Abstract
River managers strive to use the best available science to sustain biodiversity and ecosystem function. To achieve this goal requires consideration of processes at different scales. Metacommunity theory describes how multiple species from different communities potentially interact with local-scale environmental drivers to influence population dynamics and community structure. However, this body of knowledge has only rarely been used to inform management practices for river ecosystems. In this paper, we present a conceptual model outlining how the metacommunity processes of local niche sorting and dispersal can influence the outcomes of management interventions and provide a series of specific recommendations for applying these ideas as well as research needs. In all cases, we identify situations where traditional approaches to riverine management could be enhanced by incorporating an understanding of metacommunity dynamics. A common theme is developing guidelines for assessing the metacommunity context of a site or region, evaluating how that context may affect the desired outcome, and incorporating that understanding into the planning process and methods used. To maximize the effectiveness of management activities, scientists and resource managers should update the toolbox of approaches to riverine management to reflect theoretical advances in metacommunity ecology.
Keywords: spatial, dispersal, network connectivity, local sorting, biomonitoring, restoration, invasive species, conservation
Graphical Abstract
Conceptual model of how habitat connectivity influences the outcomes of management activities by altering the strength of local sorting processes on community assembly. Connectivity can be increased or decreased through human activities.
1. INTRODUCTION
The management of streams and rivers for the benefit of aquatic biodiversity and ecosystem service provisioning is grounded in community ecology (Lake, Bond, & Reich, 2007). For instance, applications of biomonitoring data in streams is based on known relationships between the taxonomic composition of local biological communities and environmental attributes such as water chemistry, habitat availability, local climate, and geology. These relationships often inform development of numeric water quality criteria intended to protect aquatic life (Davis & Simon, 1995). Similarly, the practice of stream restoration is based on the idea that by improving habitat and other environmental conditions the flora and fauna will recover toward a desired condition (i.e., “if you build it, they will come”) (Hilderbrand, Watts, & Randle, 2005; Palmer, Ambrose, & Poff, 1997). Campaigns to eradicate invasive species and diseases such as rock snot (Didymosphenia geminata; (Spaulding & Elwell, 2007) and trout whirling disease (Myxobolus cerebralis; (Hedrick, Adkison, El-Matbouli, & MacConnell, 1998) use community ecology to evaluate the effects of these species and prioritize which taxa to remove. Finally, multispecies conservation plans often focus on land acquisition and preservation to protect critical habitats (Knopf, Johnson, Rich, Samson, & Szaro, 1988). Many of these management approaches have been developed around the core concepts of niche theory, the idea that a strong association exists between local habitat conditions and community composition through processes that sort species into those that can persist and those that cannot persist under those environmental and biotic conditions. This relationship is often referred to as “local sorting” or “niche selection” (Leibold & Chase 2018).
In the past decade there has been explosion of research incorporating niche theory into a broader landscape level framework that also recognizes the effects of connectivity and dispersal on community composition. This metacommunity concept links traditional ideas of local sorting with regional processes of dispersal to better understand community dynamics and ecosystem processes at multiple scales (Mathew A. Leibold et al., 2004). Today, the metacommunity concept is an integral part of our understanding of community ecology, and the framework it provides for understanding community assembly is widely used in both theoretical and empirical investigations (Logue, Mouquet, Peter, & Hillebrand, 2011). However, practical applications of the metacommunity concept have lagged behind theoretical advances (Heino, 2013; Schiesari et al., 2019). Just as the metacommunity concept has improved our understanding of the processes shaping ecological communities, it should also guide how we use community theory in the management of natural ecosystems (B. L. Brown, Swan, C. M., Auerbach, D. A., Grant, E. H. C., Hitt, N. P., Maloney, K. O., and Patrick, C., 2011; Costello, Stagaman, Dethlefsen, Bohannan, & Relman, 2012; Van Allen & Rudolf, 2016). Here we review the basic tenets of this theory as it applies to streams and rivers, summarize where and how the theory can enhance river management, recommend an approach for applying these concepts, and identify a series a remaining research questions that remain to be addressed.
2. METACOMMUNITY THEORY IN STREAMS AND RIVERS
A major goal of metacommunity theory is to describe how local-scale processes (e.g., species interactions, environmental filtering, and demographic events) and regional processes that affect probabilities of colonization and establishment (e.g., organism dispersal abilities, distance among habitats, and dispersal barriers) interact to influence community composition. Understanding the relative role of these processes can provide insight into strategies for conservation and management. Early presentations of the metacommunity concept (Leibold et al. 2004) and its recent updates (M. A. Leibold & Chase, 2018; Logue et al., 2011) define different scenarios where the spatial variation in local-scale dynamics are produced by spatial variation in the environment, interspecific interactions, dispersal, random demographic processes, or some combination of these forces. Here, we define the combined effect of all local-scale deterministic processes (e.g. interspecific interactions driving biotic sorting, environmental sorting) on community composition in a given habitat as that habitat’s “local sorting strength”. This and other important definitions are organized in Table 1.
Table 1.
Definitions
| Category | Term | Definition |
|---|---|---|
| Biological Organization | Metacommunity | A set of local communities that are linked by dispersal of multiple interacting species |
| Community | The individuals of all species that potentially interact within a habitat at the local scale | |
| Population | All individuals of a single species within a habitat at the local scale | |
| Spatial Scale | Local Scale | Scale corresponding to a single habitat capable of holding a community |
| Regional Scale | Scale corresponding to a large area containing multiple local habitats and capable of supporting a metacommunity | |
| Biogeographic Scale | Scale corresponding to area containing multiple regions, where evolutionary processes driving patterns in diversity and composition become easily recognizable | |
| Theory | Niche Theory | Conceptual framework organized around defining an organism’s role in an ecosystem and the factors that dictate whether an organism will persist in an ecosystem. |
| Metacommunity Theory | Conceptual framework that integrates the concepts within niche theory with additional regional processes including habitat connectivity and organism dispersal ability. | |
| Local and Spatial Processes | Environmental Sorting | The process by which environmental conditions within a local scale habitat prevent colonists from establishing local populations |
| Biotic Sorting | The process by which organisms present within a local scale habitat influence whether colonists establish local populations via interspecific interactions such as competition and predation | |
| Local Sorting | The combined effects of environmental and biotic sorting occurring within a local scale habitat. Also referred to as “niche selection.” | |
| Mass Effects | The process by which continual dispersal of organisms influences population abundance of species within a local scale habitat. This process may offset local sorting processes, allowing species to persist in habitats where they would otherwise be absent. | |
| Demographic Stochasticity | Random variation in the sizes of populations due to the discrete and probabilistic nature of individual births, deaths, and dispersal movements. Important in small populations, where population variation can lead to extinctions that obscure local sorting processes. | |
| Metrics of Community State | Local Control | The state in which a community within a local scale habitat is composed of species that reflect local environmental conditions |
| Disequilibrium | The state in which a community within a local scale habitat is composed of species that do not reflect local environmental conditions | |
| Sorting Strength | The relative strength of local sorting in a local scale habitat when compared to other similar habitats | |
| Spatial Definitions | Spatial context | Comprehensive understanding of the multi-scale processes and characteristics in a region, including but not limited to, connectivity, network structure, dispersal, spatial patterns in local sorting strength, community composition, and diversity |
| Connectivity | Scale dependent measurement of potential for movement by species among local habitats, considering mode of movement, distance to travel, and ease of movement | |
| Network centrality | Measure of connectivity for a single local habitat, representing how connected a node is to other nodes within network. | |
| Colonization | Process by which populations become established at sites from which they were previously absent | |
| Dispersal | Movement of individuals from a site (emigration) to another (immigration) | |
| Regional species pool | The species inhabiting a watershed or nearby watersheds in a region that have the potential to colonize a local habitat | |
| Statistical Analysis Terms | Multivariate niche volume | The volume in n-dimensional space occupied by the species in a community at the time of sampling. A community with a large volume is inferred to have more wide spread generalist species. |
| Volume deviation | The difference between the observed multivariate niche volume for a community and that expected for the community based on random community assembly. Negative values indicate that the community is experiencing environmental filtering, reducing the volume relative to random assembly. | |
| Variation partitioning | Statistical analysis designed to partition explainable variation in a response variable into components (E, S, E-S) that can be attributed to different predictors in isolation or in tandem. E is the proportion of variation explained by the non-spatial environment. S is the proportion of variation explained by spatial variation in the response variable not shared by the measured environmental variables. E-S is the proportion of variation explained by spatially structured (non-random) environmental variation. | |
| Management Terms | Biomonitoring | Performing surveys of biological communities for the purposes of assessing the health of the surveyed sites and/or the region in which those sites occur. |
| IBI | Index of Biotic Integrity, a metric developed to assess the health of a local scale stream reach from the identity and numbers of the resident species found there. | |
| Reference Condition | Criteria used for determining whether a habitat meets the definition of a reference site. May include both environmental and community information. Is derived from data collected at undisturbed, least disturbed, or best available habitat site. | |
| Ecological Restoration | The practice of manipulating an ecosystem to achieve management goal such as returning to reference condition, restoring lost ecosystem functions, or enhancing native biodiversity. Differentiated from ecological engineering by an intent to enhance the ecology of the system rather than solely benefit human user groups. |
A concept central to metacommunity theory is that local scale processes may be constrained by regional scale processes (Poff 1997, Patrick & Swan 2011). The relationship between the relative effects of local sorting and dispersal is expected to be unimodal (Fig 1A). When the number of immigrants colonizing a habitat is very high resulting from source-sink dynamics, local-scale processes such as predation, competition, and abiotic environmental sorting may be less important in explaining local community composition than the identity of the colonizing immigrants (e.g. a mass effects assembly process). Conversely, at very low dispersal, demographic stochasticity is expected to be more important than interspecific interactions (Mathew A. Leibold et al., 2004). Furthermore, at low dispersal, inferior competitors may maintain populations in sub-optimal sites due to lack of competition with stronger competitors, causing local communities to fail to match environmental conditions and remain in a state of disequilibrium, but also allowing regional coexistence and, thus, higher gamma diversity. For example, Patrick & Swan (2011) observed that spatially isolated streams that had experienced major anthropogenic disturbance did not recover original, diverse benthic communities’ decades after water quality conditions had improved, presumably due to lack of recolonization. At intermediate levels of dispersal, the local community composition is expected to closely match local conditions. One general hypothesis for streams and rivers is that the importance of local sorting, particularly for passive in-network dispersers, declines with downstream position because connectivity and associated propagule pressure increases, leading to mass effects, while environmental conditions simultaneously become more moderate and exert less selective pressure (B.L. Brown & Swan, 2010; Swan & Brown, 2014; Tonkin et al., 2018).
