Key Points
Question
What are the associations of downstaging and treatment modalities with long-term outcomes and survival in patients with hepatocellular carcinoma after liver transplant?
Findings
In this cohort study of 2645 patients at 5 US academic centers, meaningful long-term outcomes after successful downstaging were achieved. Variables associated with outcomes were identified, and it was found that select patients with isolated and favorable biology would benefit from resection.
Meaning
This study demonstrated the utility of national downstaging policy in decision-making for liver transplant prioritization; surgical management was associated with improved survival in well-selected patients and should be pursued, if feasible.
This cohort study evaluates 10-year outcomes for patients with hepatocellular carcinoma (HCC) undergoing liver transplant and downstaging to within Milan criteria.
Abstract
Importance
National guidelines on transplant selection have adopted successful downstaging to within Milan criteria (MC) as a viable option for the treatment of hepatocellular carcinoma (HCC) before liver transplant (LT). Recurrence of HCC after LT carries a poor prognosis, and treatment modalities remain challenging.
Objective
To establish the 10-year outcomes of patients with HCC after LT in a large, multicenter US study based on individual data; provide robust data on the long-term role of downstaging; and evaluate the association of treatment modalities with postrecurrence survival.
Design, Setting, and Participants
In this cohort study, a retrospective, multicenter analysis of prospectively collected data was conducted for 2645 adults who had undergone LT for HCC at 5 US academic centers between January 2001 and December 2015. The analysis was performed from May 2019 through June 2021. Outcomes of 341 patients whose disease was downstaged to within MC were compared with those in 2122 patients whose disease was always within MC and 182 patients whose disease was not downstaged. The associations of tumor and treatment factors on postrecurrence survival were analyzed using Cox proportional hazards regression and multivariable logistic regression models.
Main Outcomes and Measures
The primary outcome was overall survival for the whole cohort and according to downstaging status. Secondary outcomes were time to recurrence, recurrence-free survival, and recurrence after specific post-LT therapies.
Results
Of the 2645 patients studied, the median age was 59.9 years (IQR, 54.7-64.7 years). The majority of the patients were men (2028 [76.7%] vs 617 [23.3%] women). The 10-year post-LT survival and recurrence rates were, respectively, 52.1% and 20.6% among those whose disease was downstaged; 61.5% and 13.3% in those always within MC; and 43.3% and 41.1% in those whose disease was not downstaged. Independent variables associated with downstaging failure were tumor size greater than 7 cm at diagnosis (OR, 2.62; 95% CI, 1.20-5.75; P = .02), more than 3 tumors at diagnosis (OR, 2.34; 95% CI, 1.22-4.50; P = .01), and α-fetoprotein response of at least 20 ng/mL with less than 50% improvement from maximum α-fetoprotein before LT (OR, 1.99; 95% CI, 1.14-3.46; P = .02). Surgically treated patients with recurrent HCC differed in clinicopathologic characteristics and had improved 5-year postrecurrence survival rates (31.6% vs 7.3%; P < .001).
Conclusions and Relevance
In a large, multicenter cohort of patients with HCC successfully downstaged to within MC, 10-year post-LT outcomes were excellent, validating national downstaging policies and showing a clear utility benefit for LT prioritization decision making. Surgical management of HCC recurrence after LT was associated with improved survival in well-selected patients and should be pursued, if feasible.
Introduction
For more than 2 decades, selection of patients with hepatocellular carcinoma (HCC) for liver transplant (LT) has been guided by the Milan criteria (MC). The rising incidence of HCC and mortality rates in the US has led to continual refinements to the selection policy.1,2,3,4,5,6,7,8,9,10 The focus has shifted from simple morphometrics to guidelines incorporating tumor biology, response to bridging therapies, and waiting times for patients within and beyond MC. Downstaging is now an option in selecting suitable LT candidates with initial tumors exceeding MC.11,12,13,14,15,16,17 Preliminary results showed promising outcomes, with a 5-year recurrence-free survival rate of 87%, leading to the integration of downstaging into national policy in 2017. Recurrence remains at 8% to 20% after LT, with overall poor prognosis.18,19 Treatment of recurrence remains challenging, with options including repeated resection, ablation, embolization, radiation, and systemic therapy.
The American Association for the Study of Liver Diseases (AASLD) guidelines recommend that patients beyond MC be considered for LT after successful downstaging to MC, but the level of evidence is very low, and the strength of the recommendation is conditional.20 Similarly, the European Association for the Study of Liver (EASL) guidelines state that consensus on expanded criteria for LT in patients with HCC has not been reached.16 Finally, an international consensus group defined the evidence for recommending a transplant after HCC downstaging as weak.21 Despite promising results, the reproducibility of these proposals on a large scale awaits confirmation. In addition, 10-year outcomes have been considered important to capture the benefit of LT in guidelines on the basis of the utility principle.16 Here, we aimed to establish the 10-year outcome of HCC after LT in a large, multicenter US study based on individual data, provide robust data on the long-term role of downstaging, and evaluate the association of treatment modalities with postrecurrence survival.
Methods
Study Design and Patient Population
In this cohort study, prospective data from 5 US academic centers (David Geffen School of Medicine at the University of California, Los Angeles; University of California, San Francisco [UCSF] School of Medicine; Weill Cornell Medical Center/Columbia University Medical Center; Washington University School of Medicine in St Louis; and Recanati/Miller Transplantation Institute, Mount Sinai Medical Center) were collected and retrospectively reviewed. All consecutive adults who underwent LT for HCC between January 2001 and December 2015 were selected. We aimed to include high-volume centers (>100 LTs per year) with similar practice patterns (adherent to the AASLD/Unified Network for Organ Sharing [UNOS]/Organ Procurement and Transplantation Network guidelines), experience (large HCC population, access to pre-LT treatments, and downstaging experience), waiting times, access to organs (live donor vs deceased donor), and LT recipient populations. Institutional review board approval was obtained in each center with a waiver of informed consent because data were collected prospectively by each center. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies.
