Abstract
Background
Venous thromboembolism (VTE) affects approximately 1–2 individuals per 1000 annually and is associated with an increased risk for pulmonary hypertension, postthrombotic syndrome, and recurrent VTE.
Objective
To determine risk factors, incidence, treatments, and outcomes of VTE through a 2‐year surveillance program initiated in Durham County, North Carolina (population approximately 280,000 at time of study).
Patients/Methods
We performed a retrospective analysis of data actively collected from three hospitals in Durham County during the surveillance period.
Results
A total of 987 patients were diagnosed with VTE, for an annual rate of 1.76 per 1000 individuals. Hospital‐associated VTE occurred in 167 hospitalized patients (16.9%) and 271 outpatients who were hospitalized within 90 days of diagnosis (27.5%). Annual incidence was 1.98 per 1000 Black individuals compared to 1.25 per 1000 White individuals (p < 0.0001), and Black individuals with VTE were younger than White individuals (p < 0.0001). Common risk factors included active cancer, prolonged immobility, and obesity, and approximately half were still taking anticoagulant therapy 1 year later. A total of 224 patients died by 1 year (28.5% of patients for whom outcomes could be confirmed), and Black patients were more likely to have recurrent VTE than White patients during the first 6 months following initial presentation (9.4% vs. 4.1%, p = 0.01).
Conclusions
Ongoing surveillance provides an effective strategy to identify patients with VTE and monitor treatment and outcomes. We demonstrated that hospital‐associated VTE continues to be a major contributor to the burden of VTE and confirmed the higher incidence of VTE in Black compared to White individuals.
Keywords: deep vein thrombosis, pulmonary embolism, racial group, risk factor, surveillance, venous thromboembolism
Essentials.
Venous thromboemboli (VTEs) are blood clots that form in the deep veins and can travel to the lungs.
We monitored for all new cases of VTE diagnosed in a racially diverse population in North Carolina.
More than 40% of patients with VTE were either hospitalized or recently discharged from the hospital.
New VTEs occurred more frequently in Black individuals than White individuals.
1. INTRODUCTION
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), has an annual incidence reported between 1.04 and 2.47 individuals per 1000 each year. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 The long‐term clinical burden associated with VTE includes chronic thromboembolic pulmonary hypertension, affecting approximately 3% of PE survivors, 10 , 11 and postthrombotic syndrome, occurring in 30%–50% of patients with proximal DVT, 12 , 13 with severe manifestations in 5%–10%. 13 , 14 For patients with no VTE risk factors at the time of the event, indefinite anticoagulation is recommended because of the high risk for recurrence. 15 , 16 Estimates of the economic burden associated with VTEs in the United States range from a conservative annual value of $7–$10 billion (2014 US dollars) for incident VTE, 17 to as high as $32.1–$69.3 billion (2011 US dollars) for all events, including indirect societal costs. 18
Despite the large disease burden and associated economic impact of VTE, there is no systematic national surveillance program for VTE currently in the United States. The surgeon general of the United States issued a Call‐to‐Action to prevent DVT and PE in 2008, suggesting that a national surveillance program would be useful. 19 A systematic surveillance of DVT and PE was also recommended by a National Workshop in 2008, sponsored by the American Society of Hematology and the Centers for Disease Control and Prevention (CDC). 20
Several limitations exist with currently available population‐based incidence data for VTE. Older studies reported lower event rates than more recent studies. 21 , 22 , 23 Whether older studies underreported VTE, however, or more recent studies included events that were of uncertain clinical relevance, is unclear. Depending on the geographic location studied, certain racial and ethnic groups were underrepresented in some reports. 2 , 4 Additional data to distinguish patients with provoked versus unprovoked VTE, as well as treatment strategies and outcomes, were not consistently collected across different studies, which would be useful when considering long‐term burdens of VTE.
This study is a retrospective analysis of results from a population‐based VTE surveillance program conducted by active review of the electronic medical record (EMR) at three hospitals within a single county in central North Carolina. We highlight how a surveillance program can be used to monitor therapeutic interventions and clinical outcomes, including reporting on different populations within the community.
2. PATIENTS, MATERIALS, AND METHODS
2.1. Materials and methods
As part of a population‐based surveillance strategy, we identified patients with VTE from Duke University Hospital (DUH), Duke Regional Hospital (DRH), and the Durham Veterans' Administration Medical Center (VAMC), the three health care institutions within Durham County, North Carolina. 24 Patients were included if they lived within Durham County at the time of diagnosis with VTE, between April 1, 2012, and March 31, 2014. This report is a retrospective review of the data collected during the study.
2.1.1. Identification of the patient cohort
In 2012, the population of Durham County, North Carolina, included ~280,000, with 52% White, 37% African American, 4% Asian, and 3% two or more races (https://www.census.gov/en.html). Patients with VTE were identified by screening the EMRs at DUH, DRH, and the Durham VAMC for new diagnoses of DVT and PE, defined by International Classification of Diseases, Ninth Revision (ICD‐9) and Current Procedural Terminology (CPT) codes, during the surveillance period. An automated search strategy using the Duke Enterprise Data Unified Content Explorer, a research portal developed to provide access to clinical data stored in the data repository for the EMR, was used at DUH and DRH. 24 All cases identified by EMR screening were confirmed by review of imaging studies and clinical records by trained research staff and clinicians. Cases were also identified by review of all autopsy records at DUH. Patients living outside Durham County at time of diagnosis, or at time of death for autopsy cases, were not included. A detailed description of the surveillance strategy, including the relevant ICD‐9 and CPT codes used to identify patients with VTE, has been previously reported. 24
2.1.2. Definition of DVT and PE
Deep vein thrombosis included all thrombotic events involving the inferior vena cava (IVC); the deep veins of the pelvis and proximal and distal lower extremity; the superior vena cava (SVC); and the deep veins of the upper thorax, neck, and upper extremity. PE included all thromboembolic events involving the pulmonary artery and its branches. VTEs were classified as being definite (identified by diagnostic imaging data as an acute DVT and/or PE, or by autopsy report), probable (identified by inconclusive imaging data but evidence for acute clinical management by thrombectomy, thrombolysis, and/or IVC filter placement), or possible (clinical diagnosis of DVT and/or PE and treatment with anticoagulation). DVT and PE were characterized as symptomatic or not, based on EMR review. Prior DVT and/or PE were defined as occurring before the surveillance period.
2.1.3. Data collection
The following information was collected from the medical record for each patient with a new VTE during the surveillance period: (1) demographic information; (2) comorbid medical conditions; (3) characteristics of the VTE, including location of DVT/PE; provoked versus unprovoked; and symptomatic or incidental finding; (4) risk factors for VTE; (5) prior VTE; (6) treatment; and (7) outcomes.