Figure 1.
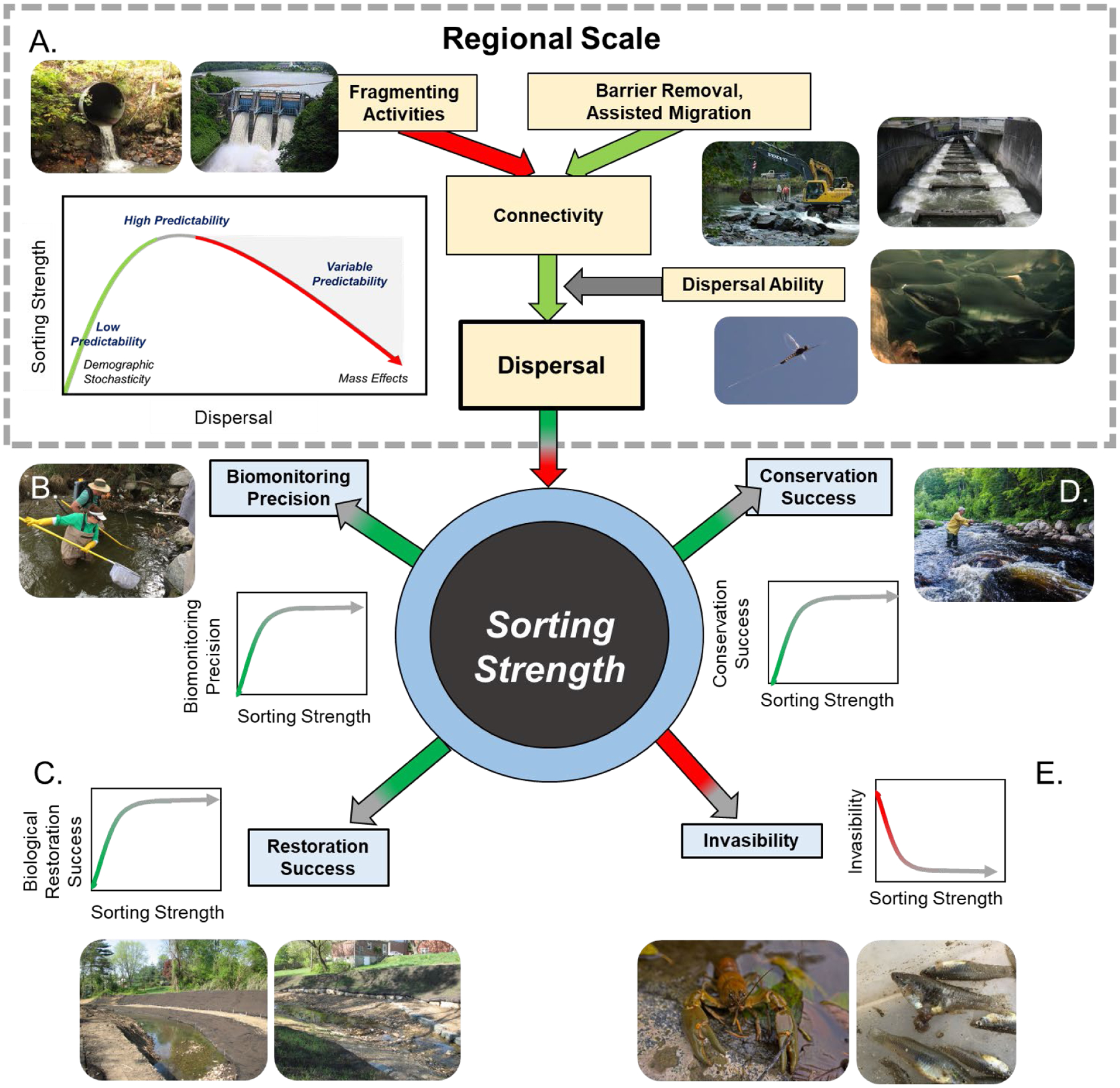
Conceptual diagram of the factors driving metacommunity dynamics and impacts on various aspects of stream and river management. A. Regional scale factors (dotted box, cream text boxes) affect connectivity among habitats which interacts with dispersal ability of resident organisms to influence the total amount of dispersal among habitats. Dispersal among habitats has a unimodal relationship with local sorting strength, defined as the amount of variation in community composition that local environmental variables can explain. Sorting strength is predicted to have positive relationships with B. biomonitoring precision (inset: electrofishing for aquatic life uses assessment), C. restoration success (inset: examples of restoration projects changing channel morphology), and D. conservation success (inset: individual enjoying ecosystem services provided by a healthy river). In contrast, local sorting is predicted to have a negative relationship with E. habitat invasibility when regional processes are not considered (inset: examples of non-native taxa, rusty crayfish (Orconectes rusticus) and western mosquitofish (Gambusia affinis). Arrow colors denote the sign of the expected relationship among factors (red = negative, green = positive) with gradient arrow indicating non-linear relationships. Inset graphs display the functional form of the expected relationships.
Applied ecologists working in riverine ecosystems are well positioned to incorporate metacommunity theory into management actions because the types of data needed are, in many cases, already being collected. Metacommunity analyses require spatially explicit species composition and environmental data from multiple communities. For example, one commonly used statistical analysis is variation partitioning, which is used to estimate the degree to which local environmental effects versus regional dispersal-driven effects account for observed patterns of community assembly (P. Legendre, Borcard, & Peres-Neto, 2005; Pierre Legendre & Legendre, 2012). The wealth of large-scale riverine biomonitoring datasets collected by local, state, and federal agencies (Carlisle, Wolock, & Meador, 2010) are ideal for metacommunity analyses (Hampton et al., 2013). As a result, metacommunity dynamics of streams and rivers encompasses a disproportionately large proportion of published metacommunity studies (B. Tornwall, Sokol, Skelton, & Brown, 2015).
Leveraging available data with these methods allows investigators to draw inferences about the spatial distribution and the importance of dispersal in shaping community assembly across landscapes, opening the door to many management applications.
3. MANAGEMENT APPLICATIONS
In this section we highlight opportunities where metacommunity theory can be used by applied ecologists and management professionals seeking to enhance the tools currently available in the field of river management. We focus on the specific areas of biomonitoring, restoration, conservation, and invasive species management, as these encompass the majority of management activities. The concepts we describe are encapsulated in a holistic conceptual diagram (Figure 1). We also provide an analysis of biomonitoring data as a case study for how these ideas may be applied. Where possible, we provide concrete suggestions for applications, and where knowledge gaps persist, we identify future areas of study we believe may be fruitful. These suggestions and research needs are summarized in Table 2. Our recommendations provide an expanded set of management strategies that capture both local and regional influences on metacommunity dynamics.
Table 2.
Research needs and potential management applications
| Category | Research Needs | Management Actions |
|---|---|---|
| Biomonitoring | Develop best practices for using variation partitioning or other similar approaches within watersheds and drainage basins to quantify strength of local sorting | Account for spatial and metacommunity context in the development of predictive niche models for biological communities. Use predications about the variation in strength of local sorting within different regions in the development of biomonitoring models and Indices of Biotic Integrity (IBI). |
| Incorporate spatial predictors, such as network position and mean distance to sources of colonists, in predictive niche models to better account for spatial context and species-specific differences in dispersal strength on site-specific probabilities of species or metric occurrence. | ||
| Devise separate models or treatments for groups of taxa that share similar dispersal behaviors or syndromes. For example, IBI’s may be enhanced if poor dispersers and univoltine species are given greater weight in index scores than strong dispersers and multivoltine species. | Apply enhanced IBI models that incorporate metacommunity principles. In the absence of such models, recognize that biomonitoring indices are sensitive to spatial processes and that scores should be interpreted in light of these potential limitations. | |
| Restoration | Develop monitoring protocols for characterizing the size and spatial distribution of regional species pools and dispersal abilities of focal taxa. | Determine whether there are suitable source populations in the regional species pool before starting a restoration project with goal of community restoration and whether those populations can disperse to the selected site. |
| Develop protocols for accurately characterizing the dispersal or connectivity of river reaches. For example, is network centrality alone a good indicator? What additional characteristics of the spatial context should be quantified? Should colonization be directly measured with drift nets? | Avoid restoration projects in areas where dispersal is too strong or too weak (Swan and Brown 2017). For example, confluences and mainstems are likely to be a low return zone for restoration efforts due to dispersal surplus (Altermatt and Fronhofer 2018). Highly disconnected areas of river corridor may fail to respond to restoration because of dispersal limitation. | |
| Develop or refine statistical approaches for quantifying the relative importance of factors impacting colonization. For example, analysis of trait distributions present among organisms within the community may provide some inference. | Determine whether restrictions to colonization are trait-based or environment-based and develop strategies accordingly.
