Abstract
Objectives
Pregnancy is associated with elevated risk for poor sleep quality, which increases the risk for poor obstetrical outcomes and parent mental health problems. The COVID-19 pandemic has seen increased reports of disturbed sleep worldwide; however, the degree this extends to pregnancy or influences pregnancy mental health outcomes has not been examined. The goal of this study was to examine changes in pregnant individuals’ sleep, anxiety, and depression during the pandemic, and to understand how sleep was associated with symptoms of anxiety and depression over time.
Methods
The Pregnancy During the COVID-19 Pandemic (PdP) study is a prospective longitudinal cohort of pregnant individuals (at enrollment) with repeated follow-ups during pregnancy and the postpartum period. About 3747 pregnant individuals participated between April and July 2020. The present analysis was restricted to participants who completed at least 2 assessments, yielding a final sample of 1842 pregnant individuals.
Results
Depression symptoms were elevated at baseline, compared to prepandemic estimates of prevalence, but declined gradually over time. Shorter sleep duration, higher sleep disturbance, and more sleep-related impairments at baseline predicted a slower decline in depression symptoms over time. More sleep disturbances at baseline also predicted slower decline in anxiety symptoms over time. In contrast, rates of depression and anxiety symptoms at baseline were not predictive of changes in any of the 3 sleep variables over time.
Conclusions
These findings highlight the importance of early intervention for sleep problems in pregnancy, in order to optimize mental health throughout pregnancy and mitigate long-term negative outcomes.
Keywords: Pregnancy, Sleep, Anxiety, Depression, COVID-19, Pandemic, Longitudinal
Sleep problems, including symptoms of insomnia and poor sleep quality, are common in pregnancy, and tend to increase as pregnancy progresses.1 , 2 Sleep problems in pregnancy are associated with poor obstetrical outcomes, including increased risk of preterm birth.3 Poor sleep quality and short sleep duration have also been shown to predict declines in mental health over time, with sleep problems being more predictive of later depression than vice versa in pregnant and nonpregnant populations.4 , 5 The direction of the relationship between sleep and anxiety during pregnancy is less clear, with some evidence that experiencing anxiety in early pregnancy is predictive of later symptoms of insomnia6 and decreasing sleep duration.7 Exposure to mental health problems during the perinatal period can negatively impact child developmental outcomes and family relationships, thus identifying potential modifiable risk factors is an important priority.8
Since being declared a worldwide pandemic, COVID-19 and the restrictions implemented to limit its spread have impacted every area of daily functioning including work changes, cancelled school/daycare, relationship strain, and social isolation. Prior to the COVID-19 pandemic, meta-analytic estimates of perinatal depression and anxiety were approximately 12–15%.9 , 10 Rapid reviews and meta analyses suggest significantly elevated rates of mental health problems across populations, including in pregnancy.11 Additionally, rates of poor sleep quality increased, with higher elevations observed in women (compared to men).12 Disturbed sleep and lower sleep quality in pregnant individuals also appear to be more common during the pandemic compared to pre pandemic levels.13 However, the research conducted on sleep in pregnancy during COVID-19 has been cross-sectional in nature and it is unclear how sleep changed as the pandemic progressed, or whether poor sleep was a risk factor for worse mental health symptoms across the perinatal period. Studies have found that, at the population level, rates of depression and anxiety were elevated at the onset of the pandemic, and decreased during the first wave.14 It is unclear whether that is also the case at the intraindividual level and in pregnant individuals. While sleep problems in pregnancy were found to be associated with increases in depression and anxiety before the pandemic, it is unclear how they associate in a historic situation where anxiety and depression levels are both abnormally elevated and decreasing. Even beyond the pandemic, it is important to better understand the temporal nature of the relationship between sleep and mental health in pregnancy in order to prioritize intervention development and delivery.
Objectives
The present study aimed to: (1) examine changes in sleep duration, sleep disturbance, sleep-related impairments, and symptoms of anxiety and depression in pregnant individuals during the first wave of the COVID 19 pandemic in Canada (ie, April to July 2020); and (2) examine how levels and change in sleep and mental health variables relate to each other over time.
