Abstract
Pseudomonas stutzeri OX1 meta pathway genes for toluene and o-xylene catabolism were analyzed, and loci encoding phenol hydroxylase, catechol 2,3-dioxygenase, 2-hydroxymuconate semialdehyde dehydrogenase, and 2-hydroxymuconate semialdehyde hydrolase were mapped. Phenol hydroxylase converted a broad range of substrates, as it was also able to transform the nongrowth substrates 2,4-dimethylphenol and 2,5-dimethylphenol into 3,5-dimethylcatechol and 3,6-dimethylcatechol, respectively, which, however, were not cleaved by catechol 2,3-dioxygenase. The identified gene cluster displayed a gene order similar to that of the Pseudomonas sp. strain CF600 dmp operon for phenol catabolism and was found to be coregulated by the tou operon activator TouR. A hypothesis about the evolution of the toluene and o-xylene catabolic pathway in P. stutzeri OX1 is discussed.
In bacteria, aerobic catabolic pathways for aromatic hydrocarbon degradation can schematically be divided into two major biochemical steps. First, early reactions, the so-called upper pathways or peripheral routes, channel the hydrocarbons towards the formation of partially oxidized aromatic intermediates. Then, dihydroxylated aromatic molecules that can undergo the cleavage of the ring are produced and further processed to give compounds that can enter the tricarboxylic acid cycle. Whereas a wide variety of very different peripheral routes for the oxidation of many different aromatic hydrocarbons exists, only a limited number of dihydroxylated compounds that can be cleaved and productively processed to enter the tricarboxylic acid cycle are known.
A good example of this is represented by the diversity of the known toluene catabolic pathways. Toluene is oxidized through different routes: via progressive oxidation of the methyl group (TOL pathway) (6), via dioxygenation (25), or via monooxygenations of the aromatic ring in different positions (18, 22, 31). Most of these pathways give rise to (methyl)catechols further processed through meta cleavage pathways. At least in one strain, Pseudomonas mendocina KR1, protocatechuate is produced and then cleaved in intradiol position (27). The genes coding for upper and lower pathways may be clustered in one (32), two (6), or more (18, 29) operons, independently but coordinately regulated.
The combination of different upper operons with one or more lower operons can thus increase not only the number of pathways through which a certain molecule can be degraded but also the range of substrates utilized for growth (10), and it is recognized as a mode for the evolution of new catabolic pathways (23, 28).
Pseudomonas stutzeri OX1 is able to utilize toluene and o-xylene as the sole carbon and energy sources. For both compounds the degradation proceeds through two successive monooxygenations of the aromatic nucleus catalyzed by toluene-o-xylene monooxygenase (ToMO) followed by extradiol ring cleavage (3). Here we investigate the organization of genes involved in the further degradation of toluene and o-xylene derivatives produced by the action of ToMO.
Identification of a P. stutzeri OX1 phenol hydroxylase activity.
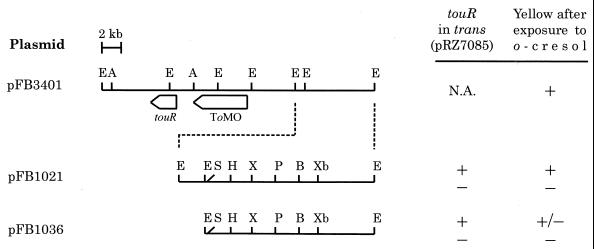
The maps of pFB3401 and its derivatives (see Table 1 for a description of strains and plasmids) are reported in Fig. 1. Previously, a catechol 2,3-dioxygenase (C2,3O) gene was roughly mapped to the 7.2-kb EcoRI fragment at the right end of the pFB3401 insert (3). As the ToMO-encoding operon did not contain genes involved in the lower pathway (1, 4), it was supposed that the C2,3O gene was part of a distinct meta operon.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strain | ||