|
|
| Identify catchment-level stressors that may override local habitat restoration efforts: are local restoration approaches swamped by regional-scale issues, such as catchment water quality. Terrestrial taxa often respond more strongly to local river habitat restoration due to instream water quality issues (Pilotto et al., 2018). | If insufficient knowledge of the regional biota and connectivity is available, first efforts of restoration should focus on sites within regions of moderate impairment to maximize the probability for biological response (Stoll et al. 2016). | |
| Conservation | Develop tools and procedures for measuring connectivity and evaluating the relationship between connectivity and the health of native communities. | Measure connectivity within and among conservation sites and then strategically increase or decrease connectivity to meet management goals. |
| Develop conservation prioritization schemes that managers can use to optimize metacommunity connectivity and maximize resilience. | Prioritize explicit maintenance or restoration of habitat connectivity in pristine areas where human perturbations are low. | |
| Develop guidelines for using multi-species connectivity and dispersal analyses, such trait-based joint species distribution models or analyses of surrogate species, to assess connectivity priorities for conservation management plans (Meurant et al., 2018). | For multi-species conservation action plans, consider how optimal levels of connectivity may differ among target species. Maximizing connectivity for the poorest dispersers in a community may subject better dispersers to mass effects conditions, resulting in loss of rare taxa. | |
| Invasive Species | Empirically evaluate theoretical predictions about the relationship between sorting strength, native dispersal pressure, and habitat invasibility. Develop best practices for using variation partitioning and similar methods to identify the spatial configuration of local sorting strength. |
Determine the relative probability of invasion success among locations in the river network and help identify areas to prioritize efforts. |
| Incorporate dispersal data in models to determine the need/frequency for maintenance eradication visits. | Focus eradication efforts in habitats or locations where local control is strong. | |
| Develop methods for inferring the relative importance of factors that affect local control and build a system for calculating trade-offs between various habitat management actions. | Perform habitat management to increase the relative strength of local sorting, to create conditions where native communities have a higher probability of resisting invasions. This can be altering local habitat conditions or decreasing connectivity. |
3.1. Biomonitoring
In biological assessments, inferences regarding ecological status are based on ecological quality indices that reflect the magnitude of departure of observed communities from those expected to occur under reference (baseline) conditions (Hawkins, Olson, & Hill, 2010). The utility of biological assessments depends on how accurately and precisely assemblage composition expected under reference conditions (or the values of derived assemblage-level metrics) can be predicted from a combination of regional (e.g., climate, geology) and local factors (e.g., water chemistry, stream size,), and whether assemblage composition changes in response to anthropogenic disturbance. Both of these requirements rely on the assumption that local environmental and habitat conditions are the primary factors determining the specific taxa that occur in each waterbody. The tighter the association between local sorting and assemblage composition, the more accurately reference values can be predicted (Figure 1B). Prediction errors are often thought to be caused by failure to include important local environmental predictors, inaccuracies in the modeling method used to make predictions, or the inherent stochastic temporal variability of assemblage composition in natural ecosystems.
However, metacommunity theory predicts that the underlying assumption of strong local sorting is only expected to be true at intermediate levels of dispersal (Figure 1A). Therefore, measurements of dispersal or site connectivity may help clarify both the sources and magnitudes of prediction error. For example, in sufficiently connected sites, strong local sorting is expected to promote high predictability, while limited dispersal in poorly connected sites may reduce the precision of predictions. Strong dispersal, including mass effects, may also decrease predictability, but the effect on model precision is likely to be more variable (Ron, Fragman-Sapir, & Kadmon, 2018; Vannette & Fukami, 2017) depending on how closely the composition of the colonizing organisms match the predicted composition of the sink community. These predictions are supported by a small but growing body of evidence. For example, recent analyses show that the precision of multitaxon distribution models used to predict the taxa expected to occur at a site under natural conditions is higher in landscapes where waterbodies are more connected (Hawkins in prep). Model precision in more connected landscapes is higher than that in less connected landscapes (Hawkins in prep). Consequently, for predictive models developed for a large region, differences among sub-regions in connectivity could result in either type I or type II errors of inference when the model is applied to specific locations. Restricting the regional extent of assessment models based on differences in within-region connectivity and considering the degree to which study areas are fragmented may improve model accuracy and precision (Heino, 2013). Furthermore, the use of statistical approaches such as variation partitioning to infer the relative strength of local sorting within watersheds can help identify regions where local sorting is likely to be strong and prediction precision is expected to be high (Table 2, see example in section 4).
Similar dynamics may occur at finer spatial scales. Simulations show that biocriteria metrics—often termed “indices of biotic integrity”—can be affected by an interaction between impairment level, dispersal intensity, and the location of a site within a stream network (Siqueira, Duraes, & Roque, 2014). Strong, local stressors can overwhelm dispersal effects (Xiong et al., 2016), and pervasive stress across a region can decrease dispersal potential from the regional species pool to individual locations (Patrick & Swan, 2011). Concordantly, strong dispersal from healthy source populations can improve the appearance of environmentally degraded sites. These observations can inform management strategies and help us interpret biomonitoring data. For example, given that stream connectivity varies according to the centrality of the site in the network (Altermatt, Seymour, & Martinez, 2013; B.L. Brown & Swan, 2010), we might predict that biomonitoring index precision declines as you move to larger streams with higher connectivity. However, the balance between local and dispersal-driven assembly processes along the river corridor also depends on characteristics such as the type of organism (Tonkin et al., 2018). For instance, less mobile species may respond more strongly to site-to-site variation in environmental factors (Beisner, Peres-Neto, Lindström, Barnett, & Longhi, 2006), whereas organisms with many generations, such as multivoltine insects with an adult flying stage, may respond less strongly to the environment because they have more dispersal opportunities per year to spread across a region (Saito, Soininen, Fonseca-Gessner, & Siqueira, 2015). Explicitly including spatial predictor variables that represent dispersal strength (Cid et al., 2020), traits that define the spatial scale of organisms’ dispersal (Guzman et al., 2019), and accounting for the spatial structure of sampling sites (Grenouillet et al., 2008) may improve empirical models predicting community composition (Table 2). The degree to which these factors can be effectively quantified in assessments relies on access to data collected from a sufficiently dense sampling network.
3.2. Restoration
River restoration is costly, and these costs are not often translated into biological gains (Palmer, Menninger, & Bernhardt, 2010). The effectiveness of river restoration depends on many different factors, including the intensity of restoration actions, the geophysical setting, and the scale of restoration (Palmer et al., 2010). One overarching cause of restoration failures is a lack of attention to ecological theory (Palmer et al., 2010). Many river restoration projects focus on improving local conditions, often with the associated goal of a subsequent improvement in aquatic life. Dispersal dynamics are rarely considered (Swan & Brown, 2017), but dispersal constraints and barriers to recolonization are a frequent cause for a lack of biological response to habitat restoration (Sundermann et al., 2011; Tonkin, Stoll, Sundermann, & Haase, 2014). Many restoration projects fall into the trap of fixing local habitat conditions and assuming unlimited recolonization potential, an operational tactic referred to as the ‘Field of Dreams’ Hypothesis (Palmer et al., 1997). The role of dispersal limitation and impoverished regional species pools in limiting restoration success has led scientists to consider the reintroduction of whole communities as a mitigation tool following restoration (Jourdan et al., 2019). However, an alternative approach would be prioritizing locations known to have adequate dispersal or to actively increase spatial connectivity among sites that will be restored. In reviewing all of these scenarios, it becomes clear that incorporating metacommunity dynamics into river restoration planning is crucial for ensuring that biological communities responds as desired after restoration.
Experimental evidence suggests that catchment-scale influences can override local-scale restoration measures. For example, using experimental flume systems, Brown et al. demonstrated that while local habitat had a strong influence on both headwater and mainstem streams, augmented dispersal only affected communities colonized from headwater streams (Bryan L. Brown, Wahl, & Swan, 2018). A lack of mainstem response to dispersal indicated that those communities were already structured by dispersal-driven dynamics, while the headwater response to dispersal indicated that those systems were dispersal limited and open to new colonists through augmented dispersal (Bryan L. Brown et al., 2018). Similarly, Tornwall et al. demonstrated that manipulations of local habitat (substrate complexity) had stronger effects on headwater macroinvertebrate communities than on mainstem communities, presumably because dispersal-driven dynamics are more important to the assembly of mainstem communities than to headwater communities (B. M. Tornwall, Swan, & Brown, 2017). Therefore, considering the spatial setting into which restorations are placed (Leps, Sundermann, Tonkin, Lorenz, & Haase, 2016), is an important first step (Table 2).