Patients were classified into 3 categories based on their radiographic tumor burden at the time of diagnosis. The within MC group included patients with 1 lesion of 5 cm or less or 2 to 3 lesions of 3 cm or less. The downstaged group included patients beyond MC at diagnosis who were successfully downstaged to within MC at the time of LT, regardless of baseline number of nodules and size. The UNOS downstaged group included patients with 1 lesion greater than 5 cm but less than or equal to 8 cm, 2 to 3 lesions of which at least 1 was greater than 3 cm but less than or equal to 5 cm with a total tumor diameter of 8 cm or less, or 4 to 5 lesions, each less than 3 cm with a total tumor diameter of 8 cm or less. Patients whose disease could not be downstaged or who had progressed beyond MC but ultimately underwent a transplant were included in the beyond MC group. Successful downstaging was defined as a reduction in viable tumor burden after locoregional therapy (LRT) to within MC. In accordance with the UNOS listing policy, extent of disease was determined by contrast imaging at least once every 3 months after listing. A minimum observation period of 3 months after downstaging was required before LT. Patients with incidental HCC, intrahepatic cholangiocarcinoma, or mixed HCC and cholangiocarcinoma were excluded.
All analyses were conducted in patients who underwent a successful transplant. Variables at diagnosis and during the waiting time were collected for patients included in the study but not for patients who ultimately did not undergo a transplant. Thus, the study provides long-term outcomes and dissects the role of downstaging and treatment of recurrences but does not provide intention-to-treat outcomes or factors associated with dropout. The latter concepts, such as wait list mortality and dropout, have been extensively analyzed in previously reported studies.8,9 Data on race and ethnicity were not collected or analyzed.
Follow-up Protocol
Median follow-up was 55.3 months (IQR, 26.4-93.5 months). Imaging was obtained every 3 to 6 months after LT. Diagnosis of recurrence was based on imaging, and pathology was obtained for confirmation as needed. Treatment for recurrence was categorized as surgical, nonsurgical locoregional, systemic therapy, or supportive care. Patients with multiple therapies were categorized according to the treatment highest on this list.
Statistical Analysis
From May 2019 to June 2021, we conducted 3 analyses: (1) long-term outcomes and independent variables associated with overall survival (OS) and recurrence, (2) outcomes according to various downstaging groups and subgroups, and (3) outcomes and independent factors associated with survival in patients in whom HCC recurrence developed after LT.
Continuous variables were compared using the Wilcoxon rank sum test. Categorical variables were compared using the χ2 test. Survival curves were calculated using the Kaplan-Meier method and compared using log-rank tests. In cases of multiple comparisons, the Bonferroni correction was used. For these analyses, significance was defined as P < .05 (2-tailed).
Cox proportional hazards regression was performed to identify variables associated with recurrence, OS, and recurrence-free survival among patients in the downstaged group. A multivariable logistic regression model was used to identify variables associated with failure to achieve downstaging to MC among patients beyond MC who received LRT before LT. To evaluate the association between α-fetoprotein (AFP) response to LRT on failure to achieve downstaging, we created a categorical variable with 3 categories: pre-LT AFP less than 20 ng/mL, pre-LT AFP greater than 20 ng/mL with at least a 50% decrease from maximum AFP, and AFP greater than 20 ng/mL with less than a 50% decrease from maximum AFP.
For both regression models, missing data underwent multiple imputation (n = 20 with 5 burn-in iterations) using the fully conditional specification method. All analyses were performed using SAS, version 9.4 (SAS Institute Inc).
Results
Baseline and Wait List Characteristics
The baseline and wait list characteristics of the cohort are listed in Table 1. The cohort included 2645 patients with HCC who underwent LT. The median age was 59.9 years (IQR, 54.7-64.7 years), 2028 (76.7%) were men, and 617 (23.3%) were women. Of 2645 patients, 2122 (80.2%) were always within MC, 341 (12.9%) were beyond MC but downstaged to within MC at LT (168 [6.4%] were within the UNOS downstaging criteria), and 182 (6.9%) exceeded MC (eFigure 1 in the Supplement).
Table 1. Baseline Patient Characteristics.
| Characteristic | No. (%) | P value | |||
|---|---|---|---|---|---|
| All patients (n = 2645) | Within MC (n = 2122) | Downstaged to within MC (n = 341) | Beyond MC (n = 182) | ||
| Age at transplant, median (IQR), y | 59.9 (54.7-64.7) | 60 (55-64.9) | 59.4 (54.8-64.2) | 59.5 (53-64.4) | .53 |
