Abstract
A late-stage functionalization (LSF) of the natural product andrographolide for the efficient assembly of a range of structurally interesting and diverse tricyclic-aza derivatives was developed. The key to the diversification is a photo-catalyzed intramolecular hydroamination reaction, and acridinium derivatives were demonstrated to be the optimal catalysts. Additionally, the synthesized tricyclic aza-andrographolide derivatives were found to inhibit human coronavirus with high potency.
Introduction
Natural products and their derivatives play a highly essential role in combating numerous diseases, especially cancer and infectious diseases.1 Data have shown that, from 1981 until 2019, 41% of anticancer and 66% of anti-infective small molecule drugs were derived from or inspired by natural sources.2 Compared with the traditional synthetic compounds, natural products have enormous structural complexity and scaffold diversity, which make them more likely to interact with biological macromolecules in a selective and specific manner.3 Driven by modern high-throughput drug discovery,4 there is an urgent and increasing demand for a large collection of bioactive natural-product-derived compounds with significant structural diversity and complexity. Developing facile and practical methods or strategies to construct them, however, remains a considerable interest in the field. Several strategies for the rapid and highly efficient generation of these structurally diverse natural-product-inspired libraries have been developed, including diversity-oriented synthesis from simple starting materials using convergent strategies such as multicomponent reaction,5 the use of natural products with high abundance as a starting point for the generation of complex natural product analogues,6 and solid-phase combinatorial synthesis using the above-mentioned strategies.7
The diterpene natural product andrographolide, isolated from Andrographis paniculata, possesses versatile biological activities, including anti-inflammatory,8 antimicrobial,9 immunomodulatory,10 antidiabetic,11 cardioprotective,12 hepatoprotective,13 and neuroprotective effects.14 Andrographolide and its derivatives were also found to exert anticancer effects on almost all types of cell lines with varied mechanisms of action.15 Moreover, it exerts broad-spectrum antivirus properties toward different virus infections.16
The outbreak of COVID-19, caused by SARS-CoV-2, has become a global pandemic, which has infected almost 519 million people and claimed over 6.2 million lives worldwide.17 Searching for effective antivirus treatments for COVID-19 from nature is needed, and thus, we are interested in creating a natural-product-like library with potential antivirus activity.18 Recent studies revealed that andrographolide is a potential inhibitor of the main protease of SARS-CoV-2 (Mpro) in an in silico approach, and it docks successfully in the binding site of SARS-CoV-2 Mpro.19 Considering its potent antiviral activity and potential interaction with the binding site of SARS-CoV-2, we chose andrographolide with high abundance as a template for analogue generation. Here we present a late-stage functionalization (LSF)20 approach to tricyclic aza-andrographolide derivatives using photo-catalyzed hydroamination as the key reaction, and their anti-HCoV activity.
Results and Discussion
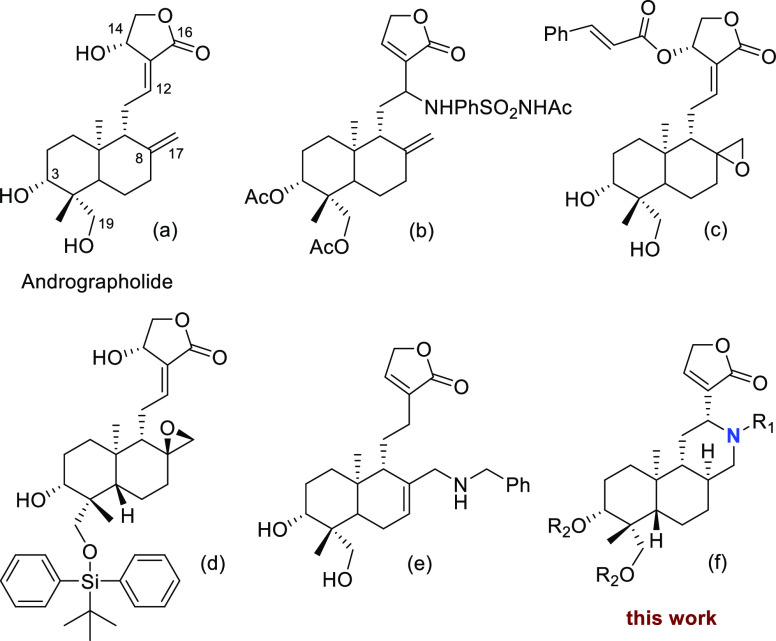
The structure modifications of andrographolide were mostly focused on the functionalization of the 3,14,19-hydroxyls, 8,17-double bond, C12–C13 double bonds, and α,β-unsaturated γ-butyrolactone moiety (Figure 1a).21 Modifications at these positions have shown improvement of antivirus or anticancer activity. For example, Golakoti and co-workers found that C-12-substituted amino 14-deoxy-andrographolide derivatives exhibited improved cytotoxic activity compared to andrographolide (Figure 1b).22 8,17-Epoxy andrographolide derivatives exhibited significant inhibitory activity toward colon cancer, breast cancer, etc. (Figure 1c,d).23,24 Andrographolide analogues with 8,17-alkene exo-to-endo isomerization (Figure 1e) displayed a potent anti-influenza virus activity, and it was approximately 1.5-fold more potent than Lianbizhi, an andrographolide analogue used clinically in China.25 Since the N-functionalization plays an important role in the activity and PK profiles of natural products, we proposed to incorporate nitrogen atom into the andrographolide skeleton to further enlarge its chemical space, providing the possibility of a novel structure of tricyclic aza-andrographolides (Figure 1f).
Figure 1.
Andrographolide (a), its representative bioactive analogues (b–e), and this work (f).
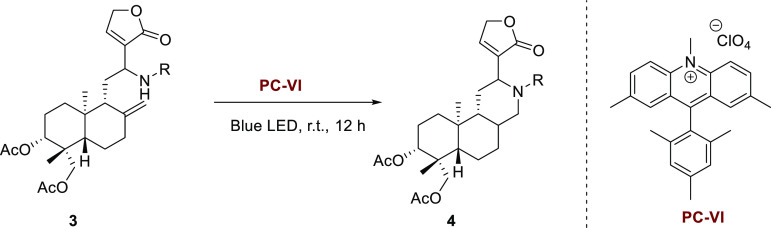
The synthetic pathways toward tricyclic aza-andrographolides mainly include two steps (Scheme 1). The acylated andrographolide 2 was aminated through a base-promoted Michael addition and elimination process, generating C-12-aminated-14-deoxy andrographolides 3,22 followed by photo-promoted intramolecular hydroamination of the 8,17-terminal double bond to yield the tricyclic aza-andrographolide derivatives. Based on previous reports,26 we initially conducted the intramolecular hydroamination of C-12-aminated-14-deoxy andrographolides with the catalysis of the Ir(III) photocatalyst (I), which was indicated to be effective for both intermolecular and intramolecular hydroamination of simple unactivated alkenes. However, the reaction proceeded with quite low yield, and some of the C-12-aminated-14-deoxy andrographolides failed to undergo Ir(III)-catalyzed hydroamination. To address this limitation, we then devoted ourselves to search for more effective catalysts.
Scheme 1. Synthetic Pathway of Tricyclic Aza-Andrographolide Derivatives.
Toward this end, C-12-benzylamino-14-deoxy andrographolide 3a was used as a model substrate for the photocatalyst screening. The reaction was carried out using 2–6% photocatalyst and 50% 2,4,6-triisopropylbenzenethiol (TRIP thiol) in toluene under blue light irradiation for 12 h at room temperature. In the presence of Ir[dF(Me)ppy]2(dtbbpy)PF6, the intramolecular hydroamination reaction produced the desired amination product 4a in 53% isolated yield (Table 1, entry 1). The related photocatalysts, such as Ir[dF(CF3)ppy]2(dtbbpy)PF6, Ir(ppy)2(dtbbpy)PF6, and Ir(dFppy)2(dtbbpy)PF6, proved less efficient, providing 4a in lower yields (entries 2–4). When using Ir(ppy)3, 4a was obtained in only a trace yield (entry 5). To our delight, the replacement of Ir catalysts with organic dye acridinium catalysts led to significantly improved yields. Three acridinium catalysts were evaluated, and catalyst VI proved superior, providing 4a in 79% yield (entries 6–8). These investigations indicated that acridinium catalyst VI was a better choice for the intramolecular hydroamination of C-12-aminated-14-deoxy andrographolides.
Table 1. Photo-catalyzed Intramolecular Hydroamination: Catalyst Evaluation.
We subsequently set out to explore the scope and potential of the photocatalyst-promoted intramolecular hydroamination. As shown in Table 2, a wide range of substrates 3 were surveyed, including those with arylmethyl, alkyl, and cycloalkyl groups. Pleasingly, most of the substrates examined underwent the cyclization smoothly to give the diverse tricyclic aza-andrographolides 4 in moderate to good yields. Electronic variation on the aryl ring, including electron-withdrawing, neutral, and electron-donating effects, did not obviously affect the efficiency of the reactions. Among these, some functional groups were well tolerated, such as nitro and hydroxyl, which could serve as handles for further synthetic manipulations. In addition, heterocycles including furan and thiophene were also compatible with the current reaction, providing the desired products in moderate yields. Lastly, a series of substrates bearing aliphatic substituents were investigated, and it was found that acyclic and cyclic amines gave diminished yields. The structures of synthesized compounds 4 were all characterized by 1H NMR, 13C NMR, and HRMS, and the stereochemistry was further confirmed by single crystal X-ray diffraction analysis of 4u (see the Supporting Information).
Table 2. Tricyclic Aza-Andrographolide Analogues Synthesized through Intramolecular Hydroaminationa.

Reaction conditions: 3 (0.05 mmol, 1 equiv), PC-VI (6 mol %), 2,4,6-triisopropylbezene-1-thiol (TRIP thiol) (0.5 equiv), toluene (2 mL), blue LED (40 W), under N2, room temperature, 12 h. Yields shown are the yields of the isolated product.
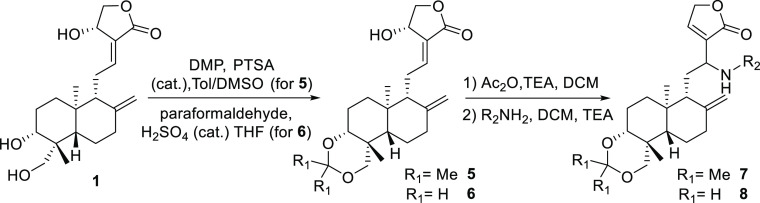
After exploring the scope of the substrates based on the 3,19-diacetate andrographolide, we next investigated other 3,19-substituted substrates, such as 3,19-ketal andrographolides, which were found to have interesting biological activity.27 To this end, two types of substrates, 7 and 8 (Scheme 2), were synthesized and subjected to the intramolecular hydroamination, leading to the rapid generation of structurally diverse aza-andrographolide derivatives 9a–p and 10a–d, as listed in Table 3.
Scheme 2. Synthetic Route for the Substrates of Intramolecular Hydroamination.
Table 3. Tricyclic Aza-Andrographolide Analogues Synthesized through Intramolecular Hydroamination.


When compounds 7 and 8 bearing arylmethyl groups served as substrates for cyclization, the desired cyclized products were produced in moderate to good yields (9a–m and 10a–d), while the substrates with branched aliphatic chains furnished the products in low yields (9o and 9p). Considering the aqueous solubility of the cyclized products, we intended to make 3,19-diol derivatives. Accordingly, the selected compound 9 was treated with acetic acid in THF and water to yield the desired product 11 in good yield (Table 3, 11a–e).
The collection of synthesized aza-andrographolide derivatives 4, 9, 10, and 11 was evaluated in the cytopathic effect (CPE) assays for their antiviral activity against the human coronavirus (HCoV) 229E. All the tested compounds were assayed at 25 μM in the MRC5 cells, and their antiviral activity was calculated based on the protection of the virus-induced CPE normalized by the virus-only control. Additionally, the cytotoxicity of compounds toward MRC5 cells was also assessed under the same conditions, but without virus infection, in parallel. The antiviral activity of compounds is expressed as % inhibition normalized by the cytotoxicity, and selected examples are depicted in Table 4 (entries 1–20). From these assessments, four types of compounds displayed a distinct inhibition profile toward HCoV 229E, with compounds 4a–y and 9a–p being potent and compounds 10a–d and 11a–e being weak. This result indicated that structural moiety at the 3,19-position is also important for activity. We found that compounds 4d, 4h, and 4l–n with halo- or CF3-substituted benzyl exhibited good inhibitory activity (∼100% inhibition) at 25 μM, whereas 4o and 4p with N-furylmethyl or N-thiophenylmethyl showed a weak inhibition effect. Bulky N-substituents (4q, 4v, and 4y) gave more favorable results compared to compounds 4r and 4w. As for compounds 9, only substituents at the benzyl-4-position with methyl (9f), tert-butyl (9 g), and nitro (9 h) demonstrated good inhibition. Noteworthily, all the tested compounds demonstrated little toxicity against the MRC5 cells (see the Supporting Information for details).