Restoration in regions with either dispersal surplus or dispersal limitation may have limited success (Stoll, Breyer, Tonkin, Früh, & Haase, 2016), with the best locations for restoration situated in regions of intermediate habitat quality that are under the strongest local control (Fig. 1C, Table 2). Restoring stream reaches at well-spaced intervals (i.e. stepping stones) may also allow dispersal among restored habitats (Gellert, Behrens, & Raschke, 2012; Kail & Hering, 2009). For instance, recent work suggests that restoration may be more ecologically successful in headwaters where a greater congruence between species’ habitat needs and local habitat availability is expected. In contrast, dispersal-driven factors are likely to be more influential in more well-connected mainstems (Swan & Brown, 2017). In some cases, connectivity of the proposed restoration site may be increased or decreased to achieve a desirable balance between local and regional control via the removal (e.g., weirs, culverts) or engineering (e.g., plunges) of barriers (Fig. 1A., Fig. 4, Table 2). Previous work has demonstrated that distance to the nearest colonist source, whether along the river network or overland, is a key indicator of chance of restoration success for benthic invertebrates (Patrick & Swan, 2011; Tonkin et al., 2014), as is the occupancy rate in the regional species pool (Tonkin et al., 2014). Thus, assessing whether desired potential colonists are present in a watershed or in nearby watersheds is an important step before starting a restoration project (Table 2). Public monitoring data are often freely available to assess the regional species pool surrounding candidate restoration sites, particularly for fish, prior to physical restoration. If these data are not available, and if restoring biodiversity is a goal, an initial region-wide rapid assessment of streams may be necessary to ensure that potential colonists are present prior to performing the restoration.
Figure 4.

Spatial distribution of sorting strength measured at regional and local scales. A. Map of local sorting strength estimated for regions and mapped at the HUC4 basin scale across the continental United States. Darker blue sites have higher sorting strength. Black areas have no available data. B-E Relationships between different estimates of sorting strength and latitude and longitude across the continental United States. Regional scale estimates of sorting strength increase slightly as you move north (B., P < 0.001, R2 = 0.022, df = 2,1220) and toward the coasts (C., P < 0.001, R2 = 0.196, df = 2,1220) with the highest values in the Pacific Northwest and the Atlantic Northeast. Local scale estimates have no relationship with latitude (D.) or longitude (E.). F. Example map of spatial distribution of local sorting strength estimated at the site scale for streams with biomonitoring data in the lower Appalachian Mountains of the United States. Sorting strength is ranked as high (red), medium (violet), or low (blue). In this region, sorting strength is lower in upstream reaches.
3.3. Conservation
Conservation efforts have historically focused on the habitats of target species of concern with the assumption that actions that benefit these “umbrella” species will benefit other taxa in the habitat. One operational strategy is conserving local environmental conditions to reflect habitat needs of the target species. This strategy is most likely to be successful where local sorting control is high (Figure 1D). However, in recent years conservation efforts have increasingly focused on multiple species (Moilanen et al., 2005) and understanding the different connectivity needs of different species is a necessary component of effective conservation action (Albert, Rayfield, Dumitru, & Gonzalez, 2017). For habitat conservation plans that cover multiple species, this process entails conserving or restoring dispersal corridors favored by different taxa (Table 2). The classic determinations of “umbrella” species whose habitat needs guarantee local persistence do not necessarily extend to metacommunities, as maintaining persistence of species distributions and temporal dynamics across a metacommunity requires identifying a different or expanded set of traits among surrogate taxa (Meurant, Gonzalez, Doxa, & Albert, 2018). In the Western Riverside Multi-species Habitat Conservation Plan (California, USA), for example, planned corridor types provide connectivity for imperiled organisms inhabiting a large range of terrestrial, riparian, and instream communities (WRCRCA, 2003). In this context, metacommunity theory may assist conservation prioritization (B.L. Brown et al., 2011), as new approaches (see section 7) have been developed for determining the extent local sites are connected to regional dynamics at different temporal and/or spatial scales (Ruhi, Datry, & Sabo, 2017; Tonkin et al., 2014). Translating this information into concrete planning for resilient meta-communities is an area of ongoing management emphasis (Isaac et al., 2018).
In addition to promoting regional stability of focal species (Cavallaro, Vivian, & Hoback, 2015), increasing or decreasing connectivity among communities affects access to food resources, either through blocking subsidies or restricting wide ranging foraging (Cloern, 2007), and alters exposure to competition, predation, or disease (Rahel, 2013). Thus, failure to consider connectivity can lead to failure of conservation efforts. For example, a multi-million-dollar riparian project in the lower Colorado River failed to restore populations of endangered Southwestern Willow Flycatcher (Empidonax traillii extimus), a riparian obligate species, when important river processes that promoted floodplain connectivity and aquatic macroinvertebrate food subsidies to the riparian zone were not established (Rubin et al., 2018).
In pristine areas, managers may focus on maintaining longitudinal connectivity by preventing flow disruptions such as dams, culverts, and excessive water extraction, or by preserving riparian corridors. In regions with patches of threatened species, conservation actions that increase connectivity may be the best course of action (Fig. 1A). For example, in Japan (Figure 2A), ladders were widely installed on small agricultural dams to increase the regional functional connectivity of threatened salamanders (Taguchi & Natuhara, 2009). In the Santa Ana River, re-wetting portions of the river corridor and restoring historical flow regime in reaches dominated by sewage treatment outflows (Fig 2B) benefits many at-risk species by increasing habitat connectivity (ICFI, 2014).
Figure 2.

A) An endangered Japanese Giant salamander (Andrias japonicus) blocked by a dam in the Kiso River drainage in Gifu Province, Japan. Courtesy of Ito Yoshihiro. B) Restoring flow connectivity to the Santa Ana River has been vital to the survival of the endangered Santa Ana Sucker (Catostomus santaanae). C) Following the restoration of flows and native fish communities in Fossil Creek, AZ, a dam was constructed on the lower creek to prevent the recolonization of invasive fish from the downstream Verde River. Natural materials were included in the design of the fish barrier in order to preserve wilderness characteristic and recreational value.
In some management scenarios, however, it may be desirable for a manager to decrease connectivity, for example to restore natural disconnections or to prevent the spread of invasive species or disease to conserve native fauna (Strecker & Brittain, 2017). Barriers, such as dams and electric fences, and alterations to flow regimes are increasingly being used strategically to reduce connectivity among communities (Rahel, 2013). After the restoration of flows and native fish populations to Fossil Creek in central Arizona, USA, a fish barrier was constructed by the United States Bureau of Reclamation to exclude invasive species common in nearby Verde Creek (Fig 2C, (Marks, Haden, O’Neill, & Pace, 2010). In the Great Lakes, a number of non-physical barriers including electric fences, bubble curtains, and acoustic deterrents have been installed to limit the dispersal of Asian Carp into bays and canals with high value to commercial fisheries (Noatch & Suski, 2012). In addition to creating artificial barriers, naturally low connectivity created by intermittent flows in xeric regions or natural debris dams in forested catchments can be conserved or restored. These types of periodic natural barriers not only limit the movement of undesirable species or diseases, but they also change local sorting strength within communities and so can be used to manipulate local control to benefit native communities (Table 2).
3.4. Invasive Species Management
One of the most pernicious threats to native aquatic biodiversity and ecosystem functioning is invasive species (Thomaz, Kovalenko, Havel, & Kats, 2015). The threats posed by aquatic invasives are well documented from both a biological and economic perspective (Lovell & Stone, 2005). Yet, management of aquatic invasives is in its infancy both in terms of development as a discipline (N’Guyen, Hirsch, Adrian-Kalchhauser, & Burkhardt-Holm, 2016), and in approaches to prevention, control, and eradication of invaders [(Sepulveda et al., 2014) but see (Chen & Olden, 2017)]. While the problems presented by aquatic invaders are multifaceted and inherently interdisciplinary (Crowely, Hinchcliff, McDonald, & lee, 2017), both theory and preliminary empirical work suggest that a metacommunity approach to understanding invasions may provide insights on prevention and management.
The only explicit metacommunity framework presented to date for aquatic invasives suggests that two factors control invasion success: empty or weakly occupied niches on landscapes and propagule pressure (Howeth & Leibold, 2010). Two major themes emerge from this framework: (i) invasibility decreases as the relative importance of local sorting strength increases (Figure 1E); (ii) invasibility decreases with increased dispersal of natives.
From the perspective of the local community, high local diversity has traditionally been assumed to be more likely to repel invaders (Elton, 1958; MacArthur & Levins, 1967), an idea that has come to be known as the Biotic Resistance Hypothesis (BRH). The BRH has clear links with the Howeth & Leibold (2010) framework, which suggests that invasion success is low where local sorting is strong. However, tests of the BRH have shown mixed support and high degrees of context dependence (Howeth, 2017; Levine & D’Antonio, 1999; Shurin, Havel, Leibold, & Pinel-Alloul, 2000). That context, in many cases, depends on rate and distance of dispersal (Shurin et al. 2000, Howeth 2017). While the widespread dispersal of invasives can be damaging (Sinclair et al. 2015), the dispersal of natives can provide resistance to invasion (Howeth, 2017; Shurin et al., 2000).
Stream and river corridors are important invasion pathways, and the structure of river systems is one factor that can dictate rate and extent of spread (Rinaldo, Gatto, & Rodriguez-Iturbe, 2018). From the perspective of preventing or controlling invasions, dispersal can act both positively and negatively in invasion scenarios, depending on which species are dispersing and how (Sinclair, Furlanetto, & Arnott, 2015). Dispersal acts positively by increasing invasion resistance in native communities (Howeth, 2017), or by promoting the recovery of native species after invasion (Sinclair et al., 2015). However, dispersal can also act negatively when invaders are rapidly spread (Sinclair et al., 2015). These results suggest that activities like dam removal, which are oft-used restoration strategies, can actually benefit invaders by removing barriers to dispersal. Thus, the use of metacommunity theory in confronting invasions relies on a) identifying whether the forces driving invasion are local or regional, and b) implementing control measures based on that relative balance (Table 2).