| Sex | |||||
| Female | 617 (23.3) | 518 (24.4) | 62 (18.2) | 37 (20.3) | .03 |
| Male | 2028 (76.7) | 1604 (75.6) | 279 (81.8) | 145 (79.7) | |
| Underlying liver disease | |||||
| Hepatitis C | 1585 (59.9) | 1299 (61.2) | 182 (53.4) | 104 (57.1) | <.001 |
| Hepatitis B | 384 (14.5) | 297 (14) | 64 (18.8) | 23 (12.6) | |
| Alcoholic liver disease | 217 (8.2) | 177 (8.3) | 28 (8.2) | 12 (6.6) | |
| Nonalcoholic steatohepatitis | 134 (5.1) | 107 (5) | 21 (6.2) | 6 (3.3) | |
| Other (ie, primary biliary cirrhosis, cryptogenic, autoimmune) | 200 (7.6) | 142 (6.7) | 22 (6.5) | 36 (19.8) | |
| Unknown | 125 (4.7) | 100 (4.7) | 24 (7) | 1 (0.6) | |
| Size of largest tumor at diagnosis, median (IQR), cm | 2.6 (2-3.6) | 2.4 (2-3) | 4.5 (3.4-5.7) | 3.7 (2.7-5.1) | <.001 |
| No. of tumors at diagnosis, median (IQR) | 1 (1-2) | 1 (1-2) | 2 (1-2) | 2 (1-3) | <.001 |
| MC at time of transplant | |||||
| Within | 2463 (93.1) | 2122 (100) | 341 (100) | NA | NA |
| Beyond | 182 (6.9) | NA | NA | 182 (100) | |
| No. of pretransplant locoregional therapies | |||||
| 0 | 388 (14.7) | 342 (16.2) | NA | 45 (24.7) | <.001 |
| 1 | 871 (32.9) | 773 (36.4) | 74 (21.7) | 24 (13.2) | |
| 2 | 760 (28.7) | 564 (26.6) | 126 (37) | 70 (38.5) | |
| ≥3 | 612 (23.1) | 435 (20.5) | 134 (39.3) | 43 (23.6) | |
| α-Fetoprotein at time of transplant, ng/mL | |||||
| Median (IQR) | 9 (4.9-32.5) | 8.4 (4.7-27.6) | 10 (5-42.6) | 24.2 (8.2-107.1) | <.001 |
| <20 | 1711 (64.7) | 1422 (67) | 212 (62.2) | 77 (42.3) | <.001 |
| 20-199 | 600 (22.7) | 456 (21.5) | 84 (24.6) | 60 (33) | |
| 200-1000 | 164 (6.2) | 123 (5.8) | 23 (6.7) | 18 (9.9) | |
| >1000 | 67 (2.5) | 44 (2.1) | 10 (2.9) | 13 (7.1) | |
| Unknown | 103 (3.9) | 77 (3.6) | 12 (3.5) | 14 (7.7) | |
| Maximum pretransplant α-fetoprotein, ng/mL | |||||
| Median (IQR) | 20.5 (7.3-107.4) | 18.8 (7-88) | 32 (10-194.3) | 41.2 (12.3-256.7) | <.001 |
| <20 | 1287 (48.7) | 1087 (51.2) | 141 (41.4) | 59 (32.4) | <.001 |
| 20-199 | 853 (32.2) | 666 (31.4) | 114 (33.4) | 73 (40.1) | |
| 200-1000 | 322 (12.2) | 249 (11.7) | 45 (13.2) | 28 (15.4) | |
| >1000 | 161 (6.1) | 104 (4.9) | 37 (10.9) | 20 (11) | |
| Laboratory MELD score, median (IQR) | 12 (9-17) | 12 (9-17) | 11 (8-15) | 13 (9-19) | <.001 |
| Unknown | 169 (6.4) | 134 (6.3) | 31 (9.1) | 4 (2.2) | |
| Neutrophil-lymphocyte ratio at time of transplant, median (IQR) | 2.7 (1.8-4.4) | 2.8 (1.8-4.4) | 2.5 (1.7-4.1) | 2.9 (1.7-4.8) | .12 |
| Unknown | 170 (6.4) | 142 (6.7) | 20 (5.9) | 8 (4.4) | |
| Donor type | |||||
| Deceased | 2535 (95.8) | 2053 (96.8) | 323 (94.7) | 159 (87.4) | <.001 |
| Living | 108 (4.1) | 68 (3.2) | 18 (5.3) | 22 (12.1) | |
| Size of largest viable tumor on explant pathology, median (IQR), cm | 2 (0.2-3.3) | 1.8 (0-3) | 2.4 (0.4-3.8) | 4 (2.3-5.5) | <.001 |
| Tumor differentiation | |||||
| Complete necrosis/no viable tumor | 626 (23.7) | 530 (25) | 77 (22.6) | 19 (10.4) | <.001 |
| Well differentiated | 516 (19.5) | 417 (19.7) | 67 (19.6) | 32 (17.6) | |
| Moderately differentiated | 1161 (43.9) | 920 (43.4) | 153 (44.9) | 88 (48.4) | |
| Poorly differentiated | 291 (11) | 216 (10.2) | 39 (11.4) | 36 (19.8) | |
| Vascular invasion on explant pathology | |||||
| None | 2048 (77.4) | 1690 (79.6) | 258 (75.7) | 100 (54.9) | <.001 |
| Microvascular | 519 (19.6) | 382 (18) | 69 (20.2) | 68 (37.4) | |
| Macrovascular | 77 (2.9) | 49 (2.3) | 14 (4.1) | 14 (7.7) | |
Abbreviations: MC, Milan criteria; MELD, Model for End-stage Liver Disease; NA, not applicable.
Recurrence and Survival
Median follow-up was 55.3 months (IQR, 26.4-93.5 months). Overall, 853 deaths (32.2%) occurred, including 116 (4.4%) within 90 days of surgery.
Median OS was 158 months (IQR, 50.2 months to not reached), with survival rates of 89.3% at 1 year, 71.3% at 5 years, and 59.0% at 10 years (eFigure 2A in the Supplement). Overall, 330 patients (12.5%) experienced recurrence. The median time to recurrence was 17 months, and the rates of recurrence were 5.1% at 1 year, 14.3% at 5 years, and 16.4% at 10 years (eFigure 2B in the Supplement). Independent variables associated with poor survival and recurrence after LT are listed in eTable 1 in the Supplement.
Downstaging
Among 454 patients presenting with disease staged beyond MC, 413 (91.0%) received LRT for the purpose of downstaging, whereas 41 (9.0%) were not treated with LRT because of prohibitive liver function. Of the 413 patients who received LRT, 341 (82.6%) were downstaged to within MC. Of note, 182 patients received a transplant with disease that exceeded MC, including 72 (39.6%) who did not respond to LRT, 41 (22.5%) unable to receive LRT, and 69 (37.9%) who were initially assessed at baseline as within MC but progressed while on the wait list beyond this HCC burden (eFigure 1 in the Supplement). The main characteristics of these groups are summarized in Table 1.
