body weight+42.8)×(22.78-(0.17×age))/1.000
Post-surgery recovery. Following surgery, the number of post-surgery complications according to Clavien-Dindo (CD) grade IIIb/IV/V (7), the number of days spent in the ICU, and the overall length of the patients’ hospital stay were documented. The complication-list frameworks of the Esophagectomy Complications Consensus Group (ECCG) and the Gastrectomy Complications Consensus Group (GCCG) were used to document post-operative complications (8,9). Post-surgery complications were further divided into 1) pulmonary, 2) cardiovascular, 3) surgical, and 4) other complications. Additionally, the body weight of patients was recorded facultatively following surgery up to the day of hospital discharge and at the first aftercare visit.
Statistical analysis. Descriptive data are presented as mean, median, range, and standard deviation. Data analyses were performed using SPSS 27 statistics software (IBM-SPSS, Inc., Armonk, NY, USA). Absolut VO2peak data were compared to the matched norm values with a paired-sample t-test. Linear regression models were calculated for the VO2peak and post-surgery recovery outcomes. In cases of patients’ death, they were excluded in the ICU and overall hospital stay analysis. In a secondary analysis, the population was separated into two subgroups according to a VO2peak cut-off value of 17 ml/min/kg and compared in post-surgery complications, ICU days, overall length of hospital stay, and body weight development. Both groups were analyzed with the Mann–Whitney-U-Test for between-group differences. The body weight change was analyzed with a repeated measures ANOVA. The p-value was set to 0.05.
Results
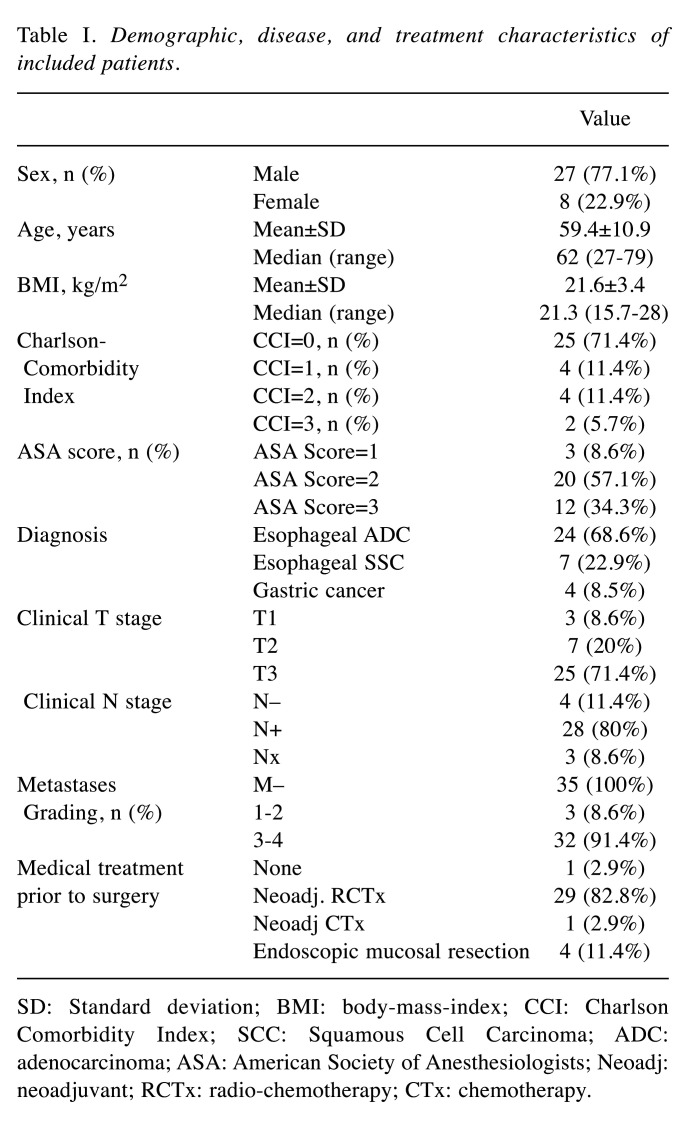
Overall, 48 patients were screened for inclusion of which 13 patients were excluded due to sole (radio-)chemotherapy treatment (recruitment rate: 69%). Therefore, 35 patients were eligible and included in the analyses. One patient died following surgery. The majority received an esophagectomy (68.6%) and were treated with neoadjuvant radiochemotherapy (82.8%). Patients’ characteristics are described in Table I.
