Abstract
This case series study describes cutaneous T-cell–rich lymphoid infiltrates characterized by papulonodules on the trunk and/or extremities in 6 patients after receiving the Pfizer/BioNTech COVID-19 mRNA vaccine.
The most common skin reactions to mRNA-based vaccination against SARS-CoV-2 have included local and delayed injection site reactions and urticaria and have been more frequently associated with the Moderna vaccine vs the Pfizer/BioNTech vaccine.1 To our knowledge, cutaneous lymphoid reactions to COVID-19 vaccination have yet to be assessed in comprehensive vaccine studies.
Methods
From March to November 21, in accordance with a Northwestern University institutional review board–approved protocol, this retrospective case series included 6 cases of T-cell–rich cutaneous lymphoid infiltrates shortly after SARS-CoV-2 vaccination, 2 of which were recently described by some of our group.2 Written informed consent was obtained. This study followed the reporting guideline for case series. R, version 2021.09.1, was used for statistical analyses.
Results
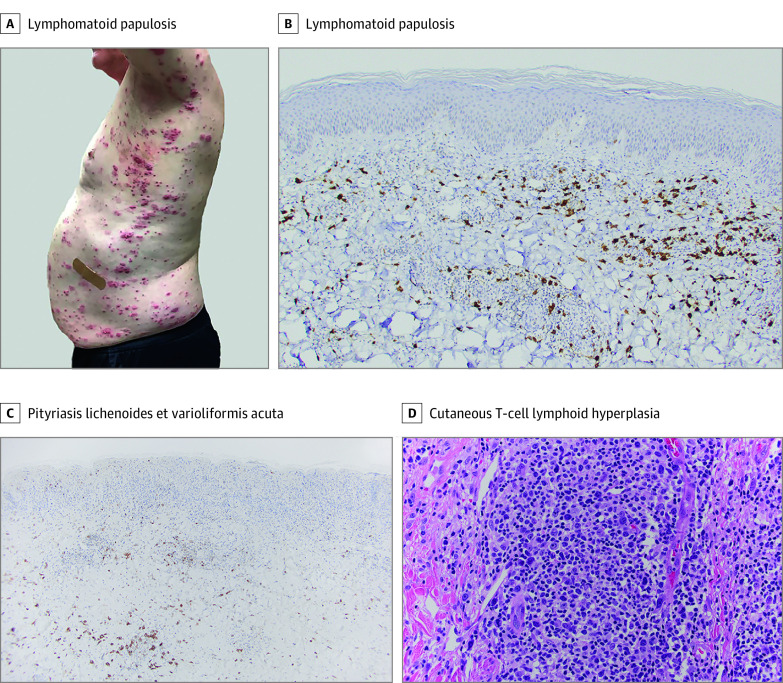
The Table summarizes patient demographics and clinical features. The cohort consisted of 3 males and 3 females (median age, 47 years [range, 18-78 years]; 100% White). One patient had a history of psoriasis and transverse myelitis, and 1 had multiple sclerosis. All patients received the Pfizer/BioNTech COVID-19 mRNA vaccine. Median time between the first vaccine dose and lesion development was 22 days (range, 4-42 days). Two patients endorsed vaccine-related constitutional symptoms, and 3 reported lesional pruritus. Cutaneous eruptions were characterized by single (n = 2) or generalized (n = 4) papulonodules on the trunk and/or extremities (Figure, A). Histologic examination revealed T-cell–predominant lymphoid infiltrates consistent with pityriasis lichenoides et varioliformis acuta (n = 2), cutaneous T-cell lymphoid hyperplasia (CLH) (n = 2), and lymphomatoid papulosis type A (n = 2) (Figure, B-D). The clinical course was indolent with resolution after various therapies. Four patients reported recrudescence after an acute viral episode; 2 had a positive SARS-CoV-2 polymerase chain reaction test result. Median follow-up time was 9.5 months (range, 7-13 months).
Table. Summary of Patient Demographics, Clinical Characteristics, and Histologic Findings of Cases From the Present Study and the Literature.
| Patient sex/age, y | Clinical history | Reaction | Time from dose 1 to onset, d | Location of lesions | Vaccine adverse effects | Diagnosis | TCR | CD4:CD8 | CD30 | Therapy | Outcome | Follow-up time, mo | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose 1 | Dose 2 | ||||||||||||
| Female/50s | Psoriasis, transverse myelitis | Multiple papules | Progressed | 14 | Trunk, extremities | None | PLEVA | – | 2:1 | + | DOX, TCS | NCR | 12 |
| Male/late teens | None | None | Multiple papules, progressive | 23 | Trunk, extremities | None | PLEVA | + | 1:3 | – | DOX, NBUVB, TCS | NCR | 10 |
| Female/70s | None | None | Single papule | 22 | Upper arm | Mild systemic symptoms | CLH | + | 2:1 | + | Surgical excision | CR | 13 |
| Male/50s | None | None | Single papule | 28 | Upper arm | Fatigue | CLH | + | 3:1 | + | Resolved with biopsy | CR | 9 |
| Male/50s | Multiple sclerosis | Multiple papules | Progressed | 4 | Trunk, extremities, palms | None | LyP type A | NA | NA | – | MTX | NCR; flare with COVID-19 | 7 |
| Female/20s | None | None | Multiple papules, progressive | 42 | Trunk, extremities | None | LyP type A | NA | NA | + | MTX | NCR; flare with non-COVID-19 viral illness | 9 |
| Male/70s (Brumfiel et al,3 2021) | PCALCL | Tumor | None | 2 | Axilla | None | CD30 LPD | + | NA | NA | None | NCR | NA |
| Female/60s (Mintoff et al,4 2021) | None | None | Single papule | 7 | Upper arm | None | CLH | NA | NA | NA | NA | NA | NA |
Abbreviations: CD30 LPD, CD30-positive lymphoproliferative disorder; CLH, cutaneous lymphoid hyperplasia; CR, complete remission; DOX, oral doxycycline; LyP, lymphomatoid papulosis; MTX, oral methotrexate; NA, not available; NBUVB, narrowband UVB light; NCR, near complete remission; PCALCL, primary cutaneous anaplastic large cell lymphoma; PLEVA, pityriasis lichenoides et varioliformis acuta; TCR, T-cell receptor (γ/β) gene rearrangement assay; TCS, topical corticosteroids.
