Abstract
The aim of this study was to determine the metabolic effects of a four-week 60% high-fat (HF) diet on mourning doves. Plasma glucose concentrations are, on average, 1.5–2 times higher in birds than in mammals of similar body mass, but birds have innate mechanisms that protect them from high blood glucose-associated pathologies normally developed in mammals. Elucidating these mechanisms may help develop therapeutics for treatment of human diabetes-related complications. A high fat (HF) diet is commonly used in rodents to investigate metabolic disease. We hypothesized that this diet in doves would elevate plasma glucose and alter metabolic physiology compared to the control (CON) diet. Following the four-week long diets, doves were euthanized, and we collected blood, liver, pectoralis muscles, and kidney samples. Contrary to the rodent-models, HF-fed birds did not have increased plasma glucose concentrations relative to CON-fed birds. Metabolomic analyses revealed no group differences in plasma, liver, pectoralis muscle, or kidney metabolites (FDR q-value>0.05 for all). Principal component analysis score plots of metabolites showed no separation between groups, and pathway analyses revealed no significantly altered metabolic pathways between groups (191 pathways across tissues, FDR q-value>0.05). Body mass, plasma uric acid, glucose, and insulin as well as liver and pectoralis muscle glycogen and triglycerides did not differ between groups (p > 0.05 for all). In conclusion, a four-week long high fat diet did not alter plasma glucose concentrations or metabolic physiology in mourning doves, indicating that these birds have mechanisms that allow them to avoid high fat diet-induced pathologies seen in mammals.
Keywords: High-fat diet, Avian, Nutrition, Metabolomics, Diabetes, Negative model
1. Introduction
Avian and mammalian nutritional physiology differ in several respects. A notable difference concerns glucose metabolism: avian plasma glucose concentrations are, on average, 1.5–2 times higher than those of mammals of similar body mass (Braun and Sweazea, 2008). Further, birds lack an insulin-responsive GLUT4-like transporter (Braun and Sweazea, 2008; Welch et al., 2013), have lower plasma insulin concentrations (Hazelwood, 1973), and a higher sensitivity to the metabolic effects of glucagon (Hazelwood, 1973) than mammals. Interestingly, glucagon administration to birds typically increases circulating lipids more than glucose (Hazelwood, 1973). Additionally, in birds, dietary fat is absorbed directly into the blood via portomicrons, instead of the lymphatic system as in mammals (Bensadoun and Rothfeld, 1972). Given these differences, the ability of birds to process (i.e., digest, absorb, and metabolize) food likely also differs from that of mammals. For example, rodents fed a high-fat (HF) diet for six weeks develop elevated fasting hyperglycemia (Sweazea et al., 2010) due to the high fat-induced upregulation of gluconeogenesis (Meng et al., 2013). However, research on effects of such diets in birds is limited and has yielded inconsistent results.
Turkey hens (Meleagris gallopavo) fed a HF diet (47% kcal from fat; 100 days) consumed more calories than controls, but this diet had no effect on body weight (Rosebrough and Steele, 1985). In addition, a high fat commercial poultry feed (22% kcal from fat; 12 weeks) had no effect on the body mass and serum triglycerides of Guineafowl (Numida meleagris) and Muscovy ducks (Cairina moschata; Donaldson et al., 2014). In contrast, male Japanese quail (Coturnix coturnix japonica) fed a moderate fat diet (22% kcal from fat or varying sources; 12 weeks) showed increased body mass and decreased serum triglycerides (Donaldson et al., 2015). Likewise, Japanese quail fed a commercial poultry feed enriched with five different types of fat (either coconut oil, palm oil, soybean oil, lard, or sunflower oil; 22.28–26.13% kcal from fat; 12 weeks) increased in body mass (Donaldson et al., 2017). In addition, the various HF diets decreased serum triglycerides but did not affect glucose tolerance or serum metabolic health markers (uric acid, total protein, albumin, total bilirubin, and calcium). Moreover, in the Taiwan country chicken, Gallus gallus, consumption of a 10% high fat diet from lard for six weeks increased body weight, as well as plasma triglycerides and cholesterol, compared to controls (Chen et al., 2018). It should be pointed out that the above studies used domesticated birds and, to date, no study has tested the effects of a high fat diet (60% kcal from fat) in wild-caught birds.
As birds naturally have relatively high plasma glucose, yet do not develop complications from hyperglycemia as seen in mammals, they can serve as a pathology-free model of diabetes: elucidating the mechanisms used to protect against these complications may help develop therapeutics for treatment of human diabetes-related pathologies (Green et al., 2018; Szwergold and Miller, 2014). In particular, determining how a high-fat diet alters the metabolic physiology of birds, if at all, may reveal potential therapeutic targets to prevent complications from diet-induced metabolic disease in mammals. Metabolic complications can be produced in as little as four weeks in rodents (Gu et al., 2015a, 2015b). The aim of this study, therefore, was to determine if a four-week 60% high-fat diet (HF) in wild-caught mourning doves (Zenaida macroura) alters metabolic physiology compared to a control (CON) diet. We hypothesized that feeding mourning doves a high fat diet for four weeks would raise plasma glucose and alter metabolic physiology.
2. Materials and methods
2.1. Animals, study design, and diet
Mourning doves commonly reside in the study area (Arizona State University [ASU] Tempe Campus; 33° 25′ 11.5” N - 111° 55′ 55.6” W; altitude: 365 m a.s.l.) and have been used for related studies (Basile et al., 2020; Jarrett et al., 2013, 2016; Smith et al., 2011; Sweazea et al., 2010). Adult mourning ddddoves (110–130 g body mass) were trapped as previously described (Basile et al., 2020; Jarrett et al., 2016; Jarrett et al., 2013; Smith et al., 2011), using a walk-in funnel trap baited with wild bird seeds. Only males were caught to limit potentially confounding effects of sex differences. Sex was determined at time of capture based on sexual dimorphic features (Petrides, 1950) and was confirmed after euthanasia yielding 13 males and 1 female to complete the study. All birds were collected from the same location and at approximately the same time of the morning, and transported in individual cloth bags with drawstring closures, to minimize stress, to the ASU Department of Animal Care and Technologies (DACT) outdoor aviaries. Week 1 consisted of trapping and acclimating animals to individual housing in either 1) metal mobile aviary cages: 60”L x 30”W x 78”H housed within a larger aviary or 2) housing pens 9′L x 5′W x 9′H within a different larger aviary.
