Abstract
Background/Aim: Transforming growth factor β (TGFβ) signaling plays a key role in modulating intestinal epithelial cell (IEC) homeostasis. The present study aimed to investigate the direct effect of tacrolimus on TGFβ signaling in IECs.
Materials and Methods: The protective effects of tacrolimus, with or without anti-TGFβ antibody, in dextran sulfate sodium (DSS)-induced colitis were evaluated.
Results: Tacrolimus ameliorated IEC apoptosis-mediated mucosal destruction despite anti-TGFβ treatment. TGFβ receptor type II (TGFβ-RII), phosphor-SMAD family members 2/3, and phosphor-extracellular signal-regulated kinase (ERK) expression in IECs was enhanced in tacrolimus-treated mice, and these positive effects were maintained despite anti-TGFβ treatment. Moreover, tacrolimus induced TGFβ-RII up-regulation through ERK activation.
Conclusion: Our data indicate that tacrolimus directly activated TGFβ–SMAD signaling via the ERK pathway in IECs, thereby providing protection against apoptosis-mediated intestinal epithelial injury.
Keywords: Tacrolimus, transforming growth factor β receptor, SMAD2/3, intestinal epithelial cell, ERK
Inflammatory bowel diseases (IBDs), including Crohn’s disease and ulcerative colitis (UC), are refractory chronic diseases with repeated relapse and remission cycles, which are mediated by multiple immunological dysfunctions (1). Transforming growth factor β (TGFβ) is a multifunctional cytokine that regulates cellular processes, including differentiation, proliferation, apoptosis, and extracellular matrix production, depending on the tissue and signal intensity (2). Deregulation of the TGFβ signaling pathway is one of the known immunological dysfunctions in IBDs. Loss of TGFβ signaling is involved in colitis development in experimental models and IBD pathogenesis in humans (3,4).
A report showed that selective loss of TGFβ signaling resulted in intestinal inflammation (5). Moreover, we reported that loss of TGFβ signaling increased apoptosis of epithelial cells (6) and cyclosporine, a calcineurin inhibitor, ameliorated dextran sulfate sodium (DSS)-induced colitis in mice by up-regulating TGFβ expression in colonic tissue and activating TGFβ signaling in intestinal epithelial cells (IECs) (7). TGFβ signaling in IECs plays a key role in maintaining the gut’s barrier function via regulation of epithelial cell apoptosis (8).
TGFβ signaling in IECs is regulated by various signaling proteins such as protein kinase C, phospholipase C, protein phosphatase 1, RAS, several mitogen-activated protein kinase (MAPK) superfamily members, and mothers against decapentaplegic (SMAD) superfamily members (9). Each signaling cascade is known to intersect intricately with others and the intersections have not been fully elucidated. Identification of signaling pathways involved in intestinal epithelial injury will contribute to the discovery of new therapeutic approaches for preventing epithelial destruction.
Like other calcineurin inhibitors, tacrolimus is a potent immunomodulator that blocks T-cell activation (10). Studies have shown that tacrolimus induces up-regulation of TGFβ signaling in renal tissue (11,12). Tacrolimus is a beneficial agent that induces remission in patients with moderate to severe corticosteroid therapy-resistant UC (13). Generally, tacrolimus has been reported as a therapeutic suppressor of T-cell proliferation and an inducer of T-cell apoptosis in vitro (10). In addition to exerting inhibitory effects on T-cells, tacrolimus was also to shown to suppress macrophage activity and induce apoptosis in vitro (14). Although it has been shown that severe ulcers in patients with UC are rapidly epithelialized on tacrolimus treatment, the beneficial effects of tacrolimus on IECs have not yet been clarified. In the present study, we aimed to clarify the protective effects of tacrolimus on the TGFβ signaling pathway in IECs and the potential underlying mechanism of action.
Materials and Methods
Animals. Eight-week-old female C57BL/6 mice (CLEA Japan, Inc., Tokyo, Japan) were maintained in a specific-pathogen-free environment. This study was performed in accordance with the Guidelines for Animal Experimentation of Hirosaki University (Permit number: M12022).
Reagents. Tacrolimus was purchased from Wako Inc. (Osaka, Japan). Recombinant human TGFβ1 and an antibody to TGFβ1, -2 and -3 were purchased from R&D Systems (Minneapolis, MN, USA). Antibodies to TGFβ receptor (TGFβ-R) types II and I and goat anti-mouse IgG were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Antibodies to SMAD2/3, phospho-SMAD2/3 and glyceraldehyde 3-phosphate dehydrogenase, and chemical inhibitors of p42/44 MAPK (U0126) and p38 MAPK (SB203580) were purchased from Santa Cruz Biotechnology Inc. Horseradish peroxidase-conjugated goat anti-rabbit IgG was purchased from R&D Systems (San Jose, CA, USA). Antibody to phospho-p44/42 MAPK was purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). Alexa Fluor 488-conjugated anti-rabbit monkey anti-rabbit IgG was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Induction of colitis. Colitis was induced in mice by the administration of 4% DSS (molecular weight, 5,000; Wako Inc.) dissolved in drinking distilled water.
