Abstract

The study of gas explosion under the influence of CO generated by spontaneous combustion of coal has practical value for preventing and controlling such accidents. The explosion limit and the explosion characteristic parameters of the CO/CH4/air mixture were measured with a 20 L explosion tank. The changes in free radical concentration and temperature sensitivity in the process of mixture explosion reaction were analyzed using the GRI-mech 3.0 mechanism. The test results show that with the increase of the CO concentration in the mixture, both the lower explosion limit and the upper explosion limit of CH4 explosion decreased, the explosion limit range became wider, and the maximum explosion pressure of the mixture decreased. The time for the H•, O•, and •OH radical molar fractions to reach the peak value was found to be prolonged with the increase of the CO ratio in the mixture. Under oxygen-enriched conditions, the •OH and O• mole fractions were larger than those under oxygen-lean conditions, while the H• concentration was reversed. The higher the proportion of CO in the premixed gas, the higher the value of the temperature sensitivity coefficient. The reaction processes R155 CH3 + O2 ⇌ O• + CH3O and R158 2•CH3 (+M) ⇌ C2H6 (+M) had the greatest influence on the temperature of the reaction process. Explosion suppression techniques can be developed for similar explosive environments based on this study.
1. Introduction
Gas explosions induced by spontaneous combustion of coal in coal mines can cause serious casualties and property damage.1−3 Coal spontaneous combustion causes an increase in ambient temperature while generating CO at concentrations up to 4–6%.4−7 Gas explosions induced by spontaneous combustion of coal are continuous and multiple,8,9 and the concentration of CO generated after the first explosion can be more than 12%.10−12 CO will change the gas explosion characteristics in the coal spontaneous combustion environment. To understand the reaction characteristics and mechanism of the CO/CH4/air mixture, it is of great significance to find the most effective way to suppress such combustion and explosion accidents.
In previous studies, research on the explosion properties of CH4 and its mixtures has focused on the flammability limit,13,14 flame propagation,15−20 explosion pressure,21−23 and chemical kinetics.24,25 Explosion experimental devices are generally used to analyze explosion characteristics directly. Mittal26 used different volumes of spherical and cubic explosion test devices to measure the CH4 explosion characteristic parameters. The measured parameters of each container were slightly different, but the rules were the same. Gieras and Klemens27 tested the effect of methane concentration on the maximum pressure of the 1.25 m3 explosion chamber. The test result of the 20 L explosive device was relatively reliable, which was used to test the explosion limit of combustible gases.28−30 The simulation method was applied to analyze the characteristics of CH4 explosion. Glarborg et al.31 modeled the methane combustion data in stirred reactors and discussed a method of first-order sensitivity of temperature to the reaction rate. Cao et al.32 used a schlieren system and CHEMKIN simulation to comprehensively analyze the explosion flame and pressure characteristics of the CH4 and air mixture and concluded that the flammability limit increased with the increase of reaction pressure. Di Sarli et al.17−20 studied the unstable propagation of flame in the presence of obstacles during premixed methane/air explosion through numerical calculations and experiments.
The mixing of CO into CH4 increases the explosion hazards of the gas mixture.33 Deng et al.34 constructed two hybrid gases CH4/CO and CH4/C2H4 for the explosion test and concluded that the impact of C2H4 on the explosion risk of CH4 was greater than that of CO. Chen35 simulated that adding different mole fractions of CO to CH4 under oxygen-lean conditions would reduce the laminar combustion velocity and affect the flame stability. Luo et al.36 used a self-made pipeline explosion platform to carry out CH4/CO mixed gas explosion and found that CO hindered the deflagration of methane under oxygen-depleted conditions, while under oxygen-enriched conditions, CO could promote the explosion of methane. Vanderstraeten et al.,37 Kondo et al.,38 and Hughes and Raybould39 have done good research on the explosion limit of flammable gas. According to the Le Chatelier criterion, the mixing of flammable gas changes the explosion limit of CH4.40,41 Le Chatelier’s formula was widely used to determine the explosion limit of flammable gas mixtures. For hydrocarbon–air mixtures, the Le Chatelier formula was relatively accurate in its predictions but not for gas mixtures containing H2 or CO.34,42,43
The above research analyzed the characteristic parameters of the CH4/CO mixture gas explosion process. However, there are few studies on the explosion limit and explosion characteristics of CH4/air mixtures under the influence of spontaneous combustion ambient temperature and CO.44 The interaction of the original CO in the fuel mixture with the products after CH4 oxidation is still unclear.45,46 In this paper, experiments were conducted to analyze the kinetic parameters of CO/CH4/air explosion using a 20 L spherical explosion tank experimental system, varying the initial reaction temperature and initial premixed CO concentration, testing the CH4 explosion limit range, and analyzing the gas mixture explosion pressure variation. Using the GRI mech 3.0 mechanism through CHEMKIN, the oxygen-lean and oxygen-enriched simulation conditions were set up to study the temperature sensitivity, evolution of free radicals, and reaction paths of CO/CH4/air mixtures. The effects of premixed CO on the CH4 explosion reaction were revealed from macroscopic and microscopic perspectives, which provided some reference for disasters under similar CO and CH4 coexistence conditions and provided a basis for the selection of related explosion suppression technologies.
2. Experimental and Simulation Methods
2.1. Experimental Method
2.1.1. Experimental System
The explosion system was capable of determining the explosion parameters of flammable gases under normal temperature and pressure and extraordinary temperature and pressure. The experiments followed the standard EN1839.47Figure 1 shows that the gas explosion test system consists of a 20 L spherical explosion tank, a gas distribution system, an ignition system, a data acquisition system, a heating system, and a control computer. The 20 L spherical explosion tank had an observation window, and threaded holes on the top were used for air intake, pressure measurement, and vacuum extraction. The explosion tank was connected to a pressure sensor and a temperature sensor to collect data. The maximum working pressure of the container was 3.5 MPa.
Figure 1.
Schematic of the 20 L spherical gas explosion experimental system.
The ignition electrode was placed in the center and the electrode was fixed with a spark plug. The pulse discharge time controlled the ignition source energy, which can be precisely controlled by PLC. The heating system heated the ambient temperature in the spherical explosion tank to the specified experimental temperature through the heating belt.
