Abstract
Background: Perioperative systemic inflammation affects the long-term oncological outcomes of patients with malignancies. We evaluated the clinical impact of the preoperative platelet-to-lymphocyte ratio (PLR) in patients with resectable esophageal cancer who received curative treatment.
Patients and Methods: This study included 168 patients who underwent curative surgery followed by perioperative adjuvant chemotherapy for esophageal cancer between 2005 and 2018. The risk factors for overall survival (OS) and recurrence-free survival (RFS) were identified.
Results: Based on the 3- and 5-year OS rates, we set the cut-off value of the PLR at 150 in the present study. Among 168 patients, 78 patients (46.4%) were categorized into the PLR-low group and 90 patients (53.6%) were categorized into the PLR-high group. The 3- and 5-year OS rates were 64.4% and 53.8%, respectively, in the PLR-low group, and 46.9% and 38.1% in the PLR-high group; the difference in OS was significant (p=0.046). PLR was therefore selected for the final multivariate analysis model (hazard ratio=1.553, 95% confidence interval=1.026-2.350, p=0.037). When the perioperative clinical course was compared between the two groups, the incidence of grade 2 or more anastomotic leakage after surgery was significantly lower in the PLR-low group at 26.9% compared to 43.3% in the PLR-high group (p=0.027).
Conclusion: The PLR had a clinical impact on the long-term oncological outcomes of patients with esophageal cancer treated with curative intent. Therefore, the PLR might be a promising prognostic factor for patients with esophageal cancer.
Keywords: Platelets, lymphocytes, survival, esophageal cancer
Worldwide, esophageal cancer is the eighth-most common cancer and the sixth leading cause of cancer-related mortality (1,2). Esophagectomy with perioperative adjuvant treatment is the standard treatment for resectable esophageal cancer (3,4). Although the survival rate after curative treatment gradually improves, more than half of patients develop recurrent disease, even after curative treatment, and the prognosis of patients with recurrence is poor (5,6). Therefore, it is necessary to determine prognostic factors and to establish which patients with prognostic factors require more aggressive treatment. Perioperative systemic inflammation is associated with both short- and long-term oncological outcomes (7,8). Previous studies demonstrated that perioperative systemic inflammation accelerated tumor invasion and enhanced micrometastasis in various malignancies (9,10). Therefore, if physicians manage and control perioperative systemic inflammation using optimal screening tools, they may be able to improve patient survival. However, screening tools that can evaluate perioperative systemic inflammation in patients with esophageal cancer are limited. Recently, the platelet-to-lymphocyte ratio (PLR) was developed and reported as a promising prognostic factor in gastrointestinal malignancies (11-13). The PLR only consists of the platelet and lymphocyte counts. Thus, the PLR has clinical advantages, including ease of implementation, preoperative accessibility, and low cost.
In the present study, we hypothesized that the preoperative PLR may have clinical impact on both short- and long-term oncological outcomes in patients who receive curative treatment for esophageal cancer. To confirm our hypothesis, we evaluated the prognostic value and clinical impact of the PLR in such patients.
Patients and Methods
Patients. Individuals were selected from the medical records of consecutive patients who were diagnosed with primary esophageal adenocarcinoma or squamous cell carcinoma and who underwent complete tumor resection at Yokohama City University from 2005 to 2018. The inclusion criteria were as follows: (i) stage I-III disease, as evaluated according to the Union for International Cancer Control classification (7th edition) (14); (ii) complete (R0) resection of esophageal cancer with lymphadenectomy; and (iii) a laboratory blood analysis performed within 1 week before surgery. Patients who underwent R1 or R2 resection were excluded from the present analysis.
Surgical procedure. In principle, subtotal esophagectomy via right thoracotomy and reconstruction with a gastric tube is the standard procedure for patients with esophageal cancer. Two-field lymph node dissection was indicated when tumors were located at the middle thoracic to lower thoracic esophagus, while three-field dissection was applied for upper thoracic tumors.
Measurement of the PLR. The PLR was calculated by dividing the platelet count by the lymphocyte count. The PLR values were assessed from blood samples taken within 7 days before surgery.
