Abstract
Background/Aim: Indoxyl sulfate is a metabolite of tryptophan and its urinary level reflects the status of bacterial flora in the intestine. Indoxyl sulfate possesses pro-oxidant properties and is implicated in various diseases including chronic kidney disease and cardiovascular diseases. However, the relation of urinary indoxyl sulfate to oxidative stress is not known.
Patients and Methods: The association of urinary indoxyl sulfate levels with urinary levels of oxidative stress markers, 15-isoprostane F2t and pteridine derivatives, was investigated in 255 patients with type 2 diabetes. Indoxyl sulfate and pteridine derivatives were measured by using spectrofluorometry.
Results: Urinary levels of indoxyl sulfate, pteridines, and 15-isoprostane F2t showed a normal distribution after logarithmic transformation but not before it, and they were thus used for parametric analysis after logarithmic transformation. Urinary indoxyl sulfate levels were significantly correlated (p<0.01) with urinary 15-isoprostane F2t and pteridine levels [Pearson’s correlation coefficients: 0.503 (15-isoprostane F2t) and 0.562 (pteridines)]. These associations were also found in multivariable analysis after adjusting for age, sex, insulin therapy for diabetes, body mass index, mean arterial pressure, hemoglobin A1c, estimated glomerular filtration rate, urinary albumin, and histories of smoking and alcohol drinking.
Conclusion: Urinary indoxyl sulfate levels showed associations with urinary levels of oxidative stress markers, and the associations were independent of age, sex, insulin therapy for diabetes, body mass index, blood pressure, glycemic status, renal function, smoking, and alcohol drinking. Indoxyl sulfate appears to be an important determinant of redox balance in patients with diabetes.
Keywords: Diabetes mellitus, indoxyl sulfate, 15-isoprostane F2t, oxidative stress, pteridines
Indole is the main metabolite of dietary tryptophan produced by gut bacteria via the action of tryptophanase. Indole is absorbed in the intestine and undergoes detoxification to indoxyl sulfate in the liver. Indoxyl sulfate mainly exists as an albumin-bound form in the blood stream and is excreted into urine through the renal proximal tubules. Indoxyl sulfate is increased in the blood of patients with renal dysfunction. It is a uremic toxin and has been shown to be involved in the progression and complications of chronic kidney disease (1). Moreover, increased levels of blood indoxyl sulfate have been shown to be associated with cardiovascular mortality (2) and severity of coronary atherosclerosis (3). Oxidative stress and deterioration of cellular antioxidant defense systems have been suggested as mechanisms of the toxic action of indoxyl sulfate (4). Indoxyl sulfate has been shown to increase oxidative stress in various cells including vascular endothelial cells (5), smooth muscle cells (6), mesangial cells (7), renal tubular cells (8), and blood mononuclear cells (9).
Urinary indoxyl sulfate levels are increased when production of indole from tryptophan is augmented by dysbiosis (10). However, it remains to be clarified whether and how urinary indoxyl sulfate level is related to a redox state in vivo. The purpose of this concise study was therefore to investigate the relations of urinary indoxyl sulfate levels to urinary oxidative stress markers including 15-isoprostane F2t and pteridine derivatives in patients with diabetes, where oxidative stress is known to be increased and involved in the pathogenesis of various complications including cardiovascular diseases (11). Urinary pteridine level has recently been proposed as a new oxidative stress biomarker in the general population (12) and in patients with diabetes (13).
Patients and Methods
Subjects. The subjects of this study were 255 outpatients (158 males and 97 females) who had been diagnosed as having type 2 diabetes mellitus. This study was approved by the ethics committees of Kobe Tokushukai Hospital (number: TGE00313-014) and Hyogo College of Medicine (number: 1766). Individual histories of medication, cigarette smoking, and alcohol drinking were surveyed by questionnaires. The subjects were divided by average alcohol consumption into three groups (nondrinkers; occasional drinkers, less than 2 days per week; regular drinkers, 2 days or more per week). The subjects were also divided into three groups by average cigarette consumption (nonsmokers; light smokers, 20 or less cigarettes per day; heavy smokers, more than 20 cigarettes per day).