| P. putida PaW340 | Cre− Dmp− C2,3O− Trp− Strr | 9 |
| P. putida PaW340::touR | TehrP. putida PaW340 derivative; contains a copy of touR integrated in the chromosome | This work |
| E. coli JM109 | recA1 hsdR17 thi Δ(lac-proAB) (F′ traD36 proAB lacIqZΔM15) | 30 |
| Plasmid | ||
| pGEM-3Z | Apr; cloning vector | Promega Corp. |
| pGEM-7Z | Apr; cloning vector, compatible with pLAFR vectors | Promega Corp. |
| pJB3Km1 | Kmr; cloning vector | 5 |
| pUC19 | Apr; cloning vector | 24 |
| pFB3401 | Tcr; 28-kb EcoRI fragment from P. stutzeri OX1 chromosome cloned in pLAFR1 | 3 |
| pFB3411 | Tcr; 22.4-kb EcoRI fragment from P. stutzeri OX1 chromosome cloned in pLAFR1 | 3 |
| pFB1021 | Tcr; partial deletion of pFB3411 | 3 |
| pFB1036 | Tcr; partial deletion of pFB3411 | 3 |
| pRZ7085 | Apr; pFB3401 8.5-kb ApaI fragment cloned in pGEM-7Z | This work |
| pJEX159 | Kmr; pFB3401 5.9-kb EcoRI-XbaI fragment cloned in pJB3Km1 | This work |
| pJSX148 | Kmr; pJEX159 4.8-kb SacI-XbaI fragment cloned in pJB3Km1 | This work |
| pBZ3120 | Apr; pFB3401 2-kb BamHI-XhoI fragment cloned in pGEM-3Z | This work |
| pVS1934 | Apr; pFB3411 3.4-kb XbaI-SacI fragment cloned in pUC19 | This work |
| pVS1921 | Apr; pFB3411 2.1-kb MluI-SmaI fragment cloned in pUC19 | This work |
Abbreviations: Cre, o-, m-, p-cresol; Dmp, 2,3-dimethylphenol and 3,4-dimethylphenol; Trp, tryptophan; Str, streptomycin; Tc, tetracycline; Km, kanamycin; Ap, ampicillin; Teh, tellurite.
FIG. 1.
Restriction endonuclease maps of the P. stutzeri OX1 chromosomal DNA fragment cloned in pFB3401 and its derivatives (3). pFB3401 allowed the host cells to convert toluene, o-xylene, and their phenolic derivatives into the corresponding ring fission products. The ToMO-encoding operon and the regulatory gene (touR) controlling its expression are indicated (1, 4). The point of the arrows indicates the direction of transcription. The ability (+) or inability (−) of the plasmids to direct the conversion of o-cresol into ring fission products in the absence (−) or in the presence (+) of touR, cloned in pRZ7085 and provided in trans, is indicated to the right of the restriction maps. +/−, mildly positive reaction after prolonged exposure. Abbreviations: A, ApaI; B, BamHI; E, EcoRI; H, HindIII; N.A., not applicable; P, PstI; S, SacI; X, XhoI; Xb, XbaI. Only relevant restriction sites are indicated.
In an attempt to verify if the C2,3O expression was controlled by the ToMO operon transcriptional activator TouR, E. coli JM109(pFB1021) cells were transformed with the compatible plasmid pRZ7085 carrying the regulatory gene touR in order to assay a possible increase in C2,3O activity upon exposure to the phenolic compounds that were demonstrated to be TouR effectors (1). Surprisingly, a yellow color, indicative of the accumulation of a ring fission product, developed in cultures exposed for 1 h to o-cresol (Fig. 1). When the same experiment was performed with cells carrying pRZ7085 and pFB1036, a faint yellow color was detectable only after prolonged incubation of more than 24 h. These results suggested that in the pFB1021 insert, a gene or genes coding for a phenol hydroxylase activity, able to catalyze the conversion of the phenolic compound into a catechol, might be present.
Mapping of meta pathway genes.