Table I. Demographic, disease, and treatment characteristics of included patients.
SD: Standard deviation; BMI: body-mass-index; CCI: Charlson Comorbidity Index; SCC: Squamous Cell Carcinoma; ADC: adenocarcinoma; ASA: American Society of Anesthesiologists; Neoadj: neoadjuvant; RCTx: radio-chemotherapy; CTx: chemotherapy.
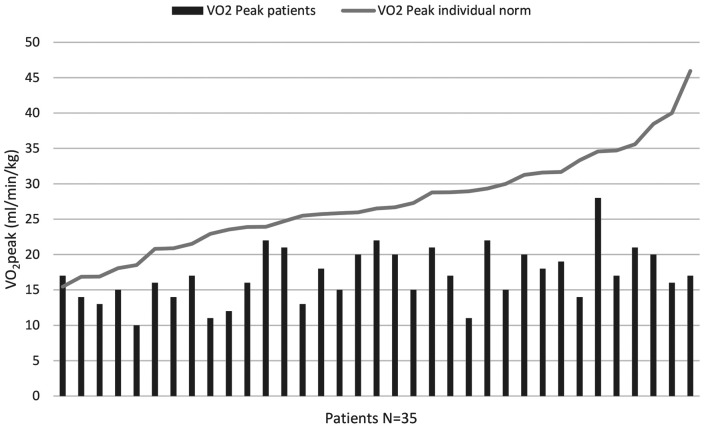
Physical performance. Overall, patients reached VO2peak values between 10-28 ml/min/kg with a mean of 17.05±3.9 ml/min/kg. Only one patient (2.9%) attained a VO2peak over the individually calculated norm value. The mean percentage of reached norm values was 64.9%±16.8%, ranging from 37% up to 110%. The differences in attained VO2peak and predicted norm values were significant (p<0.001). The VO2peak data as well as the individual norm values are displayed in Figure 1.
Figure 1. Overview of reached VO2peak values (in ml/min/kg) and the patients’ individual predicted norm value (6).
Post-surgery complications. Overall, we documented n=16 post-operative complications with Clavien-Dindo stage IIIb or above (CD≥IIIb) affecting n=12 patients (34.3%). The majority of n=23 patients did not develop CD≥IIIb complications, n=8 patients showed one CD≥IIIb complication, and n=4 patients suffered two CD≥IIIb complications. One patient (n=1) died following surgery. The most frequent complications were n=8 surgical complications (i.p. anastomotic insufficiency, pylorus-spasm), followed by n=7 pulmonary complications (i.p. respiratory insufficiency, pneumonia), and n=1 other complication (sepsis).
No correlation (r=–0.162) between the patients VO2peak and the overall number of CD≥IIIb complications or complication subgroups (pulmonary, surgical or others) was found (p=0.353). VO2peak was not a predictor (β=–0.029) of post-surgery CD≥IIIb complications (p=0.353).
Days in ICU. Following surgery, the mean duration in ICU was 5.82±8.5 days and the median ICU-stay was 2 days. Whereas the minimum was 1 day, one patient stayed for 34 consecutive days in the ICU following surgery. Neither the correlation (r=0.010) of ICU-days and VO2peak values nor the regression (β=0.023) were significant (p=0.953).
Length of hospital stay. The patients’ length of overall hospital stay varied between 11-51 days. The mean time spent in hospital was 19.62±10.8 days, and the median was 15 days. No correlation between the patients VO2peak values and length of hospital stay (r=–0.024) was found (p=0.895). The regression showed VO2peak to not be a predictor (β=–0.065) of the length of hospital stay.