Evaluation and statistical analyses. The chi-squared test was used to compare the significance of differences between the PLR and clinicopathological parameters. The Kaplan-Meier method was used to calculate the overall survival (OS) and recurrence-free survival (RFS) curves. OS was defined as the period between the date of surgery and death. RFS was defined as the period between surgery and the occurrence of an event, recurrence, or death, whichever came first. The data of patients who had not experienced an event were censored at the date of the final observation. Univariate and multivariate survival analyses were performed using a Cox proportional hazards model. p-Values of less than 0.05 were considered to indicate statistical significance of differences and associations. SPSS (v26.0 J Win; IBM, Armonk, NY, USA) was used to perform all statistical analyses. This study was approved by the Institutional Review Board of Yokohama City University (approval number: B191100037).
Results
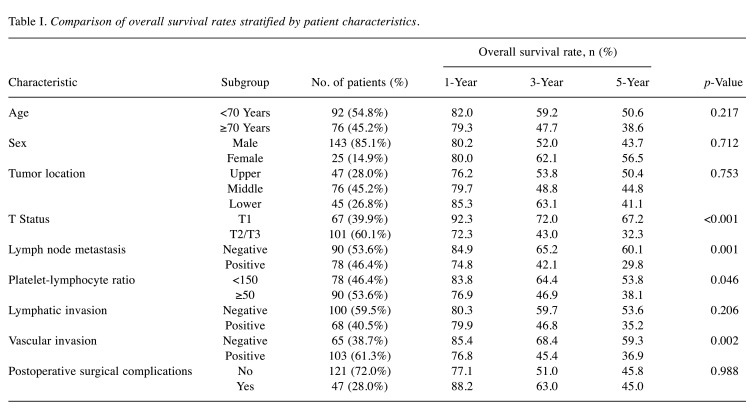
Patients. One-hundred and sixty-eight patients were evaluated in the present study. Based on the 3- and 5-year OS rates and previous reports (11-13), we set the cut-off value of the PLR at 150 in the present study (Table I). In the present study, the patients were divided into the PLR-low group (<150) and the PLR-high group (≥150). Among 168 patients, 78 patients (46.4%) were categorized into the PLR-low group and 90 patients (53.6%) into the PLR-high group. When comparing the patient background factors between these two groups, the background factors were mostly similar. The median age (67 vs. 67 years, p=0.693), male sex rate (83.3% vs. 86.6%, p=0.545), incidence of alcohol habit (87.2% vs. 87.8%, p=0.907), incidence of smoking habit (83.3% vs. 88.9%, p=0.296), incidence of hypertension (41.0% vs. 53.3%, p=0.111), incidence of diabetes mellitus (10.3% vs. 18.9%, p=0.117), and incidence of chronic obstructive pulmonary disease (32.1% vs. 31.1%, p=0.896) were similar. On the other hand, the median preoperative serum albumin (4.0 vs. 3.9 g/dl, p=0.072) and C-reactive protein (0.38 mg/dl vs. 0.85 mg/dl, p=0.030) levels in the PLR-high group were worse than those in the PLR-low group.