During the acclimation period, doves received a nutritionally balanced dove seed diet (Supreme Dove Food, Kaytee Products, Inc; Chilton, WI USA) containing 63% carbohydrate, 11% protein, and 3% lipid (% macronutrient/g). During Week 2, doves were introduced to their respective commercial pelleted experimental diets and fed a mixture of bird seed and experimental diet at increasing ratios until only the experimental diet remained. Experimental diets consisted either of a high fat (60% kcal from fat; HF) or of a control (CON) diet (see Table 1 for nutritional information). Both diets were broken into smaller pieces using a mortar and pestle to give the doves a wide selection of item sizes. The CON diet was moistened with water prior to crushing and both diets were kept refrigerated. Doves received food and water ad libitum, and both were changed daily. After the diet introduction week, doves remained on their respective diet for four weeks until euthanasia. Food intake was not recorded as birds often spilled food in and outside the cages, which would have made measurements unreliable. In total, 16 doves were captured, but two were released due to poor acclimation/self-injury. Therefore, 14 doves completed the study. Due to limitations in cage availability, doves were captured and housed in two batches (batch 1: 2/26/2018 through 4/12/2018 (46 days), n = 9 doves (CON 5, HF: 4); batch 2: 4/16/2018 through 5/29/2018 (44 days), n = 5 doves (CON: 2, HF: 3)).
Table 1.
Composition of pelleted experimental diets.
| Percent by weight | Percent kcal | ||
|---|---|---|---|
|
|
|||
| High-Fat Diet: | |||
| Kcal/g: 5.1 | Protein: | 23.5 | 18.3 |
| Carbohydrate: | 27.3 | 21.4 | |
| Fat: | 34.3 | 60.3 | |
| Ingredients (g/Kg): Casein (265.0), Maltodextrin (160.04), Sucrose (90.0), Anhydrous Milkfat (74.8), Cellulose (65.5), Soy Bean Oil (64.7), Lard (57.7), Olive Oil (57.7), Beef Tallow (51.1), TD 94046 MM AIN-93G-MX (48.0), Corn Oil (34.0), TD 94047 VM, AIN-93-VX (21.0), L-Cystine (4.0), Calcium Phosphate, dibasic (3.4), Choline Bitartrate (3.0), TBHQ (antioxidant; 0.06) | |||
| Control Diet: | |||
| Kcal/g: 3.6 | Protein: | 18.6 | 20.5 |
| Carbohydrate: | 62.6 | 69.1 | |
| Fat: | 4.2 | 10.5 | |
| Ingredients (g/Kg): Corn Starch (465.0), Casein (210.0), Maltodextrin (100.0), Sucrose (90.0), Cellulose (37.25), Mineral Mix – AIN-93G-MX (04046; 35.0), Lard (20.0), Soybean Oil (20.0), Vitamin Mix AIN-93-VX (94,047; 15.0), L-Cystine (3.0), Choline Bitartrate (2.75), Calcium Phosphate, dibasic (2.0) | |||
Experimental diet nutrition and ingredient data provided from Envigo Teklad Diets (Madison, Wisconsin, USA).
2.2. Euthanasia and tissue collection
After four weeks of CON or HF diet consumption, birds were euthanized between 8 and 10 am Mountain Standard Time with sodium pentobarbital (200 mg/kg body mass, i.p.) and ~ 2 mL of non-fasted blood was collected by cardiac puncture into ethylenediaminetetraacetic acid (EDTA) and heparin-coated vacutainers. Liver, pectoralis muscle, and kidney samples were then collected. Blood was centrifuged at 14,000 RPM for 10 min to isolate plasma. Plasma and tissue samples were stored at −80 °C until analyses. All experimental procedures were approved by the ASU Institutional Animal Care and Use Committee and were conducted under appropriate state and federal permits.
2.3. Metabolomics
Metabolomics analyses were performed at the Arizona Metabolomics Laboratory as previously described (Basile et al., 2020). Briefly, plasma, liver, pectoralis muscle, and kidney samples (HF = 7; CON = 7 for each; batches were combined for analyses) were thawed overnight at 4 °C. For plasma analysis, 50 μL of each plasma sample was placed in a 2 mL Eppendorf vial. To precipitate proteins and extract metabolites, 500 μL MeOH and 50 μL internal standard solution (containing 1810.5 μM 13C3-lactate and 142 μM 13C5-glutamic acid) were added to the samples and the mixture vortexed for 10 s, stored at −20 °C for 30 min, then centrifuged at 14,000 RPM for 10 min at 4 °C. Supernatants (450 μL) were extracted and transferred to new Eppendorf vials and dried (CentriVap Concentrator; Labconco, Fort Scott, KS, USA). Samples were reconstituted in 150 μL of 40% PBS/60% acetonitrile and centrifuged again at 14,000 RPM at 4 °C for 10 min. Supernatant (100 μL) was transferred to an LC autosampler vial for subsequent analysis. Internal quality-control (QC) samples consisted of two pooled plasma samples from all animals. The QC samples were analyzed three times during reading: beginning, middle, and end.
For tissue analyses, 20 mg samples were homogenized in 200 μL MeOH:PBS (4:1, v:v, containing 1810.5 μM 13C3-lactate and 142 μM 13C5-glutamic acid) in an Eppendorf tube (Bullet Blender homogenizer; Next Advance, Averill Park, NY, USA). Then 800 μL MeOH:PBS (4:1, v:v, containing 1810.5 μM 13C3-lactate and 142 μM 13C5-glutamic acid) was added to the homogenate and vortexed for 10 s. Homogenized samples were transferred to −20 °C for 30 min, then sonicated in an ice bath for 30 min. Samples were centrifuged at 14,000 RPM for 10 min (4 °C), and 800 μL supernatant was transferred to a new Eppendorf tube and vacuum-dried (CentriVap Concentrator; Labconco, Fort Scott, KS, USA). Prior to MS analysis, dried samples were reconstituted in 150 μL 40% PBS/60% ACN. Data were normalized to tissue weights prior to analyses.