Body weight development. The body weights of n=21 patients were documented following surgery until hospital discharge, ranging from 4-42 days after surgery with a mean of 16.1±10 days and a median of 14 days. The mean body weight change was –1.43±5.1 kg. No correlation between VO2peak and body weight change (r=0.41) as well as no predictive power of VO2peak (β=0.568) were found (p=0.068). Body weight data of n=24 patients were documented at first aftercare visit. The examination was conducted 57-335 days after surgery with a mean of 113.7±65 days and a median of 113 days. The mean body weight change was –7.9±5.7 kg. A correlation between VO2peak and body weight change (r=0.432) was observed (p=0.035). VO2peak showed predictive power (β=–0.737) as well (p=0.02).
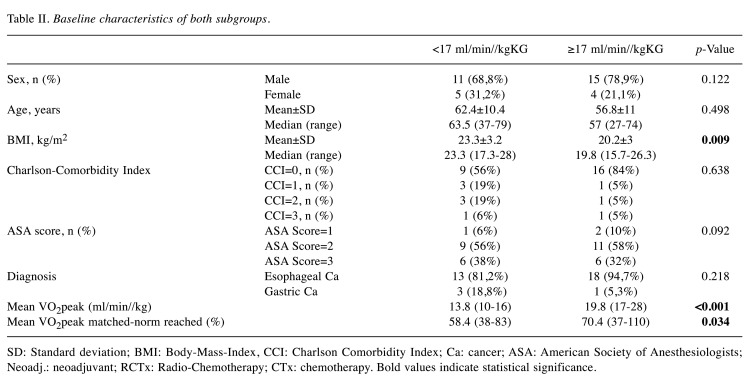
Subgroup analysis. According to the cut-off value, the patients were divided into the low-VO2p (VO2peak <17 ml/min/kg, n=16) and high-VO2p groups (VO2peak ≥17 ml/min/kg, n=19). The subgroups showed similar baseline characteristics (Table II) except for the BMI, which was higher in the low-VO2p group (23.3 vs. 20.2, p=0.009). The mean VO2peak in the low-VO2p group was 13.75±1.91 ml/min/kg compared to 19.84±2.71 ml/min/kg in the high-VO2p group (p<0.01). Also, the comparison of reached and predicted VO2peak values showed differences between the groups (p=0.03) with 58.4±14.6% (low-VO2p group) vs. 70.4±17% (high-VO2p group).
Table II. Baseline characteristics of both subgroups.
SD: Standard deviation; BMI: Body-Mass-Index, CCI: Charlson Comorbidity Index; Ca: cancer; ASA: American Society of Anesthesiologists; Neoadj.: neoadjuvant; RCTx: Radio-Chemotherapy; CTx: chemotherapy. Bold values indicate statistical significance.
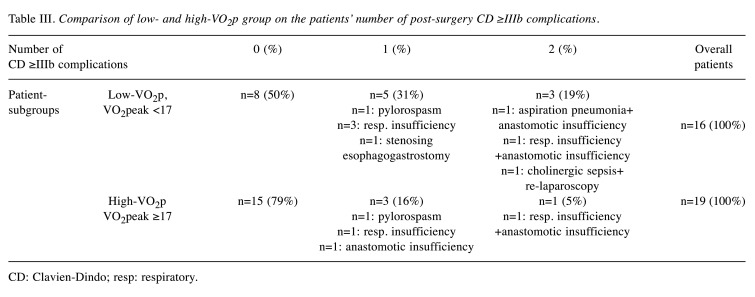
Compared to the low-VO2p group, the high-VO2p group showed reduced overall length of hospital day by 3.07 days (–14.4%), reduced ICU-stay by 3.06 days (–40.7%), and a lower CD≥IIIb complication rate by 0.43 complications/patient (–62.3%). However, none of the mentioned differences reached statistical significance (p>0.069). Figure 2 shows the differences in both groups regarding the post-operative outcomes and Table III shows the allocation and type of post-surgery CD≥IIIb complications, separately.
