Abstract
Background/Aim: Papillary thyroid cancer (PTC) is the most common endocrine malignancy with a rising incidence. There is a need for a non-invasive preoperative test to enable better patient counselling. The aim of this systematic review was to investigate the potential role of circulating microRNAs (miRNAs) in the diagnosis and prognosis of PTC.
Materials and Methods: A systematic literature search was performed using MEDLINE, Cochrane, and Scopus databases (last search date was December 1, 2021). Studies investigating the expression of miRNAs in the serum or plasma of patients with PTC were deemed eligible for inclusion.
Results: Among the 1,533 screened studies, 39 studies met the inclusion criteria. In total, 108 miRNAs candidates were identified in the serum, plasma, or exosomes of patients suffering from PTC. Furthermore, association of circulating miRNAs with thyroid cancer-specific clinicopathological features, such as tumor size (13 miRNAs), location (3 miRNAs), extrathyroidal extension (9 miRNAs), pre- vs. postoperative period (31 miRNAs), lymph node metastasis (17 miRNAs), TNM stage (9 miRNAs), BRAF V600E mutation (6 miRNAs), serum thyroglobulin levels (2 miRNAs), 131I avid metastases (13 miRNAs), and tumor recurrence (2 miRNAs) was also depicted in this study.
Conclusion: MiRNAs provide a potentially promising role in the diagnosis and prognosis of PTC. There is a correlation between miRNA expression profiles and specific clinicopathological features of PTC. However, to enable their use in clinical practice, further clinical studies are required to validate the predictive value and utility of miRNAs as biomarkers.
Keywords: miRNA, microRNA, clinicopathological features, papillary thyroid cancer, thyroid neoplasms, review
Thyroid cancer is the 20th most common cancer in the UK, and the incidence rates are projected to rise to 74% by the year 2035 (1). Papillary thyroid carcinoma (PTC) is the most common histological type, accounting for approximately 80% of the cases (2). Although, the vast majority of patients presenting with thyroid nodules that are benign, the ability to characterize the malignant nodules is quite important to ensure appropriate patient counselling when discussing curative therapy and the extent of the primary resection (3).
In addition to the cytological assessment of fine needle aspiration (FNA), molecular DNA and RNA testing of known mutations has been shown to improve the diagnostic accuracy of thyroid cancer. Kinase-activating mutations in the V-Raf murine sarcoma viral oncogene homolog B1 (BRAF) and telomerase reverse transcriptase (TERT) promoter mutations are the most common, found in nearly half of all PTCs, and in almost 80% of patients with recurrent metastatic PTC (4,5). A single amino acid substitution, from valine to glutamic acid at codon 600 (V600E), accounts for almost 90% of all BRAF mutations (6). Of clinicopathological significance, the combination of BRAF V600E and TERT promoter mutations appear to have a robust synergistic impact on the aggressiveness of PTC (7). Beyond FNA, circulating BRAF mutation in real-time liquid biopsy is now taken into account in some centres for both thyroid cancer diagnosis and to guide effective treatment strategy (8). Although these mutations as diagnostic tools significantly enhance the specificity of FNA cytology, their sensitivity to rule out cancerous nodules remains poor.
Furthermore, the prognostic value of circulating and immunohistopathology markers (including galectin-3, CK19, HBME-1, p27, p21, cyclin D1, osteopontin, and E-cadherin) have been investigated in the diagnosis and staging of PTC with promising results (9-14). However, their application is limited and further research is needed to support their clinical usefulness. For example, thyroglobulin, is an effective thyroid cancer recurrence biomarker for patients who underwent a total thyroidectomy, but it is unreliable in patients who have had a hemithyroidectomy and/or have residual normal thyroid tissue. Thyroglobulin is highly sensitive and specific for identifying patients with persistent or recurrent disease who underwent a total thyroidectomy and received radioactive iodine ablation (RAA); however, it may be unreliable in patients with circulating anti-thyroglobulin antibodies (15,16).
MicroRNAs (miRNAs) are small endogenous noncoding RNAs. Each of them targets multiple mRNAs with complementary sequences (17-20). By doing this, these small sequences of RNA negatively regulate gene expression and influence protein production by degrading, destabilizing or translationally inhibiting mRNA (20). Studies have shown that over-expression of some miRNAs can reduce the expression of tumor suppressing genes and promote oncogenesis or down-regulate oncogenes and act as tumor suppressors (19). Using many methods of isolation, such as Northern hybridization or reverse transcription – PCR (RT-PCR), miRNAs have been found in serum and plasma. Several studies have reported circulating miRNAs as cancer biomarkers (18,21). Currently, there are potential candidates that have been found to be highly expressed in PTC. Highlighting the scarcity of non-invasive diagnostic tests in thyroid cancer, there is an urgent need to identify novel biomarkers that may offer better sensitivity and specificity to distinguish between benign and malignant thyroid nodules, enabling better preoperative patient counselling and guidance to treatment. Hence, the aim of this study was to review the current literature regarding the potential role of circulating miRNAs in the diagnosis, prognosis, and staging of PTC patients.
Materials and Methods
Study design. This study was performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines for systematic reviews (22). Original clinical studies, published in English, investigating the expression of circulating miRNAs in patients with papillary thyroid cancer were deemed eligible for inclusion. Exclusion criteria were: i) articles published in languages other than English, ii) narrative or systematic reviews and meta-analyses, iii) animal and in-vitro studies, iv) case reports, errata, comments, perspectives, letters to the editor, editorials that did not provide any primary patient data, v) published abstracts with no available full text, vi) studies that report tissue instead of serum or plasma miRNA expression in thyroid cancer patients, and vii) studies that included non-PTC patients or tumors with unclear/undetermined histology. No publication date, sample size restrictions, or any other search filters were applied. Studies originating from similar institutions were included only if different miRNAs were investigated.
Search strategy. The search strategy included terms relevant to miRNAs and thyroid cancer and was conducted on three databases (MEDLINE, Cohrane, and Scopus) with an end-of-search date on 01/12/2021. The following search algorithm was used: (plasma OR biomarker OR serum OR sera OR blood OR peripheral) AND (miRNAs OR miR OR microRNA OR exosomal OR exosomes) AND (thyroid OR PTC). Two independent researchers (GG and MM) performed the literature search and a third researcher (DG) participated in the resolution of any disagreements during the selection process. In addition, the reference lists of included studies were searched for any missed study that fulfilled the inclusion criteria (snowball methodology) (23).
Data extraction. Two main researchers (GG and MM) independently extracted relevant data through a standardized data extraction template. The extraction of the following variables was performed: i) expression of serum or plasma miRNAs (upregulated, downregulated or non-statistically significant) in PTC compared to benign thyroid disease or healthy controls and ii) Tumor specific data including tumor size, tumor location, pre- and postoperative status, tumor extrathyroidal extension, lymph node metastasis, TNM stage, BRAF V600E mutation, serum thyroglobulin levels, 131I avid metastases, and tumor recurrence, and iii) sensitivity, specificity and area under the curve (AUC) as depicted by the receiver operating characteristic (ROC) curve of circulating miRNAs as a tool for PTC diagnosis. Any discrepancies during the data extraction process were solved by the rest of the authors.
Data presentation. Statistically significant differences in several miRNA expression levels in the plasma/serum of PTC patients compared to patients with benign thyroid disease or healthy controls are summarized in Supplementary Table I. Moreover, the clinicopathologic parameters of PTC patients are depicted in tabular form. The clinicopathologic features of PTC patients were also correlated with the serum/plasma miRNA expression.