2.4. Liquid chromatography-mass spectrometry
The targeted LC-MS/MS method used in the current study was modeled after previous studies (Buas et al., 2017; Carroll et al., 2015; Zhu et al., 2014; Gu et al., 2016; Gu et al., 2015a, 2015b; Li et al., 2018; Jasbi et al., 2019a, 2019b; Shi et al., 2019; He et al., 2020; Shi et al., 2020). Briefly, LC-MS/MS experiments were performed on an Agilent 1290 UPLC-6490 QQQ-MS (Santa Clara, CA, USA) system. Each sample was injected twice for analysis (10 μL using negative ionization mode, 4 μL using positive ionization mode). Chromatographic separations were performed in hydrophilic interaction chromatography (HILIC) mode on a Waters XBridge BEH Amide column (150 × 2.1 mm, 2.5 μm particle size, Waters Corporation, Milford, MA, USA). The flow rate was 0.3 mL/min, auto-sampler temperature was maintained at 4 °C, and the column compartment was set at 40 °C. The mobile phase system was composed of Solvents A (10 mM ammonium acetate, 10 mM ammonium hydroxide in 95% H2O/5% ACN) and B (10 mM ammonium acetate, 10 mM ammonium hydroxide in 95% ACN/5% H2O). After the initial 1 min isocratic elution of 90% Solvent B, the percentage of Solvent B decreased to 40% at t = 11 min. The composition of Solvent B maintained at 40% for 4 min (t = 15 min), and then the percentage of Solvent B gradually went back to 90%, to prepare for the next injection.
The mass spectrometer was equipped with an electrospray ionization (ESI) source. Targeted data acquisition was performed in multiple-reaction-monitoring (MRM) mode. We monitored 132 and 171 MRM transitions in negative and positive mode, respectively (303 transitions in total). The LC-MS system was controlled by Agilent MassHunter Workstation software (Santa Clara, CA, USA) and extracted MRM peaks were integrated using Agilent MassHunter Quantitative Data Analysis software (Santa Clara, CA, USA).
2.5. Morphometrics and plasma and tissue metabolite analyses
Body weight was not recorded at the time of capture because food within the crop of mourning doves can account for up to 10% of body weight (Hanson and Kossack, 1957). The experimental diets were not stored appreciably in the crops and body weight was, therefore, recorded at the time of euthanasia using Ohaus Triple Beam 700/00 Series Balance (linearity/sensitivity ±0.1 g; Parsippany, NJ, USA). For all assays, samples were analyzed in duplicate on the same assay plate to avoid inter-plate variations. Plasma uric acid concentrations were measured using a commercially available kit (Cat. No. DIUA-250; Bio Assay Systems, Haywood, CA, USA; sensitivity: 0.22 mg/dL; intra-assay percent coefficient variation [CV] for both groups: 1.99%). Samples were diluted 1:2 prior to the assay. Plasma insulin concentrations were determined using the Ultra-Sensitive Rat Insulin ELISA Kit (Cat No. 90060; Crystal Chem, Elk Grove Village, IL, USA; sensitivity: 50 pg/mL; CV: 4.38%; note: the structure of insulin is generally well-conserved throughout the animal kingdom (Simon et al., 2004) and this kit has been used in prior studies with avian plasma; Basile et al., 2020). Samples were diluted 1:15 prior to the assay. Liver and pectoralis glycogen concentrations were determined using published methods (sensitivity: 5 pg/mL; CV: 1.04% and 1.62%, respectively; Lo et al., 1970). Plasma glucose concentrations were measured using a commercially available kit (Cat. No. 10009582; Cayman Chemical, Ant Arbor, MI, USA; sensitivity: 5.0 mM/L; CV: 1.06%). Plasma, liver, and pectoralis muscle triglyceride concentrations were determined using a previously published method (Jouihan, 2012; sensitivity: 0.03 mM; CV: 1.06%, 0.67%, and 0.78%, respectively). Tissue samples were diluted 1:2 prior to the assay. All readings were obtained using a Thermo Fisher Scientific, Multiskan Go plate reader (Waltham, MA, USA).
2.6. Statistical analysis and metabolite and pathway interpretation
Metabolic pathways and pathway analyses, integrating enrichment analyses, and pathway topology analyses were performed, and results were visualized using the online MetaboAnalyst (metaboanalyst.ca) software package. The reported Holm adjusted p-value was used to determine significance (Xia et al., 2015). The data were log10-transformed prior to model construction. The pathway analysis was calculated from the chicken metabolic pathway library. PubChem Open Chemistry Database (https://pubchem.ncbi.nlm.nih.gov/) was used for interpretation of individual metabolites and metabolic pathways. Univariate testing was performed using SPSS 25.0 (SPSS Inc., Chicago, IL, USA). To determine significantly affected metabolites, the data were log-transformed and subjected to independent sample Student’s t-tests (HF vs. CON) for all tissues. If data were not normally distributed, a Mann-Whitney U test was used. To adjust for multiple hypothesis testing, a false discovery rate (FDR) correction was used with a significance level of p < 0.05. To determine separation between groups, a principal component analysis (PCA) was conducted on metabolites for each tissue. PCA score plots were generated for visualization. Student’s t-tests were used to compare morphometrics and biochemical variables between diet groups (SigmaStat 10.0; Systat Software, Inc.; San Jose, CA, USA). Data that were not normally distributed were log transformed prior to analysis (plasma insulin, liver glycogen, pectoralis muscle and liver triglycerides); however, non-transformed data are presented. In addition, Siegel-Tukey tests were used to compare differences in statistical dispersion (i.e., scale or spread) in ratio scale variables between study groups. A two-way analysis of variance (ANOVA) was used to determine difference in morphometrics and biochemical variables between batches. The statistical significance level of all tests was set to an α of 0.05.
3. Results
We detected numerous metabolites in all tissues: plasma: 71; liver: 151; pectoralis muscle: 159; and kidney: 187 (supplemental file 1). There were no differences in metabolite concentrations between diet groups in plasma, liver, pectoralis muscle, or kidney (FDR q-value>0.05; see supplemental file 1). PCA score plots for the plasma, liver, pectoralis muscle, and kidney metabolites showed no separation between groups (Fig. 1). Fig. 2 shows the pathway analysis enrichment and pathway topology analysis of plasma, liver, and pectoralis muscle for both groups. No metabolic pathways were significantly altered between diet groups (FDR q-value>0.05; see supplemental file 1).
Fig. 1.