2.1.2. Experimental Gas and Conditions
Explosive containers were verified before experiments. All tests were performed at an ambient pressure of 0.1 MPa with a relative humidity of 60–90%. The initial gas temperatures of the spherical tank were set to 298.15, 323.15, 348.15, and 373.15 K according to the actual temperature range of goaf environment during coal oxidation.48,49 The ignition energy was 10 J. To analyze the influence of CO on the explosion characteristics of CH4/air mixture, combined with the gas distribution accuracy of the test system and the actual situation of the mine gas, CO with volume fractions of 0, 1, 5, and 10% were used for the test. The gas distribution accuracy of the system was ±0.1%, and the purity of CH4, CO, and high-purity air used in the experiment was 99.99%. The system was equipped with gases by the method of pressure proportioning.50 Air was injected into the reaction tank and the pressure was gradually increased to 1 MPa for the pressure test before the experiment. If there was no pressure loss after closing all valves and maintaining pressure for 30 min, the experimental system was airtight. The reaction tank could then be evacuated with a vacuum pump. Because the gas pressure ratio was equal to the volume fraction ratio, CH4, air, and CO were sequentially charged into the reaction tank. After each experiment, the corresponding valve was opened and the main explosion tank was pumped with a vacuum pump to discharge the waste gas in the main tank. If the concentration detected three times in a row was the preset value, it was considered that the mixture was uniform. After the gas in the tank was mixed evenly, the heating belt outside the tank was adjusted and the heating plate at the bottom of the tank to the experimental temperature through the temperature control thermocouple.
The experimental measurement method of explosion limit refers to ASTM E681.51 After the gas configuration was completed, an explosion test was performed. An explosion can be judged by the gas pressure increasing by more than 7% after ignition. The explosive limit of CH4 was tested by the asymptotic method. For a certain concentration of gas, if three experiments under the same conditions did not cause an explosion, the gas was considered not to explode at that concentration. If an experiment resulted in an explosion, the gas mixture was considered to have exploded at that concentration. The sensor recorded the parameters of the explosion process when the explosion occurred.
2.2. Simulation Method
2.2.1. Simulation Conditions
The effect mechanism of CO on a gas explosion reaction was explored from the perspective of elementary reactions. The chemical kinetic calculation software CHEMKIN provides an effective method to study the gas explosion mechanism and its influencing factors. In the study of gas chemical kinetic mechanism, the reliability of GRI-Mech 3.0 has been confirmed by some scholars,52 and its detailed mechanism has also been widely recognized.53
The CHEMKIN built-in closed homogeneous batch reactor was selected as the constant volume reactor model for the simulation. The chemical kinetics of CH4 explosions were investigated using GRI-Mech 3.0 (53 components, 325 elementary reactions). The problem type was solving the energy equation under constant volume, adiabatic conditions, without heat loss. The gases required for the simulations were CH4, CO, O2, and N2 with an initial pressure of 1 atm and a reaction time of 0.025 s.
The stoichiometric equation for the CO/CH4 mixture is
| 1 |
In the formula, x is the mole fraction of CO in the mixed gas and n is the amount of gas substance. From this, the equivalence ratio formula of the CO/CH4 mixture can be calculated as
| 2 |
 and
and  are the actual ratio and stoichiometric
ratio of the amount of CO/CH4 mixed gas substance to the
amount of O2 substance. CH4 concentration in
air is 9.5% at φ = 1, the air content just meets the complete
combustion of CH4.54 The CH4 concentration in the simulated working condition is taken
within the explosion limit, and the initial explosion conditions are
lean combustion and oxygen-enriched combustion. Taking the CH4 volume fractions of 7 and 12% as the initial conditions of
the simulation and the specific working conditions are shown in Table 1.
are the actual ratio and stoichiometric
ratio of the amount of CO/CH4 mixed gas substance to the
amount of O2 substance. CH4 concentration in
air is 9.5% at φ = 1, the air content just meets the complete
combustion of CH4.54 The CH4 concentration in the simulated working condition is taken
within the explosion limit, and the initial explosion conditions are
lean combustion and oxygen-enriched combustion. Taking the CH4 volume fractions of 7 and 12% as the initial conditions of
the simulation and the specific working conditions are shown in Table 1.
Table 1. Initial Conditions for Simulation.
| sample | CH4 (%) | O2 (%) | N2 (%) | CO (%) | φ |
|---|---|---|---|---|---|
| 1 | 7 | 19.53 | 73.47 | 0 | 0.72 |
| 2 | 7 | 19.32 | 72.68 | 1 | 0.75 |
| 3 | 7 | 18.48 | 69.52 | 5 | 0.89 |
| 4 | 7 | 17.43 | 65.57 | 10 | 1.09 |
| 5 | 12 | 18.48 | 69.52 | 0 | 1.30 |
| 6 | 12 | 18.27 | 68.73 | 1 | 1.34 |
| 7 | 12 | 17.43 | 65.57 | 5 | 1.52 |
| 8 | 12 | 16.38 | 61.62 | 10 | 1.77 |
2.2.2. Sensitivity Analysis
The detailed
chemical reaction mechanism of the gas explosion was analyzed by using
the CHEMKIN sensitivity analysis function, and the reaction steps
that have a great influence on the reaction kinetics of the gas explosion
process were found. Sensitivity analysis is an effective method to
analyze the quantitative relationship between the solution of a model
and various parameters that appear in the model.55 Assuming that variable Z is the mass fraction or temperature
of the components in each reaction,  satisfies the following
satisfies the following
| 3 |
A is the
pre-exponential
factor for each reaction step.  When the A value
of a
certain reaction step changes, it will inevitably cause a change in
the concentration or temperature of a certain component. Sensitivity
analysis is to change the value of A in each reaction
step and analyze the degree to which the concentration or temperature
of each component changes with A.56 The larger the component concentration or temperature change,
the more it is affected by this reaction step. Its first-order sensitivity
coefficient matrix ωl,i can be calculated by the following formula
When the A value
of a
certain reaction step changes, it will inevitably cause a change in
the concentration or temperature of a certain component. Sensitivity
analysis is to change the value of A in each reaction
step and analyze the degree to which the concentration or temperature
of each component changes with A.56 The larger the component concentration or temperature change,
the more it is affected by this reaction step. Its first-order sensitivity
coefficient matrix ωl,i can be calculated by the following formula
| 4 |
After derivation
| 5 |
In formula 4, Zl is the l-th variable and Ai is the pre-exponential factor of the i-th reaction. The GRI mech 3.0 mechanism not only provides a good solution and calculation for the CH4 combustion mechanism but also includes all the combustion reaction steps of CO. Therefore, to further analyze the temperature change in the reaction process, the top 10 intermediate reactions of CO/CH4/air explosion process temperature sensitivity were analyzed.