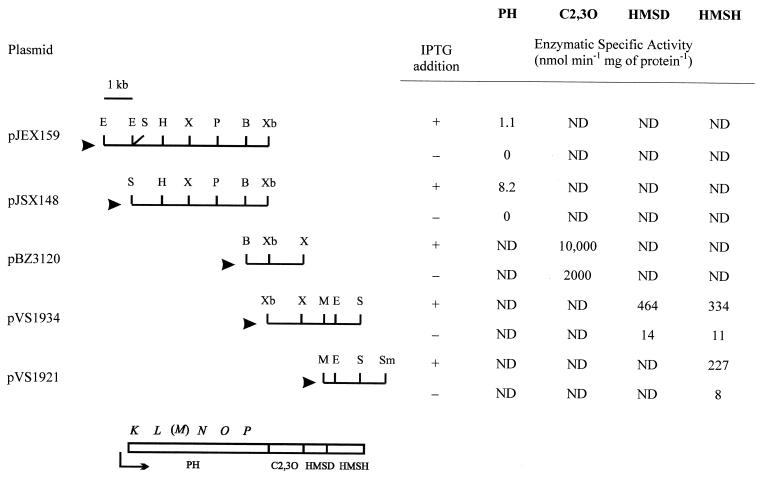
To localize genes involved in meta pathway reactions, several overlapping fragments from the insert of pFB1021 and of pFB3411 were subcloned in Escherichia coli IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible vectors. The insert of pFB3411 partially overlapped and extended beyond the right end of pFB3401, and it was unable to direct the conversion of both hydrocarbons and phenols into catechols (3). The plasmids obtained were examined for phenol hydroxylase (PH), C2,3O, 2-hydroxymuconate semialdehyde hydrolase (HMSH), and 2-hydroxymuconate semialdehyde dehydrogenase (HMSD) activities, and the encoding loci were mapped (Fig. 2). PH activity was determined in resting cells by monitoring the decrease in concentration of phenol in the medium, using a colorimetric assay described previously (3, 15). C2,3O, HMSH, and HMSD were assayed in crude extracts by measuring the formation rate (17) or the decrease (21) of the catechol ring fission product.
FIG. 2.
Restriction endonuclease maps of DNA fragments subcloned from pFB1021 (pJ and pBZ series) and from pFB3411 (pVS series). Only the plasmids containing the minimal region coding for PH, C2,3O, HMSH, and HMSD are reported. The black arrowheads indicate the position of the Plac of the vector. To the right of the restriction maps, the enzymatic activities expressed from the indicated plasmids in uninduced (−) or IPTG-induced (+) E. coli JM109 cells are reported. At the bottom, the map obtained by subcloning, Southern, and sequence analyses (see text for details) displaying the gene arrangement and the putative promoter is shown. The presence of the gene indicated in parentheses can be hypothesized but has not been investigated. Abbreviations: B, BamHI; E, EcoRI; H, HindIII; M, MluI; ND, not detectable; P, PstI; S, SacI; Sm, SmaI; X, XhoI; Xb, XbaI. Only relevant restriction sites are indicated.
Quantitative assays performed on cells carrying the plasmids depicted in Fig. 2 or plasmids carrying the same inserts cloned in the opposite direction with respect to the Plac promoter of the vector (not shown) suggested that the genes mapped are transcribed in the same direction, from the left to the right with respect to the maps shown in Fig. 2.
Substrate range of the cloned phenol hydroxylase.
Plasmids pJEX159 and pJSX148 were also examined and found not to confer upon cells the ability to convert toluene and o-xylene to phenolic compounds. The activity cloned in pJEX159 and pJSX148 is thus specific for the introduction of a hydroxyl group on phenolic compounds. The substrate range of the cloned PH was investigated in E. coli JM109(pJSX148) cells. Specific activities are reported in Table 2 and found comparable for all the phenols tested. p-Cresol, 2,4-dimethylphenol (2,4-DMP), and 3,4-DMP, due to the presence of a substituent para to the hydroxyl group, were undetectable in this assay. High-pressure liquid chromatography (HPLC) analyses, however, demonstrated that these phenols were oxidized by the cloned enzyme.
TABLE 2.
Substrate range and reaction product identification for the P. stutzeri OX1 PH from IPTG-induced E. coli JM109(pJSX148) cells
| Substrate | PH sp act (nmol min−1 mg of protein−1) | HPLC retention time of product (min) | Product identified | HPLC retention time for standard of predicted product (min) | Cleaved by C2,3O |
|---|---|---|---|---|---|
| Phenol | 8.2 ± 0.5 | 3.55 | Catechol | 3.51 | + |
| o-Cresol | 10.8 ± 1.0 | 3.44 | 3-Methylcatechol | 3.40 | + |
| m-Cresol | 8.7 ± 0.7 | 3.35 | 3-Methylcatechol | 3.40 | + |
| p-Cresol | NAa | 3.16 | 4-Methylcatechol | 3.13 | + |
| 2,3-DMP | 12.7 ± 0.5 | 4.25 | 3,4-Dimethylcatechol | 4.05 | + |
| 3,4-DMP | NA | 4.17 | 3,4-Dimethylcatechol | 4.05 | + |
| 2,4-DMP | NA | 4.10 | 3,5-Dimethylcatecholb | NAvc | − |
| 3,5-DMP | 11.4 ± 1.0 | 4.07 | Not analyzed | NAv | − |
| 2,5-DMP | 14.4 ± 2.8 | 4.21 | 3,6-Dimethylcatecholb | NAv | − |
NA, Not applicable.