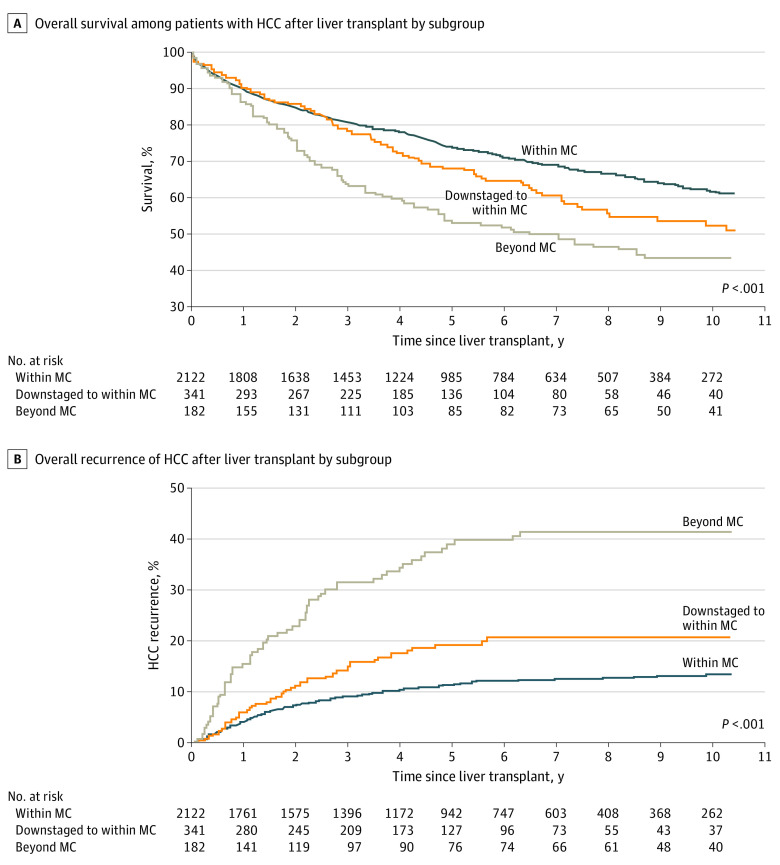
Overall, the post-LT survival rates were 89.4% at 1 year, 73.6% at 5 years, and 61.5% at 10 years in the MC group; 90.1% at 1 year, 67.9% at 5 years, and 52.1% at 10 years in the downstaged; and 86.2% at 1 year, 52.9% at 5 years, and 43.3% at 10 years in the beyond MC group (Figure 1A). The median survival rates were 172.8 months for the MC group, 126.0 months for the downstaged group, and 76.8 months for the beyond MC group. Overall survival for the MC group was significantly better than for the downstaged group (61.5% vs 52.1%; P < .001). In contrast, OS for the downstaged group was not significantly better than for the beyond MC group. Nonetheless, the actual probability of recurrence-free survival was better for the downstaged group (median, 10.3 years [IQR, 2.7 years to not reached]; 5-year survival rate, 64.3%; 10-year survival rate, 50.5%) than for the beyond MC group (median, 4.7 years [IQR, 1.2 years to not reached]; 5-year survival rate, 46.8%; 10-year survival rate, 41.5%).
Figure 1. Kaplan-Meier Estimates.
HCC indicates hepatocellular carcinoma.
A,P < .001 for within Milan criteria (MC) vs downstaged; P = .34 for downstaged vs beyond MC. B, P < .001 for within MC vs downstaged and for downstaged to within MC vs beyond MC.
Within the entire cohort, recurrence of HCC after LT was observed in 330 patients (12.5%), including 212 (10.0%) within the MC group, 54 (15.8%) in the downstaged group, and 64 (35.2%) in the beyond MC group (P < .001). The Kaplan-Meier probability of HCC recurrence was 11.3% at 5 years after LT and 13.3% at 10 years after LT for the MC group, 19.1% at 5 years and 20.6% at 10 years for the downstaged group, and 38.9% at 5 years and 41.4% at 10 years for the beyond MC group (P < .001). Overall, the probability of recurrence for the MC group (41.4%) was significantly lower than that for the downstaged group (20.6%; P < .001). Similarly, the probability of recurrence was significantly lower in the downstaged group vs the beyond MC group (13.3% vs 41.0%; P < .001) (Figure 1B).
Variables independently associated with downstaging failure were tumor size greater than 7 cm at diagnosis (OR, 2.62; 95% CI, 1.20-5.75; P = .016), more than 3 tumors at diagnosis (OR, 2.34; 95% CI, 1.22-4.50; P = .011), and failure of AFP to decrease at least 50% from maximum in patients with AFP greater than or equal to 20 ng/mL at the time of LT (OR, 1.99; 95% CI, 1.14-3.46; P = .01) (Table 2). Variables independently associated with poor survival in the downstaged group were neutrophil-to-lymphocyte (NRL) ratio greater than 5 at LT (OR, 2.15; 95% CI, 1.39-3.32; P < .001) and largest viable tumor on explant pathology greater than 5 cm (OR, 2.04; 95% CI, 1.23-3.39; P = .006). Variables independently associated with recurrence in the downstaged group were 2 vs 1 pretransplant LRTs (OR, 3.28; 95% CI, 1.17-9.18; P = .02), NLR greater than 5 at LT (OR, 2.17; 95% CI, 1.11-4.25; P = .02), AFP greater than or equal to 20 ng/mL at LT (OR, 2.19; 95% CI, 1.22-3.92; P = .009), largest viable tumor greater than 5 cm (OR, 2.97; 95% CI, 1.54-5.72; P = .001), poor tumor differentiation (OR, 3.37; 95% CI, 1.02-11.15; P = .05), and vascular invasion on explant pathologic findings (OR, 1.91; 95% CI, 1.03-3.50; P = .04). Variables independently associated with poor recurrence-free survival in the downstaged group were NLR greater than 5 at LT (OR, 2.04; 95% CI, 1.34-3.09; P < .001) and largest viable tumor on explant pathologic findings greater than 5 cm (OR, 2.18; 95% CI, 1.34-3.56; P = .002) (Table 3).