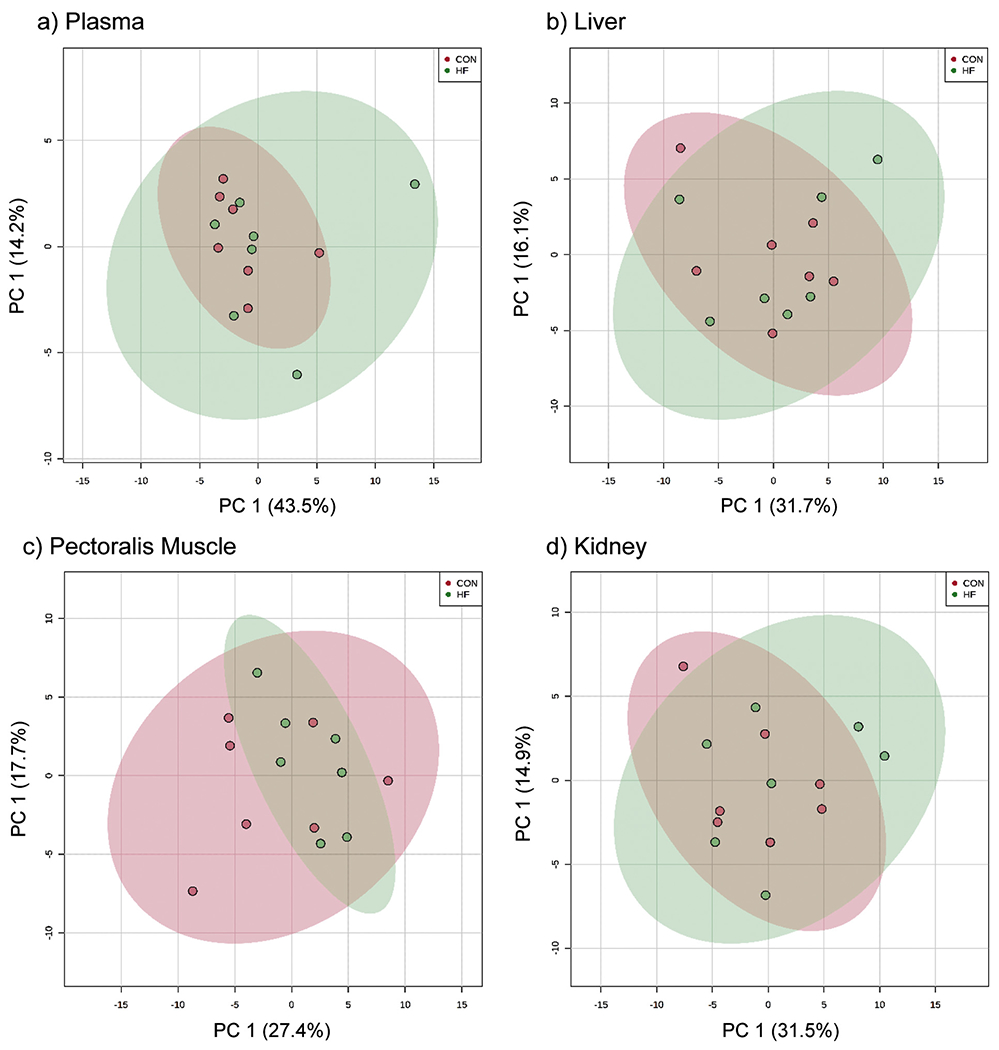
Principal Component Analysis (PCA) score plats of mourning dove plasma, liver, pectoralis muscle, and kidney show minimal separation between diet groups. PCA score plots produced via online MetaboAnalyst.ca software for plasma (number of metabolites matched to program: n = 63/71), liver (n = 138/151), pectoralis muscle (n = 145/159), and kidney (n = 167/187) between mourning doves consuming a four-week high-fat diet (HF: red circles, n = 7) or control diet (CON: green circles, n = 7). PCA X and Y Axis are principal components (PC) 1 and 2, respectively, and represent the first and second largest variation in the data. The values on the X and Y axis represent the percentage of variation explained by the PC. Note: X and Y axis vary by plot. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
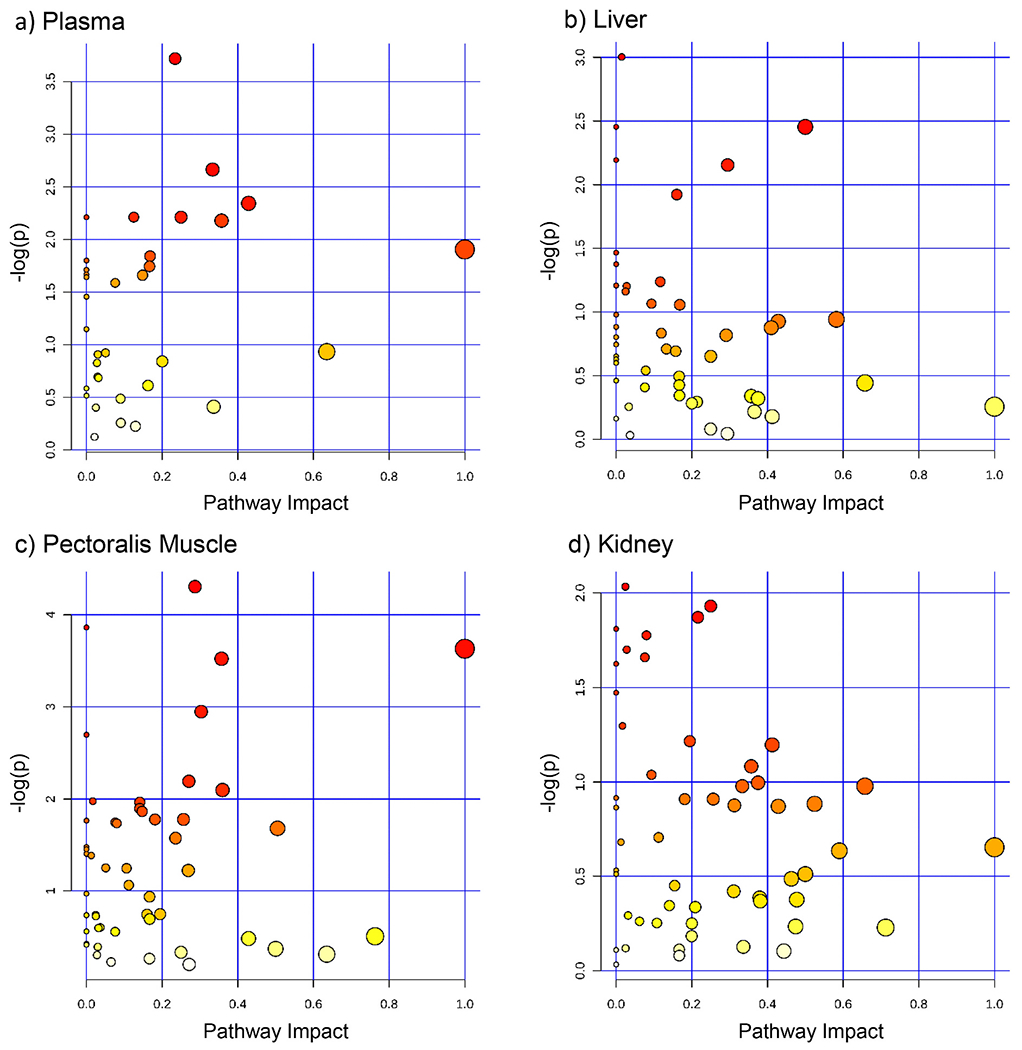
No metabolic pathways in plasma, liver, pectoralis muscle, and kidney were significantly altered between diet groups. Metabolic pathway enrichment analysis, performed using online MetaboAnalyst.ca software, for metabolite concentrations for a) plasma (metabolites matched to software: n = 63/71), b) liver (n = 138/151), c) pectoralis muscle (n = 145/159), and d) kidney (n = 167/187) between mourning doves consuming a four-week high-fat diet (HF; n = 7) or control diet (CON; n = 7). No metabolic pathways were significantly altered between diet groups (FDR adjusted p-value>0.05). Size of the dot corresponds with the pathway impact (location of metabolite in pathway, e.g. upstream or downstream, statistical significance of the set of pathway genes, and metabolite concentration fold change). Dot colour (yellow to red) corresponds with the level of significance. Note: Y axis varies by plot. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
