Abstract

Mulberry (Morus alba L.) is commonly cultivated in Asian countries as a traditional medicine and food supplement. Four Kashmiri Morus alba varieties (Zagtul, Chtattatual, Chattatual Zaingir, and Brentul Kashmir) were evaluated for their proximate composition, mineral content, total phenolic and flavonoid content, antioxidant potential, and antihyperglycemic activity. Furthermore, TLC-MS-bioautography was used for the identification of antioxidant and antidiabetic compounds in the best active extract. Lastly, UPLC-MS was employed for metabolomic profiling of the best variety of M. alba. Among all the varieties, the Zagtul variety was found to have the highest phenolic (71.10 ± 0.44 mg GAE/g DW) and flavonoid (53.22 ± 0.69 mg rutin/g DW) content. The highest antioxidant potential (DPPH) with an IC50 value of 107.88 ± 3.8 μg/mL was recorded for the Zagtul variety. Similarly, α-amylase and α-glucosidase inhibition for antidiabetic potential with IC50 74.76 ± 6.76 and 109.19 ± 5.78 μg/mL, respectively, was recorded in Zagtul variety. TLC-MS-bioautography for identification of bioactive compounds revealed the presence of chlorogenic acid for antioxidant potential and 1-deoxynojirimycin (DNJ) and syringic acid for antidiabetic potential. Further, bioactive compounds responsible for diverse functions of M. alba were confirmed by UPLC-MS in both negative and positive modes. However, major compounds in the Zagtul variety were identified as chlorogenic acid, moracin N, gallic acid, ferulic acid, morin, 1-deoxynojirimycin, and syringic acid. Hence, based on our findings, it can be concluded that M. alba leaves can be consumed as a promising dietary supplement and can be formulated as phytopharmaceutical for the management of various metabolic disorders.
Introduction
Natural plants contain a diversity of bioactive compounds with antioxidant capabilities including flavonoids, phenolics, sterols, alkaloids, carotenoids, and glucosinolates.1 In recent years, great interest has been focused on the evaluation and discovery of plant- and food-based natural antioxidants in order to eliminate the various side effects of synthetic compounds for the treatment of several diseases.2 There is a huge populace that suffers from lifestyle-related diseases such as diabetes, hyperlipidemia, and hypertension. Among these diseases, diabetes is recognized as the most important global health problem and continues to increase worldwide.3
Mulberry belongs to the genus Morus in the family Moraceae, widely cultivated in varied climatic zones. Mulberry (Morus alba L.), besides being the sole plant source for feeding Bombyx mori, is cultivated as a medicinal plant in Eastern Asian countries.4 The state of Jammu and Kashmir provides a fertile land and environment for the growth and development of bivoltine silkworm and cultivation of mulberry. Kashmir produces silk of superior quality and has gained international recognition for producing silk from indigenous varieties of M. alba due to its suitable agro-climate conditions. Furthermore, sericulture has an important place in the economy of Jammu and Kashmir.5Zagtul, Chtattatual, Chattatual Zaingir, and Brentul Kashmir are the indigenous varieties of M. alba in Kashmir. M. alba has multiple uses in Kashmir. This plant is usually used for fodder (41.46%), fruits (34.72%), fuel (12.52%), and silkworm rearing (11.30%).6 A number of studies have reported the distribution and sericultural importance of M. alba grown in Kashmir;5,7,8 however, no study is available on chemical and nutritional composition of different varieties of M. alba.
Different parts of the mulberry, including leaves, roots, stem, fruits, and bark, are reported to possess antioxidants, antihyperglycemic, antiobesity, and cardioprotective properties and inhibit certain types of cancer as well as act as an efficient diuretic.4−7 Furthermore, extracted flavonoids from M. alba in particular exhibit a wide range of biological effects, including antibacterial, antiviral, anti-inflammatory, antiallergic, antithrombotic, anticarcinogenic, hepatoprotective, and vasodilator activities.9,10
M. alba is a rich source of bioactive compounds. Flavonoids are the most abundant phenolic chemical class identified in M. alba leaves. These compounds are likely responsible for the bioactivities of the M. alba leaves. Phenolic compounds are natural antioxidants provided by the secondary metabolism of plants that protect multiple organs from oxidation.2 Other classes of compounds found in M. alba leaves are benzofurans, phenolic acids, chalcones, alkaloids, coumarins, and stilbenes. Oxyresveratrol is the only stilbene present in M. alba with proven anti-inflammatory benefits. Deoxynojirimycin and fagomine are important alkaloids that contain compounds of one or more basic nitrogen atoms in a polyhydroxylated heterocyclic ring. Other major compounds include moracins (benzofurans), caffeoylquinic acids (phenolic acids), and morachalcones. Major flavonoids are rutin, kuwanons, quercetin-3-O-b-d-glucopyranoside, quercetin-3,7-di-O-b-d-glucopyranoside, cyclomorusin, moragrols, moracinflavans, and morkotins.9 Previously reported compounds like rutin, isoquercitrin, and astragalin have been found to exhibit antioxidant activity.10 One of the earlier conducted studies found that aminosugars like 1-deoxynojirimycin, flavonoids like rutin, and phenolic acid compounds like chlorogenic acid in the M. alba leaves exert an antidiabetic effect.11 Polysaccharides extracted from M. alba leaves showed anti-obesity effect by inhibiting lipid absorption in obese mice.12
For quality control analysis of finished and raw products, separation techniques such as high-performance liquid chromatography (HPLC) gas chromatography, and thin-layer chromatography (TLC) are among the most common analytical methods of preference. However, it is complicated to associate this information with the biological (antioxidant, antibacterial, and antidiabetic) properties of medicinal plants. To overcome this difficulty, TLC-MS-bioautography has evolved as a novel technique for the biological separation of constituents due to the feasibility of separating many samples in parallel and due to the presence of an open layer permitting solvent evaporation in herbal products. Nowadays, TLC fingerprint of plant extracts is becoming a routine analytical approach because of its high sample throughput, low operational cost, and minimal sample cleanup. TLC has various advantages, including cost-effectiveness, running multiple samples simultaneously with a little amount of mobile phase, and minimum analysis time and cost per sample.13 Ultra-performance liquid chromatography coupled-mass spectrometry (UPLC-MS) has recently emerged as a powerful and reliable analytical technique for the identification of phenolic compounds, flavonoids, and other chemical metabolites.14
To the best of our knowledge, no baseline data or research is currently available on the nutritional composition of different varieties of M. alba leaves grown in Kashmir. Meanwhile, scientific evidence for their biofunctional properties and chemo profiling is lacking. Thus, keeping this in consideration, the present study aimed to investigate the in vitro antioxidant and antidiabetic potential of M. alba leaves. Furthermore, metabolomic analysis through TLC-MS-bioautography and UPLC-MS was done to identify the bioactive compounds present in indigeneous varieties of M. alba leaves. This will be the first study on the identification of hypoglycemic and antioxidant compounds from M. alba leaves using TLC-MS-bioautographic approach. The findings of the current study may provide a starting point for the research on different varieties of M. alba leaves grown in Kashmir that might be useful for designing novel nutraceutical formulations and functional foods.
Materials and Methods
Collection of Plant Material and Extract Preparation
M. alba leaves were collected from Moriculture Division Pampore, Jammu and Kashmir. The plant specimen was authenticated and identified by Pawan Saini, Scientist-B, Moriculture Division CSR and TI, Pampore, Jammu and Kashmir. The leaves were freed from any visible foreign matter, washed with distilled water, and properly shade-dried. The dried leaves were ground into fine powder, accurately weighed (50 g), and extracted through maceration with continuous stirring for 24 h using methanol.