Table 2. Variables Associated With Failure to Downstage in 413 Patients Beyond Milan Criteria Undergoing Locoregional Therapy.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| >3 vs ≤3 Tumors at diagnosis | 2.26 (1.20-4.27) | <.01 | 2.34 (1.22-4.50) | .01 |
| Largest initial tumor size, >7 vs ≤7 cm | 2.19 (1.03-4.66) | .043 | 2.62 (1.20-5.75) | .02 |
| No. of locoregional therapies | ||||
| 2 vs 1 | 1.11 (0.58-2.13) | .76 | ||
| ≥3 vs 1 | 0.75 (0.38-1.49) | .41 | ||
| α-Fetoprotein level response to locoregional therapy | ||||
| <20 ng/mL At transplant | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥20 ng/mL And improved ≥50% from maximum at transplant | 0.89 (0.26-3.03) | .85 | 0.75 (0.22-2.59) | .65 |
| ≥20 ng/mL And <50% improved from maximum at transplant | 2.04 (1.18-3.52) | .001 | 1.99 (1.14-3.46) | .01 |
Abbreviations: NA, not applicable; OR, odds ratio.
Table 3. Variables Associated With Poor Recurrence-Free Survival in 341 Patients Downstaged to Within Milan Criteria.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Pretransplant variables | ||||
| >3 vs ≤3 Tumors at diagnosis | 0.80 (0.45-1.41) | .43 | NA | NA |
| Largest initial tumor size, >7 vs ≤7 cm | 1.56 (.091-2.67) | .11 | 1.64 (0.95-2.83) | .08 |
| No. of pretransplant locoregional therapies | ||||
| 2 vs 1 | 1.35 (0.84-2.19) | .21 | NA | NA |
| ≥3 vs 1 | 1.16 (0.72-1.87) | .54 | NA | NA |
| Neutrophil-to-lymphocyte ratio at time of transplant, >5 vs <5 | 1.96 (1.31-2.93) | .001 | 2.04 (1.34-3.09) | <.001 |
| Laboratory MELD score, per unit increase | 1.01 (0.99-1.04) | .25 | NA | NA |
| α-Fetoprotein at time of transplant, ≥20 vs <20 ng/mL | 1.46 (1.02-2.09) | .04 | 1.34 (0.93-1.93) | .11 |
| Explant pathologic findings | ||||
| Largest viable tumor, >5 vs ≤5 cm | 2.58 (1.66-4.03) | <.001 | 2.18 (1.34-3.56) | .01 |
| Tumor differentiation | ||||
| Necrotic or no viable tumor | 1 [Reference] | NA | 1 [Reference] | NA |
| Well | 0.82 (0.47-1.42) | .48 | 0.74 (0.42-1.30) | .30 |
| Moderate | 1.06 (0.68-1.67) | .79 | 0.88 (0.54-1.44) | .62 |
| Poor | 2.00 (1.14-3.52) | .02 | 1.40 (0.74-2.64) | .30 |
| Vascular | ||||
| None | 1 [Reference] | NA | 1 [Reference] | NA |
| Microvascular or macrovascular | 1.55 (1.07-2.26) | .02 | 1.23 (0.82-1.86) | .32 |
Abbreviations: MELD, Model of End-stage Liver Disease; NA, not applicable.
Living Donor Liver Transplant
A total of 108 patients in the cohort (4.1%) received a transplanted liver from a living donor. Tumor burden beyond MC was higher among transplant recipients with a deceased donor (20.4% vs 6.3%, P < .001). The downstaging rate was similar between these groups.
The 10-year survival rate was similar between the living donor and deceased donor groups (64.3% vs 58.8%; P = .24); survival was slightly higher among patients within MC who received a liver from a living donor (10-year survival rate, 73% vs 61.2%), but this difference was not significant (P = .09). There were no differences in the 10-year HCC recurrence rate between transplant recipients with a living donor vs a deceased donor (12.8% vs 16.5%), whereas among patients strictly within MC, recipients with a living donor had a significantly lower 10-year recurrence rate compared with recipients with a deceased donor (3.5% vs 13.7%; P = .04). There were no differences in outcomes between recipients with a deceased vs living donor among patients whose disease was downstaged to within MC. Receiving a liver from a living donor was not independently associated with OS or recurrence.
Patterns and Treatment of Recurrence After LT
The clinicopathologic characteristics of 330 patients with HCC recurrence after LT are shown in eTable 2 in the Supplement. In patients with recurrent HCC, 74 (22.8%) had an immediate AFP greater than 200 ng/mL before LT, 91 (34.8%) had a maximum AFP greater than 200 ng/mL before LT, 283 (85.8%) had no tumor necrosis with a median maximum viable tumor diameter of 3 cm, 151 (45.8%) had vascular invasion, and 85 (26.2%) had poorly differentiated tumors.
Among the 330 patients who experienced HCC recurrence, 266 (84.2%) received therapy and 50 (15.8%) received best supportive care. Surgical resection was the first treatment approach in 101 (31.9%)cases, LRT in 83 (26.2%), and systemic therapy in 82 (25.9%). The majority of recurrences were noted to be extrahepatic (219 [66.4%]), multinodular (198 [60.9%]), and occurring more than 1 year after LT (207 [62.7%]). Characteristics in patients with recurrent HCC by treatment modality are summarized in eTable 3 in the Supplement.
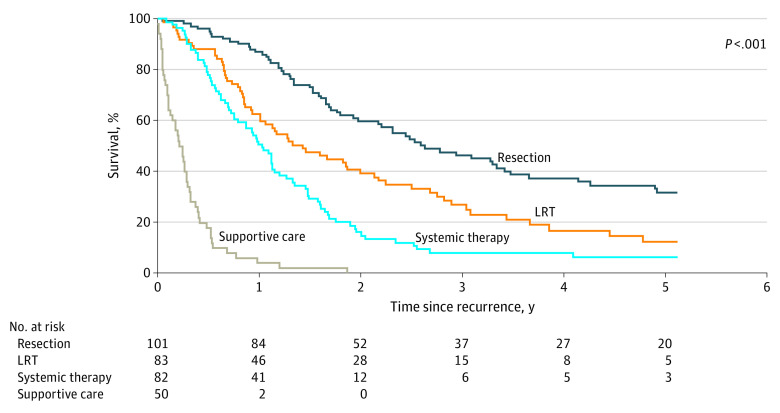
Overall, the median survival after recurrence was 14.3 months (IQR, 6.13-33.1 months), with survival rates of 56.9% at 1 year and 23.2% at 3 years. Median survival after resection (101 patients; 31.6 months [IQR, 16.1-69.4 months]) was significantly better compared with median overall survival after LRT (83 patients; 17.2 months [IQR, 8.8-36.5 months]) and systemic treatment (82 patients; 12.5 months [IQR, 6.4-19.9 months]) (P < .001) (Figure 2). Factors associated with survival after LT are shown eTable 4 in the Supplement.
