Abstract
Use of long-acting injectable antiretroviral therapy depends on patient awareness, provider discussion, and patient willingness to use. We conducted a postvisit survey with patients at 3 HIV clinics in San Francisco, Chicago, and Atlanta in May 2021 to assess for inequities in these early implementation phases.
Keywords: long-acting injectable antiretroviral therapy, implementation science
In January 2021, the US Federal Drug Administration (FDA) approved the first long-acting injectable drug regimen to treat HIV, consisting of intramuscular injections of 2 antiretroviral drugs, cabotegravir and rilpivirine. This injectable drug regimen is indicated to replace daily oral therapy in adult people with HIV (PWH) who are virologically suppressed on a stable antiretroviral regimen with no history of treatment failure and no known or suspected resistance to either cabotegravir or rilpivirine [1]. Long-acting injectable antiretroviral therapy (LAI-ART) holds promise as a means of circumventing psychosocial and structural barriers that prevent daily oral ART adherence, for example, pill forgetfulness or fatigue, unintentional disclosure and stigmatization, substance use, psychiatric illness, and housing instability [2, 3].
Limited research on patient perspectives about LAI-ART demonstrates generally positive attitudes but also potential concerns about efficacy and side effects, the need for additional medical visits, dislike of needles, medical mistrust, and cost [4–7]. Clinic-based surveys before FDA approval of LAI cabotegravir and rilpivirine found that ∼60%–70% of patients were willing to try LAI-ART [8, 9], with estimates being higher in youth/young adults (88%) [10] and those who inject illicit drugs (∼80%) [8]. Providers acknowledge the potential benefits of LAI-ART, particularly for PWH who are not virally suppressed, but caution that missed injection appointments without resumption of oral ART could result in subtherapeutic medication levels and the possibility of drug resistance, especially given the prolonged pharmacokinetic tail of LAI-ART [11].
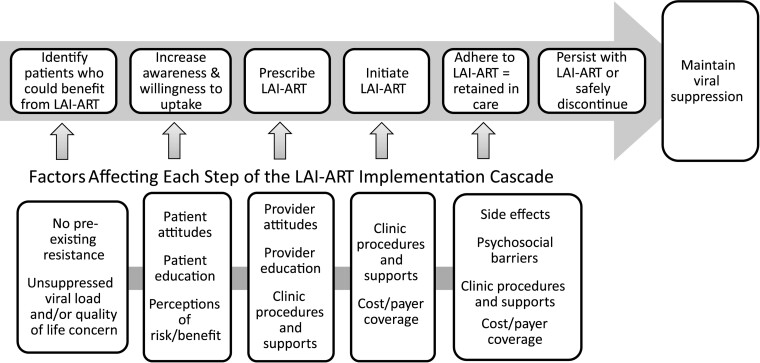
The advent of LAI-ART requires the scientific community to anticipate, understand, and address barriers and bottlenecks to implementation to realize their potential public health impact. While policy, clinic/health systems, and community-level factors play important roles in implementation, the patient–provider level of implementation is dependent on patient awareness, provider offer, and patient willingness to take up LAI-ART (Figure 1). Because past innovations in HIV pharmacotherapy have been delayed in reaching populations who may benefit the most, risking exacerbation of existing disparities [12, 13], we sought to understand “penetration” [14] into clinical visits during the early rollout of LAI-ART, monitor for inequities with regard to the patients with whom providers discuss LAI-ART, and examine whether certain patients appear less receptive to trying LAI-ART.
Figure 1.
The long-acting injectable antiretroviral therapy implementation cascade. Abbreviation: LAI-ART, long-acting injectable antiretroviral therapy.
METHODS
Over 2 weeks in May 2021, we conducted a brief survey with patients as they exited an in-person HIV provider visit at 1 of 3 clinics: Ward 86 in San Francisco, the Grady Infectious Disease Program in Atlanta, and the University of Chicago. Survey questions asked about sociodemographics, ART and viral suppression status, whether the topic of LAI-ART came up in the visit, and, if so, who raised it (provider vs patient). All participants were then asked, “If your provider thinks long-acting injectable HIV medication would be safe and effective for you, how likely are you to try it?” with a 5-point Likert response scale from “would definitely try it” to “would definitely not try it.” Participants were asked a “check all that apply” question on reasons why they would or would not try LAI-ART. The survey took ∼5–10 minutes to complete, and all participants received $5 USD for participating.