No differences between diet groups were found in body mass, plasma uric acid, insulin, and glucose, as well as liver and pectoralis muscle glycogen and triglyceride (p > 0.05 for all; Fig. 3), but a difference in scale between diet groups was found for: plasma uric acid (p = 0.031; greater scale in HF), insulin (p = 0.047; less scale in HF), and triglyceride (p = 0.047; greater scale in HF), pectoralis muscle glycogen (p = 0.047; greater scale in HF), liver glycogen (p = 0.031; greater scale in HF), and pectoralis muscle triglyceride (p = 0.016; greater scale in HF). In addition, we found no differences between batch numbers except that birds belonging to the first batch had lower pectoralis glycogen than birds belonging to the second batch (p = 0.034; batch 1: 226.75 mg/g muscle, SEM: 37.02; batch 2: 420.18 mg/g muscle, SEM: 50.38).
Fig. 3.
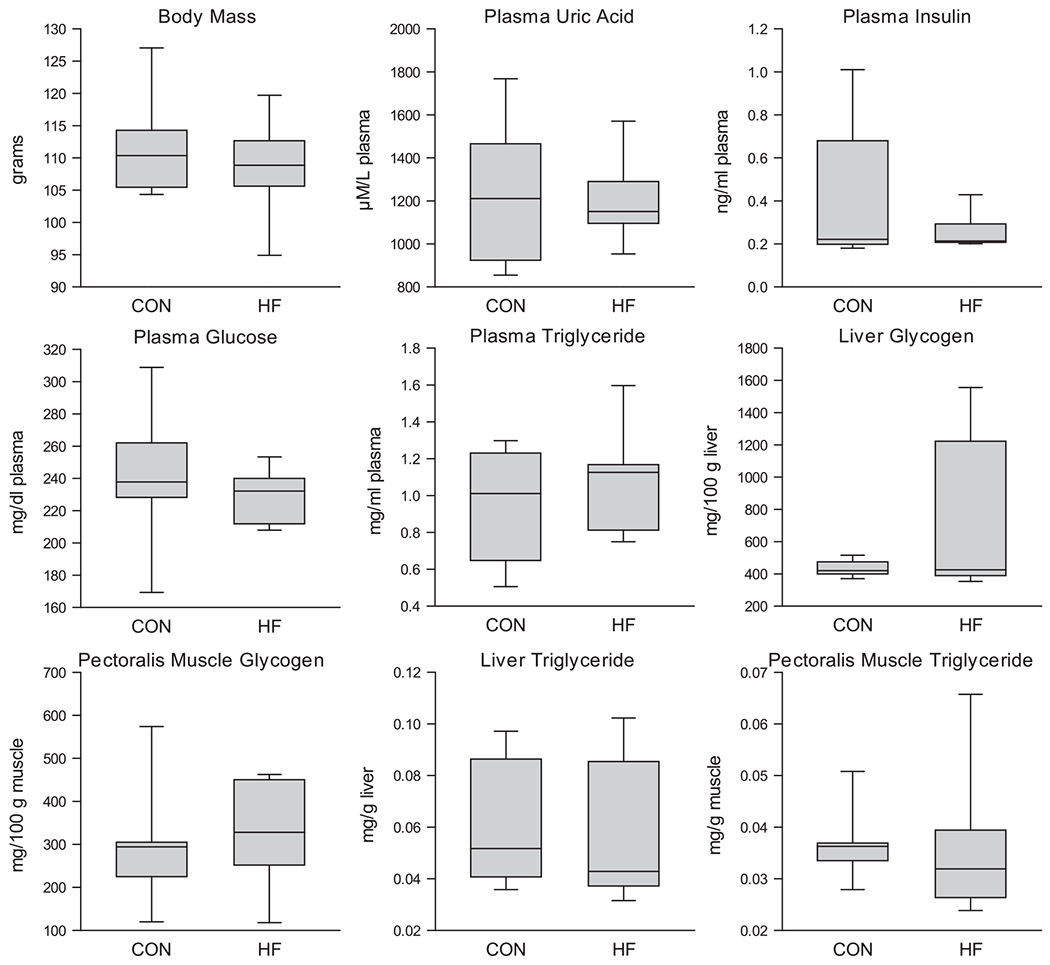
Body mass, plasma uric acid, insulin, glucose and triglyceride as well as pectoralis muscle and liver glycogen and triglycerides were not altered between diet groups. Data presented as median and upper and lower quartile values and whiskers represent upper and lower values, respectively. Batch data were combined for presentation; n = 7 for both groups except plasma glucose (CON: n = 7; HF: n = 6). No differences between diet groups (p > 0.05 for all). However, a difference in scale between diet groups was found for: plasma uric acid (p = 0.031; greater scale in HF), insulin (p = 0.047; less scale in HF), and triglyceride (p = 0.047; greater scale in HF), pectoralis muscle glycogen (p = 0.047; greater scale in HF), liver glycogen (p = 0.031; greater scale in HF), and pectoralis muscle triglyceride (p = 0.016; greater scale in HF). Note: Y axis varies by plot.
