Abstract
Objectives
To study the efficacy and safety of ixekizumab (IXE) in patients with radiographic (r-) and non-radiographic (nr-)axial spondyloarthritis (axSpA) for up to 116 weeks.
Methods
COAST-Y (NCT03129100) is the 2-year extension study following COAST-V, COAST-W and COAST-X. Patients were treated with either 80 mg IXE every 4 weeks or 2 weeks, as assigned in the originating studies. Efficacy was assessed in all participants continuously treated with IXE through week 116 and in subgroups based on disease subtype and dosing. Missing data were handled by non-responder imputation for categorical variables and modified baseline observation carried forward for continuous variables. Safety data were analysed in all patients having received ≥1 IXE dose.
Results
Of 932 patients who received ≥1 IXE dose, 773 enrolled in COAST-Y (82.9%); 665 of which (86.0%) completed week 116. Of 352 continuously treated patients, the proportion achieving Assessment of Spondyloarthritis International Society (ASAS40) at week 52 was 51.4%, which increased to 56.0% at week 116. The proportion of patients achieving ASAS40 at week 116 was 64.9% and 57.7% for biological disease-modifying antirheumatic drug (bDMARD)-naïve patients with r-axSpA and nr-axSpA, respectively, and 47.0% for TNFi-experienced patients. The proportion of patients achieving Ankylosing Spondylitis Disease Activity Score <2.1 through week 116 was 57.0% and 52.9% for bDMARD-naïve patients with r-axSpA and nr-axSpA, respectively, and 33.6% for TNFi-experienced patients. Incidences of treatment-emergent adverse events and serious adverse events were consistent with previous reports.
Conclusion
IXE treatment led to sustained long-term improvements in patients with axSpA, with similar efficacy for r-axSpA and nr-axSpA, and for patients receiving the approved every 4 weeks dose. The safety profile of IXE was consistent with previous reports. No new safety signals were identified.
Keywords: Spondylitis, Ankylosing; Patient Reported Outcome Measures; Antirheumatic Agents; Biological Therapy
WHAT IS ALREADY KNOWN ON THIS TOPIC
Ixekizumab (IXE), a high-affinity monoclonal antibody that selectively targets IL-17A, has demonstrated efficacy in treating patients within the spectrum of axial spondyloarthritis (axSpA).
WHAT THIS STUDY ADDS
This study provides data on the long-term efficacy and safety of IXE in treating patients with the whole spectrum of axSpA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
AxSpA is a chronic condition which requires chronic therapy. The data presented here support the long-term efficacy and safety of IXE when used to treat patients with r-axSpA and non-radiographic axSpA.
Introduction
Axial spondyloarthritis (axSpA) is a chronic inflammatory rheumatic disease affecting the axial skeleton, including the sacroiliac (SI) joints and spine. Two subtypes have been defined within the spectrum of axSpA.1 2 For radiographic (r-)axSpA, which is almost congruent to ankylosing spondylitis (AS),3 definite structural changes are detected on plain X-rays of the SI joints, whereas for non-radiographic (nr-)axSpA, those definite chronic SI joint changes are not visible on X-ray. The differences between the two subtypes are well known and are not the focus of this study.4 Patients with r-axSpA and nr-axSpA carry a similar disease burden,5 experiencing considerable physical, emotional and economic hurdles.6 7
Non-steroidal anti-inflammatory drugs are recommended as first-line therapy for patients with axSpA.8 9 For patients who still have high disease activity despite receiving conventional treatments, biological disease-modifying antirheumatic drugs (bDMARDs) are recommended.8 9 The first bDMARDs approved for axSpA were the tumour necrosis factor inhibitors (TNFi), which have proven long-term efficacy and safety in the treatment of axSpA,10–14 however, there is still an unmet need for those patients who, for one reason or another, do not respond or discontinue TNFi treatment.15–17 Pivotal trials with ixekizumab (IXE) led to the approval of this compound for the treatment of patients with r- and nr-axSpA, with data through 52 weeks. Here, we provide the clinical trial results for IXE from the COAST programme up until week 116 (2 years).
Three previous 52-week phase 3 studies demonstrated the efficacy of IXE in treating patients who had r-axSpA or nr-axSpA and no prior exposure to bDMARDs (COAST-V and COAST-X). COAST-W studied patients with r-axSpA who were previously treated with up to two TNFi.18–21 COAST-Y is an ongoing extension of these three originating studies. The primary objective of COAST-Y was to determine the proportion of flare-free patients during the randomised withdrawal retreatment period, data which has been previously reported.22 The goal of the present analysis was to report the pre-specified secondary objectives of the COAST-Y study: long-term efficacy and safety results for patients treated with IXE for up to 116 weeks (52 weeks of the originating studies plus 64 weeks of COAST-Y).
Methods
Participants
COAST-Y (NCT03129100) included participants from two originating studies in r-axSpA (COAST-V, NCT02696785; and COAST-W, NCT02696798) and one originating study in nr-axSpA (COAST-X, NCT02757352). Eligibility criteria for the originating studies were previously described.18–20 Patients in COAST-V and COAST-X were bDMARD-naïve, while patients in COAST-W had inadequate response or were intolerant of up to two TNFi. Moreover, patients from COAST-X (nr-axSpA), only, had objective signs of inflammation as indicated by sacroiliitis on an MRI (according to Assessment of Spondyloarthritis International Society (ASAS)/OMERACT criteria and based on central reading) or by elevated C-reactive protein (CRP). To be eligible for COAST-Y, patients must have completed the final week 52 visit in the originating study and had to reconsent in order to participate in COAST-Y. Complete eligibility criteria are provided in online supplemental data 1. To assess efficacy of IXE through 116 weeks, we analysed the subset of patients who were assigned to IXE (either every 2 weeks or every 4 weeks) at week 0 of the originating studies and who were continuously treated with the same dose of IXE through week 116 (52 weeks of the originating studies plus 64 weeks of COAST-Y) without interruptions (N=352). Therefore, patients were excluded from the continuously treated group if they (1) were randomised to placebo or adalimumab in any of the originating studies, (2) switched from IXE every 4 weeks to open-label to IXE every 2 weeks during COAST-X, (3) were withdrawn to placebo during COAST-Y or (4) were switched to open-label IXE during the randomised withdrawal period of COAST-Y. Separately, patients who were randomised to placebo or adalimumab in any of the originating studies were included in the analysis of intention-to-treat (ITT) patients who received at least one dose of IXE (N=932).
rmdopen-2021-002165supp001.pdf (43.4KB, pdf)
The safety population included patients who received at least one dose of IXE (N=932).