Identified by NMR analysis.
NAv, not available.
To identify the reaction products, the supernatants of E. coli JM109(pJSX148) cell reactions, with different phenols supplied at a final concentration of 0.5 to 3 mM depending on the compound, were extracted with a 1/5 volume of acetonitrile; the organic phase was filtered, deoxygenated with N2, and then analyzed by reverse-phase HPLC; and the retention times were compared with those of authentic standards (Table 2). The supernatants were also treated with partially purified C2,3O expressed from pBZ3120 and checked by HPLC analyses for the complete disappearance of PH reaction products. The spectral properties of the yellow compounds produced after C2,3O reaction were identical to those determined for the extradiol ring fission products obtained from authentic standards of catechol, 3-methyl-, 4-methyl-, and 3,4-dimethylcatechol (data not shown). The further data confirmed the identification of the reaction products and suggested that m-cresol and 3,4-DMP were mainly converted into 3-methyl- and 3,4-dimethylcatechol, respectively, although the presence of small amounts of other isomers could not be excluded. The reactions catalyzed by PH thus appear to be redundant with respect to those catalyzed by ToMO, at least as regards the phenols derived from toluene and o-xylene.
Identification of the products derived from 2,4-DMP and 2,5-DMP.
The P. stutzeri OX1 PH acts on a very broad range of substrates, as it was able to produce more polar compounds, presumably dimethylcatechols, even from 2,4-DMP, 3,5-DMP, and 2,5-DMP, which are not growth substrates for P. stutzeri OX1. As standards of 3,5- and 3,6-dimethylcatechols for HPLC analyses were not available, we set out to identify these compounds by nuclear magnetic resonance (NMR). The reaction products obtained upon exposure of E. coli JM109(pJSX148) cells to 3 mM 2,4-DMP and 2,5-DMP were extracted from the supernatants with ethyl acetate, separated by flash column chromatography, and analyzed by 1H-NMR. The protonic signals had the following features: δ 6.55 (s, 2H), δ 5.20 (br s, 2H, D2O); and δ 2.25 (s, 6H) for the product obtained from 2,4-DMP and δ 6.60 (s, 2H), δ 5.60 (br s, 2H, D2O), and δ 2.23 (s, 6H) for the product obtained from 2,5-DMP. Reactions of 5.20 and 5.60 protons, respectively, with D2O confirmed the assignment of the signals to hydroxyl groups. The registered spectra were consistent with those reported in literature for 3,5-dimethylcatechol (26) and for 3,6-dimethylcatechol (14). Thus, the P. stutzeri OX1 PH displays precise regioselectivity, as it produces catechols from all the phenols tested. The product from 3,5-DMP was not analyzed.
The dimethylcatechols from 2,4-DMP, 2,5-DMP, and, presumably, 3,5-DMP did not disappear in the supernatants treated with C2,3O, nor did the typical yellow color of extradiol ring fission products develop (Table 2). To further assess if the P. stutzeri C2,3O was unable to catalyze the cleavage of 3,5- and 3,6-dimethylcatechol, specific activities were measured and found to be 0.01 and 0.05%, respectively, of the activity measured using catechol as the reaction substrate.
In P. stutzeri OX1, 2,4-DMP and 2,5-DMP were derived from the ToMO-catalyzed hydroxylation of m-xylene and p-xylene and are not used for growth (2, 3). Thus, in P. stutzeri OX1, catechol 2,3-dioxygenase represents the bottleneck for m-xylene and p-xylene degradation through the monooxygenation pathway.
Arrangement of the PH genes.
The length of the minimal DNA region to detect PH activity in E. coli cells suggested that the P. stutzeri OX1 PH might be a multicomponent enzyme system. Southern hybridization analyses of pJEX159 were thus performed using the dmp genes coding for the subunits of the Pseudomonas sp. strain CF600 multicomponent PH (16) as probes. Hybridization signals were detected when pJEX159 was probed with dmpL, dmpN, dmpO, and dmpP but not when the probes were dmpK and dmpM (data not shown).