Table I. Comparison of overall survival rates stratified by patient characteristics.
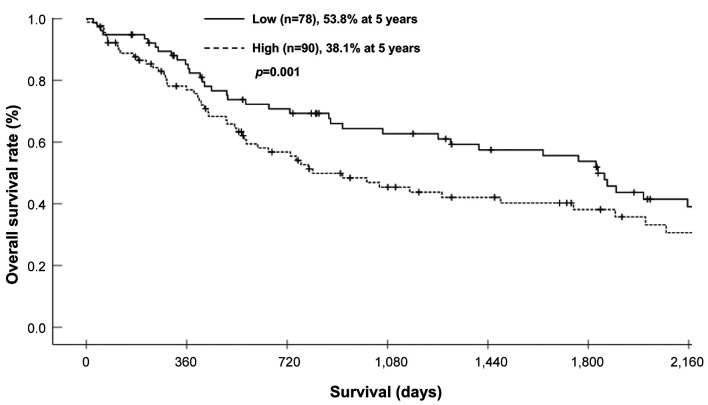
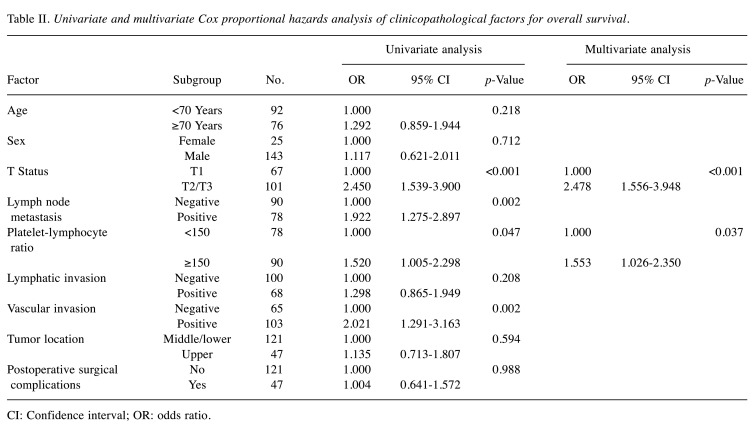
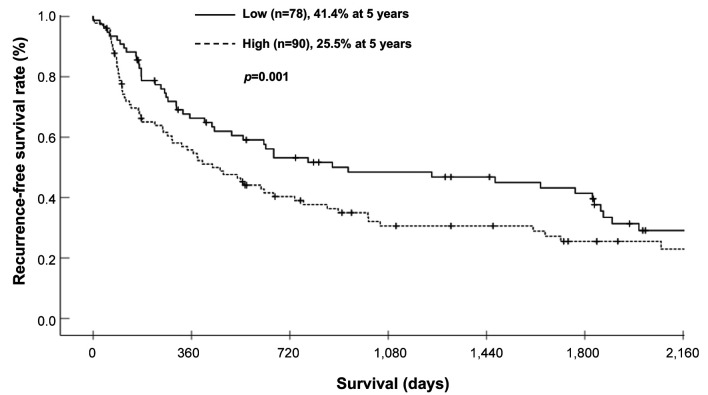
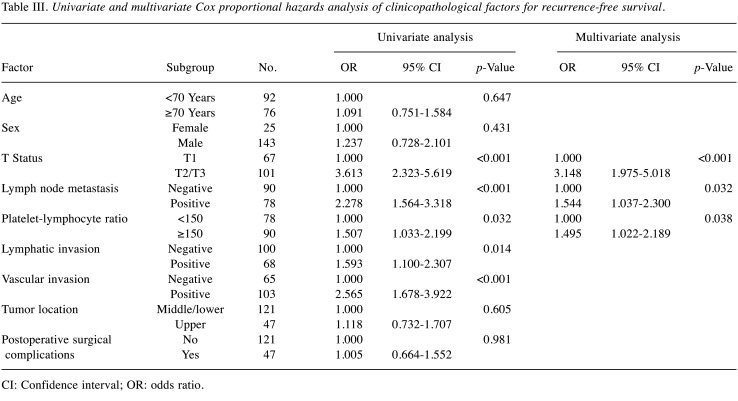
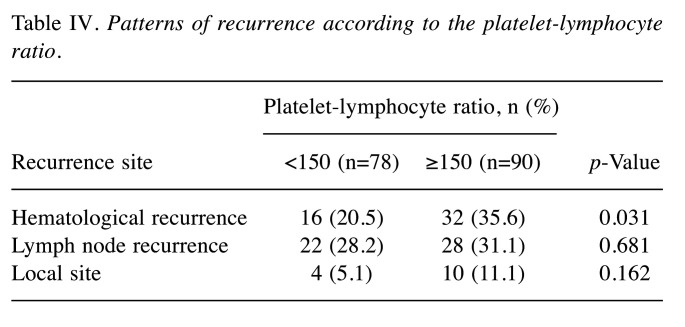
Survival analysis of the PLR-low and PLR-high groups. The 3- and 5-year OS rates were 64.4% and 53.8%, respectively, in the PLR-low group, and 46.9% and 38.1%, respectively, in the PLR-high group; these differences were significant (Figure 1) (p=0.046). Each clinicopathological factor was categorized as shown in Table II, and the prognostic significance was analyzed. The univariate analyses for OS showed that the pathological T status and PLR were significant prognostic factors. The PLR was therefore selected for the final multivariate analysis model [hazard ratio (HR)=1.553, 95% confidence interval (CI)=1.026-2.350; p=0.037]. The 3- and 5-year RFS rates were 48.4% and 41.4%, respectively, in the PLR-low group but were significantly lower (Figure 2) (p=0.032) at 30.6% and 25.5%, respectively, in the PLR-high group. Each clinicopathological factor was categorized as shown in Table III and its prognostic significance was analyzed. The univariate analyses for RFS showed that the pathological T status, lymph node metastasis, and PLR were significant prognostic factors. The PLR was also selected for the final multivariate analysis model (HR=1.495, 95% CI=1.022-2.189; p=0.038). When the sites of recurrence were compared, in the PLR-high group, hematological recurrence was significantly higher (35.6% vs. 20.5%, p=0.031) and local recurrence was approximately double that of the PLR-low group (11.1% vs. 5.1%, p=0.162) (Table IV).