Study design
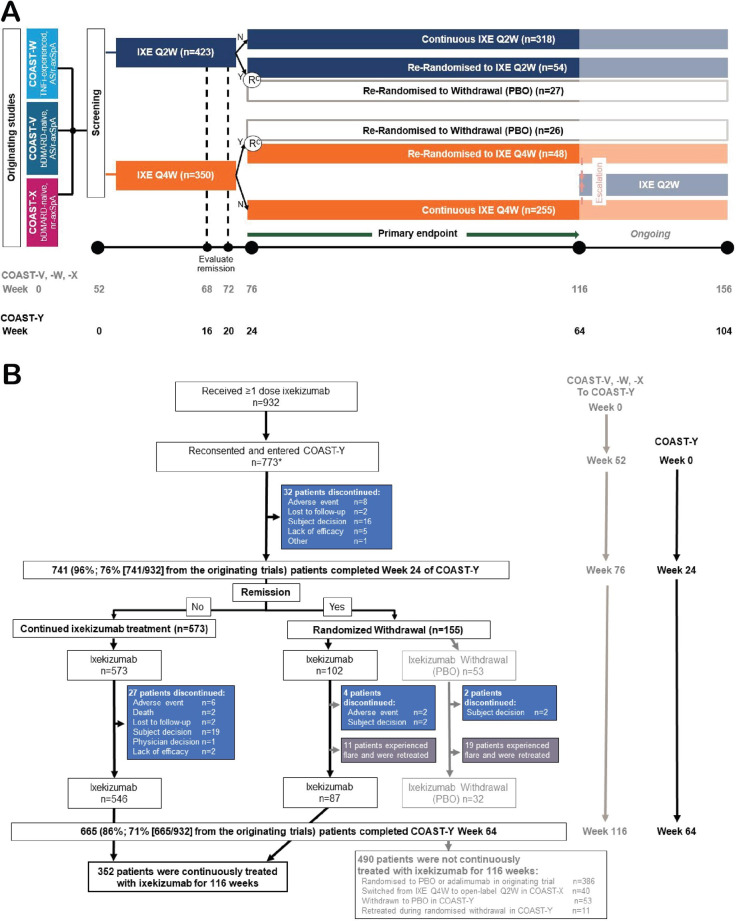
Participants who completed any of the 52-week originating studies (COAST-V, COAST-W and COAST-X) could enter COAST-Y. COAST-Y is a 2-year, ongoing, phase 3, multicentre, long-term extension study that is comprised of an open-label lead-in period and a double-blind, placebo-controlled, randomised withdrawal-retreatment period.22 In COAST-Y, patients who achieved remission could enrol into the randomised withdrawal-retreatment period (N=155, figure 1B). The study design and data from this analysis have been previously reported.22 The aim of this publication is to report data from (1) the subset of COAST-Y patients who were continuously treated with IXE for 116 weeks (52 weeks of the originating studies plus 64 weeks of COAST-Y) (figure 1A) and (2) patients from the ITT population who received at least one dose of IXE.
Figure 1.
Study design and patient disposition for the three originating studies of ixekizumab in axial spondyloarthritis and COAST-Y. (A) The study design shows that patients who completed the originating studies (COAST-V, COAST-W and COAST-X) and consented to enter the long-term extension study (COAST-Y) on the ixekizumab dose regimen they were receiving at the end of the originating study. Patients who completed COAST-X on placebo were started on ixekizumab every 4 weeks at the start of COAST-Y. (B) Patient disposition for COAST-Y. Disposition for the originating studies has been previously described.18–20 Efficacy analyses in this article focus on the patients who were continuously treated with ixekizumab for 116 weeks (52 weeks of the originating studies plus 64 weeks of the ongoing COAST-Y extension study). *Two patients who were originally randomised to placebo in COAST-X and remained on placebo throughout COAST-X entered COAST-Y but did not receive any ixekizumab injection, thus not included in the analysis population. axSpA, axial spondyloarthritis; bDMARD, biological disease-modifying anti-rheumatic drug; IXE, ixekizumab; n, n-number; N, no, did not achieve a state of sustained remission; nr-axSpA, non-radiographic axSpA; PBO, placebo; r-axSpA, radiographic axSpA; Y, yes, did achieve a state of sustained remission.
COAST-Y was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was approved by the main ethics committee Schulman Associates IRB, Cincinnati, OH, USA (IRB # 201607390), and the study was approved by the ethical review boards at each of 127 total participating sites.22 Study participants provided written informed consent prior to starting study procedures.
Outcomes
In this report, we present the results of secondary endpoints which were pre-specified in the statistical analysis plan of COAST-Y. The primary outcome has been previously reported.22 Efficacy outcomes reported from week 0 to week 116 in this analysis (online supplemental table 2), include ASAS responses (ASAS20, ASAS40 and ASAS 5/6, and ASAS partial remission), Ankylosing Spondylitis Disease Activity Score (ASDAS) status (inactive disease (<1.3), low disease activity (<2.1), clinically important improvement (≥1.1 unit of change) and major improvement (≥2.0 units of change)), 50% improvement in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI50), and CRP ≤5 mg/L, and change from baseline in the individual components of the ASAS criteria, ASDAS, CRP, BASDAI, Bath Ankylosing Spondylitis Functional Index (BASFI), Bath Ankylosing Spondylitis Metrology Index (BASMI, linear), Medical Outcomes Study 36-item Questionnaire Short-Form Health Survey (SF-36), including both the Physical Component Summary (PCS) and Mental Component Summary and ASAS Health Index. While the bDMARD-naive and TNFi-experienced studies were not designed for comparison purposes (no p values reported), we are illustrating the data in the same figure for clinical relevance.
rmdopen-2021-002165supp003.pdf (64.7KB, pdf)
Safety evaluations included vital signs, physical examinations, laboratory tests and adverse events (AEs), including treatment-emergent AEs (TEAEs), serious AEs (SAEs) and AEs of special interest. Data associated with cerebrocardiovascular events or suspected inflammatory bowel disease (IBD) were adjudicated by an external clinical events committee.
Statistical analysis
The continuously treated with IXE population included patients who were randomised to IXE at week 0 of the originating studies and continuously received the same treatment regimen through week 64 in COAST-Y (116 weeks of treatment). Both imputed and observed data for patients continuously treated with IXE were included. Post hoc analyses were performed on the continuously treated with IXE population after segmenting patients according to disease classification (r-axSpA and nr-axSpA) and TNFi-experience. This study is descriptive and any comparisons between groups are merely descriptive by nature. Missing data were imputed using non-responder imputation (NRI) for categorical data. Patients were considered non-responders for the NRI analysis if they did not meet the clinical response criteria or had missing clinical response data at any specified analysis time point. All patients who discontinued study treatment before the specified analysis time point, for any reason, were also considered non-responders. Continuous data were summarised using mean and standard deviation (SD). Missing data for continuous measures were imputed using modified baseline observation carried forward (mBOCF). Both imputed and observed data for patients continuously treated with IXE were included, with less than 2% corresponding to imputed data. Safety outcomes were analysed in the population of all patients who received at least one dose of IXE. For the purpose of the safety analysis, in COAST -X, there were 40 patients who switched from IXE every 4 weeks to open-label every 2 weeks. Those 40 patients are counted in the IXE every 4 weeks arm while in the every 4 weeks arm. On switching to every 2 weeks, those patients are also counted in the IXE every 2 weeks arm. However, for the total IXE, those patients are counted only once.
There was a potential for a selection bias favouring inclusion in COAST-Y of patients who responded well to IXE in their originator trials. To better understand this, ASAS40 outcomes at the end of the originator trials were compared in patients who enrolled versus did not enrol in COAST-Y.