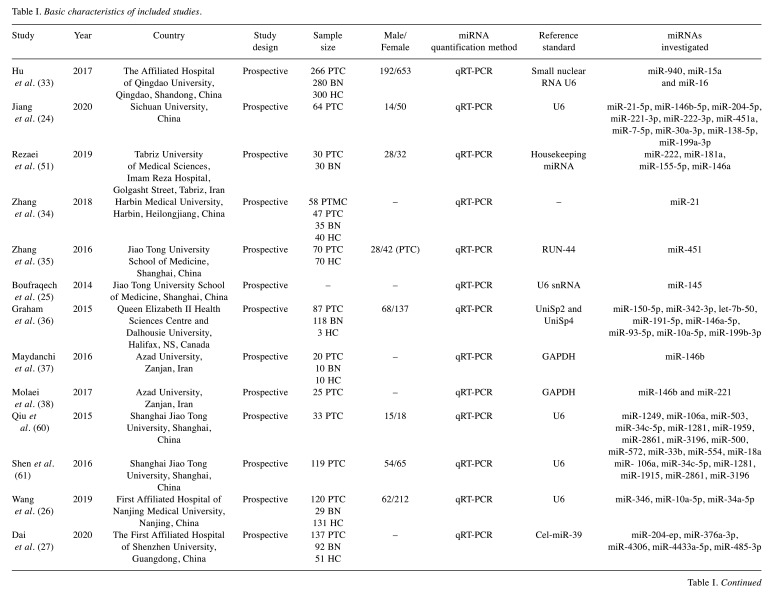
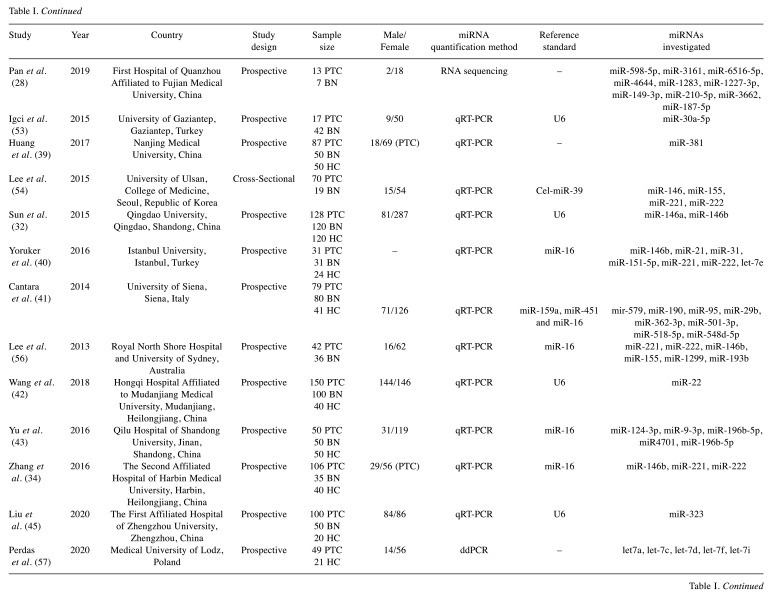
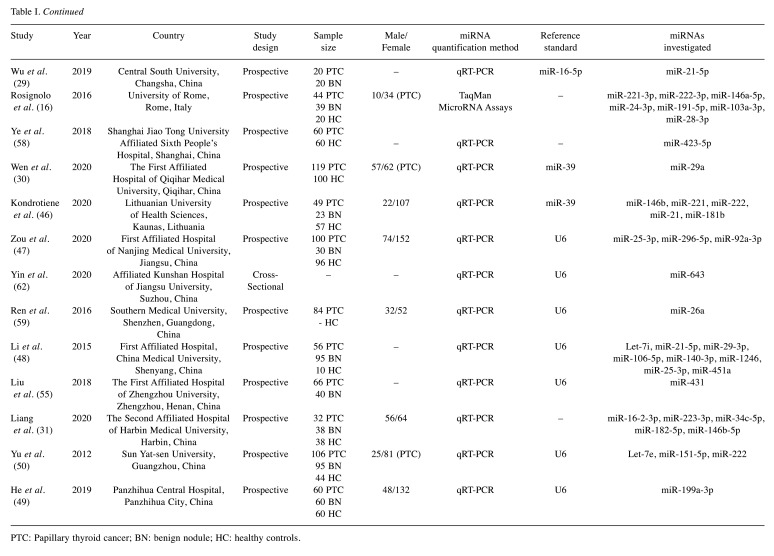
Table I. Basic characteristics of included studies.
PTC: Papillary thyroid cancer; BN: benign nodule; HC: healthy controls.
Results
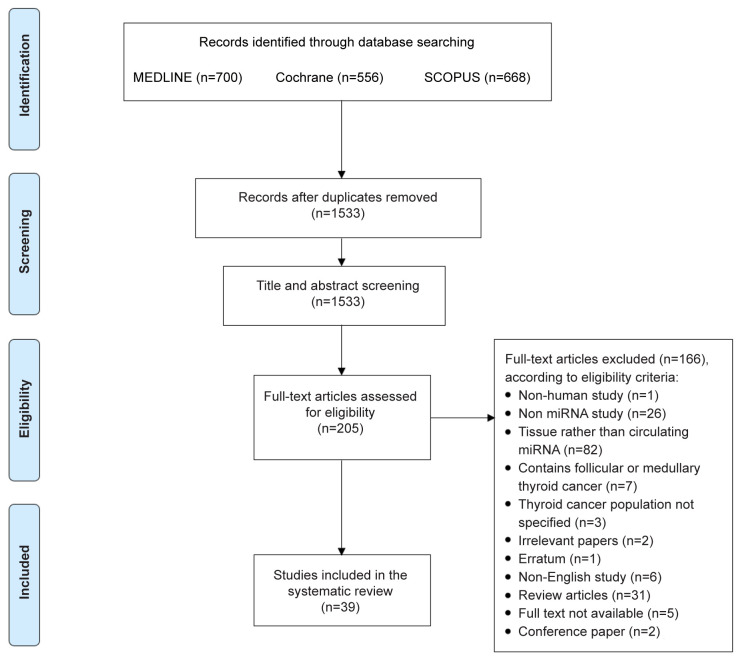
Study selection process. Our initial literature search yielded 1,533 unique articles, of which 205 articles were considered to be relevant and underwent full-text assessment. Following removal of the irrelevant, and non-eligible studies, thirty-nine studies were included in this systematic review (Figure 1 and Table I).
Figure 1. PRISMA flowchart.
MicroRNA source. In the vast majority of the included studies the miRNAs were isolated from the serum or plasma of patients. However, some studies report that miRNAs were isolated from circulating exosomes (24-31). In one study, plasma and exosomes were used (26), whereas another study did not provide data regarding the blood component that was used to isolate miRNAs (32).
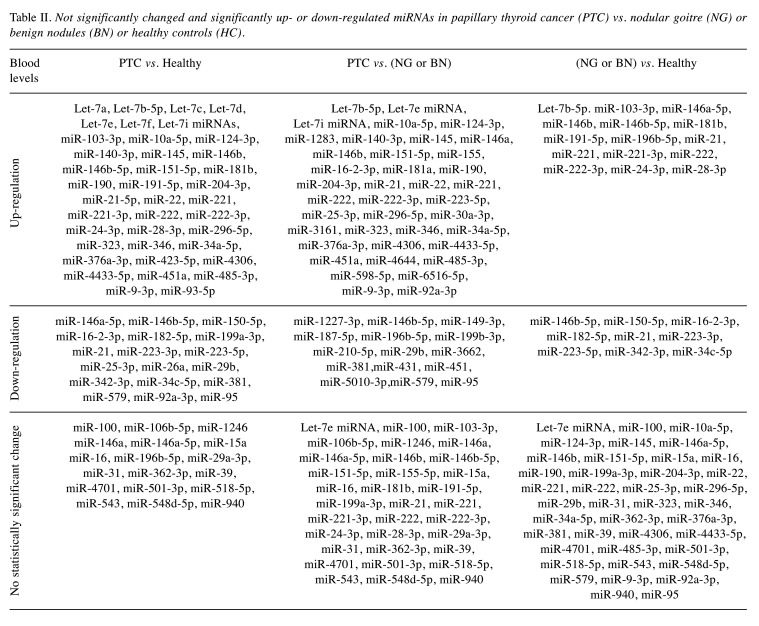
PTC vs. benign disease or healthy individuals. A total of 22 studies compared PTC against benign thyroid disease and healthy controls (16,27,31-50); six studies compared PTC to benign thyroid disease (28,51-55), seven studies compared PTC to healthy controls (26,29,30,56-59), and four studies (24,60-62) did not include healthy or benign thyroid disease comparison groups. A total of 108 miRNAs were investigated in the serum, plasma, or circulating exosomes (Table II). Between PTC and healthy subjects, 17 miRNAs did not present a significant change, while 14 were down-regulated and 40 miRNAs were up-regulated. Between PTC and benign thyroid disease, 25 miRNAs did not change significantly, while 15 and 39 miRNAs were down and up-regulated, respectively. Finally, in the comparison of benign thyroid disease with healthy controls, 37 miRNAs did not change significantly, while 8 and 12 miRNAs were down and up-regulated, respectively (Table II).
Table II. Not significantly changed and significantly up- or down-regulated miRNAs in papillary thyroid cancer (PTC) vs. nodular goitre (NG) or benign nodules (BN) or healthy controls (HC).