Using Stata, version 16 (College Station, TX, USA), we employed penalized likelihood logistic regression to account for small cell sizes [15]. We initially examined whether site was a moderator of the relationship between independent variables and dependent variables of interest and found no statistically significant interactions. Subsequently, we dropped all site–by–independent variable interactions and retained site as a covariate in bivariate analyses examining whether there were differences by sociodemographic characteristics (age, gender, race/ethnicity, housing status, substance use) or viral suppression status in whether providers raised the topic of LAI-ART or in patient willingness to try LAI-ART.
Patient Consent
The University of California San Francisco Committee on Human Research approved this study, deeming a waiver of signed consent appropriate due to its minimal risk and anonymous nature. All participants provided verbal consent before participation.
RESULTS
Among a total of 200 respondents, 18 (9%) were between the ages of 18 and 29 years, 67 (25%) were 30–49 years, and 107 (56%) were age ≥50 (8 had unknown age); 43 (22%) were cis-women, 18 (9%) were gender minority, 110 (56%) were Black/African American, 28 (14%) were Latinx/Hispanic, 63 (32%) were unstably housed or experiencing homelessness, and 40 (20%) reported use of stimulants or opiates in the past 30 days. Gender minority participants included those who identified as transgender (n = 9) or a gender different from one assigned at birth (n = 3), nonbinary (n = 4), genderqueer (n = 1), or other (n = 1). Nearly all (98%) reported being on ART, and 168 (85%) endorsed current viral suppression. One-quarter of respondents were from Atlanta, one-quarter were from Chicago, and half were from San Francisco.
The topic of LAI-ART was reported to have come up in 28% of clinical encounters, with the provider bringing it up 74% of the time. There were no statistically significant differences by sociodemographic characteristics or self-reported viral suppression status in whether providers raised the topic of LAI-ART with respondents. When asked how likely they were to try LAI-ART, 46% said they definitely would, 26% said they probably would, 16% said they might or might not, 8% said they probably would not, and 5% said they definitely would not. There was a significant positive association between participants who discussed LAI-ART with their provider and willingness to try LAI-ART (odds ratio, 2.57; 95% CI, 1.31–5.03; P = .009). There was no significant difference in willingness to try between participants with patient-initiated vs provider-initiated discussions (P = .79). There were no statistically significant differences in patient willingness to take up LAI-ART by sociodemographic characteristics or viral suppression status.
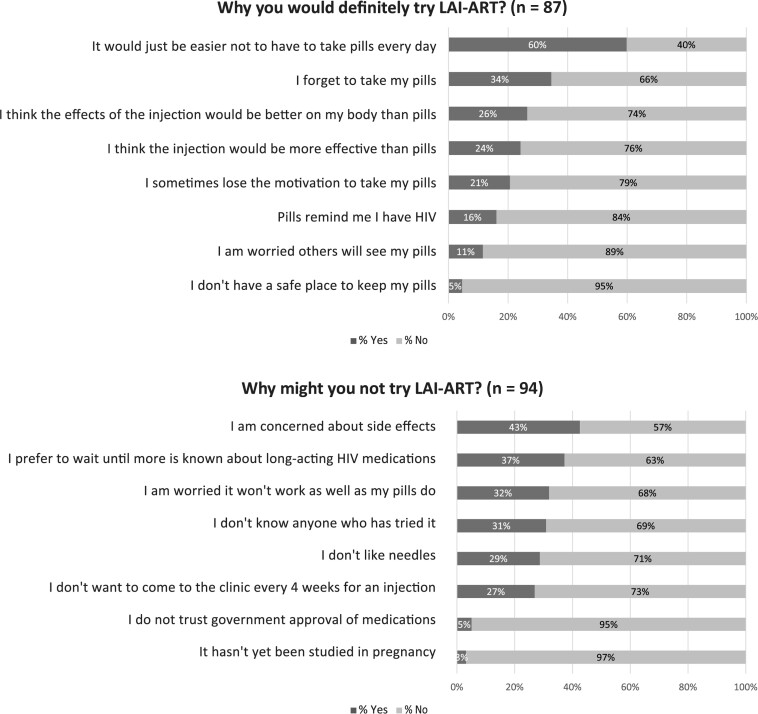
The most common reason for why respondents would definitely try LAI-ART was “It would be easier not to have to take pills every day” (60%), followed by concerns about forgetting pills (34%) and beliefs of greater tolerability (26%) and efficacy of injectables (24%) (Figure 2). Eleven percent cited worry about others seeing their pills as a motivating driver for potential future uptake. The most common reason for why patients might not try it was “I am concerned about side effects” (43%), followed by preferring to wait until more is known (37%) and concerns about efficacy (32%). Approximately a quarter (27%) cited not wanting to come to clinic every 4 weeks as a reason why they would not try LAI-ART.