Methods
Study protocol and procedures
The current investigation utilized data from the larger Pregnancy During the COVID-19 Pandemic (PdP) study,15 a prospective longitudinal cohort of pregnant individuals (at enrollment) with repeated follow ups during pregnancy and the postpartum period. Ethics approval was obtained from the Conjoint Health Research Ethics Board (CHREB) at the University of Calgary (REB20 0500), and all participants provided informed consent. Recruitment and data collection began in April 2020. Participants were eligible if they were: pregnant, ≥17 years old, ≤35 weeks gestation at study enrollment, living in Canada, and able to read and write in English or French. At enrollment, participants completed an initial online survey that gathered information on demographic and socioeconomic characteristics, and included several validated self-report questionnaires that assessed physical and mental health such as sleep, anxiety, and depression. For 3 months following the initial assessment, participants received a monthly email link to complete follow up surveys about experiences since the previous survey. This investigation followed the methods outlined by the standards set by STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines (cite; see supplementary materials for checklist).16
Sample
A total of 3747 individuals with a delivery due date after July 31, 2020 provided exclusively antepartum data between April and July 2020. The sample for the current investigation was restricted to those with data from at least 2 timepoints of 4-month monthly assessments between April and July 2020, in order to have adequate covariance coverage to conduct longitudinal analyses, yielding a final sample of 1842 participants. There were differences between individuals with data at ≥ 2 timepoints compared to those with data only at the 1 timepoint: participants were more likely to complete ≥2 questionnaires by July 2020 if they had a higher income (odds ratio [OR] = 1.13, 95% confidence interval [CI]: 1.09, 1.16, p < .001), higher education (OR = 1.61, 95% CI: 1.07, 2.42, p < .001), and were in a marriage or domestic partnership (OR = 1.61, 95% CI: 1.07, 2.42, p = .022); there were no differences in terms of previous miscarriages or depression and anxiety disorder diagnoses before pregnancy (p > .50). Furthermore, analyses examining attrition according to mental health at study onset (April (n = 1234)) showed that the odds of completing 2 or more timepoints were lower for those with higher levels of depression (OR = 0.95, 95% CI: 0.93, 0.97, p <.001) and anxiety in April (OR = 0.97, 95% CI: 0.96, 0.99, p = .009).
The average age of participants in April was 32.78 years (SD = 4.05, range = 19–49 years). The median yearly household income of participants was $100,000 124,999 CDN. About 86.1% of participants were born in Canada. And 84.5% of participants identified as White, 0.7% as First Nations, 1.0% as Metis, 0.1% as Inuit, 0.9% as Black, 2.2% as Chinese, 1.4% as Filipino, 0.2% as Korean, 0.3% as West Asian, 2.4% as South Asian, 0.5% as Southeast Asian, 1.7% as Hispanic/Latinx, and 3.9% as Mixed Race or Other. About 78.3% of participants were married and 19.8% were in a domestic partnership, with the remaining being single (1.7%), divorced (0.1%) or separated (0.2%). About 18.2% of participants had completed a trade or community college diploma, 43.4% completed a Bachelor's degree, and 32.1% had a graduate or professional degree.
Measures
Sleep-related variables
Sleep duration was self-reported at each assessment with a single item from the Pittsburgh Sleep Quality Index17 asking participants “During the PAST MONTH, how many hours of actual sleep did you get a night? (This may be different than the number of hours you spend in bed).” Skewness for sleep duration ranged from 0.05 to 0.14 across the 4 assessments, and kurtosis = 0.07–0.22.
Sleep disturbance and sleep-related impairment were self-reported at each assessment using the 4-item Patient-Reported Outcomes Measurement Information System (PROMIS®) Sleep Disturbance – Short Form 4a and the 4-item PROMIS® Sleep-Related Impairment Short Form 4a.18 Items are answered on a 5-point scale and summed scores can range from 4 to 20, where higher scores indicate more severe symptoms. Scores of 16-19 on the sleep disturbance scale and 12-15 on sleep-related impairment are indicative of moderate problems and scores of 20 on sleep disturbance and ≥16 on sleep-related impairments are considered severely elevated. In the current investigation, sleep disturbance had a skewness range of 0.25–0.34, kurtosis of 0.42–0.49, and Cronbach's alpha (α) of 0.81–-0.85 across time points, whereas sleep-related impairment had a skewness range of 0.50–0.57, kurtosis of 0.41–0.59, and α = 0.89-0.92.
Anxiety and depression symptoms
General anxiety symptoms were self-reported at each assessment using the PROMIS® Anxiety Adult 7-item short form.19 Items are answered on a 5-point scale and summed scores can range from 7 to 35, where higher scores indicate more severe symptoms. Scores of 20-27 are indicative of moderately elevated anxiety symptoms and scores ≥28 are indicative of severely elevated anxiety symptoms. Skewness ranged from 0.01 to 0.32, kurtosis from 0.57 to 0.81, and all α = 0.94.
Depression symptoms were self-reported at each assessment using the 10-item Edinburgh Postpartum Depression Scale,20 which is validated for assessments conducted during pregnancy.21 Items are answered on a 4-point scale and summed scores can range from 0 to 30, where higher scores indicate more severe symptoms. A cut-off score of ≥13 is used to identify individuals with clinically concerning depression symptoms.20 Skewness ranged from 0.37 to 0.58, kurtosis from 0.13 to 0.01, and α = 0.88-0.89.
Covariates
Potential confounding variables were accounted for in the analyses as covariates, and included baseline report of: education level (high school degree or lower vs. postsecondary education), household income, changes in household income due to COVID-19, marital status (married or in a domestic partnership vs. single, divorced, widowed or separated), number of previous births, having had a miscarriage prior to the current pregnancy, and number of weeks until delivery due date on April 1, 2020.