Figure 2. Comparison of low- and high-VO2p group on the mean number of post-surgery CD≥IIIb complications, ICU days and overall length of hospital stay. ICU: Intensive care unit; CD: Clavien-Dindo.
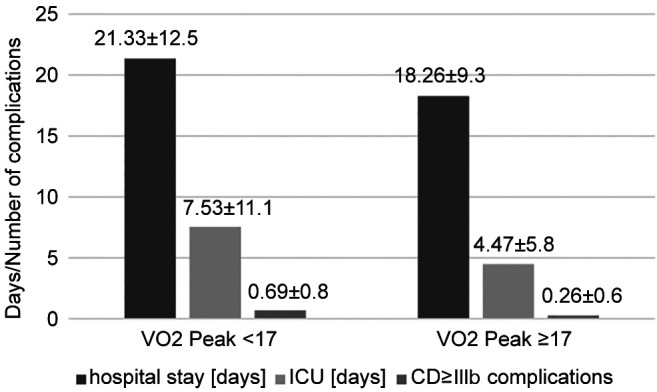
Table III. Comparison of low- and high-VO2p group on the patients’ number of post-surgery CD ≥IIIb complications.
CD: Clavien-Dindo; resp: respiratory.
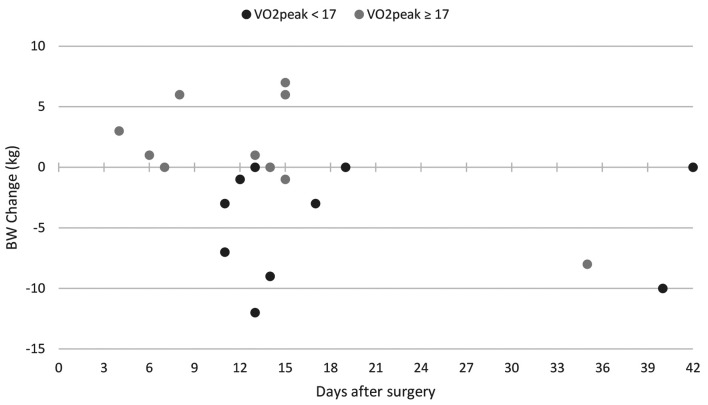
In the subset of patients with a documented body weight following surgery until hospital discharge, n=10 patients referred to the low-VO2p group and n=11 to the high-VO2p subgroup. Mean day of body weight documentation was 19.2 days (low-VO2p group) and 13.3 days (high-VO2p) with no significant differences, and the median day was 13.5 and 14, respectively. The mean body weight change was –4.5 kg in the low-VO2p group and 1.4 kg in the high-VO2p group (p=0.007). Figure 3 shows the distribution of body weight change from surgery to hospital discharge of both groups and in regard to the timing of body weight documentation.
Figure 3. Comparison of low- and high-VO2p group on the body weight change from surgery until hospital discharge. BW: Body weight.
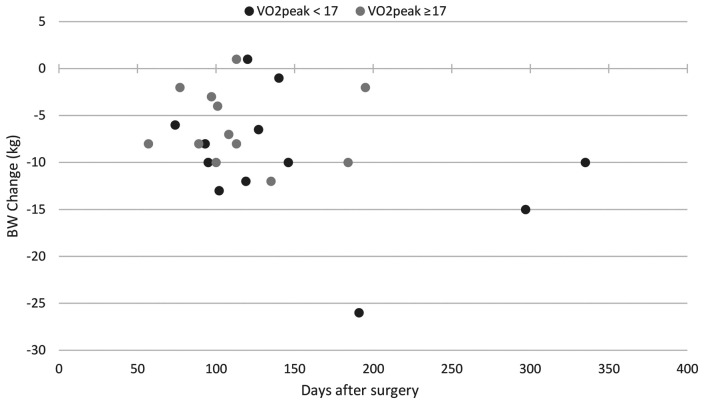
The body weight at first after care visit was collected for n=24 patients, n=12 for each subgroup. The mean duration between surgery and first aftercare visit was 153 days in the low-VO2p group and 114.1 days in the high-VO2p group with no significant differences, and the median was 123.5 and 104.5 days, respectively. Both groups showed reductions in body weight compared to baseline body weight with a mean of –9.1 kg in the low-VO2p group and –6.1 kg in the high-VO2p group (p=0.48). Figure 4 displays the body weight change at the first aftercare visit of both group and in regard to the time-interval between surgery and the first aftercare visit.