All the extracts were filtered with Whatman filter paper 41, and the filtrate was evaporated to dryness under reduced pressure. The dried extracts of different varieties of M. alba were stored at 4 °C for further analysis.
Proximate Analysis and Mineral Content
Different varieties of M. alba were analyzed for moisture, ash, crude protein, and crude fat using the methods described by the protocol of the Association of Official Analytical Chemists (AOAC, 2005). Triplicate samples were used for the determination of each parameter, and the values reported are mean ± SD.
Estimation of Ascorbic Acid Content
Ascorbic acid was estimated by the titration method with slight modifications.15 Briefly, 0.5 mL of extract was added to the titration flask containing 25 mL of glacial acetic acid (3.0%) and metaphosphoric acid (8.0%). The mixture was then titrated against 2, 6-dichloroindophenol solution (0.025%) until the pink color of the solution remained for 8–10 s. The ascorbic acid content was calculated based on the standard curve and was expressed as mg ascorbic acid/g of leaves.
Estimation of Total Phenolic Content
The total phenol content of each extract was determined by the Folin-Ciocalteu method with some modifications.16 Briefly, 0.5 mL of extract was mixed thoroughly with 2.5 mL Folin-Ciocalteu (10%) and 2.5 mL sodium carbonate in a test tube. The tubes were vortexed for a few seconds and incubated in a dark place for 45 min for color development. After incubation, the absorbance was measured by a UV spectrophotometer at 760 nm. Different concentrations of gallic acid were used to make a calibration curve. The results of the total phenolic content of extracts were expressed as mg/g gallic acid equivalent (GAE) of dried extract. Each sample was assayed in triplicate.
Estimation of Total Flavonoid Content
The total flavonoid content (TFC) of extracts was determined by the aluminum chloride method with some modifications.13 Briefly, 0.5 mL of extract solution was added in 0.1 mL sodium acetate (0.1 mM) and 0.5 mL aluminum chloride. The mixture was made up 5.0 mL of distilled water and incubated at room temperature for 30 min. After incubation, the absorbance was measured at 415 nm. Different concentrations of rutin were used to make a calibration curve, and TFC in extracts was expressed as mg/g rutin equivalent of dried extract.
In Vitro Antioxidant Activity
DPPH Scavenging Potential
The free radical scavenging potential of the extracts was determined by a 1,1-diphenyl-2-picryl-hydrazyl (DPPH) assay.17 A solution of 0.1 mM of DPPH in methanol was prepared, and 1.5 mL of this solution was mixed with 0.5 mL of different concentrations of sample extract (100–500 μg/mL). Furthermore, the reaction mixture was left in a dark place for 30 min, and finally, the absorbance of the mixture was measured at 517 nm in a UV–visible spectrophotometer. Ascorbic acid was used as a reference standard.
Free radical scavenging activity was calculated by the following equation
where A0 is the absorbance of the control, and A1 is the absorbance of the extracts and standard. The concentration of sample required to scavenge 50% of the DPPH free radical (IC50) was determined from the graph of sample against the respective concentration.
Reducing Power Assay
The reducing power of all extracts was determined by evaluating the transformation of Fe3+to Fe2+ according to the protocol of Zahiruddin et al.13 Different concentrations of the sample extract (100–500 μg/mL) were mixed with 2 mL of phosphate buffer maintained at pH 6.7 and 2 mL of potassium ferricyanide solution. The whole mixture was incubated for 30 min at 45 °C. After incubation, 2 mL of trichloroacetic acid (10 g/100 mL) was added to each sample and centrifuged for 10 min. After centrifugation, 5 mL from the upper layer of each sample was taken and mixed with 5 mL of distilled water and 1 mL of ferric chloride (0.1 g/100 mL). Then, after continuous shaking, the absorbance of the solution was measured at 700 nm in a UV–visible spectrophotometer. The higher the absorbance, the greater the reducing power.
In Vitro α-Amylase Inhibitory Potential
α-Amylase activity was carried out as per the reported method with slight modifications.18 Briefly, 1.0 mL of sample and standard (acarbose) together with 1.0 mL α-amylase (4 units/mL prepared in sodium phosphate buffer and maintained at a pH 6.8) were mixed and incubated for 25 min at 37 °C. Further, 1.0 mL of 1% w/v starch was added to the mixture, and the whole mixture was then incubated for 45 min at 37 °C. Further, 100 μL of supernatant was taken out and glucose concentration was measured by glucose reagent. The α-amylase activity of all extracts was determined by estimating the glucose concentration according to the mentioned protocol. Different concentrations of all extracts (100–500 μg/mL) were prepared in methanol and tested for α-amylase inhibition potential and % inhibition of enzyme activity and calculated as
In Vitro α-Glucosidase Inhibitory Potential
The α-glucosidase inhibitory studies were performed spectrophotometrically according to a reported method with slight modifications.19 The assay was performed in triplicate, and the results were expressed as the sample concentration required to inhibit 50% of the enzyme activity (IC50). Briefly, 120 μL of different concentrations of each extract and 20 μL of 1 unit/mL α-glucosidase in 0.1 M potassium phosphate buffer (pH 6.8) were incubated for 15 min at 37 °C. The reaction was initiated by adding 20 μL of 5 mM para-nitrophenyl-α-D-glucopyranoside prepared in 0.1 M potassium phosphate buffer, and the mixture was further incubated for 15 min. Finally, the reaction was terminated by adding 80 μL of 0.2 M sodium bicarbonate in 0.1 M potassium phosphate buffer, and then, absorbance was measured at 405 nm. The results were calculated as % inhibition of enzyme activity and calculated as
TLC Fingerprinting Profile of the Best Active Extract
TLC was used to separate the metabolites of the best active extracts (Zagtul). 30 mg of the extract was dissolved in 1 mL of HPLC-grade methanol separately to obtain 30 mg/mL working stock solutions. The stock solution was then filtered, and 4 μL of extract solution was separately applied on silica gel 60 F254 precoated TLC plates, 10 × 10 cm (Merck, Germany) with the help of Camag Linomat V (Camag, Switzerland) applicator. The sample solution was applied with a 6 mm wide band using a Camag Linomat V automated TLC applicator with the nitrogen flow providing a delivery speed of 150 nL/s from the syringe. Plates were developed vertically, in a Camag twin trough glass chamber previously saturated with mobile phase for 25 min at room temperature with a linear ascending mode of up to 80 mm. Different types of mobile phases with varied ratios were involved for better separation of metabolites present in the extract by the hit-and-trial method. However, using the solvent system toluene:ethyl acetate:formic acid (5:4:1, v/v/v) as a mobile phase, maximum separation of metabolites was achieved based on optimal bands with compactness in terms of resolution in TLC plates. The optimized saturation time for the mobile phase was 25 min at room temperature (25 ± 2 °C) and 60 ± 5% relative humidity. Finally, the developed plate was scanned at two different wavelengths, that is, 254 and 366 nm, with a TLC scanner III (Camag, Switzerland), with a slit dimension of 6 × 0.45 mm, and the scanning speed was 10 mm/s13
Rapid Screening of DPPH Scavenging Metabolites from the Best Variety of M. alba by TLC-MS-Bioautography
TLC-MS-bioautography was done to identify the DPPH scavenging metabolites in the best variety of M. alba. The same mobile phase that was used in TLC was utilized for bioautography analysis. The air-dried plate was dipped in a 5 mM methanolic solution of DPPH solution for DPPH scavenging metabolite. The presence of antioxidant compounds was detected by yellowish spots against a purple background. Finally, the parallel track of the yellowish band was scrapped from the second plate. The scrapped spot of silica gel was dissolved in LC-MS-grade methanol. The dissolved silica gel was centrifuged at 3000 rpm for 10 min, and the supernatant was separated and filtered through a 0.2 μM PTFE membrane filter. The filtered samples were processed for MS.