Experimental protocols for in vivo studies. Tacrolimus was dissolved in pharmaceutical grade olive oil via sonication before use. Tacrolimus (15 mg/kg) was then injected intraperitoneally 1 day before DSS initiation. A monoclonal antibody to TGFβ1, -2 and -3 was injected intraperitoneally on day 1 of tacrolimus treatment. After DSS treatment was initiated, mice were injected with tacrolimus on alternate days until the ninth day. Control mice were injected only with olive oil.
Evaluation of colitis. Colonic tissue sections at day 9 after DSS treatment were obtained, embedded in paraffin, and analyzed via hematoxylin and eosin (H&E) staining and TdT-mediated dUTP nick-end labeling (TUNEL) staining. H&E staining was performed to assess histological severity of colitis, which was graded on a scale from 0 to 3 (0, normal; 1, slight; 2, moderate; 3, severe) based on cell infiltration into the lamina propria, the appearance of erosion, and a decrease in crypts and glands, as described elsewhere (15). TUNEL staining was performed using the ApopTaq Apoptosis Detection Kit (Merck Millipore, Darmstadt, Germany), according to the manufacturer’s instructions. TUNEL-positive cells were observed under a microscope and scored. The results were expressed as the number of TUNEL-positive cells per 100 crypt cells.
Isolation of IECs. Mice were anesthetized via isoflurane inhalation, and the abdomen was opened under aseptic conditions. The thoracic cavity was opened and perfused with 30 mmol/l EDTA in Hanks’ balanced salt solution (HBSS) (15 ml) through the left ventricle. At the end of perfusion, the entire colon, excluding the cecum, was removed, inverted, and placed in a cold tube with cold HBSS (2 ml). The tube was shaken using a Mini Beadbeater, and the tissue remnants were discarded. The separated crypts from colonic tissues in the supernatant were collected and washed thrice with HBSS (16).
Cell culture. Caco-2 cells were purchased from the American Type Culture Collection (Teddington, UK). Caco-2 cells were grown in T-75 culture flasks with fresh culture medium, which was replaced on alternate days. Cells were maintained in Dulbecco’s modified Eagle’s medium (Thermo Fisher, Waltham, MA, USA) supplemented with glucose (4.5 g/l) and 10% fetal bovine serum (Thermo Fisher) and incubated at 37˚C in a humidified atmosphere with 5% CO2/95% air. Confluent Caco-2 cells were subcultured using 0.25% trypsin-EDTA at a ratio of 1:3 in T-75 culture flasks or 1:12 in 35-mm2 culture dishes. Caco-2 cells were seeded on 35-mm2 culture dishes and maintained for 21 days in fresh medium, which was replaced on alternate days after seeding. The cells were used between 45 and 60 passages in this study.
Caco-2 cells were treated with or without tacrolimus (0.03 mg/ml) for 24 h. For the analysis of TGFbR-II expression, Caco-2 cells were pretreated with the vehicle, U0126 (0.1 mM) or SB203580 (0.1 mM) for 1 h before tacrolimus treatment.
Protein extraction. Freshly isolated IECs, tissue remnants (lamina propria), and Caco-2 cells were lysed in a lysis buffer containing 50 mM Tris-HCl, 1 mM EDTA, 0.5% NP-40, 10% glycerol, 2 mM phenylmethylsulphonyl fluoride (Sigma-Aldrich, St. Louis, MO, USA), 50 mM sodium fluoride (Sigma-Aldrich), 1.1 mM sodium vanadate (Sigma-Aldrich), 10 mM sodium pyrophosphate (Sigma-Aldrich), and protease and phosphatase inhibitor cocktail (Roche Molecular Biochemicals, Mannheim, Germany) and incubated on ice for 30 min. Protein content was measured using a BCA Protein Assay Kit (Thermo Fisher).
Western blotting. Protein from isolated IECs or Caco-2 cells after 24-h tacrolimus treatment was separated by 4-20% TGX gradient gel electrophoresis (Bio-Rad, Hercules, CA, USA) and transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 0.05% ECL blocking agent (GE Healthcare, Amersham, Buckinghamshire, UK). Blots were incubated with primary antibody (anti-TGFbR-I, TGFbR-II, phosphor-SMAD family members 2/3, SMAD2/3 and glyceraldehyde 3-phosphate dehydrogenase) at a dilution of 1:1000 at room temperature for 10 min. After incubation, the membrane was washed four times with Tris-buffered saline with Tween 20 using SNAP i.d. 2.0 Protein Detection System (Merck Millipore) and incubated with secondary antibody at a dilution of 1:2000 at room temperature for 10 min. Immunoreactive bands were visualized using Clarity Western ECL Substrate (Bio-Rad). The densitometric analysis of the protein bands was performed using Image Lab 3.0 (Bio-Rad).
