Abstract
Background/Aim: Inhalation toxicity tests of glycolic acid, which is used in many household products, have been reported, but the pulmonary toxicity of glycolic acid has not been confirmed. Here, the lung damage caused by glycolic acid was investigated in rats.
Materials and Methods: An intratracheal instillation test was performed with glycolic acid in male rats. Bronchoalveolar lavage fluid (BALF) and histopathological analysis were conducted to identify the pulmonary toxicities.
Results: Intratracheal instillation of glycolic acid caused weight loss in animals and increased the content of lactate dehydrogenase, total protein, polymorphonuclear neutrophils, and inflammatory cytokines in BALF. In addition, pulmonary edema, alveolar/interstitial inflammation, and necrosis and desquamation of bronchial/bronchiolar epithelia were confirmed via histopathological examination.
Conclusion: Exposure to glycolic acid can be harmful and toxic to the lungs.
Keywords: Glycolic acid, intratracheal instillation, lung, toxicity
Glycolic acid, also known as hydroxyacetic acid, is widely used in the cosmetic, textile, food, and pharmaceutical industries (1-3). The global glycolic acid market is expected to grow gradually every year (4). This means people will be more exposed to glycolic acid, via skin contact, oral intake, or inhalation. Workers dealing with glycolic acid, in particular, are more likely to be exposed to it (5).
Glycolic acid was assigned to the hazard statement H332 for acute inhalation toxicity (6). The inhalation LC50 (4-h exposure) of glycolic acid for rats has been reported to be 7.1 mg/l, and the no observed adverse effect concentration values were 0.16 mg/l and 0.05 mg/l or higher for 14- (7) and 28-day inhalation exposure (8), respectively.
There are many differences between humans and rodents. One of them is that rodents are obligatory nasal breathers, whereas humans are oronasal breathers. This means that the lower respiratory tract of humans may be more exposed to substances than that of rodents (9,10). Animal inhalation experiments with glycolic acid have been performed, but the damage to the lower respiratory tract or the toxic effects derived from acute inhalation have not been clearly identified. Therefore, we conducted intratracheal instillation experiments to confirm the pulmonary toxicities of glycolic acid in Sprague-Dawley rats.
Materials and Methods
Reagents. Glycolic acid (Cas no. 79-14-1) was obtained from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s Phosphate Buffered Saline (DPBS) was purchased from Lonza (Basel, Switzerland). 10% neutral buffered formalin was purchased from BBC Biochemical (McKinney, TX, USA). Isoflurane was purchased from Piramal Critical Care (Bethlehem, PA, USA).
Animals and treatments. The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of the National Institute of Environmental Research (NIER) (Republic of Korea) and an IACUC-approved test protocol was followed. Male Sprague-Dawley (6-week-old) rats were purchased from Orient Bio Inc., (Seongnam, Republic of Korea). Animals were housed at room temperature, 22˚C (±3˚C) and a humidity of 50% (±20%), with a 12-h light-dark cycle. A laboratory diet (LabDiet 5053, Orient Bio Inc., Sungnam, Republic of Korea) and water were freely available during the whole experiment. After a week of acclimatization, the animals were randomly divided into four groups (20 rats/group). The body weights of the animals were measured twice a week and the clinical signs and symptoms were observed during the experiment period. Glycolic acid was intratracheally instilled into the rats and the injection volume was 0.2 ml/kg body weight. For the pulmonary toxicity test, different concentrations of glycolic acid (0.1, 1, and 10 mg/kg) were administered into the low-, middle-, and high-exposure groups. The concentrations of glycolic acid in the administered solution were 0.05%-5% (w/v). The rats were autopsied 1 and 7 days after exposure, and bronchoalveolar lavage fluid (BALF) samples and lung tissues were collected.