Risk factors were separated into major (e.g., active cancer, hospitalization, and/or major surgery/procedure within 90 days, and prolonged immobility, defined as confinement to bed for 3 or more consecutive days) and minor (e.g., minor surgery, estrogen‐containing oral contraceptives, or hormone replacement therapy). 25 Active cancer was defined as having a positive pathology report for cancer and current evidence of disease (based on imaging, biopsy, etc.). 25 , 26 Major surgery was defined as a surgery or procedure that lasted 60 min or more, and minor surgery lasted less than 60 min. Other minor risk factors included obesity (body mass index [BMI] of 30 or higher), current tobacco use, extended travel (more than 4 h), pregnancy and the postpartum period, central venous catheter, transvenous pacemaker, systemic corticosteroids, myocardial infarction within previous 3 months, ischemic stroke, nephrotic syndrome, superficial vein thrombosis/thrombophlebitis, nonfracture trauma o‐r tissue injury, and an active autoimmune disorder. 25 , 27 , 28 , 29 , 30 , 31 Patients with ≥1 major and/or minor risk factors were considered to have a provoked VTE, in contrast to patients with no risk factors, who were considered to have an unprovoked VTE.
Inpatient VTEs were defined as thrombotic events that were diagnosed 2 or more days after admission and while the patient was still in the hospital. Outpatient VTEs were defined as thrombotic events diagnosed either (i) within 2 days of hospital admission (i.e., clinically suspected on admission) or (ii) in the outpatient setting, including in the emergency department and observation unit but not admitted to the hospital. Hospital‐associated VTE included (i) all inpatient VTE, and (ii) the subset of outpatient VTE associated with a prior hospitalization within 90 days of the VTE diagnosis.
Outcome data were collected by reviewing the EMR at prespecified intervals after diagnosis. All events occurring within 7 days of diagnosis were defined as occurring at the time of diagnosis. Events occurring more than 7 days after diagnosis and by the 6‐month time point were included in the outcomes at 6 months, and all events occurring after the 6‐month time point and by the 12‐month time point were included in the outcomes at 12 months. Data collected included whether anticoagulant therapy was still being administered and whether the patient had developed a recurrent VTE, defined as a new DVT/PE or clear demonstration of thrombosis extension on a subsequent imaging study; major bleeding complications as defined by the ISTH 32 ; and/or all‐cause death. Patients who were not seen by a prespecified time point, and when death could not be confirmed, were considered lost to follow‐up and not included in the subsequent analyses.
All data were reviewed by research staff for accuracy, duplicate entries removed, and final data entered into a REDCap database before analysis. 33
2.1.4. Data analysis
Summary statistics, means/standard deviations/medians/ranges for continuous variables, and counts/percentages for categorical variables were used to summarize data. Comparison between groups and continuous variables were conducted using either t test or the Kruskal‐Wallis test, depending on whether the variables were normally or nonnormally distributed, respectively. Associations between any categorical variables were examined using chi‐squared tests or Fisher's exact test. No adjustment was made for multiple testing. Statistical significance was examined at alpha = 0.05. The statistical significance of the difference in incidence rates between any two groups was assessed using Fisher's exact test as well as based on Z‐score test. Statistical analyses were performed using SAS 9.4 statistical software (SAS Institute Inc.).
2.1.5. Institutional Review Board Review
This population‐based surveillance study, a public health activity performed according to guidance from the CDC and the U.S. Department of Health and Human Services, 34 was deemed exempt from review by the Duke Institutional Review Board, and patient informed consent was waived.
3. RESULTS
3.1. Patient cohort
A total of 987 patients were diagnosed with VTE affecting the pelvis, limbs, neck, upper thorax, SVC, and/or IVC while living in Durham County during the surveillance period (Table 1). Twelve patients were diagnosed with VTE at autopsy only; an additional five patients were diagnosed before death by imaging studies, with autopsy confirming the imaging findings. Thirty‐two patients were diagnosed with VTE at the Durham VAMC, four of whom were separately identified through surveillance at DUHS. All other patients were diagnosed at DUH or DRH. A total of 975 patients met criteria for definite VTE (98.8%), with a single probable event (0.1%) and 11 possible events (1.1%), primarily related to a diagnosis made at another location, with the report unavailable to providers at the Durham County facility.
TABLE 1.
Baseline characteristics of all patients with VTE in Durham County, North Carolina, 2012–2014
| Characteristic | N (Total n = 987) |
|---|---|
| Age, years, mean (range) | 60.2 (<1 to 101 ) |
| Sex, female, n (%) | 512 (51.9) |
| Race, n (%) | |
| Black | 508 (51.5) |
| White | 452 (45.8) |
| Asian | 4 (0.4) |
| Native Hawaiian or Pacific Islander | 3 (0.3) |
| American Indian or Alaska Native | 1 (0.1) |
| Unknown/Other | 19 (1.9) |
| BMI (n = 857), mean (SD) | 29.7 (9.5) |
| Resident at a long‐term health care facility, n (%) | 95 (9.6) |
| Diagnosis at autopsy, n (%) | 17 (1.7) |
| Diagnosis at autopsy only | 12 (1.2) |
| History of prior venous thromboembolism, n (%) | 183 (18.5) |
Abbreviations: BMI, body mass index; VTE, venous thromboembolism.
Among individuals diagnosed with VTE, slightly more than half were women, and the mean age was 60.2 years (Table 1). About 10% were residents in a long‐term care facility, and 18.5% had a history of VTE before the surveillance period (Table 1). Most individuals were Black or White, representing over 97% of all patients with VTE during the surveillance period. Only four individuals were Asian (0.4%), and most of the remaining individuals were identified as unknown race or “other” (Table 1).
3.2. Comorbid conditions and VTE characteristics
Black patients were younger than White patients with VTE, and were more likely to have clinical diagnoses of hypertension, renal insufficiency, or obesity, whereas White patients were more likely to have an underlying respiratory disease (Table 2). Almost half the patients had PE (with or without DVT) at presentation, and most patients with PE were symptomatic at time of diagnosis. PE was an incidental finding in 7.4% of patients. Most DVTs involved the lower extremities, although 25% affected an upper extremity (Table 2).
TABLE 2.