Magnetic bead assay. Freshly isolated IECs or tissue remnants were lysed in the lysis buffer and measured as described above. Phosphor-ERK1/2 (Thr202/Tyr204, Thr185/Tyr187), phosphor-p38MAPK (Thr 180/Tyr182) were measured using the Bio-Plex Pro Cell Signaling Assay Kit (MAPK 9-Plex and custom panel; Bio-Rad), according to the manufacturer’s instructions. TGFβ expression in IECs or lamina propria were measured using Bio-Plex Pro TGFβ 3-Plex panel (Bio-Rad).
Immunohistochemistry. The colonic sections acquired at 24 h after treatment with tacrolimus or vehicle were stained with an anti-phospho-ERK rabbit polyclonal antibody (1:200) for 1 h, and Alexa Fluor 488 anti-rabbit IgG (1:500) for 30 min. Thereafter, samples were mounted with Prolong Gold antifade medium (Thermo Fisher) and observed using an Olympus IX73 inverted microscope system (Olympus Co., Tokyo, Japan).
Statistical analysis. Data are expressed as the mean±standard deviation. When the data were not normally distributed, they were analyzed with a Mann-Whitney U-test to determine the significance of the differences between the vehicle- and tacrolimus-treated mice using Graph Pad Prism v6.0 (Graph Pad, La Jolla, CA, USA). p-Values <0.05 or 0.01 were considered to indicate statistical significance.
Results
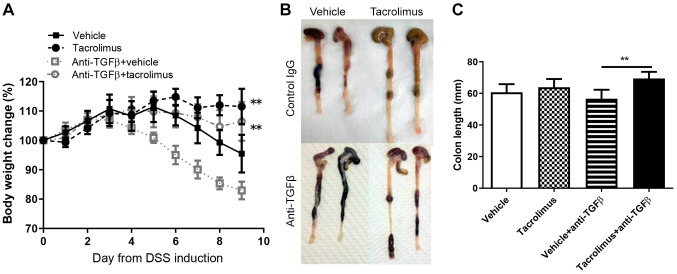
Tacrolimus provided protection against DSS-induced mucosal injury in a TGFβ expression-independent manner. To confirm the protective effects of tacrolimus on DSS-induced colitis, we assessed the body weight loss in mice after DSS administration. Vehicle-treated mice began losing weight on day 5, whereas tacrolimus-treated mice significantly maintained their weight until day 9 after DSS initiation (Figure 1A). Efficacy of tacrolimus was maintained in anti-TGFβ-treated mice. Severe body weight loss due to anti-TGFβ treatment was abrogated in tacrolimus-treated mice compared with that in vehicle-treated mice (Figure 1A). Mice were sacrificed on day 9, and the colon length was measured. Although there was no significant difference in colon length between mice that were and were not treated with anti-TGFβ, the colon length of tacrolimus-treated mice was significantly more than that of vehicle-treated mice after anti-TGFβ treatment (Figure 1B and C).
Figure 1. Tacrolimus provides protection against dextran sulfate sodium (DSS)-induced colitis in a transforming growth factor (TGF)β expressionindependent manner. A: Graphical representation of the average percentage of body weight change during the experiment is shown. The data are shown as the mean±SD (n=8 mice per group). B: Macroscopic view of colons on day 9 after DSS treatment in tacrolimus- and vehicle-treated mice that were or were not treated with anti-transforming growth factor β (TGFβ) are presented. C: Colon length in tacrolimus- and vehicle-treated mice on day 9 after DSS treatment, with and without anti-TGFβ. **Significantly different at p<0.01 vs. vehicle-treated mice.
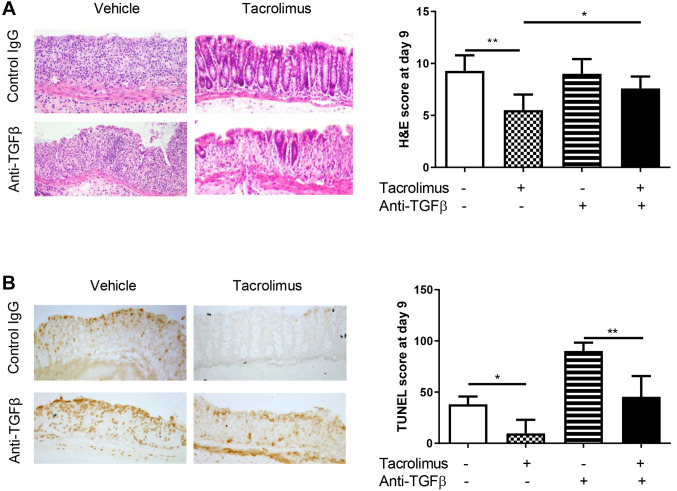
The degree of mucosal injury was assessed based on a previously described scoring system (14). Severe cell infiltration and extensive erosion were observed in vehicle-treated mice, whereas mild inflammatory cell infiltration and less severe erosion were observed in tacrolimus-treated mice. The histological score was significantly lower (less severe colitis) in tacrolimus-treated mice than in vehicle-treated mice. In anti-TGFβ-treated mice, tacrolimus exerted a partial protective effect, and the total histological score was not significantly lower in tacrolimus-treated mice than in vehicle-treated mice after anti-TGFβ treatment (Figure 2A).