Table 4. Evaluation of the In Vitro Inhibitory Activity of Test Compounds against Human Coronavirus 229E.
| entry | CPD no. | inhibition % (25 μM) | cytotoxicity % (25 μM) | normalized inhibition % (25 μM) |
|---|---|---|---|---|
| 1 | 4a | 82.4 | 15.5 | 66.8 |
| 2 | 4d | 106.9 | –3.0 | 110.0 |
| 3 | 4h | 113.5 | –5.4 | 118.9 |
| 4 | 4j | 50.4 | –5.2 | 55.6 |
| 5 | 4l | 128.4 | –19.5 | 148.0 |
| 6 | 4m | 116.5 | –13.2 | 129.8 |
| 7 | 4n | 96.6 | –4.9 | 101.5 |
| 8 | 4o | 53.9 | –4.5 | 58.4 |
| 9 | 4p | 31.2 | 4.2 | 26.9 |
| 10 | 4q | 106.4 | –14.7 | 121.2 |
| 11 | 4r | 45.8 | –5.6 | 51.4 |
| 12 | 4v | 104.9 | 3.2 | 101.7 |
| 13 | 4w | 6.8 | –4.5 | 11.3 |
| 14 | 4y | 116.8 | –18.2 | 135.0 |
| 15 | 9f | 98.8 | –1.6 | 100.5 |
| 16 | 9g | 103.1 | –0.2 | 103.3 |
| 17 | 9h | 97.5 | –4.7 | 102.3 |
| 18 | 10d | 45.1 | –13.4 | 58.6 |
| 19 | 11a | 34.9 | –4.8 | 39.7 |
| 20 | andrographolide 1 | 58.4 | 23.6 | 34.8 |
| EC50 (μM) | CC50 (μM) | |||
| 21 | 4l | 18.7 | >100 | |
| 22 | 4m | 15.7 | >100 | |
| 23 | 4y | 10.1 | >100 | |
| 24 | 9f | 23.1 | >100 | |
Four compounds—4l, 4m, 4y, and 9f—were then chosen to assess the anti-HCoV 229E activity through full dose–response curves. These compounds exhibited great potency with a half-maximal effective concentration (EC50) ranging from 10.1 to 23.1 μM (Table 4, entries 21–24), and compound 4y was identified as the most promising one, with an EC50 of 10.1 μM. The four tested compounds displayed no signs of cytotoxicity, with a half-maximal cytotoxic concentration (CC50) of >100 μM. This result indicated that our synthesized compounds have a potential as anti-HCoV hits for further development.
Conclusions
In conclusion, we have developed a concise approach to the structurally diverse and bioactive tricyclic aza-andrographolide derivatives through photo-redox catalyzed intramolecular hydroamination, in which the acridinium catalyst VI was proven to be the most effective. We anticipate that this method or strategy will find a variety of applications in the late-stage functionalization of natural products and drugs. Preliminary screening of the generated library of aza-andrographolide derivatives led to the identification of small molecules with anti-HCoV activity, and they can serve as promising anti-HCoV hits for further drug development.
Experimental Section
General Information
Unless noted otherwise, all reactions were performed under a nitrogen atmosphere, and materials obtained from commercial suppliers were used without further purification. The purification of products was conducted by flash column chromatography on a silica gel (200–300 mesh). 1H NMR spectra were recorded on 300–500 MHz spectrometers using the residual solvent (δ (CDCl3) = 7.26) as internal standard. All the coupling constants are reported in hertz. 13C NMR spectra were recorded on the same instruments, and chemical shifts were measured relative to solvent resonances (δ (CDCl3) = 77.0). High-resolution mass spectra were obtained on a quadrupole time-of-flight (QqTOF) mass spectrometer utilizing the electrospray ionization (ESI) method.
General Procedure for Photocatalytic Intramolecular Hydroamination
A 16 × 125 mm screw cap culture tube with a Teflon septum was equipped with a Teflon stir bar and charged with the secondary amine (0.05 mmol), 9-mesityl-2,7,10-trimethylacridinium perchlorate (1.5 mg, 0.0030 mmol, 6 mol %), and 2,4,6-triisopropylbezene-1-thiol (6 mg, 0.025 mmol, 0.5 equiv).The reaction vessel was placed under a vacuum and backfilled with argon three times. Dry toluene (2 mL, 0.05 M) was then added. The resulting pale green solution was then irradiated by two 40 W Kessil KSH150B blue LED lamps and stirred vigorously until starting material consumption was observed by TLC. Throughout the reaction, a fan was positioned to keep the culture tube at room temperature. Upon completion as determined by GC analysis, the reaction mixture was concentrated and then purified by silica gel column chromatography to obtain the desired product.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-benzyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4a)
79% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.42 (s, 1H), 7.37–7.18 (m, 5H), 4.78 (s, 2H), 4.57 (dd, J = 10.2, 5.6 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.10 (d, J = 12.0 Hz, 1H), 3.82 (s, 1H), 3.15 (d, J = 9.9 Hz, 1H), 2.96 (d, J = 13.9 Hz, 1H), 2.87–2.73 (m, 1H), 2.02 (d, J = 2.3 Hz, 6H), 1.60 (qdd, J = 26.7, 12.8, 2.8 Hz, 10H), 1.11 (dd, J = 27.1, 7.5 Hz, 2H), 0.98 (s, 3H), 0.88–0.74 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.65, 171.15, 170.68, 146.01, 139.28, 137.42, 130.11, 128.50, 127.09, 80.21, 77.58, 77.26, 76.94, 70.59, 65.18, 60.47, 59.48, 59.41, 55.37, 53.89, 41.34, 36.79, 36.11, 35.06, 33.23, 31.99, 29.96, 29.88, 29.51, 27.40, 23.97, 22.79, 21.92, 21.38, 21.28, 21.24, 14.63; HRMS (m/z) calcd for C31H42NO6 (+) 524.3012, found 524.3004.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(4-fluorobenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4b)
63% yield, yellow solid; 1H NMR (500 MHz, CDCl3) δ 7.40 (s, 1H), 7.22 (dd, J = 8.4, 5.6 Hz, 2H), 6.97 (t, J = 8.7 Hz, 2H), 4.79 (s, 2H), 4.56 (dd, J = 11.1, 4.8 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.12–4.07 (m, 1H), 3.77 (d, J = 13.8 Hz, 1H), 3.14 (d, J = 9.3 Hz, 1H), 2.92 (d, J = 13.8 Hz, 1H), 2.77 (dd, J = 11.1, 3.2 Hz, 1H), 2.02 (d, J = 3.8 Hz, 6H), 1.80–1.42 (m, 10H), 1.16–1.04 (m, 2H), 0.98 (s, 3H), 0.85–0.76 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 173.26, 170.87, 170.42, 162.85, 160.90, 145.78, 137.22, 134.71, 134.68, 129.78, 129.72, 115.15, 114.98, 80.02, 70.30, 64.96, 60.18, 59.24, 58.51, 55.22, 53.77, 41.18, 36.63, 35.93, 34.90, 32.97, 31.82, 23.78, 22.58, 21.74, 21.12, 21.02, 14.43; HRMS (m/z) calcd for C31H41FNO6 (+) 542.2918, found 542.2918.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(4-chlorobenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4c)
60% yield, yellow solid; 1H NMR (500 MHz, CDCl3) δ 7.39 (s, 1H), 7.28–7.26 (m, 1H), 7.22 (t, J = 7.8 Hz, 2H), 7.17 (d, J = 7.0 Hz, 1H), 4.79 (s, 2H), 4.57 (dd, J = 11.1, 4.6 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.11 (d, J = 11.8 Hz, 1H), 3.78 (d, J = 14.0 Hz, 1H), 3.15 (d, J = 9.6 Hz, 1H), 2.93 (d, J = 14.0 Hz, 1H), 2.76 (dd, J = 11.1, 3.2 Hz, 1H), 2.03 (d, J = 4.3 Hz, 6H), 1.78–1.38 (m, 10H), 1.17–1.03 (m, 2H), 0.99 (s, 3H), 0.85 (s, 5H); 13C NMR (125 MHz, CDCl3) δ 173.36, 170.99, 170.53, 145.91, 137.17, 132.59, 129.65, 129.07, 128.48, 128.26, 125.33, 80.05, 77.33, 77.07, 76.82, 70.40, 65.02, 60.27, 59.25, 58.61, 55.22, 53.74, 41.19, 36.64, 35.96, 34.91, 32.99, 31.82, 23.80, 22.63, 21.76, 21.21, 21.11, 14.47; HRMS (m/z) calcd for C31H41ClNO6 (+) 558.2622, found 558.2620.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(4-bromobenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4d)
50% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.41 (d, J = 8.2 Hz, 3H), 7.16 (d, J = 8.2 Hz, 2H), 4.79 (s, 2H), 4.57 (dd, J = 10.1, 5.6 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.14–4.07 (m, 1H), 3.76 (d, J = 14.0 Hz, 1H), 3.15 (d, J = 10.1 Hz, 1H), 2.92 (d, J = 14.0 Hz, 1H), 2.76 (d, J = 8.3 Hz, 1H), 2.03 (d, J = 2.8 Hz, 6H), 1.82–1.36 (m, 10H), 1.17–1.02 (m, 2H), 0.98 (s, 3H), 0.88–0.71 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.56, 171.21, 170.74, 146.14, 138.34, 137.31, 131.63, 130.24, 120.86, 80.22, 77.58, 77.26, 76.94, 70.62, 65.22, 60.44, 59.42, 58.82, 55.39, 53.90, 41.37, 36.82, 36.15, 35.07, 33.16, 31.99, 23.98, 22.82, 21.94, 21.41, 21.32, 14.66; HRMS (m/z) calcd for C31H41BrNO6 (+) 602.2117, found 602.2110.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-3-(4-methylbenzyl)-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4e)
53% yield, yellow solid; 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H), 7.15 (d, J = 7.9 Hz, 2H), 7.10 (d, J = 7.8 Hz, 2H), 4.79 (s, 2H), 4.57 (dd, J = 11.2, 4.8 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.11 (d, J = 11.7 Hz, 1H), 3.80 (d, J = 13.7 Hz, 1H), 3.14 (d, J = 10.3 Hz, 1H), 2.94 (d, J = 13.8 Hz, 1H), 2.83 (dd, J = 11.1, 2.7 Hz, 1H), 2.32 (s, 3H), 2.02 (d, J = 3.7 Hz, 6H), 1.80–1.40 (m, 10H), 1.09 (dd, J = 21.0, 19.2 Hz, 2H), 0.98 (s, 3H), 0.85–0.76 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 173.64, 171.14, 170.68, 145.93, 137.53, 136.73, 136.06, 129.22, 128.54, 80.29, 77.51, 77.26, 77.01, 70.59, 65.22, 60.41, 59.41, 59.19, 55.47, 53.99, 41.43, 36.87, 36.17, 35.13, 33.29, 32.06, 29.91, 24.03, 22.83, 21.99, 21.38, 21.29, 14.67, 14.31; HRMS (m/z) calcd for C32H44NO6 (+) 538.3169, found 538.3159.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(4-(tert-butyl)benzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4f)