Measurements of urinary indoxyl sulfate, pteridine derivatives, and 15-isoprostane F2t. Levels of indoxyl sulfate and the oxidized-form of pteridine derivatives in urine were estimated by using spectrofluorometry as described previously (12,14). The excitation and emission wavelengths used were 280 nm and 390 nm, respectively, for indoxyl sulfate and 360 nm and 450 nm, respectively, for pteridine derivatives. Levels of 15-isoprostane F2t were measured by an enzyme-linked immunoassay using a commercial kit, Urinary Isoprostane EIA Kit (Oxford Biochemical Research Inc., Oxford, MI, USA). Albumin concentrations in urine were measured by immune-nephelometry using an auto-analyzer (JCA-BM8000 series, JEOL Ltd., Tokyo, Japan).
Measurements of other variables. Height and body weight were measured with each subject wearing light clothes. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Arterial pressure of the right brachial artery at rest was recorded using VaSera VS-1500 (Fukuda Denshi, Tokyo, Japan).
Fasting blood was collected from each patient in the morning, and serum was separated by centrifugation and stored at –20˚C until measurements. Hemoglobin A1c in the blood was measured by using an automatic glycol-hemoglobin analyzer based on high-performance liquid chromatography. Since the standards of hemoglobin A1c used for measurement are different in the National Glycohemoglobin Standardization Program (NGSP) method and Japan Diabetes Society (JDS) method, hemoglobin A1c values were calibrated by using a formula proposed by the JDS (15): hemoglobin A1c (NGSP) (%)=1.02 × hemoglobin A1c (JDS) (%) + 0.25%. Subjects with diabetes were defined as those receiving drug therapy for diabetes and/or those showing high hemoglobin A1c levels (≥6.5%), according to the criteria for diagnosis of diabetes by the American Diabetes Association (16). Serum creatinine was measured by an enzymatic method using a commercial kit, CRE-CL (Serotec Co., Ltd., Sapporo, Japan). Estimated glomerular filtration rate (eGFR) was calculated by using the following equation (Cre: serum creatinine) developed by the Japanese Society of Nephrology (17): eGFR (ml/min/1.73 m2)=194× Cre (mg/dl)−1.094× age (years)−0.287.
Statistical analysis. Statistical analyses were performed using a computer software program (IBM SPSS Statistics for Windows, Version 25.0., Armonk, NY, USA). Pearson’s correlation coefficients (r) and standardized partial regression coefficients (β) were calculated in univariable analysis and multivariable analysis, respectively. Age, sex, insulin therapy, smoking, alcohol drinking, BMI, mean arterial pressure, hemoglobin A1c, eGFR, and urinary albumin were adjusted in multivariable analysis. Probability (p) values less than 0.05 were defined as significant.
Results
Characteristics of the subjects. The means with standard deviations of age (years) and hemoglobin A1c (%) were 69.1±10.4 and 7.16±1.24, respectively. Thus, a large proportion of the subjects were elderly patients and patients with mild diabetes. About 30% and 45% of the subjects were habitual smokers and drinkers, respectively. The percentage of the subjects receiving insulin injection was 14.5%. The means with standard deviations of BMI (kg/m2), mean arterial pressure (mmHg), and eGFR (ml/min/1.73 m2) were 24.9±4.5, 104.3±13.1, and 77.9±28.7, respectively. The medians with interquartile ranges of urinary indoxyl sulfate (μM), 15-isoprostane F2t (ng/ml), pteridines (μM), and albumin (mg/l) were 288.0 (174.7, 490.9), 1.38 (0.75, 2.43), 13.6 (8.8, 21.3), and 19.0 (6.7, 69.1), respectively.