Rapid Screening of α-Glucosidase Inhibitor Metabolites from the Best Variety of M. alba by TLC-MS-Bioautography
α-Glucosidase (100 U) was dissolved in acetate buffer, and substrate solution was prepared by dissolving p-nitrophenyl α-d-glucopyranoside (p-NPG) in 50% aqueous ethanol and fast blue in distilled water. The same mobile phase that was used in TLC was utilized for bioautography analysis. The air-dried plate was dipped in an enzyme solution and incubated in a desiccator for 1.5 h. After incubation, the plate was dipped in a substrate solution (p-NPG:fast blue in 1:1 ratio). Glucosidase inhibition was visible on the TLC plate by the appearance of a white spot on light purple/violet background within 5 min. Similarly, the parallel track of the whitish spot was scrapped from the second plate. The dissolved silica gel was centrifuged at 3000 rpm for 10 min, and the supernatant was separated and filtered through a 0.2 μM PTFE membrane filter. The filtered samples were processed for MS.
In the present study, MS was performed on a Waters ACQUITY UPLC system equipped with a binary solvent delivery system, an autosampler, a column manager, and a tunable MS detector. Separated metabolites present in different samples were tentatively identified based on their m/z value from the mass data bank.
UPLC-MS Analysis of the Extract
Waters ACQUITY UPLC system (serial no. #F09 UPB 920M; model code # UPB; Waters Corp., MA, USA), which is equipped with a binary solvent delivery system, a column manager, an autosampler, and a tunable MS detector (serial no # JAA 272; Synapt; Waters, Manchester, UK), was used for UPLC-MS analysis of extract. The extract was chromatographically separated in the previously degassed mobile phase consisting of 0.5% v/v formic acid in water (A) and acetonitrile (B) in gradient elution mode (initially 100% A and held for 5 min; further, decreased to 5% A in 20 min). Waters ACQUITY UPLC BEH C18 (100 × 2.1 mm × 1.7 μm) column was used, and the flow rate of the mobile phase was 0.4 mL/min. The column manager and sample manager temperature were set to 35 ± 2 and 25 ± 2 °C, respectively. About 10 μL of the sample was injected with a split mode of 5:1 with the help of an auto-injector, and the pressure of the system was set to 15,000 psi. The separated metabolites were detected by the MS detector. The nebulizer gas and cone gas were set to 500 and 50 L/h, respectively. The source temperature of the MS detector was set to 100 °C. The capillary voltage and cone voltage were set to 3.0 and 40 kV, respectively. For collision of ions, argon gas was used at a pressure of 5.3 × 10–5 Torr. Both UPLC and the mass detector were operated by using MassLynxV4.1 software incorporated with the instrument. The separated compounds were identified based on their m/z value through a literature survey.20
Statistical Analysis
For each measurement, three replicates of samples were taken and mean ± standard deviation values were reported. The results were analyzed using Student’s t-test procedure to determine the level of significance; p values of less than 0.05 were considered to be statistically significant. The analysis was performed using GraphPad prism software (GraphPad software Inc., version 7, Chicago, IL, USA).
Results
Extraction is an important step in the route of phytochemical processing for the discovery of bioactive constituents from plant extract. The selection of an appropriate extraction technique is important for the standardization of herbal products.13 The dried leaves of different varieties of mulberry extracts were extracted using methanol by cold maceration method on a mechanical shaker for 24 h at 1600 rpm. The highest extractive yield was found in Zagtul (28.44 ± 3.52%) followed by Chattatual (25.93 ± 2.84%), Chattatual Zaingir (18.76 ± 2.22%), and Brentul Kashmir (18.34 ± 2.07%).
Proximate Analysis of Different Varieties of M. alba
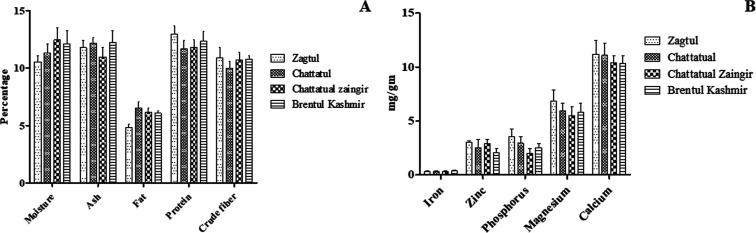
Proximate analysis is usually done for determining the values of means the components of moisture, crude protein, ash, crude fiber, fat, and macronutrients in food samples. The mean values of percentage proximate composition for the leaves of four different varieties of M. alba are summarized in Figure 1A. The statistical analysis of data indicated non-significant differences among the varieties for all proximate parameters analyzed.
Figure 1.
(A) Proximate analysis of four different Kashmiri varieties of M. alba. (B) Mineral content of different varieties of M. alba.
Among all varieties, moisture content was found to be the highest in Chattatual Zaingir (12.43 ± 1.03%) on a dry basis and lowest in Zagtul (10.53 ± 0.51%). The minimum and maximum ash contents were observed in Chattatual Zaingir (10.95 ± 0.83%) and Brentul Kashmir (12.22 ± 1.01%), respectively, on a dry weight basis. A higher ash value indicates the presence of heavy amounts of inorganic nutrients in plant materials.21 However, low moisture content may lead to roughness of leaves.15
Lipid estimation is among the key factors for the nutritional assessment of any material. The different varieties of M. alba exhibited considerable variation in the content of lipids; Chattatual (6.51 ± 0.51%) contained the highest and Zagtul (4.8 ± 0.33%) contained the lowest percentage of lipid content. However, these values were lower as compared to previously reported by Iqbal et al.15 There was a small difference in protein content between different varieties of M. alba. Zagtul variety had the highest content of protein (12.92 ± 0.77%) and Chattatual had the lowest protein content (11.68 ± 0.71%). The trend of protein content in leaves from all the investigated varieties was observed in the following order: Zagtul > Brentul Kashmir > Chattatual Zaingir > Chattatual.
Dietary fibers are non-starch polysaccharides that bind minerals and accelerate their passage through the digestive tract; as a result, bioavailability and absorption of nutrients is reduced. All the varieties contained an appreciable amount of crude fiber. Like protein content, there was a small difference in crude fiber between different varieties, which was almost similar to that reported earlier by Yen et al.(22)M. alba is also an important source of some minerals. The mineral content of different varieties is shown in Figure 1B. In our study among all the minerals, the amount of calcium was present in higher quantities followed by magnesium, zinc, and iron. The iron content was in the range of 0.32 ± 0.07–0.4 ± 0.06 mg/g, and the highest was found in Brentul Kashmir and the lowest in Zagtul and Chattatual. The calcium content was in the range of 10.34 ± 0.69–11.22 ± 1.23 mg/g. Among all the varieties, calcium content was highest in Zagtul (11.22 ± 1.23) and lowest in Brentul Kashmir (10.34 ± 0.69) variety. The magnesium content was highest in Zagtul (6.88 ± 0.99) and lowest in Chattatual Zaingir (5.52 ± 0.79 mg/g).