Figure 2. Kaplan-Meier Estimates of Survival After Recurrence Stratified by Type of Treatment.
LRT indicates locoregional therapy.
Overall, patients receiving surgical treatment for recurrence had generally favorable recurrent tumor characteristics. The majority of surgically treated recurrences were solitary (70 [69.3%]) and extrahepatic (90 [89.1%]), and AFP was most often less than 200 ng/mL (71 [73.2%]) at the time of recurrence.
Discussion
This cohort study provides meaningful data to support recommendations in clinical practice guidelines. It establishes the 10-year outcome of HCC in more than 2600 patients who underwent LT based on individual data from multiple academic centers, provides robust data on the long-term outcomes of downstaging to MC, and evaluates the association of treatment modalities with postrecurrence survival.
For this cohort, we found a median survival time of 158 months, with 5-year and 10-year survival rates of 71.3%, and 59.0%, respectively. The probability of recurrence at 10 years was 16.4%. Most importantly, 10-year survival and recurrence rates were 61.5% and 13.3%, respectively, for patients with HCC within MC. These outcomes were obtained after a median follow-up 53 months, with more than 350 patients at risk at 10 years, and can be considered an important benchmark when assessing benefits of treatments and recommendations in guidelines.
This study also provides relevant information about the long-term role of downstaging to MC after LT. As previously mentioned, the AASLD and EASL reported a low level of evidence in their guidelines.16,20 Here, we show that patients beyond MC were nevertheless able to achieve meaningful long-term outcomes after successful downstaging. Indeed, the 5- and 10-year OS rates were 67.9% and 52.1%, respectively, and the 10-year recurrence rate was 20.6%. We consider these outcomes, even if lower than for patients with tumors staged within MC, to represent a new benchmark for use in future studies evaluating the utility principle. Of note, in another study, a median survival of 50 to 60 months was the best outcome reported for patients with similarly staged disease treated with LRT alone.17
Previous studies have highlighted the important variables associated with clinicopathologic outcomes for patients with HCC. Recent scoring systems, including the French AFP model, Metroticket 2.0, Model for Tumor Recurrence of After Living Donor Liver Transplantation score, and New York/California score, have shown that a 5-year recurrence-free survival rate of greater than 70% is achievable in patients with disease staged beyond MC.19,20,21,22 In recent years, The Transplantation Society expanded the LT eligibility criteria to maximize survival benefit. Yao and Fidelman12 reported comparable outcomes for patients with the proposed UCSF downstaging criteria to those within MC at the outset. As a result, UNOS implemented a new policy that would allow priority listing for LT in patients meeting the UCSF downstaging criteria. Our study provides robust information to support the concept of more flexible inclusion criteria, taking into account additional biological indicators of positive outcomes before LT.
Also of note, our study found improved recurrence-free survival and time to recurrence among patients with tumors downstaged to MC, emphasizing the need to achieve this goal as a part of the neoadjuvant treatment provided to wait-listed patients. In another study, the intention-to-treat outcomes showed a significantly lower LT probability (1-year dropout rate of 25% vs 54%) and inferior intention-to-treat survival rate (56% vs 21% at 5 years) among patients whose cancer was downstaged according to the UCSF criteria compared with the all-comers group.23 In that study, the cumulative probability of downstaging decreased from 68% to 38%, with a greater sum of tumor number and diameter of 8 tumors and 14 cm, respectively. Patients in the all-comers downstaged group had the highest post-LT rates of recurrence at 3 years (16.7%), microvascular invasion (18%), and understaging on explant pathologic findings beyond MC (41%).23 Therefore, the need for strict LT selection criteria for patients whose disease is downstaged is crucial.
A previous multicenter study24 revealed that the probability of successful downstaging to within MC was 87.7%. Among patients achieving downstaging, the 2-year probability of dropping out was 37.3%. In addition, no significant differences were found when transarterial chemoembolization was compared with yttrium-90 as the type of the first LRT received.24
We identified several variables that were independently associated with downstaging failure, which can aid in decision making and risk-benefit assessment. Lack of AFP response to LRT is a surrogate of aggressive tumor biology and carries a worse prognosis. Rather than using static numbers, evaluating the dynamics of the response to LRT has become a novel approach in assessing tumor biology.13,18,19,25,26,27,28 A new national policy requires that an AFP greater than 1000 ng/mL must decrease to less than 500 ng/mL before LT, which is associated with a more than 2-fold reduction in mortality after LT and a 3-fold reduction in HCC recurrence.13 Nonresponders are more likely to have a radiographic tumor burden beyond MC, increased tumor numbers and diameters, and failed radiographic and pathologic responses. High NLR has been reported to be a poor prognostic indicator in HCC29,30 and was confirmed to be independently associated with poor outcomes in this study.
We also provide new information on treatment of subsequent HCC recurrence after LT, an area that is still poorly defined. Despite the overall poor prognosis, our study reveals that meaningful long-term survival can be achieved in a subset of patients with recurrence after LT. Time to recurrence was associated with poor prognosis and an observed risk of “fast tracking” candidates, presumably with aggressive tumors.12,31,32,33,34 Halazun et al8 reported that OS is significantly better in patients with a long waiting time before LT. In 2015, UNOS policy mandated a delay of 6 months before granting listing priority. In our study, variables of recurrent tumors independently associated with survival from the time of recurrence are defined by the characteristics of the recurrence itself but not the explant pathologic factors of the initial tumor. We also found that treatment of the recurrent tumor is independently associated with outcome. Recipients who underwent surgical treatment for their recurrence achieved a median survival of 31.6 months. We found that a subgroup of patients with isolated and favorable tumor biology would benefit from resection.