Results
Study populations
Overall, 932 patients received at least one dose of IXE in the originating studies (COAST-V, COAST-W, or COAST-X) and COAST-Y (figure 1B), and 824 completed their originating trial. Of these, 773 patients from 22 countries enrolled in COAST-Y beginning on 9 May 2017, 86% (665/773 including 71% (665/932) of patients from the originating studies) completed week 116 of treatment (weeks 0–52 of one of the originating studies and 64 weeks of COAST-Y). Among the 824 patients who completed their originator trial, a comparison of patients who re-consented and enrolled (n=773) versus those who did not reconsent and enrol (n=51) in COAST-Y demonstrated that ASAS40 was more frequently achieved by patients who enrolled (50.3% vs 19.6%, respectively) (online supplemental table 1). Data for the 932 ITT patients who received at least one dose of IXE, including the patients who were initially randomised to placebo or adalimumab in an originating study, patients who switched from IXE every 4 weeks to open-label to IXE every 2 weeks during COAST-X, and patients who were withdrawn to placebo during COAST-Y are presented in online supplemental table 5 for efficacy and table 3 and online supplemental table 6 for safety.
rmdopen-2021-002165supp002.pdf (27.2KB, pdf)
rmdopen-2021-002165supp006.pdf (50.7KB, pdf)
rmdopen-2021-002165supp007.pdf (28.5KB, pdf)
Demographics and baseline disease activity
At entry into the originating studies (baseline), the continuously treated population had a mean age of 41.9 years, and 65.3% had elevated CRP >5 mg/L) levels. A total of 352 patients were grouped according to disease classification and TNFi-experience, with equal proportions of each of the following subgroups within each treatment arm: 114 (32.4%) patients had r-axSpA and were bDMARD-naïve, 104 (29.5%) patients had nr-axSpA and were bDMARD-naïve, and 134 (38.1%) patients had r-axSpA and prior treatment with TNFi (TNFi-experienced) (table 1). Generally, the demographic characteristics were similar when patients were grouped according to axSpA classification and prior TNFi treatment (table 1), except patients with r-axSpA had longer mean symptom duration than patients with nr-axSpA (15.1 years for bDMARD-naïve r-axSpA and 17.2 years for TNFi-experienced r-axSpA compared with 10.1 years for nr-axSpA). Additionally, there was a significantly greater proportion of male patients in the r-axSpA studies, compared with the nr-axSpA study (82.1% of TNFi-experienced patients with r-axSpA were male and 83.3% of bDMARD-naïve patients with r-axSpA were male compared with 49% of patients with nr-axSpA). The TNFi-experienced patients with r-axSpA had numerically greater disease activity (ASDAS, BASDAI), greater functional impairment (BASFI and BASMI) and lower health-related quality of life (SF-36 PCS) at baseline. Additionally, 32.1% of the TNFi-experienced patients had previously been treated with two TNFi (table 1).
Table 1.
Baseline demographics and disease characteristics for patients continuously treated with ixekizumab for 116 weeks (N=352)
| Biological DMARD-naïve | TNFi-experienced | ||
| r-axSpA N=114 |
nr-axSpA N=104 |
r-axSpA N=134 |
|
| Age (years) | 40.3 (10.4) | 39.6 (12.8) | 45.9 (11.8) |
| Male gender, n (%) | 95 (83.3) | 51 (49.0) | 110 (82.1) |
| Race, n (%) | |||
| White | 70 (61.4) | 81 (77.9) | 108 (81.2) |
| Asian | 36 (31.6) | 16 (15.4) | 16 (12.0) |
| Other | 8 (7.0) | 7 (6.7) | 9 (6.8) |
| BMI (kg/m2) | 26.0 (3.8) | 27.0 (5.4) | 28.4 (6.1) |
| axSpA symptom duration (years) | 15.1 (9.7) | 10.1 (9.9) | 17.2 (10.0) |
| axSpA diagnosis duration (years) | 7.6 (8.3) | 3.5 (4.8) | 10.6 (7.8) |
| HLA-B27 positive, n (%) | 109 (95.6) | 81 (78.6) | 110 (82.1) |
| Concomitant baseline medication, n (%) | |||
| NSAIDs | 109 (95.6) | 95 (91.3) | 104 (77.6) |
| Sulfasalazine | 40 (35.1) | 31 (29.8) | 24 (17.9) |
| Methotrexate | 8 (7.0) | 17 (16.3) | 14 (10.4) |
| Glucocorticoids | 12 (10.5) | 18 (17.3) | 16 (11.9) |
| Prior TNFi, n (%)* | |||
| Used 0 | 114 (100) | 104 (100) | 0 |
| Used 1 | 0 | 0 | 91 (67.9) |
| Used 2 | 0 | 0 | 43 (32.1) |
| CRP (mg/L) | 12.9 (14.2) | 12.2 (16.4) | 18.9 (31.3) |
| Elevated CRP (>5.0 mg/L), n (%) | 76 (66.7) | 60 (57.7) | 94 (70.1) |
| ASDAS | 3.8 (0.7) | 3.8 (0.8) | 4.1 (0.8) |
| BASDAI score | 6.8 (1.4) | 7.1 (1.3) | 7.4 (1.2) |
| BASDAI inflammation† | 6.7 (1.6) | 6.9 (1.6) | 7.3 (1.6) |
| BASFI score | 6.2 (1.9) | 6.3 (1.9) | 7.4 (1.4) |
| BASMI score | 3.9 (1.3) | 3.17 (1.2) | 4.8 (1.4) |
| PatGA | 7.0 (1.5) | 7.3 (1.6) | 7.8 (1.6) |
| SF-36 PCS | 36.4 (6.9) | 33.1 (6.7) | 31.5 (6.7) |
| SF-36 MCS | 48.8 (11.3) | 47.1 (11.9) | 46.8 (11.7) |
| Spinal pain due to AS | 7.2 (1.4) | 7.3 (1.7) | 7.8 (1.5) |
| Spinal pain at night due to AS | 7.1 (1.4) | 7.2 (1.8) | 7.7 (1.5) |
Data are presented as mean (SD) unless otherwise specified. Baseline was defined as week 0 of originating study.
*Excludes adalimumab taken as study drug in COAST-V.
†Mean of BASDAI questions 5 and 6.
AS, ankylosing spondylitis; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Score; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BMI, body mass index; CRP, C reactive protein; DMARD, disease-modifying anti-rheumatic drug; HLA-B27, human leucocyte antigen B27; IXE, ixekizumab; MCS, Mental Component Summary; nr-axSpA, non-radiographic axSpA; NSAIDs, non-steroidal anti-inflammatory drugs; PatGA, Patient Global Assessment of Disease Activity; PCS, Physical Component Summary; r-axSpA, radiographic axSpA; SF-36, 36-item Questionnaire Short-Form Health Survey; TNFi, tumour necrosis factor inhibitor.
Clinical outcomes
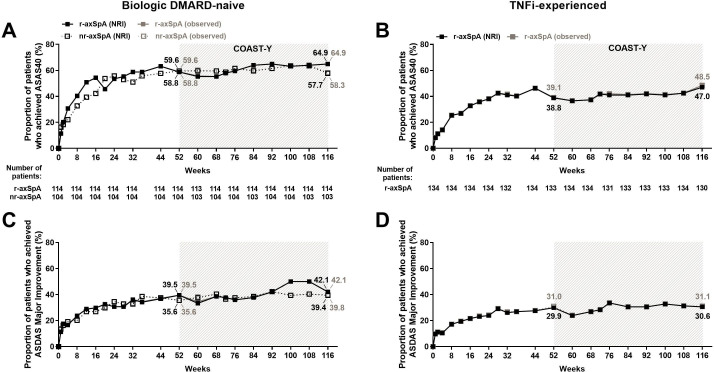
Patients receiving IXE in either of the two different dose regimens (every 4 weeks or every 2 weeks, online supplemental table 3) showed similar efficacy. Thus, the remaining analyses were conducted by pooling the two dose regimens. Patients who were continuously treated with IXE for 116 weeks (52 weeks of the originating studies plus 64 weeks of COAST-Y) showed improvements in axSpA disease activity by week 16, and the reduction was sustained through week 116 (table 2, figure 2). Overall, the proportion of patients continuously treated with IXE achieving ASAS40 at weeks 52 and 116 (week 0 and 64 of COAST-Y, respectively) were 51.4% (181/352) and 56.0% (197/352), respectively (table 2). To evaluate the responses by classification as r-axSpA or nr-axSpA and TNFi-experience, patients were stratified accordingly by these criteria. Almost half of patients achieved ASAS40 by week 16 and sustained responses through week 116, the proportion of patients achieving ASAS40 was 64.9% (74/114) and 57.7% (60/104) for bDMARD-naïve patients with r-axSpA and nr-axSpA, respectively (figure 2A, table 2). For the TNFi-experienced patients with r-axSpA, 47.0% (63/134) achieved ASAS40 at week 116 (figure 2B, table 2). The proportion of patients achieving low disease activity (ASDAS <2.1) through week 116 was 46.9% (165/352) overall, and 57.0% (65/114) and 52.9% (55/104) for bDMARD-naïve patients with r-axSpA and nr-axSpA, respectively (table 2; online supplemental figure 1A); for TNFi-experienced patients with r-axSpA, the proportion achieving ASDAS <2.1 low disease activity through 116 weeks was 33.6% (45/134) (table 2; online supplemental figure 1). The proportion of patients achieving ASDAS major improvement (change from baseline ≥2.0) through week 116 was 36.9% (130/352) overall, and 42.1% (48/114) and 39.4% (41/104) for bDMARD-naïve patients with r-axSpA and nr-axSpA, respectively (figure 2C; table 2); for TNFi-experienced patients with r-axSpA, the proportion of patients achieving ASDAS major improvement through week 116 was 30.6% (41/134) (figure 2D; table 2). Similar trends were observed for BASDAI50, and other clinical measures (table 2, online supplemental figure 1).