3. Results and Discussion
3.1. Influence of Initial Temperature on the Explosion Limit of CH4
The explosion limit of gas is the concentration range of methane in the air that can maintain the continuous reaction and the spread of flames. A lower LEL (lower explosion limit) with a higher UEL (upper explosion limit) means a wider range of explosion limits and a greater explosion hazard. Because the spontaneous combustion of coal caused the temperature of the fire zone to be changeable, the gas explosion characteristics at different temperatures were necessary. Figure 2 shows the variation of the explosion limit of CO/CH4/air mixtures with the initial temperature. It can be seen from Figure 2 that the pure CH4 LEL and UEL measured at a room temperature of 298.15 K were consistent with other studies.37,38 The explosion limit was in a range of 5–16%, and the experimental error was small. Therefore, the experimental system in this study can be used to accurately determine the explosion limit of methane/air mixtures. At 298.15 K, the UEL of pure gas explosion in the air was 16.07%, and the LEL was 5.52%. When the initial temperature was up to 373.15 K, the UEL increased to 17.79% and the LEL decreased to 4.98%. When the CO concentration was up to 10%, at an initial temperature of 298.15 K, the CH4 UEL and LEL were 14.06 and 1.58%.
Figure 2.
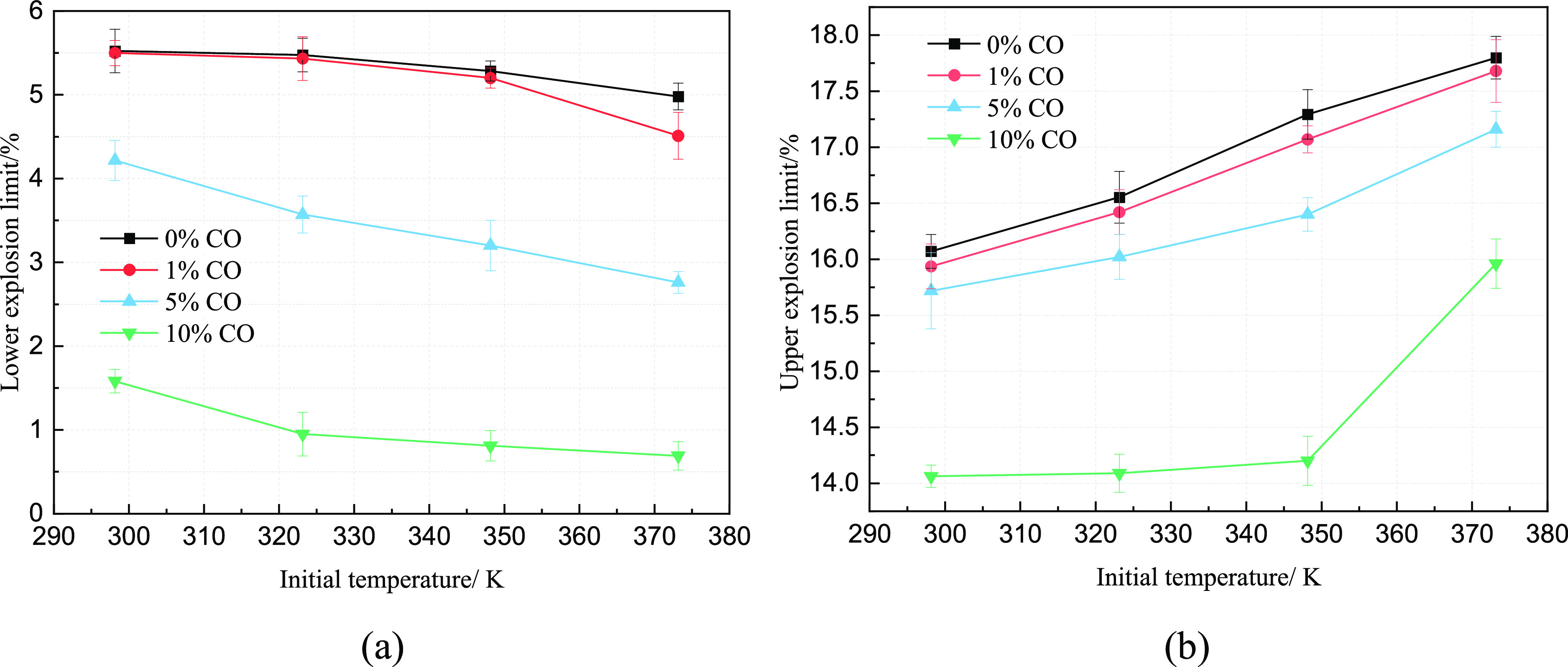
Relationship between the CH4 explosion limit and temperature. (a) Variation of LEL with initial temperature and (b) variation of UEL with initial temperature.
When the temperature increased to 373.15 K, the CH4 UEL and LEL were 16.00 and 0.70%, respectively, with a change range of 12.12 and 55.69%, respectively. When the volume fraction of CO was the same, with the increase of temperature, the UEL increased, the LEL decreased, and the explosion limits became wider. Due to the high temperature and the CO atmosphere, more molecules were activated in the system, and more free radicals were formed. The elementary reaction made it easier for the chain reaction to continue so that a higher concentration of CH4/air mixture can be maintained for the detonation reaction.
3.2. Effect of CO Concentration on the Explosion Limit of CH4
Based on Figure 2, the variation of CH4 explosion limit with CO concentration in the mixture is shown in Figure 3. At an initial temperature of 298.15 K, the proportion of CO in the mixture increased from 0 to 10%, the UEL decreased from 16.07 to 14.06%, and the LEL decreased from 5.52 to 1.58%. It was consistent with the research results of Deng.57 When the initial temperature was 373.15 K, the proportion of CO in the mixture increased from 0 to 10%, the UEL of CH4 decreased from 17.79 to 16.00%, and the LEL decreased from 4.98 to 0.7%. Under different initial temperatures, the UEL and LEL of CH4 decreased with the increase of the mixed CO concentration, showing an approximately linear relationship.