Hematology. Blood samples were obtained through the ventral aorta under anesthesia, as described in a previous study (8) and transferred to tubes containing K2 EDTA (BD vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ, USA). Hematological analyses were carried out using Microsemi LC-662G (HORIBA, Ltd., Kyoto, Japan). The following blood parameters were tested: i) hematocrit (Hct), ii) hemoglobin (Hgb) level, iii) mean corpuscular hemoglobin, iv) mean corpuscular hemoglobin concentration (MCHC), v) mean corpuscular volume, vi) plateletcrit (PCT), vii) platelet (PLT) count, viii) plate volume distribution width, ix) red blood cell (RBC) count, x) red blood cell volume distribution width (RDW), xi) mean platelet volume, and xii) white blood cell count.
Serum biochemistry. Serum samples were obtained via centrifugation at 2,000 rpm (740×g) for 10 min and stored at –80˚C in a deep freezer, prior to analysis. A biochemistry autoanalyzer (FUJI DRI-CHEM 4000ie, Tokyo, Japan) was used to measure the levels of the following compounds: i) albumin (ALB), ii) alkaline phosphatase (ALP), iii) blood urea nitrogen, iv) creatinine (CRE), v) glucose (GLU), vi) glutamic-oxaloacetic transaminase, vii) glutamic-pyruvic transaminase, viii) lactate dehydrogenase (LDH), ix) total cholesterol (T-CHO), x) triglyceride (TG), and xi) total bilirubin (T-BIL).
BALF analysis. BALF analysis was performed as described in a previous study (11). BALF samples were obtained via three lavages of the lung with a total of 12 ml of calcium- and magnesium-free phosphate-buffered saline (PBS, pH 7.4). The BALF samples were centrifuged at 1,500 rpm (470×g) for 10 min using a Hanil Union 32R centrifuge (Incheon, Korea) and the supernatant of the first lavage was stored at –80˚C prior to the assay. The cell pellets were resuspended in PBS, and the total cell count was measured using a Vi-Cell® XR analyzer (Beckman Coulter, Brea, CA, USA). The cells were immobilized on a glass slide using Shandon Cytospin (Shandon, Pittsburgh, PA, USA) and stained with Diff-Quik (International Reagents, Kobe, Japan). Polymorphonuclear leukocytes (PMNs) were counted using light microscopy (Olympus, Tokyo, Japan). The total protein, LDH, and inflammation cytokine levels were measured in the supernatant of the first lavage. The total protein and LDH levels were measured using a bicinchoninic acid (BCA) protein assay kit (Intron Biotechnology, Seongnam, Republic of Korea) and an EZ-LDH cell cytotoxicity assay kit (Daeil Lab Service, Cheongwon, Republic of Korea). The levels of interleukin-1beta (IL-1β), interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and tumor necrosis factor-alpha (TNF-α) were determined using a Quantikine enzyme-linked immunosorbent assay (ELISA) kit (R&D systems; Minneapolis, MN, USA), and macrophage inflammatory protein-2 (MIP-2) level was determined using Invitrogen Novex MIP-2 Rat ELISA kit (Invitrogen, Carlsbad, CA, USA).
Histopathological analysis. After gross examination, the lungs were fixed in 10% neutral buffered formalin. Following the routine tissue processing (8), the tissues were embedded in paraffin and sectioned in 3-mm-thick sections. The sections were then stained with hematoxylin and eosin (H&E) for histological examination using a light microscope (Olympus BX41). The lesions were graded depending on the severity by pathologists.
Statistical analysis. All values are expressed as the mean±standard error (SE). Means of different groups were compared by one-way analysis of variance and Student’s t-test, using GraphPad Prism version 5.01 (GraphPad Software Inc., San Diego, CA, USA), and p<0.05 was used for establishing statistical significance.
Results
Changes in the body and organ weight. The rats were observed for 7 days after intratracheal instillation (0.1, 1, and 10 mg/kg). No rats died during the observation period, and it was confirmed that the body weight of the high-exposure group decreased significantly after 7 days (p=0.008) (Figure 1). There were no significant differences in the weight of the organs, including the lungs, kidneys, heart, liver, and spleen, between the control and exposure group rats (data not shown). No treatment-related changes in the hematological and serum biochemical parameters were observed (data not shown).
Figure 1. Changes in body weight following intratracheal instillation of glycolic acid in rats.