Clinical characteristics of patients with VTE
| Condition | Total (n = 987) | Black (n = 508) | White (n = 452) | p |
|---|---|---|---|---|
| Age, years, mean (range) | 60.2 (<1–101) | 58.3 (1–99) | 63.2 (<1–101) | <0.0001* |
| Female sex, n (%) | 512 (51.9) | 273 (53.7) | 224 (49.6) | 0.20 |
| Comorbid conditions, n (%) | ||||
| Diabetes mellitus | 249 (25.2) | 141 (27.8) | 102 (22.6) | 0.08 |
| Heart disease | 175 (17.7) | 80 (15.7) | 91 (20.1) | 0.06 |
| Congestive heart failure | 102 (10.3) | 51 (10.0) | 49 (10.8) | 0.69 |
| Hemoglobinopathy | 11 (1.1) | 9 (1.8) | 1 (0.2) | 0.02 |
| Hypertension | 582 (59.0) | 330 (65.0) | 243 (53.9) | 0.0004 |
| Renal insufficiency | 116 (11.8) | 82 (16.1) | 32 (7.1) | <0.0001 |
| Obesity | 243 (24.6) | 146 (28.7) | 93 (20.6) | 0.004 |
| Peripheral arterial disease | 32 (3.2) | 17 (3.3) | 15 (3.3) | 0.98 |
| Respiratory/pulmonary disease | 240 (24.3) | 109 (21.5) | 126 (27.9) | 0.02 |
| Venous varicosities | 8 (0.8) | 2 (0.4) | 5 (1.1) | 0.20 |
| Venous thromboembolic event, n (%) | ||||
| PE (with or without DVT) | 461 (46.7) | 246 (48.4%) | 203 (44.9%) | 0.28 |
| Symptomatic | 427 (92.6) | 229 (93.1%) | 189 (93.1%) | 0.99 |
| DVT (with or without PE) | 638 (64.6) | 318 (62.6%) | 301 (66.6%) | 0.20 |
| Symptomatic | 585 (91.7) | 289 (90.9%) | 279 (92.7%) | 0.41 |
| Location a | ||||
| Right upper extremity | 100 (10.1) | 51 (10.0) | 45 (10.0) | 0.97 |
| Left upper extremity | 62 (6.3) | 28 (5.5) | 30 (6.6) | 0.47 |
| Right lower extremity | 220 (22.3) | 110 (21.7) | 104 (23.0) | 0.61 |
| Left lower extremity | 260 (26.3) | 125 (24.6) | 132 (29.2) | 0.11 |
| Other b | 41 (4.2) | 22 (4.3) | 16 (3.5) | 0.52 |
Note: Diagnoses are clinical diagnoses applied to the patient. *All p values, comparing results for Black and White patients, were calculated by chi‐square test for homogeneity except for those marked by (*), which were determined by the Kruskal–Wallis test.
Abbreviations: DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Individual patients may have had more than one location of DVT on presentation.
Other thrombotic events included: internal jugular (24); inferior vena cava/pelvic (12); superior vena cava/right atrium (3).
3.3. Hospital‐associated VTE
One‐hundred sixty‐seven inpatients were hospitalized at one of the three hospitals in Durham County for ≥2 days before being diagnosed with VTE (16.9% of all patients with VTE; Table 3). More than half were not receiving thromboprophylaxis, pharmacologic or mechanical, at the time of diagnosis. Aspirin was frequently administered, although we did not determine whether this was for VTE prophylaxis or an alternative clinical indication (Table 3).
TABLE 3.
Patients hospitalized for ≥2 days at the time of diagnosis with a new VTE
| Characteristic | Total (n = 167) | Black (n = 80) | White (n = 75) | p |
|---|---|---|---|---|
| Venous thromboembolic event, n (%) | ||||
| DVT only | 117 (70.1) | 54 (67.5) | 54 (72.0) | 0.54 |
| PE with or without DVT | 50 (29.9) | 26 (32.5) | 21 (28.0) | |
| Days from admission, mean (SD) | 11.3 (10.9) | 10.8 (9.8) | 12.1 (12.5) | 0.77† |
| Antithrombotic strategy at time of event, n (%) a | 76 (45.5) | 37 (46.3) | 34 (45.3) | 0.91 |
| Intermittent compression device | 4 (5.3) | 4 (10.8) | 0 | |
| Graduated compression stockings | 3 (3.9) | 1 (2.7) | 2 (5.9) | |
| Enoxaparin | 10 (13.2) | 2 (5.4) | 6 (17.6) | |
| Unfractionated heparin | 13 (17.1) | 5 (13.5) | 6 (17.6) | |
| Fondaparinux | 1 (1.3) | 1 (2.7) | 0 | |
| Warfarin | 7 (9.2) | 2 (5.4) | 5 (14.7) | |
| Aspirin | 50 (65.8) | 27 (73.0) | 22 (64.7) | |
| Other | 3 (3.9) | 2 (5.4) | 1 (2.9) | |
Note: Defined as hospitalized for 2 days or longer, and still in the hospital, at the time of the diagnosis by imaging data. All p values, comparing results for Black and White patients, were calculated by Chi square test for homogeneity except for those marked by (†), which were determined by the Kruskal Wallis test.
Abbreviations: DVT, deep venous thrombosis; PE, pulmonary embolism; SD, standard deviation; VTE, venous thromboembolism.
Patients could be on one or more of the treatment options listed below at the time of diagnosis with VTE.
An additional 271 patients (27.5% of all patients with VTE) who were diagnosed in the outpatient setting had been hospitalized within 90 days of VTE diagnosis (Table 4), resulting in 438 patients (44.4%) with new VTE in Durham County during the surveillance period being hospital‐associated events. Most prior hospitalizations were for nonsurgical indications (Table 4).
TABLE 4.
Major and minor risk factors for VTE in patients diagnosed with VTE in the outpatient setting
| Characteristic | Total (n = 820) | Black (n = 428) | White (n = 377) | p |
|---|---|---|---|---|
| Major risk factors, n (%) | ||||
| Active cancer | 151/744 (20.3) | 86/398 (21.9) | 62/332 (18.7) | 0.33 |
| Major surgery | 130/743 (17.5) | 64/397 (16.1) | 64/332 (19.3) | 0.22 |
| Hospitalization within 90 days | 271/741 (36.6) | 148/396 (37.4) | 117/331 (35.3) | 0.57 |
| Nonsurgical hospitalization | 186/271 (68.6) | 105/148 (70.9) | 76/117 (20.2) | 0.14 |
| Prolonged or permanent immobility | 179/743 (24.1) | 90/397 (22.7) | 85/332 (25.6) | 0.36 |
| No major risk factors, n (%) | 401 (48.9) | 201 (47) | 193 (51.2) | 0.23 |
| Minor risk factors, n (%) | ||||
| Minor surgery | 3/743 (4.7) | 19/397 (4.8) | 13/332 (3.9) | 0.22 |
| Hormonal contraceptive therapy a | 36/389 (9.3) | 18/214 (8.4) | 16/168 (9.5) | 0.70 |
| Hormonal replacement therapy a | 3/388 (0.8) | 1/213 (0.5) | 2/168 (1.2) | 0.43 |
| Pregnancy or postpartum a | 12/424 (2.8) | 7/228 (3.1) | 5/188 (2.7) | 0.80 |
| BMI ≥30 | 343/744 (46.1) | 214/398 (53.8) | 124/332 (37.3) | <0.0001 |
| Current tobacco use | 142/744 (19.1) | 97/398 (24.4) | 45/332 (13.6) | 0.0002 |
| Extended travel (>4 h) | 54/738 (7.3) | 14/394 (3.6) | 39/330 (11.8) | <0.0001 |
| CVC within affected limb | 40/744 (5.4) | 26/398 (6.5) | 13/332 (3.9) | 0.12 |
| Transvenous pacemaker | 15/739 (2.0) | 7/395 (1.8) | 8/330 (2.4) | 0.54 |
| Autoimmune disorder | 74/744 (9.9) | 46/398 (11.6) | 26/332 (7.8) | 0.09 |
| No major or minor risk factors | 76/820 (9.3) | 30/398 (7.5) | 45/322 (14.0) | <0.0001 |
Note: Patients included in this table were either (i) outpatients at the time of diagnosis, or (ii) diagnosed with VTE within 48 h of hospitalization. All p values, comparing results for Black and White patients, calculated by chi‐square test for homogeneity.