Statistical approaches that assess the degree of local control in ecosystems can be used in conjunction with riverine biomonitoring data to map out areas of high and low invasion risk (Table 2, see example section 7.). A high degree of resistance to invasion would be expected where natives are the primary dispersers (Howeth, 2017). Mouillot (2007) suggests that when local sorting is the major driving force in structuring fish metacommunities, habitat restoration and preservation are the most effective strategies for dealing with invasive species. However, in systems where community dynamics are more dependent on dispersal, focusing on actions that alter landscape-scale connectivity to the benefit of native species is more important (Mouillot 2007).
4. PUTTING IT TOGETHER: THEORY AND PRACTICE
Looking across these areas of riverine management, several key themes emerge. Regardless of the goal, projects should begin with an assessment of the spatial context of the focal habitats. The first step of this process is defining the boundaries of the region that include the site or sites of interest (e.g., watershed; catchment) and then assembling community data that is spatially distributed across the region. If no such data exists, new spatially balanced surveys can be designed and implemented, however the importance of stream invertebrates for aquatic ecosystem health assessments usually results in such data already being available from government or non-profit entities for these taxa. Once assembled, the data are used to assess spatial connectivity such as network density or centrality (Estrada & Bodin, 2008), the presence and distribution of dispersal barriers such as dams and road crossings (Clarkin et al., 2003; Januchowski-Hartley et al., 2013), the dispersal modes and abilities of the target species (Poff et al., 2006; Radinger & Wolter, 2014) and the size and compositional identity of the regional species pool. Multivariate analyses may be used to assess the relative strength of local and regional processes in driving community assembly (P. Legendre et al., 2005). Armed with this information, managers can determine how metacommunity context may influence the likelihood of success for different management actions in different locations and use that information to inform decision making (Figure 3). If the metacommunity context is unfavorable for a proposed project – e.g., local sorting is relatively weak or spatial connectivity is low, managers can then choose to move the spatial location of the project, change their strategy, identify ways to alter the metacommunity context to maximize success, or understand at the outset that the ecological community may not change or “improve” (Figure 3).
Figure 3.

Decision tree for incorporating metacommunity theory into management activities. 1) Work begins with surveys or statistical analysis of existing biomonitoring datasets to determine the spatial context of the proposed project. This may include assessing factors like connectivity, dispersal ability of organisms, regional species pools, and the relative importance of local sorting for target communities. 2) Determine whether the current spatial context will prevent management goals from being met. For example, a stream restoration in a site that is under strong regional control (dispersal) from degraded sites is unlikely to result in the assembly of desirable biotic community after the restoration is complete. 3) If there are no problems presented by spatial context, proceed with management plan (3a); however, if there are issues managers can either attempt to change the spatial context (3b-1), change the plan (3b-2), or move the project location (3b-3). In each of these cases (3b-1–3), the managers must then return to step 1 or step 2. For example, if spatial context is manipulated by removing barriers to increase connectivity, post-removal conditions should be assessed to determine how much the manipulation changed connectivity. This is a crucial step before proceeding to 3a because not all manipulations may be successful.
Some of these suggested analyses are conceptually easy to implement, consisting of visually assessing spatially mapped data to evaluate patterns in taxonomic richness, biodiversity, and composition and locating dispersal barriers. However, quantitative assessment of local sorting strength and dispersal is more statistically complex. In the next sections we demonstrate how spatially distributed community and environmental data can inform both regional and site-level estimates of the strength of local sorting in streams and discuss basic considerations in the choice of statistical analysis. We showcase two different example analytical frameworks: variation partitioning, a common approach to assessing regional variation in the strength of local sorting (B.A. Hawkins 2012), and a newer framework developed by Blonder et al. to evaluate the degree of local sorting strength within an individual habitat (2015). These analyses are by no means exhaustive and many advanced methods of analysis exist. However, they do represent an introduction to commonly used techniques and illustrate how signals of metacommunity processes may emerge in commonly collected data.
4.1. Example of regional scale analysis of sorting strength
To illustrate the utility of quantitative analyses of sorting strength, we apply variance partitioning to continental-scale biomonitoring data collected from streams of the United States. Variation partitioning is used in spatial analysis to separate variation in biological data into parts explained by E) the non-spatial environment, E-S) spatial structured environmental variation, S) spatial variation of the target variables that is not shared by the environmental variables, with the remainder referred to as unexplained variation (Pierre Legendre, 1993). (Gilbert & Bennett, 2010, Clappe, Dray & Peres Neto, 2018). In metacommunity analysis, portions of variation explained by the environment are often interpreted as being caused by species sorting, but two aspects of the analysis should be considered when interpreting the results. First, portion S is often interpreted as representing “pure space” or “spatial processes” but the S fraction of variation also includes variation associated with unmeasured, spatially structured environmental gradients (B.A. Hawkins 2012). Second, portion E-S can capture spatial correlations between species distributions caused by dispersal limitation and the spatial environmental that are not causally linked, leading researchers to incorrectly infer that all variation in this category is caused by spatially structured environment (Clappe, Dray, & Peres-Neto, 2018). This can increase proportion of variation explained in E-S portion to be on average 25% greater than that actually caused by the spatially structured environment (Gilbert & Bennett, 2010). Despite these issues, variation partitioning remains preferable to other statistical approaches (Gilbert & Bennett, 2010), and recent updates to the method can help with interpretation of the E-S fraction of variation (Clappe et al., 2018). For example, Gilbert & Bennett recommend using the approach but paying close attention to the chosen scale of the analysis to ensure it biologically meaningful and running secondary analyses or experiments to confirm the observed results (2010).
We applied variance partitioning to overlapping regional zones resampled from a continental-scale data set (Patrick & Yuan 2018). Application of variance partitioning to regional zones allows us to investigate dynamics at spatial scales that are much smaller than the size of the study area. We analysed biomonitoring data from the United States Environmental Protection Agency (USEPA) National Rivers and Streams Assessment (NRSA) 2007 (USEPA, 2016). NRSA employs a spatially balanced sampling design to survey wadeable, perennial streams of the continental United States and is a robust dataset for evaluating large scale patterns in sorting strength. The dataset included 1269 stream sites with both macroinvertebrate community data and associated environmental data (2010). We used the complete suite of environmental variables provided in the NRSA dataset, including habitat and water quality measurements collected in-situ. We estimated variance components at a spatial scale of 500km. We used the varpart() and pcnm() functions in the vegan library in the statistical program R (R Core Team, 2016) for the variation partitioning analysis performed on each replicate sub-sample (Oksanen et al., 2014). We averaged the output values by river basins that were classified as level four according to the United States Geological Survey Hydrologic Unit Code system.
4.2. Example of local scale analysis of sorting strength
For the local-scale analysis, we used the same dataset but applied an analysis framework developed by Blonder et al. (2015) to infer the relative strength of local control on stream communities at the site level. The approach uses the distributional data for species and environmental conditions to develop expected niche distributions for each species in multivariate environmental space. Expected environmental conditions in each location are generated from the observed environmental distributions of each observed species and then the volume of niche space is calculated from distance among species in n-dimensional environmental space (Blonder et al. 2015). Predicted environmental conditions are compared to the observed environmental conditions to determine the degree of match between community and environment. Deviations between the expected and observed environmental conditions and the multivariate volume of niche space occupied by the community can then be used to infer whether the community is being constrained by local environmental conditions or composed of taxa that we would not have expected to observe (Blonder et al. 2015).
For this analysis we focused on a subset of environmental variables [conductivity (μs/cm), turbidity (NTU), pH, dissolved oxygen (mg/L), NO3− (mg/L), and NH4+ (mg/L)] because they often exhibit strong associations with macroinvertebrate community composition. Within each level III ecoregion, a separate model using all local scale observations (n=96–250 per region) was developed for taxa – habitat associations. These associations were then used to calculate the volume deviation for each stream community. Values greater than one indicate that the community was representing a larger niche volume than was expected by the observed environmental conditions, suggesting that the site was environmentally permissive, i.e. local conditions were not controlling community composition. Statistical distributions of the volume deviation were compared among ecoregions. The relationship between volume deviation and watershed size within and among regions were then assessed to evaluate the previously mentioned hypothesis that strength of local sorting decreases as stream size increases. All analyses were performed using functions in the comclim library (Blonder et al. 2015) in the statistical program R.
4.3. Interpretation and application
At the regional scale, we observed significant variation in the estimated degree of local sorting (Fig 4A). Sorting strength was more variable in western regions with separation between southern areas (low sorting) and northern areas (high sorting). In eastern regions, sorting strength increased toward the east and north (Fig 4B, 4C). In contrast, for local scale measurements sorting strength did not follow broad geographic patterns (Fig 4D, 4E), but did exhibit spatial structuring within regions (Fig 4F). For 36% of the streams in the dataset, processes other than local sorting such as dispersal pressure were determined to be the dominant forces structuring the local communities (Fig 5A). Within ecoregions, we observed that local sorting strength declined with increasing stream order in five (Northern Appalachians, Northern Plains, Southern Plains, Western Mountains, and Xeric) of the nine ecoregions, comprising approximately 60% of the coterminous United States (Fig 5C).
Figure 5.