Changes in the LDH and total protein contents in BALF. To evaluate the pulmonary toxic effects of glycolic acid, the levels of LDH and total protein in BALF were measured. No differences in the LDH contents were identified in the low- and middle-exposure group rats. The LDH contents in the high-exposure group were increased at 1 day post exposure and significantly increased 7 days post exposure (p=0.013). The total protein contents increased in the high-exposure group rats at 1 and 7 days post exposure, but the differences were not statistically significant (Figure 2).
Figure 2. The pulmonary toxicities induced by the intratracheal instillation test of glycolic acid in rats. (A) Lactate dehydrogenase (LDH) activity, (B) Total protein level in bronchoalveolar lavage fluid (BALF). The values are expressed as mean±SE of pentaplicate samples.
Inflammatory responses in BALF. The levels of inflammatory cytokines, including IL-1β, IL-6, MCP-1, MIP-2, and TNF-α, were measured in rats. There was a slight significant increase in the IL-1β level in the middle-exposure group after 1 day of exposure (p=0.019). Moreover, after 7 days of exposure, significant increases in the MCP-1 (p=0.035 or p=0.0001), MIP-2 (p=0.012), and TNF-α (p=0.035 or p=0.0001) levels were observed in the middle- or high-exposure group rats. The PMN content increased in a dose-dependent manner after 1 day of exposure, but the difference was not significant. After 7 days of exposure, the PMN content increased more than that observed after 1 day of exposure, and a significant increase was confirmed in the high-exposure group rats (p=0.012) (Figure 3).
Figure 3. Change in pulmonary inflammation induced by glycolic acid in the intratracheal instillation test. (A) Tumor necrosis factor-alpha (TNF-α), (B) Monocyte chemoattractant protein-1 (MCP-1), (C) Macrophage inflammatory protein-2 (MIP-2), (D) Interleukin-6 (IL-6), (E) Interleukin-1beta (IL-1β), (F) Polymorphonuclear leukocyte (PMN) count in BALF, (G) Diff-quick staining of BALF cells. The values are expressed as mean±SE of pentaplicate samples.
Histopathological changes in lung tissues. Histopathological changes in the lungs were observed at 1 day post exposure to glycolic acid in a dose-dependent manner. The toxic effects of glycolic acid in the lungs included pulmonary edema, alveolar/interstitial inflammation, and necrosis and desquamation of the bronchial/bronchiolar epithelia (Table I). Pulmonary edema was observed in 3/5 cases in the high-exposure group and its severity was mild to moderate. Pulmonary edema was observed in the localized to sublobar range, and the alveolar spaces were filled with fibrotic inflammatory exudate; hemorrhage was also observed. Multifocal inflammation in the pulmonary parenchyma indicated acute alveolitis, with damage to the alveolar epithelial cells and neutrophil infiltration. Some bronchioles were filled with neutrophils and desquamated cell debris. The multifocal alveolitis was dose-dependent in its frequency and severity (Figure 4).
Table I. The histopathological results of rats after intratracheal instillation of glycolic acid.
The parentheses represent the percentage of the cases with the lesions of the total examined number of tissues.
Figure 4. Histological features of representative lungs in each group of rats 1 day after intratracheal instillation of glycolic acid. In the Lowexposure group, note the acute alveolitis with neutrophil infiltration and hemorrhage. In the Middle-exposure group, multifocal inflammation is observed, which is expanding from the terminal bronchioles (b) (circles). Note the fibrinous pulmonary edema in High, characterized by the alveolar spaces filled with exudate fluid and fibrin. Also, in the High-exposure group, note the severe necrosis of epithelial cells of bronchioles (b) (the arrow). (a) alveolar space, (b) bronchioles. H&E. Magnification: 100× for Middle and High, 200× for Control and Low.