Figure 1. Comparison of the overall survival of patients of the platelet-lymphocyte ratio (PLR)-high and PLR-low groups.
Table II. Univariate and multivariate Cox proportional hazards analysis of clinicopathological factors for overall survival.
CI: Confidence interval; OR: odds ratio.
Figure 2. Comparison of the recurrence-free survival of patients of the platelet-lymphocyte ratio (PLR)-high and PLR-low groups.
Table III. Univariate and multivariate Cox proportional hazards analysis of clinicopathological factors for recurrence-free survival.
CI: Confidence interval; OR: odds ratio.
Table IV. Patterns of recurrence according to the platelet-lymphocyte ratio.
Comparison of the postoperative clinical course between the PLR-low and PLR-high groups. When the perioperative clinical course was compared between the two groups, they were similar. The median postoperative hospital stay (43 vs. 46 days, p=0.638), median operative time (628 vs. 647 mins, p=0.563), and median intraoperative blood loss (639 ml vs. 752 ml, p=0.273) were similar between the PLR-low and PLR-high groups respectively. In addition, the incidence of postoperative complications was also similar between the PLR-low and PLR-high groups (71.8% vs. 72.2%, p=0.951). On the other hand, the incidence of Clavien-Dindo grade 2 or more anastomotic leakage was significantly lower at 26.9% in the PLR-low group, while it was 43.3% in PLR-high group (p=0.027).
Discussion
The present study aimed to clarify whether the platelet-to-lymphocyte ratio (PLR) has a clinical impact in patients with esophageal cancer who receive curative treatment. The major finding was that the preoperative PLR did appear to affect the long-term oncological outcomes in patients with esophageal cancer who underwent curative treatment. Therefore, our results suggest that the perioperative PLR is a promising prognostic factor for patients with esophageal cancer.
Firstly, we wish to discuss the clinical impact of the PLR on long-term oncological outcomes. In the present study, the 5-year OS rate was 53.8% in the PLR-low group, and 38.1% in the PLR-high group. Moreover, the HR of a PLR>150 for OS was 1.553. Similar results were observed in limited studies. Feng et al. evaluated the prognostic value of the pretreatment PLR in 483 patients with esophageal squamous cell carcinoma (15). They demonstrated that the 5-year OS rate was 63.5% in their PLR-low group, and 32.7% in their PLR-high group. In addition, they also showed that a high PLR was a prognostic factor, with an HR of the PLR (cut-off value at 150) for OS was 1.840 (95% CI=1.407-2.407, p<0.001). Xie et al. investigated the clinical impact of the preoperative PLR in 317 patients with esophageal squamous cell carcinoma (16). They demonstrated that the 3-year disease-specific survival rate was 71.7% in their PLR-low group, and 54.3% in their PLR-high group. A high-PLR was a negative prognostic factor, with an HR of 1.776 (95% CI=1.224-2.578, p=0.003). Taken together, the preoperative PLR was considered to be a promising prognostic factor for patients who received curative treatment for esophageal cancer.
Why does the preoperative PLR affect long-term oncological outcomes? The most likely reason is that the preoperative PLR is related to the occurrence of infectious complications, such as anastomotic leakage or fistula. In the present study, the incidence of postoperative anastomotic leakage in the PLR-high group was significantly higher than that in the PLR-low group (43.3% vs. 26.9%, p=0.027). Recently, we reported that postoperative anastomotic leakage was a significant prognostic factor in patients with esophageal cancer (17). The 3- and 5-year OS rates were 63.9% and 53.2%, respectively, in the group without, and 43.9% and 40.2% in that with anastomotic leakage (p=0.0049). Similarly to our study, Han et al. evaluated the clinical impact of the PLR as a predictor of fistula formation in 317 patients who underwent esophagectomy for esophageal cancer (18). They found that the incidence of fistula formation was significantly higher in their PLR-high group than in their PLR-low group (60.5% vs. 39.5%, p=0.001). Moreover, the PLR was identified as an independent risk factor for fistula formation (odds ratio=2.359, 95% CI=1.096-5.080, p=0.028). In addition, Paliogiannis et al. evaluated the clinical relationship between the PLR and postoperative anastomotic leakage in 1,432 patients with colorectal cancer (19). They found that the PLR in patients with postoperative anastomotic leakage was significantly higher than that in patients without it. Therefore, the preoperative PLR status may be involved in the occurrence of postoperative complications and it may be necessary to develop treatment or postoperative management strategies based on the preoperative PLR status.