Ascorbic Acid Content of Different Varieties of M. alba
Ascorbic acid is a naturally occurring organic compound with antioxidant properties. The ascorbic acid content was calculated based on the standard curve and was expressed as mg ascorbic acid/g of powdered leaves. Ascorbic acid content was found to be the highest in Zagtul (2.18 ± 0.33 mg/g) followed by Chattatual (2.06 ± 0.30 mg/g) and Brentul Kashmir (2.03 ± 0.29 mg/g), while the lowest content was found in Chattatual Zaingir (1.89 ± 0.21 mg/g). The ascorbic acid content of all the varieties of M. alba was in accordance with the previously reported study by Erscisli and Orhan (2007).23
Total Phenolic and Flavonoid Content of Different Varieties of M. alba
Total phenolic and flavonoid contents of different varieties of M. alba are expressed in terms of GAEs and rutin equivalents, respectively. Among all varieties, Zagtul had the highest phenolic and flavonoids content and Chattatual had the lowest phenolic and flavonoid content. The mean values of four different varieties of M. alba are presented (n = 3). All varieties do not showed statistically significant differences (p < 0.001). The reported phenolic and flavonoid contents of different varieties of M. alba were higher than those reported in the study by Iqbal et al.(16) The antioxidant activity is related to the production of phenolic compounds and flavonoids. However, we observed significant differences in phenolic and flavonoid contents of the same species grown in Kashmir (Figure 2). Based on the phenolic and flavonoid content, it could be inferred that M. alba varieties are a good source of health-promoting bioactive compounds. In the present study, the reported amounts of phenolic and flavonoid contents of different M. alba varieties are sufficient to achieve various biological activities.
Figure 2.
Total phenolic and flavonoid contents of four different Kashmiri varieties of M. alba.
Antioxidant Potential of Different Varieties of M. alba
Searching and identifying natural and safe antioxidants, especially those of plant origin, have notably increased in recent years. M. alba leaves are rich in secondary metabolites, including phenolics and flavonoids, which have antioxidant activity due to their redox properties. Effects of different varieties of M. alba on DPPH free radical scavenging activity are shown in Figure 3. Maximum DPPH scavenging activity of 80.33% at a concentration of 500 μg/mL and an IC50 of 107.88 ± 3.8 was observed for Zagtul variety, whereas free radical scavenging potential for ascorbic acid was 93.59% with an IC50 of 81.78 ± 8.8 μg/mL. The lowest DPPH scavenging activity was observed in Chattatual variety with an IC50 value of 307.19 μg/mL. The DPPH scavenging potential of M. alba varieties increased with respect to the concentration of extract up to 400 μg/mL. Further, the increment of extract concentration (up to 500 μg/mL) showed no increment in DPPH scavenging potential. The scavenging effect of all the varieties of M. alba was compared with ascorbic acid.
Figure 3.
Free radical scavenging and reducing power capacity of four different Kashmiri varieties of M. alba.
Ferric reducing power was also significantly higher for Zagtul followed by Brentul Kashmir, Chattatual Zaingir, and Chattatual (Figure 3). The enhancement in antioxidant activity of M. alba varieties could be attributed to the presence of higher amounts of phenolic and flavonoid contents. The reducing power potential of different varieties of M. alba was linearly proportionate to DPPH scavenging activity. The mean values of four different varieties of M. alba are presented (n = 3). All varieties were found to possess a significant antioxidant potential with P-value summary*** and p-value (one-tailed) <0.0001.
Overall, the order of antioxidant potential of M. alba varieties for all the above antioxidant assays was as follows: Zagtul > Brentul Kashmir > Chattatual Zaingir > Chattatual. Hence, it may concluded that the M. alba leaves may be used as alternatives to synthetic antioxidants.
Determination of In Vitro α-Amylase Inhibitory Activity of Different Varieties of M. alba
The α-amylase is a calcium-containing metalloenzyme that breaks down the α-1,4 linkages of polysaccharide to monosaccharide in the oral cavity.21 α-Inhibitory potential of different varieties showed significant dose-dependent inhibition with an average inhibition of 78.55 ± 2.53% at a concentration of 500 μg/mL and an IC50 of 74.76 ± 6.76 μg/mL, as shown in Figure 4. However, the average inhibition of acarbose at 500 μg/mL was 87.67 ± 3.67% and IC50 was 35.34 ± 4.87 μg/mL. The mean values of four different varieties of M. alba are presented (n = 3). All varieties were found to possess the significant α glucosidase inhibitory potential with p-value summary*** and p-value (one-tailed) <0.0001.
Figure 4.
α-Amylase and α-glucosidase inhibitor activity of four different Kashmiri varieties of M. alba.
Determination of In Vitro α-Glucosidase Inhibitory Activity of Different Varieties of M. alba
α-Glucosidase is a sequence of enzymes present on the intestinal brush border. The important carbohydrates in food such as sucrose and starch are hydrolyzed to monosaccharides, such as glucose and fructose by an α-glucosidase enzyme and subsequently absorbed into the blood, thereby raising blood glucose value.24 The results of α-glucosidase inhibitor activity of different varieties of M. alba are presented in Figure 4. In the present study, all varieties of M. alba exhibited excellent α-glucosidase inhibition. The results showed a significant dose-dependent inhibitory potential with an average inhibition of 73.03 ± 1.23% at a concentration of 500 μg/mL and an IC50 of 109.19 ± 5.78 μg/mL for Zagtul and 62.45 ± 3.24 at a concentration of 500 μg/mL with an IC50 of 241.25 ± 8.98 μg/mL for Chattatual. The mean values of four different varieties of M. alba are presented (n = 3). All varieties were found to possess a significant α glucosidase inhibitory potential with p-value summary*** and p-value (one-tailed) <0.0001.
This enzyme inhibition ability might be attributed to the polyhydroxylated alkaloids and specific phenolic compounds presented in different M. alba varieties from Kashmir. Based on the above studies, among all the varieties, Zagtul was found to be the best variety, and it was selected for further analysis.
TLC Fingerprinting Profile of Best Active Extract for Quality Control Analysis
TLC fingerprint is a commonly used method to obtain the patterns of metabolites from plant materials. If any plant material has the same TLC fingerprint pattern, it may have the same biological activity.13 TLC was used to separate the metabolites of extract, various mobile phases with varied ratios were used for the separation of the compound in extract, and toluene:ethyl acetate:formic acid (5:4:1, v/v/v) was the best solvent system for maximum separation of bioactive compounds. The result of TLC fingerprinting of the best variety of M. alba (Zagtul variety) showed 6 bands at each wavelength with Rf values of 0.06, 0.54, 0.56, 0.63, 0.70, and 0.96 at 254 nm and Rf values of 0.06, 0.51, 0.56, 0.63, 0.70, and 0.96 at 366 nm (Figure 5). The bands at Rf values of 0.06, 0.56, 0.63, and 0.96 were found to be common at both wavelengths.
Figure 5.
Developed TLC plate photograph of Zagtul variety of M. alba at (A) 254 nm and (B) 366 nm.
Identification of DPPH Scavenging Metabolite by TLC-MS-Bioautography
TLC-bioautography links separation on the adsorbent layer with biological tests performed directly on it in order to identify DPPH scavenging metabolite. Under visible light, the derivatized plate with DPPH solution was analyzed, and a yellowish band on a purple background indicated the presence of DPPH scavenging metabolite; chlorogenic acid (Rf 0.63) was confirmed by mass spectroscopy (Figure 6). The study conducted by Memon et al. reported that chlorogenic acid is a major phenolic compound in M. alba leaves.25
Figure 6.
Developed TLC-bioautography plate photograph of Zagtul variety of M. alba and mass spectrometry chromatogram of identified metabolites after being stained with DPPH and α-glucosidase.
Identification of α-Glucosidase Inhibitor Metabolite by TLC-MS-Bioautography
The α-glucosidase inhibitor metabolite present in the best variety of M. alba was separated on a developed TLC plate using the same mobile phase and determined the responsible α-glucosidase inhibitor metabolite with their inhibition zones. Under visible light, the derivatized plate with α-glucosidase inhibitor was analyzed, and a light purple/violet background and detected by comparing experimental data for the m/z ratio of molecular ion peak and with those of the literature reports and mass bank library. Two metabolites were identified as α-glucosidase inhibitors by mass spectroscopy: DNJ (Rf 0.70) and syringic acid (Rf 0.56) (Figure 6).