Data analyses
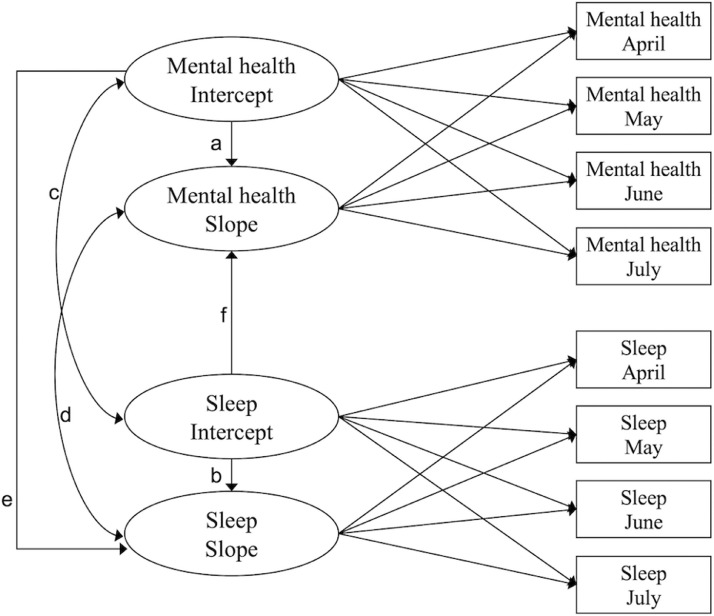
Latent growth models (LGMs) were constructed in Mplus 8.522 using maximum likelihood estimation. Analyses were conducted in 2 steps. First, unconditional LGMs were estimated separately for sleep duration, sleep disturbance, sleep-related impairments, anxiety, and depression. These univariate models estimate 2 latent growth parameters: (1) an intercept, which was centered in April as an estimate of the initial levels; and (2) a linear slope, which captures change over time. Second, parallel (ie, bivariate) LGMs were conducted to examine the associations between the sleep, anxiety, and depression growth factors. Six bivariate LGMs were estimated, including sleep duration, sleep disturbance, and sleep-related impairments each with anxiety and depression. Fig. 1 shows the associations tested in these models. The models estimate: autoregressive paths for the mental health variable intercepts predicting mental health variable slopes (a) and for sleep variable intercepts predicting sleep variable slopes (b); correlations between sleep and mental health variable intercepts (c) and between sleep and mental health variable slopes (d); and regression paths for mental health variable intercepts predicting sleep variable slopes (e) and sleep variable intercepts predicting mental health variables’ slopes (f). All covariates (see measures section) were included in the bivariate LGMs by regressing the intercepts and slopes on these variables. The Benjamini-Hochberg procedure23 was used to correct for multiple testing.
Fig. 1.
Associations tested in the bivariate (parallel) latent growth models (LGMs). Mental health refers to depression or anxiety. Sleep refers to sleep duration, sleep disturbance, or sleep-related impairments.
Tests of goodness of model fit included the model chi square (χ2), the Comparative Fit Index (CFI), the Tucker Lewis Index (TLI), the Root Mean Square Error of Approximation (RMSEA), and the Standardized Root Mean Residual (SRMR). An adequate fit is suggested by a nonsignificant χ2, a CFI and TLI > 0.90 and a RMSEA and SRMR < 0.08, and close fit is suggested by a CFI and TLI > 0.95 and a RMSEA and SRMR < 0.05.24
Full information maximum likelihood was used to account for missing data under the missing completely at random and missing at random (MAR) assumptions.25 Missing data analyses showed that of the 1842 participants, 358 had data at all 4 timepoints. 1163 enrolled in May or June and therefore had missing data for previous assessments, which was considered missing completely at random,25 but had complete data thereafter. Three hundred twenty-one withdrew or missed assessments after joining the study. Missing data rates for each variable are provided in supplementary materials. Household income, number of children, previous miscarriages, marital status, education, anxiety and depression diagnoses before pregnancy, temporary or permanent job loss due to COVID-19, and income change due to COVID-19 were examined as potential predictors of withdrawal and missed assessments (ie, potential predictors of MAR25 within the analyzed sample). All were found to be nonsignificant predictors of missingness (p > .10) and were thus not included as auxiliary variables (variables that are not in the analytical model but are included for missing data estimation). Anxiety and depression at study onset were related to attrition (see sample section). As these variables were already in the models, this was taken into account in full information maximum likelihood estimation under the MAR assumption.
Results
Descriptive statistics
Descriptive statistics of the study variables are presented in Table 1 . Cross tabulations conducted in SPSS showed that in April, participants with moderately to severely elevated sleep disturbance had higher rates of severely elevated anxiety symptoms compared to participants with lower sleep disturbance levels (82% vs. 47%; χ2(1) = 37.93, p < .001). The same was found for participants with moderate to severe sleep-related impairments (72% vs. 40%; χ2(1) = 73.72, p < .001). Similarly, participants with moderately to severely elevated sleep disturbance and sleep-related impairments had higher rates of clinically concerning depression symptoms compared to participants with lower sleep disturbance and impairment (disturbance 68% vs. 26%; χ2(1) = 63.55, p < .001; impairments 54% vs. 19%; χ2(1) = 107.00, p < .001). Correlations between all sleep and mental health variables across the 4 assessments can be found in Supplementary Materials.
Table 1.