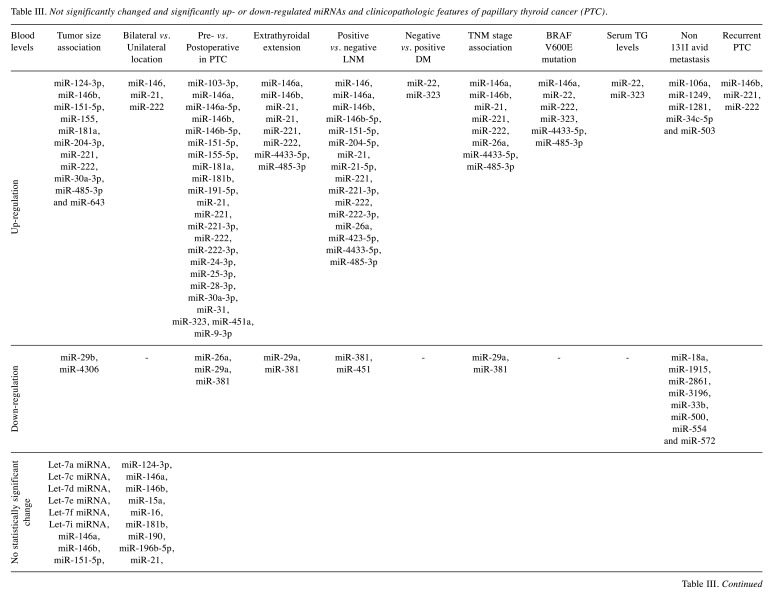
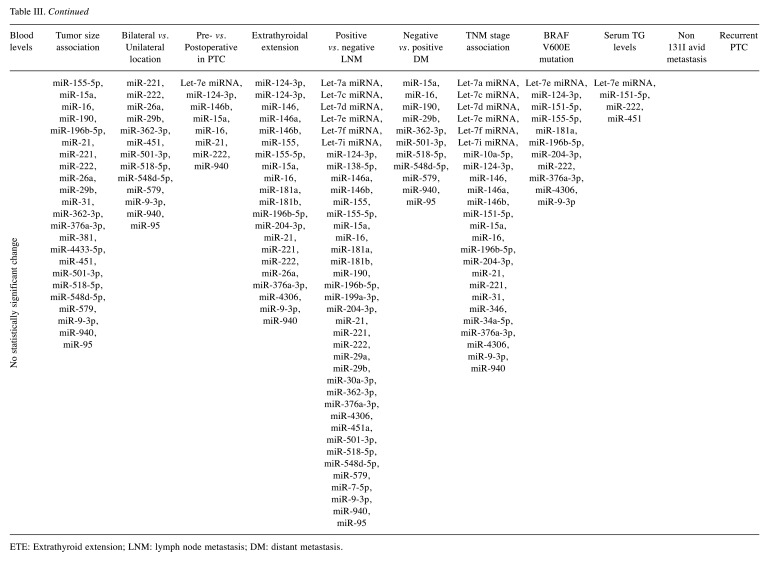
Tumor size. In total, 17 studies investigated the association of circulating miRNAs with the thyroid tumor size (27,30,32-35,37,38,44,45,51-54,57,59,62). The included studies reported a non-significant association between 27 miRNAs, while 11 (miR-124-3p, miR-146b, miR-151-5p, miR-155, miR-181a, miR-204-3p, miR-221, miR-222, miR-30a-3p, miR-485-3p and miR-643) and 2 (miR-29b and miR-4306) miRNAs were found to be up- and down-regulated in patients with increased thyroid tumor size, respectively (Table III).
Table III. Not significantly changed and significantly up- or down-regulated miRNAs and clinicopathologic features of papillary thyroid cancer (PTC).
ETE: Extrathyroid extension; LNM: lymph node metastasis; DM: distant metastasis.
The cut-off tumor size varied among the included studies. Ten studies set the tumor diameter cut-ff at 2 cm (30,32-34,38,44,45,51,53,62), four studies at 1 cm (27,52,59), while a two-level cut-off (1 cm and 2 cm) was reported by one study (37). The cut-off was not reported by two studies (35,57). The linear correlation of miRNA expression and tumor size was investigated in one study (54). While miR-222 and miR146b demonstrated a strong correlation between their expression and tumor size, miR-155 showed the strongest correlation (54).
Bilateral vs. unilateral and multifocality of the tumor. Six studies investigated the association of circulating miRNAs with bilateral or unilateral PTC (32,35,37,45,46,59) and 20 miRNAs did not present significant changes between patients with bilateral and unilateral thyroid lesions. Further, three miRNAs (miR-146, miR-21, and miR-222) were found to be up-regulated in bilateral thyroid lesions, whereas no miRNAs were identified to be down-regulated in bilateral thyroid lesions (Table III).
PTC multifocality and miRNA expression were assessed by six studies (32,35,37,45,46,59). PTC multifocality correlated with significantly increased expression of miR15a, miR16, miR-222, miR-146, and miR-21 compared to unifocal PTC cases (45,46) (Table III). The expression of miR-940, miR-221, miR-146a, miR-146b, miR-124-3p, miR-9-3p, miR-196b-5p, and let-7e did not present significant changes in multifocal vs. unifocal PTC cases (32,35,37,46,63). Furthermore, the expression of miR-222 and miR-146b in patients with papillary thyroid microcarcinoma was not associated with multifocality (46). In addition, miR-26a did not present significant changes in multifocal vs. non multifocal thyroid cancer patients (59).
Lastly, patient with tumors located in thyroid isthmus, did not present significant changes in serum expression of miR-451 compared to right, left, and bilateral thyroid tumor location (52).
Pre- vs. postoperative changes. The change in circulating miRNAs occurring after thyroid surgery was investigated by 17 studies (16,30,33,34,37-40,44-46,49-51,53,56,59). Out of 37 miRNAs, the preoperative levels of 28 (miR-103-3p, miR-146a, miR-146a-5p, miR-146b, miR-146b-5p, miR-151-5p, miR-155-5p, miR-181a, miR-181b, miR-191-5p, miR-21, miR-221, miR-221-3p, miR-222, miR-222-3p, miR-24-3p, miR-25-3p, miR-28-3p, miR-30a-3p, miR-31, miR-323, miR-451a and miR-9-3p) and 3 (miR-26a, miR-29b and miR-381) miRNAs were up and down-regulated, respectively, compared to postoperative levels in PTC patients (Table III).
The interval between the pre- and postoperative serum/plasma sampling of miRNA levels was 30 (16,33,37,46), 14 (39,45), 14-42 (51,56) and 5-15 days (44), respectively. Two studies reported more than one postoperative serum/plasma miRNA measurements; at 1, 3, 6, and 12 months postoperatively (38) or at 1 and 3 months (30). The interval between pre- and post-operative sampling was not reported by the rest of the studies.
In a longitudinal study by Rosignolo et al., the authors collected serum at 1-2 years post thyroidectomy and despite an initial decrease in miRNA levels, tumor recurrence following surgery resulted in an increased expression of miR-146a-5p and miR-221-3p compared to pre-surgery levels. In contrast, the expression of miR-146a-5p and miR-221-3p remained stable and at low levels in recurrence-free patients (16).
The postoperative expression of miR-124-3p, miR-9-3p, miR-222, and miR-151-5p postoperatively was not significantly different in PTC patients compared to healthy controls (37,44). Moreover, Hurthle cell cancer presented reduced miR-30a-5p levels postoperatively similar to non-Hurthle cell cancer (53).
Finally, hemithyroidectomy compared to total thyroidectomy did not affect significantly the expression of miR-221, miR-222, and miR-146b at 1, 3, 6, and 12 months postoperatively (38). Similarly, Kondrotient et al. supports the significantly reduced expression of miR-221 following hemi-thyroidectomy in PTC patients (46).
Lastly, four studies investigated the effect of surgery on the expression of circulating miRNAs in control groups (benign nodules) and reported that three miRNAs (miR-146a, miR-146b-5p and miR-222-3p) were significantly down-regulated in the postoperative period (38,45,51,56).
Extrathyroidal extension. Twelve studies investigated the association between miRNA levels and extrathyroid extension of PTC (27,30,32,33,37,38,40,45,46,51,54,59). Eight miRNAs did not change significantly in tumors with extrathyroidal extension compared to those without extension. However, seven (miR-146b, miR-21, miR-221, miR-222, miR-4433-5p, miR-485-3p) and two (miR-29b and miR-381) miRNAs were found to be up and down-regulated, respectively, in the serum or plasma of patients with extrathyroidal tumor extension compared to patients with limited disease (Table III).
Lymph node metastasis. A total of 18 studies examined the association between circulating miRNAs and lymph node metastasis (LNM) in PTC patients (24,27,30,32,33,35,37,38,40,44-46,51,52,54,57-59). A total of 39 miRNAs were not associated with LNM. However, 15 (miR-146, miR-146b, miR-146b-5p, miR-151-5p, miR-204-5p, miR-21, miR-21-5p, miR-221, miR-221-3p, miR-222, miR-222-3p, miR-26a, miR-423-5p, miR-4433-5p and miR-485-3p) and 2 (miR-381 and miR-451) miRNAs were found to be significantly up and down-regulated, respectively, in the serum/plasma of patient with LNM positive PTC (Table III).
The compartment of involved lymph nodes was reported by one study (54) in which the expression of miR-146b, miR-221, and miR-222 did not differ significantly between the central and lateral LNM compartment groups (54).
Distant metastasis and recurrence. The presence of distant metastasis in PTC patients and its association with aberrant expression of miRNAs was assessed by four studies (35,36,39,45). Eleven miRNAs were not associated with PTC metastasis. However, two miRNAs (miR-22 and miR-323) were significantly up-regulated in PTC metastasis (Table III).