Figure 4. Comparison of low- and high-VO2p group on the body weight change from surgery to the first aftercare visit. BW: Body weight.
Discussion
This study examined the physical performance of esophageal and gastric cancer patients prior to their planned surgery and investigated the correlation between physical performance and the number of post-surgery complications, ICU-days, length of hospital stay and post-operative body weight change. The overall physical performance measured with a spiroergometry was below the individually calculated norm values in 34 of 35 patients. No correlations between physical performance and post-surgery complications, ICU-duration, and length of hospital stay were found nor could a prognostic value of VO2peak be identified. In a smaller subset, with a reported body weight change at first aftercare visit, a correlation with VO2peak was found with a predictive power of VO2peak. In a direct comparison of the low- and high-VO2p patient subgroups, noticeable differences on post-operative outcomes could be observed.
A first important finding of this investigation was the low physical performance of the investigated patients. Except for one patient, the remaining 34 patients showed reduced relative VO2peak data in relation to age, sex, and body-mass matched norm values (p<0.01). Seven patients did not reach 50% of their calculated VO2peak norm, illustrating severely reduced physical performance prior to surgery. Heavily decreased physical performance was found as a predictor of poor overall survival in patients undergoing esophago-gastric surgery and should be considered as a target for specific therapeutic approaches to increase functional capacity, such as pre-surgery exercise (10). Even short-term exercise may be enough to prevent post-operative complications following esophagectomy (11).
One major factor surely contributing to the patients’ low physical performance is neoadjuvant medical treatment. (Radio-)chemotherapy regimens were administered in 30 of our patients before esophagectomy or gastrectomy. They are known to reduce cardiovascular fitness and therefore, increase postoperative morbidity and mortality rates in esophageal or gastric cancer patients (12,13). Several studies have suggested that esophageal cancer patients undergoing neoadjuvant chemotherapy may benefit from an exercise prehabilitation approach with improved functional capacity before and after esophagectomy (14) and reduced readmission rates during neoadjuvant chemotherapy and following surgery (15). One study even showed positive impacts in functional capacity, rates of post-surgery complications, and atelectasis following esophagectomy due to a 7-day exercise program before surgery (16). Our results, however, indicate no significant correlations between physical VO2peak capacity and post-surgery complications, ICU-days, and overall length of hospital stay. That is contrary to existing studies that determine VO2peak values or results of a 6-min walking test as a predictor of post-operative outcomes (17,18). Compared to both studies with 91 and 111 patients, our pilot study was limited by the small number of examined patients. A larger cohort would be more fitting in regard to regression and correlation analyses. Nonetheless, a small but potentially meaningful descriptive indicator of the differences of post-operative outcomes according to their VO2peak values can be assumed on the basis of the cut-off value comparison of the low- and high-VO2peak-groups. The fitter group showed a mean of –3.07 days (–14.4%) in the overall length of hospital stay, –3.06 days (–40.7%) in ICU-days and –0.43 (–62.3%) post-operative CD≥IIIb complications/person compared to the low-VO2p group. The overall number of pulmonary CD≥IIIb complications in the high- VO2p group was n=2, whereas in the low-VO2p fit group was n=5. However, none of these descriptive differences reached statistical significance (p>0.069). Moreover, the potential impact of physical performance on (pulmonary) post-surgery CD≥IIIb complications was reported in comparable studies, which may have affected ICU-days and overall hospital stay as well (17,18). Also, the potential impact of VO2peak on post-operative outcomes suggests potential economic savings through the better physical function of patients before surgery, as well as specific exercise regimes prior surgery (19).