Descriptive statistics of study variables
| April (n = 825) |
May (n = 1316) |
June (n = 1520) |
July (n = 1316) |
|||||
|---|---|---|---|---|---|---|---|---|
| Variable | M (SD) | % | M (SD) | % | M (SD) | % | M (SD) | % |
| Anxiety symptoms | 19.20 (6.12) | 17.81 (6.02) | 17.16 (5.95) | 16.91 (5.99) | ||||
| Moderately elevated | 42.3 | 35.1 | 31.6 | 30.6 | ||||
| Severely elevated | 7.8 | 5.4 | 4.3 | 4.0 | ||||
| Depression symptoms | 9.83 (5.20) | 9.20 (5.21) | 8.67 (5.32) | 8.38 (5.35) | ||||
| Clinically concerning | 30.1 | 26.4 | 23.1 | 21.6 | ||||
| Sleep duration (hours) | 7.19 (1.17) | 7.13 (1.26) | 7.02 (1.22) | 6.83 (1.29) | ||||
| Sleep disturbance | 10.74 (3.38) | 10.78 (3.55) | 10.90 (3.53) | 11.30 (3.53) | ||||
| Moderately elevated | 9.8 | 10.8 | 11.3 | 12.3 | ||||
| Severely elevated | 0.4 | 0.7 | 0.8 | 1.5 | ||||
| Sleep-related impairments | 9.80 (3.72) | 9.90 (3.97) | 9.74 (3.88) | 10.08 (4.01) | ||||
| Moderately elevated | 23.9 | 22.8 | 21.8 | 24.0 | ||||
| Severely elevated | 8.5 | 11.2 | 10.4 | 11.8 | ||||
Note. M, mean; SD, standard deviation.
Univariate growth models
Table 2 shows model fit indices and parameter estimates for the univariate LGMs. For all models, the chi square test was significant. However, this test is sensitive to sample size, and with a large sample size such as in the present study, large power leads the chi square test to be significant.24 All other model fit indices showed acceptable to close fit.
Table 2.
Model fit indices and parameters of univariate latent growth models for mental health and sleep-related variables
| Model fit |
Parameter estimates |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 (df = 5) | CFI | TLI | RMSEA | SRMR | Intercept M | Intercept v | Slope M | Slope v | Intercept-Slope r | |
| Anxiety symptoms | 49.19⁎⁎⁎ | .98 | .98 | .07 | .04 | 19.11⁎⁎⁎ | 25.52⁎⁎⁎ | -0.82⁎⁎⁎ | 0.94⁎⁎⁎ | -0.18* |
| Depression symptoms | 15.14⁎⁎ | .99 | .99 | .03 | .02 | 10.12⁎⁎⁎ | 20.86⁎⁎⁎ | -0.63⁎⁎⁎ | 0.90⁎⁎⁎ | -0.26⁎⁎⁎ |
| Sleep duration | 17.02⁎⁎ | .99 | .99 | .04 | .05 | 7.29⁎⁎⁎ | 1.09⁎⁎⁎ | -0.16⁎⁎⁎ | 0.06⁎⁎⁎ | -0.29⁎⁎⁎ |
| Sleep disturbance | 30.64⁎⁎⁎ | .99 | .99 | .05 | .05 | 10.79⁎⁎⁎ | 9.30⁎⁎⁎ | 0.13⁎⁎⁎ | 0.50⁎⁎⁎ | -0.35⁎⁎⁎ |
| Sleep-related impairments | 27.77⁎⁎⁎ | .99 | .99 | .05 | .04 | 10.01⁎⁎⁎ | 10.54⁎⁎⁎ | -0.03 | 0.58⁎⁎⁎ | -0.27⁎⁎⁎ |
Note. CFI, comparative fit index; TLI, Tucker-Lewis index; RMSEA, root-mean-square error of approximation; SRMR, standardized root-mean residual; M, mean; v, variance; r, correlation.
p < .05.
p < .001.
p < .001.
On average, initial sleep duration was 7 h 17 min and significantly decreased by 10 min per month. Sleep disturbance had a significantly increasing slope. While the slope mean of sleep-related impairments was nonsignificant, the slope variance was significant. Accordingly, while on average there was no significant change across participants, there was significant within participant variance where some had increasing and some had decreasing sleep-related impairments. Results (Table 2) showed that both anxiety and depression symptoms had a significant decreasing slope between April and July. All intercept and slope parameters had significant variance.
Bivariate growth models
As can be seen in Table 3 , all bivariate LGMs had adequate model fit. Results of the bivariate LGMs are presented in Table 4 . Initial levels of sleep duration and anxiety symptoms were negatively associated and initial levels of sleep-related impairments and anxiety symptoms were positively associated, but there were no longitudinal associations between these 2 sleep-related variables and anxiety (ie, intercepts did not predict slopes in either direction). Initial levels of sleep disturbance and anxiety symptoms were positively associated and higher initial levels of sleep disturbance predicted a slower decline in anxiety symptoms, whereas anxiety symptoms did not predict the slope of sleep disturbance.
Table 3.