Figure 2. Tacrolimus provides protection against dextran sulfate sodium (DSS)-induced colitis, involving intestinal epithelial apoptosis, independently of transforming growth factor β (TGFβ) expression. A: Hematoxylin and eosin staining of the colonic tissue of vehicle- and tacrolimus-treated mice that were or were not treated with anti-TGFβ was performed on day 9 after DSS induction. The stained tissues were observed at ×200 magnification. The histological scores of colitis are expressed as the mean±standard deviation (n=6 mice per group). B: TdT-mediated dUTP nick-end labeling (TUNEL) staining of colonic tissues of vehicle- and tacrolimus-treated mice that were or were not treated with anti-TGFβ antibody was observed at ×200 magnification. The apoptotic score was calculated as the number of TUNEL-positive cells per 100 consecutive crypts. Data are expressed as the mean±standard deviation (n=5 mice per group). Significantly different at: *p<0.05 and **p<0.01.
TUNEL staining was performed to assess IEC apoptosis. The apoptotic score was expressed as the number of TUNEL-positive epithelial cells per 100 crypt cells. The TUNEL score was significantly lower in tacrolimus-treated mice than in vehicle-treated mice. The anti-apoptotic effect of tacrolimus was also maintained in mice that were treated with anti-TGFβ (Figure 2B).
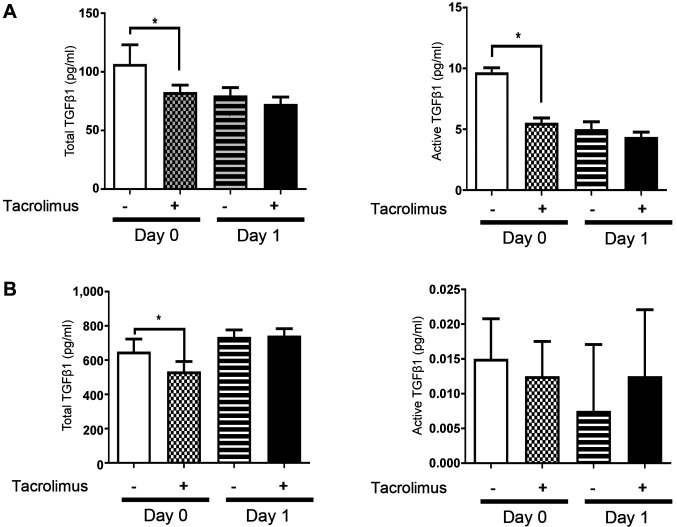
Tacrolimus did not up-regulate TGFβ1 expression in IECs and the lamina propria. To elucidate the involvement of TGFβ expression in the anti-apoptotic effect of tacrolimus, TGFβ expression in IECs and the lamina propria of tacrolimus- and vehicle-treated mice was evaluated by magnetic bead assay. Expression of total and activated TGFβ1 in IECs was significantly lower in tacrolimus-treated mice than in vehicle-treated mice (Figure 3A). Total TGFβ1 expression in the lamina propria was lower in tacrolimus-treated mice (Figure 3B). Tacrolimus did not up-regulate TGFβ1 expression at any location or phase of the experiment.
Figure 3. Tacrolimus does not up-regulate expression of transforming growth factor β (TGFβ) in intestinal epithelial cells and the lamina propria. A: Magnetic bead assay of TGFβ1 expression in intestinal epithelial cells was performed. Expression of total and activated TGFβ1 was suppressed in tacrolimus-treated mice compared with vehicle-treated mice, on day 0 after dextran sulfate sodium feeding. Data are expressed as the mean±standard deviation (n=4 mice per group). B: Magnetic bead assay of TGFβ1 expression in the intestinal lamina propria was performed. No difference in the expression of total and activated TGFβ1 was observed between the tacrolimus- and vehicle-treated mice. Data are expressed as the mean±standard deviation (n=4 mice per group). *Significantly different at p<0.05.
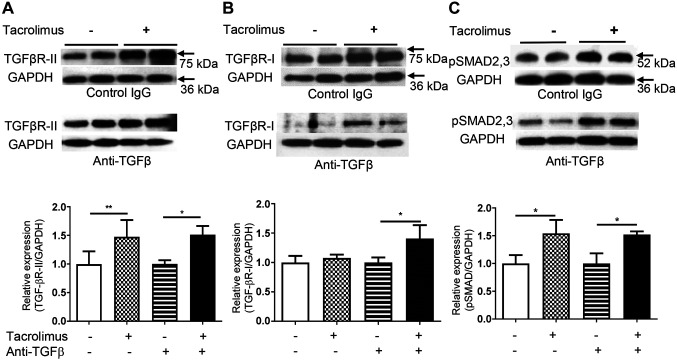
Tacrolimus up-regulated TGFβ-RII expression and SMAD2/3 phosphorylation in IECs in vivo. TGFβ-RI and TGFβ-RII expression in IECs was evaluated by western blot analysis. TGFβ-RII expression was up-regulated in tacrolimus-treated mice compared with vehicle-treated mice, 24 h after tacrolimus treatment. This positive effect was also observed in mice that were treated with anti-TGFβ (Figure 4A). TGFβ-RI expression did not differ between tacrolimus- and vehicle-treated mice that were not treated with anti-TGFβ antibody. However, after anti-TGFβ treatment, TGFβ-RI expression was up-regulated in tacrolimus-treated mice compared with vehicle-treated mice, 24 h after tacrolimus treatment (Figure 4B). SMAD2/3 phosphorylation in IECs was significantly increased in tacrolimus-treated mice compared with vehicle-treated mice, 6 h after tacrolimus treatment (Figure 4C).