Identification of Metabolites of Best Variety of M. alba by UPLC-MS
Massbank, chemical library, and literature survey were used to identify the metabolites present in the extract. The main abundant metabolites of the extract are summarized in Table 1, and the structure of separated metabolites is shown in Figure 7. The UPLC-MS chromatogram examination revealed different peaks, indicating the presence of 19 phytochemical components. The isolated compounds are shikimic acid (Rt 0.170), chlorogenic acid (Rt 3.6), coumarin (Rt 4.56), 8-hydroxyquinoline (Rt 3.03), gentisic acid (Rt 1.66), ferulic acid (Rt 0.95), moracin N (Rt 3.60), 1-deoxynojirimycin (Rt 3.18), gallic acid (Rt 5.75), 3′,4′,6-trimethoxyflavanone (Rt 0.88), morusimic acid E (Rt 2.02), atalantoflavone (Rt 0.42), 2′,5-dimethoxyflavone (Rt 4.27), morin (Rt 4.00), 1,3-dicaffeoylquinic acid (Rt 3.69), and vanillin (Rt 0.73).
Table 1. List of Some Major Metabolites in M. alba Identified through UPLC-MS.
| S. N. | Rt (min) | accurate mass | tentative mass | mass ID | compound Name | chemical formula |
|---|---|---|---|---|---|---|
| 1 | 0.17 | 174.05 | 174.90 | PR100485 | shikimic acid | C7H10O5 |
| 2 | 0.42 | 336.3 | 336.56 | PubChem14162621 | atalantoflavone | C20H16O5 |
| 3 | 0.54 | 712.70 | 712.65 | PubChem 10117838 | sanggenon T | C40H40O12 |
| 4 | 0.73 | 152.04 | 153.98 | ML005951 | vanillin | C8H8O3 |
| 5 | 0.88 | 314.34 | 314.95 | NGA03341 | 3′,4′,6-trimethoxyflavanone | C18H18O5 |
| 6 | 0.95 | 194.18 | 194.93 | PM000409 | ferulic acid | C10H10O4 |
| 7 | 1.66 | 154.86 | 154.02 | KO000574 | gentisic acid | C7H6O4 |
| 8 | 2.02 | 507.30 | 508.30 | PR305805 | morusimic acid E | C24H45NO10 |
| 9 | 3.03 | 145.05 | 145.04 | LU099402 | 8-hydroxyquinoline | C9H7NO |
| 10 | 3.18 | 163.17 | 164.94 | PubChem 29435 | 1-deoxynojirimycin | C6H13NO4 |
| 11 | 3.60 | 354.31 | 353.07 | KO002577 | chlorogenic acid | C16H18O9 |
| 12 | 3.60 | 310.3 | 311.24 | PubChem 641376 | moracin N | C19H18O4 |
| 13 | 3.62 | 342.30 | 343.37 | PubChem 6124135 | 1-o-caffeoylglucose | C15H18O9 |
| 14 | 3.69 | 516.4 | 515.75 | PubChem 6474640 | 1,3-dicaffeoylquinic acid | C25H24O12 |
| 15 | 4.00 | 303.23 | 301.16 | PubChem 5281670 | morin | C15H10O7 |
| 16 | 4.27 | 282.08 | 282.51 | BML01757 | 2′,5-dimethoxyflavone | C17H14O4 |
| 17 | 4.56 | 146.03 | 146.93 | NA002586 | coumarin | C9H6O2 |
| 18 | 5.72 | 542.67 | 542.00 | PR300530′ | puberanidine | C30H42N2O7 |
| 19 | 5.75 | 170.12 | 171.05 | PM000401 | gallic acid | C7H6O5 |
Figure 7.
Chemical structures of bioactive metabolites identified through UPLC-MS in M. alba leaves.
Discussion
Traditional medicinal plants have a long history and are still used in basic healthcare by indigenous peoples. These medicinal plants are gaining popularity as medical options in both developed and developing countries to cure a variety of ailments.26 Natural antioxidants that are present in the leaf and other parts of a plant are responsible for inhibiting or preventing the harmful costs of oxidative stress.27 The herbal metabolites and derived constituents are extensively used globally due to their efficacy with rarer side effects.28
Mulberry is a superb natural matrix because of its wide range of bioactive properties. M. alba contains a significant amount of alkaloids, glycosides, lipids, organic acids, volatile oils, proteins, carbohydrates, fibers, mineral contents, and some vitamins or their precursors.29,30 Traditionally, it has been used in Asia as a medicine to treat a variety of infectious and internal disorders. Unexplored information regarding mulberry is valuable to researchers, and its utilization in functional foods and medicines is the need of the hour. However, due to a lack of approved and standardized techniques for its examination, it is yet to be recognized in the scientific world.31 India is the second-largest cultivator of mulberry in terms of acreage, mainly in its northern Himalayan region. In India, mulberry is known as “Kalpa Vruksha” as all the parts are used in the traditional system of Ayurvedic medicine.32 Kashmir is a fertile land for mulberry production. It is a known fact that the mulberry silkworm reared in the state of Jammu and Kashmir produces superior quality cocoon. However, limited information is available on the medicinal benefits of mulberry varieties cultivated there, and till date, no baseline research is available. Therefore, we carried out a study to identify the key therapeutic candidates from a phytochemical matrix of M. alba leaves grown in the Kashmir region of North India. Furthermore, based on conducted investigations, a comparison was made among leaves of selected species regarding their proximate composition, antioxidant activity, and antidiabetic and nutraceutical potential.
Four varieties of M. alba cultivated in Kashmir, viz, Zagtul, Chattatual, Chattatual Zaingir, and Brentul Kashmir, were examined for proximate analysis and in vitro antioxidant and antidiabetic potential. The extraction of bioactive constituents from plant materials with strong antioxidant activity is affected by several factors. Among them, the method and choice of solvent are of foremost significance.13Zagtul variety contained the highest amount of protein (12.92 ± 0.77%) and dietary fiber and the lowest amount of fat (4.8 ± 0.33%) among all other varieties. Previously, a study reported that dietary fiber of mulberry may decrease lipids in the liver and increase the activity of low-density lipoprotein receptor.33 The hypoglycemic activity of mulberry leaves may be attributed to the high fiber content. Thus, the presence of an appreciable lower content of lipids and significant crude fiber content demonstrates that different varieties of M. alba of the Jammu and Kashmir belt of India can be a healthy food choice for consumers to utilize as a nutraceutical or functional food. For a wide range of physiological functions, a huge number of minerals are necessary in trace amounts. Mulberry leaves were also reported to possess important minerals such as iron, magnesium, and calcium, which are also required for the normal growth of humans.
A higher amount of phenolic and flavonoid content corresponds to their stronger antioxidant activity.19 Many phenolic and flavonoid compounds in M. alba have been reported to have antioxidant and antidiabetic properties.11 The flavonoid content in different varieties ranged from 34.91 ± 7.63 to 108.27 ± 6.64 mg rutin/g DW. Previously, one study reported 57.83 ± 4.64 mg rutin/g in mulberry leaves.34 However, Radojković et al., Thabti et al., and Wang et al. reported a lower flavonoid content than that reported in the present study (up to 33.3 mg/g).35−37 Phenolic content in different varieties of M. alba varied from 32.91 ± 5.09 to 53.44 ± 2.23 mg GAE/g DW. A study conducted earlier reported a total phenolic content of 66.8 ± 0.8 mg/g in M. alba extract.35 Significant variation was found among different M. alba varieties regarding their antioxidant activity. Zagtul variety was characterized by the highest antioxidant activity among all varieties. However, Chattatual variety showed the least antioxidant activity. The differences in antioxidant activity among different varieties could be preliminarily attributed to their different polyphenol compositions and concentrations.