Abbreviations: BMI, body mass index; CVC, central venous catheter; VTE, venous thromboembolism.
Relevant to women only; for postpartum VTE, defined as within 12 weeks of delivery.
3.4. Other risk factors for VTE
A variety of additional risk factors were present in individuals diagnosed with VTE in the outpatient setting (Table 4). Approximately half had at least one major risk factor (Table 4). Estrogen‐containing oral contraceptives or hormonal replacement therapy were being used by 9.3% and 0.8% of women with VTE, respectively, and 2.8% of women were diagnosed while pregnant or during the postpartum period. Black patients were more likely to have a BMI ≥30 and current tobacco use, whereas White patients were more likely to have recently been on a trip with a travel time of more than 4 h (Table 4).
3.5. Prior VTE
One or more prior DVTs/PEs had occurred in 183 patients (18.5%) diagnosed with a new VTE during the surveillance period (Table 5). Most patients had a single prior event. Approximately half of the patients with prior VTE had one or more events that occurred in the same vascular distribution as the event diagnosed during the surveillance period (Table 5). For those patients in whom a date for the prior event could be identified (n = 99), the most recent event occurred a mean of 4.8 years (range, 1.8 months to 52 years) before the VTE diagnosed during the surveillance period. At the time of diagnosis with VTE, 9.5% of patients were being treated with an anticoagulant, but less than half were being treated for prior VTE (Table 5).
TABLE 5.
Prior VTE and treatment in patients presenting with a new VTE during the surveillance period
| Characteristic | Total (n = 987) | Black (n = 508) | White (n = 452) | p |
|---|---|---|---|---|
| Prior VTE | ||||
| History of prior PE/DVT, n (%) | 183 (18.5) | 97 (19.1) | 83 (18.5) | 0.77 |
| Number of prior PE/DVT episodes | ||||
| N = 1 | 143 (14.5) | 71 (14.0) | 70 (15.5) | 0.30 |
| N = 2 | 28 (2.8) | 17 (3.3) | 11 (2.4) | |
| N = 3 | 5 (0.5) | 4 (0.8) | 0 (0.0) | |
| N = 4 | 5 (0.5) | 4 (0.8) | 1 (0.2) | |
| N = 5 | 2 (0.2) | 1 (0.2) | 1 (0.2) | |
| Prior event in same location as current VTE, a n (%) | 92 (50.3) | 48 (49.5) | 43 (51.8) | 0.48 |
| Antithrombotic therapy on presentation with current VTE, n (%) | ||||
| Anticoagulant therapy (any drug) | 94 (9.5) | 48 (9.4) | 44 (9.7) | 0.88 |
| For prior VTE | 42 (4.3) | 24 (4.7) | 17 (3.8) | 0.46 |
| Antiplatelet therapy (any drug) | 239 (24.2) | 126 (24.8) | 110 (24.3) | 0.87 |
| Initial treatment for current event, n (%) | ||||
|---|---|---|---|---|
| Anticoagulant therapy | 894 (90.6) | 468 (92.1) | 404 (89.4) | 0.14 |
| Warfarin b | 525 (53.2) | 286 (56.3) | 224 (49.6) | 0.04 |
| Rivaroxaban c | 63 (6.4) | 26 (5.1) | 36 (8.0) | 0.07 |
| Enoxaparin only d | 262 (26.5) | 133 (26.2) | 123 (27.2) | 0.72 |
| Other e | 20 (2.0) | 7 (1.4) | 12 (2.7) | 0.15 |
| Thrombolytic therapy, n (%) | 20 (2.0) | 10 (2.0) | 9 (2.0) | 0.57 |
| IVC filter placement, n (%) | 69 (7.0) | 41 (8.1) | 26 (5.8) | 0.30 |
| Thrombectomy/embolectomy, n (%) | 7 (0.7) | 4 (0.8) | 3 (0.7) | 0.82 |
Note: All p values compare characteristics in Black compared to White patients, and were determined by chi‐ squared test for homogeneity.
Abbreviations: DVT, deep vein thrombosis; IVC, inferior vena cava; PE, pulmonary embolism; VTE, venous thromboembolism.
Same distribution defined as being in the same limb for patients with a new DVT, or a prior PE in patients with a new PE, and could be any prior event.
Patient typically started on enoxaparin or unfractionated heparin, then transitioned to warfarin.
Patient may have initially received enoxaparin or unfractionated heparin; five of these patients also received warfarin, either before or after rivaroxaban.
Patients may have also received unfractionated heparin but did not receive warfarin or rivaroxaban during the initial encounter.
Includes argatroban (n = 3), apixaban (n = 4), bivalirudin (n = 3), dabigatran (n = 3), dalteparin (n = 1), and fondaparinux (n = 6).
3.6. Annual incidence of VTE
Given the estimated population in Durham County at the start of the surveillance period, the annual rate for VTE was 1.76 per 1000 individuals (including patients with initial and recurrent events). Removing patients with prior VTE, the annual incidence of VTE during the surveillance period was 1.44 per 1000 individuals living in Durham County. A total of 411 Black, 369 White, and 4 Asian individuals were diagnosed with a first event during the 2‐year surveillance period, for an annual incidence of 1.98 per 1000 Black, 1.27 per 1000 White, and 0.18 per 1000 Asian individuals. The increased incidence of VTE in Black individuals compared to White individuals was observed across all adult age groups (Figure 1).
FIGURE 1.