Figure 3.

Relationship between the CH4 explosion limit and the CO concentration. (a) Variation of LEL with CO concentration and (b) variation of UEL with CO concentration.
The effect of temperature and CO concentration on LEL was greater than that on UEL. The oxygen concentration in the upper limit reaction was lower than the oxygen concentration in the lower limit reaction. When the CH4 concentration was close to LEL, and the same proportion of CO was premixed to participate in the reaction, the proportion of oxygen consumed by CO was higher, which reduced the effective collision between CH4 and O2 molecules, thereby affecting the reaction limit. A comprehensive comparison showed that the range of gas explosion limits increases significantly under the coupling effect of initial temperature and CO. When judging whether the CH4 concentration in the goaf of a coal mine meets the explosion conditions, the influence of the ambient temperature and CO concentration in the goaf on the CH4 explosion limits should be comprehensively considered.
3.3. CH4 Explosion Pressure Changes with Temperature and Concentration
Figure 4 records the variation law of Pmax (maximum explosion pressure) with the initial reaction temperature and the gas concentration of the mixture components in the process of approaching the CH4 explosion limit. When the concentration of gas components was the same, the temperature increased and the maximum explosion pressure showed a downward trend. Increasing the temperature at a constant initial pressure would increase the thermal conductivity of the gas and decrease the density of the fuel. This would result in a reduction in the amount of heat released during the reaction and thus reduce the maximum explosion pressure. When the initial temperature of the premixed gas was 323.15 K, the CH4 concentration was 5%, and when the CO concentration increased from 5 to 10%, the Pmax during the mixture explosion increased from 0.82 to 1.04 MPa, an increase of 26.8%. The CO concentration was 10%, and the Pmax was 0.25 MPa when the CH4 concentration was reduced to UEL 0.9%.
Figure 4.
Variation of Pmax with CH4 concentration and CO concentration at different initial temperatures. (a) CH4 concentration close to LEL and (b) CH4 concentration close to UEL.
Observing the change law of Pmax: in the process of CH4 concentration approaching the LEL, under the same initial temperature and the same CO concentration, the higher the CH4 concentration and the CO concentration, the greater the Pmax. When the initial temperature of the premixed gas was 323.15 K, the CH4 concentration was 14%, and when the mixed CO concentration decreased from 10 to 5%, the peak pressure of the mixture during the explosion process increased from 0.64 to 0.68 MPa. When the CO concentration was 5%, the Pmax decreased to 0.55 MPa and when the CH4 concentration increased to UEL16%. For the CH4 concentration approaching the UEL process, under the same initial temperature and the same CO concentration, Pmax decreased with the increase of CH4 concentration. At the same CH4 concentration, Pmax decreased with the increase of CO concentration.
When the CH4 concentration was close to LEL and was below its stoichiometric concentration, the increase of CO can increase the concentration of combustibles and increased the gas explosion reaction rate. The explosion pressure increased with the increase of CO concentration, and the growth rate gradually decreased. When the CH4 concentration was close to UEL and exceeded its stoichiometric concentration, the O2 concentration decreased with the increase of the mixed CO concentration. The addition of CO suppressed the gas explosion reaction, and the explosion pressure would be reduced accordingly.
3.4. Effect of Mixed CO on the Free Radical Mole Fraction
In the reaction process, the intermediate products were free radicals, especially H•, O•, and •OH, although the concentration was low and the retention time was short, they maintain and dominate the chain reaction, and the chemical reaction rate of these free radicals determines the explosive strength of the premixed gas.58 When the composition of the premixed system was changed, the effect of different concentrations of H•, O•, and other free radicals on the chain reaction will also change greatly. To analyze the reaction process, the relationship of H•, O•, and •OH radical concentration with reaction time is shown in Figure 5. The gas had a chain reaction at high temperatures, and the generated H•, O•, and other highly reactive free radicals, due to the accumulation of energy, sharply increased in concentration and reached a peak at a certain moment, and then caused an explosion. The free radicals collided, part of the free radicals was consumed in the subsequent chain reaction, and their concentration rapidly decreased to a certain value and remained stable. For the clear expression, some curves were not shown in the stage where the concentration of free radicals was almost unchanged after 0.02 s.
Figure 5.

Variation of free radical mole fraction with time. (a) Variation of H• mole fraction, (b) variation of O• mole fraction, and (c) variation of •OH mole fraction.
The time for the concentration of free radicals to reach the peak was prolonged with the increase of the CO ratio in the mixture. The time required for the reaction energy accumulation process increased with the increase of the fuel concentration, and the macroscopic manifestation was that the ignition delay time of the explosion reaction increased.
The •OH and O• mole fractions in oxygen-enriched conditions were larger than in oxygen-lean conditions, while the H• concentration was the opposite. Combined with each elementary reaction, H• + O2 ⇌ O• + •OH generated two highly active oxidation units at the same time, and •OH can react with CO to generate H• to supplement the consumption of this reaction. The concentrations of •OH and O• depended on the reactions that O2 participated in, while the formation of H• was more dependent on hydrocarbon groups. The O2 concentration in the reaction affected the formation of oxygen-containing free radicals in the reaction process.
Figure 6 shows the relationship between the peak molar fraction of free radicals and the premixed CO concentration. The volume fractions of CH4 were 7% and 12%, and the H• concentration was greater than •OH and O• concentration. Figure 6a shows that when the volume fraction of CH4 was 7%, with the increase of the volume fraction of CO, the peak value of the mole fraction of H• radicals continue to rise. O• and •OH radicals were close to the maximum when the volume fraction of CO was 5% and decreased when the CO volume fraction was 10%. At this time, the increase of CO ratio would increase the highly active H• radicals produced by the reaction, and some H• and O• could react to generate •OH, thereby accelerating the oxidation reaction and promoting the explosion of CH4. Figure 6b shows that when the volume fraction of CH4 was 12%, the maximum mole fraction of H• increased slightly at a CO concentration of 0–1%. The maximum mole fraction of H• continued to decrease when the CO concentration increased from 1 to 10%. With the increase of CO volume fraction, the peak value of O• and •OH mole fraction continued to decrease. When CH4 and CO provided too much fuel, oxygen consumption increased, decreasing the peak concentrations of O• and •OH.