4. Discussion
Based on the mammalian response to a HF diet, we used mourning doves as an experimental model (i.e., negative model of hyperglycemia complications) to test the hypothesis that this diet also alters plasma glucose concentrations in mourning doves. Contrary to this hypothesis, we found no HF diet-induced effects on plasma glucose concentration or metabolic physiology compared to the control group. Moreover, metabolomics analyses also revealed no significant differences between groups in plasma, liver, pectoralis muscle, or kidney metabolites. Pathway analyses displayed no alterations in metabolic pathways in plasma, liver, pectoralis muscle, or kidney between diet groups. Lastly, body mass, plasma uric acid and insulin, and liver and pectoralis muscle glycogen and triglyceride did not differ between diet groups. However, the plasma levels of uric acid and triglyceride, the levels of pectoralis muscle glycogen and triglyceride, and the levels of liver glycogen had a larger scale in the HF than the CON group. In addition, doves in batch 2 had a higher pectoralis muscle glycogen concentration than birds in batch 1. Average daily ambient temperature was lower when batch 1 birds were tested than when batch 2 birds were tested (batch 1: 69.22 °F, SD: 8.57; batch 2: 81.82 °F, SD: 6.83; p < 0.001; see Supplemental File). This temperature difference may have caused a difference between birds of the two batches in activity and consequently also in pectoralis muscle glycogen. In contrast, plasma insulin concentrations have a smaller scale in the HF than the control group of doves. Overall, compared to mammalian physiology, mourning dove metabolic physiology appears to be minimally altered by a high fat diet.
Previous research found that high fat diets can alter avian physiology, but these studies differed from the present investigation with respect to duration and to percentage of kcal from fat in the diet, and they used poultry as model species. For example, prior studies were typically of longer duration than this study (6–14 weeks; Chen et al., 2018; Donaldson et al., 2014, 2015; Donaldson et al., 2017; Rosebrough and Steele, 1985). This difference may have contributed to our finding no diet-related changes in metabolic physiology. However, the HF diet used in current study had a higher percentage of kcals from fat (60%) than the diet used in previous studies (10%–47% kcal from fat; Chen et al., 2018; Donaldson et al., 2014, 2015; Donaldson et al., 2017; Rosebrough and Steele, 1985). Although Jia et al. (2012) used a 50% kcal from animal fat diet in female Guangdong Yuehuang chickens, these researchers did not examine metabolic physiology (Jia et al., 2012). The use of poultry as model organisms in the above studies does not inform about the normal physiological response of wild birds to a high fat diet. Further, to our knowledge, there are no studies examining the effect of a high fat diet in laboratory raised animals compared to wild. Similarly, laboratory rodents do not reflect wild rodents due to conditions of captivity (e.g., limited environmental stimulation, restricted physical activity, and ad libitum access to food; Martin et al., 2010). In addition, laboratory and wild animals may have evolved differences in metabolic physiology, altering their physiological response to a high fat diet. Therefore, the inclusion of wild birds in the present study widens the understanding of the effects of a high fat diet on avian physiology.
Consistent with previous work, body mass (Donaldson et al., 2014; Rosebrough and Steele, 1985), plasma uric acid (Donaldson et al., 2017), liver glycogen (Rosebrough and Steele, 1985), and liver triglycerides (Donaldson et al., 2014) were not altered by the HF diet. Conversely, this diet given to poultry can decrease liver lipids (Rosebrough and Steele, 1985), and increase body mass and serum triglycerides (Chen et al., 2018; Donaldson et al., 2014, 2017). Overall, the effects of a high fat diet on birds are variable and may be species- or diet type-specific. We also found no group difference in body weight and plasma or tissue metabolite concentrations, but the HF diet resulted in either a smaller or larger scale for some metabolite concentrations compared to CON. This result suggests that the physiology of HF diet-fed doves changed in complex and individually variable ways compared to that CON diet-fed doves, but the factors that account for this difference (e.g., eating behavioral variation) have not been identified.
Birds can serve as a negative model (see Green et al., 2018) in which to identify potential therapeutic targets for mammalian diabetes/hyperglycemia complications (Szwergold and Miller, 2014). The present study contributes to this research tool in two ways. First, a better understanding of the metabolic physiology of a wild-caught model can help understand avian resistance to metabolic complications. Second, the present results demonstrate that mourning doves can maintain metabolic homeostasis while consuming a HF diet for four weeks. This diet in mammals induces pathological effects. For example, Sprague-Dawley rats develop dyslipidemia after four weeks of a 48% HF diet consumption (Gu et al., 2015a, 2015b). The present results indicate that doves have a physiological plasticity to avoid changes in metabolic physiology when consuming a diet known to induce pathology in mammals. The mechanisms that doves use to elude this effect of HF diet consumption are unclear and warrant additional research. Whether this dietary plasticity extends to other wild bird species is unclear. Prior research in our laboratory tested the effects of an extremely high carbohydrate diet (white bread; Basile et al., 2020) and similar to the present study, it showed minimal changes in the metabolic physiology of the animals, although endothelium dependent vasodilation and liver glycogen were altered by the refined carbohydrate diet. Together, the findings from these two dietary studies on mourning doves suggest that these birds have considerable plasticity with regard to their ability to consume diets comprised of extreme macronutrient ratios that produce pathologies in mammals (de Oliveira et al., 2015; Spadaro et al., 2015; Sweazea et al., 2010).
Limitations of the current study include the following: 1) not all metabolites were matched to the software for PCA and pathway analysis; 2) the pathway analysis was conducted using the chicken pathway library, which may vary from that of mourning doves; 3) the present study was of shorter duration than previous investigations examining the effect of HF diet in poultry; 4) doves were caught and housed in two separate groups separated by seven weeks; 5) doves were captured in an urban environment and so may have already been acclimated to consuming anthropogenic foods that are high in fat. This possibility is, however, unlikely because anthropogenic foods are typically diverse and commonly carbohydrate/starch dense (e.g., bread and fruit; Galbraith et al., 2014); 6) mourning doves are granivorous and omnivorous, and so how the HF used here would affect birds that normally consume other diet types is unknown. Fatty acids are the predominant metabolic fuel used by omnivorous migratory species, such as mourning doves (Gannes, 2001; Guglielmo, 2010; Jenni-Eiermann et al., 2002) which may help explain the lack of significant effects of the HF diet on metabolic physiology in the current study.