45% yield, yellow solid; 1H NMR (500 MHz, CDCl3) δ 7.42 (s, 1H), 7.32 (d, J = 8.2 Hz, 2H), 7.20 (d, J = 8.1 Hz, 2H), 4.77 (d, J = 4.4 Hz, 2H), 4.57 (dd, J = 11.3, 4.7 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.12 (d, J = 11.7 Hz, 1H), 3.81 (d, J = 13.9 Hz, 1H), 3.15 (d, J = 10.1 Hz, 1H), 2.96 (d, J = 13.9 Hz, 1H), 2.87 (dd, J = 11.1, 2.8 Hz, 1H), 2.03 (d, J = 2.9 Hz, 6H), 1.82–1.50 (m, 10H), 1.31 (s, 9H), 1.08 (d, J = 11.1 Hz, 2H), 0.99 (s, 3H), 0.86–0.78 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 173.66, 171.14, 170.68, 150.08, 145.91, 137.60, 136.19, 128.21, 125.44, 77.52, 77.26, 77.01, 60.56, 59.43, 59.13, 55.50, 54.00, 41.46, 36.90, 36.21, 35.18, 34.70, 33.34, 32.10, 31.64, 24.06, 22.86, 22.02, 21.39, 21.28, 14.69; HRMS (m/z) calcd for C35H50NO6 (+) 580.3638 found 580.3629.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(4-methoxybenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4g)
53% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.44 (s, 1H), 7.16 (d, J = 8.5 Hz, 2H), 6.83 (d, J = 8.6 Hz, 2H), 4.80 (s, 2H), 4.56 (dd, J = 10.0, 5.6 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.09 (d, J = 11.8 Hz, 1H), 3.78 (s, 4H), 3.12 (d, J = 9.7 Hz, 1H), 2.92 (d, J = 13.6 Hz, 1H), 2.81 (d, J = 8.3 Hz, 1H), 2.02 (d, J = 2.5 Hz, 6H), 1.80–1.37 (m, 10H), 1.17–1.03 (m, 2H), 0.97 (s, 3H), 0.86–0.71 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.85, 171.21, 170.73, 158.81, 146.14, 137.33, 130.79, 129.81, 113.86, 77.68, 77.26, 76.83, 65.18, 60.13, 59.26, 58.74, 55.47, 55.34, 53.85, 41.32, 36.77, 36.09, 34.98, 33.19, 32.01, 23.96, 22.78, 21.92, 21.41, 21.31, 14.63; HRMS (m/z) calcd for C32H44FNO7 (+) 554.3118, found 554.3110.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)-3-(4-(trifluoromethyl)benzyl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4h)
57% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.55 (d, J = 8.1 Hz, 2H), 7.41 (d, J = 7.4 Hz, 3H), 4.79 (s, 2H), 4.57 (dd, J = 10.2, 5.6 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.11 (d, J = 11.6 Hz, 1H), 3.86 (d, J = 14.3 Hz, 1H), 3.19 (d, J = 9.7 Hz, 1H), 3.03 (d, J = 14.4 Hz, 1H), 2.76 (dd, J = 10.7, 2.5 Hz, 1H), 2.03 (d, J = 2.2 Hz, 6H), 1.87–1.34 (m, 10H), 1.21–1.12 (m, 1H), 1.12–1.04 (m, 1H), 0.99 (s, 3H), 0.91–0.75 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.53, 171.21, 170.75, 146.17, 143.71, 137.31, 129.64, 129.26, 128.63, 128.45, 125.50, 125.46, 80.22, 77.58, 77.26, 76.94, 70.60, 65.22, 60.61, 59.44, 59.03, 55.40, 53.90, 41.37, 36.82, 36.16, 35.10, 33.15, 31.98, 23.98, 22.82, 21.94, 21.41, 21.31, 14.66; HRMS (m/z) calcd for C32H41F3NO6 (+) 592.2886, found 592.2880.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-3-(4-nitrobenzyl)-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4i)
40% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 8.16 (d, J = 8.6 Hz, 2H), 7.48 (d, J = 8.5 Hz, 2H), 7.40 (s, 1H), 4.80 (s, 2H), 4.57 (dd, J = 10.0, 5.5 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.12 (d, J = 11.7 Hz, 1H), 3.89 (d, J = 14.7 Hz, 1H), 3.22 (d, J = 9.4 Hz, 1H), 3.09 (d, J = 14.9 Hz, 1H), 2.77–2.64 (m, 1H), 2.03 (d, J = 1.9 Hz, 6H), 1.74–1.57 (m, 10H), 1.08 (d, J = 11.0 Hz, 2H), 0.99 (s, 3H), 0.87 (s, 5H); 13C NMR (75 MHz, CDCl3) δ 173.41, 171.23, 170.78, 147.51, 147.33, 146.34, 137.22, 129.01, 123.87, 80.22, 77.59, 77.27, 76.95, 70.60, 65.23, 60.78, 59.50, 58.92, 55.41, 53.92, 41.39, 36.84, 36.19, 35.14, 33.07, 31.98, 23.99, 22.84, 21.94, 21.44, 21.34, 14.69; HRMS (m/z) calcd for C31H41N2O8 (+) 569.2863, found 569.2857.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(4-hydroxybenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4j)
57% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.26 (s, 1H), 7.11 (d, J = 8.1 Hz, 2H), 6.75 (d, J = 8.4 Hz, 2H), 4.84 (s, 2H), 4.56 (dd, J = 10.0, 5.8 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.13–4.07 (m, 1H), 3.79 (s, 1H), 3.00 (m, 4H), 2.03 (d, J = 2.6 Hz, 6H), 1.84–1.38 (m, 10H), 1.15–1.02 (m, 2H), 0.98 (s, 3H), 0.86–0.76 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 172.92, 171.04, 170.59, 152.64, 135.92, 125.06, 124.16, 115.29, 105.83, 80.05, 67.11, 66.68, 65.03, 60.41, 59.10, 58.54, 55.18, 41.18, 36.62, 35.96, 33.63, 31.85, 29.73, 23.78, 22.62, 21.74, 21.21, 21.11, 14.43; HRMS (m/z) calcd for C31H43NO7 (+) 540.2961, found 540.2956.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-3-(2-methylbenzyl)-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4k)
47% yield, yellow solid; 1H NMR (500 MHz, CDCl3) δ 7.43 (d, J = 7.1 Hz, 1H), 7.36 (s, 1H), 7.14 (dt, J = 16.7, 7.1 Hz, 3H), 4.75 (d, J = 8.5 Hz, 2H), 4.59 (dd, J = 11.3, 4.9 Hz, 1H), 4.38 (d, J = 11.7 Hz, 1H), 4.16–4.11 (m, 1H), 3.68 (d, J = 14.5 Hz, 1H), 3.19 (d, J = 10.3 Hz, 1H), 3.07–2.98 (m, 1H), 2.83 (d, J = 9.6 Hz, 1H), 2.24 (s, 3H), 2.03 (d, J = 3.5 Hz, 6H), 1.79–1.58 (m, 10H), 1.09 (d, J = 10.7 Hz, 2H), 1.00 (s, 3H), 0.91–0.85 (m, 5H); 13C NMR (125 MHz, CDCl3) δ 173.83, 171.22, 170.75, 145.98, 137.46, 136.64, 130.35, 128.41, 126.82, 126.07, 77.58, 77.26, 76.94, 70.61, 65.28, 60.82, 59.83, 57.26, 55.44, 54.00, 41.39, 36.85, 36.17, 35.18, 33.42, 32.04, 24.02, 22.84, 21.98, 21.43, 21.33, 19.60, 14.68; HRMS (m/z) calcd for C32H44NO6 (+) 538.3169, found 538.3176.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(2-fluorobenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4l)
59% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.48 (s, 1H), 7.34 (t, J = 7.4 Hz, 1H), 7.24 7.15 (m, 1H), 7.09 (t, J = 7.1 Hz, 1H), 7.0–6.94 (m, 1H), 4.83 (s, 2H), 4.57 (dd, J = 10.3, 5.4 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.10 (d, J = 11.8 Hz, 1H), 3.81 (d, J = 13.8 Hz, 1H), 3.12 (dd, J = 22.3, 12.2 Hz, 2H), 2.85 (dd, J = 11.0, 2.8 Hz, 1H), 2.02 (d, J = 1.8 Hz, 6H), 1.83–1.37 (m, 10H), 1.11 (d, J = 11.4 Hz, 2H), 0.98 (s, 3H), 0.86–0.75 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.59, 171.11, 170.65, 162.54, 160.58, 146.14, 137.15, 131.26, 128.88, 124.12, 115.61, 115.44, 80.25, 77.51, 77.26, 77.01, 70.63, 65.20, 60.37, 59.58, 55.44, 53.86, 52.68, 41.42, 36.85, 36.16, 35.06, 33.31, 32.07, 24.02, 22.83, 21.97, 21.37, 21.27, 14.65; HRMS (m/z) calcd for C31H41FNO6 (+) 542.2918, found 542.2910.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(3-chlorobenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4m)
64% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.42 (s, 1H), 7.30 (s, 1H), 7.21 (d, J = 5.7 Hz, 2H), 7.17–7.10 (m, 1H), 4.80 (s, 2H), 4.58 (d, J = 9.6 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.11 (d, J = 11.6 Hz, 1H), 3.80 (d, J = 14.1 Hz, 1H), 3.16 (d, J = 10.1 Hz, 1H), 2.93 (d, J = 14.1 Hz, 1H), 2.78 (d, J = 8.2 Hz, 1H), 2.03 (d, J = 3.0 Hz, 6H), 1.84–1.34 (m, 10H), 1.10 (dd, J = 17.6, 16.0 Hz, 2H), 0.99 (s, 3H), 0.90–0.76 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.61, 171.21, 170.74, 146.13, 141.65, 137.31, 134.51, 129.81, 128.35, 127.34, 126.66, 80.23, 77.58, 77.26, 76.94, 70.64, 65.25, 60.56, 59.38, 58.96, 55.39, 53.90, 41.36, 36.82, 36.16, 35.04, 33.20, 32.00, 23.98, 22.83, 21.95, 21.41, 21.32, 14.66; HRMS (m/z) calcd for C31H41ClNO6 (+) 558.2622, found 558.2618.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(3-chloro-4-fluorobenzyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4n)
60% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.41 (s, 1H), 7.34 (d, J = 6.7 Hz, 1H), 7.09 (dd, J = 13.6, 6.6 Hz, 2H), 4.81 (s, 2H), 4.58 (d, J = 9.2 Hz, 1H), 4.37 (d, J = 11.6 Hz, 1H), 4.11 (d, J = 11.5 Hz, 1H), 3.75 (d, J = 13.9 Hz, 1H), 3.15 (d, J = 10.1 Hz, 1H), 2.89 (d, J = 14.0 Hz, 1H), 2.74 (d, J = 9.0 Hz, 1H), 2.03 (s, 6H), 1.64 (m, J = 48.5, 31.1, 19.0 Hz, 10H), 1.08 (d, J = 12.1 Hz, 2H), 0.99 (s, 3H), 0.86 (s, 5H); 13C NMR (75 MHz, CDCl3) δ 173.52, 171.21, 170.75, 158.51, 156.05, 146.15, 137.29, 136.50, 130.26, 128.10, 128.03, 121.12, 120.95, 116.65, 116.44, 80.22, 77.58, 77.27, 76.95, 70.62, 65.23, 60.42, 59.39, 58.34, 55.39, 53.92, 41.36, 36.82, 36.16, 35.05, 33.14, 32.00, 23.98, 22.83, 21.94, 21.41, 21.32, 14.66; HRMS (m/z) calcd for C31H40ClFNO6 (+) 576.2528, found 576.2525.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-(furan-2-ylmethyl)-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4o)
53% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H), 7.35 (d, J = 1.1 Hz, 1H), 6.28 (dd, J = 3.1, 1.9 Hz, 1H), 6.13 (d, J = 3.0 Hz, 1H), 4.82 (s, 2H), 4.57 (s, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.10 (d, J = 11.6 Hz, 1H), 3.69 (d, J = 14.5 Hz, 1H), 3.28 (d, J = 14.5 Hz, 1H), 3.09 (d, J = 9.7 Hz, 1H), 2.92 (dd, J = 11.3, 3.4 Hz, 1H), 2.02 (d, J = 6.3 Hz, 6H), 1.77–1.38 (m, 10H), 1.06 (d, J = 12.6 Hz, 2H), 0.98 (s, 3H), 0.83 (s, 3H), 0.80–0.67 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 173.60, 171.20, 170.74, 146.44, 142.38, 130.34, 110.38, 108.92, 80.23, 77.51, 77.26, 77.01, 60.65, 58.65, 55.40, 53.74, 51.81, 41.40, 36.82, 36.15, 35.08, 33.07, 32.06, 24.00, 22.83, 21.97, 21.42, 21.33, 14.66; HRMS (m/z) calcd for C29H40NO7 (+) 514.2805, found 514.2800.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)-3-(thiophen-2-ylmethyl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4p)
60% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.51 (s, 1H), 7.20 (d, J = 4.9 Hz, 1H), 6.93 (dd, J = 5.0, 3.5 Hz, 1H), 6.84 (d, J = 3.2 Hz, 1H), 4.82 (s, 2H), 4.56 (dd, J = 10.1, 5.7 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.14–4.08 (m, 1H), 3.89 (d, J = 14.5 Hz, 1H), 3.36 (d, J = 14.5 Hz, 1H), 3.17 (d, J = 9.6 Hz, 1H), 2.95 (dd, J = 11.1, 3.3 Hz, 1H), 2.03 (d, J = 3.5 Hz, 6H), 1.83–1.42 (m, 10H), 1.09 (s, 2H), 0.98 (s, 3H), 0.87–0.73 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.65, 171.21, 170.73, 146.09, 142.71, 137.10, 126.91, 125.65, 124.84, 80.23, 77.58, 77.47, 77.26, 76.94, 70.74, 65.23, 60.42, 58.72, 55.39, 53.93, 53.82, 41.38, 36.82, 36.15, 35.13, 33.33, 32.02, 23.99, 22.83, 21.95, 21.42, 21.32, 14.65; HRMS (m/z) calcd for C29H40NO6S (+) 530.2576, found 530.2574.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-3-(naphthalen-1-ylmethyl)-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4q)
53% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 8.14–8.06 (m, 1H), 7.88–7.80 (m, 1H), 7.74 (d, J = 8.1 Hz, 1H), 7.61 (d, J = 6.9 Hz, 1H), 7.52–7.39 (m, 3H), 7.37 (s, 1H), 4.64 (dt, J = 16.0, 11.9 Hz, 3H), 4.38 (d, J = 11.7 Hz, 1H), 4.12 (dd, J = 13.3, 5.6 Hz, 2H), 3.55 (d, J = 14.6 Hz, 1H), 3.28 (d, J = 9.9 Hz, 1H), 2.87 (dd, J = 11.1, 2.6 Hz, 1H), 2.03 (s, 6H), 1.86–1.42 (m, 10H), 1.11 (s, 2H), 0.99 (s, 3H), 0.87 (s, 5H); 13C NMR (75 MHz, CDCl3) δ 173.82, 171.22, 170.75, 146.02, 137.38, 135.01, 133.90, 132.13, 128.80, 127.65, 126.01, 125.97, 125.83, 125.73, 123.98, 80.27, 77.58, 77.26, 76.94, 70.57, 65.27, 61.21, 60.10, 57.76, 55.42, 54.01, 41.38, 36.85, 36.17, 35.20, 33.41, 32.01, 24.01, 22.83, 21.96, 21.42, 21.32, 14.70; HRMS (m/z) calcd for C35H44NO6 (+) 574.3169, found 574.3138.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)-3-propyltetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4r)
43% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 4.87–4.78 (m, 2H), 4.57 (dd, J = 10.8, 5.3 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.12 (d, J = 11.7 Hz, 1H), 3.16–3.02 (m, 2H), 2.51 (dd, J = 11.6, 5.4 Hz, 1H), 2.03 (d, J = 8.3 Hz, 8H), 1.71–1.42 (m, 10H), 1.13–1.04 (m, 2H), 0.99 (s, 3H), 0.83 (d, J = 16.0 Hz, 10H); 13C NMR (100 MHz, CDCl3) δ 173.66, 171.18, 170.73, 139.16, 114.38, 80.20, 77.51, 77.26, 77.00, 70.71, 65.20, 59.95, 59.12, 57.07, 55.38, 53.79, 41.38, 36.79, 36.15, 34.80, 32.73, 32.11, 23.97, 22.82, 21.99, 21.41, 21.33, 19.26, 14.61, 12.04; HRMS (m/z) calcd for C27H42NO66 (+) 476.3012, found 476.3005.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-isopropyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4s)
30% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.66 (s, 1H), 4.95–4.76 (m, 2H), 4.65–4.48 (m, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.12 (d, J = 11.6 Hz, 1H), 3.51 (d, J = 10.2 Hz, 1H), 3.02 (dd, J = 39.3, 8.4 Hz, 2H), 2.04 (d, J = 6.4 Hz, 7H), 1.71 (ddd, J = 16.5, 10.0, 4.6 Hz, 10H), 1.16 (d, J = 6.6 Hz, 3H), 1.10 (s, 2H), 1.00 (s, 3H), 0.98–0.93 (m, 3H), 0.91–0.77 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.16, 171.13, 170.77, 149.90, 139.99, 80.11, 77.51, 77.26, 77.01, 70.86, 65.24, 56.39, 55.36, 53.91, 51.02, 41.37, 36.80, 36.27, 32.05, 29.94, 29.52, 27.51, 23.94, 22.91, 21.97, 21.41, 21.33, 14.58; HRMS (m/z) calcd for C27H42NO6 (+) 476.3012, found 476.3012.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-butyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4t)
50% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.41 (s, 1H), 4.93–4.78 (m, 2H), 4.56 (dd, J = 10.1, 5.9 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.11 (dd, J = 9.5, 2.2 Hz, 1H), 3.11–2.99 (m, 2H), 2.59–2.46 (m, 1H), 2.03 (d, J = 6.4 Hz, 8H), 1.80–1.32 (m, 13H), 1.14–1.04 (m, 2H), 0.99 (s, 3H), 0.86 (d, J = 7.3 Hz, 4H), 0.85–0.74 (m, 5H); 13C NMR (75 MHz, CDCl3) δ 173.70, 171.19, 170.73, 146.57, 136.77, 77.58, 77.26, 76.94, 70.64, 65.18, 60.12, 59.24, 55.39, 55.06, 53.85, 41.38, 36.79, 36.13, 35.01, 32.93, 32.15, 28.53, 23.98, 22.81, 22.00, 21.41, 21.33, 20.87, 14.62, 14.21; HRMS (m/z) calc. For C28H44NO6 (+) 490.3169, found 490. 3164.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-isobutyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4u)
47% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.32 (s, 1H), 4.91–4.71 (m, 2H), 4.57 (dd, J = 10.7, 5.3 Hz, 1H), 4.37 (d, J = 11.7 Hz, 1H), 4.12 (d, J = 11.7 Hz, 1H), 2.97 (dd, J = 18.2, 8.9 Hz, 2H), 2.10 (t, J = 12.0 Hz, 1H), 2.03 (d, J = 7.6 Hz, 6H), 1.81–1.43 (m, 12H), 1.12–1.04 (m, 2H), 0.99 (s, 3H), 0.85 (d, J = 8.7 Hz, 8H), 0.78 (d, J = 6.3 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ 173.92, 171.22, 170.73, 146.18, 137.79, 80.28, 77.58, 77.26, 76.94, 70.54, 65.24, 63.57, 60.65, 59.84, 55.43, 53.81, 41.40, 36.80, 36.11, 35.10, 33.46, 32.24, 25.92, 24.00, 22.83, 22.03, 21.51, 21.43, 21.34, 20.80, 14.66; HRMS (m/z) calcd for C28H44NO6 (+) 490.3169, found 490. 3164.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-isopentyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4v)
39% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.43 (s, 1H), 4.91–4.75 (m, 2H), 4.56 (dd, J = 10.7, 5.4 Hz, 1H), 4.36 (d, J = 11.7 Hz, 1H), 4.17–4.09 (m, 1H), 3.16–3.01 (m, 2H), 2.62–2.52 (m, 1H), 2.10–1.97 (m, 8H), 1.77–1.39 (m, 11H), 1.38–1.28 (m, 2H), 1.16–1.05 (m, 2H), 0.99 (s, 3H), 0.88–0.72 (m, 11H); 13C NMR (100 MHz, CDCl3) δ 173.55, 171.07, 170.64, 146.42, 136.39, 80.24, 77.51, 77.26, 77.01, 70.62, 65.17, 60.56, 60.11, 59.27, 55.46, 53.90, 53.55, 41.45, 36.85, 36.18, 35.21, 34.99, 32.83, 32.15, 29.90, 26.72, 24.01, 23.06, 22.83, 22.59, 22.03, 21.35, 21.27, 14.63; HRMS (m/z) calcd for C29H46NO6 (+) 504.3325, found 504.3323.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-cyclopentyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4w)
45% yield, yellow solid; 1H NMR (500 MHz, MeOD) δ 7.73 (s, 1H), 4.94 (dd, J = 3.6, 1.4 Hz, 2H), 4.57 (dd, J = 11.6, 4.6 Hz, 1H), 4.46 (d, J = 11.7 Hz, 1H), 4.14 (d, J = 11.7 Hz, 1H), 3.55 (d, J = 10.5 Hz, 1H), 3.44–3.33 (m, 1H), 3.03 (dd, J = 11.5, 3.4 Hz, 1H), 2.27–2.10 (m, 1H), 2.03 (d, J = 8.8 Hz, 6H), 1.83–1.47 (m, 18H), 1.21 (d, J = 13.8 Hz, 2H), 1.03 (s, 3H), 0.98–0.85 (m, 5H); 13C NMR (125 MHz, MeOD) δ 173.83, 171.37, 170.93, 151.14, 139.40, 80.12, 71.09, 64.53, 62.01, 58.10, 54.80, 53.11, 51.59, 48.11, 47.94, 47.77, 47.60, 47.43, 47.26, 47.09, 41.01, 36.10, 35.61, 34.02, 31.32, 28.22, 24.27, 23.96, 23.36, 22.18, 21.55, 21.47, 19.68, 19.59, 13.28; HRMS (m/z) calcd for C29H44NO6 (+) 502.3169, found 502.3163.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-cyclohexyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4x)
37% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.39 (s, 1H), 4.85 (d, J = 6.2 Hz, 2H), 4.66–4.49 (m, 1H), 4.38 (d, J = 11.7 Hz, 1H), 4.11 (d, J = 11.7 Hz, 1H), 3.47 (d, J = 9.5 Hz, 1H), 2.88 (d, J = 10.3 Hz, 1H), 2.42 (s, 1H), 2.04 (d, J = 5.9 Hz, 6H), 1.75–1.46 (m, 21H), 1.11–1.05 (m, 2H), 1.00 (s, 4H), 0.86 (s, 5H); 13C NMR (100 MHz, CDCl3) δ 171.54, 171.10, 170.79, 146.49, 133.32, 80.01, 77.56, 77.25, 76.93, 75.48, 65.32, 63.69, 55.26, 54.67, 54.01, 52.72, 41.29, 36.79, 36.28, 34.40, 32.17, 31.88, 29.93, 29.55, 24.40, 23.86, 22.93, 21.92, 21.41, 21.32, 14.53, 14.30; HRMS (m/z) calcd for C30H46NO6 (+) 516.3325, found 516.3326.