Sequencing of approximately 1,000 nucleotides (accession no. AJ309239) at the 5′ end of the PH locus revealed a sequence identical to the −12, −24 consensus of ς54-dependent promoters followed by a putative open reading frame (ORF) (nucleotides 247 to 507) whose deduced amino acid sequence shared 62.6% identity (74% similarity) to the Pseudomonas sp. strain CF600 PH DmpK subunit. The map obtained is depicted in Fig. 2 and evidenced a conserved gene order with respect to that of the Pseudomonas sp. strain CF600 PH gene cluster (16). In the Pseudomonas sp. strain CF600 PH, DmpK is dispensable for the enzyme activity in vitro and it was suggested to play a role in assembly of the active form of the enzyme (20). In pJSX148, which expresses a high level of PH activity, the DmpK-like ORF is partially deleted, suggesting that its integrity is not essential for the enzyme to be assembled correctly enough to function.
The P. stutzeri meta operon putative promoter is located in the 1.1-kb EcoRI fragment deleted in pFB1036 (Fig. 1), and its deletion may explain the scarce and delayed production of extradiol ring fission products observed in cells carrying pFB1036 and touR in trans and exposed to o-cresol.
PH and C2,3O are controlled by TouR.
The ability of pFB1021 to direct the conversion of phenols into ring cleavage products when in trans with pRZ7085 (Fig. 1) together with the presence of a putative ς54-dependent promoter upstream from the PH-encoding genes (Fig. 2) suggested that the expression of the identified meta pathway genes could also be controlled by TouR, the phenol-responsive regulator of the tou upper operon (1). The regulatory gene touR was thus integrated in the Pseudomonas putida PaW340 chromosome by means of minitransposon (8, 11), and the recombinant strain was transformed with pFB3411. Upon induction with o-cresol, the specific activities of PH and C2,3O increased 30- and 8-fold, respectively, in comparison to uninduced samples (Table 3). The inability of P. putida PaW340(pFB3411) to convert phenolic compounds into ring cleavage products (3) can thus be ascribed to the absence of a suitable transcriptional activator.
TABLE 3.
PH and C2,3O activity in P. putida PaW340(pFB3411) cells expressing or not expressing TouR
| Strain | Induction with o-cresol | Sp act (nmol min−1 mg of protein−1)
|
|
|---|---|---|---|
| PH | C2,3O | ||
| PaW340::touR(pFB3411) | − | 2 | 232 |
| + | 61 | 1,870 | |
| PaW340(pFB3411) | − | 1 | 420 |
| + | 2 | 300 | |
Conclusive remarks.
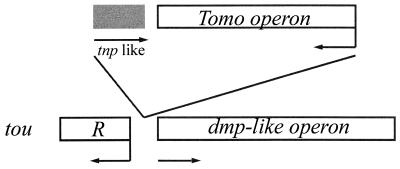
The P. stutzeri OX1 genes coding for toluene and o-xylene catabolism are organized into at least two operons, one coding for the toluene-o-xylene-monooxygenase (4) and another for phenol catabolism, the latter organized as the Pseudomonas sp. strain CF600 dmp operon in which the multicomponent PH genes are followed by C2,3O, HMSH, and HMSD encoding genes (19). Both operons are inducible by phenols under the control of a ς54-dependent promoter and TouR. The presence of a dmp-like operon together with a phenol-responsive regulator in the P. stutzeri OX1 genome suggests that, in this strain, the toluene and o-xylene catabolic pathway evolved by vertical expansion of a preexisting route for phenol catabolism through the incorporation of the ToMO gene cluster (Fig. 3). In this perspective, the redundancy between the hydroxylation of phenols catalyzed by ToMO and the PH activity can be fortuitous due to the acquisition of a monooxygenase endowed with a very broad range of substrates. The scarce specificity of ς54-dependent promoters (13), which makes the PToMO promoter activatable by TouR, may be a factor that contributed to the successful acquisition of the ToMO gene cluster.
FIG. 3.
A model for the evolution of the toluene and o-xylene catabolic pathway in P. stutzeri OX1. See text for details. The arrows indicate the direction of transcription. tnp, transposase-like ORF (4).
P. stutzeri OX1 is the first toluene-degrading strain in which the toluene monooxygenase-encoding operon has been found associated to a dmp-like operon. Toluene monooxygenases similar to the P. stutzeri OX1 ToMO have been found in other bacterial strains (7, 31), but they are associated with very different lower pathways (12, 29). These observations further confirm the modular organization of aromatic hydrocarbon catabolic pathways and suggest that the toluene monooxygenase operon represents an independent module.