Model fit indices of bivariate latent growth models between mental health and sleep-related variables
| Model fit |
||||||
|---|---|---|---|---|---|---|
| Bivariate model between: | χ2 (df = 46) | CFI | TLI | RMSEA | SRMR | |
| Anxiety | Sleep duration | 114.00⁎⁎⁎ | .99 | .97 | .03 | .02 |
| Anxiety | Sleep disturbance | 109.05⁎⁎⁎ | .99 | .98 | .03 | .02 |
| Anxiety | Sleep-related impairments | 102.00⁎⁎⁎ | .99 | .98 | .03 | .02 |
| Depression | Sleep duration | 71.18* | .99 | .99 | .02 | .02 |
| Depression | Sleep disturbance | 73.46⁎⁎ | .99 | .99 | .02 | .02 |
| Depression | Sleep-related impairments | 64.24* | .99 | .99 | .02 | .02 |
Note. CFI, Comparative Fit Index; TLI, Tucker-Lewis Index; RMSEA, Root-Mean-Square Error of Approximation; SRMR, Standardized Root-Mean Residual.
p < .05.
p < .001.
p < .001.
Table 4.
Results of bivariate latent growth models between mental health and sleep-related variables
| Anxiety |
Depression |
|||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI |
95% CI |
|||||||
| Estimate(SE) | LL | UL | β/r | Estimate(SE) | LL | UL | β/r | |
| Sleep duration | ||||||||
| a. Mental health intercept → mental health slope | -0.04 (0.02) | -0.07 | 0.00 | -0.19 | -0.07 (0.02)* | -0.10 | -0.03 | -0.31 |
| b. Sleep intercept → sleep slope | -0.08 (0.02)* | -0.25 | 0.07 | -0.32 | -0.08 (0.02)* | -0.35 | -0.07 | -0.32 |
| c. Mental health intercept ↔ sleep intercept | -1.27 (0.22)* | -1.70 | -0.84 | -0.26 | -1.27 (0.19)* | -1.64 | -0.90 | -0.29 |
| d. Mental health slope ↔ sleep slope | -0.04 (0.03) | -0.10 | 0.03 | -0.18 | -0.02 (0.03) | -0.07 | 0.04 | -0.08 |
| e. Mental health intercept → sleep slope | 0.00 (0.01) | -0.01 | 0.00 | -0.06 | -0.01 (0.00) | -0.01 | 0.00 | -0.09 |
| f. Sleep intercept → mental health slope | -0.09 (0.08) | -0.25 | 0.07 | -0.10 | -0.21 (0.07)* | -0.35 | -0.07 | -0.23 |
| Sleep disturbance | ||||||||
| a. Mental health intercept → mental health slope | -0.05 (0.02)* | -0.09 | -0.01 | -0.26 | -0.08 (0.02)* | -0.12 | -0.04 | -0.40 |
| b. Sleep intercept → sleep slope | -0.10 (0.02)* | -0.14 | -0.06 | -0.45 | -0.10 (0.02)* | -0.15 | -0.06 | -0.44 |
| c. Mental health intercept ↔ sleep intercept | 6.65 (0.65)* | 5.38 | 7.92 | 0.46 | 6.82 (0.57)* | 5.71 | 7.93 | 0.53 |
| d. Mental health slope ↔ sleep slope | 0.14 (0.09) | -0.03 | 0.32 | 0.25 | 0.14 (0.07) | -0.01 | 0.28 | 0.25 |
| e. Mental health intercept → sleep slope | 0.03 (0.01) | 0.01 | 0.05 | 0.22 | 0.03 (0.01) | 0.00 | 0.05 | 0.19 |
| f. Sleep intercept → mental health slope | 0.07 (0.03)* | 0.00 | 0.13 | 0.21 | 0.09 (0.03)* | 0.04 | 0.15 | 0.30 |
| Sleep-related impairment | ||||||||
| a. Mental health intercept → mental health slope | -0.05 (0.02) | -0.10 | -0.01 | -0.28 | -0.08 (0.02)* | -0.13 | -0.04 | -0.41 |
| b.Ssleep intercept → sleep slope | -0.07 (0.03) | -0.12 | -0.01 | -0.30 | -0.07 (0.03) | -0.13 | -0.01 | -0.29 |
| c. Mental health intercept ↔ sleep intercept | 7.73 (0.72)* | 6.33 | 9.13 | 0.51 | 7.71 (0.63)* | 6.48 | 8.93 | 0.58 |
| d. Mental health slope ↔ sleep slope | 0.25 (0.11) | 0.03 | 0.47 | 0.43 | 0.21 (0.10)* | 0.02 | 0.40 | 0.36 |
| e. Mental health intercept → sleep slope | 0.03 (0.02) | 0.01 | 0.06 | 0.22 | 0.03 (0.02) | 0.00 | 0.07 | 0.20 |
| f. sleep Intercept → mental health Slope | 0.08 (0.04) | 0.01 | 0.15 | 0.26 | 0.09 (0.03)* | 0.03 | 0.15 | 0.30 |
Note. See Fig. 1 for visualization of a-f paths. Estimate = unstandardized estimate (unstandardized beta for regression paths); SE, standard error; CI, confidence interval; LL, lower limit; UL, upper limit; β, standardized beta for regression paths; r, correlation coefficient for correlation paths; mental health refers to anxiety or depression; models control for education level), household income, changes in household income due to COVID-19, marital status, number of live children, having had a miscarriage prior to the current pregnancy, and number of weeks until delivery due date.
p < .05 after correction for multiple testing.