α-Glucosidase is a key enzyme in releasing glucose by hydrolyzing oligosaccharides; it can then cause postprandial hyperglycemia and result in type 2 diabetes. Therefore, identifying inhibitors of α-glucosidase is important. Natural compounds, primarily from foods or medicinal plants, have recently gained a lot of attention as alternative α -glucosidase inhibitors. Mulberry leaves, a Chinese herbal remedy, are an efficient α-glucosidase inhibitor.38 In our case, the α-glucosidase and α-amylase inhibitory activity of all M. alba leaves increased dose dependently. Lower IC50 values indicate higher α-glucosidase and α-amylase inhibitory activity. As shown in Figure 4, the α-amylase and α-glucosidase inhibitory sequence was Zagtul > Brentul Kashmir > Chattatual > Chattatual Zaingir. Therefore, the present result indicated that the methanolic extracts of M. alba are an effective α-glucosidase and α-amylase inhibitors. Hence, the present study implied that the mulberry grown in the Kashmir region can be utilized as an antidiabetic food supplement and a good alternative to synthetic antidiabetic drugs.
The TLC fingerprint of the plant materials can be used for quality control to confirm product quality and safety.18 This approach, however, can also be used to differentiate between closely related species of this plant.39 TLC profiling is a typical method for attaining metabolite patterns from plant extracts.
TLC-MS-bioautography assay being a simple and versatile approach was used to determine the active antioxidant in a mixture of compounds. Earlier, a study resulted in identification of constituents with antimicrobial and radical scavenging activities from M. alba roots by TLC-MS-bioautography technique.35 The current study is the first report on identification of hypoglycemic compounds from M. alba leaves using TLC-MS-bioautography approach.
On the TLC plates, antioxidant and antidiabetic compounds were seen as yellow spots on a purple background and whitish spots on a light purple/violet background, respectively (Figure 6). Chlorogenic acid was the antioxidant compound identified in Zagtul variety by TLC-MS-bioautography based on its mass and charge ratio obtained from MS analysis. Arfan et al. confirmed the presence of chlorogenic acid as the predominant phenolic constituent by HPLC method in mulberry.40 However, DNJ and syringic acid were the main antidiabetic compounds present in M. alba leaves as identified through TLC-MS-bioautography analysis. DNJ is a unique polyhydroxylated alkaloid present in M. alba leaves. Besides being a potent α-glucosidase inhibitor, it also encourages weight loss by increasing adiponectin levels, which plays a significant role in energy intake and in the prevention of diet-induced obesity.41 Syringic acid is a major phenolic acid present in M. alba leaves. Syringic acid can increase insulin secretion of pancreatic β-cells and regulate the plasma glucose level. The TLC bioautographic approach presented here is very simple and fast and can be utilized to differentiate the biological activity of M. alba extracts obtained by different extraction techniques or solvents.
Furthermore, to identify the diverse metabolites present in the extract, UPLC-MS analysis was performed. UPLC-MS is the most acceptable approach for the identification of both polar and nonpolar metabolites. Chlorogenic acid (Rt 3.34) is among the phenolic compounds from the hydroxy cinnamic family, which is recognized for its antioxidant properties against free radicals. It has been shown to play a function in reducing oxidative and antidiabetic complications in both in vitro and in vivo studies.37,38 This phenolic acid is identified in other conducted studies as constituents of M. alba leaves.36 Gentisic acid (Rt 1.66) is a diphenolic compound and a derivative of benzoic acid, which was previously isolated from M. alba fruit by Natić et al.42 DNJ (Rt 3.18) is an alkaloid and a biologically active natural compound that exists mainly in M. alba leaves. DNJ has been shown to inhibit intestinal alpha glucosidase.43 Atalantoflavone (Rt 0.42) is a potent prenylated cytotoxic flavonoid identified in M. alba. It has an anticancerous activity against human cervical carcinoma HeLa, human breast carcinoma MCF-7, and human hepatocarcinoma Hep3B cells.44 Similarly, another secondary metabolite, viz, Moracin N (Rt 3.60), is a potent anticancer agent and phosphodiesterase-4 inhibitor.42,43 Previously, Aelenei et al. identified Moracin M, Moracin P pentoside, Moracin P, and Moracin U in mulberry extracts by HPLC-DAD-ESI-Q-TOF-MS/MS.34 Morin (Rt 4.00), a pentahydroxyflavone, is an important phytochemical in M. albawith strong antioxidant, antibacterial, and antiviral effects previously reported by Baliga et al.45 1,3-Dicaffeoylquinic (Rt 3.69) and 1-o-caffeoylglucose acid (Rt 3.62) are a type of hydrocinnamic acid that were also separated in the present study. Earlier, both these compounds were separated by Li et al. using UHPLC-MS/MS in mulberry.46 Sanggenon T (Rt 0.54) is an antimicrobial compound identified in our study. Previously, Sanggenon T was identified in M. alba root bark by UPLC–MS/MS.47 Sanggenol M was previously identified by Aelenei et al. in mulberry extracts by HPLC-DAD-ESI-Q-TOF-MS/MS.34
In lieu of the hypothesized relation between diabetes and antioxidants, polyphenols present in our extract have the potential to protect against the progression of diabetes. Further, we chromatographically characterized the methanolic extract of different varieties of M. alba leaves and investigated bioactive principles. The results of this study will be helpful for the development of food supplements and phytopharmaceuticals that can be used for the management of diabetes and related complications.
Conclusions
Based on the current findings, it can be stated that all the varieties of M. alba are a good source of nutrients and minerals, as well as having antioxidant and antidiabetic properties. Among all the varieties, Zagtul showed the highest antioxidant and antidiabetic potential. Comparative nutritional and antioxidant properties of different varieties of M. alba grown in Kashmir were evaluated for the first time. The overall study on M. alba exhibited potent radical scavenging and antidiabetic inhibition activities. The TLC-MS-bioautography screening of compounds led to the identification of chlorogenic acid, DNJ, and syringic acid as the major antioxidant and antidiabetic bioactive principles of M. alba leaves, which was confirmed by MS. Furthermore, UPLC-MS analysis led to the identification of some important metabolites with versatile bio-functions. Our results suggested that M. alba could be a promising source of natural antioxidants for use in the food, pharmaceutical, and cosmetic industries. These findings will be crucial in the design of future dietary intervention studies investigating the function of mulberry leaves in disease risk reduction, which further justifies its traditional use. Moreover, this information will be of considerable value to the commercial producers of mulberry trees in Kashmir. However, further in vivo and clinical studies are required for its complete efficacy and to identify the actual dosage to establish specific biological effects against diabetic condition.
Acknowledgments
Authors would like to acknowledge the Indian Council of Medical Research (ICMR), New Delhi, India, for providing scholarship to B.J. (sanction no. 3/1/2/161/2019) to carry out the present research work.
The authors declare no competing financial interest.