In patients with PTC and 131I avid metastasis, two studies reported significant up-regulation of five (miR-106a, miR-1249, miR-1281, miR-34c-5p, and miR-503) and down-regulation of eight (miR-18a, miR-1915, miR-2861, miR-3196, miR-33b, miR-500, miR-554, and miR-572) miRNAs (60,61).
Lastly, thyroid tumor recurrence was assessed by two studies (38,56). The circulating levels of miR-146b, miR-221, and miR-222 were significantly elevated in patients with recurrent PTC compared to the control group and non-recurrent PTC group (38).
TNM stage. TNM stage (Comparison of stage I/II vs. III/IV in all studies except two) and circulating miRNA association was examined by 13 studies (26,27,30,32-34,37,38,44-46,57,59). Advanced TNM stage (III/IV) was associated with significant up or down-regulation in the serum/plasma of seven (miR-146b, miR-21, miR-221, miR-222, miR-26a, miR-4433-5p, and miR-485-3p) and two (miR-29b and miR-381) miRNAs, respectively (Table III). Twenty-two miRNAs presented no significant association with PTC TNM stage. Lastly, one study compared discrete stages (I, II, III, and IV) without finding any significant change in the expression levels of miRNAs (45) (Table III).
BRAF V600E mutation and TG serum correlation. The association between the BRAF V600E mutation and circulating miRNAs was assessed by six studies (27,36,37,39,44,51). Out of 18 miRNAs, six miRNAs (miR-146a, miR-22, miR-222, miR-323, miR-4433-5p, and miR-485-3p) were significantly up-regulated in patients with BRAF V600E mutation positive tumors (Table III).
The association between serum thyroglobulin (TG) levels and circulating miRNAs was examined by three studies (36,39,44). Two out of six investigated miRNAs (miR-22 and miR-323) were significantly up-regulated in patients with increased serum TG levels.
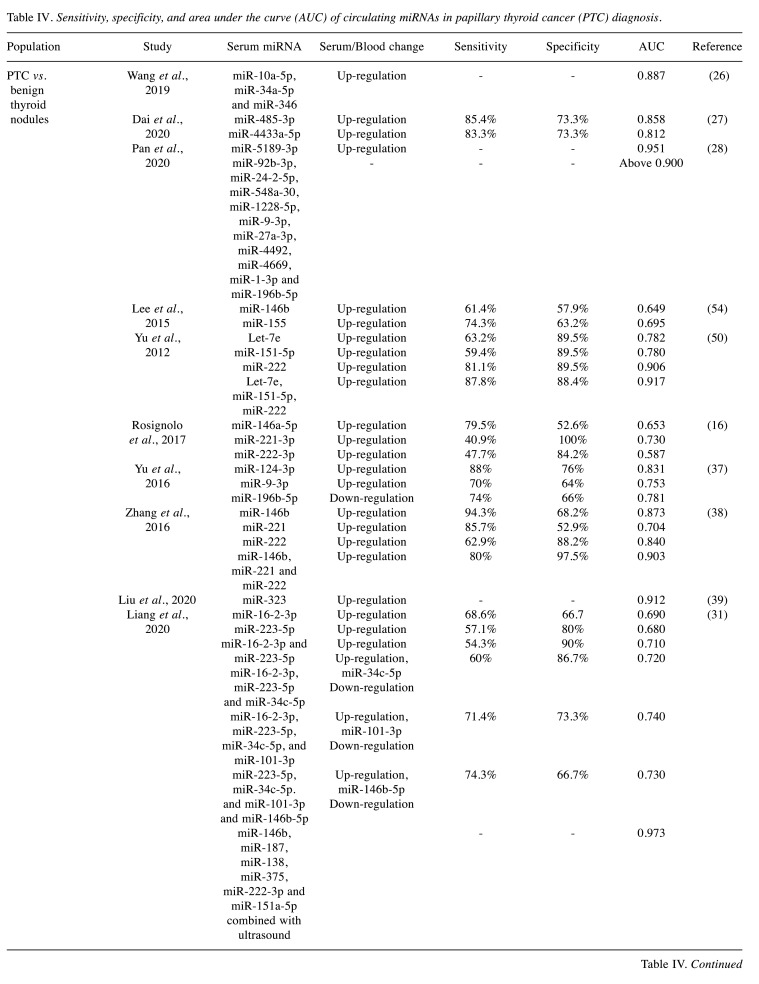
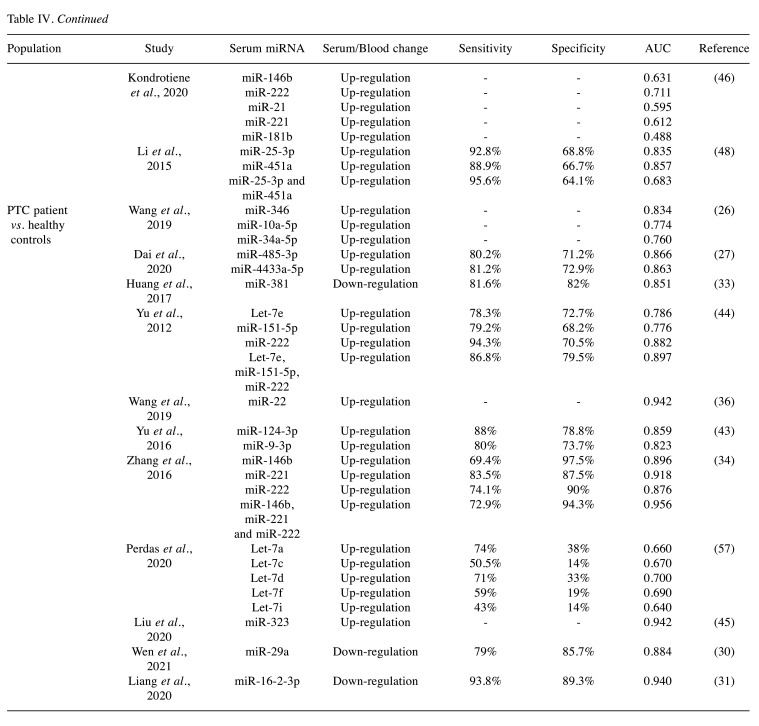
Diagnostic accuracy. Among the included studies, twenty studies investigated the diagnostic accuracy of circulating miRNAs (Table II) (16,24,26-28,30,31,33,36-42,44,46,52,54,57). Circulating miRNAs could differentiate PTC from benign thyroid nodules with a sensitivity, specificity, and AUC of 40.9-95.6%, 52.6-100%, and 0.488-0.973, respectively. In contrast, the differentiation of PTC from healthy controls based on circulating miRNAs had a sensitivity, specificity, and AUC of 43-94.3%, 14-97.5%, and 0.640-0.980, respectively. Lastly, nine studies reported the diagnostic accuracy of circulating miRNAs as markers of PTC clinical characteristics, including lymph node metastasis (24,52), papillary thyroid microcarcinoma (46), TNM stage (26), advanced clinical stage (28,30), metastatic disease (39,54), and recurrent disease (30) with variable results (Table IV).
Table IV. Sensitivity, specificity, and area under the curve (AUC) of circulating miRNAs in papillary thyroid cancer (PTC) diagnosis.
Discussion
To the best of our knowledge, this is the first systematic review that focuses on whole blood circulating miRNAs and the clinicopathologic features of PTC. A recently published meta-analysis included studies with other subtypes of differentiated thyroid cancer as well as focused on exosomal miRNAs, excluding studies that investigate the whole serum or plasma (64). Several miRNAs were identified to differentiate healthy controls or patients with benign thyroid nodules from PTC patients. Moreover, our results show that specific PTC tumor characteristics could be associated with statistically significant up or down-regulation of circulating miRNAs: tumor size (13 miRNAs), tumor location (3 miRNAs), extrathyroid extension (9 miRNAs), lymph node metastasis (17 miRNAs), distant metastasis (2 miRNAs), TNM (9 miRNAs), BRAF V600E positivity (6 miRNAs), or serum TG levels (2 miRNAs). Furthermore, the thyroidectomy operation results in significant changes in 31 circulating miRNAs. Two miRNAs (miR-146a-5p and miR-221-3p) presented an association with PTC recurrence at one or two years following thyroidectomy. The aforementioned findings suggest that circulating miRNAs are a promising tool for thyroid cancer classification, diagnosis, and management.
The presence of a thyroid nodule in an otherwise anatomically and functionally normal thyroid alerts clinicians to order an array of further diagnostic tests. Thyroid assessment includes medical history, clinical examination, thyroid ultrasound, thyroid scintigraphy, and serum thyroid function tests. In the vast majority of the patients, these tests are used to determine if FNA is appropriate and whether a patient is a surgical candidate. Thyroid ultrasound findings that are suspicious for malignancy and thyroid nodule size are the main factors that determine the need for FNA examination (3,65). However, it should be noted than up to 16% of the FNAs performed are non-diagnostic and of those, only 8% has been proven to be a PTC (66). Adjuvant immunocytochemical markers for PTC diagnosis including PAX 8, HBME1 positivity, CK19 positivity, galectin-3 positivity, and CD56 negativity have been investigated, but limitations in sensitivity and specificity preclude their widespread use (67). Lastly, given the aforementioned marker’s high cost compared to qRT-PCR, miRNA detection could replace these modalities and potentially assist to PTC diagnosis and classification (68).