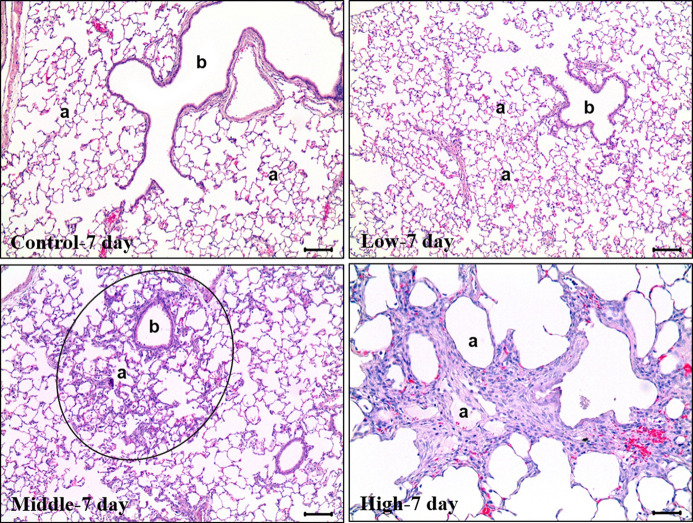
The histopathological results of the lungs obtained on day 7 after instillation of glycolic acid showed that the toxic effects had changed to chronic. In the control and low-exposure groups, no abnormal findings were observed, except for a slight focal alveolar macrophage aggregation and mononuclear cell infiltration, which were observed in some animals. Severe pulmonary edema was observed in 1/5 cases in the high-exposure group, and chronic alveolar inflammation was observed in 2/5 and 5/5 cases in the middle- and high-exposure groups, respectively. Chronic alveolar inflammation was observed in a dose-dependent manner. Histopathologically, lymphocytes and histiocytes were the main inflammatory cells, and alveolar fibrosis was also clearly observed (Figure 5).
Figure 5. Histological features of representative lungs in each group of rats 7 days after intratracheal instillation of glycolic acid. In the Control and Low-exposure groups, no specific abnormal findings were observed. Focal chronic alveolitis with fibrosis was evident in M1. Note the thickened alveolar walls with fibrosis and mild accumulation of histiocytes around the terminal bronchiole (b) and in the alveolar spaces (a). In the Highexposure group, the pulmonary fibrosis is evident. H&E. Magnification: 100× for Control, Low and Middle, 200× for High.
Discussion
Our results showed that the animals did not die in the 7 days after intratracheal instillation of glycolic acid and that the body weight of the rats gradually decreased. It was thought that the toxic effects of the substance lasted for 7 days. Lactate dehydrogenase (LDH) and total protein are indicators of lung injury (11,12). LDH and total protein contents in BALF also increased over time after exposure in the high-exposure group. This tendency was also observed for the inflammation-related factors. There were no changes in the PMN and inflammatory cytokine contents in all exposure groups compared to those of the control group 1 day after exposure; however, after 7 days, the PMN, MCP-1, MIP-2, and TNF-α contents in the high-exposure group were significantly changed (p<0.05).
PMNs are one of the main factors responsible for an acute inflammatory response during lung damage and play a role in the progression of chronic inflammation (13,14). MCP-1 is one of the most important cytokines for monocyte/macrophage recruitment in inflammatory responses (15). MIP-2 is produced by monocytes and neutrophils and is involved in attracting PMNs (16). Our results show that the trends observed for MIP-2 and PMNs were similar. TNF-α, a strong inflammatory cytokine, is associated with leukocyte adhesion to the epithelium, edema formation, and vasodilatation (17,18). Similar to the results of this study, a previous study showed that toxicity due to acute exposure to hydrochloric acid, a highly irritating and corrosive chemical, persisted for several days, resulting in sustained or increased inflammation marker levels (19).
Glycolic acid is considered to cause pulmonary edema, acute hemorrhagic alveolitis, and injury of bronchial and bronchiolar epithelial cells. The chronic bronchopneumonia observed in the control group 1 day after exposure was not observed in the lungs of normal rats; thus, it was considered to have occurred during the administration of an excipient. Considering that the interstitial inflammation with slight fibrosis observed in the low-exposure group 1 day after exposure occurred immediately after the exposure, it was not considered to be a change due to exposure to glycolic acid. As per the inflammation-related markers, the toxicity persisted for 7 days after exposure, as confirmed by the lung histopathological findings.
It has been reported that glycolic acid, a corrosive substance, can increase irritation depending on its concentration and pH (5,20). Various acidic substances that are corrosive like glycolic acid have been reported to cause lung damage, such as pulmonary edema and alveolitis (21-23), which is in accordance with the pathological results of this study.