Acknowledgments
This work was supported by the Consiglio Nazionale delle Ricerche (Rome), grant no. CT99.00287.PF49 of the Target Project on Environmental Biotechnology, and by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Rome) under the Programma di Ricerca di Interesse Nazionale, contract “Characterization of biodegradative enzymatic systems: oxidases and oxygenases” to P.B.
We are grateful to V. De Lorenzo and V. Shingler, who kindly provided the mini-Tn5 transposition systems and the dmp probes, and to V. Valota and R. Macchi for collaboration on the experimental work. We also thank G. Baggi for providing 3,4-dimethylcatechol.
REFERENCES
- 1.Arenghi F L G, Pinti M, Galli E, Barbieri P. Identification of the Pseudomonas stutzeri OX1 toluene-o-xylene monooxygenase regulatory gene (touR) and of its cognate promoter. Appl Environ Microbiol. 1999;65:4057–4063. doi: 10.1128/aem.65.9.4057-4063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri P, Palladino L, Di Gennaro P, Galli E. Alternative pathways for o-xylene or m-xylene and p-xylene degradation in a Pseudomonas stutzeri strain. Biodegradation. 1993;4:71–80. [Google Scholar]
- 3.Bertoni G, Bolognese F, Galli E, Barbieri P. Cloning of the genes for and characterization of the early stages of toluene and o-xylene catabolism in Pseudomonas stutzeri OX1. Appl Environ Microbiol. 1996;62:3704–3711. doi: 10.1128/aem.62.10.3704-3711.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertoni G, Martino M, Galli E, Barbieri P. Analysis of the gene cluster encoding toluene/o-xylene monooxygenase from Pseudomonas stutzeri OX1. Appl Environ Microbiol. 1998;64:3626–3632. doi: 10.1128/aem.64.10.3626-3632.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blatny J M, Brautaset T, Winther-Larsen H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burlage R S, Hooper S W, Sayler G S. The TOL (pWW0) catabolic plasmid. Appl Environ Microbiol. 1989;55:1323–1328. doi: 10.1128/aem.55.6.1323-1328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne A M, Kukor J J, Olsen R H. Sequence analysis of the gene cluster encoding toluene-3-monooxygenase from Pseudomonas pickettii PKO1. Gene. 1995;154:65–70. doi: 10.1016/0378-1119(94)00844-i. [DOI] [PubMed] [Google Scholar]
- 8.De Lorenzo V, Timmis K M. Analysis and construction of stable phenotypes in Gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 1994;235:386–405. doi: 10.1016/0076-6879(94)35157-0. [DOI] [PubMed] [Google Scholar]
- 9.Franklin F C H, Williams P A. Construction of a partial diploid for the degradative pathway encoded by the TOL plasmid (pWW0) from Pseudomonas putida mt-2: evidence for a positive nature of the regulation by the xylR gene. Mol Gen Genet. 1980;177:321–328. doi: 10.1007/BF00267445. [DOI] [PubMed] [Google Scholar]
- 10.Haigler B E, Pettigrew C A, Spain J C. Biodegradation of mixtures of substituted benzenes by Pseudomonas sp. strain JS150. Appl Environ Microbiol. 1992;58:2237–2244. doi: 10.1128/aem.58.7.2237-2244.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrero M, De Lorenzo V, Timmis K M. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kukor J J, Olsen R H. Genetic organization and regulation of a meta cleavage pathway for catechols produced from catabolism of toluene, benzene, phenol, and cresols by Pseudomonas pickettii PKO1. J Bacteriol. 1991;173:4587–4594. doi: 10.1128/jb.173.15.4587-4594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leahy J G, Johnson G R, Olsen R H. Cross-regulation of toluene monooxygenases by the transcriptional activators TbmR and TbuT. Appl Environ Microbiol. 1997;63:3736–3739. doi: 10.1128/aem.63.9.3736-3739.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutolf V F, Prewo R, Bieri J H, Ruedi P, Eugster C H. Synthesen von Dibenzo[b,e][1,4]dioxin-2,3-chinonen, einschliesslich der Ecklonochinone A and B sowie der Isoecklonochinone A and B′. Helv Chim Acta. 1985;68:860–881. [Google Scholar]