Currently, the histopathologic examination of thyroid is the gold standard for a definitive diagnosis (69). However, thyroidectomy is not a complication-free procedure, since all patients who require a total thyroidectomy require postoperative lifelong thyroidal hormone replacement therapy, as well as complications associated with damage to the recurrent laryngeal nerve have a significant impact in patients quality of life (44,70). Based on these findings, the circulating miRNAs added to the existing diagnostic modalities may potentiate the discrimination between malignant and benign thyroid disease and reduce the unnecessary thyroidectomies.
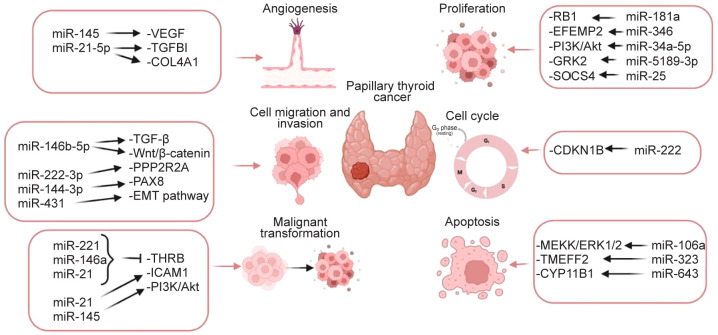
Although microRNAs constitute about 3% of the whole genome, their function is of utmost importance in the regulation of gene expression (71). Following a maturation process taking place in the nucleus, miRNAs are transferred to the cytoplasm where they form complexes with proteins and mediate their regulatory action (20,72). Their ribonucleotide sequence is usually complementary with more than one messenger RNA (mRNA) and their interaction results in the inhibition of mRNA translation. As a result, miRNAs may act indirectly as tumor suppressors, or oncogenic factors, through their interference with the expression of oncogenes, or tumor suppressor genes, respectively (73) (Figure 2).
Figure 2. The role of circulating microRNAs in the cellular pathways involved in papillary thyroid carcinoma. Protein phosphatase 2 regulatory subunit B alpha (PPP2R2A) (51), tumor growth factor β (TGF-β) (2), WNT/β-catenin (24), thyroid hormone receptor β (THRB) (51), Retinoblastoma 1 gene (51), intracellular cell adhesion molecular-1 (ICAM1) (34), PI3K/Akt (25), vascular endothelial growth factor (25), MEKK/ERK1/2 (61), EGF-containing fibulin-like extracellular matrix protein 2 (EFEMP2) (26), complex G protein-coupled receptor kinase 2 (GRK2) (28), PAX 8 (55), transmembrane protein with EGF-like and 2 follistatin domain (TMEFF2) (55), gene encoding type IV collagen α1 (COL4A1) (29), cyclin-dependent kinase inhibitor 1B (CDKN1B) (46), suppressor of cytokine signaling 4 (SOCS4) (47), cytochrome P450 Family 11 Subfamily B Member 1 (CYP11B1) (62), epithelial to mesenchymal transition (EMT) (31) (Created with BioRender.com).
Recently, further evidence on the importance of miRNA dysregulation in thyroid tumorigenesis has been uncovered. The DICER1 gene product, DICER1, is a protein that normally functions as an endoribonuclease that plays a major role in regulating miRNA processing and maturation (74). It has been found that when DICER1 gene is mutated, through inherited or acquired mutation, patients develop an increased predisposition to thyroid tumor development. Patients with germline inherited DICER1 loss-of-function mutations could suffer from a wide variety of rare life-threatening childhood-onset malignancies, such as benign and malignant thyroid neoplasms including PTC, collectively called DICER1 syndrome (75). Also, acquired somatic mutations or down-regulation of DICER1 expression have been found in early-adulthood-onset well differentiated thyroid cancer (PTC and follicular thyroid cancer) and poorly differentiated thyroid carcinoma. The DICER1 mutated thyroid tumors in both adult and pediatric patients have been shown by several groups to be mutually exclusive of any of the well-known driver mutations of thyroid cancer (including BRAF and RAS among others). This provides evidence that DICER1 mutation can be a driver of thyroid tumor development (76,77) and establishes another link between miRNA dysregulation and thyroid cancer. Furthermore, the role of DICER1 down-regulation and subsequently miRNA dysregulation has been shown in vitro. Ramírez-Moya et al., found that DICER1 protein expression was lower in thyroid cancer cell lines in comparison to normal thyroid follicular cell lines. In their study, silencing of DICER1 in PTC cell lines led to a reduction in several miRNA levels including miR-221-3p, miR-30a-5p, miR-21-5p, miR-146b-5p, miR-100-5p, and miR-204-5p, which ultimately led to an increase in proliferation, invasion, migration, and epithelial-mesenchymal transition of PTC cells (78). In conclusion, DICER1 acts as a tumor suppressor of thyroid cancer through increased miRNA expression (78).
Deregulation of gene expression by de novo or preexisting genetic mutations is a common characteristic of carcinogenesis. Chromosomal instability and genetic alterations commonly seen in cancer cells may affect miRNA expression (79). Contrarywise, the deregulation of miRNA expression may affect the expression of genes. This vicious cycle is part of the deranged gene expression seen in tumorigenesis (20). Several studies have pointed out the association of deregulated miRNA expression and cancer development as well as the association of miRNA signatures/profile with human tumors including thyroid cancer (54,80,81).
The underlying mechanisms behind the secretion of miRNAs in body fluids is still under investigation. Several theories have been proposed, including miRNA release following tumor cell death, secretion of tumor exosomes, microvesicles, or secretion induced by tumor-targeting immune response (82,83). In the serum, miRNAs may be present either as free miRNAs combined with protective proteins or in envelopes consisting of microparticles, such as exosomes or apoptotic bodies. These proteins facilitate the exosome-cell membrane interaction and RNA material exchange (49). High density lipoprotein (HDL) and nucleophosmin 1 (Npm1) belong to the serum protein microRNA stabilizers (57). However, the measured amount of circulating miRNAs has to be interpreted with caution, as additional sources of circulating miRNAs should also be considered. Circulating tumor cells as well as other cells in the bloodstream may contribute to the total circulating volume of miRNAs. These miRNAs could act as confounders and mistakenly attributed to the actual tumor activity (84,85). Several methods have been proposed to avoid this restriction, including the division of the tumor-derived miRNAs and the bloodstream-derived ones, without any clinical application at present (85).
Furthermore, the expression and/or aberrant regulation of miRNAs in serum and thyroid neoplastic tissue (including PTC) and the evaluation of their role as diagnostic biomarkers have also been reported (48). Although the association between tissue and serum miRNA expression is yet to be determined, important differences have been previously described (54,86,87). Cantara et al. compared the expression of four miRNAs (miR-29b, miR-579, miR-95, and miR-190) in tissue and serum, and all except miR-29b presented significant changes in both the serum and tissue specimens of patients diagnosed with PTC (41). Further, Yu et al. reported that the serum expression of let-7e was not consistent with that in tissue, (50). Similar differences were described by Wang et al., who reported that the miR-10a-5p, in contrast to miR-346 and miR-34a-5p, was not significantly increased in the tissue samples of PTC patients (26). Lastly, interesting findings emerged upon the investigation of miR-146 expression in tissue and serum (54). Two isoforms of miR-146 have been described; namely miR-146a and miR-146b, which are located in different chromosomes. Although the structural difference of the protein products is only two nucleotides, it is hypothesized that the over-expression of miR-146b is associated with poor thyroid cancer outcomes compared with the expression of miR-146a (25,50). Sun et al. reported that the expression of miR-146a and miR146b were significantly higher in PTC tissue compared to normal goiter and perineoplastic tissue specimens (32).
The ability of miRNAs to interact with multiple mRNA transcripts and the dysregulation of several miRNAs in thyroid cancer give rise to a molecular network that affects a wide group of gene products. This network could drive multiple cellular functions and pathways towards tumorigenesis (19,20,88). However, knowledge of these PTC miRNA “signatures” could be associated with specific oncogenic pathways facilitating the implementation of novel PTC therapeutic targets. In addition, future guidelines may include panels of circulating miRNAs that are associated with specific thyroid cancer subtypes and characteristics, such as extrathyroidal extension, lymph node infiltration, vascular invasion, and distant metastasis. The hypothesis that miRNAs are secreted by tumor cells enhances the potential use of miRNAs as thyroid cancer biomarkers (50). Although some of them may not be tumor-derived, such as let-7e, their presence in plasma could be used as a tumor fingerprint for the differential diagnosis of thyroid nodules (50).