Table 2.
Efficacy outcomes at weeks 52 and 116 during continuous ixekizumab treatment (N=352; NRI and mBOCF)
| All patients N=352 |
Biological DMARD-naïve | TNFi-experienced | ||||||
| r-axSpA N=114 |
nr-axSpA N=104 |
r-axSpA N=134 |
||||||
| Week 52 | Week 116 | Week 52 | Week 116 | Week 52 | Week 116 | Week 52 | Week 116 | |
| Response: n (%) | ||||||||
| ASAS20 | 254 (72.2) | 260 (73.9) | 90 (78.9) | 92 (80.7) | 80 (76.9) | 80 (76.9) | 84 (62.7) | 88 (65.7) |
| ASAS40 | 181 (51.4) | 197 (56.0) | 67 (58.8) | 74 (64.9) | 62 (59.6) | 60 (57.7) | 52 (38.8) | 63 (47.0) |
| ASAS partial remission | 69 (19.6) | 70 (19.9) | 30 (26.3) | 31 (27.2) | 25 (24.0) | 29 (27.9) | 14 (10.4) | 10 (7.5) |
| ASAS 5/6 | 208 (59.1) | 210 (59.7) | 77 (67.5) | 80 (70.2) | 67 (64.4) | 65 (62.5) | 64 (47.8) | 65 (48.5) |
| CRP ≤5 mg/L | 241 (68.5) | 260 (73.9) | 90 (78.9) | 94 (82.5) | 79 (76.0) | 81 (77.9) | 72 (53.7) | 85 (63.4) |
| ASDAS LDA (<2.1) | 163 (46.3) | 165 (46.9) | 67 (58.8) | 65 (57.0) | 56 (53.8) | 55 (52.9) | 40 (29.9) | 45 (33.6) |
| ASDAS ID (<1.3) | 48 (13.6) | 53 (15.1) | 25 (21.9) | 22 (19.3) | 15 (14.4) | 23 (22.1) | 8 (6.0) | 8 (6.0) |
| BASDAI50 | 161 (45.7) | 174 (49.4) | 65 (57.0) | 69 (60.5) | 51 (49.0) | 56 (53.8) | 45 (33.6) | 49 (36.6) |
| ASDAS clinically important improvement (≥1.1 CFB) | 233 (66.2) | 247 (70.2) | 81 (71.1) | 84 (73.7) | 74 (71.2) | 74 (71.2) | 78 (58.2) | 89 (66.4) |
| ASDAS major improvement (≥2.0 CFB) | 122 (34.7) | 130 (36.9) | 45 (39.5) | 48 (42.1) | 37 (35.6) | 41 (39.4) | 40 (29.9) | 41 (30.6) |
| Change from baseline: mean (SD) | ||||||||
| ASDAS | −1.63 (1.04) | −1.70 (1.09) | −1.76 (0.98) | −1.82 (0.99) | −1.71 (1.06) | −1.79 (1.21) | −1.46 (1.06) | −1.54 (1.07) |
| BASDAI | −3.24 (2.18) | −3.39 (2.23) | −3.56 (2.21) | −3.66 (2.18) | −3.52 (2.07) | −3.75 (2.32) | −2.76 (2.16) | −2.88 (2.13) |
| BASDAI inflammation* | −3.52 (2.45) | −3.64 (2.42) | −3.79 (2.47) | −3.84 (2.38) | −3.79 (2.51) | −3.91 (2.56) | −3.09 (2.35) | −3.25 (2.31) |
| CRP (mg/L) | −10.79 (22.30) | −11.15 (22.15) | −9.70 (13.45) | −9.96 (13.36) | −8.70 (16.33) | −8.87 (16.38) | −13.34 (30.67) | −13.94 (30.38) |
| PatGA | −3.3 (2.5) | −3.4 (2.6) | −3.5 (2.4) | −3.6 (2.5) | −3.6 (2.4) | −3.8 (2.9) | −2.9 (2.6) | −3.0 (2.5) |
| BASFI | −2.85 (2.35) | −2.98 (2.37) | −3.06 (2.19) | −3.23 (2.18) | −3.15 (2.60) | −3.31 (2.63) | −2.44 (2.22) | −2.50 (2.24) |
| BASMI | −0.54 (0.93) | −0.59 (0.97) | −0.61 (0.86) | −0.67 (0.90) | −0.59 (0.95) | −0.56 (0.91) | −0.45 (0.97) | −0.53 (1.06) |
| ASAS HI | −3.19 (3.43) | −3.37 (3.84) | −3.20 (3.15) | −3.42 (3.69) | −3.77 (3.68) | −3.95 (3.97) | −2.72 (3.40) | −2.87 (3.81) |
| SF-36 PCS | 8.94 (8.04) | 9.22 (8.58) | 8.89 (7.15) | 9.13 (7.63) | 10.36 (9.08) | 10.61 (10.70) | 7.89 (7.80) | 8.22 (7.32) |
| SF-36 MCS | 4.43 (9.66) | 3.88 (10.44) | 4.02 (9.49) | 3.35 (9.93) | 4.77 (9.44) | 4.26 (10.75) | 4.51 (10.02) | 4.05 (10.67) |
| Spinal pain due to AS | −3.5 (2.5) | −3.7 (2.6) | −4.0 (2.3) | −4.1 (2.4) | −3.6 (2.6) | −3.8 (2.8) | −3.0 (2.5) | −3.1 (2.5) |
| Spinal pain at night due to AS | −3.7 (2.6) | −3.8 (2.6) | −4.0 (2.4) | −4.3 (2.5) | −3.9 (2.7) | −4.0 (2.7) | −3.2 (2.7) | −3.2 (2.5) |
Baseline was defined as week 0 of originating study.
*Mean of BASDAI questions 5 and 6.
AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis International Society; ASAS HI, ASAS Health Index; ASASx, x% improvement of ASAS criteria; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CFB, change from baseline; CRP, C reactive protein; ID, inactive disease; LDA, low disease activity; mBOCF, modified baseline observation carried forward; MCS, Mental Component Summary; nr-axSpA, non-radiographic axSpA; NRI, non-responder imputation; PatGA, Patient Global Assessment of Disease Activity; PCS, Physical Component Summary; r-axSpA, radiographic axSpA; SF-36, 36-item Questionnaire Short-Form Health Survey; TNFi, tumour necrosis factor inhibitor.