- 15.Martin R W. Rapid colorimetric estimation of phenol. Anal Chem. 1949;21:1419–1420. [Google Scholar]
- 16.Nordlund I, Powlowski J, Shingler V. Complete nucleotide sequence and polypeptide analysis of multicomponent phenol hydroxylase from Pseudomonas sp. strain CF600. J Bacteriol. 1990;172:6826–6833. doi: 10.1128/jb.172.12.6826-6833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nozaki M, Kotani S, Ono K, Seno S. Metapyrocatechase. Substrate specificity and mode of ring fission. Biochim Biophys Acta. 1970;220:1419–1420. doi: 10.1016/0005-2744(70)90007-0. [DOI] [PubMed] [Google Scholar]
- 18.Olsen R H, Kukor J J, Kaphammer B. A novel toluene-3-monooxygenase pathway cloned from Pseudomonas pickettii PKO1. J Bacteriol. 1994;176:3749–3756. doi: 10.1128/jb.176.12.3749-3756.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powlowski J, Shingler V. Genetics and biochemistry of phenol degradation by Pseudomonas sp. CF600. Biodegradation. 1994;5:219–236. doi: 10.1007/BF00696461. [DOI] [PubMed] [Google Scholar]
- 20.Powlowski J, Sealy J, Shingler V, Cadieux E. On the role of DmpK, an auxiliary protein associated with multicomponent phenol hydroxylase from Pseudomonas sp. strain CF600. J Biol Chem. 1997;272:945–951. doi: 10.1074/jbc.272.2.945. [DOI] [PubMed] [Google Scholar]
- 21.Sala-Trepat J M, Murray K, Williams P A. The metabolic divergence in the meta cleavage of catechols by Pseudomonas putida NCIB 10015. Physiological significance and evolutionary implications. Eur J Biochem. 1972;28:347–356. doi: 10.1111/j.1432-1033.1972.tb01920.x. [DOI] [PubMed] [Google Scholar]
- 22.Shields M S, Montgomery S O, Chapman P J, Cuskey S M, Pritchard P H. Novel pathway of toluene catabolism in the trichloroethylene-degrading bacterium G4. Appl Environ Microbiol. 1989;55:1624–1629. doi: 10.1128/aem.55.6.1624-1629.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Meer J, de Vos W M, Harayama S, Zehnder A J. Molecular mechanisms of genetic adaptation to xenobiotic compounds. Microbiol Rev. 1992;56:677–694. doi: 10.1128/mr.56.4.677-694.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vieira J, Messing J. The pUC plasmid, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 25.Wackett L P. Toluene dioxygenase from Pseudomonas putida F1. Methods Enzymol. 1990;188:39–45. doi: 10.1016/0076-6879(90)88010-8. [DOI] [PubMed] [Google Scholar]
- 26.Wellr D D, Stirchak E P. Quassinoid synthesis via o-quinone Diels-Alder reactions. J Org Chem. 1983;48:4873–4879. [Google Scholar]
- 27.Whited G M, Gibson D T. Separation and partial characterization of the enzymes of the toluene-4-monooxygenase catabolic pathway in Pseudomonas mendocina KR1. J Bacteriol. 1991;173:3017–3020. doi: 10.1128/jb.173.9.3017-3020.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams P A, Sayers J R. The evolution of pathways for aromatic hydrocarbon oxidation in Pseudomonas. Biodegradation. 1994;5:195–217. doi: 10.1007/BF00696460. [DOI] [PubMed] [Google Scholar]
- 29.Wright A, Olsen R H. Self-mobilization and organization of the genes encoding the toluene metabolic pathway of Pseudomonas mendocina KR1. Appl Environ Microbiol. 1994;60:235–242. doi: 10.1128/aem.60.1.235-242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yanish-Perron C, Vieira J, Messing J. Improved M13 phage cloning vector and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 31.Yen K M, Karl M R, Blatt L M, Simon M J, Winter R B, Fausset P R, Lu H S, Harcourt A A, Chen K K. Cloning and characterization of a Pseudomonas mendocina KR1 gene cluster encoding toluene-4-monooxygenase. J Bacteriol. 1991;173:5315–5327. doi: 10.1128/jb.173.17.5315-5327.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zylstra G J, McCombie W R, Gibson T D, Finette B A. Toluene degradation by Pseudomonas putida F1: genetic organization of the tod operon. Appl Environ Microbiol. 1988;54:1498–1503. doi: 10.1128/aem.54.6.1498-1503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]