An interesting diagnostic approach was proposed by Zhang et al., who combined the serum expression of four miRNAs (miR-222, miR-221, miR-146b, and miR-21) with ultrasound. This novel diagnostic combination achieved a cumulative sensitivity of 91.4% and specificity of 91.1% in the diagnosis of PTC. The study concluded that the combination of these four miRNAs and thyroid ultrasound, improved the PTC diagnosis when compared to thyroid ultrasound alone (34). Several other studies combined multiple circulating miRNAs for PTC diagnosis and reported that sensitivity and specificity markedly improved compared to single miRNA use (26,28,31,38,44,46). However, in the study of Perdas et al., the combination of the let family of miRNAs did not provide an additional increase in sensitivity and specificity of PTC diagnosis (57). Table II summarizes the sensitivity, specificity and AUC of several miRNAs and their combination as reported in the literature. These results could support the hypothesis that a combination of the miRNAs reported in this study could enhance the sensitivity and specificity of PTC diagnosis.
Despite its strengths (systematic literature search, high number of included studies), our study has several limitations. The high heterogeneity of the included studies precludes the quantification and generalizability of our findings. Our analysis focused on the most common thyroid cancer subtype and did not investigate the role of miRNAs in other less common subtypes, including follicular, anaplastic, and medullary thyroid cancer. Caution should be taken when interpreting our results as 27 out of 39 included studies originated from China. This issue may increase the risk of generalizability bias and preclude the application of our findings to other ethnic groups. Furthermore, some included studies follow the World Health Organization (WHO) 2004 TNM staging criteria as well as the American Joint Committee on Cancer (AJCC) 7th edition for extrathyroid tumor extension characterization while newer versions of both AJCC and WHO staging criteria have been published thereafter. Lastly, the variation in the specimens that were used to extract miRNAs, such as serum, plasma, and thyroid tissues, further complicates the interpretation of our results and the development of an ideal biomarker diagnostic panel.
In conclusion, our study focused on the miRNA expression patterns that may be utilized in the diagnosis of thyroid nodules with a PTC potential. The expression of miRNAs was shown to be associated with PTC clinical characteristics, including lymph node or distant metastasis, extrathyroidal extension, bilateral location, BRAF mutation, TNM stage, serum thyroglobulin levels, and/or 131I avid thyroid cancer metastasis. Specifically, among the miRNAs investigated in this study, circulating miR-146, miR-221, and miR-222 were frequently encountered in the literature and presented the strongest association with PTC. Among the included studies and identified miRNAs, it is reported that in some populations miR-221-3p presented 100% specificity, miR-146b presented 94.3% sensitivity, while the combination of miR-146b, miR-187, miR-138, miR-375, miR-222-3p, miR-151a-3p and thyroid ultrasound had the highest AUC. The development of a miRNA assay for the detection of the most common aberrantly regulated miRNAs in the serum could be a promising diagnostic minimally invasive tool for the early diagnosis of PTC. This approach may increase the diagnostic sensitivity and specificity and improve preoperative patient counselling and guidance to treatment.
Supplementary Material
Available at: https://docs.google.com/document/d/1kjxRohBlKG0YpeUw_mLmT_h1U05r7srE/
Conflicts of Interest
The Authors have no conflicts of interest to declare that are relevant to the content of this article.
Authors’ Contributions
All Authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by GG, MP, KP, NMA, KK, MM and DG. The first draft of the manuscript was written by GG, ME, and DG and all Authors commented on previous versions of the manuscript. All Authors read and approved the final manuscript. The final editing was performed by ETP, DG, TEP, KS, TEA, and ME.
References
- 1.Thyroid Cancer Statistics: Cancer Research UK, 2021. Available at: https://www.cancerresearchuk.org/health-professional/cancerstatistics/statistics-by-cancer-type/thyroid-cancer. [Last accessed on December 20, 2021]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth MY, Witt RL, Steward DL. Molecular testing for thyroid nodules: Review and current state. Cancer. 2018;124(5):888–898. doi: 10.1002/cncr.30708. [DOI] [PubMed] [Google Scholar]
- 5.Elisei R, Ugolini C, Viola D, Lupi C, Biagini A, Giannini R, Romei C, Miccoli P, Pinchera A, Basolo F. BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93(10):3943–3949. doi: 10.1210/jc.2008-0607. [DOI] [PubMed] [Google Scholar]
- 6.Vasko V, Hu S, Wu G, Xing JC, Larin A, Savchenko V, Trink B, Xing M. High prevalence and possible de novo formation of BRAF mutation in metastasized papillary thyroid cancer in lymph nodes. J Clin Endocrinol Metab. 2005;90(9):5265–5269. doi: 10.1210/jc.2004-2353. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Xing M. TERT promoter mutations in thyroid cancer. Endocr Relat Cancer. 2016;23(3):R143–R155. doi: 10.1530/ERC-15-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khatami F, Larijani B, Nasiri S, Tavangar SM. Liquid biopsy as a minimally invasive source of thyroid cancer genetic and epigenetic alterations. Int J Mol Cell Med. 2019;8(Suppl1):19–29. doi: 10.22088/IJMCM.BUMS.8.2.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang W, Huang C, Tang C, Xu J, Wang H. Galectin-3 may serve as a potential marker for diagnosis and prognosis in papillary thyroid carcinoma: a meta-analysis. Onco Targets Ther. 2016;9:455–460. doi: 10.2147/OTT.S94514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z, Yu P, Xiong Y, Zeng W, Li X, Maiaiti Y, Wang S, Song H, Shi L, Liu C, Cheng B, Zhang B, Ming J, Dong F, Ge H, Nie X, Huang T. Significance of CK19, TPO, and HBME-1 expression for diagnosis of papillary thyroid carcinoma. Int J Clin Exp Med. 2015;8(3):4369–4374. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodolico V, Cabibi D, Pizzolanti G, Richiusa P, Gebbia N, Martorana A, Russo A, Amato MC, Galluzzo A, Giordano C. BRAF V600E mutation and p27 kip1 expression in papillary carcinomas of the thyroid <or=1 cm and their paired lymph node metastases. Cancer. 2007;110(6):1218–1226. doi: 10.1002/cncr.22912. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Yang C, Chong D. E-cadherin expression is associated with susceptibility and clinicopathological characteristics of thyroid cancer: A PRISMA-compliant meta-analysis. Medicine (Baltimore) 2019;98(30):e16187. doi: 10.1097/MD.0000000000016187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siironen P, Nordling S, Louhimo J, Haapiainen R, Haglund C. Immunohistochemical expression of Bcl-2, Ki-67, and p21 in patients with papillary thyroid cancer. Tumour Biol. 2005;26(1):50–56. doi: 10.1159/000084340. [DOI] [PubMed] [Google Scholar]
- 14.Pesutić-Pisac V, Punda A, Gluncić I, Bedeković V, Pranić-Kragić A, Kunac N. Cyclin D1 and p27 expression as prognostic factor in papillary carcinoma of thyroid: association with clinicopathological parameters. Croat Med J. 2008;49(5):643–649. doi: 10.3325/cmj.2008.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta M, Chia SY. Circulating thyroid cancer markers. Curr Opin Endocrinol Diabetes Obes. 2007;14(5):383–388. doi: 10.1097/MED.0b013e3282eeb2f4. [DOI] [PubMed] [Google Scholar]
- 16.Rosignolo F, Sponziello M, Giacomelli L, Russo D, Pecce V, Biffoni M, Bellantone R, Lombardi CP, Lamartina L, Grani G, Durante C, Filetti S, Verrienti A. Identification of thyroid-associated serum microRNA profiles and their potential use in thyroid cancer follow-up. J Endocr Soc. 2017;1(1):3–13. doi: 10.1210/js.2016-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32(4):189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Aravin A, Tuschl T. Identification and characterization of small RNAs involved in RNA silencing. FEBS Lett. 2005;579(26):5830–5840. doi: 10.1016/j.febslet.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor. Cancer Metastasis Rev. 2009;28(3-4):369–378. doi: 10.1007/s10555-009-9188-5. [DOI] [PubMed] [Google Scholar]