Another interesting finding was the patient-subset with documented body weight, showing body weight reductions in the post-surgery period until hospital discharge, but especially in the aftercare follow-up visits. In this subset, the VO2peak seemed to predict a body weight decrease at the first aftercare visit (p=0.02). The heavy body weight decline [–9.1 kg (low-VO2p) and –6.1 kg (high-VO2p)] from surgery to the first after care visit strengthens the rationale for nutrition and exercise programs following surgery to reduce the risk of cachexia development or progression. Also, the subgroup comparison of low- and high-VO2p patient groups revealed significant differences in the body weight after surgery until hospital discharge, favoring the high-VO2p patients (p=0.007). However, a mean body weight change of 1.4 kg following surgery compared to –4.5 kg in the low-VO2p group may suggest potential water retentions. Nevertheless, these observations may influence other post-operative outcomes such as complications, ICU-days, and length of stay. Noteworthy, the small sample size with documented body weight and the heterogeneous timing of body weight assessment limit these findings.
Despite our findings, the question whether the functional capacity of patients undergoing esophago-gastric surgery has prognostic value remains. Skeletal muscle mass and strength serve as important factors of physical performance. Skeletal muscle mass and presence of sarcopenia were shown to be independent risk factors of post-operative pulmonary complications (20) and seem to be a prognostic factors for relevant survival after esophagectomy (21). Additionally, the skeletal muscle mass seems to correlate with neoadjuvant chemoradiation-toxicities and may influence the following post-operative complication rate in esophageal cancer patients (22). Handgrip strength was also identified as a prognostic factor of post-operative complications (23) and mortality rates following esophagectomy (24) or gastrectomy (25). Therefore, assessment of muscle mass and strength should be included in the pre-operative prognostic assessment of esophageal and gastric cancer patients, additionally to a functional capacity assessment.
In conclusion, this cohort study reported a heavily reduced pre-surgery physical performance but could not confirm VO2peak as a prognostic factor for post-operative outcomes, except for body weight change. The low sample size may limit the significance of our analyses, since noticeable differences in the post-operative outcomes between low and high-VO2p patient groups were observed, favoring the high-VO2peak patients. Nonetheless, our results indicate the need of approaches that will help maintain the physical performance of esophageal and gastric cancer patients during their neoadjuvant chemoradiation/chemotherapy and in the field of prehabilitation. Exercise and nutrition programs may be a promising approach.
Conflicts of Interest
The Authors have no conflicts of interest to declare in relation to this study.
Authors’ Contributions
Conceptualization: FTB, CTB, AHH, BE, UF; methodology: FTB, CTB, UF; formal analysis: TN, GK, JHN.; investigation: FTB, CTB, GK.; resources: FTB; writing—original draft preparation: TN; writing—review and editing: TN, FTB, CTB, AHH, BE, UF, JHN; supervision: FTB, CTB; project administration: FTB, CTB.
Acknowledgements
FT Baumann is funded by the German Cancer Aid, Bonn, Germany.