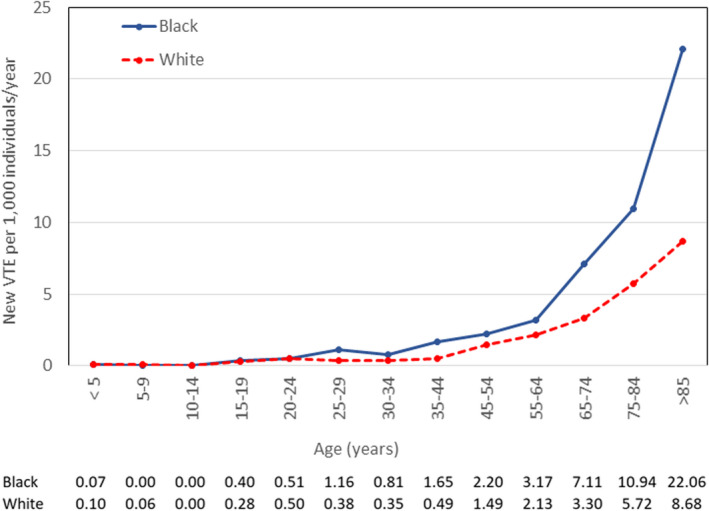
Incidence of VTE by age group among Black individuals and White individuals in Durham County, North Carolina, 2012–2014. Incidence for Black patients is shown with the solid line, and for White patients with the dashed line. The values for incidence of new VTE per 1000 individuals per year for each age range are shown at the bottom of the figure
3.7. Treatment
Most patients were treated with an anticoagulant, with warfarin being used most commonly, slightly more frequently in Black individuals than White individuals (56.3% vs. 49.6%, p = 0.04; Table 5). Rivaroxaban was the most commonly used direct oral anticoagulant, reflecting approval by the Food and Drug Administration during the surveillance period. Slightly more than a quarter of the patients were treated with enoxaparin only at the time of initial data collection (Table 5). Few patients received thrombolytic therapy or thrombectomy, but an IVC filter was implanted in 7% of patients (Table 5).
3.8. Outcomes
Within 7 days of diagnosis, hemorrhagic complications occurred in 22 patients (2.2%) and recurrent thromboembolic events in 25 patients (2.5%; Table 6). In addition to the 12 patients found to have VTE at autopsy, 31 patients died within 7 days of VTE diagnosis (Table 6). At the 6‐month time point, follow‐up information was available for 817 patients (82.8% of the total population with VTE; Table 6). The proportions of men compared to women, and Black patients compared to White patients, without a 6‐month encounter were similar (Table S1). Almost two‐thirds were still taking anticoagulant therapy at the time of the first 6‐month follow‐up (Table 6). Black patients were more likely to have recurrent VTE than White patients during this period (9.4% vs. 4.1%, p = 0.01; Table 6), a difference driven by a higher risk of recurrent VTE among Black male patients than White male patients (25/191 Black males compared to 13/205 White males). There were otherwise no differences in the sexes between the two groups. An additional 147 patients died during the first 6 months after the initial period (Table 6).
TABLE 6.
Outcome events occurring around the time of diagnosis with VTE and at 6 and 12 months after diagnosis
| Characteristic | Total (n = 987) | Black (n = 508) | White (n = 452) | p |
|---|---|---|---|---|
| Outcomes within 7 days of diagnosis, n (%) | ||||
| Major bleeding | 22 (2.2) | 11 (2.2) | 8 (1.8) | 0.66 |
| Heparin‐induced thrombocytopenia | 6 (0.6) | 4 (0.8) | 2 (0.4) | 0.50 |
| Recurrent VTE | 25 (2.5) | 13 (2.6) | 12 (2.7) | 0.93 |
| Death | 43 (4.4) | 28 (5.5) | 15 (3.3) | 0.10 |
| 6‐month outcomes, n (%) | ||||
| Individuals known alive at the beginning of the first 6‐month block of time | 944 (95.6) | 480 (94.5) | 437 (96.7) | |
| Follow‐up encounter by 6 months | 817 (82.8) | 407 (80.1) | 388 (85.8) | 0.14 |
| Patients taking anticoagulant therapy | 544/817 (66.6) | 268/407 (65.8) | 264/388 (68.0) | 0.51 |
| Major bleeding | 34/817 (3.6) | 18/407 (4.4) | 15/388 (3.9) | 0.69 |
| Recurrent VTE | 54/806 (6.7) | 38/405 (9.4) | 16/384 (4.2) | 0.01 |
| Deaths since initial encounter | 147/817 (18.0) | 80/407 (19.7) | 62/388 (16.0) | 0.30 |
| 12‐month outcomes, n (%) | ||||
| Individuals known alive at the beginning of the second 6‐month block | 670 (67.9) | 327 (64.4) | 326 (72.1) | |
| Follow‐up encounter by 12 months | 596 (60.4) | 307 (60.4) | 274 (60.6) | 0.24 |
| Patients taking anticoagulant therapy | 302/595 (50.8) | 159/307 (51.8) | 140/273 (51.3) | 0.90 |
| Major bleeding | 12/596 (1.8) | 5/307 (1.6) | 7/274 (2.6) | 0.43 |
| Recurrent VTE | 24/596 (3.6) | 12/307 (3.9) | 12/274 (4.4) | 0.78 |
| Deaths since 6‐month encounter | 34/596 (5.7) | 15/307 (3.6) | 19/274 (5.8) | 0.37 |
Note: All p values determined by chi‐squared testing. For calculation of the percentages, the denominator is the number of patients at baseline in each column unless provided with the entry.
Abbreviation: VTE, venous thromboembolism.
At the 12‐month time point, follow‐up information was available for 596 patients (60.4% of the total population with VTE; Table 6). The proportions of men compared to women, and Black patients compared to White patients, without a 12‐month encounter were similar (Table S1). At the 12‐month follow‐up, 302 patients (50.8%) were still taking an anticoagulant. There were no differences in the proportions of Black and White patients who were diagnosed with major bleeding events, recurrent VTE, and/or who died during the second 6‐month interval (Table 6). In total, 224 patients died within 1 year of the VTE event (28.5% of patients for whom death or follow‐up through the 12‐month surveillance period could be confirmed).
4. DISCUSSION
A key objective of this study was to determine whether our VTE surveillance program could provide incidence and overall rate data for VTE in Durham County, North Carolina, as well as a means to identify comorbid conditions, potential risk factors, and document therapeutic management and outcomes. We identified several areas where adaptations to the program could provide additional information to monitor results associated with management changes. This report summarizes data collected during this 2‐year surveillance period.
The annual incidence of VTE in Durham County was 1.44 per 1000 individuals, similar to previously reported rates, which have ranged from 1.04 to 2.47 per 1000. 1 , 2 , 5 , 7 , 8 , 9 , 21 Hospitalization, particularly in association with major surgery, is one of the strongest risk factors for VTE. 26 Almost 17% of the patients in our surveillance cohort were hospitalized at the time of diagnosis, and one‐third of individuals diagnosed in the outpatient setting had been hospitalized within 90 days of diagnosis. Multiple studies have documented the value of thromboprophylaxis during hospitalization, yet many patients still do not receive recommended preventive therapy. 35 , 36 Of the patients who were diagnosed with VTE while hospitalized, half were not receiving thromboprophylaxis at the time of diagnosis (Table 3). The increased risk for VTE associated with hospitalization has been shown to persist for up to several months after discharge, 37 , 38 and certain high‐risk patients may benefit from an extended course of thromboprophylaxis following discharge from the hospital. 39 , 40 These results confirm that our surveillance program captured a population of VTE patients similar to prior reported cohorts, and that hospital‐associated VTE continues to represent a substantial number of VTE patients.