Overall, mourning doves appear to have remarkable ability to maintain homeostasis in response to consuming a high fat (60% kcal from fat) diet compared to a control diet. Future studies on this topic may improve our understanding of avian physiology and the potential of birds as negative, pathology-free models of mammalian diabetes.
Supplementary Material
Acknowledgements
We would like to acknowledge Michael Renner and Monique Bertin for their assistance with husbandry and analyses.
Grants
This project was funded by the ASU School of Life Sciences and Office of Knowledge Enterprise Development Research Investment Initiative, United States (KLS and PD) and the National Science Foundation under grant number DEB-1832016, Central Arizona-Phoenix Long-Term Ecological Research Program (CAP LTER), United States (AJB).
Footnotes
Disclosures
AM is currently a graduate student at Arizona State University and is employed by Isagenix International, LLC. Isagenix was not involved in any aspect of the current study. All other authors have no financial disclosures or conflict of interests to declare.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cbpa.2020.110820.
References
- Basile AJ, Jasbi P, Clark W, Shi X, Gu H, Deviche P, Sweazea KL, 2020. A four-week white bread diet does not alter plasma glucose concentrations, metabolic or vascular physiology in mourning doves, Zenaida macroura. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol 247, 110718 10.1016/j.cbpa.2020.110718. [DOI] [PubMed] [Google Scholar]
- Bensadoun A, Rothfeld A, 1972. The form of absorption of lipids in the chicken, Gallus domesticus. Proc. Soc. Exp. Biol. Med 141, 814–817. [DOI] [PubMed] [Google Scholar]
- Braun EJ, Sweazea KL, 2008. Glucose regulation in birds. Comp. Biochem. Physiol. B Biochem. Mol. Biol 151, 1–9. 10.1016/j.cbpb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Buas MF, Gu H, Djukovic D, Zhu J, Onstad L, Reid BJ, Raftery D, Vaughan TL, 2017. Candidate serum metabolite biomarkers for differentiating gastroesophageal reflux disease, Barrett’s esophagus, and high-grade dysplasia/esophageal adenocarcinoma. Metabolomics 13, 23. 10.1007/si1306-016-1154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll PA, Diolaiti D, McFerrin L, Gu H, Djukovic D, Du J, Cheng PF, Anderson S, Ulrich M, Hurley JB, Raftery D, Ayer DE, Eisenman RN, 2015. Deregulated Myc requires MondoA/mlx for metabolic reprogramming and tumorigenesis. Cancer Cell 27, 271–285. 10.1016/j.ccell.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Chen YJ, Ding ST, Lin YY, 2018. Expression profile of adiponectin and adiponectin receptors in high-fat diet feeding chickens. J. Anim. Physiol. Anim. Nutr. (Berl) 102, 1585–1592. 10.1111/jpn.12979. [DOI] [PubMed] [Google Scholar]
- de Oliveira MC, Menezes-Garcia Z, Arifa RD, do N, de Paula TP, Andrade JMO, Santos SHS, de Menezes GB, de Souza D, da G, Teixeira MM, Ferreira AVM, 2015. Platelet-activating factor modulates fat storage in the liver induced by a high-refined carbohydrate-containing diet. J. Nutr. Biochem 26, 978–985. 10.1016/j.jnutbio.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Dangarembizi R, Mtetwa B, Madziva MT, Erlwanger KH, 2014. The progressive effects of a high-fat diet on erythrocyte osmotic fragility, growth performance and serum triglyceride and cholesterol levels in Guinea fowl (Numida meleagris) and Muscovy duck (Cairina moschata). J. Anim. Physiol. Anim. Nutr. (Berl) 98, 867–874. 10.1111/jpn.12149. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Pillay K, Madziva MT, Erlwanger KH, 2015. The effect of different high-fat diets on erythrocyte osmotic fragility, growth performance and serum lipid concentrations in male, Japanese quail (Coturnix coturnix japonica). J. Anim. Physiol. Anim. Nutr. (Berl) 99, 281–289. 10.1111/jpn.12250. [DOI] [PubMed] [Google Scholar]
- Donaldson J, Madziva MT, Erlwanger KH, 2017. The effects of high-fat diets composed of different animal and vegetable fat sources on the health status and tissue lipid profiles of male Japanese quail (Coturnix coturnix japonica). Asian-Australasian J. Anim. Sci 30, 700–711. 10.5713/ajas.16.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith JA, Beggs JR, Jones DN, McNaughton EJ, Krull CR, Stanley MC, 2014. Risks and drivers of wild bird feeding in urban areas of New Zealand. Biol. Conserv 180, 64–74. 10.1016/j.biocon.2014.09.038. [DOI] [Google Scholar]
- Gannes LZ, 2001. Comparative fuel use of migrating passerines: effects of fat stores, migration distance, and diet. Auk 118, 665–677. 10.1093/auk/118.3.665. [DOI] [Google Scholar]
- Green S, Dietrich MR, Leonelli S, Ankeny RA, 2018. ‘Extreme’ organisms and the problem of generalization: interpreting the Krogh principle. Hist Philos Life Sci 40, 1–22. 10.1007/s40656-018-0231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J, Jing L, Ma X, Zhang Z, Guo Q, Li Y, 2015a. GC-TOF-MS-based serum metabolomic investigations of naked oat bran supplementation in high-fat-diet-induced dyslipidemic rats. J. Nutr. Biochem 26, 1509–1519. 10.1016/j.jnutbio.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Gu H, Zhang P, Zhu J, Raftery D, 2015b. Globally optimized targeted mass spectrometry: reliable metabolomics analysis with broad coverage. Anal. Chem 87, 12355–12362. 10.1021/acs.analchem.5b03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Carroll PA, Du J, Zhu J, Neto FC, Eisenman RN, Raftery D, 2016. Quantitative method to investigate the balance between metabolism and proteome biomass: starting from glycine. Angew. Chem. Int. Ed 55, 15646–15650. 10.1002/anie.201609236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmo CG, 2010. Move that fatty acid: fuel selection and transport in migratory birds and bats. Integr. Comp. Biol 50, 336–345. 10.1093/icb/icq097. [DOI] [PubMed] [Google Scholar]
- Hanson HC, Kossack CW, 1957. Weight and body-fat relationships of mourning doves in Illinois, 21. J. Wildl. Manage, pp. 169–181 [Google Scholar]