Figure 6.

Variation of maximum radical molar fraction with CO concentration. (a) Volume fraction of 7% CH4 and (b) volume fraction of 12% CH4.
Increasing CO concentration under oxygen-lean conditions can strengthen some elementary reactions, such as O• + CO (+M) ⇌ CO2 (+M). At this time, the energy of a large number of free radicals was transferred to CO molecules, increasing the loss of energy and reducing the collision frequency of other free radicals in the CH4 explosion chain reaction, which reduced the reaction capacity of the system.59,60 This was consistent with the law in Figure 4 that the Pmax of UEL decreased with the increase of premixed CO concentration. When the CH4 concentration is in the fuel-rich state, the addition of CO hinders the formation of free radicals. The reaction activity between combustible gas and oxygen decreases. The explosion intensity of the mixture also decreases significantly. Spontaneous combustion of coal will cause a relative lack of oxygen concentration in the environment. Therefore, if the CH4 explosion occurs when the CO concentration in the coal spontaneous combustion environment is high, the explosion pressure will be relatively reduced. Explosion-proof devices should be designed considering the explosion effect in the actual reaction environment.
3.5. Temperature Sensitivity Analysis
It was difficult to record the transient temperature completely through experiments, so the effect of each elementary reaction on the temperature in the CO/CH4 explosion reaction was analyzed in the CHEMKIN simulation. The sensitivity coefficients of each reaction under different working conditions were different and cannot be directly compared in the same figure. To facilitate comparison, the top 10 TSCs (temperature sensitivity coefficients) of elementary elements under different reaction conditions with the same CH4 concentration were normalized. The results of TSC under different CO concentrations are shown in Figure 7, and the normalized comparison is shown in Figure 8. The higher the concentration of premixed CH4 and CO, the greater the TSC of each element reaction, and the greater the influence on the reaction temperature. Comparing Figures 7 and 8, it can be seen that the negative reaction R158, which absorbs heat, and the positive reaction R155, which releases heat, have the largest TSC values under each condition. When the CH4 concentration was 7%, the top 10 elementary reactions of the TSC under the conditions of 0 and 1% CO concentration were consistent, which were positive reactions R155, R156, R38, R119, R32, R161, and R170, and negative reactions R158, R53, and R98.
Figure 7.
Variation of TSCs with time under different conditions. (a) Volume fractions of 7% CH4 and 0% CO, (b) volume fractions of 7% CH4 and 1% CO, (c) volume fractions of 7% CH4 and 5% CO, (d) volume fractions of 7% CH4 and 10% CO, (e) volume fractions of 12% CH4 and 0% CO, (f) volume fractions of 12% CH4 and 1% CO, (g) volume fractions of 12% CH4 and 5% CO, and (h) volume fractions of 12% CH4 and 10% CO.
Figure 8.

Normalized TSCs under different reaction conditions. (a) 7% volume fraction of CH4 and (b) 12% volume fraction of CH4.
When the concentration of CO increased to more than 5% and the concentrations of CH4 were 7 and 12%, the TSC of the R120 reaction gradually increased, and the TSC of the R170 reaction CH3O + O2 ⇌ HO2 + CH2O gradually weakened until it withdrew from the top 10. The R120 negative reaction consumed CO to produce •OH. The increase of CO promoted the heat absorption process of the R120 reaction. With the increase of CO concentration, the oxygen deficiency of the reaction system gradually increased, the temperature sensitivity coefficient of R170 positive reaction decreased, and the effect of heat release on the temperature weakened. When the CO concentration was 10%, the elementary reaction •OH + CO ⇌ H• + CO2 of R99 entered the top 10 TSC values. 10% CO reacted with •OH to enhance the effect of heat release. R98 was the CH4 dehydrogenation process •OH + CH4 ⇌ •CH3 + H2O. R99 and R98 caused •OH to be consumed together, which was consistent with the change law of •OH mole fraction in Figure 6. Comparing Figure 7e,f, when the CH4 concentration was 12% and the CO concentrations were 0 and 1%, R57 H• + CH2O (+M) ⇌ CH3O (+M) appeared in the top 10 TSC values. However, when the CO concentration increased above 5%, R98 replaced R57 again. It was proved that the reaction of low concentrations of CO and H• had a strong effect on temperature under the oxygen-lean reaction conditions. The increase in CO concentration promoted the endothermic process of the CH4 dehydrogenation reaction. Mining flame retardant and explosion suppression materials can reduce the damage of high temperatures caused by CO/CH4 mixture explosion during coal spontaneous combustion by inhibiting the key reaction steps.
4. Conclusions
This paper aims to reveal the effect of CO on the CH4 explosion reaction process and characteristic parameters through experiments and numerical simulations. The explosion limit and pressure change characteristics of the CO/CH4/air mixed gas were recorded using a 20 L explosion tank. With the increase of CO concentration in the gas mixture, both UEL and LEL of CH4 decreased, and the explosion limit range became wider; at the same CO concentration, with the increase of initial reaction temperature, UEL increased and LEL decreased. As the initial temperature increased, the Pmax of the CO/CH4/air mixture decreased. At the same initial temperature and with a CH4 concentration close to the LEL, Pmax decreased as the CH4 and CO concentrations decreased. As CH4 concentrations approached UEL, the Pmax became lower as the concentrations of CH4 and CO increased.
The CO/CH4/air reaction process was simulated under oxygen-lean and oxygen-enriched conditions, respectively. The time when the mole fractions of H•, O•, and •OH reach their peaks increases with the increase of the CO ratio in the mixture. The mole fractions of •OH and O• during the reaction in the oxygen-enriched conditions were larger than those in the oxygen-depleted conditions, while the H• concentration was the opposite. In oxygen-enriched reaction conditions, CO increased the concentration of radicals and promoted the explosive reaction; while in oxygen-deprived conditions, it had the opposite effect and weakened the explosive reaction. Normalized TSC values of each reaction process showed that the higher the proportion of CO in the mixture, the higher the TSC value, and the greater the effect on the temperature during the reaction.