Racial differences in the incidence of VTE have been noted in several studies, 9 , 41 , 42 , 43 although one study observed that the rate in Black individuals varied in different regions within the United States. 42 During the surveillance period, we found that Black individuals in Durham County had a higher rate of VTE compared to White individuals (1.98 per 1000 vs. 1.27 per 1000). There were no differences related to the proportions of Black and White individuals with hospital‐associated VTE, however, or whether thromboprophylaxis was used or not. We found that Black individuals with VTE were younger, and were more likely to have clinical diagnoses of hypertension and kidney disease as well as a higher BMI than White individuals with VTE. We had previously observed increased rates of hypertension, renal disease, and obesity in Black patients with VTE compared to White patients in a cross‐sectional study conducted through the CDC Thrombosis and Hemostasis Research and Prevention Network. 44 This increased frequency of VTE risk factors, including hypertension and a higher BMI, has been identified as contributing to the increased frequency of VTE in Black individuals. 45
Several studies have reported a lower rate of VTE in Asian individuals, with event rates in Asians living in the United States ranging from 0.29 to 0.63 per 1000. 9 , 41 A recent systematic review reported that the incidence of VTE in Asian populations was approximately 15%–20% of the level reported in Western countries. 46 The observed rate in Asian individuals was 14% of what was observed in White individuals in Durham County, consistent with this finding. We also observed an increase in the risk for VTE with aging, as noted in prior studies, 1 , 2 , 4 , 5 , 9 , 46 which occurred similarly for Black and White individuals. There were too few individuals from other races identified during the surveillance period to include in this analysis.
We also documented that a surveillance program could be enhanced by monitoring therapeutic management and clinical outcomes in patients with VTE. Anticoagulant therapy is the preferred treatment for patients with VTE, 47 , 48 and over 90% of patients in our cohort were treated this way. Unfractionated heparin or a low‐molecular‐weight heparin bridged to warfarin was the primary therapeutic approach for most patients. Several of the direct oral anticoagulants were approved for treatment of VTE during the surveillance period, and we observed an increased use of these agents over time. IVC filters were implanted in 7% of patients, most commonly in those not receiving anticoagulant therapy or with bleeding complications, consistent with guideline recommendations. 47 More aggressive interventions were infrequently used.
A primary variable that is critical for decision making concerning the duration of anticoagulant therapy is whether the VTE is considered provoked, particularly by a transient risk factor, or unprovoked. 25 , 47 In our cohort, about half had one (or more) transient major risk factors, including recent hospitalization, as noted above. However, a significant proportion of the patients had chronic major risk factors, including cancer and/or permanent or prolonged immobility, for which continued anticoagulation is typically recommended (Table 4). Many patients had one or more minor transient risk factors, primarily nonsurgical, which are associated with a higher risk for recurrent VTE than surgical transient risk factors. 49 Incorporating a strategy that targets key time points for decisions concerning duration of therapy in addition to identifying risk factors for recurrence would facilitate decision making concerning duration of anticoagulant therapy for individual patients. Similarly, this approach could integrate follow‐up to optimize the rate of retrieval of IVC filters, given the continued problem of not retrieving filters originally placed for a temporary indication. 50
Recurrent thromboembolism, major bleeding, and death are common complications following the diagnosis of VTE. 1 , 4 , 8 , 9 , 51 Several studies have described differences in the frequency of adverse outcomes in Black and White patients with VTE. 52 One study reported that Black individuals with PE were more likely to present with a more clinically severe event, 53 and a second study noted that Black individuals were more likely to be hospitalized than White individuals with PE. 54 Although we did not collect information about hospitalization following a new diagnosis of PE, we did not observe a difference in the use of thrombolytic therapy or embolectomy in Black compared to White patients. Mortality has been reported to be higher in Black patients following VTE than in White patients in some studies, 55 , 56 but not others. 9 , 57 We did not observe a difference between Black and White patients with VTE in mortality or major bleeding over the 12 months following diagnosis. We did observe an increase in recurrent VTE in Black patients compared to White patients during the first 6‐month block following initial diagnosis, but this difference was not significant at the other time points or for all recurrent VTEs during the entire 12‐months of follow‐up. Black female patients have been reported to have an increased risk for recurrent VTE compared to White female patients, a difference not observed for male patients. 58 , 59 We observed more recurrent VTE in Black male patients compared to White male patients during the first 6‐month block following initial diagnosis, but otherwise no differences in recurrent VTE in Black and White patients by sex. Additional studies are needed to clarify differences in clinical outcomes in Black and White patients with VTE.
Strengths of our study include that we were able to combine a VTE surveillance strategy with efforts to monitor comorbid conditions, chronic and transient risk factors, prevention efforts, treatment, and outcomes. An EMR‐driven strategy that provides ongoing identification of new patients with VTE becomes a key component of this effort. Expanding to a nationwide surveillance program would require coordination between programs to ensure that new patients are uniquely identified, a problem that we observed in patients identified at both DUH and the Durham VAMC. Recent reports of the increased risk for VTE in hospitalized patients with COVID‐19 60 , 61 could be rapidly documented with a VTE surveillance program in place.
There were several limitations to our surveillance program that need to be addressed. In particular, while our surveillance program was designed around an electronic strategy, it still required considerable manual review and data collection to confirm all VTEs. 24 Our electronic search strategy used ICD‐9 and CPT codes, and concerns about the validity of using these codes to identify patients with VTE have been raised. 62 , 63 Some behavioral risk factors may be underreported in medical records, such as extended travel (>4 h). In addition, information about comorbid medical conditions was collected from diagnoses made in the medical record, which has the potential to introduce misclassification errors in the data set. Future strategies that incorporate natural language processing and machine learning may decrease the need for manual oversight, 64 , 65 , 66 allowing data extraction to be more efficient and more accurate. Another limitation is that a subset of individuals who live within Durham County will get their health care at a facility in another county, and would therefore not be captured in our surveillance program. Similarly, patients who lived within Durham County at the time of diagnosis but subsequently moved out of the county or transitioned their health care to a facility outside of Durham County would have been lost to follow‐up. A broader strategy with cross‐reference of patients with VTE across different health care systems would be required to address this limitation.
In conclusion, we have shown that implementation of a population‐based strategy VTE surveillance strategy can provide detailed data that can be integrated with strategies designed to improve patient outcomes.
AUTHOR CONTRIBUTIONS
I. Saber, A. Adamski, K. Abe, M. Beckman, N. Reyes, and T.L. Ortel contributed to the design of the study. I. Saber, R. Schulteis, B. Pendurthi Singh, A. Sitlinger, and E.H. Thames collected the surveillance data. All authors contributed to analysis and interpretation of the data. M. Kuchibhatla performed the statistical analyses. T.L. Ortel wrote the first draft of the manuscript. All authors reviewed and contributed to the final draft of the manuscript and approve of the submitted manuscript.