Associations between initial levels of the sleep variables and symptoms of depression were similar to those found for anxiety, but there was stronger evidence of longitudinal associations. Initial sleep duration and levels of depression symptomatology were negatively associated, and lower initial sleep duration predicted a slower decline in symptoms of depression. Initial levels of sleep disturbance and symptoms of depression were positively associated, and slopes of sleep disturbance and symptoms of depression were positively associated, indicating that a steeper increase in sleep disturbance was associated with a slower decline in depression symptomatology. Higher initial levels of sleep disturbance also predicted a slower decline in depression symptomatology. Similarly, higher initial levels of sleep-related impairments and symptoms of depression and were positively associated, and a steeper increase in sleep-related impairments was associated with a slower decline in depression symptoms. Higher initial levels of sleep-related impairments also predicted a slower decline in depression symptoms. In contrast, initial levels of depression symptoms did not predict the 3 sleep-related slopes.
Discussion
Here, we provide important insight into links between pregnant individuals’ sleep problems and mental health difficulties in a large longitudinal sample during the COVID-19 pandemic. Shorter sleep duration, more disturbances, and sleep-related impairment, were associated with higher symptoms of anxiety and depression among pregnant individuals during the first wave of the COVID-19 pandemic. Consistent with other studies of nonpregnancy cohorts, from April to July 2020, rates of anxiety and depression decreased within the overall sample,14 although they remained higher when compared to meta-analytic estimates of pre-pandemic levels.11 Over the course of the study, sleep disturbance increased and sleep duration declined—a pattern in line with the broader pregnancy literature.1 , 2 , 6 Shorter sleep duration, higher sleep disturbance and more sleep-related impairments at baseline predicted a slower decline in depression symptoms. Increasing sleep-related impairment and disturbances over time were also associated with a slower decline in symptoms of depression. In contrast, initial depression symptoms were not predictive of changes in sleep. At baseline, shorter sleep duration, more sleep-related impairments and disturbance were associated with higher anxiety. Whereas there were no longitudinal associations between sleep duration and sleep-related impairments with anxiety, higher sleep disturbance at baseline was predictive of a slower decrease in anxiety over the course of the study. Similar to depression, initial rates of anxiety did not predict the slope of any of the sleep variables. While previous studies in children, adolescents and adults have found that sleep is predictive of later mental health problems26 , 27; these findings suggests that poor sleep quality was also associated with slower recovery from high rates of anxiety and depression during early phases of the pandemic and that sleep may be a key target for intervention.
Sleep problems in pregnancy are common and given their serious long-term impact when left untreated, they should be considered part of standard perinatal mental health screening protocols. Pregnant individuals show a strong preference for psychotherapy (ie, cognitive behavioral therapy for insomnia (CBT I)) versus pharmacotherapy for the treatment of insomnia.28 Recent studies suggest that both in person and online CBT-I delivered in pregnancy significantly reduce symptoms of insomnia.29., 30., 31., 32. Data from our research group also shows that CBT I reduced symptoms of insomnia during the COVID-19 pandemic when delivered via telehealth.33 Treating insomnia in pregnancy also appears to reduce symptoms of depression, with evidence showing benefit up to 2 years postpartum.30 , 32 Data from this study, and others longitudinal cohorts add to a burgeoning literature showing the impact of untreated sleep problems on later symptoms of depression, and point to an urgent need for accessible treatment options to be disseminated.4 , 5 , 34
Treating sleep problems in pregnancy may also be an important prevention-based strategy for promoting child health and development. Poor sleep quality in pregnancy has been shown to predict shorter infant sleep duration and longer wake after sleep onset.35 Poor sleep in infancy and early childhood has itself been linked to important child health and developmental outcomes.36., 37., 38., 39. Additionally, perinatal depression is associated with risk for many negative child outcomes.40 When maternal depression persists into the postpartum period or beyond, further impacts are seen on child development at both biological (ie altered brain development) and behavioral (ie, delays in socioemotional and cognitive development) levels.41 , 42 Such negative impacts of maternal depression are exacerbated in the presence of postpartum sleep difficulties and other stressors, such as domestic conflict or poverty related stress, given the high demands of caring for infants while managing high burdens of internal distress alongside inadequate psychosocial support.
Strengths and limitations
The current investigation utilized data from a large national prospective pregnancy cohort study conducted during the COVID-19 pandemic. While this provides an unprecedented opportunity to examine the sleep and mental health of pregnant individuals within the context of adversity, the results should be interpreted with limitations in mind. Although there were no significant differences in previous anxiety or depression diagnoses among participants who were excluded from the study for withdrawing after the initial assessment, similar to other pandemic studies, later attrition was higher among those with elevated symptoms of anxiety and depression at baseline.43 This may have contributed to the lack of significant findings for anxiety and depression predicting later sleep problems; it may also suggest that the strength of association between sleep and mental health was underestimated. Furthermore, all data were self-reported, raising the possibility of shared method variance accounting for a portion of the associations.44 The PROMIS sleep tools are commonly used measures of sleep quality; however, although informative, self-report measures of sleep are poorly correlated with objective measurements.45 The sample was also highly educated and had high household income, and while sociodemographic factors were accounted for in the analyses, the results may not be generalizable to specific subpopulations with more vulnerabilities such as job or food insecurity, lack of access to health care, adequate housing, transportation and language barriers. The experience of pregnancy, labor and delivery may have differed depending on the phase of the pandemic, and these differences are not addressed in our analyses. Finally, other important variables known to influence both mental health and sleep, such as social isolation or illness (eg, COVID infection) may have influenced our results.