((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Acetoxy-3-cycloheptyl-7,10a-dimethyl-2-(2-oxo-2,5-dihydrofuran-3-yl)tetradecahydrobenzo[f]isoquinolin-7-yl)methyl Acetate (4y)
43% yield, yellow solid; 1H NMR (500 MHz, MeOD) δ 7.73 (s, 1H), 5.01–4.90 (m, 2H), 4.57 (dd, J = 11.6, 4.6 Hz, 1H), 4.46 (d, J = 11.7 Hz, 1H), 4.14 (d, J = 11.7 Hz, 1H), 3.66 (s, 1H), 3.03 (d, J = 9.1 Hz, 1H), 2.81 (s, 1H), 2.31–2.09 (m, 1H), 2.03 (d, J = 8.5 Hz, 6H), 1.91–1.37 (m, 20H), 1.28–1.08 (m, 4H), 1.02 (s, 3H), 0.98–0.82 (m, 5H); 13C NMR (125 MHz, MeOD) δ 173.78, 171.39, 170.94, 148.73, 143.94, 80.13, 75.62, 71.16, 64.52, 55.97, 54.82, 53.23, 51.88, 41.01, 36.09, 35.63, 34.22, 32.57, 31.30, 27.45, 27.27, 25.10, 24.47, 23.36, 21.54, 21.45, 19.67, 19.59, 13.32; HRMS (m/z) calcd for C31H49NO6 (+) 530.3482, found 530.3474.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-Benzyl-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9a)
67% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.44 (s, 1H), 7.7–7.32 (m, 5H), 4.82(s, 2H), 3.99(d, J = 11.7 Hz, 1H), 3.84 (d, J = 13.8 Hz,1H), 3.44–3.50 (m, 1H), 3.17–3.21 (m, 2H), 3.95 (d, J = 14.1 Hz, 1H), 2.83 (d, J = 8.4 Hz, 1H), 1.93–2.02 (m, 2H), 1.59–1.86 (m, 7H), 1.43 (s, 3H), 1.37 (s, 3H), 1.17 (s, 3H), 1.03 (s, 3H), 0.76–1.03 (m, 4H); 13CNMR (75 MHz, CDCl3) δ 173.52, 145.67, 137.22, 130.10, 128.31, 126.90, 98.91, 70.44, 63.94, 60.40, 59.36, 59.26, 53.63, 52.34, 37.53, 35.21, 34.09, 33.24, 31.37, 29.29, 27.32, 26.10, 25.49, 24.73, 20.39, 15.93, 14.10; HRMS (m/z) calcd for C30H42NO4 (+) 480.3108, found 480.3111.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(4-Methoxybenzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9b)
57% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.44 (s, 1H), 7.19 (d, J = 8.3 Hz, 2H), 6.85 (d, J = 8.6 Hz, 2H), 4.83 (s, 2H), 4.02 (d, J = 11.5 Hz, 1H), 3.80 (s, 3H), 3.77 (s, 1H), 3.46 (dd, J = 9.1, 4.1 Hz, 1H), 3.19 (d, J = 11.6 Hz, 1H), 3.13 (d, J = 11.7 Hz, 1H), 2.93 (d, J = 14.5 Hz, 1H), 2.83 (d, J = 9.5 Hz, 1H), 2.10–1.88 (m, 2H), 1.82 (d, J = 12.8 Hz, 1H), 1.78–1.61 (m, 7H), 1.43 (s, 3H), 1.37 (s, 3H), 1.18 (s, 3H), 1.03 (s, 3H), 0.98–1.01 (m, 1H), 0.96–0.68 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.52, 159.05, 145.78, 129.89, 129.86, 129.61, 129.59, 113.70, 98.91, 70.47, 60.23, 59.26, 58.59, 55.25, 53.63, 52.34, 37.53, 35.21, 34.10, 33.37, 32.46, 31.39, 29.67, 29.30, 27.33, 27.21, 26.10, 25.49, 24.73, 22.93, 20.39, 15.93, 14.34; HRMS (m/z) calcd for C31H44NO5 (+) 510.3214, found 510.3209.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-2,2,4a,10b-Tetramethyl-8-(4-(trifluoromethyl)benzyl) tetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9c)
67% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.55 (t, J = 8.8 Hz, 2H), 7.42 (d, J = 8.8 Hz, 3H), 4.94–4.66 (m, 2H), 4.01 (d, J = 11.6 Hz, 1H), 3.88 (d, J = 14.4 Hz, 1H), 3.47 (dd, J = 9.0, 4.1 Hz, 1H), 3.19 (d, J = 11.5 Hz, 1H), 3.05 (d, J = 14.4 Hz, 1H), 2.77 (d, J = 8.6 Hz, 1H), 2.09–1.90 (m, 1H), 1.84 (d, J = 12.8 Hz, 1H), 1.78–1.49 (m, 6H), 1.43 (s, 3H), 1.37 (s, 3H), 1.18 (s, 3H), 1.04 (s, 3H), 1.03–0.97 (m, 1H), 0.96–0.70 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 177.47, 173.39, 145.73, 144.06, 137.14, 128.39, 125.22, 98.95, 70.39, 63.96, 60.58, 59.38, 58.82, 53.64, 52.32, 37.56, 35.29, 35.22, 34.07, 33.20, 31.34, 29.77, 27.27, 26.11, 25.50, 24.71, 20.38, 15.94; HRMS (m/z) calc. For C31H41F3NO4 (+) 548.2982, found 548.2982.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(4-Bromobenzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9d)
40% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.50–7.35 (m, 3H), 7.17 (d, J = 8.0 Hz, 2H), 4.83 (d, J = 9.7 Hz, 2H), 4.01 (d, J = 11.7 Hz, 1H), 3.78 (d, J = 13.7 Hz, 1H), 3.46 (dd, J = 9.0, 4.1 Hz, 1H), 3.30–3.04 (m, 1H), 2.93 (d, J = 14.2 Hz, 1H), 2.77 (d, J = 8.8 Hz, 1H), 2.06–1.89 (m, 2H), 1.82 (d, J = 13.0 Hz, 1H), 1.70 (dd, J = 11.2, 6.8 Hz, 3H), 1.65–1.49 (m, 6H), 1.43 (s, 3H), 1.37 (s, 3H), 1.17 (s, 3H), 1.03 (s, 3H), 0.97 (dd, J = 8.2, 4.8 Hz, 2H), 0.93–0.73 (m, 4H); 13C NMR(100 MHz, CDCl3) δ 173.32, 145.97, 137.16, 131.48, 131.28, 129.89, 98.83, 70.30, 63.85, 60.30, 59.26, 58.52, 53.55, 52.24, 37.45, 35.11, 33.98, 33.09, 31.80, 31.26, 29.57, 29.19, 27.19, 26.01, 25.40, 24.62, 22.57, 20.29, 15.83, 14.00; HRMS (m/z) calcd for C30H41BrNO4 (+) 558.2213, found 558.2213.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(4-Iodobenzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9e)
58% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.63 (d, J = 8.2 Hz, 2H), 7.39 (s, 1H), 7.05 (d, J = 8.2 Hz, 2H), 4.87–4.73 (m, 2H), 4.01 (d, J = 11.6 Hz, 1H), 3.77 (d, J = 14.2 Hz, 1H), 3.46 (dd, J = 9.0, 4.2 Hz, 1H), 3.17 (dd, J = 16.1, 10.7 Hz, 2H), 2.92 (d, J = 14.1 Hz, 1H), 2.77 (d, J = 8.1 Hz, 1H), 2.09–1.89 (m, 2H), 1.82 (d, J = 12.8 Hz, 1H), 1.71 (dd, J = 13.3, 5.0 Hz, 2H), 1.67–1.61 (m, 6H), 1.43 (s, 3H), 1.37 (s, 3H), 1.22 (dd, J = 11.6, 4.4 Hz, 2H), 1.18 (s, 3H), 1.13 (dd, J = 8.5, 5.4 Hz, 1H), 1.03 (s, 3H), 1.00 (d, J = 13.0 Hz, 1H), 0.9–0.71 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.42, 145.65, 138.85, 137.36, 137.19, 130.28, 98.93, 92.05, 70.40, 63.95, 60.45, 59.35, 58.71, 53.65, 52.33, 37.55, 35.29, 35.21, 34.08, 33.21, 31.35, 29.68, 27.30, 26.11, 25.50, 24.72, 20.39, 15.94; HRMS (m/z) calcd for C30H41INO4 (+) 606.2075, found 606.2076.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-2,2,4a,10b-Tetramethyl-8-(4-methylbenzyl)tetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9f)
67% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.44 (s, 1H), 7.17 (d, J = 7.9 Hz, 2H), 7.12 (d, J = 7.9 Hz, 2H), 4.82 (s, 2H), 4.02 (d, J = 11.6 Hz, 1H), 3.82 (d, J = 13.6 Hz, 1H), 3.46 (dd, J = 9.1, 4.1 Hz, 1H), 3.19 (d, J = 8.9 Hz, 1H), 3.14 (d, J = 10.2 Hz, 1H), 2.94 (d, J = 13.7 Hz, 1H), 2.84 (d, J = 8.2 Hz, 1H), 2.34 (s, 3H), 2.08–1.90 (m, 2H), 1.78–1.67 (m, 2H), 1.66–1.60 (m, 5H), 1.43 (s, 3H), 1.37 (s, 3H), 1.17 (s, 3H), 1.02 (s, 3H), 0.82–0.98 (m, 5H); 13C NMR (100 MHz, CDCl3) δ 173.45, 145.49, 137.80, 136.59, 136.00, 128.89, 128.22, 98.80, 70.35, 63.84, 60.28, 59.21, 58.84, 53.53, 52.26, 37.43, 35.11, 34.00, 33.13, 31.27, 29.57, 29.37, 27.24, 26.00, 25.39, 24.64, 20.97, 20.30, 15.83; HRMS (m/z) calcd for C31H44NO4 (+) 494.3265, found 494.3270.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(4-(tert-Butyl)benzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9g)
63% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.44 (s, 1H), 7.33 (d, J = 8.2 Hz, 2H), 7.21 (d, J = 8.0 Hz, 2H), 4.92–4.68 (m, 2H), 4.02 (d, J = 11.6 Hz, 1H), 3.82 (d, J = 13.9 Hz, 1H), 3.46 (dd, J = 9.1, 4.1 Hz, 1H), 3.24–3.08 (m, 2H), 2.97 (d, J = 14.0 Hz, 1H), 2.88 (d, J = 8.2 Hz, 1H), 2.11–1.91 (m, 2H), 1.84 (d, J = 13.2 Hz, 1H), 1.78–1.63 (m, 6H), 1.43 (s, 3H), 1.36 (s, 3H), 1.32 (s, 9H), 1.18 (s, 3H), 1.03 (s, 3H), 0.99 (s, 1H), 0.95–0.65 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.92, 145.84, 137.54, 136.08, 127.97, 125.20, 98.91, 70.43, 63.96, 60.42, 59.29, 58.92, 55.57, 53.63, 52.36, 37.54, 35.22, 34.44, 34.10, 33.37, 31.36, 29.68, 29.39, 27.34, 26.11, 25.50, 24.74, 22.74, 20.40, 15.94; HRMS (m/z) calcd for C34H50NO4 (+) 536.3734, found 536.3735.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-2,2,4a,10b-Tetramethyl-8-(4-nitrobenzyl)tetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9h)
70% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 8.17 (d, J = 8.7 Hz, 2H), 7.49 (d, J = 8.6 Hz, 2H), 7.41 (s, 1H), 4.91–4.71 (m, 2H), 4.02 (d, J = 11.6 Hz, 1H), 3.90 (d, J = 14.9 Hz, 1H), 3.47 (dd, J = 9.1, 4.1 Hz, 1H), 3.21 (t, J = 10.1 Hz, 1H), 3.10 (d, J = 14.9 Hz, 1H), 2.76–2.65 (m, 1H), 2.07–1.89 (m, 2H), 1.84 (d, J = 13.0 Hz, 1H), 1.58–1.72 (m, 6H), 1.43 (s, 3H), 1.37 (s, 3H), 1.18 (s, 3H), 1.05 (s, 3H), 1.01 (d, J = 10.9 Hz, 1H), 0.95–0.74 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.34, 147.31, 147.02, 145.95, 137.03, 128.77, 123.62, 114.06, 98.98, 70.43, 63.96, 60.73, 59.42, 58.71, 53.65, 53.46, 52.30, 37.57, 35.31, 35.23, 34.07, 33.12, 31.32, 29.70, 27.30, 26.12, 25.51, 24.71, 20.37, 15.97; HRMS (m/z) calcd for C30H41N2O6 (+) 525.2959, found 525.2951.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(4-Hydroxybenzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9i)
53% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.27 (s, 1H), 7.14 (d, J = 8.4 Hz, 2H), 6.78 (d, J = 7.8 Hz, 2H), 4.87 (s, 2H), 4.00 (d, J = 11.7 Hz, 1H), 3.82–3.92 (m, 1H), 3.35–3.58 (m, 2H), 3.22–3.32 (m, 1H), 3.17 (d, J = 11.6 Hz, 2H), 2.90 (d, J = 7.8 Hz, 1H), 1.86–2.05 (m, 2H), 1.83 (d, J = 13.2 Hz, 1H), 1.79–1.47 (m, 7H), 1.42 (s, 3H), 1.37 (s, 3H), 1.17 (s, 3H), 1.04 (s, 3H), 0.59–1.02 (m, 6H); 13C NMR (100 MHz, CDCl3) δ 173.59, 155.70, 130.40, 129.75, 115.35, 98.92, 70.74, 65.75, 63.80, 59.23, 58.52, 53.21, 52.13, 37.42, 35.09, 34.61, 33.92, 31.13, 29.57, 29.19, 27.19, 25.98, 25.38, 22.56, 20.22, 15.76, 15.11, 14.00; HRMS (m/z) calcd for C30H42NO5 (+) 496.3057, found 496.3058.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(2-Cyclopropylbenzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9j)
70% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 6.7 Hz, 1H), 7.35 (s, 1H), 7.21–7.12 (m, 2H), 7.03–6.96 (m, 1H), 4.83–4.69 (m, 2H), 4.03 (d, J = 11.7 Hz, 1H), 3.82 (d, J = 14.8 Hz, 1H), 3.47 (dd, J = 9.1, 4.1 Hz, 1H), 3.36 (d, J = 14.7 Hz, 1H), 3.22 (t, J = 12.4 Hz, 2H), 2.90–2.85 (m, 1H), 2.08–1.81 (m, 3H), 1.80–1.61 (m, 8H), 1.44 (s, 3H), 1.38 (s, 3H), 1.19 (s, 3H), 1.04 (s, 3H), 1.02 (d, J = 12.8 Hz, 1H), 0.93–0.78 (m, 6H), 0.70–0.59 (m, 1H), 0.57–0.47 (m, 1H); 13C NMR (100 MHz, CDCl3) δ 173.67, 145.42, 141.00, 138.73, 137.35, 127.64, 126.54, 125.79, 125.62, 98.89, 70.41, 63.95, 60.94, 59.85, 56.37, 53.75, 52.44, 37.54, 35.36, 35.25, 34.16, 33.39, 31.40, 29.68, 27.38, 26.14, 25.54, 24.80, 20.42, 15.93, 12.69, 7.44, 6.60; HRMS (m/z) calcd for C33H46NO4 (+) 520.3421, found 520.3414.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(3-Chloro-4-fluorobenzyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9k)
68% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 1H), 7.35 (d, J = 5.3 Hz, 1H), 7.16–7.10 (m, 1H), 7.07 (t, J = 8.6 Hz, 1H), 4.95–4.73 (m, 2H), 4.02 (d, J = 11.6 Hz, 1H), 3.77 (d, J = 14.3 Hz, 1H), 3.47 (dd, J = 9.0, 4.0 Hz, 1H), 3.24–3.12 (m, 2H), 2.91 (d, J = 14.1 Hz, 1H), 2.76 (d, J = 7.7 Hz, 1H), 2.06–1.88 (m, 2H), 1.82 (d, J = 12.8 Hz, 1H), 1.57–1.72 (m, 6H), 1.43 (s, 3H), 1.37 (s, 3H), 1.18 (s, 3H), 1.04 (s, 3H), 1.00 (d, J = 10.8 Hz, 1H), 0.82–0.88 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.38, 145.70, 137.15, 136.60, 130.02, 127.85, 122.61, 116.41, 98.94, 70.41, 63.95, 60.40, 59.34, 58.14, 53.66, 52.32, 37.55, 35.22, 34.08, 33.18, 31.36, 29.67, 27.29, 26.12, 25.50, 24.72, 20.38, 15.94; HRMS (m/z) calc. For C30H40ClFNO4 (+) 532.2624, found 532.2624.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(Furan-2-ylmethyl)-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9l)
57% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H), 7.36 (s, 1H), 6.29 (s, 1H), 6.14 (s, 1H), 4.85 (s, 2H), 4.01 (d, J = 11.7 Hz, 1H), 3.71 (d, J = 14.7 Hz, 1H), 3.45 (dd, J = 8.8, 4.1 Hz, 1H), 3.27 (d, J = 14.2 Hz, 1H), 3.18 (d, J = 11.6 Hz, 1H), 3.09 (d, J = 11.4 Hz, 1H), 2.92 (d, J = 10.3 Hz, 1H), 2.12–1.98 (m, 1H), 1.94 (dd, J = 13.8, 5.1 Hz, 1H), 1.86–1.75 (m, 2H), 1.62–1.57 (m, 4H), 1.42 (s, 3H), 1.36 (s, 3H), 1.17 (s, 3H), 1.13–1.10 (m, 2H), 1.01 (s, 3H), 0.97–0.91 (m, 1H), 0.91–0.82 (m, 2H), 0.78 (t, J = 10.1 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 173.44, 145.99, 142.15, 129.89, 110.12, 108.98, 98.93, 70.54, 63.94, 60.55, 58.60, 53.46, 52.27, 51.53, 37.55, 35.18, 34.03, 33.02, 31.40, 29.68, 27.29, 26.08, 25.43, 24.66, 20.41, 15.97; HRMS (m/z) calcd for C28H40NO5 (+) 470.2901, found 470.2906.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-2,2,4a,10b-Tetramethyl-8-(thiophen-2-ylmethyl)tetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9m)
66% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H), 7.21 (d, J = 4.8 Hz, 1H), 6.97–6.89 (m, 1H), 6.85 (s, 1H), 4.84 (s, 2H), 4.02 (d, J = 11.6 Hz, 1H), 3.90 (d, J = 14.4 Hz, 1H), 3.46 (dd, J = 9.1, 4.1 Hz, 1H), 3.37 (d, J = 14.5 Hz, 1H), 3.18 (t, J = 9.7 Hz, 2H), 2.96 (dd, J = 11.0, 2.8 Hz, 1H), 2.04–1.87 (m, 1H), 1.84 (d, J = 12.8 Hz, 1H), 1.78–1.51 (m, 7H), 1.43 (s, 3H), 1.37 (s, 3H), 1.18 (s, 3H), 1.02 (s, 3H), 0.95–0.64 (m, 4H); 13C NMR (100 MHz, CDCl3) δ 173.49, 145.66, 142.53, 137.92, 126.67, 125.37, 124.57, 98.92, 70.53, 63.95, 60.40, 58.68, 53.72, 53.64, 52.34, 37.54, 35.31, 35.26, 34.10, 33.32, 31.38, 29.68, 27.33, 26.11, 25.50, 24.73, 20.39, 15.93; HRMS (m/z) calcd for C28H40NO4S (+) 486.2673, found 486.2673.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-Butyl-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9n)
50% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.26 (s, 1H), 4.99–4.76 (m, 2H), 4.01 (d, J = 11.6 Hz, 1H), 3.47 (dd, J = 8.7, 3.7 Hz, 2H), 3.34–3.23 (m, 1H), 3.20 (d, J = 11.6 Hz, 1H), 2.89–2.65 (m, 1H), 2.44 (dd, J = 27.2, 7.9 Hz, 2H), 2.12–1.91 (m, 4H), 1.88–1.67 (m, 5H), 1.65–1.49 (m, 4H), 1.42 (s, 3H), 1.37 (s, 3H), 1.19 (s, 3H), 1.09 (s, 3H), 0.90 (dd, J = 14.3, 7.1 Hz, 9H); 13C NMR (100 MHz, CDCl3) δ 173.11, 129.86, 129.12, 99.08, 76.43, 70.98, 63.87, 59.01, 54.40, 53.03, 52.07, 37.60, 35.90, 35.25, 33.95, 31.49, 30.13, 29.68, 29.29, 27.16, 26.08, 25.40, 24.51, 22.66, 20.26, 15.93, 14.10, 13.68; HRMS (m/z) calcd for C27H44NO4 (+) 446.3265, found 446.3269.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-Isobutyl-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9o)
16% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.33 (s, 1H), 4.91–4.76 (m, 2H), 4.03 (d, J = 11.6 Hz, 1H), 3.46 (dd, J = 8.9, 4.0 Hz, 1H), 3.20 (d, J = 11.6 Hz, 1H), 2.98 (dd, J = 20.1, 9.3 Hz, 2H), 2.12 (s, 1H), 2.06–1.89 (m, 3H), 1.85–1.66 (m, 6H), 1.65–1.47 (m, 4H), 1.43 (s, 3H), 1.37 (s, 3H), 1.19 (s, 3H), 1.15–1.08 (m, 2H), 1.02 (s, 3H), 0.99–0.93 (m, 2H), 0.87 (d, J = 6.8 Hz, 6H), 0.79 (d, J = 5.1 Hz, 4H); 13C NMR (100 MHz, CDCl3) δ 173.74, 145.82, 129.86, 98.90, 70.34, 63.95, 63.42, 60.61, 59.78, 53.70, 52.96, 37.54, 35.55, 34.09, 31.90, 31.59, 29.67, 29.30, 27.28, 26.12, 25.57, 24.76, 21.98, 20.53, 15.91, 14.10; HRMS (m/z) calcd for C27H44NO4 (+) 446.3265, found 446.3268.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-Isopentyl-2,2,4a,10b-tetramethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (9p)
36% yield, yellow solid; 1H NMR (300 MHz, MeOD) δ 7.84 (s, 1H), 5.00 (s, 2H), 4.06 (d, J = 11.6 Hz, 1H), 3.82 (d, J = 7.4 Hz, 1H), 3.50 (dd, J = 8.6, 3.9 Hz, 1H), 3.22 (d, J = 11.7 Hz, 1H), 2.94 (d, J = 12.3 Hz, 1H), 2.68 (t, J = 12.1 Hz, 1H), 2.58–2.29 (m, 2H), 2.00 (dd, J = 13.1, 5.1 Hz, 2H), 1.94–1.75 (m, 5H), 1.75–1.60 (m, 5H), 1.58–1.48 (m, 3H), 1.41 (s, 3H), 1.33 (s, 3H), 1.19 (s, 3H), 1.13 (s, 3H), 0.91 (dd, J = 12.4, 5.8 Hz, 9H); 13C NMR (100 MHz, CDCl3) δ 172.95, 129.60, 128.49, 99.90, 99.11, 76.36, 71.09, 63.83, 58.75, 52.91, 52.05, 37.60, 35.27, 33.95, 33.47, 30.98, 29.47, 29.24, 27.08, 26.23, 26.05, 25.40, 24.48, 22.57, 22.03, 20.14, 15.91; HRMS (m/z) calcd for C28H46NO4 (+) 460.3421, found 460.3422.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-Benzyl-4a,10b-dimethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (10a)
56% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.42 (s, 1H), 7.31 (dd, J = 11.7, 4.7 Hz, 4H), 7.23 (d, J = 6.5 Hz, 1H), 4.93 (d, J = 6.3 Hz, 1H), 4.83 (d, J = 6.5 Hz, 1H), 4.80 (s, 2H), 4.03 (d, J = 11.4 Hz, 1H), 3.86 (d, J = 13.9 Hz, 1H), 3.51–3.36 (m, 2H), 3.16 (d, J = 10.1 Hz, 1H), 2.97 (d, J = 13.9 Hz, 1H), 2.84 (dd, J = 10.7, 2.7 Hz, 1H), 2.30–2.17 (m, 1H), 1.91–1.80 (m, 1H), 1.79–1.72 (m, 1H), 1.63 (d, J = 10.5 Hz, 4H), 1.37 (s, 3H), 1.22–1.14 (m, 2H), 1.05 (td, J = 13.3, 2.8 Hz, 1H), 0.95 (d, J = 12.8 Hz, 1H), 0.91 (d, J = 4.7 Hz, 3H), 0.89–0.68 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.47, 145.64, 139.01, 137.27, 128.30, 126.90, 87.66, 79.62, 70.41, 69.28, 60.40, 59.27, 54.79, 53.41, 37.48, 35.82, 35.44, 34.79, 33.05, 31.40, 29.68, 25.75, 20.42, 19.92, 15.10; HRMS (m/z) calcd for C28H38NO4 (+) 452.2795, found 452.2792.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(4-Fluorobenzyl)-4a,10b-dimethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (10b)
60% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.41 (s, 1H), 7.24 (dd, J = 8.3, 5.6 Hz, 2H), 6.99 (t, J = 8.7 Hz, 2H), 4.93 (d, J = 6.3 Hz, 1H), 4.82 (s, 2H), 4.80 (s, 1H), 4.03 (d, J = 11.4 Hz, 1H), 3.79 (d, J = 13.8 Hz, 1H), 3.49–3.39 (m, 2H), 3.15 (d, J = 9.4 Hz, 1H), 2.94 (d, J = 13.8 Hz, 1H), 2.78 (dd, J = 10.6, 2.4 Hz, 1H), 2.28–2.19 (m, 1H), 1.85–1.77 (m, 1H), 1.74 (dt, J = 13.3, 3.4 Hz, 1H), 1.62 (t, J = 15.0 Hz, 7H), 1.37 (s, 3H), 1.23–1.10 (m, 3H), 1.08–1.02 (m, 1H), 0.95 (d, J = 11.0 Hz, 1H), 0.91 (d, J = 4.7 Hz, 3H), 0.89–0.67 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.37, 160.63, 145.70, 137.20, 134.54, 129.76, 115.19, 114.98, 87.65, 79.60, 70.38, 69.26, 60.23, 59.23, 58.50, 54.79, 53.42, 37.47, 35.81, 35.44, 34.77, 32.97, 31.40, 29.67, 25.74, 20.41, 19.91, 15.08; HRMS (m/z) calcd for C28H37FNO4 (+) 470.2701, found 470.2701.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-8-(Furan-2-ylmethyl)-4a,10b-dimethyltetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (10c)
60% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.49 (s, 1H), 7.36 (d, J = 1.1 Hz, 1H), 6.30 (dd, J = 3.0, 1.9 Hz, 1H), 6.14 (d, J = 2.9 Hz, 1H), 4.93 (d, J = 6.3 Hz, 1H), 4.85 (s, 2H), 4.80 (d, J = 6.1 Hz, 1H), 4.02 (d, J = 11.4 Hz, 1H), 3.71 (d, J = 14.5 Hz, 1H), 3.44 (dd, J = 12.2, 5.5 Hz, 2H), 3.26 (d, J = 14.5 Hz, 1H), 3.09 (d, J = 10.2 Hz, 1H), 2.92 (dd, J = 11.1, 3.2 Hz, 1H), 2.28–2.20 (m, 1H), 2.07–1.92 (m, 1H), 1.80 (dd, J = 12.8, 3.0 Hz, 1H), 1.77–1.69 (m, 3H), 1.61–1.52 (m, 2H), 1.37 (s, 3H), 1.23–1.08 (m, 3H), 1.04 (dd, J = 13.6, 2.8 Hz, 1H), 1.00–0.91 (m, 2H), 0.89 (s, 3H), 0.81–0.65 (m, 2H); 13C NMR (100 MHz, CDCl3) δ 151.46, 145.91, 142.10, 136.51, 110.10, 87.66, 79.61, 70.51, 69.26, 60.54, 58.46, 54.76, 53.22, 51.59, 37.47, 35.80, 35.43, 34.78, 32.83, 31.42, 29.67, 25.75, 20.39, 19.93, 15.06; HRMS (m/z) calc. For C26H36NO5 (+) 442.2588, found 442.2590.