Figure 4. Tacrolimus up-regulates expression of transforming growth factor β receptor type II (TGFβ-RII) and phosphorylation of SMAD family members 2/3 (p SMAD2/3) in intestinal epithelial cells. Western blot analysis of expression of TGFβ-RII (A), TGFβ-RI (B) and SMAD2/3 phosphorylation (C) in intestinal epithelial cells 24 h after tacrolimus treatment. Data are expressed as the mean±standard deviation (n=4 mice per group). GAPDH: Glyceraldehyde 3-phosphate dehydrogenase. Significantly different at: *p<0.05 and **p<0.01.
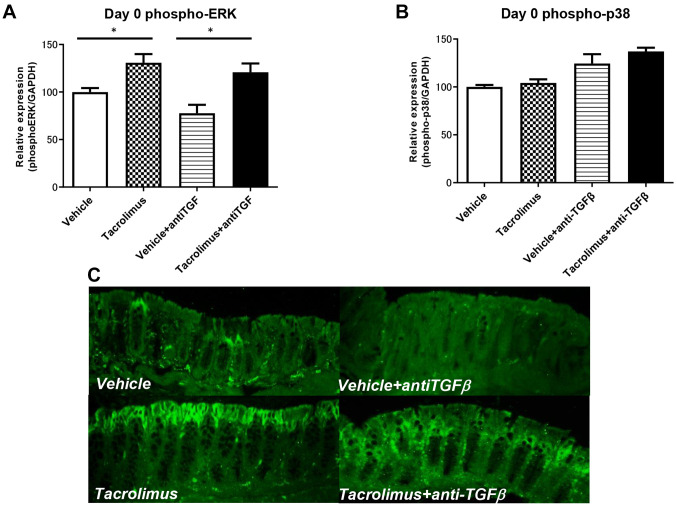
Tacrolimus enhanced phosphorylation of ERK in IECs but not of p38. To evaluate MAPK signaling in IECs, phospho-MAPKs were analyzed by magnetic bead assay of purified IECs and immunohistochemical analysis of the colonic sections. As shown in Figure 5, phospho-ERK but not p38, was up-regulated after tacrolimus treatment (Figure 5A and B). Tacrolimus-mediated up-regulation of phospho-ERK was also observed after anti-TGF treatment (Figure 5A and C).
Figure 5. Tacrolimus enhances phosphorylation of extracellular-signal-regulated kinase (ERK) but not p38 in intestinal epithelial cells in a transforming growth factorβ1 expression-independent manner. Magnetic bead assays of phospho-ERK (A) and phospho-p38 were performed (B). Immunohistochemical analysis of phospho-ERK was also performed (C). Data are expressed as the mean±standard deviation (n=4 mice per group). *Significantly different at p<0.05.
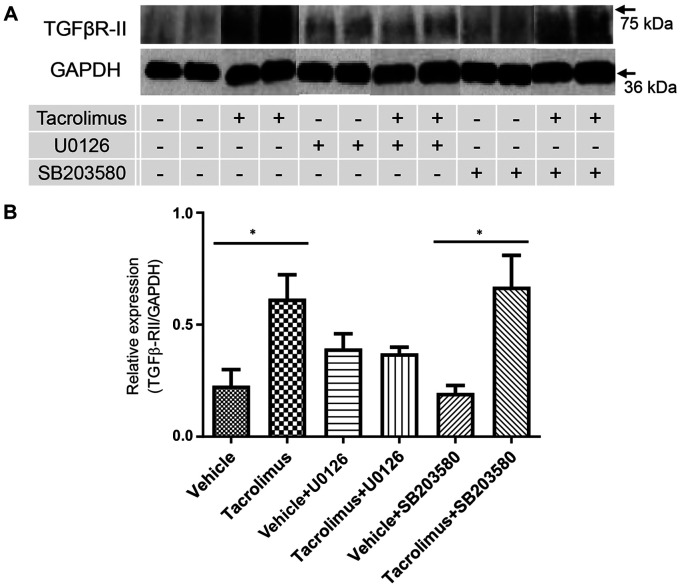
Tacrolimus up-regulated TGFβ-RII expression in Caco-2 cells via ERK activation. To evaluate the direct effect of tacrolimus on TGFβ-RII expression, Caco-2 cells were treated with tacrolimus, and TGFβ-RII expression was analyzed by western blot analysis. As shown in Figure 6A and B, TGFβ-RII expression was significantly up-regulated in tacrolimus-treated cells (compared with vehicle-treated cells). Next, to confirm MAPK involvement in TGFβ-RII up-regulation, Caco-2 cells were pretreated with U0126 (0.1 mM) or SB203580 (0.1 mM). As shown in Figure 6A and B, pretreatment with U0126, but not SB203580, abolished tacrolimus-induced up-regulation of TGFβ-RII expression.