Relationships between stream size, ecoregion, and strength of local sorting. A. Distribution of the deviation from expected community based on species-habitat associations among ecoregions of the United States (CPL = Coastal Plains, NAP = Northern Appalachians, NPL = North Plains, SAP = Southern Appalachians, SPL = Southern Plains, TPL = Temperate Plains, UMW = Upper Midwest, WMT = Western Mountains, XER = Xeric). Values with a magnitude greater than zero indicate that communities are not being structured by local environmental variables. B. Map of ecoregions of the United States. Ecoregion colors correspond to colors in other panels. C. Relationship between stream watershed size and community habitat volume deviation within ecoregions (individual lines) of the United States. Left plot shows ecoregions with positive relationships, indicating that, as predicted by theory, sorting strength declines with stream size (NAP, NPL, SPL, WMT, UMW, XER). Right plot shows ecoregions with negative relationships, indicating that sorting strength increases with stream size (CPL, SAP, TPL).
The results demonstrate that stream communities are not always dominated by local sorting processes and considering the dependence of these processes on spatial scale. These results also provide some actionable information that could be useful when planning management activities. Most US wadeable streams were found to be under sufficiently strong local sorting to be considered under “local control”, but in a substantial proportion of streams, environmental conditions were not the dominant force shaping local communities. In this second group of streams, classic biomonitoring methods may be less informative and approaches that incorporate additional spatial predictors may be useful additions to the management toolbox. Interpretation of this analysis supports the notion that application of metacommunity theory could be an important enhancement to existing methods, particularly in the south western United States where local control was observed to be weakest. Similarly, based on these analyses it appears that in at least some ecoregions, restoration activities are more likely to be effective in headwater streams than mainstems, agreeing with published case studies (Swan & Brown 2017).
While these data show some biogeographic patterns in sorting strength, we caution that the estimates are likely scale dependent. While regional estimates show some agreement with local estimates, individual streams may be more influenced by dispersal even within regions where local control is more prevalent. Hence, consideration of multi-scale processes is critical when applying these approaches to enhance ecosystem management. Furthermore, characteristics of each system can be unique, and we therefore suggest that practitioners design a study specifically for their region and study question to develop an understanding of the unique spatial context that is relevant for their project and goals.
Conclusion
Incorrect inferences regarding ecological condition and the causes of degradation can result in management actions that do not yield the desired outcomes. Explicit incorporation of the metacommunity framework can improve inferences by helping increase our understanding of the causes of community assembly within and among streams, and ultimately enhance many aspects of riverine management including bioassessment, restoration, conservation, and invasive species management. Understanding all of the causes of variability in biological reference condition among sites, watersheds, and regions improves our ability to characterize the uncertainty in biological assessments. Understanding the processes that structure communities allows us to determine when, where, and how to implement management interventions for conservation, restoration, and invasive species management, such that the actions have a higher probability of successful outcomes and a greater return on investment. This improved understanding will also allow us to ascertain when project goals for a given system may be unachievable due to logistical and financial constraints.
One of the challenges to better bridge metacommunity theory to river management is developing tools that can effectively operationalize metacommunity theory. We have described some analytical and practical approaches that can be employed immediately, but also a series of research questions that remain to be answered, showing that there is much work that remains to be done. For example, adjusting scoring tools can help account for differences among waterbodies in the relative importance of regional vs local processes. Similarly, modifications might be made to indices of biotic integrity that incorporate organism dispersal ability into the weighting and scoring formulas. However, we might also need new approaches and analytical tools that better incorporate metacommunity dynamics into river management. The ideas presented in Table 2 and throughout the manuscript are a first step down that path of expanding our toolbox of approaches, and in reviewing them, there are clear commonalities for how metacommunity theory influences management (Figure 3). In all cases, the first steps are the evaluation of metacommunity processes in the system using available or newly collected data and using the information gained from those investigations to inform the next steps in the process.
The field of applied metacommunity ecology is still emerging, and many questions remain regarding how to use these ideas to enhance existing management strategies. Operationalizing many of the recommendations in Table 2 will require applied ecologists and ecosystem managers to work together to develop specific approaches and tools for different situations. There must be an open flow of information between practitioners and theoretical ecologists, and as the theory and analysis frameworks continue to develop, so too must the applications. This will be a challenging task and change will not occur overnight. Rather we foresee a slow and incremental shift occurring in steps. Early partnerships that apply these ideas and demonstrate successful outcomes will act as case studies for others to emulate. Indeed, several of these already exist in terrestrial ecosystems such as the incorporation of metacommunity theory into the development of Montreal’s plan for a network of protected habitats (Albert et al. 2017), but there is room for more innovative trail blazers in freshwater ecosystems. Ultimately we believe that this effort will increase the effectiveness of riverine ecosystem management and that the lessons learned will have value across a wide variety of other ecosystem types.
Acknowledgments
Special thanks to the Society for Freshwater Science Annual Meeting for supporting the special session “Practical applications of metacommunity theory in the management of stream and riverine ecosystems” at the 2017 meeting in Raleigh, North Carolina. This manuscript would not have been possible without the session to bring the authors and co-presenters together and the collegial environment of the meeting to facilitate brainstorming and new collaborations. We thank Peter Ode for reviewing an early version of this manuscript. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Funding Information
TS thanks the São Paulo Research Foundation (FAPESP, grant #2013/50424-1) that partially funded his travel costs to attend the meeting. BLB, CMN, and KEA acknowledge funding support from NSF award DEB-1655764. CJP acknowledges support from NASEM Gulf Research Fellowship Program and support from NSF award DEB-1927645. JDT is supported by a Rutherford Discovery Fellowship administered by the Royal Society Te Apārangi (RDF-18-UOC-007). CPH was supported by NSF award IOS-1456278NSF.
Contributor Information
Christopher J. Patrick, Department of Biological Sciences, Virginia Institute of Marine Science, College of William and Mary, 1370 Greate Rd., Gloucester Point, VA 23062.
Kurt E. Anderson, Department of Evolution, Ecology, and Organismal Biology, 900 University Ave., University of California, Riverside, CA, 92521, USA.
Brown L. Brown, Department of Biological Sciences, Virginia Tech, Blacksburg, Virginia 24060, USA
Charles P. Hawkins, Department of Watershed Sciences, Ecology Center, and National Aquatic Monitoring Center, Utah State University, Logan, Utah, USA
Anya Metcalfe, United States Geological Survey, Grand Canyon Monitoring and Research Center, 2255 North Gemini Drive, Flagstaff, AZ 86001.
Parsa Saffarinia, Department of Wildlife, Fish, and Conservation Biology, University of California, Davis, CA, 95616, USA.
Tadeu Siqueira, Institute of Biosciences, São Paulo State University (Unesp), Av. 24A 1515, Rio Claro, São Paulo 13506-900 Brazil.
Christopher M. Swan, University of Maryland, Baltimore County, Baltimore, MD 21250 USA
Jonathan D. Tonkin, School of Biological Sciences, University of Canterbury, Private Bag 4800, Christchurch 8140, New Zealand
Lester L. Yuan, United States Environmental Protection Agency – Office of Water
References
- Albert CH, Rayfield B, Dumitru M, & Gonzalez A (2017). Applying network theory to prioritize multispecies habitat networks that are robust to climate and land-use change. Conservation Biology, 31(6), 1383–1396. doi:doi: 10.1111/cobi.12943 [DOI] [PubMed] [Google Scholar]
- Altermatt F, Seymour M, & Martinez N (2013). River network properties shape α-diversity and community similarity patterns of aquatic insect communities across major drainage basins. Journal of Biogeography, 40(12), 2249–2260. doi:doi: 10.1111/jbi.12178 [DOI] [Google Scholar]
- Beisner BE, Peres-Neto PR, Lindström ES, Barnett A, & Longhi ML (2006). THE ROLE OF ENVIRONMENTAL AND SPATIAL PROCESSES IN STRUCTURING LAKE COMMUNITIES FROM BACTERIA TO FISH. Ecology, 87(12), 2985–2991. doi:doi: 10.1890/0012-9658(2006)87[2985:TROEAS]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Brown BL, & Swan CM (2010). Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology, 79(3), 571–580. [DOI] [PubMed] [Google Scholar]
- Brown BL, Swan CM, Auerbach DA, Grant EHC, Hitt NP, Maloney KO, & Patrick C (2011). Metacommunity theory as a multispecies, multiscale framework for studying the influence of river network structure on riverine communities and ecosystems. Journal of the North American Benthological Society, 30(1), 310–327. [Google Scholar]