Figure. Clinical Images and Histologic Findings Consistent With Lymphomatoid Papulosis, Pityriasis Lichenoides et Varioliformis Acuta, and Cutaneous Lymphoid Hyperplasia.
A, Generalized eruption of hemorrhagic-necrotic papules and plaques. B, Infiltrate composed of CD30-positive atypical cells (original magnification ×20). C, Infiltrate with scattered CD30 expression (original magnification ×10). D, Dense infiltrate composed of medium to large, immunoblastic and pleomorphic lymphocytes, with well-visualized atypia (hematoxylin-eosin stain; original magnification ×40).
Discussion
This case series found cutaneous T-cell–rich lymphoid infiltrates to be rare but potential complications after Pfizer/BioNTech COVID-19 mRNA vaccination. Although the Moderna and Pfizer/BioNTech mRNA vaccines have demonstrated similar efficacy and immunogenicity, reporting of vaccine-related cutaneous lymphoid proliferations has been more common after the latter.3,4 It is difficult to comment on this association owing to the small and anecdotal character of our study and lack of supportive literature. Furthermore, this study is limited in its ability to assess true incidence owing to a scarcity of reported reactions and possible confirmation bias intrinsic to self-reported data.
This cohort had 3 clinicopathological presentations characterized by T-cell–rich infiltrates, including pityriasis lichenoides et varioliformis acuta, lymphomatoid papulosis type A, and cases reported as CLH with overlapping features of primary cutaneous small to medium CD4-positive T-cell lymphoproliferative disorder. To date, cutaneous lymphoid infiltrates have yet to be thoroughly discussed in the literature on mRNA vaccine reactions. Most common cutaneous reactions to COVID-19 vaccines may occur secondary to immune activation and/or T-cell–mediated responses to vaccine components, including mRNA1; as such, we hypothesize that cutaneous lymphoid infiltrates are associated with immune system stimulation. Of note, Moderna and Pfizer/BioNTech engineered different solutions to the challenge of RNA instability inherent to mRNA vaccine technology, which may explain the differences observed between the 2 formulations’ adverse effect profiles.5 Nonetheless, in contrast to the T-cell activation of mRNA vaccines, previous studies of vaccine-induced CLH have suggested that the antigenic presence of foreign materials (eg, vaccine adjuvants, viral proteins) induces local B-cell hyperplasia.6
Our observations should raise awareness about low-grade cutaneous lymphoid reactions after COVID-19 vaccination. These findings should not preclude vaccination, and continued follow-up will help elucidate the chronicity and disease course.
References
- 1.Kroumpouzos G, Paroikaki ME, Yumeen S, Bhargava S, Mylonakis E. Cutaneous complications of mRNA and AZD1222 COVID-19 vaccines: a worldwide review. Microorganisms. 2022;10(3):624. doi: 10.3390/microorganisms10030624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeWitt T, Chung C, Manton J, et al. Rare lymphomatoid reactions following SARS-CoV-2 vaccination. JAAD Case Rep. 2022;20:26-30. doi: 10.1016/j.jdcr.2021.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brumfiel CM, Patel MH, DiCaudo DJ, Rosenthal AC, Pittelkow MR, Mangold AR. Recurrence of primary cutaneous CD30-positive lymphoproliferative disorder following COVID-19 vaccination. Leuk Lymphoma. 2021;62(10):2554-2555. doi: 10.1080/10428194.2021.1924371 [DOI] [PubMed] [Google Scholar]
- 4.Mintoff D, Scerri L, Betts A. SARS-CoV-2 mRNA vaccine injection site pseudolymphoma. J Eur Acad Dermatol Venereol. 2022;36(1):e20-e22. doi: 10.1111/jdv.17680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung YH, Beiss V, Fiering SN, Steinmetz NF. COVID-19 vaccine frontrunners and their nanotechnology design. ACS Nano. 2020;14(10):12522-12537. doi: 10.1021/acsnano.0c07197 [DOI] [PubMed] [Google Scholar]
- 6.Cerroni L, Borroni RG, Massone C, Chott A, Kerl H. Cutaneous B-cell pseudolymphoma at the site of vaccination. Am J Dermatopathol. 2007;29(6):538-542. doi: 10.1097/DAD.0b013e3181591bea [DOI] [PubMed] [Google Scholar]