Figure 6. Transforming growth factorβ receptor type II (TGFβ-RII) expression is up-regulated in Caco-2 cells by tacrolimus via the extracellularsignal-regulated kinase pathway. A: Western blot analysis of TGFβ-RII expression in Caco-2 cells. Caco-2 cells were pretreated with the vehicle, or p42/44 mitogen-activated protein kinase inhibitor U0126 or p38 mitogen-activated protein kinase inhibitor SB203580 (0.1 mM) before tacrolimus treatment. Data are expressed as the mean±standard deviation (n=6 per group). GAPDH: Glyceraldehyde 3-phosphate dehydrogenase. *Significantly different at p<0.05.
Discussion
Our in vivo study indicated that tacrolimus exerted a beneficial effect against apoptosis-mediated epithelial cell injury by activating the TGFβ–SMAD signaling pathway. Tacrolimus activated the TGFβ-SMAD signaling pathway in IECs via up-regulation of TGFβ-RII expression. Furthermore, our in vitro study revealed that tacrolimus up-regulated TGFβ-RII expression through the ERK signaling pathway in a TGFβ expression-independent manner.
Tacrolimus is a macrolide isolated from Streptomyces tsukubaensis. The immunosuppressive effect of tacrolimus was first reported in 1987. Kino et al. showed that mixed lymphocyte reaction, cytotoxic T-cell regeneration, and T-cell-derived soluble mediator and receptor production were suppressed by tacrolimus in vitro (10). Tacrolimus was also reported to exert immunosuppressive effects on macrophage activity and induce apoptosis in 2010 (14). Clinically, tacrolimus has a rapid action with a high trough level and induces ulcer epithelialization (17). Our data indicate that in addition to exerting regulatory effects on immune cells, tacrolimus directly acts on IECs, possibly contributing to the rapid improvement of UC.
TGFβ plays a critical role in intestinal mucosal healing (2,8). When TGFβ1 interacts with TGFβ-RII, TGFβ-RI is phosphorylated and TGFβ-RI and TGFβ-RII form complexes. TGFβ-RI and TGFβ-RII work together as a kinase that phosphorylates SMAD2/3. Phospho-SMAD2/3 forms complexes with SMAD4 and is translocated into the nucleus to modulate cell apoptosis (18). To evaluate the activity of the TGFβ–SMAD signaling pathway, we analyzed SMAD2/3 and MAPK phosphorylation in purified IECs. Phospho-SMAD2/3 was enhanced after tacrolimus injection regardless of anti-TGFβ treatment. On the other hand, there were no differences in SMAD7 expression between the vehicle- and tacrolimus-treated mice (data not shown). Active and total TGFβ1 expression in both IECs and the lamina propria was not up-regulated on tacrolimus treatment. However, TGFβ-RII expression was significantly enhanced in tacrolimus-treated mice even after anti-TGFβ treatment. These results suggest that tacrolimus directly enhanced phosphor-SMAD2/3 and up-regulated TGFβ-RII expression in IECs regardless of SMAD7 or TGFβ expression.
ERK is a major MAPK which is involved in cytoprotective signaling in IECs in processes such as wound healing or anti-apoptotic effects (19). Our data showed that tacrolimus increased phosphorylation of ERK1/2 but not of p38 in IECs. Additionally, the increase in phosphorylated ERK was preserved in anti-TGFβ-treated mice. ERK inhibitor abolished the tacrolimus-induced up-regulation of TGFβ-RII, indicating that tacrolimus induced TGFβ-RII up-regulation by activating the ERK1/2 pathway. A previous report indicated that ERK regulated TGFβ-RII expression in lung cancer cells (20). However, to our knowledge, the regulatory effects of ERK on TGFβ-RII expression in IECs have not yet been reported. The ERK signaling pathway is one of the key mediators in the regulation of IEC apoptosis (21). Our data indicated that ERK-mediated TGFβ-RII up-regulation induced by tacrolimus in IECs might play a key role in providing protection against apoptosis-mediated epithelial cell injury. Moreover, ERK phosphorylation in IECs might be a candidate biomarker for the effects of tacrolimus in patients with UC.