Figure 2.
Proportion of patients achieving ASAS40 and ASDAS major improvement responses for 116 weeks of continuous ixekizumab treatment for (A, C) biological DMARD (bDMARD)-naïve patients and (B, D) TNFi-experienced patients. Missing data among the patients continuously treated with ixekizumab were imputed using non-responder imputation (NRI). The study was not designed to allow comparison between bDMARD-naïve patients with r-axSpA and bDMARD-naïve patients with nr-axSpA. Data are presented as proportion (%) of patients, both NRI (black) and observed (grey) values. ASAS40, 40% improvement in Assessment of Spondyloarthritis International Society criteria; ASDAS major improvement, ≥2.0 change from baseline in Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; DMARD, disease-modifying antirheumatic drug; nr-axSpA, non-radiographic axSpA; r-axSpA, radiographic axSpA; TNFi, tumour necrosis factor inhibitor.
rmdopen-2021-002165supp004.pdf (48.9KB, pdf)
rmdopen-2021-002165supp008.pdf (227.3KB, pdf)
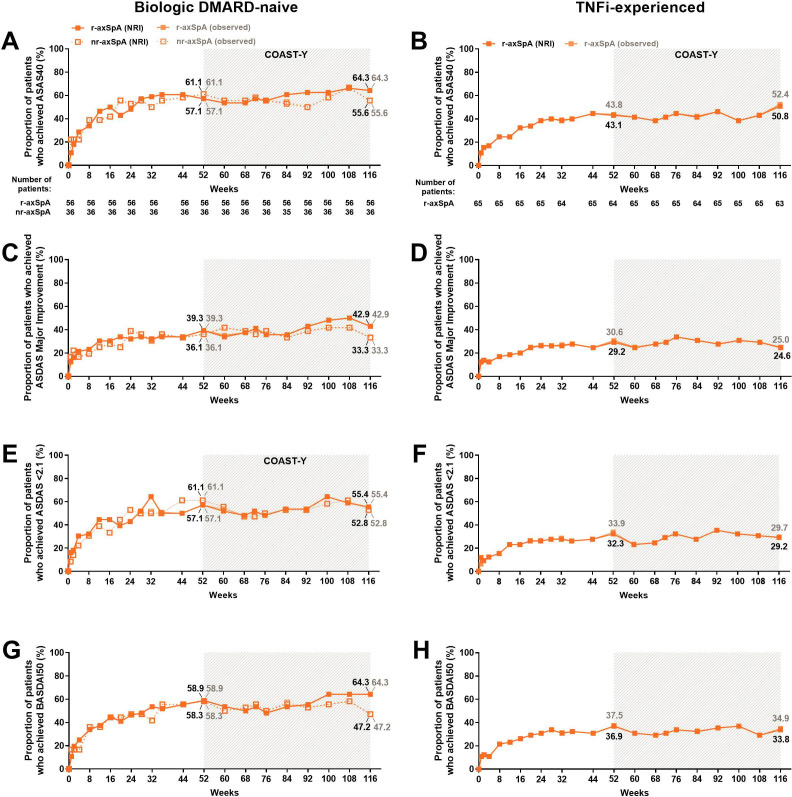
In addition to the improvements in ASAS40, ASDAS major improvement, and BASDAI50 observed in the combined IXE population (figure 2, table 2), similar response rates were observed for patients who received IXE every 4 weeks at weeks 52 and 116 (figure 3). Similar results were obtained when missing data from COAST-Y were imputed by NRI (figure 2; table 2) and when the data were summarised as observed (online supplemental table 4). Additionally, similar results were observed regardless of whether the patients started on IXE, placebo or adalimumab during the originating studies (online supplemental table 5). As expected, the imputed efficacy estimates were lower than the observed efficacy estimates in the analyses of all patients with ≥1 IXE dose in any COAST trial (including patients who did not participate in COAST-Y for any reason).
Figure 3.
Proportion of patients achieving ASAS40 and ASDAS major improvement, ASDAS <2.1 and BASDAI50 responses, for 116 weeks of IXE Q4W treatment for (A, C, E, G) biological DMARD (bDMARD)-naïve patients and (B, D, F, H) TNFi-experienced patients. Missing data among the IXE Q4W treated patients were imputed using NRI. The study was not designed to allow comparison between bDMARD-naïve patients with r-axSpA and bDMARD-naïve patients with nr-axSpA. Data are presented as the proportion (%) of patients, both NRI (black) and observed (grey) values. ASAS40, 40% improvement in Assessment of Spondyloarthritis International Society criteria; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI50, 50% improvement in Bath Ankylosing Spondylitis Disease Activity Index; DMARD, disease-modifying antirheumatic drug; IXE, ixekizumab; nr-axSpA, non-radiographic axSpA; r-axSpA, radiographic axSpA; TNFi, tumour necrosis factor inhibitor.
rmdopen-2021-002165supp005.pdf (48.9KB, pdf)
Patient-reported outcomes
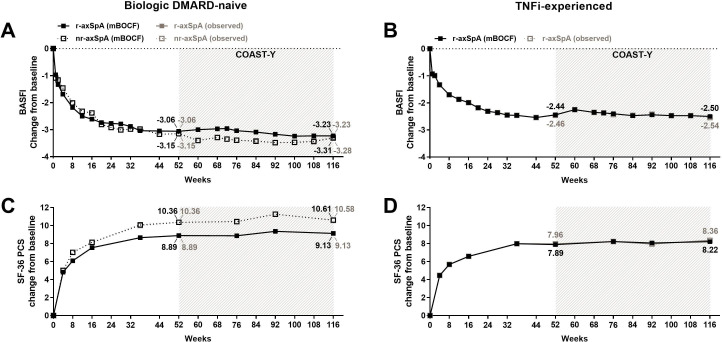
Examining the mean change from baseline from weeks 0 to 116 (52 weeks of the originating studies plus 64 weeks of COAST-Y), we demonstrated that patients had continuous improvements in patient-reported outcomes through week 52 and these clinically relevant improvements were sustained through week 116 (figure 4 and table 2). To determine the long-term effect of IXE on patients’ overall function, we examined the mean change from baseline for BASFI through week 116. The change from baseline for BASFI through week 116 was −2.98 overall, and −3.23 and −3.31 for bDMARD-naïve patients in r-axSpA and nr-axSpA groups, respectively (figure 4A, table 2). The TNFi-experienced patients who had r-axSpA achieved −2.50 improvement in BASFI through week 116 (figure 4B, table 2). Furthermore, a measure of patients’ quality of life as it relates to physical aspects (SF-36 PCS), was also improved, and these improvements were sustained through 116 weeks. The mean change from baseline for SF-36 PCS through week 116 was 9.22 overall, and 9.13 and 10.61 for bDMARD-naïve patients with r-axSpA and nr-axSpA, respectively (figure 4C). The change from baseline for TNFi-experienced patients with r-axSpA showed marked improvement in SF-36 PCS through week 116, (8.22; Figure 4D, table 2). Similar results were observed for other patient-reported outcomes at weeks 52 and 116 (table 2).
Figure 4.