R155 CH3 + O2 ⇌ O• + CH3O in the positive reaction and R158 2•CH3 (+M) ⇌ C2H6 (+M) in the negative reaction have the greatest effect on the temperature change. The TSC of R120 increased gradually with the CO concentration above 5%, and the TSC of R170 was gradually decreased. Increasing CO concentration under oxygen-lean reaction conditions can promote R98, the endothermic process of dehydrogenation of CH4.
The research results are significant for the risk assessment and prevention of explosions caused by combustible gas mixtures in coal mines. However, there are relatively few studies on the explosion reaction conditions and the explosion initiation process in this paper. Future works could focus on the induction mechanism of the explosive response. The spectral distribution of key radicals obtained by using planar laser-induced fluorescence technology can reveal the microscopic mechanism of combustible gas explosion.
Acknowledgments
This work was financially supported by the Liaoning Revitalization Talents Program (no. XLYC2008021) and the National Natural Science Foundation of China (nos. 51704147, 51874161).
Author Contributions
∥ X.Z. and G.B. contributed equally to this work.
The authors declare no competing financial interest.
References
- Wachowicz J. Analysis of Underground Fires in Polish Hard Coal Mines. J. China Univ. Min. Technol. 2008, 18, 332–336. 10.1016/S1006-1266(08)60070-X. [DOI] [Google Scholar]
- De Rosa M. I.Analysis of Mine Fires for All US Metal/Nonmetal Mining Categories: 1990-2001. 2004. [Google Scholar]
- Bai G.; Su J.; Zhang Z.; Lan A.; Zhou X.; Gao F.; Zhou J. Effect of CO2 Injection on CH4 Desorption Rate in Poor Permeability Coal Seams: An Experimental Study. Energy 2022, 238, 121674. 10.1016/j.energy.2021.121674. [DOI] [Google Scholar]
- Hu X.; Yang S.; Zhou X. Investigation on Indicator Gas of Coal Spontaneous Combustion. Coal Technol. 2012, 31, 94. [Google Scholar]
- Wojtacha-Rychter K.; Smoliński A. Profile of CO2, CO, and H2 Emissions from Thermal Oxidation of Polish Coals. Materials 2020, 13, 848. 10.3390/ma13040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracher G. B.Gases Generated during the Low-Temperature Oxidation and Pyrolysis of Coal and the Effects on Methane-Air Flammable Limits. Coal and Peat Fires: A Global Perspective; Elsevier, 2019; pp 157–171. [Google Scholar]
- Pan R.; Cheng Y.; Yu M.; Lu C.; Yang K. New Technological Partition for “Three Zones” Spontaneous Coal Combustion in Goaf. Int. J. Min. Sci. Technol. 2013, 23, 489–493. 10.1016/j.ijmst.2013.07.005. [DOI] [Google Scholar]
- Qiang C.; Zhong-jun S.; Xin-quan Z.; Hai-yan W. Multi-Field Coupling Laws of Mixed Gas in Goaf. Procedia Eng. 2011, 26, 204–210. 10.1016/j.proeng.2011.11.2159. [DOI] [Google Scholar]
- Qin B. T.; Zhang L. L.; Wang D. M.; Yao Y. Mechanism and Restraining Technology on Spontaneous Combustion of Coal Detonating Gas in Goaf. J. China Coal Soc. 2009, 34, 1655–1659. [Google Scholar]
- Ma Q.; Zhang Q.; Li D.; Chen J.; Ren S.; Shen S. Effects of Premixed Methane Concentration on Distribution of Flame Region and Hazard Effects in a Tube and a Tunnel Gas Explosion. J. Loss Prev. Process Ind. 2015, 34, 30–38. 10.1016/j.jlp.2015.01.018. [DOI] [Google Scholar]
- Jiang Y.; Zhao Y.; Wang H.; Zhu J. A Review of Mechanism and Prevention Technologies of Coal Bumps in China. J. Rock Mech. Geotech. Eng. 2017, 9, 180–194. 10.1016/j.jrmge.2016.05.008. [DOI] [Google Scholar]
- Sun Y.; Zhou X.; Bai G.; Teng Y.; Xin T.; Xiao M. Removal of CO Generated by a Gas Explosion Using a Cu-Mn Elimination Agent. ACS Omega 2021, 6, 16140–16150. 10.1021/acsomega.1c02019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharagheizi F.; Ilani-Kashkouli P.; Mohammadi A. H. Estimation of Lower Flammability Limit Temperature of Chemical Compounds Using a Corresponding State Method. Fuel 2013, 103, 899–904. 10.1016/j.fuel.2012.06.101. [DOI] [Google Scholar]
- Lin S.; Liu Z.; Qian J.; Li X.; Zhang Q. Flammability and Explosion Risk of Post-Explosion CH4/Air and CH4/Coal Dust/Air Mixtures. Combust. Sci. Technol. 2021, 193, 1279–1292. 10.1080/00102202.2019.1688313. [DOI] [Google Scholar]
- Dold J.; Joulin G. An evolution equation modeling inversion of tulip flames,. Combust. Flame 1995, 100, 450–456. 10.1016/0010-2180(94)00156-m. [DOI] [Google Scholar]
- Law C. K. Propagation, Structure, and Limit Phenomena of Laminar Flames at Elevated Pressures. Combust. Sci. Technol. 2006, 178, 335–360. 10.1080/00102200500290690. [DOI] [Google Scholar]
- Di Sarli V.; Di Benedetto A.; Russo G. Large Eddy Simulation of transient premixed flame-vortex interactions in gas explosions. Chem. Eng. Sci. 2012, 71, 539–551. 10.1016/j.ces.2011.11.034. [DOI] [Google Scholar]
- Di Sarli V.; Di Benedetto A.; Russo G. Sub-Grid Scale Combustion Models for Large Eddy Simulation of Unsteady Premixed Flame Propagation around Obstacles. J. Hazard. Mater. 2010, 180, 71–78. 10.1016/j.jhazmat.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Di Sarli V.; Di Benedetto A.; Russo G. Using Large Eddy Simulation for Understanding Vented Gas Explosions in the Presence of Obstacles. J. Hazard. Mater. 2009, 169, 435–442. 10.1016/j.jhazmat.2009.03.115. [DOI] [PubMed] [Google Scholar]