Glycolic acid is industrially used at high concentrations, such as 30%, 60%, 70%, and 99% (24). Therefore, workers dealing with glycolic acid are more likely to be exposed to a high concentration of this substance than consumers. Although the results of inhalation experiments of glycolic acid using rats are available (7,8), they have limitations since rats are nasal breathers, unlike humans, who are oronasal breathers (10). Oral breathing leads to the deposition of more substances in the bronchi and lungs than nasal breathing (25,26). The main target organ of glycolic acid in acute inhalation exposure using rats was the upper respiratory tract (27).
Intratracheal instillation also has limitations such as the non-physiological route of exposure and uneven distribution of the substance (28,29). It has recently been reported that the intratracheal instillation test can be useful for acidic compounds that are difficult to test with the inhalation test (30). In addition, many studies on the toxic effects of nanomaterials using intratracheal instillation have been published (31-33). One study reported that the intratracheal instillation experiment can be used to rank the harmfulness of nanomaterials (34).
In this study, an intratracheal instillation technique was used to confirm the pulmonary toxicities of glycolic acid. However, this method has a limitation in that it is not the common exposure route for humans (35). Therefore, it is necessary to develop inhalation exposure experiments designed for human inhalation routes.
In conclusion, this study confirmed the pulmonary toxicities of glycolic acid, such as pulmonary edema, alveolitis, and necrosis and desquamation of bronchial and bronchiolar epithelial cells, with an increase in the levels of inflammatory mediators through intratracheal instillation, and showed that the toxicities were similar to those of corrosive substances. In addition, pulmonary toxicity may appear stronger in the case of human inhalation exposure than in the case of rat inhalation exposure, which shows that more studies on models suitable for human exposure are needed.
Conflicts of Interest
The Authors declare that there are no conflicts of interest in relation to this study.
Authors’ Contributions
Pilje Kim, and Ilseob Shim conceived and designed this study. Seong Kwang Lim, Byung-Il Yoon, and Ig-Chun Eom wrote the manuscript. Seong Kwang Lim, Haewon Kim, Jean Yoo, Woong Kim, Ilseob Shim, and Ig-Chun Eom did experiments of cytotoxicity and inhalation toxicity. Byung-Il Yoon performed the experiments of histopathology in rats.
Acknowledgements
This work was supported by the National Institute of Environmental Research, Incheon, Republic of Korea (NIER-2018-01-01-008).
References
- 1.Atzori L, Brundu MA, Orru A, Biggio P. Glycolic acid peeling in the treatment of acne. J Eur Acad Dermatol Venereol. 1999;12(2):119–122. [PubMed] [Google Scholar]
- 2.Green BA, Yu RJ, Van Scott EJ. Clinical and cosmeceutical uses of hydroxyacids. Clin Dermatol. 2009;27(5):495–501. doi: 10.1016/j.clindermatol.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Pubchem Compound summary-glycolic acid. (2022) Available at: https://pubchem.ncbi.nlm.nih.gov/compound/757. [Last accessed on January 14, 2022]
- 4.Data Bridge Market Research (DBMR) Global glycolic acid market – industry trends and forecast to 2027 (2020) Available at: https://www.databridgemarketresearch.com/reports/globalglycolic-acid-market. [Last accessed on January 14, 2022]
- 5.National Industrial Chemicals Notification and Assessment Scheme (NICNAS) Glycolic acid, priority existing chemical assessment report no. 12 (2000) Available at: https://www.industrialchemicals.gov.au/sites/default/files/PEC12-Glycolic-acid.pdf. [Last accessed on January 14, 2022]