References
- Saranya B.; Sulfikarali T.; Chindhu S.; Muneeb A. M.; Leela N. K.; Zachariah T. J. Turmeric and Cinnamon Dominate in Antioxidant Potential among Four Major Spices. J. Spices Aromat. Crop 2017, 26, 27. 10.25081/josac.2017.v26.i1.803. [DOI] [Google Scholar]
- Stefanucci A.; Zengin G.; Llorent-Martinez E. J.; Dimmito M. P.; Della Valle A.; Pieretti S.; Ak G.; Sinan K. I.; Mollica A. Chemical Characterization, Antioxidant Properties and Enzyme Inhibition of Rutabaga Root’s Pulp and Peel (Brassica Napus L.). Arab. J. Chem. 2020, 13, 7078. 10.1016/j.arabjc.2020.07.013. [DOI] [Google Scholar]
- Asano N.; Yamashita T.; Yasuda K.; Ikeda K.; Kizu H.; Kameda Y.; Kato A.; Nash R. J.; Lee H. S.; Ryu K. S. Polyhydroxylated Alkaloids Isolated from Mulberry Trees (Morus Alba L.) and Silkworms (Bombyx Mori L.). J. Agric. Food Chem. 2001, 49, 4208. 10.1021/jf010567e. [DOI] [PubMed] [Google Scholar]
- Jan B.; Parveen R.; Zahiruddin S.; Khan M. U.; Mohapatra S.; Ahmad S. Nutritional Constituents of Mulberry and Their Potential Applications in Food and Pharmaceuticals: A Review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. 10.1016/j.sjbs.2021.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabhat A.; Malik M. A.; Malik F. A.; Sofi A. M.; Mir M. R. Nutritional Efficiency of Selected Silkworm Breeds of Bombyx Mori L. Reared on Different Varieties of Mulberry under Temperate Climate of Kashmir. Afr. J. Agric. Res. 2011, 6, 120–126. 10.5897/AJAR1. [DOI] [Google Scholar]
- Rafeeq J.; Mughal A. H.; Fayaz S. Studies on Identification and Uses of Morus Alba : An Important Multi-Purpose Tree Species in Kashmir Valley. J. Pharmacogn. Phytochem. 2020, 9, 295–297. 10.22271/phyto.2020.v9.i5sa.12333. [DOI] [Google Scholar]
- Bhat M. A.; Buhroo Z. I.; Aziz A.; Qadir J.; Azam M. An Overview of Current Scenario of Sericulture Industry in Jammu and Kashmir. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 3813–3824. 10.20546/ijcmas.2020.906.452. [DOI] [Google Scholar]
- Dar K. A.; H G. A.; Na G.; Farhat S.; Sharma R. K.; Salib S.; Omaise A.; Rafique A. A.; Parviz S.. Jammu and Kashmir Silk Industry : Problems and Prospects, 2021; Vol. 10 (6), pp 369–371.
- Chen Y.-C.; Tien Y.-J.; Chen C.-H.; Beltran F. N.; Amor E. C.; Wang R.-J.; Wu D.-J.; Mettling C.; Lin Y.-L.; Yang W.-C. Morus Alba and Active Compound Oxyresveratrol Exert Anti-Inflammatory Activity via Inhibition of Leukocyte Migration Involving MEK/ERK Signaling. BMC Complementary Altern. Med. 2013, 13, 45. 10.1186/1472-6882-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsube T.; Imawaka N.; Kawano Y.; Yamazaki Y.; Shiwaku K.; Yamane Y. Antioxidant Flavonol Glycosides in Mulberry (Morus Alba L.) Leaves Isolated Based on LDL Antioxidant Activity. Food Chem. 2006, 97, 25–31. 10.1016/j.foodchem.2005.03.019. [DOI] [Google Scholar]
- Hunyadi A.; Martins A.; Hsieh T.-J.; Seres A.; Zupkó I. Chlorogenic Acid and Rutin Play a Major Role in the In Vivo Anti-Diabetic Activity of Morus Alba Leaf Extract on Type II Diabetic Rats. PLoS One 2012, 7, e50619 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R.; Xue Z.; Jia Y.; Wang Y.; Li S.; Zhou J.; Liu J.; Zhang M.; He C.; Chen H. Polysaccharides from Mulberry (Morus Alba L.) Leaf Prevents Obesity by Inhibiting Pancreatic Lipase in High-Fat Diet Induced Mice. Int. J. Biol. Macromol. 2021, 192, 452. 10.1016/j.ijbiomac.2021.10.010. [DOI] [PubMed] [Google Scholar]
- Zahiruddin S.; Parveen A.; Khan W.; Parveen R.; Ahmad S. TLC-Based Metabolite Profiling and Bioactivity-Based Scientific Validation for Use of Water Extracts in AYUSH Formulations. Evid Based Complement Alternat Med. 2021, 2021, 2847440. 10.1155/2021/2847440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Rivera G.; Ballesteros-Vivas D.; Parada-Alfonso F.; Ibañez E.; Cifuentes A. Recent Applications of High Resolution Mass Spectrometry for the Characterization of Plant Natural Products. TrAC, Trends Anal. Chem. 2019, 112, 87–101. 10.1016/j.trac.2019.01.002. [DOI] [Google Scholar]
- Iqbal S.; Younas U.; Sirajuddin; Chan K. W.; Sarfraz R. A.; Uddin M. K. Proximate Composition and Antioxidant Potential of Leaves from Three Varieties of Mulberry (Morus Sp.): A Comparative Study. Int. J. Mol. Sci. 2012, 13, 6651–6664. 10.3390/ijms13066651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C.; Kapoor H. C. Anti-Oxidant Activity and Total Phenolic Content of Some Asian Vegetables. Int. J. Food Sci. Technol. 2002, 37, 153–161. 10.1046/j.1365-2621.2002.00552.x. [DOI] [Google Scholar]
- Zahiruddin S.; Parveen A.; Khan W.; Ibrahim M.; Want M. Y.; Parveen R.; Ahmad S. Metabolomic Profiling and Immunomodulatory Activity of a Polyherbal Combination in Cyclophosphamide-Induced Immunosuppressed Mice. Front. Pharmacol. 2022, 12, 1–15. 10.3389/fphar.2021.647244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W.; Parveen R.; Chester K.; Parveen S.; Ahmad S. Hypoglycemic Potential of Aqueous Extract of Moringa Oleifera Leaf and in Vivo GC-MS Metabolomics. Front. Pharmacol. 2017, 8, 577. 10.3389/fphar.2017.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaurav; Zahiruddin S.; Parveen B.; Ibrahim M.; Sharma I.; Sharma S.; Sharma A. K.; Parveen R.; Ahmad S. TLC-MS Bioautography-Based Identification of Free-Radical Scavenging, Α-amylase, and Α-glucosidase Inhibitor Compounds of Antidiabetic Tablet BGR-34. ACS Omega 2020, 5, 29688. 10.1021/acsomega.0c02995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahiruddin S.; Khan W.; Nehra R.; Alam M. J.; Mallick M. N.; Parveen R.; Ahmad S. Pharmacokinetics and Comparative Metabolic Profiling of Iridoid Enriched Fraction of Picrorhiza Kurroa—An Ayurvedic Herb. J. Ethnopharmacol. 2017, 197, 157–164. 10.1016/j.jep.2016.07.072. [DOI] [PubMed] [Google Scholar]
- Aletor O.; Oshodi A. A.; Ipinmoroti K. Chemical Composition of Common Leafy Vegetables and Functional Properties of Their Leaf Protein Concentrates. Food Chem. 2002, 78, 63–68. 10.1016/S0308-8146(01)00376-4. [DOI] [Google Scholar]
- Yen G.-C.; Wu S.-C.; Duh P.-D. Extraction and Identification of Antioxidant Components from the Leaves of Mulberry (Morus Alba L.). J. Agric. Food Chem. 1996, 44, 1687–1690. 10.1021/jf9503725. [DOI] [Google Scholar]