FUNDING INFORMATION
This article was supported by Grant/Cooperative Agreement U50DD000897 from the Centers for Disease Control and Prevention.
RELATIONSHIP DISCLOSURE
None of the authors have any conflicts of interest to report.
Supporting information
Table S1
ACKNOWLEDGMENTS
The authors thank Kristin Hudd‐Byrne, Kristen Bagby, RN, and Sheila Lambert‐Adams for assistance with manual review of the DUH/DRH EMR reports; and Alan Proia, MD, PhD, and Brenda Dudzinski for their assistance with review of the Duke autopsy reports. This article was supported by Grant/Cooperative Agreement U50DD000897 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Saber I, Adamski A, Kuchibhatla M, et al. Racial differences in venous thromboembolism: A surveillance program in Durham County, North Carolina. Res Pract Thromb Haemost. 2022;6:e12769. doi: 10.1002/rth2.12769
Handling Editor: Dr Cihan Ay
REFERENCES
- 1. Cushman M, Tsai AW, White RH, et al. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117(1):19‐25. [DOI] [PubMed] [Google Scholar]
- 2. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158(6):585‐593. [DOI] [PubMed] [Google Scholar]
- 3. Oger E. Incidence of venous thromboembolism: a community‐based study in Western France. EPI‐GETBP study group. Groupe d'Etude de la thrombose de Bretagne Occidentale. Thromb Haemost. 2000;83(5):657‐660. [PubMed] [Google Scholar]
- 4. Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population‐based study. J Thromb Haemost. 2007;5(4):692‐699. [DOI] [PubMed] [Google Scholar]
- 5. Puurunen MK, Gona PN, Larson MG, Murabito JM, Magnani JW, O'Donnell CJ. Epidemiology of venous thromboembolism in the Framingham heart study. Thromb Res. 2016;145:27‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spencer FA, Emery C, Lessard D, et al. The Worcester venous thromboembolism study: a population‐based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21(7):722‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delluc A, Tromeur C, Le Ven F, et al. Current incidence of venous thromboembolism and comparison with 1998: a community‐based study in Western France. Thromb Haemost. 2016;116(5):967‐974. [DOI] [PubMed] [Google Scholar]
- 8. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. Secular trends in incidence and mortality of acute venous thromboembolism: the AB‐VTE population‐based study. Am J Med. 2016;129(8):879.e19‐879.e25. [DOI] [PubMed] [Google Scholar]
- 9. Wendelboe AM, Campbell J, Ding K, et al. Incidence of venous thromboembolism in a racially diverse population of Oklahoma County, Oklahoma. Thromb Haemost. 2021;121(6):816‐825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350(22):2257‐2264. [DOI] [PubMed] [Google Scholar]
- 11. Ende‐Verhaar YM, Cannegieter SC, Vonk Noordegraaf A, et al. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49(2):1601792. [DOI] [PubMed] [Google Scholar]
- 12. Roumen‐Klappe EM, den Heijer M, Janssen MC, van der Vleuten C, Thien T, Wollersheim H. The post‐thrombotic syndrome: incidence and prognostic value of non‐invasive venous examinations in a six‐year follow‐up study. Thromb Haemost. 2005;94(4):825‐830. [DOI] [PubMed] [Google Scholar]
- 13. Ende‐Verhaar YM, Tick LW, Klok FA, et al. Post‐thrombotic syndrome: short and long‐term incidence and risk factors. Thromb Res. 2019;177:102‐109. [DOI] [PubMed] [Google Scholar]
- 14. Kahn SR, Kearon C, Julian JA, et al. Predictors of the post‐thrombotic syndrome during long‐term treatment of proximal deep vein thrombosis. J Thromb Haemost. 2005;3(4):718‐723. [DOI] [PubMed] [Google Scholar]
- 15. Boutitie F, Pinede L, Schulman S, et al. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. BMJ. 2011;342:d3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007;92(2):199‐205. [DOI] [PubMed] [Google Scholar]
- 17. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital‐acquired and preventable costs utilising long‐term attack rates. Thromb Haemost. 2012;108(2):291‐302. [DOI] [PubMed] [Google Scholar]
- 19. (HHS) UDoHaHS . Surgeon General's call to action to prevent deep vein thrombosis and pulmonary embolism. 2008. Accessed June 01, 2022. https://www.ncbi.nlm.nih.gov/books/NBK44178/ [PubMed]
- 20. Raskob GE, Silverstein R, Bratzler DW, Heit JA, White RH. Surveillance for deep vein thrombosis and pulmonary embolism: recommendations from a national workshop. Am J Prev Med. 2010;38(4 suppl):S502‐S509. [DOI] [PubMed] [Google Scholar]
- 21. Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985‐2009). Am J Med. 2014;127(9):829‐39 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dentali F, Ageno W, Pomero F, Fenoglio L, Squizzato A, Bonzini M. Time trends and case fatality rate of in‐hospital treated pulmonary embolism during 11 years of observation in northwestern Italy. Thromb Haemost. 2016;115(2):399‐405. [DOI] [PubMed] [Google Scholar]
- 24. Ortel TL, Arnold K, Beckman M, et al. Design and implementation of a comprehensive surveillance system for venous thromboembolism in a defined region using electronic and manual approaches. Appl Clin Inform. 2019;10(3):552‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kearon C, Ageno W, Cannegieter SC, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost. 2016;14(7):1480‐1483. [DOI] [PubMed] [Google Scholar]
- 26. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Arch Intern Med. 2000;160(6):809‐815. [DOI] [PubMed] [Google Scholar]
- 27. Konstantinides SV, Meyer G, Becattini C, et al. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543‐603. [DOI] [PubMed] [Google Scholar]
- 28. Orsi FA, Lijfering WM, Geersing GJ, et al. Glucocorticoid use and risk of first and recurrent venous thromboembolism: self‐controlled case‐series and cohort study. Br J Haematol. 2021;193(6):1194‐1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogers MA, Levine DA, Blumberg N, Flanders SA, Chopra V, Langa KM. Triggers of hospitalization for venous thromboembolism. Circulation. 2012;125(17):2092‐2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahmoodi BK, ten Kate MK, Waanders F, et al. High absolute risks and predictors of venous and arterial thromboembolic events in patients with nephrotic syndrome: results from a large retrospective cohort study. Circulation. 2008;117(2):224‐230. [DOI] [PubMed] [Google Scholar]
- 31. Mi Y, Yan S, Lu Y, Liang Y, Li C. Venous thromboembolism has the same risk factors as atherosclerosis: a PRISMA‐compliant systemic review and meta‐analysis. Medicine (Baltimore). 2016;95(32):e4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost. 2005;3(4):692‐694. [DOI] [PubMed] [Google Scholar]
- 33. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)‐‐a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention . HIPAA privacy rule and public health: Guidance from CDC and the U.S. Department of Health and Human Services. Vol. 52. MMWR; 2003:1‐17. [PubMed] [Google Scholar]