Figure 2.
Reasons for trying or not trying long-acting injectable antiretroviral therapy. Participants were asked to “check all that apply” for reasons they would definitely try or why they might not try LAI-ART. The above percentages will not sum to 100%. Of the n = 88/192 who would definitely try LAI-ART, 1 declined to answer why they would definitely try it. Of the n = 104/192 who reported that they “would probably,” “might or might not,” “would probably not,” or “would definitely not” try LAI-ART, 10 declined to answer why they might not try it. Abbreviation: LAI-ART, long-acting injectable antiretroviral therapy.
DISCUSSION
Discussion of LAI-ART occurred in ∼1 in 4 patient–provider encounters, with providers raising the topic in most of these cases and no obvious disparities in the kinds of patients with whom providers discussed LAI-ART. Patient willingness to try LAI-ART was high, with 72% stating they would “definitely” or “probably” try LAI-ART, and, concordant with other clinic-based studies, there were no differences by gender or race/ethnicity; we also found no differences by site.
The patient populations who may stand to benefit the most from LAI-ART are those that experience disparities in HIV outcomes, for example, racial/ethnic minorities, those who use substances, and those who are experiencing homelessness. In this study, the most common reasons for being willing to try LAI-ART (ie, that it is easier than taking daily pills and because of concern about forgetting to take daily pills) highlight the challenges this innovation is meant to address. Further, the positive association between having a discussion with a provider and willingness to try LAI-ART suggests, not surprisingly, that the patient–provider interaction may be an important driver of uptake. Yet the patients who currently experience the most disparities may face barriers to discussion of LAI-ART and subsequent use, including lower awareness, not meeting the package insert eligibility criterion of viral suppression, less appointment attendance, lack of insurance coverage, and provider bias in willingness to prescribe (as has been seen with HIV preexposure prophylaxis) [16]. Indeed, qualitative work with providers in phase 3 trials found that clinicians were hesitant to prescribe to those who might be at risk of loss to follow-up [17]. While it is promising that we did not find differences in provider discussion of LAI-ART by housing status and substance use, all participants were surveyed after attending a clinical visit, and most self-reported being on ART (98%) and virally suppressed (85%), indicating some level of care engagement.
A limitation of this analysis is a potential bias toward well-engaged patients, as it was conducted after attended clinic visits. Patients not well represented in this study due to less access or engagement in care may still benefit from LAI-ART, and future work should target this population to better understand their interest in LAI-ART. In addition, patients were sampled at in-person rather than telehealth visits, though telehealth comprised a minority of visits during the study period (∼10%), and as logistic regression with small to moderately sized samples may bias odds ratios away from the null, results should be interpreted with this limitation in mind. The reported frequency of discussion of LAI-ART may have been influenced by provider knowledge of this study, although providers were only told that the survey was about HIV treatment in general. Additionally, the content of the discussions of LAI-ART is unknown, and thus we cannot infer that discussion of LAI-ART is equivalent to a recommendation to use LAI-ART. Further, viral suppression status was self-reported. Finally, the 3 clinics in this study are affiliated with academic institutions; findings may vary in community settings.
This study provides data on penetration of the topic of LAI-ART into clinical encounters during the early real-world rollout and is strengthened by the inclusion of 3 clinic sites in different geographic areas. While sociodemographic and viral suppression differences in provider discussion of LAI-ART were not apparent and patient willingness to use it was high, LAI-ART was discussed in a minority of patient–provider encounters. Ongoing effort to assess awareness across a range of populations, as well as provider prescribing patterns across diverse care settings, will be crucial to monitor for inequities in LAI-ART delivery and potential administrative, policy, and insurance barriers to its uptake.
Acknowledgments
Financial support. This work was funded by the National Institutes of Health R01 MH123396 (K.A.C.).
Potential conflicts of interest. Dr. Christopoulos has received investigator-initiated grant support from Gilead Sciences and served as a medical advisory board member for Gilead Sciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Katerina A Christopoulos, Division of HIV, ID and Global Medicine, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Jonathan Colasanti, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
Mallory O Johnson, Center for AIDS Prevention Studies, Division of Prevention Science, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Manami Diaz Tsuzuki, Division of HIV, ID and Global Medicine, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Xavier A Erguera, Division of HIV, ID and Global Medicine, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Rey Flores, Chicago Center for HIV Elimination, University of Chicago, Chicago, Illinois, USA; Section of Infectious Diseases and Global Health, Department of Medicine, University of Chicago, Chicago, Illinois, USA.