Improvement of BASFI and Medical Outcomes Study 36-item Questionnaire Short-Form Health Survey (SF-36) PCS with up to 116 weeks of continuous ixekizumab treatment. For (A, C) biological DMARD (bDMARD)-naïve patients and (B, D) TNFi-experienced patients. Missing data among the patients continuously treated with ixekizumab were imputed using the mBOCF method. The study was not designed to allow comparison between bDMARD-naïve patients with r-axSpA and bDMARD-naïve patients with nr-axSpA. Data are presented as the proportion (%) of patients, both mBOCF (black) and observed (grey) values. axSpA, axial spondyloarthritis; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARD, biological disease-modifying antirheumatic drug; mBOCF, modified baseline observation carried forward; nr-axSpA, non-radiographic axSpA; PCS, Physical Component Summary; r-axSpA, radiographic axSpA; TNFi, tumour necrosis factor inhibitor.
Safety
TEAEs through 116 weeks (52 weeks of the originating studies plus 64 weeks of COAST-Y) of treatment were analysed for the 932 patients with axSpA who received at least one dose of IXE, with 454 patients who received IXE every 4 weeks and 518 patients who received IXE every 2 weeks, representing 713.3 and 885.6 patient-years (PY) of exposure for the groups, respectively. Safety outcomes for patients who received either IXE every 4 weeks or IXE every 2 weeks were analysed separately.
Overall, the long-term safety profile of IXE was consistent with what has been previously reported and no new safety signals were identified (table 3, online supplemental table 6). The incidence rates (IRs) for TEAEs were 52.4 and 47.8 cases per every 100 PY of treatment for IXE every 4 weeks and IXE every 2 weeks, respectively (table 3). Most TEAEs were mild or moderate in severity (table 3). Three deaths occurred in patients treated with IXE (total IR 0.2 per 100 PY): one patient died by homicide, one patient committed suicide and the third patient developed sepsis (probable pneumonia with multiorgan failure) after receiving IXE every 4 weeks for 108 weeks. The IRs were 5.0–5.7 per 100 PY for SAEs. The IRs were 1.2–1.3 per 100 PY for serious infections. There were no active cases of tuberculosis. There were 15 cases of IBD in total (IR 0.9 per 100 PY), confirmed by adjudication; of which, 6 were Crohn’s disease (5 of which were new-onset) and 9 were ulcerative colitis (of which 6 were new-onset). Anterior uveitis occurred in 52 patients (IR 3.3 per 100 PY). Two major adverse cerebro cardiovascular events occurred (IR 0.1 per 100 PY). Candidiasis occurred in 11 patients (IR 0.7 per 100 PY) (online supplemental table 6).
Table 3.
Safety data for all patients who received ≥1 dose of ixekizumab
| Safety population (n=932) (weeks 0–116) |
||||
| IXE Q4W* N=454 |
IXE Q2W* N=518 |
|||
| n (%) | IR (95% CI) PY=713.3 |
n (%) | IR (95% CI) PY=885.6 |
|
| TEAE | 374 (82.4) | 52.4 (47.4 to 58.0) | 423 (81.7) | 47.8 (43.4 to 52.5) |
| Mild | 150 (33.0) | 21.0 (17.9 to 24.7) | 165 (31.9) | 18.6 (16.0 to 21.7) |
| Moderate | 186 (41.0) | 26.1 (22.6 to 30.1) | 207 (40.0) | 23.4 (20.4 to 26.8) |
| Severe | 38 (8.4) | 5.3 (3.9 to 7.3) | 51 (9.8) | 5.8 (4.4 to 7.6) |
| SAE | 41 (9.0) | 5.7 (4.2 to 7.8) | 44 (8.5) | 5.0 (3.7 to 6.7) |
| Discontinuation due to AE | 26 (5.7) | 3.6 (2.5 to 5.4) | 34 (6.6) | 3.8 (2.7 to 5.4) |
| Death | 2 (0.4) | 0.3 (0.1 to 1.1) | 1 (0.2) | 0.1 (0.0 to 0.8) |
| TEAEs of special interest | ||||
| Infections | 238 (52.4) | 33.4 (29.4 to 37.9) | 280 (54.1) | 31.6 (28.1 to 35.5) |
| Serious infections | 9 (2.0) | 1.3 (0.7 to 2.4) | 11 (2.1) | 1.2 (0.7 to 2.2) |
| Tuberculosis (active cases) | 0 | 0 | 0 | 0 |
| IBD (adjudicated)† | 10 (2.2) | 1.4 (0.8 to 2.6) | 5 (1.0) | 0.6 (0.2 to 1.4) |
| Crohn’s disease | 4 (0.9) | 0.6 (0.2 to 1.5) | 2 (0.4) | 0.2 (0.1 to 0.9) |
| Ulcerative colitis | 6 (1.3) | 0.8 (0.4 to 1.9) | 3 (0.6) | 0.3 (0.1 to 1.1) |
| Anterior uveitis | 25 (5.5) | 3.5 (2.4 to 5.2) | 27 (5.2) | 3.0 (2.1 to 4.4) |
| Injection-site reactions | 53 (11.7) | 7.4 (5.7 to 9.7) | 107 (20.7) | 12.1 (10.0 to 14.6) |
| Mild | 40 (8.8) | 5.6 (4.1 to 7.6) | 79 (15.3) | 8.9 (7.2 to 11.1) |
| Moderate | 12 (2.6) | 1.7 (1.0 to 3.0) | 23 (4.4) | 2.6 (1.7 to 3.9) |
| Severe | 1 (0.2) | 0.1 (0.0 to 1.0) | 5 (1.0) | 0.6 (0.2 to 1.4) |
| Confirmed cerebrocardiovascular events | 7 (1.5) | 1.0 (0.5 to 2.1) | 7 (1.4) | 0.8 (0.4 to 1.7) |
| MACE‡ | 1 (0.2) | 0.1 (0.0 to 1.0) | 1 (0.2) | 0.1 (0.0 to 0.8) |
*During COAST-X, 40 patients switched from IXE Q4W to Q2W. AEs for these patients were counted for the dose regimen they were receiving when the AE occurred. These patients were counted in the total for both dose regimens.
†Events of suspected IBD were confirmed by adjudication by an external clinical events committee with expertise in IBD. EPIdemiologique des Maladies de l’Appareil Digestif criteria for adjudication of suspected IBD define ‘probable’ and ‘definite’ classifications as confirmed cases; two additional cases of IBD occurred, one during the long-term extension period (weeks 64–104) and one case during the post-treatment follow-up period.
‡Includes confirmed cases of vascular death (including cardiovascular and cerebrovascular deaths and excluding haemorrhagic deaths outside of the CNS), nonfatal myocardial infarction and nonfatal stroke.
AE, adverse event; IBD, inflammatory bowel disease; IR, incidence rate per 100 patient-years; IXE, ixekizumab; MACE, major adverse cardiovascular event; PY, patient-years; Q2W, every 2 weeks; Q4W, every 4 weeks; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
Of the 345 patients continuously treated with IXE for 116 weeks who were evaluated for antidrug antibodies, 17 developed antidrug antibodies during weeks 52–116, and none were positive for neutralising antibodies. Of these 17 patients, 13 achieved ASAS40 at week 116, compared with 183 of 328 of patients without antidrug antibodies.
Discussion
Previous studies have already demonstrated that IXE is efficacious in patients with axSpA, whether they were bDMARD-naïve or had been previously treated with TNFi. In the initial placebo phase of the studies, patients with axSpA did have definite improvements in disease activity, function, and quality of life on treatment with IXE by week 16.18–20 Here, we demonstrate that these improvements were sustained for over 2 years (116 weeks).
Unlike other long-term studies in patients with axSpA, the COAST-Y extension study included patients with both r- and nr-axSpA. Regardless of disease subtype, patients with axSpA responded to IXE treatment; ASAS40 (figure 2A), ASDAS low disease activity and ASDAS major improvement responses were sustained in the long-term among patients with r-axSpA and nr-axSpA. Improvements from baseline were very similar for clinical outcomes and patient-reported outcomes in both subgroups.