- Hazelwood RL, 1973. The avian endocrine pancreas. Am. Zool 13, 699–709. [Google Scholar]
- He H, Shi X, Lawrence A, Hrovat J, Turner C, Cui JY, Gu H, 2020. 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) induces wide metabolic changes including attenuated mitochondrial function and enhanced glycolysis in PC12 cells. Ecotoxicol. Environ. Saf 201, 110849 10.1016/j.ecoenv.2020.110849. [DOI] [PubMed] [Google Scholar]
- Jarrett C, Lekic M, Smith CL, Pusec CM, Sweazea KL, 2013. Mechanisms of acetylcholine-mediated vasodilation in systemic arteries from mourning doves (Zenaida macroura). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol 183, 959–967. 10.1007/S00360-013-0757-0. [DOI] [PubMed] [Google Scholar]
- Jarrett CL, Ahmed Z, Faust JJ, Sweazea KL, 2016. High glucose impairs acetylcholine-mediated vasodilation in isolated arteries from Mourning doves (Z. macroura). Comp. Biochem. Physiol. -Part A Mol. Integr. Physiol 201, 141–145. 10.1016/j.cbpa.2016.07.010. [DOI] [PubMed] [Google Scholar]
- Jasbi P, Mitchell NM, Shi X, Grys TE, Wei Y, Liu L, Lake DF, Gu H, 2019a. Coccidioidomycosis detection using targeted plasma and urine metabolic profiling. J. Proteome Res 18, 2791–2802. 10.1021/acs.jproteome.9b00100. [DOI] [PubMed] [Google Scholar]
- Jasbi P, Wang D, Cheng SL, Fei Q, Cui JY, Liu L, Wei Y, Raftery D, Gu H, 2019b. Breast cancer detection using targeted plasma metabolomics. J. Chromatogr. B Anal. Technol. Biomed. Life Sci 1105, 26–37. 10.1016/j.jchromb.2018.11.029. [DOI] [PubMed] [Google Scholar]
- Jenni-Eiermann S, Jenni L, Kvist A, Lindstrom A, Piersma T, Visser GH, 2002. Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long-distance migrant shorebird. J. Exp. Biol 205, 2453–2460. [DOI] [PubMed] [Google Scholar]
- Jia X, Nie Q, Lamont SJ, Zhang X, 2012. Variation in sequence and expression of the avian FTO, and association with glucose metabolism, body weight, fatness and body composition in chickens. Int. J. Obes 36, 1054–1061. 10.1038/ijo.2011.221. [DOI] [PubMed] [Google Scholar]
- Jouihan H, 2012. Measurement of liver triglyceride content. BIO-PROTOCOL 2. 10.21769/BioProtoc.223. [DOI] [Google Scholar]
- Li R, Grimm SA, Mav D, Gu H, Djukovic D, Shah R, Merrick BA, Raftery D, Wade PA, 2018. Transcriptome and DNA methylome analysis in a mouse model of diet-induced obesity predicts increased risk of colorectal cancer. Cell Rep. 22, 624–637. 10.1016/j.celrep.2017.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S, Russell JC, Taylor AW, 1970. Determination of glycogen in small tissue samples. J. Appl. Physiol 28 (2), 234–236. 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- Martin B, Ji S, Maudsley S, Mattson MP, 2010. “Control” laboratory rodents are metabolically morbid: why it matters. Proc. Natl. Acad. Sci 107, 6127–6133. 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng R, Zhu D, Bi Y, Yang D, Wang Y, 2013. Erythropoietin inhibits gluconeogenesis and inflammation in the liver and improves glucose intolerance in high-fat diet-fed mice. PLoS One 8, e53557. 10.1371/journal.pone.0053557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides GA, 1950. Notes on determination of sex and age in the woodcock and mourning dove. Auk 67, 357–360. 10.2307/4080924. [DOI] [Google Scholar]
- Rosebrough RW, Steele NC, 1985. Effect of dietary fat and environment on lipogenesis by large white breeder turkeys. Poult. Sci 64, 1170–1176. 10.3382/ps.0641170. [DOI] [PubMed] [Google Scholar]
- Shi X, Wang S, Jasbi P, Turner C, Hrovat J, Wei Y, Liu J, Gu H, 2019. Database-assisted globally optimized targeted mass spectrometry (dGOT-MS): broad and reliable metabolomics analysis with enhanced identification. Anal. Chem 91, 13737–13745. 10.1021/acs.analchem.9b03107. [DOI] [PubMed] [Google Scholar]
- Shi X, Xi B, Jasbi P, Turner C, Jin Y, Gu H, 2020. Comprehensive isotopic targeted mass spectrometry: reliable metabolic flux analysis with broad coverage. Anal. Chem 92, 11728–11738. 10.1021/acs.analchem.0c01767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J, Laurent S, Grolleau G, Thoraval P, Soubieux D, Rasschaert D, 2004. Evolution of preproinsulin gene in birds. Mol. Phylogenet. Evol 30, 755–766. 10.1016/S1055-7903(03)00254-9. [DOI] [PubMed] [Google Scholar]
- Smith CL, Toomey M, Walker BR, Braun EJ, Wolf BO, McGraw K, Sweazea KL, 2011. Naturally high plasma glucose levels in mourning doves (Zenaida macroura) do not lead to high levels of reactive oxygen species in the vasculature. Zoology 114, 171–176. 10.1016/j.zool.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadaro PA, Naug HL, Du Toit EF, Donner D, Colson NJ, 2015. A refined high carbohydrate diet is associated with changes in the serotonin pathway and visceral obesity. Genet. Res. (Camb) 97. 10.1017/S0016672315000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweazea KL, Lekic M, Walker BR, 2010. Comparison of mechanisms involved in impaired vascular reactivity between high sucrose and high fat diets in rats. Nutr. Metab. (Lond.) 7, 48. 10.1186/1743-7075-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwergold BS, Miller CB, 2014. Potential of birds to serve as a pathology-free model of type 2 diabetes, part 1: is the apparent absence of the rage gene a factor in the resistance of avian organisms to chronic Hyperglycemia? Rejuvenation Res. 17, 54–61. 10.1089/rej.2013.1498. [DOI] [PubMed] [Google Scholar]
- Welch KC, Allalou A, Sehgal P, Cheng J, Ashok A, 2013. Glucose transporter expression in an avian Nectarivore: the ruby-throated hummingbird (Archilochus colubris). PLoS One 8. 10.1371/journal.pone.0077003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS, 2015. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257. 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Djukovic D, Deng L, Gu H, Himmati F, Chiorean E, Raftery D, 2014. Colorectal cancer detection using targeted serum metabolic profiling. J. Proteome Res 13, 4120–4130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