- 6.European Chemecals Agency (ECHA) Brief profile-glycolic acid (2022) Available at: https://echa.europa.eu/brief-profile/-/briefprofile/100.001.073. [Last accessed on January 14, 2022]
- 7.Kennedy G, Burgess B. Inhalation toxicology of glycolic acid. Inhalation Toxicology. 2021;9(5):435–447. doi: 10.1080/089583797198114. [DOI] [Google Scholar]
- 8.Lim SK, Yoo J, Kim H, Kim W, Shim I, Yoon BI, Kim P, DO Yu S, Eom IC. Acute and 28-day repeated inhalation toxicity study of glycolic acid in male sprague-dawley rats. In Vivo. 2019;33(5):1507–1519. doi: 10.21873/invivo.11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LC, Lippmann M. Inhalation toxicology methods: the generation and characterization of exposure atmospheres and inhalational exposures. Curr Protoc Toxicol. 2015;63:24.4.1–24.4.23. doi: 10.1002/0471140856.tx2404s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Movia D, Bruni-Favier S, Prina-Mello A. In vitro alternatives to acute inhalation toxicity studies in animal models-a perspective. Front Bioeng Biotechnol. 2020;8:549. doi: 10.3389/fbioe.2020.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim YM, Kim H, Lim SK, Yoo J, Lee JY, Eom IC, Yoon BI, Kim P, Yu SD, Shim I. In vitro and in vivo evaluation of the toxic effects of dodecylguanidine hydrochloride. Toxics. 2020;8(3):76. doi: 10.3390/toxics8030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cesta MF, Ryman-Rasmussen JP, Wallace DG, Masinde T, Hurlburt G, Taylor AJ, Bonner JC. Bacterial lipopolysaccharide enhances PDGF signaling and pulmonary fibrosis in rats exposed to carbon nanotubes. Am J Respir Cell Mol Biol. 2010;43(2):142–151. doi: 10.1165/rcmb.2009-0113OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 14.Scoville DK, Botta D, Galdanes K, Schmuck SC, White CC, Stapleton PL, Bammler TK, MacDonald JW, Altemeier WA, Hernandez M, Kleeberger SR, Chen LC, Gordon T, Kavanagh TJ. Genetic determinants of susceptibility to silver nanoparticle-induced acute lung inflammation in mice. FASEB J. 2017;31(10):4600–4611. doi: 10.1096/fj.201700187R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matzer SP, Baumann T, Lukacs NW, Röllinghoff M, Beuscher HU. Constitutive expression of macrophage-inflammatory protein 2 (MIP-2) mRNA in bone marrow gives rise to peripheral neutrophils with preformed MIP-2 protein. J Immunol. 2001;167(8):4635–4643. doi: 10.4049/jimmunol.167.8.4635. [DOI] [PubMed] [Google Scholar]
- 17.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 18.Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62(7):641–651. doi: 10.1007/s00011-013-0633-0. [DOI] [PubMed] [Google Scholar]
- 19.Marinova M, Solopov P, Dimitropoulou C, Colunga Biancatelli RML, Catravas JD. Acute exposure of mice to hydrochloric acid leads to the development of chronic lung injury and pulmonary fibrosis. Inhal Toxicol. 2019;31(4):147–160. doi: 10.1080/08958378.2019.1624895. [DOI] [PubMed] [Google Scholar]
- 20.Andersen F. Final report on the safety assessment of glycolic acid, ammonium, calcium, potassium, and sodium glycolates, methyl, ethyl, propyl, and butyl glycolates, and lactic acid, ammonium, calcium, potassium, sodium, and tea-lactates, methyl, ethyl, isopropyl, and butyl lactates, and lauryl, myristyl, and cetyl lactates. International Journal of Toxicology. 2021;17(1_suppl):1–241. doi: 10.1177/109158189801700101. [DOI] [Google Scholar]
- 21.Czuppon AB, Kaplan V, Speich R, Baur X. Acute autoimmune response in a case of pyromellitic acid dianhydride-induced hemorrhagic alveolitis. Allergy. 1994;49(5):337–341. doi: 10.1111/j.1398-9995.1994.tb02278.x. [DOI] [PubMed] [Google Scholar]
- 22.Hajela R, Janigan DT, Landrigan PL, Boudreau SF, Sebastian S. Fatal pulmonary edema due to nitric acid fume inhalation in three pulp-mill workers. Chest. 1990;97(2):487–489. doi: 10.1378/chest.97.2.487. [DOI] [PubMed] [Google Scholar]