For the patients with r-axSpA who were previously treated with up to two TNFi, we observed that the proportion of patients achieving ASAS40 at week 16 was lower than the other groups. Although these studies were not designed to compare between these groups, this result is in accordance with what has been reported in previous studies.21 23 TNFi-experienced patients usually have longer symptom duration and more severe disease at baseline in comparison to bDMARD-naïve patients. COAST-W is presently the only study that exclusively studied patients with r-axSpA who were previously treated with up to two TNFi. These patients represent a group that is typically more difficult to treat. We demonstrated sustained improvements in efficacy outcomes through week 116.
Similar to previous reports, here, we observe that the demographics of patients with r-axSpA and nr-axSpA were different.24–26 Particularly, differences were noted in the gender ratio (83% vs 49% male gender), HLA-B27 positivity (96% vs 79%), symptom duration (16 vs 10 years) and the time to diagnosis. However, the treatment responses were similar between subgroups with only a trend for better response rates in r-axSpA. The somewhat better response rates in patients with r-axSpA are in agreement with what has been previously reported for other biologics.25 27 28 This may potentially be due to differences in demographics and disease characteristics, such as disease duration and peripheral involvement, between these two populations.
Safety data reported in this analysis were consistent with previously published reports for IXE in axSpA.29 There were no new safety signals; of the TEAEs, the majority observed were mild or moderate in severity and only 9.5% of TEAEs were severe. The most frequently reported TEAE was infection, affecting 54.7% (510/932) of patients in the safety population, with only 3.9% (20/510) of all infections being serious.
One strength of the study is that COAST-Y is a multicentre study with geographically diverse patient population with approximately equal proportions representing bDMARD-naïve patients with r-axSpA, bDMARD-naïve patients with nr-axSpA, and TNFi-experienced patients with r-axSpA. These characteristics support the generalisability of the results for axSpA patients worldwide. Finally, the large axSpA population in the COAST programme allowed reporting of safety with over 1500 PY of exposure, with populations that reflect in great proportion what is seen in clinical practice.
This study was limited by the relatively short duration of the comparator arms (16 weeks for placebo in all originator studies, as well as for adalimumab in COAST-V). Another limitation is that patients were required to re-consent in order to transition from their originator study into COAST-Y. While multiple factors inevitably influenced patients’ decisions to participate in COAST-Y, there was a selection bias, such that patients who responded favourably to IXE in an originator trial participated in COAST-Y more frequently than patients with less favourable responses. It is possible that this bias may, in part, explain the difference seen in the response rates among the continuously treated with IXE population (N=352) and the ITT population of patients who received at least one dose of IXE (N=932).
In summary, improvements in disease activity, function and quality of life were achieved early on into the treatment and were sustained through 116 weeks of IXE treatment for patients with r-axSpA and nr-axSpA. This was noted in patients without bDMARD exposure, as well as in patients who were TNFi-experienced. In addition, treatment responses in measures of disease activity were comparable in both the r-axSpA and nr-axSpA patient populations. No new safety signals were identified in patients treated with IXE. Overall, the sustained efficacy and safety results in this selected patient population demonstrate that IXE could be a treatment option for r-axSpA and nr-axSpA. Further confirmation is needed from real-life data.
Acknowledgments
The authors thank Emily Blue, PhD, and Edel Hughes, PhD, medical writers and employees of Eli Lilly and Company, for writing and editorial support. The authors also thank Ann Leung, MS, a biostatistician and employee of Syneos Health, for statistical review and support. Eli Lilly and Company was involved in study design, collection, analysis, and interpretation of data, and preparation of the submitted manuscript. All authors had full access to all data in the study and had the final responsibility for the decision to submit for publication.
Footnotes
Contributors: JB was an investigator for the COAST-Y trial, contributed to the interpretation of data, and revised the draft paper. UK contributed to the interpretation of data, and revised the draft paper. AD was an investigator for the COAST-Y trial, contributed to the interpretation of data and revised the draft paper. TT was an investigator for the COAST-Y trial, contributed to the interpretation of data, and revised the draft paper. MD contributed to the interpretation of data, and revised the draft paper. RB contributed to the analysis and interpretation of data, revised the draft paper, and is responsible for the overall content as guarantor. DS contributed to the conception of the work, the interpretation of data, and revised the draft paper. C-YL contributed to the analysis and interpretation of data, and revised the draft paper. JW contributed to the interpretation of data, and revised the draft paper.
Funding: This study was funded by Eli Lilly and Company (grant number: not applicable).
Competing interests: JB has received compensation as a speaker, adviser, or consultant for, or has received grant support from Abbvie (Abbott), Amgen, Baxter, Biogen, BMS, Boehringer, Celgene, Celltrion, Centocor, Chugai, Eli Lilly and Company, Fresenius, GlaxoSmithKline, Gilead, Hexal, Janssen, Medac, MSD (Schering-Plough), Mylan, Mundipharma, Novartis, Pfizer (Wyeth, Hospira), Roche, Sanofi-Aventis and UCB. MD has received compensation as a consultant or adviser for, and has received grant support from Abbvie, Biogen, Eli Lilly and Company, Galapagos, Merck, Pfizer and UCB. UK has received compensation as a consultant, adviser, or speaker for Abbvie, Eli Lilly and Company, Hexal, Janssen, MSD, Novartis, Pfizer, UCB and Viatris; and has received grant support from Amgen, Hexal and Novartis. AD has received compensation as a consultant, adviser or speaker for Abbvie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, GlaxoSmithKilne, Janssen, Novartis, Pfizer and UCB; has received travel, grant, or research support from Abbvie, Eli Lilly and Company, GlaxoSmithKline, Novartis, Pfizer and UCB; and serves as a member of the GRAPPA steering committee. TT has received compensation as a consultant and adviser for Eli Lilly and Company; and as a speaker for Abbvie, Bristol Myers Squibb, Eli Lilly and Company, Janssen, Mitsubishi-Tanabe, Novartis, Pfizer. JW has received compensation as a consultant for Abbvie, Amgen, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB; has received grant or travel support from Abbvie, Eli Lilly and Company, Merck, and Pfizer. DS, and CL are full-time employees and shareholders of Eli Lilly and Company. RB is an employee and shareholder of Eli Lilly and Company; has received travel support for and has received compensation as an adviser for Eli Lilly and Company.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available on reasonable request. Lilly provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by COAST-Y was conducted in accordance with the ethical principles of the Declaration of Helsinki. The study was approved by the main ethics committee Schulman Associates IRB, Cincinnati, Ohio, USA (IRB # 201607390), and the study was approved by the ethical review boards at each of 127 total participating sites (Landewé 2021). Participants gave informed consent to participate in the study before taking part.