Jared Kerman, Chicago Center for HIV Elimination, University of Chicago, Chicago, Illinois, USA; Section of Infectious Diseases and Global Health, Department of Medicine, University of Chicago, Chicago, Illinois, USA.
Kaylin Dance, Division of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia, USA.
John A Sauceda, Center for AIDS Prevention Studies, Division of Prevention Science, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Torsten B Neilands, Center for AIDS Prevention Studies, Division of Prevention Science, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Samantha E Dilworth, Center for AIDS Prevention Studies, Division of Prevention Science, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Kimberly A Koester, Center for AIDS Prevention Studies, Division of Prevention Science, Department of Medicine, University of California San Francisco, San Francisco, California, USA.
Jose Gutierrez, National Clinician Scholars Program, University of California San Francisco, San Francisco, California, USA.
John A Schneider, Chicago Center for HIV Elimination, University of Chicago, Chicago, Illinois, USA; Section of Infectious Diseases and Global Health, Department of Medicine, University of Chicago, Chicago, Illinois, USA.
Elizabeth Montgomery, Women's Global Health Imperative, RTI International, Berkeley, California, USA; Department of Epidemiology and Biostatistics, School of Medicine, University of California San Francisco, San Francisco, California, USA.
Moira C McNulty, Chicago Center for HIV Elimination, University of Chicago, Chicago, Illinois, USA; Section of Infectious Diseases and Global Health, Department of Medicine, University of Chicago, Chicago, Illinois, USA.
References
- 1. Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr 2007;46:395–401. [DOI] [PubMed] [Google Scholar]
- 2. Scarsi KK, Swindells S. The promise of improved adherence with long-acting antiretroviral therapy: what are the data? J Int Assoc Provid AIDS Care 2021;20:23259582211009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kanazawa JT, Saberi P, Sauceda JA, Dubé K. The LAIs are coming! Implementation science considerations for long-acting injectable antiretroviral therapy in the United States: a scoping review. AIDS Res Hum Retroviruses 2021;37:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Philbin MM, Parish CL, Kinnard EN, et al. Multisite study of women living with HIV’s perceived barriers to, and interest in, long-acting injectable antiretroviral therapy. J Acquir Immune Defic Syndr 2020;84:263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerrigan D, Mantsios A, Gorgolas M, et al. Experiences with long acting injectable ART: a qualitative study among PWHIV participating in a phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018;13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mantsios A, Murray M, Karver TS, et al. “I feel empowered”: women's perspectives on and experiences with long-acting injectable antiretroviral therapy in the USA and Spain. Cult Health Sex 2021;23:1066–1078. [DOI] [PubMed] [Google Scholar]
- 7. Simoni JM, Beima-Sofie K, Mohamed ZH, et al. Long-acting injectable antiretroviral treatment acceptability and preferences: a qualitative study among US providers, adults living with HIV, and parents of youth living with HIV. AIDS Patient Care STDs 2019;33:104–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams J, Sayles HR, Meza JL, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013;8:1807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dandachi D, Dang BN, Lucari B, et al. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2021;33:801–9. [DOI] [PubMed] [Google Scholar]
- 10. Weld ED, Rana MS, Dallas RH, et al. Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019;80:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koester KA, Colasanti J, McNulty M, et al. “It’s going to get complicated quick.” Providers and staff discuss implementation of long-acting injectable anti-retroviral therapy. Paper presented at: 16th International Conference on Treatment and Prevention Adherence 2021; November 7–9, Orlando, FL.
- 12. Harris NS, Johnson AS, Huang YA, et al. Vital signs: status of human immunodeficiency virus testing, viral suppression, and HIV preexposure prophylaxis - United States, 2013–2018. MMWR Morb Mortal Wkly Rep 2019;68:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang YA, Zhu W, Smith DK, et al. HIV preexposure prophylaxis, by race and ethnicity – United States, 2014–2016. MMWR Morb Mortal Wkly Rep 2018;67:1147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Mental Health 2011;38:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21:2409–19. [DOI] [PubMed] [Google Scholar]
- 16. Edelman EJ, Moore BA, Calabrese SK, et al. Primary care physicians’ willingness to prescribe HIV pre-exposure prophylaxis for people who inject drugs. AIDS Behav 2017;21:1025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mantsios A, Murray M, Karver TS, et al. Multi-level considerations for optimal implementation of long-acting injectable antiretroviral therapy to treat people living with HIV: perspectives of health care providers participating in phase 3 trials. BMC Health Services Res 2021;21:255. [DOI] [PMC free article] [PubMed] [Google Scholar]