- 23.Huh G, Ha H, Park J, Jang S. Fatal inhalation injury by sulfuric acid fumes: case report. Korean Journal of Legal Medicine. 2019;37(4):216. doi: 10.7580/kjlm.2013.37.4.216. [DOI] [Google Scholar]
- 24.Market Research Future (MRF) Glycolic acid market: Information by source (synthetic, natural), purity level (70% purity, 99% purity, 30% purity, 60% purity and others), grade (cosmetic grade, industrial grade, technical grade and medical), application (skin care & facial rejuvenation, household cleaners, industrial cleaners, medical, oil field and petroleum refining, textile dyeing & finishing, electropolishing and others) and region (North America, Europe, Asia-Pacific, Latin America, Middle East & Africa) - forecast till 2030 (2019) Available at: https://www.marketresearchfuture.com/reports/glycolic-acidmarket-3141. [Last accessed on January 14, 2022]
- 25.Cheng YS. Mechanisms of pharmaceutical aerosol deposition in the respiratory tract. AAPS PharmSciTech. 2014;15(3):630–640. doi: 10.1208/s12249-014-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1(4):315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 27.European Chemecals Agency (ECHA) Registry dossier. Glycolic acid. Acute toxicity (2022) Available at: https://echa.europa.eu/registration-dossier/-/registered-dossier/14561/7/3/3. [Last accessed on January 14, 2022]
- 28.Fröhlich E, Salar-Behzadi S. Toxicological assessment of inhaled nanoparticles: role of in vivo, ex vivo, in vitro, and in silico studies. Int J Mol Sci. 2014;15(3):4795–4822. doi: 10.3390/ijms15034795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Yoo J, Lim YM, Kim EJ, Yoon BI, Kim P, Yu SD, Eom IC, Shim I. Comprehensive pulmonary toxicity assessment of cetylpyridinium chloride using A549 cells and Sprague-Dawley rats. J Appl Toxicol. 2021;41(3):470–482. doi: 10.1002/jat.4058. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi K, Kuroda Y, Numano T, Kimura M, Hayashi S, Furukawa S. Comparison of acute inhalation toxicity of sulfuric acid by the inhalation and intratracheal instillation methods. J Toxicol Pathol. 2021;34(3):269–273. doi: 10.1293/tox.2020-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi T, Oshima Y, Tsubokura Y, Muroi T, Ajimi S, Nakai M, Kawaguchi K, Sasaki T, Shinohara N, Imatanaka N. Time-course comparison of pulmonary inflammation induced by intratracheal instillation of four different nickel oxide nanoparticles in male Fischer rats. J Toxicol Pathol. 2021;34(1):43–55. doi: 10.1293/tox.2020-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park EJ, Kim SN, Yoon C, Cho JW, Lee GH, Kim DW, Park J, Choi I, Lee SH, Song J, Lim HJ, Kang MS, Lee HS. Repeated intratracheal instillation of zinc oxide nanoparticles induced pulmonary damage and a systemic inflammatory response in cynomolgus monkeys. Nanotoxicology. 2021;15(5):621–635. doi: 10.1080/17435390.2021.1905899. [DOI] [PubMed] [Google Scholar]
- 33.Guo T, Fang X, Liu Y, Ruan Y, Hu Y, Wang X, Hu Y, Wang G, Xu Y. Acute lung inflammation induced by zinc oxide nanoparticles: Evolution and intervention via NRF2 activator. Food Chem Toxicol. 2022;162:112898. doi: 10.1016/j.fct.2022.112898. [DOI] [PubMed] [Google Scholar]
- 34.Morimoto Y, Izumi H, Yoshiura Y, Fujishima K, Yatera K, Yamamoto K. Usefulness of intratracheal instillation studies for estimating nanoparticle-induced pulmonary toxicity. Int J Mol Sci. 2016;17(2):165. doi: 10.3390/ijms17020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osier M, Oberdörster G. Intratracheal inhalation vs. intratracheal instillation: differences in particle effects. Fundam Appl Toxicol. 1997;40(2):220–227. doi: 10.1006/faat.1997.2390. [DOI] [PubMed] [Google Scholar]