- 35. Cohen AT, Tapson VF, Bergmann JF, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross‐sectional study. Lancet. 2008;371(9610):387‐394. [DOI] [PubMed] [Google Scholar]
- 36. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the international medical Prevention registry on venous thromboembolism. Chest. 2007;132(3):936‐945. [DOI] [PubMed] [Google Scholar]
- 37. Spencer FA, Lessard D, Emery C, Reed G, Goldberg RJ. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167(14):1471‐1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Amin AN, Varker H, Princic N, Lin J, Thompson S, Johnston S. Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med. 2012;7(3):231‐238. [DOI] [PubMed] [Google Scholar]
- 39. Chiasakul T, Evans CR, Spyropoulos AC, Raskob G, Crowther M, Cuker A. Extended vs. standard‐duration thromboprophylaxis in acutely ill medical patients: a systematic review and meta‐analysis. Thromb Res. 2019;184:58‐61. [DOI] [PubMed] [Google Scholar]
- 40. Bajaj NS, Vaduganathan M, Qamar A, et al. Extended prophylaxis for venous thromboembolism after hospitalization for medical illness: a trial sequential and cumulative meta‐analysis. PLoS Med. 2019;16(4):e1002797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. White RH, Zhou H, Murin S, Harvey D. Effect of ethnicity and gender on the incidence of venous thromboembolism in a diverse population in California in 1996. Thromb Haemost. 2005;93(2):298‐305. [DOI] [PubMed] [Google Scholar]
- 42. Zakai NA, McClure LA, Judd SE, et al. Racial and regional differences in venous thromboembolism in the United States in 3 cohorts. Circulation. 2014;129(14):1502‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schneider D, Lilienfeld DE, Im W. The epidemiology of pulmonary embolism: racial contrasts in incidence and in‐hospital case fatality. J Natl Med Assoc. 2006;98(12):1967‐1972. [PMC free article] [PubMed] [Google Scholar]
- 44. Heit JA, Beckman MG, Bockenstedt PL, et al. Comparison of characteristics from White‐ and black‐Americans with venous thromboembolism: a cross‐sectional study. Am J Hematol. 2010;85(7):467‐471. [DOI] [PubMed] [Google Scholar]
- 45. Folsom AR, Basu S, Hong CP, et al. Reasons for differences in the incidence of venous thromboembolism in black versus White Americans. Am J Med. 2019;132(8):970‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee LH, Gallus A, Jindal R, Wang C, Wu CC. Incidence of venous thromboembolism in Asian populations: a systematic review. Thromb Haemost. 2017;117(12):2243‐2260. [DOI] [PubMed] [Google Scholar]
- 47. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence‐based clinical practice guidelines. Chest. 2012;141(2 suppl):e419S‐e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4(19):4693‐4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010;170(19):1710‐1716. [DOI] [PubMed] [Google Scholar]
- 50. Jia Z, Fuller TA, McKinney JM, et al. Utility of retrievable inferior vena cava filters: a systematic literature review and analysis of the reasons for nonretrieval of filters with temporary indications. Cardiovasc Intervent Radiol. 2018;41(5):675‐682. [DOI] [PubMed] [Google Scholar]
- 51. Ageno W, Farjat A, Haas S, et al. Provoked versus unprovoked venous thromboembolism: findings from GARFIELD‐VTE. Res Pract Thromb Haemost. 2021;5(2):326‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aujesky D, Long JA, Fine MJ, Ibrahim SA. African American race was associated with an increased risk of complications following venous thromboembolism. J Clin Epidemiol. 2007;60(4):410‐416. [DOI] [PubMed] [Google Scholar]
- 53. Phillips AR, Reitz KM, Myers S, et al. Association between black race, clinical severity, and management of acute pulmonary embolism: a retrospective cohort study. J Am Heart Assoc. 2021;10(17):e021818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martin KA, McCabe ME, Feinglass J, Khan SS. Racial disparities exist across age groups in Illinois for pulmonary embolism hospitalizations. Arterioscler Thromb Vasc Biol. 2020;40(9):2338‐2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ibrahim SA, Stone RA, Obrosky DS, Sartorius J, Fine MJ, Aujesky D. Racial differences in 30‐day mortality for pulmonary embolism. Am J Public Health. 2006;96(12):2161‐2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barco S, Valerio L, Ageno W, et al. Age‐sex specific pulmonary embolism‐related mortality in the USA and Canada, 2000‐18: an analysis of the WHO mortality database and of the CDC multiple cause of death database. Lancet Respir Med. 2021;9(1):33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Minges KE, Bikdeli B, Wang Y, Attaran RR, Krumholz HM. National and regional trends in deep vein thrombosis hospitalization rates, discharge disposition, and outcomes for Medicare beneficiaries. Am J Med. 2018;131(10):1200‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. White RH, Dager WE, Zhou H, Murin S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006;96(3):267‐273. [DOI] [PubMed] [Google Scholar]
- 59. Burwen DR, Wu C, Cirillo D, et al. Venous thromboembolism incidence, recurrence, and mortality based on Women's Health Initiative data and medicare claims. Thromb Res. 2017;150:78‐85. [DOI] [PubMed] [Google Scholar]
- 60. Smilowitz NR, Subashchandran V, Yuriditsky E, et al. Thrombosis in hospitalized patients with viral respiratory infections versus COVID‐19. Am Heart J. 2021;231:93‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sridharan GK, Vegunta R, Rokkam VRP, et al. Venous thromboembolism in hospitalized COVID‐19 patients. Am J Ther. 2020;27(6):e599‐e610. [DOI] [PubMed] [Google Scholar]
- 62. Fang MC, Fan D, Sung SH, et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care. 2017;55(12):e137‐e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bilimoria KY, Chung J, Ju MH, et al. Evaluation of surveillance bias and the validity of the venous thromboembolism quality measure. JAMA. 2013;310(14):1482‐1489. [DOI] [PubMed] [Google Scholar]
- 64. Zheng S, Lu JJ, Ghasemzadeh N, Hayek SS, Quyyumi AA, Wang F. Effective information extraction framework for heterogeneous clinical reports using online machine learning and controlled vocabularies. JMIR Med Inform. 2017;5(2):e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dantes RB, Zheng S, Lu JJ, et al. Improved identification of venous thromboembolism from electronic medical records using a novel information extraction software platform. Med Care. 2018;56(9):e54‐e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selby LV, Narain WR, Russo A, Strong VE, Stetson P. Autonomous detection, grading, and reporting of postoperative complications using natural language processing. Surgery. 2018;164(6):1300‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