Clinically, tacrolimus efficiently induces remission in patients with steroid-resistant UC (22). We reported that TGFβ-RII mRNA expression was significantly lower in patients with steroid-resistant UC than in those with steroid-responsive UC (23). Another study showed that down-regulation of TGFβ–SMAD signaling, despite abundant TGFβ levels in patients with IBD, was involved in the mechanism underlying refractory chronic inflammation (7). These findings suggest that TGFβ-RII up-regulation in a TGFβ-independent manner might be one of the mechanisms underlying the therapeutic effect of tacrolimus in patients with steroid-resistant UC.
Both tacrolimus and cyclosporine are calcineurin inhibitors that block T-cell activation (24). Both agents exert a strong therapeutic effect in patients with refractory UC. We previously demonstrated that cyclosporine provided protection against apoptosis-mediated intestinal epithelial injury via up-regulation of colonic TGFβ expression (7). Interestingly, tacrolimus did not up-regulate colonic TGFβ expression, but instead directly activated TGFβ–SMAD signaling via TGFβ-RII and phospho-SMAD2/3 up-regulation. Tacrolimus directly acted on IECs and inhibited apoptosis-mediated intestinal epithelial injury. These findings suggest that tacrolimus acted via a site of action that was different from that of cyclosporine to exert therapeutic effects in patients with UC.
One puzzling issue is that the precise mechanism of up-regulation of TGFβ-RII expression in IECs via ERK activation by tacrolimus is still unclear. A previous study demonstrated correlation between TGFβ-R and FK506 binding protein (12 kDa), suggesting that tacrolimus might exert its effect on this pathway (25). However, its precise mechanism of action remains unclear. Further studies are needed to elucidate the underlying mechanism. Moreover, whether tacrolimus functions similarly in humans, especially in patients with UC, remains unknown. Further investigation on human IECs may clarify the protective mechanism of tacrolimus in patients with UC.
In conclusion, our results indicate that tacrolimus directly exerts its effects on IECs, including up-regulation of TGFβ-RII expression and activation of TGFβ–SMAD signaling, thereby providing protection against mucosal injury induced by intestinal epithelial apoptosis in DSS-induced colitis. These mechanisms might contribute to the rapid improvement of clinical symptoms induced by tacrolimus in patients with UC.
Conflicts of Interest
The Authors have no conflicts of interest to declare.
Authors’ Contributions
Conceptualization: Hirotake Sakuraba, Miwa Satake. Performed most of the experiments: Miwa Satake, Shukuko Yoshida, Hiroto Hiraga, Keisuke Hasui and Yasuhisa Murai. Designed the experiments and drafted the article: Miwa Satake, Hirotake Sakuraba, Shukuko Yoshida, Shinji Ota and Hidezumi Kikuchi. Histological and immunohistochemical analysis: Shukuko Yoshida, Yui Akemoto, Yasuhisa Murai and Shogo Kawaguchi. Supervision: Shinsaku Fukuda. Approval of final article: all Authors.
Acknowledgements
The Authors thank all the staff of our laboratories. Editage (app.editage.jp) provided English writing assistance for this article.
References
- 1.Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therap Adv Gastroenterol. 2016;9(4):606–625. doi: 10.1177/1756283X16644242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sedda S, Marafini I, Dinallo V, Di Fusco D, Monteleone G. The TGF-β/Smad system in IBD pathogenesis. Inflamm Bowel Dis. 2015;21(12):2921–2925. doi: 10.1097/MIB.0000000000000542. [DOI] [PubMed] [Google Scholar]
- 3.Fiocchi C. TGF-beta/Smad signaling defects in inflammatory bowel disease: mechanisms and possible novel therapies for chronic inflammation. J Clin Invest. 2001;108(4):523–526. doi: 10.1172/JCI13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuss IJ, Boirivant M, Lacy B, Strober W. The interrelated roles of TGF-beta and IL-10 in the regulation of experimental colitis. J Immunol. 2002;168(2):900–908. doi: 10.4049/jimmunol.168.2.900. [DOI] [PubMed] [Google Scholar]
- 5.Hahm KB, Im YH, Parks TW, Park SH, Markowitz S, Jung HY, Green J, Kim SJ. Loss of transforming growth factor beta signalling in the intestine contributes to tissue injury in inflammatory bowel disease. Gut. 2001;49(2):190–198. doi: 10.1136/gut.49.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakuraba H, Ishiguro Y, Yamagata K, Munakata A, Nakane A. Blockade of TGF-beta accelerates mucosal destruction through epithelial cell apoptosis. Biochem Biophys Res Commun. 2007;359(3):406–412. doi: 10.1016/j.bbrc.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 7.Satoh Y, Ishiguro Y, Sakuraba H, Kawaguchi S, Hiraga H, Fukuda S, Nakane A. Cyclosporine regulates intestinal epithelial apoptosis via TGF-beta-related signaling. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G514–G519. doi: 10.1152/ajpgi.90608.2008. [DOI] [PubMed] [Google Scholar]