References
- 1.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the new York criteria. Arthritis Rheum 1984;27:361–8. 10.1002/art.1780270401 [DOI] [PubMed] [Google Scholar]
- 2.Rudwaleit M, Landewé R, van der Heijde D, et al. The development of assessment of spondyloarthritis International Society classification criteria for axial spondyloarthritis (Part I): classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–6. 10.1136/ard.2009.108217 [DOI] [PubMed] [Google Scholar]
- 3.Boel A, Molto A, van der Heijde D, et al. Do patients with axial spondyloarthritis with radiographic sacroiliitis fulfil both the modified New York criteria and the ASAS axial spondyloarthritis criteria? results from eight cohorts. Ann Rheum Dis 2019;78:1545–9. 10.1136/annrheumdis-2019-215707 [DOI] [PubMed] [Google Scholar]
- 4.Deodhar A, Strand V, Kay J, et al. The term 'non-radiographic axial spondyloarthritis' is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann Rheum Dis 2016;75:791–4. 10.1136/annrheumdis-2015-208852 [DOI] [PubMed] [Google Scholar]
- 5.López-Medina C, Ramiro S, van der Heijde D, et al. Characteristics and burden of disease in patients with radiographic and non-radiographic axial spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open 2019;5:e001108. 10.1136/rmdopen-2019-001108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilic G, Kilic E, Ozgocmen S. Relationship between psychiatric status, self-reported outcome measures, and clinical parameters in axial spondyloarthritis. Medicine 2014;93:e337. 10.1097/MD.0000000000000337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Heijde D, Purcaru O, Kavanaugh A. FRI0439 High economic burden of axial spondyloarthritis related to paid work and household productivity at baseline in the rapid-axspa study: differences and similarities between ankylosing spondylitis and non-radiographic axial spondyloarthritis. Ann Rheum Dis 2013;72:A523.2–4. 10.1136/annrheumdis-2013-eular.1566 [DOI] [Google Scholar]
- 8.van der Heijde D, Ramiro S, Landewé R, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis 2017;76:978–91. 10.1136/annrheumdis-2016-210770 [DOI] [PubMed] [Google Scholar]
- 9.Ward MM, Deodhar A, Gensler LS, et al. 2019 update of the American College of Rheumatology/Spondylitis association of America/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and Nonradiographic axial spondyloarthritis. Arthritis Rheumatol 2019;71:1599–613. 10.1002/art.41042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glintborg B, Ostergaard M, Krogh NS, et al. Predictors of treatment response and drug continuation in 842 patients with ankylosing spondylitis treated with anti-tumour necrosis factor: results from 8 years' surveillance in the Danish nationwide DANBIO registry. Ann Rheum Dis 2010;69:2002–8. 10.1136/ard.2009.124446 [DOI] [PubMed] [Google Scholar]
- 11.Heiberg MS, Koldingsnes W, Mikkelsen K, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Arthritis Rheum 2008;59:234–40. 10.1002/art.23333 [DOI] [PubMed] [Google Scholar]
- 12.Inman RD, Davis JC, Heijde DVD, et al. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum 2008;58:3402–12. 10.1002/art.23969 [DOI] [PubMed] [Google Scholar]
- 13.van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 2006;54:2136–46. 10.1002/art.21913 [DOI] [PubMed] [Google Scholar]
- 14.Dougados M, Baeten D. Spondyloarthritis. Lancet 2011;377:2127–37. 10.1016/S0140-6736(11)60071-8 [DOI] [PubMed] [Google Scholar]
- 15.Braun J, Baraliakos X, Brandt J, et al. Persistent clinical response to the anti-TNF-alpha antibody infliximab in patients with ankylosing spondylitis over 3 years. Rheumatology 2005;44:670–6. 10.1093/rheumatology/keh584 [DOI] [PubMed] [Google Scholar]
- 16.van der Heijde D, Sieper J, Baeten DL, et al. SAT0337 Clinical Response and Remission in Patients with Non-Radiographic Axial Spondyloarthritis after Three Years of Adalimumab Therapy: Table 1. Ann Rheum Dis 2014;73:714.1–714. 10.1136/annrheumdis-2014-eular.1696 [DOI] [Google Scholar]
- 17.George MD, Baker JF, Ogdie A. Comparative persistence of methotrexate and tumor necrosis factor inhibitors in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol 2020;47:826–34. 10.3899/jrheum.190299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deodhar A, Poddubnyy D, Pacheco-Tena C, et al. Efficacy and Safety of Ixekizumab in the Treatment of Radiographic Axial Spondyloarthritis: Sixteen-Week Results From a Phase III Randomized, Double-Blind, Placebo-Controlled Trial in Patients With Prior Inadequate Response to or Intolerance of Tumor Necrosis Factor Inhibitors. Arthritis Rheumatol 2019;71:599–611. 10.1002/art.40753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deodhar A, van der Heijde D, Gensler LS, et al. Ixekizumab for patients with non-radiographic axial spondyloarthritis (COAST-X): a randomised, placebo-controlled trial. Lancet 2020;395:53–64. 10.1016/S0140-6736(19)32971-X [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D, Cheng-Chung Wei J, Dougados M, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. Lancet 2018;392:2441–51. 10.1016/S0140-6736(18)31946-9 [DOI] [PubMed] [Google Scholar]
- 21.Dougados M, Wei JC-C, Landewé R, et al. Efficacy and safety of ixekizumab through 52 weeks in two phase 3, randomised, controlled clinical trials in patients with active radiographic axial spondyloarthritis (COAST-V and COAST-W). Ann Rheum Dis 2020;79:176–85. 10.1136/annrheumdis-2019-216118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landewé RBM, Gensler LS, Poddubnyy D, et al. Continuing versus withdrawing ixekizumab treatment in patients with axial spondyloarthritis who achieved remission: efficacy and safety results from a placebo-controlled, randomised withdrawal study (COAST-Y). Ann Rheum Dis 2021;80:1022–30. 10.1136/annrheumdis-2020-219717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieper J, Deodhar A, Marzo-Ortega H, et al. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the measure 2 study. Ann Rheum Dis 2017;76:571–92. 10.1136/annrheumdis-2016-210023 [DOI] [PubMed] [Google Scholar]
- 24.Kiltz U, Baraliakos X, Karakostas P, et al. Do patients with non-radiographic axial spondylarthritis differ from patients with ankylosing spondylitis? Arthritis Care Res 2012;64:1415–22. 10.1002/acr.21688 [DOI] [PubMed] [Google Scholar]
- 25.Landewé R, Braun J, Deodhar A, et al. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis 2014;73:39–47. 10.1136/annrheumdis-2013-204231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dougados M, van der Heijde D, Sieper J, et al. Symptomatic efficacy of etanercept and its effects on objective signs of inflammation in early nonradiographic axial spondyloarthritis: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 2014;66:2091–102. 10.1002/art.38721 [DOI] [PubMed] [Google Scholar]
- 27.van der Heijde D, Dougados M, Landewé R, et al. Sustained efficacy, safety and patient-reported outcomes of certolizumab pegol in axial spondyloarthritis: 4-year outcomes from RAPID-axSpA. Rheumatology 2017;56:1498–509. 10.1093/rheumatology/kex174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei JC-C, Kim T-H, Kishimoto M, et al. Efficacy and safety of brodalumab, an anti-IL17RA monoclonal antibody, in patients with axial spondyloarthritis: 16-week results from a randomised, placebo-controlled, phase 3 trial. Ann Rheum Dis 2021. 10.1136/annrheumdis-2020-219406. [Epub ahead of print: 07 Apr 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese MC, Mysler E, Tomita T, et al. Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatology 2020;59:3834–44. 10.1093/rheumatology/keaa189 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002165supp001.pdf (43.4KB, pdf)
rmdopen-2021-002165supp003.pdf (64.7KB, pdf)
rmdopen-2021-002165supp002.pdf (27.2KB, pdf)
rmdopen-2021-002165supp006.pdf (50.7KB, pdf)
rmdopen-2021-002165supp007.pdf (28.5KB, pdf)
rmdopen-2021-002165supp004.pdf (48.9KB, pdf)
rmdopen-2021-002165supp008.pdf (227.3KB, pdf)
rmdopen-2021-002165supp005.pdf (48.9KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available on reasonable request. Lilly provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.