- Di Sarli V.; Di Benedetto A.; Russo G.; Jarvis S.; Long E. J.; Hargrave G. K. Large Eddy Simulation and PIV Measurements of Unsteady Premixed Flames Accelerated by Obstacles. Flow, Turbul. Combust. 2009, 83, 227–250. 10.1007/s10494-008-9198-3. [DOI] [Google Scholar]
- Mitu M.; Prodan M.; Giurcan V.; Razus D.; Oancea D. Influence of inert gas addition on propagation indices of methane-air deflagrations. Process Saf. Environ. Prot. 2016, 102, 513–522. 10.1016/j.psep.2016.05.007. [DOI] [Google Scholar]
- Zanganeh J.; Ajrash Al-Zuraiji M. J.; Moghtaderi B. Capture and Mitigation of Fugitive Methane: Examining the Characteristics of Methane Explosions in an Explosion Chamber Connected to a Venting Duct. Energy Fuels 2019, 34, 645–654. 10.1021/acs.energyfuels.9b02942. [DOI] [Google Scholar]
- Luo Z.; Li D.; Su B.; Zhang S.; Deng J. On the Time Coupling Analysis of Explosion Pressure and Intermediate Generation for Multiple Flammable Gases. Energy 2020, 198, 117329. 10.1016/j.energy.2020.117329. [DOI] [Google Scholar]
- Liberman M.; Wang C.; Qian C.; Liu J. Influence of Chemical Kinetics on Spontaneous Waves and Detonation Initiation in Highly Reactive and Low Reactive Mixtures. Combust. Theory Modell. 2019, 23, 467–495. 10.1080/13647830.2018.1551578. [DOI] [Google Scholar]
- Wang C.; Qian C.; Liu J.; Liberman M. A. Influence of Chemical Kinetics on Detonation Initiating by Temperature Gradients in Methane/Air. Combust. Flame 2018, 197, 400–415. 10.1016/j.combustflame.2018.08.017. [DOI] [Google Scholar]
- Mittal M. Explosion Pressure Measurement of Methane-Air Mixtures in Different Sizes of Confinement. J. Loss Prev. Process Ind. 2017, 46, 200–208. 10.1016/j.jlp.2017.02.022. [DOI] [Google Scholar]
- Gieras M.; Klemens R. Experimental Studies of Explosions of Methane-Air Mixtures in a Constant Volume Chamber. Combust. Sci. Technol. 2009, 181, 641–653. 10.1080/00102200802665102. [DOI] [Google Scholar]
- Zlochower I. A.; Green G. M. The Limiting Oxygen Concentration and Flammability Limits of Gases and Gas Mixtures. J. Loss Prev. Process Ind. 2009, 22, 499–505. 10.1016/j.jlp.2009.03.006. [DOI] [Google Scholar]
- Ajrash M. J.; Zanganeh J.; Moghtaderi B. Effects of Ignition Energy on Fire and Explosion Characteristics of Dilute Hybrid Fuel in Ventilation Air Methane. J. Loss Prev. Process Ind. 2016, 40, 207–216. 10.1016/j.jlp.2015.12.014. [DOI] [Google Scholar]
- Li Q.; Lin B.; Li W.; Zhai C.; Zhu C. Explosion characteristics of nano-aluminum powder-air mixtures in 20L spherical vessels. Powder Technol. 2011, 212, 303–309. 10.1016/j.powtec.2011.04.038. [DOI] [Google Scholar]
- Glarborg P.; Miller J. A.; Kee R. J. Kinetic Modeling and Sensitivity Analysis of Nitrogen Oxide Formation in Well-Stirred Reactors. Combust. Flame 1986, 65, 177–202. 10.1016/0010-2180(86)90018-0. [DOI] [Google Scholar]
- Cao Y.; Dahari M.; Tlili I.; Raise A. Investigation on the Laminar Flame Speed of CH4/CO2/Air Mixture at Atmospheric and High Pressures Using Schlieren Photography. Int. J. Hydrogen Energy 2020, 45, 31151–31161. 10.1016/j.ijhydene.2020.08.061. [DOI] [Google Scholar]
- Luo Z.; Li R.; Wang T.; Cheng F.; Liu Y.; Yu Z.; Fan S.; Zhu X. Explosion Pressure and Flame Characteristics of CO/CH4/Air Mixtures at Elevated Initial Temperatures. Fuel 2020, 268, 117377. 10.1016/j.fuel.2020.117377. [DOI] [Google Scholar]
- Deng J.; Luo Z.; Wu X.; Hu Y. Explosive Limits of Mixed Gases Containing CH4, CO and C2H4 in the Goaf Area. Min. Sci. Technol. 2010, 20, 557–562. 10.1016/S1674-5264(09)60243-X. [DOI] [Google Scholar]
- Chen W.-T. Effects of CO Addition on the Lean Premixed CH4/Air Flame. Acta Phys.-Chim. Sin. 2010, 26, 1481–1487. [Google Scholar]
- Luo Z.; Su Y.; Li R.; Chen X.; Wang T. Effect of Inert Gas CO2 on Deflagration Pressure of CH4/CO. ACS Omega 2020, 5, 23002–23008. 10.1021/acsomega.0c02686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstraeten B.; Tuerlinckx D.; Berghmans J.; Vliegen S.; Van’t Oost E.; Smit B. Experimental Study of the Pressure and Temperature Dependence on the Upper Flammability Limit of Methane/Air Mixtures. J. Hazard. Mater. 1997, 56, 237–246. 10.1016/s0304-3894(97)00045-9. [DOI] [Google Scholar]
- Kondo S.; Takizawa K.; Takahashi A.; Tokuhashi K. On the Temperature Dependence of Flammability Limits of Gases. J. Hazard. Mater. 2011, 187, 585–590. 10.1016/j.jhazmat.2011.01.037. [DOI] [PubMed] [Google Scholar]
- Hughes A. J.; Raybould W. E. The Rapid Determination of the Explosibility of Mine Fire Gases. Min. Eng. 1960, 120, 37–53. [Google Scholar]
- Liu Z.-K.; Ågren J.; Hillert M. Application of the Le Chatelier Principle on Gas Reactions. Fluid Phase Equilib. 1996, 121, 167–177. 10.1016/0378-3812(96)02994-9. [DOI] [Google Scholar]
- Luo Z.; Liang H.; Wang T.; Cheng F.; Su B.; Liu L.; Liu B. Evaluating the Effect of Multiple Flammable Gases on the Flammability Limit of CH4: Experimental Study and Theoretical Calculation. Process Saf. Environ. Prot. 2021, 146, 369–376. 10.1016/j.psep.2020.09.023. [DOI] [Google Scholar]
- Zheng L. G.; Fan S. J.; Yu M. G.; Yu S.; Zuo Q.. Predictive Model on Explosion Limits of Explosive Gas Mixture Containing H2, CH4 and CO Based on Neural Network. International Symposium on Safety Science and Technology, 5th, Changsha, China, 2006; pp 1227–1231.