- Brown BL, Swan CM, Auerbach DA, Grant EHC, Hitt NP, Maloney KO, and Patrick C. (2011). Metacommunity theory as a multispecies, multiscale framework for studying the influence of river network structure on riverine communities and ecosystems. Journal of the North American Benthological Society 30, 310–327. [Google Scholar]
- Brown BL, Wahl C, & Swan CM (2018). Experimentally disentangling the influence of dispersal and habitat filtering on benthic invertebrate community structure. Freshwater Biology, 63(1), 48–61. doi: 10.1111/fwb.12995 [DOI] [Google Scholar]
- Carlisle DM, Wolock DM, & Meador MR (2010). Alteration of streamflow magnitudes and potential ecological consequences: a multiregional assessment. Frontiers in Ecology and the Environment, 9(5), 264–270. [Google Scholar]
- Cavallaro MC, Vivian LA, & Hoback WW (2015). Aquatic vertebrate predation threats to the Platte River caddisfly (Trichoptera: Limnephilidae). Florida Entomologist, 98(1), 152–156. Retrieved from <Go to ISI>://WOS:000352087500025 [Google Scholar]
- Chen W, & Olden JD (2017). Designing flows to resolve human and environmental water needs in a dam-regulated river. Nature Communications, 8(1), 2158. doi: 10.1038/s41467-017-02226-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cid N, Bonada N, Heino J, Cañedo-Argüelles M, Crabot J, Sarremejane R, … Datry T (2020). A Metacommunity Approach to Improve Biological Assessments in Highly Dynamic Freshwater Ecosystems. Bioscience. doi: 10.1093/biosci/biaa033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clappe S, Dray S, & Peres-Neto PR (2018). Beyond neutrality: disentangling the effects of species sorting and spurious correlations in community analysis. Ecology, 99(8), 1737–1747. doi: 10.1002/ecy.2376 [DOI] [PubMed] [Google Scholar]
- Clarkin K, Connor A, Furniss MJ, Gubernik B, Love M, & Moynan K (2003). National Inventory and Assessment Procedure for Identifying Barriers to Aquatic Organism Passage at Road-stream Crossings. Retrieved from San Dimas Technology and Development Center, San Dimas, CA: [Google Scholar]
- Cloern JE (2007). Habitat connectivity and ecosystem productivity: Implications from a simple model. American Naturalist, 169(1), E21–E33. doi: 10.1086/510258 [DOI] [PubMed] [Google Scholar]
- Costello EK, Stagaman K, Dethlefsen L, Bohannan BJM, & Relman DA (2012). The Application of Ecological Theory Toward an Understanding of the Human Microbiome. Science, 336(6086), 1255–1262. doi: 10.1126/science.1224203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowely S, Hinchcliff S, McDonald R, & lee T (2017). Invasive species management will benefit from social impact assessment. Journal of Applied Ecology, 54, 351–357. [Google Scholar]
- Davis W, & Simon T (1995). Biological Assessment and Critera: Tools for Water Resource Planning and Decision Making. Boca Raton: CRC Press. [Google Scholar]
- Elton CS (1958). The ecology of invasions by animals and plants: The University of Chicago Press. [Google Scholar]
- Estrada E, & Bodin Ö (2008). USING NETWORK CENTRALITY MEASURES TO MANAGE LANDSCAPE CONNECTIVITY. Ecological Applications, 18(7), 1810–1825. doi:doi: 10.1890/07-1419.1 [DOI] [PubMed] [Google Scholar]
- Gellert G, Behrens S, & Raschke M (2012). The return of degraded stream ecosystems by using positive impacts from near-natural sections: a new practical guide for restorations. Water and Environment Journal, 26(3), 415–421. doi:doi: 10.1111/j.1747-6593.2012.00307.x [DOI] [Google Scholar]
- Gilbert B, & Bennett JR (2010). Partitioning variation in ecological communities: do the numbers add up? Journal of Applied Ecology, 47(5), 1071–1082. [Google Scholar]
- Grenouillet G, Brosse S, Tudesque L, Lek S, Baraillé Y, & Loot G (2008). Concordance among stream assemblages and spatial autocorrelation along a fragmented gradient. Diversity and Distributions, 14(4), 592–603. doi:doi: 10.1111/j.1472-4642.2007.00443.x [DOI] [Google Scholar]
- Guzman LM, Germain RM, Forbes C, Straus S, O’Connor MI, Gravel D, … Thompson PL (2019). Towards a multi-trophic extension of metacommunity ecology. Ecology Letters, 0(0). doi:doi: 10.1111/ele.13162 [DOI] [PubMed] [Google Scholar]
- Hampton SE, Holmes EE, Scheef LP, Scheuerell MD, Katz SL, Pendleton DE, & Ward EJ (2013). Quantifying effects of abiotic and biotic drivers on community dynamics with multivariate autoregressive (MAR) models. Ecology, 94(12), 2663–2669. [DOI] [PubMed] [Google Scholar]
- Hawkins CP, Olson JR, & Hill RA (2010). The reference condition: predicting benchmarks for ecological and water-quality assessments. Journal of the North American Benthological Society, 29(1), 312–343. doi: 10.1899/09-092.1 [DOI] [Google Scholar]
- Hedrick RP, Adkison MA, El-Matbouli M, & MacConnell E (1998). Whirling disease: re-emergence among wild trout. Immunological Reviews, 166(1), 365–376. doi:doi: 10.1111/j.1600-065X.1998.tb01276.x [DOI] [PubMed] [Google Scholar]
- Heino J (2013). The importance of metacommunity ecology for environmental assessment research in the freshwater realm. Biological Reviews, 88(1), 166–178. doi: 10.1111/j.1469-185X.2012.00244.x [DOI] [PubMed] [Google Scholar]
- Hilderbrand RH, Watts AC, & Randle AM (2005). The Myths of Restoration Ecology. Ecology and Society, 10(1). [Google Scholar]
- Howeth JG (2017). Native species dispersal reduces community invasibility by increasing species richness and biotic resistance. Journal of Animal Ecology, 86(6), 1380–1393. doi: 10.1111/1365-2656.12733 [DOI] [PubMed] [Google Scholar]
- Howeth JG, & Leibold MA (2010). Species dispersal rates alter diversity and ecosystem stability in pond metacommunities. Ecology, 91, 2727–2741. [DOI] [PubMed] [Google Scholar]
- ICFI. (2014). Phase 1 Report: Upper Santa Ana River Habitat Conservation Plan. March. (ICF 00455.13) Redlands, CA. Prepared for San Bernardino Valley Municipal Water District, San Bernardino, CA. Retrieved from [Google Scholar]
- Isaac NJB, Brotherton PNM, Bullock JM, Gregory RD, Boehning-Gaese K, Connor B, … Mace GM (2018). Defining and delivering resilient ecological networks: Nature conservation in England. Journal of Applied Ecology, 55(6), 2537–2543. doi: 10.1111/1365-2664.13196 [DOI] [Google Scholar]
- Januchowski-Hartley SR, McIntyre PB, Diebel M, Doran PJ, Infante DM, Joseph C, & Allan JD (2013). Restoring aquatic ecosystem connectivity requires expanding inventories of both dams and road crossings. Frontiers in Ecology and the Environment, 11(4), 211–217. doi:doi: 10.1890/120168 [DOI] [Google Scholar]
- Jourdan J, Plath M, Tonkin JD, Ceylan M, Dumeier AC, Gellert G, … Haase P (2019). Reintroduction of freshwater macroinvertebrates: challenges and opportunities. Biological Reviews, 0(0). doi:doi: 10.1111/brv.12458 [DOI] [PubMed] [Google Scholar]
- Kail J, & Hering D (2009). The influence of adjacent stream reaches on the local ecological status of Central European mountain streams. River Research and Applications, 25(5), 537–550. doi:doi: 10.1002/rra.1238 [DOI] [Google Scholar]
- Knopf FL, Johnson RR, Rich T, Samson FB, & Szaro RC (1988). Conservation of Riparian Ecosystems in the United States. The Wilson Bulletin, 100(2), 272–284. Retrieved from http://www.jstor.org/stable/4162566 [Google Scholar]
- Lake PS, Bond N, & Reich P (2007). Linking ecological theory with stream restoration. Freshwater Biology, 52(4), 597–615. doi:doi: 10.1111/j.1365-2427.2006.01709.x [DOI] [Google Scholar]
- Legendre P (1993). Spatial Autocorrelation: Trouble or New Paradigm? Ecology, 74(6), 1659–1673. doi: 10.2307/1939924 [DOI] [Google Scholar]
- Legendre P, Borcard D, & Peres-Neto PR (2005). Analyzing beta diversity: partitioning the spatial variation of community composition data. Ecological Monographs, 75, 435–450. [Google Scholar]
- Legendre P, & Legendre LFJ (2012). Numerical ecology (Vol. 24): Elsevier. [Google Scholar]
- Leibold MA, & Chase JM (2018). Metacommunity Ecology (Vol. 59): Princeton University Press. [Google Scholar]
- Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, … Tilman D (2004). The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters, 7(7), 601–613. [Google Scholar]
- Leps M, Sundermann A, Tonkin JD, Lorenz AW, & Haase P (2016). Time is no healer: increasing restoration age does not lead to improved benthic invertebrate communities in restored river reaches. Science of the Total Environment, 557–558, 722–732. doi: 10.1016/j.scitotenv.2016.03.120 [DOI] [PubMed] [Google Scholar]
- Levine J, & D’Antonio C (1999). Elton Revisited: A Review of Evidence Linking Diversity and Invasibility. Oikos, 87(1), 15–26. [Google Scholar]
- Logue JB, Mouquet N, Peter H, & Hillebrand H (2011). Empirical approaches to metacommunities: a review and comparison with theory. Trends in Ecology & Evolution, 26(9), 482–491. doi: 10.1016/j.tree.2011.04.009 [DOI] [PubMed] [Google Scholar]
- Lovell S, & Stone S (2005). The economic impacts of aquatic invasive species: A review of the literature. Retrieved from Washington, DC: [Google Scholar]
- MacArthur RH, & Levins R (1967). Limiting Similarity Convergence and Divergence of Coexisting Species. American Naturalist, 101(921), 377–385. Retrieved from <Go to ISI>://A1967A307700002 [Google Scholar]
- Marks JC, Haden GA, O’Neill M, & Pace C (2010). Effects of Flow Restoration and Exotic Species Removal on Recovery of Native Fish: Lessons from a Dam Decommissioning. Restoration Ecology, 18(6), 934–943. doi:doi: 10.1111/j.1526-100X.2009.00574.x [DOI] [Google Scholar]
- Meurant M, Gonzalez A, Doxa A, & Albert CH (2018). Selecting surrogate species for connectivity conservation. Biological Conservation, 227, 326–334. doi: 10.1016/j.biocon.2018.09.028 [DOI] [Google Scholar]
- Moilanen A, Franco AMA, Early RI, Fox R, Wintle B, & Thomas CD (2005). Prioritizing multiple-use landscapes for conservation: methods for large multi-species planning problems. Proceedings of the Royal Society B: Biological Sciences, 272(1575), 1885–1891. doi: 10.1098/rspb.2005.3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Guyen A, Hirsch PE, Adrian-Kalchhauser I, & Burkhardt-Holm P (2016). Improving invasive species management by integrating priorities and contributions of scientists and decision makers. Ambio, 45(3), 280–289. doi: 10.1007/s13280-015-0723-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noatch MR, & Suski CD (2012). Non-physical barriers to deter fish movements. Environmental Reviews, 20(1), 71–82. doi: 10.1139/a2012-001 [DOI] [Google Scholar]
- Oksanen J, Guillaume Blanchet F, Kindt R, Legendre P, Minchin PR, O’Hara RB, … Wagner H (2014). Vegan: community ecology package. Version 2.1–41. In (pp. http://r-forge.r-project.org/projects/vegan).