3-((4aR,4bS,6aS,9R,10aR,10bS,12aR)-4a,10b-Dimethyl-8-(thiophen-2-ylmethyl)tetradecahydro-4H-[1,3]dioxino[5′,4′:3,4]benzo[1,2-f]isoquinolin-9-yl)furan-2(5H)-one (10d)
53% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.51 (s, 1H), 7.21 (d, J = 4.9 Hz, 1H), 6.94 (dd, J = 5.0, 3.5 Hz, 1H), 6.84 (d, J = 3.2 Hz, 1H), 4.93 (d, J = 6.3 Hz, 1H), 4.84 (s, 2H), 4.81 (d, J = 6.4 Hz, 1H), 4.03 (d, J = 11.4 Hz, 1H), 3.90 (d, J = 14.4 Hz, 1H), 3.47–3.40 (m, 2H), 3.36 (d, J = 14.5 Hz, 1H), 3.17 (d, J = 9.4 Hz, 1H), 2.96 (dd, J = 11.2, 3.4 Hz, 1H), 2.29–2.19 (m, 1H), 1.88–1.80 (m, 1H), 1.78–1.70 (m, 2H), 1.69–1.63 (m, 3H), 1.58–1.54 (m, 1H), 1.37 (s, 3H), 1.21–1.08 (m, 3H), 1.04 (td, J = 13.4, 3.0 Hz, 1H), 0.97–0.93 (m, 1H), 0.91 (s, 3H), 0.90–0.73 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 173.43, 145.65, 142.52, 136.95, 126.66, 125.35, 124.55, 87.66, 79.61, 70.51, 69.28, 60.34, 58.53, 54.78, 53.73, 53.32, 37.48, 35.82, 35.44, 34.84, 33.11, 31.40, 29.68, 25.75, 20.40, 19.91, 15.09; HRMS (m/z) calcd for C26H36NO4S (+) 458.2360, found 458.2357.
3-((2R,4aS,6aS,7R,8R,10aS,10bR)-3-Benzyl-8-hydroxy-7-(hydroxymethyl)-7,10a-dimethyltetradecahydrobenzo[f]isoquinolin-2-yl)furan-2(5H)-one (11a)
A solution of 9a (0.03 mmol) in 1 mL of AcOH/THF/H2O (4:1:2) was stirred at 45 °C for 2 h. The reaction mixture was then concentrated under reduced pressure, and the residue was purified by prep-TLC using MeOH/DCM (1:15) as a developing agent to yield the desired product 11a as a yellow solid in 53% yield. 1H NMR (400 MHz, CDCl3) δ 7.42 (s, 1H), 7.37–7.28 (m, 4H), 7.25–7.24 (m, 1H), 4.80 (s, 2H), 4.21 (d, J = 11.1 Hz, 1H), 3.85 (d, J = 14.0 Hz, 1H), 3.45 (dd, J = 11.8, 3.9 Hz, 1H), 3.32 (d, J = 10.7 Hz, 1H), 3.15 (d, J = 11.0 Hz, 1H), 2.97 (d, J = 13.4 Hz, 1H), 2.84 (d, J = 11.2 Hz, 2H), 2.02 (d, J = 5.3 Hz, 1H), 1.86–1.76 (m, 2H), 1.76–1.64 (m, 4H), 1.21 (s, 3H), 1.19–1.01 (m, 3H), 0.96 (d, J = 10.9 Hz, 1H), 0.87 (dt, J = 7.3, 5.2 Hz, 3H), 0.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.54, 131.49, 129.91, 128.55, 128.38, 128.30, 80.76, 70.53, 64.22, 59.22, 55.05, 53.59, 42.77, 36.53, 35.79, 31.57, 29.68, 29.30, 27.69, 27.19, 22.67, 22.51, 20.84, 14.99, 14.10. HRMS (m/z) calcd for C27H38NO4 (+) 440.2795, found 440.2796.
3-((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Hydroxy-7-(hydroxymethyl)-3-(4-methoxybenzyl)-7,10a-dimethyltetradecahydrobenzo[f]isoquinolin-2-yl)furan-2(5H)-one (11b)
The synthetic procedure of 11a was applied to 11b using 9b as substrate. 56% yield, yellow solid; 1H NMR (300 MHz, CDCl3) δ 7.26 (s, 1H), 7.23 (d, J = 8.1 Hz, 2H), 6.87 (d, J = 8.4 Hz, 2H), 4.89 (s, 2H), 4.19 (d, J = 11.0 Hz, 1H), 4.10–3.83 (m, 1H), 3.81 (d, J = 3.6 Hz, 3H), 3.7–3.55 (m, 1H), 3.47–3.25 (m, 3H), 2.93 (s, 1H), 2.16–1.94 (m, 2H), 1.88–1.68 (m, 6H), 1.62 (s, 2H), 1.20 (s, 3H), 1.11–1.02 (m, 2H), 0.97 (d, J = 6.8 Hz, 2H), 0.89 (t, J = 8.9 Hz, 4H), 0.80 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.40, 129.94, 114.22, 114.17, 114.07, 114.00, 80.60, 70.82, 64.15, 58.99, 55.28, 54.98, 53.41, 42.70, 36.50, 35.80, 31.90, 31.41, 29.67, 27.61, 22.53, 20.73, 14.97, 14.10; HRMS (m/z) calc. For C28H40NO5 (+) 470.2901, found 470.2903.
3-((2R,4aS,6aS,7R,8R,10aS,10bR)-3-(3-Chloro-4-fluorobenzyl)-8-hydroxy-7-(hydroxymethyl)-7,10a-dimethyltetradecahydrobenzo[f]isoquinolin-2-yl)furan-2(5H)-one (11c)
The synthetic procedure of 11a was applied to 11c using 9k as substrate. 93% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 1H), 7.34 (d, J = 7.3 Hz, 1H), 7.17–7.10 (m, 1H), 7.06 (t, J = 8.6 Hz, 1H), 4.82 (s, 2H), 4.21 (d, J = 11.1 Hz, 1H), 3.76 (d, J = 14.1 Hz, 1H), 3.45 (dd, J = 11.1, 4.6 Hz, 1H), 3.32 (d, J = 11.1 Hz, 1H), 3.14 (d, J = 10.2 Hz, 1H), 2.90 (d, J = 14.0 Hz, 1H), 2.75 (d, J = 8.2 Hz, 1H), 2.07 (d, J = 16.1 Hz, 1H), 1.87–1.76 (m, 2H), 1.76–1.62 (m, 8H), 1.22 (s, 3H), 1.19–1.00 (m, 3H), 0.97 (d, J = 10.6 Hz, 1H), 0.92–0.82 (m, 2H), 0.79 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.37, 155.72, 145.77, 136.93, 129.96, 127.79, 120.79, 116.32, 116.11, 80.57, 70.38, 65.73, 64.12, 60.15, 59.05, 57.97, 54.95, 53.54, 42.61, 36.41, 35.68, 34.57, 32.94, 31.47, 29.57, 27.54, 22.45, 20.73, 15.13, 14.90; HRMS (m/z) calcd for C27H36ClFNO4 (+) 492.2311, found 492.2321.
3-((2R,4aS,6aS,7R,8R,10aS,10bR)-3-(Furan-2-ylmethyl)-8-hydroxy-7-(hydroxymethyl)-7,10a-dimethyltetradecahydrobenzo[f]isoquinolin-2-yl)furan-2(5H)-one (11d)
The synthetic procedure of 11a was applied to 11d using 9l as substrate. 66% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.50 (s, 1H), 7.36 (s, 1H), 6.30 (s, 1H), 6.15 (s, 1H), 4.91–4.75 (m, 2H), 4.20 (d, J = 11.1 Hz, 1H), 3.71 (d, J = 14.9 Hz, 1H), 3.44 (dd, J = 11.3, 4.4 Hz, 1H), 3.30 (t, J = 14.8 Hz, 2H), 3.08 (d, J = 10.0 Hz, 1H), 2.93 (d, J = 11.5 Hz, 1H), 2.06–1.98 (m, 1H), 1.81–1.64 (m, 9H), 1.22 (s, 3H), 1.05 (ddd, J = 13.0, 10.2, 6.2 Hz, 3H), 0.96 (d, J = 10.6 Hz, 1H), 0.91–0.83 (m, 3H), 0.76 (s, 3H), 0.73 (d, J = 7.6 Hz, 1H); 13C NMR (125 MHz, CDCl3) δ 173.52, 142.19, 129.93, 110.16, 100.82, 80.81, 70.61, 64.25, 60.50, 58.45, 55.06, 53.47, 51.57, 42.80, 36.53, 35.79, 34.73, 31.65, 29.72, 29.34, 27.71, 22.63, 20.89, 15.01, 14.15; HRMS (m/z) calcd for C25H36NO5 (+) 430.2588, found 430.2591.
3-((2R,4aS,6aS,7R,8R,10aS,10bR)-8-Hydroxy-7-(hydroxymethyl)-7,10a-dimethyl-3-(thiophen-2-ylmethyl)tetradecahydrobenzo[f]isoquinolin-2-yl)furan-2(5H)-one (11e)
The synthetic procedure of 11a was applied to 11e using 9m as substrate. 94% yield, yellow solid; 1H NMR (400 MHz, CDCl3) δ 7.52 (s, 1H), 7.21 (t, J = 5.6 Hz, 1H), 6.97–6.91 (m, 1H), 6.85 (s, 1H), 4.84 (s, 2H), 4.21 (d, J = 11.1 Hz, 1H), 3.90 (d, J = 13.1 Hz, 1H), 3.48–3.42 (m, 1H), 3.38 (d, J = 14.2 Hz, 1H), 3.32 (d, J = 11.1 Hz, 1H), 3.16 (d, J = 10.5 Hz, 1H), 2.96 (d, J = 8.4 Hz, 1H), 2.01 (d, J = 5.7 Hz, 1H), 1.80 (dd, J = 16.0, 6.5 Hz, 4H), 1.75–1.62 (m, 7H), 1.21 (s, 3H), 1.14–1.00 (m, 3H), 0.96 (d, J = 12.4 Hz, 1H), 0.91–0.83 (m, 2H), 0.78 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 173.51, 145.76, 136.64, 126.65, 125.54, 124.45, 80.71, 70.48, 64.17, 60.12, 58.33, 54.96, 53.45, 42.59, 36.36, 35.67, 34.55, 33.07, 31.42, 29.57, 27.56, 22.44, 20.75, 14.89; HRMS (m/z) calcd for C25H36NO4S (+) 446.2360, found 446.2351.
Protocol for Evaluation of the In Vitro Inhibitory Activity of Test Compounds against Human Coronavirus 229E
MRC5 cells are seeded in 96-well plates, at 100 μL per well of the assay medium, at an appropriate density of 20,000 cells per well and cultured at 37 °C and 5% CO2 overnight. Next day, the test compound is diluted with the assay medium and then added into the cells at 50 μL per well. Then 50 μL per well of the assay medium diluted virus is added. The final volume of the cell culture is 200 μL per well. The final concentration of DMSO in the cell culture is 0.5%. The resulting cell culture is incubated for an additional 3 days until the virus infection in the virus control (cells infected with virus, without compound treatment) displays significant CPE. The CPEs are measured by CellTiter Glo following the manufacturer’s manual. The antiviral activity of compounds is calculated based on the protection of the virus-induced CPE at each concentration normalized by the virus control. The cytotoxicity of compounds is assessed under the same conditions, but without virus infection, in parallel. Cell viability is measured with CellTiter Glo following the manufacturer’s manual. EC50 and CC50 values are calculated using the GraphPad Prism software using the nonlinear regression model of log(inhibitor) vs response-variable slope (four parameters) (see Supporting Information S53–S55 for details).
Acknowledgments
This work was financially supported by the Natural Science Foundation of China (No. 21977008), Natural Science Foundation of Guangdong Province (2018A0303130052), and Shenzhen Basic Research Project (JCYJ20180503182116931). We would like to thank professor Zhen Yang (Peking University) for his support and valuable suggestions.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02979.