- 8.Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162(2):597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartsough MT, Mulder KM. Transforming growth factor-beta signaling in epithelial cells. Pharmacol Ther. 1997;75(1):21–41. doi: 10.1016/s0163-7258(97)00020-x. [DOI] [PubMed] [Google Scholar]
- 10.Kino T, Hatanaka H, Miyata S, Inamura N, Nishiyama M, Yajima T, Goto T, Okuhara M, Kohsaka M, Aoki H. FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot (Tokyo) 1987;40(9):1256–1265. doi: 10.7164/antibiotics.40.1256. [DOI] [PubMed] [Google Scholar]
- 11.Akool el-S, Doller A, Babelova A, Tsalastra W, Moreth K, Schaefer L, Pfeilschifter J, Eberhardt W. Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506. J Immunol. 2008;181(4):2831–2845. doi: 10.4049/jimmunol.181.4.2831. [DOI] [PubMed] [Google Scholar]
- 12.Chiasson VL, Jones KA, Kopriva SE, Mahajan A, Young KJ, Mitchell BM. Endothelial cell transforming growth factor-β receptor activation causes tacrolimus-induced renal arteriolar hyalinosis. Kidney Int. 2012;82(8):857–866. doi: 10.1038/ki.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellermann K, Ludwig D, Stahl M, David-Walek T, Stange EF. Steroid-unresponsive acute attacks of inflammatory bowel disease: immunomodulation by tacrolimus (FK506) Am J Gastroenterol. 1998;93(10):1860–1866. doi: 10.1111/j.1572-0241.1998.539_g.x. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino T, Nakase H, Honzawa Y, Matsumura K, Yamamoto S, Takeda Y, Ueno S, Uza N, Masuda S, Inui K, Chiba T. Immunosuppressive effects of tacrolimus on macrophages ameliorate experimental colitis. Inflamm Bowel Dis. 2010;16(12):2022–2033. doi: 10.1002/ibd.21318. [DOI] [PubMed] [Google Scholar]
- 15.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 16.Mizoguchi E, Mizoguchi A, Takedatsu H, Cario E, de Jong YP, Ooi CJ, Xavier RJ, Terhorst C, Podolsky DK, Bhan AK. Role of tumor necrosis factor receptor 2 (TNFR2) in colonic epithelial hyperplasia and chronic intestinal inflammation in mice. Gastroenterology. 2002;122(1):134–144. doi: 10.1053/gast.2002.30347. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Inoue T, Murano M, Narabayashi K, Nouda S, Ishida K, Abe Y, Nogami K, Hida N, Yamagami H, Watanabe K, Umegaki E, Nakamura S, Arakawa T, Higuchi K. Effects of oral tacrolimus as a rapid induction therapy in ulcerative colitis. World J Gastroenterol. 2015;21(6):1880–1886. doi: 10.3748/wjg.v21.i6.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill CS. Nucleocytoplasmic shuttling of Smad proteins. Cell Res. 2009;19(1):36–46. doi: 10.1038/cr.2008.325. [DOI] [PubMed] [Google Scholar]
- 19.Seidelin JB, Coskun M, Vainer B, Riis L, Soendergaard C, Nielsen OH. ERK controls epithelial cell death receptor signalling and cellular FLICE-like inhibitory protein (c-FLIP) in ulcerative colitis. J Mol Med (Berl) 2013;91(7):839–849. doi: 10.1007/s00109-013-1003-7. [DOI] [PubMed] [Google Scholar]
- 20.Shang L, Jia SS, Jiang HM, Wang H, Xu WH, Lv CJ. Simvastatin downregulates expression of TGF-βRII and inhibits proliferation of A549 cells via ERK. Tumour Biol. 2015;36(6):4819–4824. doi: 10.1007/s13277-015-3134-7. [DOI] [PubMed] [Google Scholar]
- 21.Karrasch T, Steinbrecher KA, Allard B, Baldwin AS, Jobin C. Wound-induced p38MAPK-dependent histone H3 phosphorylation correlates with increased COX-2 expression in enterocytes. J Cell Physiol. 2006;207(3):809–815. doi: 10.1002/jcp.20626. [DOI] [PubMed] [Google Scholar]
- 22.Wu B, Tong J, Ran Z. Tacrolimus therapy in steroid-refractory ulcerative colitis: a review. Inflamm Bowel Dis. 2020;26(1):24–32. doi: 10.1093/ibd/izz068. [DOI] [PubMed] [Google Scholar]
- 23.Shimaya K, Ishiguro Y, Kawaguchi S, Satoh Y, Hiraga H, Yamaguchi S, Sakuraba H, Fujita H, Yamagata K, Fukuda S. Transforming growth factor (TGF)β receptors are suppressed in the refractory cases with ulcerative colitis. Hirosaki Med J. 2008;59:90–97. [Google Scholar]
- 24.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191(12):5785–5791. doi: 10.4049/jimmunol.1390055. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Li BY, Danielson PD, Shah PC, Rockwell S, Lechleider RJ, Martin J, Manganaro T, Donahoe PK. The immunophilin FKBP12 functions as a common inhibitor of the TGF beta family type I receptors. Cell. 1996;86(3):435–444. doi: 10.1016/s0092-8674(00)80116-6. [DOI] [PubMed] [Google Scholar]