- 20.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 21.Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32(2):326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wohlin C. New York, NY, USA, ACM. 2014. Guidelines for Snowballing in Systematic Literature Studies and a Replication in Software Engineering. In: Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering; p. pp. 1. [Google Scholar]
- 24.Jiang K, Li G, Chen W, Song L, Wei T, Li Z, Gong R, Lei J, Shi H, Zhu J. Plasma exosomal miR-146b-5p and miR-222-3p are potential biomarkers for lymph node metastasis in papillary thyroid carcinomas. Onco Targets Ther. 2020;13:1311–1319. doi: 10.2147/OTT.S231361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boufraqech M, Zhang L, Jain M, Patel D, Ellis R, Xiong Y, He M, Nilubol N, Merino MJ, Kebebew E. miR-145 suppresses thyroid cancer growth and metastasis and targets AKT3. Endocr Relat Cancer. 2014;21(4):517–531. doi: 10.1530/ERC-14-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Lv J, Zou X, Huang Z, Zhang H, Liu Q, Jiang L, Zhou X, Zhu W. A three plasma microRNA signature for papillary thyroid carcinoma diagnosis in Chinese patients. Gene. 2019;693:37–45. doi: 10.1016/j.gene.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Dai D, Tan Y, Guo L, Tang A, Zhao Y. Identification of exosomal miRNA biomarkers for diagnosis of papillary thyroid cancer by small RNA sequencing. Eur J Endocrinol. 2020;182(1):111–121. doi: 10.1530/EJE-19-0524. [DOI] [PubMed] [Google Scholar]
- 28.Pan Q, Zhao J, Li M, Liu X, Xu Y, Li W, Wu S, Su Z. Exosomal miRNAs are potential diagnostic biomarkers between malignant and benign thyroid nodules based on next-generation sequencing. Carcinogenesis. 2020;41(1):18–24. doi: 10.1093/carcin/bgz160. [DOI] [PubMed] [Google Scholar]
- 29.Wu F, Li F, Lin X, Xu F, Cui RR, Zhong JY, Zhu T, Shan SK, Liao XB, Yuan LQ, Mo ZH. Exosomes increased angiogenesis in papillary thyroid cancer microenvironment. Endocr Relat Cancer. 2019;26(5):525–538. doi: 10.1530/ERC-19-0008. [DOI] [PubMed] [Google Scholar]
- 30.Wen Q, Wang Y, Li X, Jin X, Wang G. Decreased serum exosomal miR-29a expression and its clinical significance in papillary thyroid carcinoma. J Clin Lab Anal. 2021;35(1):e23560. doi: 10.1002/jcla.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang M, Yu S, Tang S, Bai L, Cheng J, Gu Y, Li S, Zheng X, Duan L, Wang L, Zhang Y, Huang X. A panel of plasma exosomal miRNAs as potential biomarkers for differential diagnosis of thyroid nodules. Front Genet. 2020;11:449. doi: 10.3389/fgene.2020.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun M, Fang S, Li W, Li C, Wang L, Wang F, Wang Y. Associations of miR-146a and miR-146b expression and clinical characteristics in papillary thyroid carcinoma. Cancer Biomark. 2015;15(1):33–40. doi: 10.3233/CBM-140431. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Li C, Liu C, Zhao S, Wang Y, Fu Z. Expressions of miRNAs in papillary thyroid carcinoma and their associations with the clinical characteristics of PTC. Cancer Biomark. 2017;18(1):87–94. doi: 10.3233/CBM-161723. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Pan J, Xu D, Yang Z, Sun J, Sun L, Wu Y, Qiao H. Combination of serum microRNAs and ultrasound profile as predictive biomarkers of diagnosis and prognosis for papillary thyroid microcarcinoma. Oncol Rep. 2018;40(6):3611–3624. doi: 10.3892/or.2018.6776. [DOI] [PubMed] [Google Scholar]
- 35.Boufraqech M, Zhang L, Jain M, Gulati N, Nilubol N, Kitano M, Xiong Y, Kebebew E. MiRNA-145 is a master regulator of the hallmarks of thyroid cancer. Cancer Res. 2013;73(S8):3098. doi: 10.1158/1538-7445.AM2013-3098. [DOI] [Google Scholar]
- 36.Graham ME, Hart RD, Douglas S, Makki FM, Pinto D, Butler AL, Bullock M, Rigby MH, Trites JR, Taylor SM, Singh R. Serum microRNA profiling to distinguish papillary thyroid cancer from benign thyroid masses. J Otolaryngol Head Neck Surg. 2015;44:33. doi: 10.1186/s40463-015-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maydanchi M, Mirzaahmadi S. Investigating the expression levels of miR-146b as a tumor marker for early diagnosis of thyroid cancer. I J Pharm Res Allied Sci. 2016;5(3):500–506. [Google Scholar]
- 38.Molaei J, Meidanchi M, Mirzaahmadi S. The stature of human thyroid cancer related to over expressed mir-221 and mir-146b. Journal of Molecular Biomarkers & Diagnosis. 2017;8(s2):1–8. doi: 10.4172/2155-9929.S2-030. [DOI] [Google Scholar]
- 39.Huang T, Yi D, Xu L, Bu E, Zhu C, Sang J, Zhang Y. Downregulation of miR-381 is associated with poor prognosis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 2017;10(12):11610–11616. [PMC free article] [PubMed] [Google Scholar]
- 40.Yoruker EE, Terzioglu D, Teksoz S, Uslu FE, Gezer U, Dalay N. MicroRNA expression profiles in papillary thyroid carcinoma, benign thyroid nodules and healthy controls. J Cancer. 2016;7(7):803–809. doi: 10.7150/jca.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cantara S, Pilli T, Sebastiani G, Cevenini G, Busonero G, Cardinale S, Dotta F, Pacini F. Circulating miRNA95 and miRNA190 are sensitive markers for the differential diagnosis of thyroid nodules in a Caucasian population. J Clin Endocrinol Metab. 2014;99(11):4190–4198. doi: 10.1210/jc.2014-1923. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Guo C, Kong T, Mi G, Li J, Sun Y. Serum miR-22 may be a biomarker for papillary thyroid cancer. Oncol Lett. 2019;17(3):3355–3361. doi: 10.3892/ol.2019.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu S, Liu X, Zhang Y, Li J, Chen S, Zheng H, Reng R, Zhang C, Chen J, Chen L. Circulating microRNA124-3p, microRNA9-3p and microRNA196b-5p may be potential signatures for differential diagnosis of thyroid nodules. Oncotarget. 2016;7(51):84165–84177. doi: 10.18632/oncotarget.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Xu D, Pan J, Yang Z, Chen M, Han J, Zhang S, Sun L, Qiao H. Dynamic monitoring of circulating microRNAs as a predictive biomarker for the diagnosis and recurrence of papillary thyroid carcinoma. Oncol Lett. 2017;13(6):4252–4266. doi: 10.3892/ol.2017.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Li L, Liu Z, Yuan Q, Lu X. Plasma miR-323 as a biomarker for screening papillary thyroid cancer from healthy controls. Front Med (Lausanne) 2020;7:122. doi: 10.3389/fmed.2020.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kondrotienė A, Daukša A, Pamedytytė D, Kazokaitė M, Žvirblienė A, Daukšienė D, Simanavičienė V, Klimaitė R, Golubickaitė I, Stakaitis R, Šarauskas V, Verkauskienė R, Žilaitienė B. Plasma-derived miRNA-222 as a candidate marker for papillary thyroid cancer. Int J Mol Sci. 2020;21(17):6445. doi: 10.3390/ijms21176445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zou X, Gao F, Wang ZY, Zhang H, Liu QX, Jiang L, Zhou X, Zhu W. A three-microRNA panel in serum as novel biomarker for papillary thyroid carcinoma diagnosis. Chin Med J (Engl) 2020;133(21):2543–2551. doi: 10.1097/CM9.0000000000001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li M, Song Q, Li H, Lou Y, Wang L. Circulating miR-25-3p and miR-451a may be potential biomarkers for the diagnosis of papillary thyroid carcinoma. PLoS One. 2015;10(7):e0132403. doi: 10.1371/journal.pone.0132403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He T, Wang H, Sun J, Wu J, Gong F, Li S, Wang H, Li Y. Altered expression of DLG1-AS1 distinguished papillary thyroid carcinoma from benign thyroid nodules. BMC Endocr Disord. 2019;19(1):122. doi: 10.1186/s12902-019-0440-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu S, Liu Y, Wang J, Guo Z, Zhang Q, Yu F, Zhang Y, Huang K, Li Y, Song E, Zheng XL, Xiao H. Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97(6):2084–2092. doi: 10.1210/jc.2011-3059. [DOI] [PubMed] [Google Scholar]
- 51.Rezaei M, Khamaneh AM, Zarghami N, Vosoughi A, Hashemzadeh S. Evaluating pre- and post-operation plasma miRNAs of papillary thyroid carcinoma (PTC) patients in comparison to benign nodules. BMC Cancer. 2019;19(1):690. doi: 10.1186/s12885-019-5849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M, Wu W, Gao M, Fei Z. MicroRNA-451 as a prognostic marker for diagnosis and lymph node metastasis of papillary thyroid carcinoma. Cancer Biomark. 2017;19(4):437–445. doi: 10.3233/CBM-170059. [DOI] [PubMed] [Google Scholar]