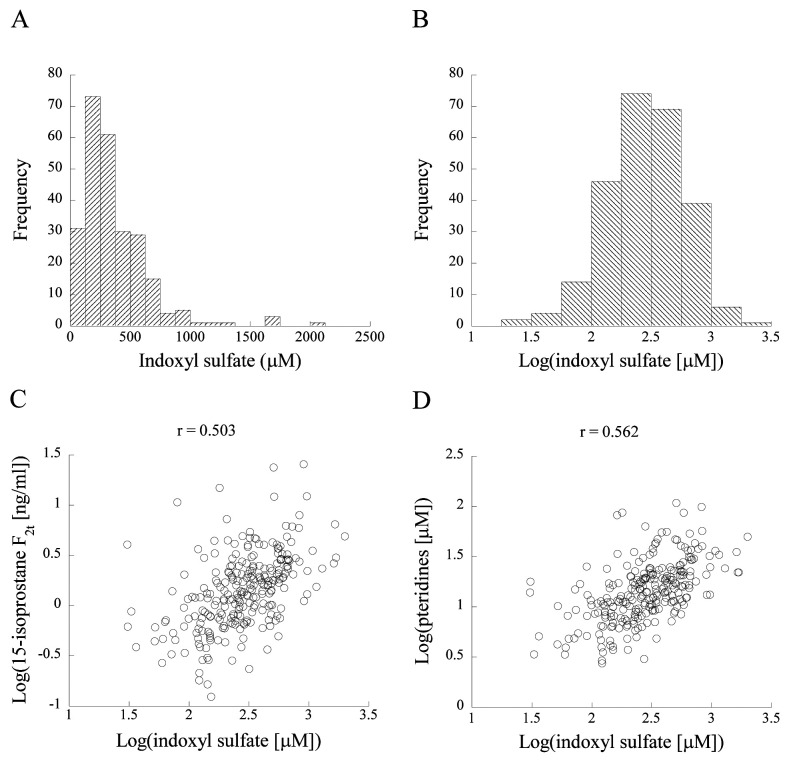
Distribution of urinary indoxyl sulfate levels. Figure 1A and B show histograms of urinary indoxyl sulfate levels. Indoxyl sulfate displayed a normal distribution after logarithmic transformation with a base of 10 but not before it. Similarly, levels of urinary pteridines and 15-isoprostane F2t showed a normal distribution only after logarithmic transformation (data not shown). Therefore, we used log-transformed levels of these variables in parametric analysis such as linear regression analysis.
Figure 1. Histograms of indoxyl sulfate before and after logarithmic transformation with a base of 10 (A, B) and scatter plots for relationships of log-transformed indoxyl sulfate with log-transformed 15-isoprostane F2t (C) and log-transformed pteridines (D). Pearson’s correlation coefficients (r) are shown in the figures.
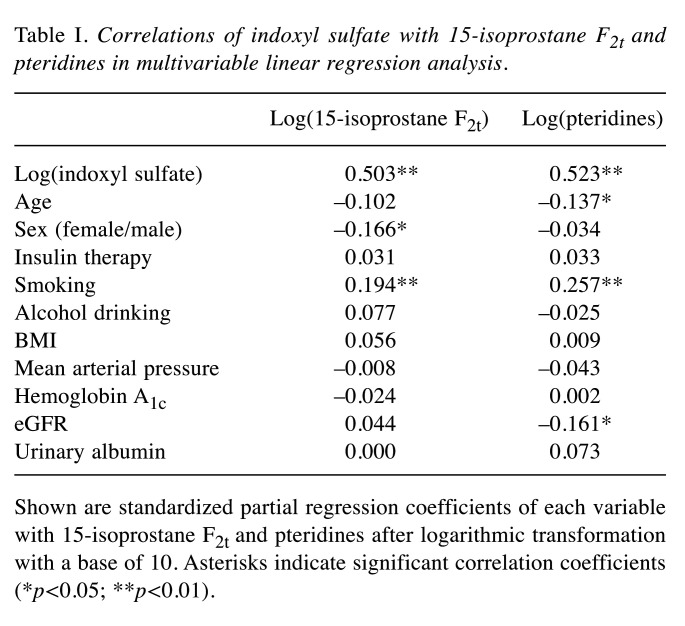
Correlations of indoxyl sulfate levels with 15-isoprostane F2t and pteridine levels. Figure 1C and D show scatter plots of the relationships of indoxyl sulfate levels with 15-isoprostane F2t and pteridine levels. Indoxyl sulfate was significantly correlated with 15-isoprostane F2t (r=0.503, p<0.01) and pteridines (r=0.562, p<0.01). These correlations remained significant (15-isoprostane F2t: β=0.503, p<0.01; pteridines: β=0.523, p<0.01) in multivariable linear regression analysis after adjustment for age, sex, insulin therapy for diabetes, BMI, mean arterial pressure, hemoglobin A1c, eGFR, urinary albumin, and histories of smoking and alcohol drinking (Table I).
Table I. Correlations of indoxyl sulfate with 15-isoprostane F2t and pteridines in multivariable linear regression analysis.
Shown are standardized partial regression coefficients of each variable with 15-isoprostane F2t and pteridines after logarithmic transformation with a base of 10. Asterisks indicate significant correlation coefficients (*p<0.05; **p<0.01).