Author Contributions
§ D.J. and J.Z. made equal contributions to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Atanasov A. G.; Zotchev S. B.; Dirsch V. M.; Supuran C. T. Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. 10.1038/s41573-020-00114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- Galloway W. R. J. D.; Isidro-Llobet A.; Spring D. R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. 10.1038/ncomms1081. [DOI] [PubMed] [Google Scholar]
- a Nguyen G. T. H.; Bennett J. L.; Liu S.; Hancock S. E.; Winter D. L.; Glover D. J.; Donald W. A. Multiplexed screening of thousands of natural products for protein-ligand binding in native mass spectrometry. J. Am. Chem. Soc. 2021, 143, 21379–21387. 10.1021/jacs.1c10408. [DOI] [PubMed] [Google Scholar]; b Schneider G. Automating drug discovery. Nat. Rev. Drug Discov. 2018, 17, 97–113. 10.1038/nrd.2017.232. [DOI] [PubMed] [Google Scholar]; c Gesmundo N. J.; Sauvagnat B.; Curran P. J.; Richards M. P.; Andrews C. L.; Dandliker P. J.; Cernak T. Nanoscale synthesis and affinity ranking. Nature 2018, 557, 228–232. 10.1038/s41586-018-0056-8. [DOI] [PubMed] [Google Scholar]
- a Tourá B. B.; Hall D. G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486. 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; b Lai X.; Che C. Synthesis of chromeno[4,3-b]pyrrol-4(1h)-ones through a multicomponent reaction and cyclization strategy. ACS Omega 2020, 5, 21968–21977. 10.1021/acsomega.0c03589. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Pando O.; Stark S.; Denkert A.; Porzel A.; Preusentanz R.; Wessjohann L. A. The multiple multicomponent approach to natural product mimics: Tubugis, N-substituted anticancer peptides with picomolar activity. J. Am. Chem. Soc. 2011, 133, 7692–7695. 10.1021/ja2022027. [DOI] [PubMed] [Google Scholar]; d Morton D.; Leach S.; Cordier C.; Warriner S.; Nelson A. Synthesis of natural-product-like molecules with over eighty distinct scaffolds. Angew. Chem., Int. Ed. 2009, 48, 104–109. 10.1002/anie.200804486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Morrison K. C.; Hergenrothe P. J. Natural products as starting points for the synthesis of complex and diverse compounds. Nat. Prod. Rep. 2014, 31, 6–14. 10.1039/C3NP70063A. [DOI] [PubMed] [Google Scholar]; b Llabani E.; Hicklin R. W.; Lee H. Y.; Motika S. E.; Crawford L. A.; Weerapana E.; Hergenrother P. J. Diverse compounds from pleuromutilin lead to a thioredoxin inhibitor and inducer of ferroptosis. Nat. Chem. 2019, 11, 521–532. 10.1038/s41557-019-0261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Huigens R. W. III; Morrison K. C.; Hicklin R. W.; Flood T. A. Jr.; Richter M. F.; Hergenrother P. J. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nat. Chem. 2013, 5, 195–202. 10.1038/nchem.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nicolaou K. C.; Pfefferkorn J. A.; Roecker A. J.; Cao G. Q.; Barluenga S.; Mitchell H. J. Natural Product-like Combinatorial Libraries Based on Privileged Structures. 1. General principles and solid-phase synthesis of benzopyrans. J. Am. Chem. Soc. 2000, 122, 9939–9953. 10.1021/ja002033k. [DOI] [Google Scholar]; b Xiao X. Y.; Parandoosh Z.; Nova M. P. Design and synthesis of a taxoid library using radiofrequency encoded combinatorial chemistry. J. Org. Chem. 1997, 62, 6029–6033. 10.1021/jo9708699. [DOI] [Google Scholar]; c Nicolaou K. C.; Winssinger N.; Vourloumis D.; Ohshima T.; Kim S.; Pfefferkorn J.; Xu J. Y.; Li T. Solid and solution phase synthesis and biological evaluation of combinatorial Sarcodictyin libraries. J. Am. Chem. Soc. 1998, 120, 10814–10826. 10.1021/ja9823870. [DOI] [Google Scholar]
- Suebsasana S.; Pongnaratorn P.; Sattayasai J.; Arkaravichien T.; Tiamkao S.; Aromdee C. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Arch. Pharmacol. Res. 2009, 32, 1191–1200. 10.1007/s12272-009-1902-x. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Yu P.; Zhang G.; Xu L.; Wang D.; Wang L.; Zeng X.; Wang Y. Design, synthesis and antibacterial activity of novel andrographolide derivatives. Bioorg. Med. Chem. 2010, 18, 4269–4274. 10.1016/j.bmc.2010.04.094. [DOI] [PubMed] [Google Scholar]
- Wang W.; Wang J.; Dong S.; Liu C.; Italiani P.; Sun S.; Xu J.; Boraschi D.; Ma S.; Qu D. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 2010, 31, 191–201. 10.1038/aps.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. W.; Dai G. F.; Liu G. Z.; Wang J. F.; Liu H. M. Synthesis of andrographolide derivatives: A new family of α-glucosidase inhibitors. Bioorg. Med. Chem. 2007, 15, 4247–4255. 10.1016/j.bmc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Woo A. Y. H.; Waye M. M. Y.; Tsui S. K. W.; Yeung S. T. W.; Cheng C. H. K. Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J. Pharmacol. Exp. Ther. 2008, 325, 226–235. 10.1124/jpet.107.133918. [DOI] [PubMed] [Google Scholar]
- Tang C.; Gu G.; Wang B.; Deng X.; Zhu X.; Qian H.; Huang W. Design, synthesis, and biological evaluation of andrographolide derivatives as potent hepatoprotective agents. Chem. Biol. Drug Des. 2014, 83, 324–333. 10.1111/cbdd.12246. [DOI] [PubMed] [Google Scholar]
- Ahmed S.; Kwatra M.; Panda S. R.; Murty U. S. N.; Naidu V. G. M. Andrographolide suppresses NLRP3 inflammasome activation in microglia through induction of parkin-mediated mitophagy in in-vitro and in-vivo models of Parkinson disease. Brain, Behav. Immun. 2021, 91, 142–158. 10.1016/j.bbi.2020.09.017. [DOI] [PubMed] [Google Scholar]
- a Islam M. T.; Ali E. S.; Uddin S. J.; Islam M. A.; Shaw S.; Khan I. N.; Saravi S. S. S.; Ahmad S.; Rehman S.; Vijai Kumar Gupta V. K.; Gǎman M.-A.; Amelia Maria Gǎman A. M.; Santosh Yele S.; Das A. K.; Sousa J. M. C.; Dantas S. M. M. M.; Rolim H. M. L.; Melo-Cavalcante A. A. C.; Mohammad S. M. S.; Yarla N. S.; Shilpi J. A.; Mishra S. K.; Atanasov A. G.; Kamal M. A. Andrographolide, a diterpene lactone from Andrographis paniculata and its therapeutic promises in cancer. Cancer Lett. 2018, 420, 129–145. 10.1016/j.canlet.2018.01.074. [DOI] [PubMed] [Google Scholar]; b Soo H. L.; Quah S. Y.; Sulaiman I.; Sagineedu S. R.; Lim J. C. W.; Stanslas J. Advances and challenges in developing andrographolide and its analogues as cancer therapeutic agents. Drug Discovery Today 2019, 24, 1890–1898. 10.1016/j.drudis.2019.05.017. [DOI] [PubMed] [Google Scholar]
- a Gupta S.; Mishra K. P.; Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017, 162, 611–623. 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]; b Lee J.-C.; Tseng C.-K.; Young K.-C.; Sun H.-Y.; Wang S.-W.; Chen W.-C.; Lin C.-K.; Wu Y.-H. Andrographolide exerts anti-hepatitis C virus activity by up-regulating haeme oxygenase-1 via the p38 MAPK/Nrf2 pathway in human hepatoma cells. Br. J. Pharmacol. 2014, 171, 237–252. 10.1111/bph.12440. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Chen J.-X.; Xue H.-J.; Ye W.-C.; Fang B.-H.; Liu Y.-H.; Yuan S.-H.; Yu P.; Wang Y.-Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. 10.1248/bpb.32.1385. [DOI] [PubMed] [Google Scholar]; d Calabrese C.; Berman S. H.; Babish J. G.; Ma X.; Shinto L.; Dorr M.; Wells K.; Wenner C. A.; Standish L. J. A Phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother. Res. 2000, 14, 333–338. . [DOI] [PubMed] [Google Scholar]; e Lin T.-P.; Chen S.-Y.; Duh P.-D.; Chang L.-K.; Liu Y.-N. Inhibition of the Epstein–Barr Virus lytic cycle by andrographolide. Biol. Pharm. Bull. 2008, 31, 2018–2023. 10.1248/bpb.31.2018. [DOI] [PubMed] [Google Scholar]
- Data from the website: COVID Live Update: 257,514,223 Cases and 5,165,832 Deaths from the Coronavirus - Worldometer (worldometers.info)
- Christy M. P.; Uekusa Y.; Gerwick L.; Gerwick W. H. Natural products with potential to treat RNA virus pathogens including SARS-CoV-2. J. Nat. Prod. 2021, 84, 161–182. 10.1021/acs.jnatprod.0c00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enmozhi S. K.; Raja K.; Sebastine I.; Joseph J. Andrographolide as a potential inhibitor of SARS-CoV-2 main protease: an in silico approach. J. Biomol. Struct. Dyn. 2021, 39, 3092–3098. 10.1080/07391102.2020.1760136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernak T.; Dykstra K. D.; Tyagarajan S.; Vachalb P.; Krska S. W. The medicinal chemist’s toolbox for late-stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. 10.1039/C5CS00628G. [DOI] [PubMed] [Google Scholar]
- a Zhang H.; Li S.; Si Y.; Xu H. Andrographolide and its derivatives: current achievements and future perspectives. Eur. J. Med. Chem. 2021, 224, 113710 10.1016/j.ejmech.2021.113710. [DOI] [PubMed] [Google Scholar]; b Peng Y.; Sun Y.; Wang D.; Wei P.; Ouyang P.; Zhou G. Recent progress in synthesis of andrographolide derivatives with anti-tumor activities. Chin. J. Org. Chem. 2015, 35, 1451–1468. 10.6023/cjoc201501027. [DOI] [Google Scholar]
- Kandanur S. G. S.; Golakoti N. R.; Nanduri S. Synthesis and in vitro cytotoxicity of novel C-12 substituted-14- deoxy-andrographolide derivatives as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2015, 25, 5781–5786. 10.1016/j.bmcl.2015.10.053. [DOI] [PubMed] [Google Scholar]
- Nanduri S.; Nyavanandi V. K.; Thunuguntla S. S.; Kasu S.; Pallerla M. K.; Ram P. S.; Rajagopal S.; Kumar R. A.; Ramanujam R.; Babu J. M.; Vyas K.; Devi A. S.; Reddy G. O.; Akella V. Synthesis and structure-activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorg. Med. Chem. Lett. 2004, 14, 4711–4717. 10.1016/j.bmcl.2004.06.090. [DOI] [PubMed] [Google Scholar]
- Reabroia S.; Chairoungdua A.; Saeeng R.; Kasemsuk T.; Saengsawang W.; Zhu W.; Piyachaturawat P. A silyl andrographolide analogue suppresses Wnt/β-catenin signaling pathway in colon cancer. Biomed. Pharmacother. 2018, 101, 414–421. 10.1016/j.biopha.2018.02.119. [DOI] [PubMed] [Google Scholar]
- Yuan L.; Zhang C.; Sun H.; Liu Q.; Huang J.; Sheng L.; Lin B.; Wang J.; Chen L. The semi-synthesis of novel andrographolide analogues and anti-influenza virus activity evaluation of their derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 769–773. 10.1016/j.bmcl.2015.12.100. [DOI] [PubMed] [Google Scholar]
- Musacchio A. J.; Lainhart B. C.; Zhang X.; Naguib S. G.; Sherwood T. C.; Knowles R. R. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 2017, 355, 727–730. 10.1126/science.aal3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Jada S. R.; Matthews C.; Saad M. S.; Hamzah A. S.; Lajis N. H.; Stevens M. F. G.; Stanslas J. Benzylidene derivatives of andrographolide inhibit growth of breast and colon cancer cells in vitro by inducing G1 arrest and apoptosis. Br. J. Pharmacol. 2008, 155, 641–654. 10.1038/bjp.2008.368. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wong C. C.; Lima S. H.; Sagineedua S. R.; Lajis N. H.; Stanslas J. SRJ09, a promising anticancer drug lead: Elucidation of mechanisms of antiproliferative and apoptogenic effects and assessment of in vivo antitumor efficacy. Pharmacol. Res. 2016, 107, 66–78. 10.1016/j.phrs.2016.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.