- Ercisli S.; Orhan E. Chemical Composition of White (Morus Alba), Red (Morus Rubra) and Black (Morus Nigra) Mulberry Fruits. Food Chem. 2007, 103, 1380. 10.1016/j.foodchem.2006.10.054. [DOI] [Google Scholar]
- Bischoff H. Pharmacology of Alpha-Glucosidase Inhibition. Eur. J. Clin. Invest. 1994, 24, 3–10. [PubMed] [Google Scholar]
- Memon A. A.; Memon N.; Luthria D. L.; Bhanger M. I.; Pitafi A. A. Phenolic Acids Profiling and Antioxidant Potential of Mulberry (Morus Laevigata W., Morus Nigra L., Morus Alba L.) Leaves and Fruits Grown in Pakistan. Pol. J. Food Nutr. Sci. 2010, 60, 25. [Google Scholar]
- Yuan H.; Ma Q.; Ye L.; Piao G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Ranjan B.; Kumar R.; Verma N.; Mittal S.; Pakrasi P. Evaluation of the Antidiabetic Properties of S-1708 Mulberry Variety. Pharmacogn. Mag. 2017, 13, S280–S288. 10.4103/pm.pm_490_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester K.; Paliwal S.; Khan W.; Ahmad S. UPLC-ESI-MS/MS and HPTLC Method for Quantitative Estimation of Cytotoxic Glycosides and Aglycone in Bioactivity Guided Fractions of Solanum Nigrum L. Front. Pharmacol. 2017, 8, 434. 10.3389/fphar.2017.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt M. S.; Nazir A.; Sultan M. T.; Schroën K. Nature’s Functional Tonic. Trends Food Sci. Technol. 2008, 19, 505. 10.1016/j.tifs.2008.06.002. [DOI] [Google Scholar]
- Paul A.; Rajiung M.; Zaman K.; Chaudhary S. K.; Shakya A. Quantification of the Bioactive Marker Resveratrol in Morus Alba Linn. Fruits by High–performance Thin–layer Chromatography. J. Planar Chromatogr.--Mod. TLC 2020, 33, 481–487. 10.1007/s00764-020-00062-9. [DOI] [Google Scholar]
- He H.; Lu Y.-H. Comparison of Inhibitory Activities and Mechanisms of Five Mulberry Plant Bioactive Components against α-Glucosidase. J. Agric. Food Chem. 2013, 61, 8110–8119. 10.1021/jf4019323. [DOI] [PubMed] [Google Scholar]
- Rohela G. K.; Shukla P.; Muttanna; Kumar R.; Chowdhury S. R. Mulberry (Morus Spp.): An Ideal Plant for Sustainable Development. Trees, Forests and People 2020, 2, 100011. 10.1016/j.tfp.2020.100011. [DOI] [Google Scholar]
- Venkatesan N.; Niranjali Devaraj S.; Devaraj H. Increased Binding of LDL and VLDL to Apo B,E Receptors of Hepatic Plasma Membrane of Rats Treated with Fibernat. Eur. J. Nutr. 2003, 42, 262–271. 10.1007/s00394-003-0420-8. [DOI] [PubMed] [Google Scholar]
- Aelenei P.; Luca S. V.; Horhogea C. E.; Rimbu C. M.; Dimitriu G.; Macovei I.; Silion M.; Aprotosoaie A. C.; Miron A. Morus Alba Leaf Extract: Metabolite Profiling and Interactions with Antibiotics against Staphylococcus Spp. Including MRSA. Phytochem. Lett. 2019, 31, 217–224. 10.1016/j.phytol.2019.04.006. [DOI] [Google Scholar]
- Radojković M.; Zeković Z.; Mašković P.; Vidović S.; Mandić A.; Mišan A.; D̵urović S. Biological Activities and Chemical Composition of Morus Leaves Extracts Obtained by Maceration and Supercritical Fluid Extraction. J. Supercrit. Fluids 2016, 117, 50–58. 10.1016/j.supflu.2016.05.004. [DOI] [Google Scholar]
- Thabti I.; Elfalleh W.; Hannachi H.; Ferchichi A.; Campos M. D. G. Identification and Quantification of Phenolic Acids and Flavonol Glycosides in Tunisian Morus Species by HPLC-DAD and HPLC-MS. J. Funct. Foods 2012, 4, 367–374. 10.1016/j.jff.2012.01.006. [DOI] [Google Scholar]
- Wang W.; Zu Y.; Fu Y.; Efferth T. Vitro Antioxidant and Antimicrobial Activity of Extracts from Morus Alba L. Leaves, Stems and Fruits. Am. J. Chin. Med. 2012, 40, 349. 10.1142/S0192415X12500279. [DOI] [PubMed] [Google Scholar]
- Kim G.-N.; Kwon Y.-I.; Jang H.-D. Mulberry Leaf Extract Reduces Postprandial Hyperglycemia with Few Side Effects by Inhibiting α-Glucosidase in Normal Rats. J. Med. Food 2011, 14, 712–717. 10.1089/jmf.2010.1368. [DOI] [PubMed] [Google Scholar]
- Annegowda H. V.; Mordi M. N.; Ramanathan S.; Hamdan M. R.; Mansor S. M. Effect of Extraction Techniques on Phenolic Content, Antioxidant and Antimicrobial Activity of Bauhinia Purpurea: HPTLC Determination of Antioxidants. Food Anal. Methods 2012, 5, 226–233. 10.1007/s12161-011-9228-y. [DOI] [Google Scholar]
- Arfan M.; Khan R.; Rybarczyk A.; Amarowicz R. Antioxidant Activity of Mulberry Fruit Extracts. Int. J. Mol. Sci. 2012, 13, 2472–2480. 10.3390/ijms13022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Lv W.; Gu Y.; Yu S. 1-Deoxynojirimycin in Mulberry (Morus Indica L.) Leaves Ameliorates Stable Angina Pectoris in Patients with Coronary Heart Disease by Improving Antioxidant and Anti-Inflammatory Capacities. Front. Pharmacol. 2019, 10, 569. 10.3389/fphar.2019.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natić M. M.; Dabić D. Č.; Papetti A.; Fotirić Akšić M. M.; Ognjanov V.; Ljubojević M.; Tešić Ž. L. Analysis and Characterisation of Phytochemicals in Mulberry (Morus Alba L.) Fruits Grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. 10.1016/j.foodchem.2014.08.101. [DOI] [PubMed] [Google Scholar]
- Hansawasdi C.; Kawabata J. α-Glucosidase Inhibitory Effect of Mulberry (Morus Alba) Leaves on Caco-2. Fitoterapia 2006, 77, 568. 10.1016/j.fitote.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Dat N. T.; Binh P. T. X.; Quynh L. T. P.; Van Minh C.; Huong H. T.; Lee J. J. Cytotoxic Prenylated Flavonoids from Morus Alba. Fitoterapia 2010, 81, 1224–1227. 10.1016/j.fitote.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Baliga M. S.; Shivashankara A. R.; Venkatesh S.; Bhat H. P.; Palatty P. L.; Bhandari G.; Rao S.. Phytochemicals in the Prevention of Ethanol-Induced Hepatotoxicity: A Revisit. Dietary Interventions in Liver Disease: Foods; Nutrients, and Dietary Supplements, 2019, pp 79–89. [Google Scholar]
- Li F.; Zhang B.; Chen G.; Fu X. The Novel Contributors of Anti-Diabetic Potential in Mulberry Polyphenols Revealed by UHPLC-HR-ESI-TOF-MS/MS. Food Res. Int. 2017, 100, 873. 10.1016/j.foodres.2017.06.052. [DOI] [PubMed] [Google Scholar]
- Ristivojević P. M.; Tahir A.; Malfent F.; Opsenica D. M.; Rollinger J. M. High-Performance Thin-Layer Chromatography/bioautography and Liquid Chromatography-Mass Spectrometry Hyphenated with Chemometrics for the Quality Assessment of Morus Alba Samples. J. Chromatogr. A 2019, 1594, 190–198. 10.1016/j.chroma.2019.02.006. [DOI] [PubMed] [Google Scholar]