The present study offers some future suggestions. Firstly, there was a clinical relationship between the PLR status and the pattern of recurrence. In the present study, the rate of hematological recurrence was significantly higher in the PLR-high group than in the PLR-low group. However, the underlying mechanism through which the PLR status significantly affected hematological recurrence is unclear. In addition, as far as we are aware, no reports have focused on the relationship between the PLR status and the recurrence pattern. Secondly, the optimal cut-off value of the PLR was unclear. In the present study, we set the cut-off value of the PLR at 150 according to the 3- and 5-year OS rates. However, previous studies set various cut-off values for the PLR (15,16,20,21). Similarly to our study, Chen et al. set a cut-off value of 150 for 107 patients with esophageal cancer according to a previous study (20), while Feng et al. set a cut-off value of 150 in 483 patients with esophageal cancer according to the results of a receiver operating characteristics (ROC) analysis (15). On the other hand, Xie et al. set a cut-off value of 103 in 317 patients according to an ROC analysis (16), while Wang et al. set a cut-off value of 183 in 113 patients according to an ROC analysis (21). The differences in the cut-off values for the PLR were due to the different numbers of patients, background characteristics, and treatment strategies. To utilize the preoperative PLR for esophageal cancer treatment, it is necessary to set and determine the optimal cut-off value of the PLR. Further studies should focus on these issues.
In conclusion, the preoperative PLR affects the long-term oncological outcomes of patients with esophageal cancer who receive curative treatment. Therefore, the perioperative PLR is a promising prognostic factor for esophageal cancer.
Conflicts of Interest
The Authors declare no conflicts of interest in association with the present study.
Authors’ Contributions
TA, MJ and KK made substantial contributions to the concept and design. TA, AO, TK, MF, KE, IH, HC, AT, KS, HT, SO, JM, TO, NY and YR made substantial contributions to the acquisition of data and the analysis and interpretation of the data. TA, HW, AT, HT, TO, NY and YR were involved in drafting the article or revising it critically for important intellectual content. TA, NM, AO and TO give their final approval of the version to be published.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 21K08688.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D, ESMO Guidelines Committee Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 4.Muro K, Lordick F, Tsushima T, Pentheroudakis G, Baba E, Lu Z, Cho BC, Nor IM, Ng M, Chen LT, Kato K, Li J, Ryu MH, Zamaniah WIW, Yong WP, Yeh KH, Nakajima TE, Shitara K, Kawakami H, Narita Y, Yoshino T, Van Cutsem E, Martinelli E, Smyth EC, Arnold D, Minami H, Tabernero J, Douillard JY. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic oesophageal cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):34–43. doi: 10.1093/annonc/mdy498. [DOI] [PubMed] [Google Scholar]
- 5.Okada M, Kato K, Cho BC, Takahashi M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Matsumura Y, Takazawa A, Kitagawa Y. Three-year follow-up and response-survival relationship of nivolumab in previously treated patients with advanced esophageal squamous cell carcinoma (ATTRACTION-3) Clin Cancer Res. 2002 doi: 10.1158/1078-0432.CCR-21-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y, CheckMate 648 Trial Investigators Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 7.Atsumi Y, Kawahara S, Kakuta S, Onodera A, Hara K, Kazama K, Numata M, Aoyama T, Tamagawa A, Tamagawa H, Oshima T, Yukawa N, Rino Y. Low preoperative albumin-to-globulin ratio is a marker of poor prognosis in patients with esophageal cancer. In Vivo. 2021;35(6):3555–3561. doi: 10.21873/invivo.