- Palmer MA, Ambrose RF, & Poff NL (1997). Ecological Theory and Community Restoration Ecology. Restoration Ecology, 5(4), 291–300. doi:doi: 10.1046/j.1526-100X.1997.00543.x [DOI] [Google Scholar]
- Palmer MA, Menninger HL, & Bernhardt E (2010). River restoration, habitat heterogeneity and biodiversity: a failure of theory or practice? Freshwater Biology, 55(s1), 205–222. doi:doi: 10.1111/j.1365-2427.2009.02372.x [DOI] [Google Scholar]
- Patrick CJ, & Swan CM (2011). Reconstructing the assembly of a stream-insect metacommunity. Journal of the North American Benthological Society, 30, 259–272. [Google Scholar]
- Pilotto F, Tonkin JD, Januschke K, Lorenz AW, Jourdan J, Sundermann A, … Haase P (2018). Diverging response patterns of terrestrial and aquatic species to hydromorphological restoration. Conservation Biology, 0(0). doi:doi: 10.1111/cobi.13176 [DOI] [PubMed] [Google Scholar]
- Poff NL, Olden JD, Vieira NKM, Finn DS, Simmons MP, & Kondratieff BC (2006). Functional trait niches of North American lotic insects: traits-based ecology applications in light of phylogenetic relationships. Journal of the North American Benthological Society, 25(4), 730–755. [Google Scholar]
- R Core Team. (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. In: ISBN 3-900051-07-0. [Google Scholar]
- Radinger J, & Wolter C (2014). Patterns and predictors of fish dispersal in rivers. Fish and Fisheries, 15(3), 456–473. doi: 10.1111/faf.12028 [DOI] [Google Scholar]
- Rahel FJ (2013). Intentional Fragmentation as a Management Strategy in Aquatic Systems. BioScience, 63(5), 362–372. doi: 10.1525/bio.2013.63.5.9 [DOI] [Google Scholar]
- Rinaldo A, Gatto M, & Rodriguez-Iturbe I (2018). River networks as ecological corridors: A coherent ecohydrological perspective. Advances in Water Resources, 112, 27–58. doi: 10.1016/j.advwatres.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron R, Fragman-Sapir O, & Kadmon R (2018). Dispersal increases ecological selection by increasing effective community size. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1812511115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin Z, Rios-Touma B, Kondolf GM, Power ME, Saffarinia P, & Natali J (2018). Using prey availability to evaluate Lower Colorado River riparian restoration. Restoration Ecology, 0(0). doi:doi: 10.1111/rec.12829 [DOI] [Google Scholar]
- Ruhi A, Datry T, & Sabo JL (2017). Interpreting beta diversity components over time to conserve metacommunities in highly-dynamic ecosystems. Conservation Biology. [DOI] [PubMed] [Google Scholar]
- Saito VS, Soininen J, Fonseca-Gessner AA, & Siqueira T (2015). Dispersal traits drive the phylogenetic distance decay of similarity in Neotropical stream metacommunities. Journal of Biogeography, 42(11), 2101–2111. doi:doi: 10.1111/jbi.12577 [DOI] [Google Scholar]
- Schiesari L, Matias MG, Prado PI, Leibold MA, Albert CH, Howeth JG, … Vázquez DP (2019). Towards an applied metaecology. Perspectives in Ecology and Conservation, 17(4), 172–181. doi: 10.1016/j.pecon.2019.11.001 [DOI] [Google Scholar]
- Sepulveda A, Ray A, Al-Chokhachy R, Muhlfeld C, Gresswell R, Gross J, & Kershner J (2014). Aquatic invasive species: Lessons from cancer research. American Scientist 100, 234–242. [Google Scholar]
- Shurin JB, Havel JE, Leibold MA, & Pinel-Alloul B (2000). Local and Regional Zooplankton Species Richness: A Scale-Independent Test for Saturation. Ecology, 81(11), 3062–3073. doi: 10.2307/177401 [DOI] [Google Scholar]
- Sinclair JS, Furlanetto KJ, & Arnott SE (2015). Dispersal acts as both bane and balm for invaded zooplankton communities. Journal of Plankton Research, 37(2), 462–471. doi: 10.1093/plankt/fbv007 [DOI] [Google Scholar]
- Siqueira T, Duraes LD, & Roque FD (2014). Predictive Modelling of Insect Metacommunities in Biomonitoring of Aquatic Networks. In C. F & W. G (Eds.), Ecological Modelling Applied to Entomology. Entomology in Focus (Vol. Vol 1.): Springer. [Google Scholar]
- Spaulding S, & Elwell L (2007). Increase in nuisance blooms and geographic expansion of the freshwater diatom Didymosphenia geminata: Recommendations for response. Retrieved from Denver, Colorado: [Google Scholar]
- Stoll S, Breyer P, Tonkin JD, Früh D, & Haase P (2016). Scale-dependent effects of river habitat quality on benthic invertebrate communities — Implications for stream restoration practice. Science of the Total Environment, 553, 495–503. doi: 10.1016/j.scitotenv.2016.02.126 [DOI] [PubMed] [Google Scholar]
- Strecker AL, & Brittain JT (2017). Increased habitat connectivity homogenizes freshwater communities: historical and landscape perspectives. Journal of Applied Ecology, 54(5), 1343–1352. doi:doi: 10.1111/1365-2664.12882 [DOI] [Google Scholar]
- Sundermann A, Antons C, Cron N, Lorenz AW, Hering D, & Haase P (2011). Hydromorphological restoration of running waters: effects on benthic invertebrate assemblages. Freshwater Biology. [Google Scholar]
- Swan CM, & Brown BL (2014). Using rarity to infer how dendritic network structure shapes biodiversity in riverine communities. Ecography, 37(10), 993–1001. doi:doi: 10.1111/ecog.00496 [DOI] [Google Scholar]
- Swan CM, & Brown BL (2017). Metacommunity theory meets restoration: isolation may mediate how ecological communities respond to stream restoration. Ecological Applications, 27(7), 2209–2219. doi: 10.1002/eap.1602 [DOI] [PubMed] [Google Scholar]
- Taguchi Y, & Natuhara Y (2009). Requirements for small agricultural dams to allow the Japanese giant salamander (Andrias japonicus) to move upstream. . Japanese Journal of Conservation Ecology, 14(2), 165–172. [Google Scholar]
- Thomaz SM, Kovalenko KE, Havel JE, & Kats LB (2015). Aquatic invasive species: general trends in the literature and introduction to the special issue. Hydrobiologia, 746(1), 1–12. doi: 10.1007/s10750-014-2150-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonkin JD, Altermatt F, Finn DS, Heino J, Olden JD, Pauls SU, & Lytle DA (2018). The role of dispersal in river network metacommunities: Patterns, processes, and pathways. Freshwater Biology, 63(1), 141–163. doi:doi: 10.1111/fwb.13037 [DOI] [Google Scholar]
- Tonkin JD, Stoll S, Sundermann A, & Haase P (2014). Dispersal distance and the pool of taxa, but not barriers, determine the colonisation of restored river reaches by benthic invertebrates. Freshwater Biology, 59(9), 1843–1855. doi: 10.1111/fwb.12387 [DOI] [Google Scholar]
- Tornwall B, Sokol E, Skelton J, & Brown B (2015). Trends in Stream Biodiversity Research since the River Continuum Concept. Diversity, 7(1), 16. Retrieved from http://www.mdpi.com/1424-2818/7/1/16 [Google Scholar]
- Tornwall BM, Swan CM, & Brown BL (2017). Manipulation of local environment produces different diversity outcomes depending on location within a river network. Oecologia, 184(3), 663–674. doi: 10.1007/s00442-017-3891-7 [DOI] [PubMed] [Google Scholar]
- USEPA. (2016). National Rivers and Streams Assessment 2008–2009: A Collaborative Survey Retrieved from
- Van Allen BG, & Rudolf VHW (2016). Carryover effects drive competitive dominance in spatially structured environments. Proceedings of the National Academy of Sciences, 113(25), 6939–6944. doi: 10.1073/pnas.1520536113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannette RL, & Fukami T (2017). Dispersal enhances beta diversity in nectar microbes. Ecology Letters, 20(7), 901–910. doi: 10.1111/ele.12787 [DOI] [PubMed] [Google Scholar]
- WRCRCA. (2003). Riverside County multiple species habitat conservation plan. Retrieved from http://www.wrc-rca.org/about-rca/.
- Xiong W, Li J, Chen Y, Shan B, Wang W, & Zhan A (2016). Determinants of community structure of zooplankton in heavily polluted river ecosystems. Scientific Reports, 6, 22043. doi:10.1038/srep22043 https://www.nature.com/articles/srep22043#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]