Conclusions
The current findings suggest that poor sleep during pregnancy leads to more persistent symptoms of anxiety and depression across the perinatal period during chronic stressful events such as the COVID-19 pandemic. Poor sleep in pregnancy is highly responsive to treatment and broader screening and treatment may improve parental mental health outcomes.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgments
The authors gratefully acknowledge the Pregnancy during the Pandemic study team and the study participants and their families. We would like to acknowledge funding from the Alberta Children's Hospital Research Foundation and the Owerko Center for the Developing Child, which supported the PdP cohort. The Canadian Child Health Clinician Scientist Program (LTM) funding from the Canadian Institutes of Health Research through a fellowship (CR) and early career Investigator award in Maternal, Reproductive, Child and Youth Health (LTM), Fonds de Recherche du Québec-Santé fellowship (CR) and a Social Sciences and Humanities Research Council fellowship (AM).
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.sleh.2022.05.011.
Appendix. Supplementary materials
References
- 1.Sedov I, Cameron EE, Madigan S, Tomfohr-Madsen LM. Sleep quality during pregnancy: a meta-analysis. Sleep Med Rev. 2017;38:168–176. doi: 10.1016/j.smrv.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Sedov ID, Anderson NJ, Dhillon AK, Tomfohr-Madsen LM. Insomnia symptoms during pregnancy: a meta-analysis. J Sleep Res. 2021;30(1):e13207. doi: 10.1111/jsr.13207. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Zhu Z, Wang C, Zhang F, Zeng H. Association between adverse perinatal outcomes and sleep disturbances during pregnancy: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2022;35(1):166–174. doi: 10.1080/14767058.2020.1711727. [DOI] [PubMed] [Google Scholar]
- 4.González-Mesa E, Cuenca-Marín C, Suarez-Arana M, et al. Poor sleep quality is associated with perinatal depression. A systematic review of last decade scientific literature and meta-analysis. J Perinat Med. 2019;47(7):689–703. doi: 10.1515/jpm-2019-0214. doi:0.1515/jpm-2019-0214. [DOI] [PubMed] [Google Scholar]
- 5.Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety. 2015;32(9):664–670. doi: 10.1002/da.22386. [DOI] [PubMed] [Google Scholar]
- 6.Sedov ID, Tomfohr-Madsen LM. Trajectories of insomnia symptoms and associations with mood and anxiety from early pregnancy to the postpartum. Behav Sleep Med. 2020;12(1):395–406. doi: 10.1080/15402002.2020.1771339. [DOI] [PubMed] [Google Scholar]
- 7.van der Zwan JE, de Vente W, Tolvanen M, et al. Longitudinal associations between sleep and anxiety during pregnancy, and the moderating effect of resilience, using parallel process latent growth curve models. Sleep Med. 2017;40:63–68. doi: 10.1016/j.sleep.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 8.Pires GN, Benedetto L, Cortese R, et al. Effects of sleep modulation during pregnancy in the mother and offspring: evidences from preclinical research. J Sleep Res. 2020:e13135. doi: 10.1111/jsr.13135. [DOI] [PubMed] [Google Scholar]
- 9.Dennis CL, Falah-Hassani K, Shiri R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br J Psychiatry. 2017;210(5):315–323. doi: 10.1192/bjp.bp.116.187179. [DOI] [PubMed] [Google Scholar]
- 10.Woody CA, Ferrari AJ, Siskind DJ, Whiteford HA, Harris MG. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord. 2017;219(May):86–92. doi: 10.1016/j.jad.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Tomfohr-Madsen LM, Racine N, Giesbrecht GF, Lebel C, Madigan S. Depression and anxiety in pregnancy during COVID-19: a rapid review and meta-analysis. Psychiatry Res. 2021 doi: 10.1016/j.psychres.2021.113912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuksel D, McKee GB, Perrin PB, et al. Sleeping when the world locks down: correlates of sleep health during the COVID-19 pandemic across 59 countries. Sleep Health. 2021;7(2):134–142. doi: 10.1016/j.sleh.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demissie DB, Bitew ZW. Mental health effect of COVID-19 pandemic among women who are pregnant and/or lactating: a systematic review and meta-analysis. SAGE Open Med. 2021;9 doi: 10.1177/20503121211026195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daly M, Robinson E. Psychological distress and adaptation to the COVID-19 crisis in the United States. J Psychiatr Res. 2021;136:603–609. doi: 10.1016/j.jpsychires.2020.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giesbrecht GF, Bagshawe M, Van Sloten M, et al. Protocol for the Pregnancy During the COVID-19 Pandemic (PdP) study: a longitudinal cohort study of mental health among pregnant Canadians during the COVID-19 pandemic and developmental outcomes in their children. JMIR Res Protoc. 2021;10(4):e25407. doi: 10.2196/25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(suppl 1):S31–S34. doi: 10.4103/sja.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS (TM) sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2012;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pilkonis PA, Choi SW, Reise SP, et al. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS (R)): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 21.Bergink V, Kooistra L, Lambregtse-van den Berg MP, et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosomat Res. 2011;70(4):385–389. doi: 10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Muthén LK, Muthén BO.Mplus User's Guide. 8th ed. Muthén & Muthén; 1998-2017.