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiwitkeyoonwong J, Jiarpinitnun C, Hiranyatheb P, Ngamphaiboon N. Impact of weight loss on patients with locally advanced esophageal and esophagogastric junction cancers treated with chemoradiotherapy. Asian Pac J Cancer Prev. 2021;22(12):3847–3855. doi: 10.31557/APJCP.2021.22.12.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Q, Song Q, Cheng Y, Wang N. Prognostic significance of preoperative prognostic nutritional index for overall survival and postoperative complications in esophageal cancer patients. Cancer Manag Res. 2021;13:8585–8597. doi: 10.2147/CMAR.S333190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroda K, Toyokawa T, Miki Y, Yoshii M, Tamura T, Tanaka H, Lee S, Muguruma K, Yashiro M, Ohira M. Prognostic impact of postoperative systemic inflammatory response in patients with stage II/III gastric cancer. Sci Rep. 2022;12(1):3025. doi: 10.1038/s41598-022-07098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Z, Gao J, Liu Z, Li C, Xue Y. Preoperative platelet-to-lymphocyte ratio (PLR) for predicting the survival of stage I-III gastric cancer patients with a MGC component. Biomed Res Int. 2021;2021:9678363. doi: 10.1155/2021/9678363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen R, Wang L, Zhao Q, Li Z, Chen M, Lian G, Zhang J. Platelet-to-lymphocyte ratio and C-reactive protein as markers for colorectal polyp histological type. BMC Cancer. 2021;21(1):556. doi: 10.1186/s12885-021-08221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Y, Cheng S, Fathy AH, Qian H, Zhao Y. Prognostic value of platelet-to-lymphocyte ratio in pancreatic cancer: a comprehensive meta-analysis of 17 cohort studies. Onco Targets Ther. 2018;11:1899–1908. doi: 10.2147/OTT.S154162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C. Hoboken, NJ, Wiley-Blackwell. 2009. TNM Classification of Malignant Tumours, Seventh Edition. [Google Scholar]
- 15.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie X, Luo KJ, Hu Y, Wang JY, Chen J. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus. 2016;29(1):79–85. doi: 10.1111/dote.12296. [DOI] [PubMed] [Google Scholar]
- 17.Aoyama T, Kazama K, Atsumi Y, Tamagawa H, Tamagawa A, Komori K, Machida D, Maezawa Y, Kano K, Hara K, Murakawa M, Numata M, Oshima T, Yukawa N, Masuda M, Rino Y. Clinical influence of anastomotic leakage on esophageal cancer survival and recurrence. Anticancer Res. 2020;40(1):443–449. doi: 10.21873/anticanres.13972. [DOI] [PubMed] [Google Scholar]
- 18.Han D, Zhang J, Zhao J, Lei T, Chen X, Zhang T, Wei H, Guan Y, Wang J, Zhang W, Zhao L, Wang J, Yuan Z, Song Y, Liu N, Pang Q, Wang P. Platelet-to-lymphocyte ratio is an independent predictor of chemoradiotherapy-related esophageal fistula in esophageal cancer patients. Ann Transl Med. 2020;8(18):1163. doi: 10.21037/atm-20-4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paliogiannis P, Deidda S, Maslyankov S, Paycheva T, Farag A, Mashhour A, Misiakos E, Papakonstantinou D, Mik M, Losinska J, Scognamillo F, Sanna F, Feo CF, Cherchi G, Xidas A, Zinellu A, Restivo A, Zorcolo L. Blood cell count indexes as predictors of anastomotic leakage in elective colorectal surgery: a multicenter study on 1432 patients. World J Surg Oncol. 2020;18(1):89. doi: 10.1186/s12957-020-01856-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen LC, Li SH, Lo CM, Chen YH, Huang SC, Wang YM, Chou SY, Lu HI. Platelet-to-lymphocyte ratio is an independent prognosticator in patients with esophageal squamous cell carcinoma receiving esophagectomy. J Thorac Dis. 2019;11(11):4583–4590. doi: 10.21037/jtd.2019.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Tong J, Tang M, Lu Y, Liang G, Zhang Z, Chen T. Pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as prognostic factors and reference markers of treatment options for locally advanced squamous cell carcinoma located in the middle and upper esophagus. Cancer Manag Res. 2021;13:1075–1085. doi: 10.2147/CMAR.S294344. [DOI] [PMC free article] [PubMed] [Google Scholar]