- 53.Igci YZ, Ozkaya M, Korkmaz H, Bozgeyik E, Bayraktar R, Ulasli M, Erkilic S, Eraydin A, Oztuzcu S. Expression levels of miR-30a-5p in papillary thyroid carcinoma: a comparison between serum and fine needle aspiration biopsy samples. Genet Test Mol Biomarkers. 2015;19(8):418–423. doi: 10.1089/gtmb.2015.0062. [DOI] [PubMed] [Google Scholar]
- 54.Lee YS, Lim YS, Lee JC, Wang SG, Park HY, Kim SY, Lee BJ. Differential expression levels of plasma-derived miR-146b and miR-155 in papillary thyroid cancer. Oral Oncol. 2015;51(1):77–83. doi: 10.1016/j.oraloncology.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Li L, Liu Z, Yuan Q, Lu X. Downregulation of MiR-431 expression associated with lymph node metastasis and promotes cell invasion in papillary thyroid carcinoma. Cancer Biomark. 2018;22(4):727–732. doi: 10.3233/CBM-181253. [DOI] [PubMed] [Google Scholar]
- 56.Lee JC, Zhao JT, Clifton-Bligh RJ, Gill A, Gundara JS, Ip JC, Glover A, Sywak MS, Delbridge LW, Robinson BG, Sidhu SB. MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer. 2013;119(24):4358–4365. doi: 10.1002/cncr.28254. [DOI] [PubMed] [Google Scholar]
- 57.Perdas E, Stawski R, Kaczka K, Zubrzycka M. Analysis of let-7 family miRNA in plasma as potential predictive biomarkers of diagnosis for papillary thyroid cancer. Diagnostics (Basel) 2020;10(3):130. doi: 10.3390/diagnostics10030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye W, Deng X, Fan Y. Exosomal miRNA423-5p mediated oncogene activity in papillary thyroid carcinoma: a potential diagnostic and biological target for cancer therapy. Neoplasma. 2019;66(4):516–523. doi: 10.4149/neo_2018_180824N643. [DOI] [PubMed] [Google Scholar]
- 59.Ren G, Li H, He X, Zhang J. Downregulation of serum miR-26a predicts poor clinical outcome of papillary thyroid carcinoma. Int J Clin Exp Pathol. 2017;10(8):9042–9047. [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu ZL, Shen CT, Song HJ, Wei WJ, Luo QY. Differential expression profiling of circulation microRNAs in PTC patients with non-131I and 131I-avid lungs metastases: a pilot study. Nucl Med Biol. 2015;42(5):499–504. doi: 10.1016/j.nucmedbio.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Shen CT, Qiu ZL, Song HJ, Wei WJ, Luo QY. miRNA-106a directly targeting RARB associates with the expression of Na(+)/I(-) symporter in thyroid cancer by regulating MAPK signaling pathway. J Exp Clin Cancer Res. 2016;35(1):101. doi: 10.1186/s13046-016-0377-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin H, Shao J. MicroRNA-643 promotes proliferation and inhibits apoptosis of papillary thyroid carcinoma by down-regulating the cytochrome P450 family member 11B1. Transl Cancer Res. 2020;9(3):1465–1475. doi: 10.21037/tcr.2020.01.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Z, Wang X, Kwak KJ, Hu J, Lee JL. Novel platform for extracellular vesicle mRNA characterisation and mutation detection in cancer patient blood. Abstract Book: ISEV2017. J Extracell Vesicles. 2017;6(sup1):OF15.02. doi: 10.1080/20013078.2017.1310414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toraih EA, Elshazli RM, Trinh LN, Hussein MH, Attia AA, Ruiz EML, Zerfaoui M, Fawzy MS, Kandil E. Diagnostic and prognostic performance of liquid biopsy-derived exosomal microRNAs in thyroid cancer patients: a systematic review and meta-analysis. Cancers (Basel) 2021;13(17):4295. doi: 10.3390/cancers13174295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guidelines N: Thyroid Carcinoma, 2021. Available at: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1470. [Last accessed on December 20, 2021]
- 66.Jack GA, Sternberg SB, Aronson MD, Mukamal KJ, Oshin A, Hennessey JV. Nondiagnostic fine-needle aspiration biopsy of thyroid nodules: outcomes and determinants. Thyroid. 2020;30(7):992–998. doi: 10.1089/thy.2019.0140. [DOI] [PubMed] [Google Scholar]
- 67.Xin Y, Guan D, Meng K, Lv Z, Chen B. Diagnostic accuracy of CK-19, Galectin-3 and HBME-1 on papillary thyroid carcinoma: a meta-analysis. Int J Clin Exp Pathol. 2017;10(8):8130–8140. [PMC free article] [PubMed] [Google Scholar]
- 68.Khan TM, Zeiger MA. Thyroid nodule molecular testing: is it ready for prime time. Front Endocrinol (Lausanne) 2020;11:590128. doi: 10.3389/fendo.2020.590128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oertel YC, Oertel JE. Thyroid cytology and histology. Baillieres Best Pract Res Clin Endocrinol Metab. 2000;14(4):541–557. doi: 10.1053/beem.2000.0102. [DOI] [PubMed] [Google Scholar]
- 70.Mehanna HM, Jain A, Morton RP, Watkinson J, Shaha A. Investigating the thyroid nodule. BMJ. 2009;338:b733. doi: 10.1136/bmj.b733. [DOI] [PubMed] [Google Scholar]
- 71.Butz H, Patócs A. MicroRNAs in endocrine tumors. EJIFCC. 2019;30(2):146–164. [PMC free article] [PubMed] [Google Scholar]
- 72.Curtis HJ, Sibley CR, Wood MJ. Mirtrons, an emerging class of atypical miRNA. Wiley Interdiscip Rev RNA. 2012;3(5):617–632. doi: 10.1002/wrna.1122. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302(1):1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 74.Foulkes WD, Priest JR, Duchaine TF. DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer. 2014;14(10):662–672. doi: 10.1038/nrc3802. [DOI] [PubMed] [Google Scholar]
- 75.Theotoki EI, Pantazopoulou VI, Georgiou S, Kakoulidis P, Filippa V, Stravopodis DJ, Anastasiadou E. Dicing the disease with dicer: the implications of dicer ribonuclease in human pathologies. Int J Mol Sci. 2020;21(19):7223. doi: 10.3390/ijms21197223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chong AS, Nikiforov YE, Condello V, Wald AI, Nikiforova MN, Foulkes WD, Rivera B. Prevalence and spectrum of DICER1 mutations in adult-onset thyroid nodules with indeterminate cytology. J Clin Endocrinol Metab. 2021;106(4):968–977. doi: 10.1210/clinem/dgab025. [DOI] [PubMed] [Google Scholar]
- 77.Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, Song YS, Cho SW, Won JK, Shin JY, Park do J, Kim JI, Lee KE, Park YJ, Seo JS. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016;12(8):e1006239. doi: 10.1371/journal.pgen.1006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramírez-Moya J, Wert-Lamas L, Riesco-Eizaguirre G, Santisteban P. Impaired microRNA processing by DICER1 downregulation endows thyroid cancer with increased aggressiveness. Oncogene. 2019;38(27):5486–5499. doi: 10.1038/s41388-019-0804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 80.Svoboda M, Izakovicova Holla L, Sefr R, Vrtkova I, Kocakova I, Tichy B, Dvorak J. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. Int J Oncol. 2008;33(3):541–547. [PubMed] [Google Scholar]
- 81.Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J Endocrinol. 2005;187(3):327–332. doi: 10.1677/joe.1.06426. [DOI] [PubMed] [Google Scholar]
- 82.Liu R, Zhang C, Hu Z, Li G, Wang C, Yang C, Huang D, Chen X, Zhang H, Zhuang R, Deng T, Liu H, Yin J, Wang S, Zen K, Ba Y, Zhang CY. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47(5):784–791. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 83.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 84.Mompeón A, Ortega-Paz L, Vidal-Gómez X, Costa TJ, Pérez-Cremades D, Garcia-Blas S, Brugaletta S, Sanchis J, Sabate M, Novella S, Dantas AP, Hermenegildo C. Disparate miRNA expression in serum and plasma of patients with acute myocardial infarction: a systematic and paired comparative analysis. Sci Rep. 2020;10(1):5373. doi: 10.1038/s41598-020-61507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krell J, Frampton AE, Stebbing J. MicroRNAs in the cancer clinic. Front Biosci (Elite Ed) 2013;5(1):204–213. doi: 10.2741/e608. [DOI] [PubMed] [Google Scholar]
- 86.He H, Jazdzewski K, Li W, Liyanarachchi S, Nagy R, Volinia S, Calin GA, Liu CG, Franssila K, Suster S, Kloos RT, Croce CM, de la Chapelle A. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U.S.A. 2005;102(52):19075–19080. doi: 10.1073/pnas.0509603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chou CK, Chen RF, Chou FF, Chang HW, Chen YJ, Lee YF, Yang KD, Cheng JT, Huang CC, Liu RT. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAF(V600E) mutation. Thyroid. 2010;20(5):489–494. doi: 10.1089/thy.2009.0027. [DOI] [PubMed] [Google Scholar]
- 88.Liu X, He M, Hou Y, Liang B, Zhao L, Ma S, Yu Y, Liu X. Expression profiles of microRNAs and their target genes in papillary thyroid carcinoma. Oncol Rep. 2013;29(4):1415–1420. doi: 10.3892/or.2013.2263. [DOI] [PubMed] [Google Scholar]