Discussion
Indoxyl sulfate in urine was moderately correlated with urinary oxidative stress markers including 15-isoprostane F2t and pteridines independently of various factors including age, sex, BMI, blood pressure, glycemic status, renal function, and smoking and alcohol drinking. Thus, the results suggest that urinary indoxyl sulfate level reflects a redox status in vivo. This is, to the best of our knowledge, the first study showing the significance of urinary indoxyl sulfate level in relation to oxidative stress in patients with diabetes. Our findings are reasonable since indoxyl sulfate has recently been reported to cause the production of oxygen radicals in blood mononuclear cells and in the vascular endothelium and kidney (5,7,9) and is thought to be involved in the pathogenesis of various diseases including chronic kidney disease and cardiovascular disease (1-3). Moreover, serum indoxyl sulfate levels in patients receiving hemodialysis have been reported to show significant correlations with serum carbonyl stress markers including pentosidine and carboxymethyllysine, which are also associated with oxidative stress (18). Thus, indoxyl sulfate levels both in the blood and urine are associated with oxidative stress.
Urinary levels of oxidative stress markers including 15-isoprostane F2t and pteridines are thought to represent total levels of oxidative stress in vivo (12,19). Since indoxyl sulfate is known to stimulate production of reactive oxygen species and reduce antioxidant capacity (4), the associations between urinary indoxyl sulfate and oxidative stress markers suggest that indoxyl sulfate is considerably involved in redox balance in the body. This hypothesis needs to be proven by further epidemiological and experimental studies. In the present study, smoking also showed associations with 15-isoprostane F2t and pteridines (Table I), which are reasonable since smoking causes oxidative stress. Interestingly, the correlations of indoxyl sulfate with these oxidative stress markers were much stronger than the correlations of smoking with them. This supports the above hypothesis of indoxyl sulfate as an important determinant of the redox state.
Indoxyl sulfate is excreted from the blood to urine through renal tubules and has been shown to be retained in the blood as a nephrotoxin in patients with chronic kidney disease (1). However, the associations between indoxyl sulfate and oxidative stress markers were not confounded by renal function (Table I). In this study, indoxyl sulfate level in urine was measured by using spectrofluorometry, which is a simple and economically beneficial method. A strong correlation (r=0.987) was obtained between urinary levels of indoxyl sulfate measured by using spectrofluorometry and high-performance liquid chromatography (14). Therefore, urinary indoxyl sulfate level is suggested to be a clinically useful biomarker of the risk for vascular complications in patients with diabetes.
There are limitations to this study. Blood indoxyl sulfate levels were not available in this study, although the associations of urinary indoxyl sulfate level with oxidative stress markers were independent of renal function. The subjects of this study were outpatients with type 2 diabetes, and the significance of urinary indoxyl sulfate level in relation to oxidative stress should be elucidated in the general population. In addition, a large proportion of the subjects were elderly patients and patients with mild diabetes. There were no significant correlations between urinary levels of indoxyl sulfate and renal function evaluated by measuring eGFR and urinary albumin (data not shown). Therefore, further studies using cohorts of younger subjects and patients at a more progressed stage of diabetes are needed to confirm the findings of the present study. Although various factors were adjusted in multivariable analysis, information on the status of intestinal flora, which potently influences indoxyl sulfate level, was not available in this study. In fact, urinary indoxyl sulfate was reported to be increased in patients with diabetes and patients with diabetic neuropathy manifested as steatorrhea (20).
Conclusion
Urinary indoxyl sulfate was associated with urinary levels of oxidative stress markers including 15-isoprostane F2t and pteridine derivatives, and these associations were independent of age, sex, insulin therapy for diabetes, BMI, blood pressure, glycemic status, renal function, smoking, and alcohol drinking in patients with diabetes. Therefore, indoxyl sulfate is thought to be an important factor to determine redox state in vivo.
Conflicts of Interest
The Authors declare no competing interests regarding this study.
Authors’ Contributions
All Authors have contributed significantly to this work. IW conceptualized and designed the study. MM acquired the source data, and IW and MM prepared them for use in this study. IW performed a series of data analyses. IW wrote the manuscript, and MM reviewed it.
Acknowledgements
The Authors are grateful to Dr. Mamoru Nakanishi and Mr. Makoto Ohki for their excellent technical support in the measurements of indoxyl sulfate and urinary pteridines.
Funding
This study was supported by a Grant-in-Aid for Scientific Research (No. 21H03386) from the Japan Society for the Promotion of Science (Tokyo, Japan).