In our study, transplant patients who received a liver from a living donor had oncologic outcomes comparable to those of patients with a deceased donor. Some studies question the concept of equipoise among patients with disease staged beyond MC undergoing live-donor liver transplant.35 In answer to this question, a meta-analysis revealed similar outcomes among patients with disease staged within MC undergoing live-donor vs deceased-donor liver transplant.36 Furthermore, investigators analyzed the safety and effectiveness of live-donor transplant in patients with disease staged beyond MC. In a prospective pilot study involving patients with HCC with Barcelona Clinic Liver Cancer extended criteria, an overall 5-year survival rate of 80% and an actual probability of recurrence at 5 years of 23.8% were reported.37 Other groups have advocated for further expansion, including the extended Toronto criteria, National Cancer Center Korea criteria, and Kyoto criteria.38,39,40 Another large-scale series found that patients listed with a potential live donor had a 33% reduction in the risk of death from the time of listing.41
Although AASLD guidelines have adopted downstaging to MC, the EASL guidelines state that patients beyond MC can be considered for LT after successful downstaging within defined protocols (recommendation level weak).16 The outcomes provided in the current study of more than 2600 patients with HCC who underwent LT (ie, a 10-year survival rate >50% and an acceptable recurrence rate) can certainly increase the level of recommendation for the downstaging policy on a global basis. In addition, these results align with the first published phase 2b/3 randomized clinical trial that supports downstaging in Italian centers.18
Strengths and Limitations
The major strength of our study is the inclusion of more than 2600 patients with HCC who underwent LT at 5 large US transplant centers with relatively equal waiting times and long-term follow-ups, permitting us to draw universally applicable conclusions from our results. Access to detailed individual data while on the wait list allowed us to compare post-LT outcomes, evaluate factors associated with HCC recurrence after LT, and identify factors associated with downstaging failure.
This study had several limitations, including a lack of information on patients dropped from the wait list because of progression, the preclusion of an intention-to-treat analysis, and the study’s retrospective nature and associated biases. However, to apply this study prospectively, the short available study period would not allow for the drawing of substantive conclusions.
Conclusions
Liver transplant for patients with HCC has changed dramatically over more than 25 years as new eligibility criteria and prognostic factors have been identified. In this large, multicenter US cohort study, we establish excellent 10-year post-LT outcomes and validate national downstaging policy. We confirm a clear utility benefit for decision-making on LT prioritization. Tumor characteristics and lack of AFP response before LT were associated with failure to achieve downstaging. Although recurrence of HCC after LT remains a challenge, surgical management is associated with improved survival in well-selected patients and should be pursued, if feasible.
eTable 1. Factors Associated With Posttransplant HCC Recurrence and Poor Overall Survival
eTable 2. Clinicopathologic Characteristics of Liver Transplant Recipients by Tumor Recurrence
eTable 3. Comparison of Recurrent Tumor Characteristics by Treatment Modality
eTable 4. Factors Associated With Survival After Liver Transplantation
eFigure 1. Cohort Diagram
eFigure 2. Overall Survival and Recurrence of the Whole Cohort of 2645 Patients With HCC After Transplantation
References
- 1.Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 3.Global Cancer Observatory. International Agency for Research on Cancer . 2020. Accessed November 1, 2021 https://gco.iacr.fr
- 4.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699. doi: 10.1056/NEJM199603143341104 [DOI] [PubMed] [Google Scholar]
- 5.Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403. doi: 10.1053/jhep.2001.24563 [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Llovet JM, Miceli R, et al. ; Metroticket Investigator Study Group . Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43. doi: 10.1016/S1470-2045(08)70284-5 [DOI] [PubMed] [Google Scholar]
- 7.OPTN/UNOS Liver and Intestinal Organ Transplantation Committee . Changes to HCC criteria for auto approval. Accessed March 1,2018. https://optn.transplant.hrsa.gov/media/1922/liver_hcc_criteria_for_auto_approval_20160815.pdf
- 8.Halazun KJ, Patzer RE, Rana AA, et al. Standing the test of time: outcomes of a decade of prioritizing patients with hepatocellular carcinoma, results of the UNOS natural geographic experiment. Hepatology. 2014;60(6):1957-1962. doi: 10.1002/hep.27272 [DOI] [PubMed] [Google Scholar]
- 9.Ishaque T, Massie AB, Bowring MG, et al. Liver transplantation and waitlist mortality for HCC and non-HCC candidates following the 2015 HCC exception policy change. Am J Transplant. 2019;19(2):564-572. doi: 10.1111/ajt.15144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pomfret EA, Washburn K, Wald C, et al. Report of a national conference on liver allocation in patients with hepatocellular carcinoma in the United States. Liver Transpl. 2010;16(3):262-278. doi: 10.1002/lt.21999 [DOI] [PubMed] [Google Scholar]
- 11.Yao FY, Breitenstein S, Broelsch CE, Dufour JF, Sherman M. Does a patient qualify for liver transplantation after the down-staging of hepatocellular carcinoma? Liver Transpl. 2011;17(suppl 2):S109-S116. doi: 10.1002/lt.22335 [DOI] [PubMed] [Google Scholar]
- 12.Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: Where do we stand with tumor down-staging? Hepatology. 2016;63(3):1014-1025. doi: 10.1002/hep.28139 [DOI] [PubMed] [Google Scholar]
- 13.Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968-1977. doi: 10.1002/hep.27752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta N, Guy J, Frenette CT, et al. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: a multicenter study. Clin Gastroenterol Hepatol. 2018;16(6):955-964. doi: 10.1016/j.cgh.2017.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta N, Dodge JL, Roberts JP, Hirose R, Yao FY. Alpha-fetoprotein decrease from > 1,000 to < 500 ng/mL in patients with hepatocellular carcinoma leads to improved posttransplant outcomes. Hepatology. 2019;69(3):1193-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 17.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using Drug Eluting Beads. Implications for clinical practice and trial design. J Hepatol. 2012;56(6):1330-1335. doi: 10.1016/j.jhep.2012.01.008 [DOI] [PubMed] [Google Scholar]