References
- 1.Europe WHO ROF: World Cancer Report. Cancer research for cancer development. IARC 2020. Available at: https://publications.iarc.fr/Non-Series-Publications/WorldCancer-Reports/World-Cancer-Report-Cancer-Research-ForCancer-Prevention-2020. [Last accessed on May 10, 2022]
- 2.Valkenet K, Trappenburg JC, Gosselink R, Sosef MN, Willms J, Rosman C, Pieters H, Scheepers JJ, de Heus SC, Reynolds JV, Guinan E, Ruurda JP, Rodrigo EH, Nafteux P, Fontaine M, Kouwenhoven EA, Kerkemeyer M, van der Peet DL, Hania SW, van Hillegersberg R, Backx FJ. Preoperative inspiratory muscle training to prevent postoperative pulmonary complications in patients undergoing esophageal resection (PREPARE study): study protocol for a randomized controlled trial. Trials. 2014;15:144. doi: 10.1186/1745-6215-15-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heger P, Probst P, Wiskemann J, Steindorf K, Diener MK, Mihaljevic AL. A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366) J Gastrointest Surg. 2020;24(6):1375–1385. doi: 10.1007/s11605-019-04287-w. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society , American College of Chest Physicians ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 5.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, Januel JM, Sundararajan V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 6.Wasserman K. Philadelphia, PA, USA, Lippincott Williams & Wilkins. 1999. Principles of exercise testing & interpretation. Including pathophysiology and clinical applications, 3th ed. [Google Scholar]
- 7.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Low DE, Alderson D, Cecconello I, Chang AC, Darling GE, DʼJourno XB, Griffin SM, Hölscher AH, Hofstetter WL, Jobe BA, Kitagawa Y, Kucharczuk JC, Law SY, Lerut TE, Maynard N, Pera M, Peters JH, Pramesh CS, Reynolds JV, Smithers BM, van Lanschot JJ. International Consensus on standardization of data collection for complications associated with esophagectomy: Esophagectomy Complications Consensus Group (ECCG) Ann Surg. 2015;262(2):286–294. doi: 10.1097/SLA.0000000000001098. [DOI] [PubMed] [Google Scholar]
- 9.Baiocchi GL, Giacopuzzi S, Marrelli D, Reim D, Piessen G, Matos da Costa P, Reynolds JV, Meyer HJ, Morgagni P, Gockel I, Lara Santos L, Jensen LS, Murphy T, Preston SR, Ter-Ovanesov M, Fumagalli Romario U, Degiuli M, Kielan W, Mönig S, Kołodziejczyk P, Polkowski W, Hardwick R, Pera M, Johansson J, Schneider PM, de Steur WO, Gisbertz SS, Hartgrink H, van Sandick JW, Portolani N, Hölscher AH, Botticini M, Roviello F, Mariette C, Allum W, De Manzoni G. International consensus on a complications list after gastrectomy for cancer. Gastric Cancer. 2019;22(1):172–189. doi: 10.1007/s10120-018-0839-5. [DOI] [PubMed] [Google Scholar]
- 10.Whibley J, Peters CJ, Halliday LJ, Chaudry AM, Allum WH. Poor performance in incremental shuttle walk and cardiopulmonary exercise testing predicts poor overall survival for patients undergoing esophago-gastric resection. Eur J Surg Oncol. 2018;44(5):594–599. doi: 10.1016/j.ejso.2018.01.242. [DOI] [PubMed] [Google Scholar]
- 11.Yamana I, Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Shimaoka H, Shiota E, Yamashita Y. Randomized controlled study to evaluate the efficacy of a preoperative respiratory rehabilitation program to prevent postoperative pulmonary complications after esophagectomy. Dig Surg. 2015;32(5):331–337. doi: 10.1159/000434758. [DOI] [PubMed] [Google Scholar]