- 23.Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. J Educ Behav Stat. 2002;27(1):77–83. doi: 10.3102/10769986027001077. [DOI] [Google Scholar]
- 24.Little TD. Methodology in the Social Sciences. The Guildford Press; 2013. Longitudinal structural equation modeling; p. 385. [Google Scholar]
- 25.Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- 26.Marino C, Andrade B, Campisi SC, et al. Association between disturbed sleep and depression in children and youths. JAMA Netw Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, Wu C, Gan Y, Qu X, Lu Z. Insomnia and the risk of depression: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2016;16(1) doi: 10.1186/s12888-016-1075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sedov I, Goodman SH, Tomfohr-Madsen LM. Insomnia treatment preferences among pregnant women. JOGNN. 2017;46(3):e95–e104. doi: 10.1016/j.jogn.2017.01.005. doi. [DOI] [PubMed] [Google Scholar]
- 29.Arnedt JT, Conroy DA, Mooney A, Furgal A, Sen A, Eisenberg D. Telemedicine versus face-to-face delivery of cognitive behavioral therapy for insomnia: a randomized controlled noninferiority trial. Sleep. 2021;44(1) doi: 10.1093/sleep/zsaa136. [DOI] [PubMed] [Google Scholar]
- 30.Bei B, Pinnington DM, Quin N, et al. Improving perinatal sleep via a scalable cognitive behavioural intervention: findings from a randomised controlled trial from pregnancy to 2 years postpartum. Psychol Med. 2021:1–11. doi: 10.1017/s0033291721001860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silang KA, Sohal PR, Bright KS, et al. eHealth interventions for treatment and prevention of depression, anxiety, and insomnia during pregnancy: systematic review and meta-analysis. JMIR Ment Health. 2022;9(2):e31116. doi: 10.2196/31116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manber R, Bei B, Simpson N, et al. Cognitive behavioral therapy for prenatal insomnia: a randomized controlled trial. Obstet Gynecol. 2019;133(5):911–919. doi: 10.1097/AOG.0000000000003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacKinnon AL, Guadagni V, Donnici C, et al. Presented at: National Conference of the Canadian Sleep Society. 2021. Sleep, emotions and mood disorders during the COVID 19 pandemic across countries and populations. Virtual. [Google Scholar]
- 34.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1-3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 35.Burdayron R, Pennestri MH, Keys EM, et al. Maternal sleep quality in pregnancy and infant sleep: a longitudinal investigation. In review
- 36.Wu Y, Gong Q, Zou Z, Li H, Zhang X. Short sleep duration and obesity among children: a systematic review and meta-analysis of prospective studies. Obes Res Clin Pract. 2017;11(2):140–150. doi: 10.1016/j.orcp.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 37.Chaput JP, Gray CE, Poitras VJ, et al. Systematic review of the relationships between sleep duration and health indicators in school-aged children and youth. Appl Physiol Nutr Metab. 2016;41(6 suppl 3):S266–S282. doi: 10.1139/apnm-2015-0627. [DOI] [PubMed] [Google Scholar]
- 38.Matricciani L, Paquet C, Galland B, Short M, Olds T. Children's sleep and health: a meta-review. Sleep Med Rev. 2019;46:136–150. doi: 10.1016/j.smrv.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Short MA, Blunden S, Rigney G, et al. Cognition and objectively measured sleep duration in children: a systematic review and meta-analysis. Sleep Health. 2018;4(3):292–300. doi: 10.1016/j.sleh.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Stein A, Pearson RM, Goodman SH, et al. Effects of perinatal mental disorders on the fetus and child. Lancet North Am Ed. 2014;384(9956):1800–1819. doi: 10.1016/S0140-6736(14)61277-0. [DOI] [PubMed] [Google Scholar]
- 41.Lebel C, Walton M, Letourneau N, Giesbrecht GF, Kaplan BJ, Dewey D. Prepartum and postpartum maternal depressive symptoms are related to children's brain structure in preschool. Biol Psychiatry. 2016;80(11):859–868. doi: 10.1016/j.biopsych.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Slomian J, Honvo G, Emonts P, Reginster JY, Bruyère O. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Women’s Health. 2019;15 doi: 10.1177/1745506519844044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czeisler M, Wiley J, Czeisler C, Rajaratnam S, Howard M. Uncovering survivorship bias in longitudinal mental health surveys during the COVID-19 pandemic. Epidemiol Psychiatric Sci. 2021;30:1–26. doi: 10.1017/s204579602100038x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grandner MA, Kripke DF, Yoon IY, Youngstedt SD. Criterion validity of the Pittsburgh Sleep Quality Index: investigation in a non-clinical sample. Sleep Biol Rhythms. 2006;4(2):129–136. doi: 10.1111/j.1479-8425.2006.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol. 2012;22(5):462–468. doi: 10.2188/jea.je20120012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.