- Wierzba I.; Ale B. B. Rich Flammability Limits of Fuel Mixtures Involving Hydrogen at Elevated Temperatures. Int. J. Hydrogen Energy 2000, 25, 75–80. 10.1016/s0360-3199(99)00009-9. [DOI] [Google Scholar]
- Gieras M.; Klemens R.; Rarata G.; Wolański P. Determination of Explosion Parameters of Methane-Air Mixtures in the Chamber of 40 Dm3 at Normal and Elevated Temperature. J. Loss Prev. Process Ind. 2006, 19, 263–270. 10.1016/j.jlp.2005.05.004. [DOI] [Google Scholar]
- Metghalchi M.; Keck J. C. Laminar Burning Velocity of Propane-Air Mixtures at High Temperature and Pressure. Combust. Flame 1980, 38, 143–154. 10.1016/0010-2180(80)90046-2. [DOI] [Google Scholar]
- Sung C. J.; Huang Y.; Eng J. A. Effects of Reformer Gas Addition on the Laminar Flame Speeds and Flammability Limits of N-Butane and Iso-Butane Flames. Combust. Flame 2001, 126, 1699–1713. 10.1016/s0010-2180(01)00284-x. [DOI] [Google Scholar]
- EN B.. Determination of Explosion Limits of Gases and Vapours. London, U.K.: British Standard; 2003. [Google Scholar]
- Wang Y.; Shi G.; Wang D. Numerical Study on Thermal Environment in Mine Gob Under Coal Oxidation Condition. Ecol. Chem. Eng. S 2013, 20, 567–578. 10.2478/eces-2013-0041. [DOI] [Google Scholar]
- Yuan L.; Smith A. C. Numerical Study on Effects of Coal Properties on Spontaneous Heating in Longwall Gob Areas. Fuel 2008, 87, 3409–3419. 10.1016/j.fuel.2008.05.015. [DOI] [Google Scholar]
- Bai G.; Zhou X.; Song D. Experimental Study on the Coupling Influence of Temperature and CO Concentration on CH4 Explosion Limit. Chin. J. High Pressure Phys. 2019, 33, 045203. [Google Scholar]
- E681-01 A. Standard test method for concentration limits of flammability of chemicals (vapors and gases). West Conshohocken: PA: ASTM International; 2001. [Google Scholar]
- Yuntao L.; Liancong W.; Haizhu L. Computational Model of Reaction Kinetic for Gas Explosion in Constant Volume Combustion Reactor. J. China Coal Soc. 2015, 40, 1853–1858. [Google Scholar]
- Rozenchan G.; Zhu D. L.; Law C. K.; Tse S. D. Outward Propagation, Burning Velocities, and Chemical Effects of Methane Flames up to 60 Atm. Proc. Combust. Inst. 2002, 29, 1461–1470. 10.1016/s1540-7489(02)80179-1. [DOI] [Google Scholar]
- Lapalme D.; Lemaire R.; Seers P. Assessment of the method for calculating the Lewis number of H 2 /CO/CH 4 mixtures and comparison with experimental results. Int. J. Hydrogen Energy 2017, 42, 8314–8328. 10.1016/j.ijhydene.2017.01.099. [DOI] [Google Scholar]
- Carrasco N.; Alcaraz C.; Dutuit O.; Plessis S.; Thissen R.; Vuitton V.; Yelle R.; Pernot P. Sensitivity of a Titan Ionospheric Model to the Ion-Molecule Reaction Parameters. Planet. Space Sci. 2008, 56, 1644–1657. 10.1016/j.pss.2008.04.007. [DOI] [Google Scholar]
- Rodat S.; Abanades S.; Coulie J.; Flamant G. Kinetic Modelling of Methane Decomposition in a Tubular Solar Reactor. Chem. Eng. J. 2009, 146, 120–127. 10.1016/j.cej.2008.09.008. [DOI] [Google Scholar]
- Deng J.; Cheng F.; Song Y.; Luo Z.; Zhang Y. Experimental and Simulation Studies on the Influence of Carbon Monoxide on Explosion Characteristics of Methane. J. Loss Prev. Process Ind. 2015, 36, 45–53. 10.1016/j.jlp.2015.05.002. [DOI] [Google Scholar]
- Nie B.; Yang L.; Ge B.; Wang J.; Li X. Chemical Kinetic Characteristics of Methane/Air Mixture Explosion and Its Affecting Factors. J. Loss Prev. Process Ind. 2017, 49, 675–682. 10.1016/j.jlp.2017.02.021. [DOI] [Google Scholar]
- Di Benedetto A.; Di Sarli V.; Salzano E.; Cammarota F.; Russo G. Explosion Behavior of CH4/O2/N2/CO2 and H2/O2/N2/CO2 Mixtures. Int. J. Hydrogen Energy 2009, 34, 6970–6978. 10.1016/j.ijhydene.2009.05.120. [DOI] [Google Scholar]
- Luo Z.; Li D.; Su B.; Wang T.; Li K.; Li Q.; Deng J. Thermodynamic Effects of the Generation of H*/OH*/CH2O* on Flammable Gas Explosion. Fuel 2020, 280, 118679. 10.1016/j.fuel.2020.118679. [DOI] [Google Scholar]