- 12.Sinclair R, Navidi M, Griffin SM, Sumpter K. The impact of neoadjuvant chemotherapy on cardiopulmonary physical fitness in gastro-oesophageal adenocarcinoma. Ann R Coll Surg Engl. 2016;98(6):396–400. doi: 10.1308/rcsann.2016.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack S, West MA, Raw D, Marwood S, Ambler G, Cope TM, Shrotri M, Sturgess RP, Calverley PM, Ottensmeier CH, Grocott MP. The effect of neoadjuvant chemotherapy on physical fitness and survival in patients undergoing oesophagogastric cancer surgery. Eur J Surg Oncol. 2014;40(10):1313–1320. doi: 10.1016/j.ejso.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of exercise and nutrition prehabilitation on functional capacity in esophagogastric cancer surgery: a randomized clinical trial. JAMA Surg. 2018;153(12):1081–1089. doi: 10.1001/jamasurg.2018.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dewberry LC, Wingrove LJ, Marsh MD, Glode AE, Schefter TE, Leong S, Purcell WT, McCarter MD. Pilot prehabilitation program for patients with esophageal cancer during neoadjuvant therapy and surgery. J Surg Res. 2019;235:66–72. doi: 10.1016/j.jss.2018.09.060. [DOI] [PubMed] [Google Scholar]
- 16.Akiyama Y, Sasaki A, Fujii Y, Fujisawa R, Sasaki N, Nikai H, Endo F, Baba S, Hasegawa Y, Kimura T, Takahara T, Nitta H, Otsuka K, Koeda K, Nishimura Y, Iwaya T. Efficacy of enhanced prehabilitation for patients with esophageal cancer undergoing esophagectomy. Esophagus. 2021;18(1):56–64. doi: 10.1007/s10388-020-00757-2. [DOI] [PubMed] [Google Scholar]
- 17.Nagamatsu Y, Shima I, Yamana H, Fujita H, Shirouzu K, Ishitake T. Preoperative evaluation of cardiopulmonary reserve with the use of expired gas analysis during exercise testing in patients with squamous cell carcinoma of the thoracic esophagus. J Thorac Cardiovasc Surg. 2001;121(6):1064–1068. doi: 10.1067/mtc.2001.113596. [DOI] [PubMed] [Google Scholar]
- 18.Inoue T, Ito S, Kanda M, Niwa Y, Nagaya M, Nishida Y, Hasegawa Y, Koike M, Kodera Y. Preoperative six-minute walk distance as a predictor of postoperative complication in patients with esophageal cancer. Dis Esophagus. 2020;33(2):doz050. doi: 10.1093/dote/doz050. [DOI] [PubMed] [Google Scholar]
- 19.Baltin CT, Bludau M, Kron F, Zander T, Hallek M, Hölscher AH, Schröder W. [Profit center analysis of esophagectomy : Economical analysis of transthoracic esophagectomy depending on postoperative complications] Chirurg. 2018;89(3):229–236. doi: 10.1007/s00104-018-0590-9. [DOI] [PubMed] [Google Scholar]
- 20.Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K. Prognostic significance of sarcopenia in patients undergoing esophagectomy for superficial esophageal squamous cell carcinoma. Dis Esophagus. 2019;32(7):doy104. doi: 10.1093/dote/doy104. [DOI] [PubMed] [Google Scholar]
- 21.Boshier PR, Heneghan R, Markar SR, Baracos VE, Low DE. Assessment of body composition and sarcopenia in patients with esophageal cancer: a systematic review and meta-analysis. Dis Esophagus. 2018;31(8):doy047. doi: 10.1093/dote/doy047. [DOI] [PubMed] [Google Scholar]
- 22.Panje CM, Höng L, Hayoz S, Baracos VE, Herrmann E, Garcia Schüler H, Meier UR, Henke G, Schacher S, Hawle H, Gérard MA, Ruhstaller T, Plasswilm L, Swiss Group for Clinical Cancer Research (SAKK) Skeletal muscle mass correlates with increased toxicity during neoadjuvant radiochemotherapy in locally advanced esophageal cancer: A SAKK 75/08 substudy. Radiat Oncol. 2019;14(1):166. doi: 10.1186/s13014-019-1372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato S, Nagai E, Taki Y, Watanabe M, Watanabe Y, Nakano K, Yamada H, Chiba T, Ishii Y, Ogiso H, Takagi M. Hand grip strength as a predictor of postoperative complications in esophageal cancer patients undergoing esophagectomy. Esophagus. 2018;15(1):10–18. doi: 10.1007/s10388-017-0587-3. [DOI] [PubMed] [Google Scholar]
- 24.Chen CH, Ho-Chang , Huang YZ, Hung TT. Hand-grip strength is a simple and effective outcome predictor in esophageal cancer following esophagectomy with reconstruction: a prospective study. J Cardiothorac Surg. 2011;6:98. doi: 10.1186/1749-8090-6-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato T, Aoyama T, Hayashi T, Segami K, Kawabe T, Fujikawa H, Yamada T, Yamamoto N, Oshima T, Rino Y, Masuda M, Ogata T, Cho H, Yoshikawa T. Impact of preoperative hand grip strength on morbidity following gastric cancer surgery. Gastric Cancer. 2016;19(3):1008–1015. doi: 10.1007/s10120-015-0554-4. [DOI] [PubMed] [Google Scholar]