- 18.Mazzaferro V, Citterio D, Bhoori S, et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21(7):947-956. doi: 10.1016/S1470-2045(20)30224-2 [DOI] [PubMed] [Google Scholar]
- 19.Halazun KJ, Najjar M, Abdelmessih RM, et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 2017;265(3):557-564. doi: 10.1097/SLA.0000000000001966 [DOI] [PubMed] [Google Scholar]
- 20.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology. 2018;68(2):723-750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 21.Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A; OLT for HCC Consensus Group . Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012;13(1):e11-e22. doi: 10.1016/S1470-2045(11)70175-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta N, Yao FY. Moving past “one size (and number) fits all” in the selection of candidates with hepatocellular carcinoma for liver transplantation. Liver Transpl. 2013;19(10):1055-1058. doi: 10.1002/lt.23730 [DOI] [PubMed] [Google Scholar]
- 23.Ravaioli M, Grazi GL, Piscaglia F, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8(12):2547-2557. doi: 10.1111/j.1600-6143.2008.02409.x [DOI] [PubMed] [Google Scholar]
- 24.Mehta N, Frenette C, Tabrizian P, et al. Downstaging outcomes for hepatocellular carcinoma: results from the Multicenter Evaluation of Reduction in Tumor Size before Liver Transplantation (MERITS-LT) Consortium. Gastroenterology. 2021;161(5):1502-1512. doi: 10.1053/j.gastro.2021.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duvoux C, Roudot-Thoraval F, Decaens T, et al. ; Liver Transplantation French Study Group . Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986-994.e3. doi: 10.1053/j.gastro.2012.05.052 [DOI] [PubMed] [Google Scholar]
- 26.Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology. 2018;154(1):128-139. doi: 10.1053/j.gastro.2017.09.025 [DOI] [PubMed] [Google Scholar]
- 27.Halazun KJ, Tabrizian P, Najjar M, et al. Is it time to abandon the Milan criteria?: results of a bicoastal US collaboration to redefine hepatocellular carcinoma liver transplantation selection policies. Ann Surg. 2018;268(4):690-699. doi: 10.1097/SLA.0000000000002964 [DOI] [PubMed] [Google Scholar]
- 28.Sinha J, Mehta N, Dodge JL, Poltavskiy E, Roberts J, Yao F. Are there upper limits in tumor burden for down-staging of hepatocellular carcinoma to liver transplant? Analysis of the all-comers protocol. Hepatology. 2019;70(4):1185-1196. doi: 10.1002/hep.30570 [DOI] [PubMed] [Google Scholar]
- 29.Halazun KJ, Hardy MA, Rana AA, et al. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250(1):141-151. doi: 10.1097/SLA.0b013e3181a77e59 [DOI] [PubMed] [Google Scholar]
- 30.Templeton AJ, McNamara MG, Šeruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 31.Sherman M. The resurrection of alphafetoprotein. J Hepatol. 2010;52(6):939-940. doi: 10.1016/j.jhep.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 32.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20(8):945-951. doi: 10.1002/lt.23904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiNorcia J, Florman SS, Haydel B, et al. Pathologic response to pretransplant locoregional therapy is predictive of patient outcome after liver transplantation for hepatocellular carcinoma: analysis from the US Multicenter HCC Transplant Consortium. Ann Surg. 2020;271(4):616-624. doi: 10.1097/SLA.0000000000003253 [DOI] [PubMed] [Google Scholar]
- 34.Mehta N, Heimbach J, Lee D, et al. Wait time of less than 6 and greater than 18 months predicts hepatocellular carcinoma recurrence after liver transplantation: proposing a wait time “sweet spot”. Transplantation. 2017;101(9):2071-2078. doi: 10.1097/TP.0000000000001752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pomfret EA, Lodge JP, Villamil FG, Siegler M. Should we use living donor grafts for patients with hepatocellular carcinoma?: ethical considerations. Liver Transpl. 2011;17(suppl 2):S128-S132. doi: 10.1002/lt.22356 [DOI] [PubMed] [Google Scholar]
- 36.Liang W, Wu L, Ling X, et al. Living donor liver transplantation versus deceased donor liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18(10):1226-1236. doi: 10.1002/lt.23490 [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Pavel M, Rimola J, et al. Pilot study of living donor liver transplantation for patients with hepatocellular carcinoma exceeding Milan criteria (Barcelona Clinic Liver Cancer extended criteria). Liver Transpl. 2018;24(3):369-379. doi: 10.1002/lt.24977 [DOI] [PubMed] [Google Scholar]
- 38.Sapisochin G, Goldaracena N, Laurence JM, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: a prospective validation study. Hepatology. 2016;64(6):2077-2088. doi: 10.1002/hep.28643 [DOI] [PubMed] [Google Scholar]
- 39.Lee SD, Lee B, Kim SH, et al. Proposal of new expanded selection criteria using total tumor size and (18)F-fluorodeoxyglucose—positron emission tomography/computed tomography for living donor liver transplantation in patients with hepatocellular carcinoma: the National Cancer Center Korea criteria. World J Transplant. 2016;6(2):411-422. doi: 10.5500/wjt.v6.i2.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaido T, Ogawa K, Mori A, et al. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154(5):1053-1060. doi: 10.1016/j.surg.2013.04.056 [DOI] [PubMed] [Google Scholar]
- 41.Goldaracena N, Gorgen A, Doyle A, et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J Hepatol. 2019;70(4):666-673. doi: 10.1016/j.jhep.2018.12.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Factors Associated With Posttransplant HCC Recurrence and Poor Overall Survival
eTable 2. Clinicopathologic Characteristics of Liver Transplant Recipients by Tumor Recurrence
eTable 3. Comparison of Recurrent Tumor Characteristics by Treatment Modality
eTable 4. Factors Associated With Survival After Liver Transplantation
eFigure 1. Cohort Diagram
eFigure 2. Overall Survival and Recurrence of the Whole Cohort of 2645 Patients With HCC After Transplantation