References
- 1.Niwa T. Indoxyl sulfate is a nephro-vascular toxin. J Ren Nutr. 2010;20(5 Suppl):S2–S6. doi: 10.1053/j.jrn.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA, European Uremic Toxin Work Group (EUTox) Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4(10):1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu CC, Lu YC, Chiu CA, Yu TH, Hung WC, Wang CP, Lu LF, Chung FM, Lee YJ, Tsai IT. Levels of indoxyl sulfate are associated with severity of coronary atherosclerosis. Clin Invest Med. 2013;36(1):E42–E49. doi: 10.25011/cim.v36i1.19404. [DOI] [PubMed] [Google Scholar]
- 4.Lu CL, Zheng CM, Lu KC, Liao MT, Wu KL, Ma MC. Indoxyl-sulfate-induced redox imbalance in chronic kidney disease. Antioxidants (Basel) 2021;10(6):936. doi: 10.3390/antiox10060936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lano G, Burtey S, Sallée M. Indoxyl sulfate, a uremic endotheliotoxin. Toxins (Basel) 2020;12(4):229. doi: 10.3390/toxins12040229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo J, Lu L, Hua Y, Huang K, Wang I, Huang L, Fu Q, Chen A, Chan P, Fan H, Liu ZM, Wang BH. Vasculopathy in the setting of cardiorenal syndrome: roles of protein-bound uremic toxins. Am J Physiol Heart Circ Physiol. 2017;313(1):H1–H13. doi: 10.1152/ajpheart.00787.2016. [DOI] [PubMed] [Google Scholar]
- 7.Gelasco AK, Raymond JR. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am J Physiol Renal Physiol. 2006;290(6):F1551–F1558. doi: 10.1152/ajprenal.00281.2004. [DOI] [PubMed] [Google Scholar]
- 8.Cheng TH, Ma MC, Liao MT, Zheng CM, Lu KC, Liao CH, Hou YC, Liu WC, Lu CL. Indoxyl sulfate, a tubular toxin, contributes to the development of chronic kidney disease. Toxins (Basel) 2020;12(11):684. doi: 10.3390/toxins12110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pieniazek A, Gwozdzinski L, Hikisz P, Gwozdzinski K. Indoxyl sulfate generates free radicals, decreases antioxidant defense, and leads to damage to mononuclear blood cells. Chem Res Toxicol. 2018;31(9):869–875. doi: 10.1021/acs.chemrestox.8b00065. [DOI] [PubMed] [Google Scholar]
- 10.Lord RS, Bralley JA. Clinical applications of urinary organic acids. Part 2. Dysbiosis markers. Altern Med Rev. 2008;13(4):292–306. [PubMed] [Google Scholar]
- 11.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118(11):1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wakabayashi I, Nakanishi M, Ohki M, Suehiro A, Uchida K. A simple and useful method for evaluation of oxidative stress in vivo by spectrofluorometric estimation of urinary pteridines. Sci Rep. 2021;10(1):11223. doi: 10.1038/s41598-020-67681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marumo M, Ekawa K, Wakabayashi I. Urinary pteridines as a discriminator of atherosclerotic risk in patients with diabetes. Atherosclerosis Plus. 2021;46:27–34. doi: 10.1016/j.athplu.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakanishi M, Ohki M, Mukai J, Uchida K, Harano Y. Development of simple measurement method of urinary indoxyl sulfate reflecting intestinal environment. J Jpn Mibyou Assoc. 2017;23(3):1–5. [Google Scholar]
- 15.Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes Society International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig. 2012;3(1):39–40. doi: 10.1111/j.2040-1124.2012.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Japan Nephrology Society [Special issue: Clinical practice guidebook for diagnosis and treatment of chronic kidney disease 2012] Nihon Jinzo Gakkai Shi. 2012;54(8):1034–1191. [PubMed] [Google Scholar]
- 18.Kato A, Odamaki M, Hishida A. Association between blood indoxyl sulfate and carbonyl stress marker in hemodialysis patients. Clin Nephrol. 2003;60(3):161–167. doi: 10.5414/cnp60161. [DOI] [PubMed] [Google Scholar]
- 19.Cracowski JL, Durand T, Bessard G. Isoprostanes as a biomarker of lipid peroxidation in humans: physiology, pharmacology and clinical implications. Trends Pharmacol Sci. 2002;23(8):360–366. doi: 10.1016/s0165-6147(02)02053-9. [DOI] [PubMed] [Google Scholar]
- 20.Patney NL, Saxena SK, Mehrotra MP, Khanna HK, Kumar A. Urinary indican in diabetes mellitus. J Indian Med Assoc. 1977;68(5):94–97. [PubMed] [Google Scholar]