Abstract
Introduction
Clinical research and treatment of childhood obesity is challenging, and objective biomarkers obtained in a home-setting are needed. The aim of this study was to determine the potential of novel digital endpoints gathered by a home-monitoring platform in pediatric obesity.
Methods
In this prospective observational study, 28 children with obesity aged 6–16 years were included and monitored for 28 days. Patients wore a smartwatch, which measured physical activity (PA), heart rate (HR), and sleep. Furthermore, daily blood pressure (BP) measurements were performed. Data from 128 healthy children were utilized for comparison. Differences between patients and controls were assessed via linear mixed effect models.
Results
Data from 28 patients (average age 11.6 years, 46% male, average body mass index 30.9) and 128 controls (average age 11.1 years, 46% male, average body mass index 18.0) were analyzed. Patients were recruited between November 2018 and February 2020. For patients, the median compliance for the measurements ranged from 55% to 100% and the highest median compliance was observed for the smartwatch-related measurements (81–100%). Patients had a lower daily PA level (4,597 steps vs. 6,081 steps, 95% confidence interval [CI] 862–2,108) and peak PA level (1,115 steps vs. 1,392 steps, 95% CI 136–417), a higher nighttime HR (81 bpm vs. 71 bpm, 95% CI 6.3–12.3) and daytime HR (98 bpm vs. 88 bpm, 95% CI 7.6–12.6), a higher systolic BP (115 mm Hg vs. 104 mm Hg, 95% CI 8.1–14.5) and diastolic BP (76 mm Hg vs. 65 mm Hg, 95% CI 8.7–12.7), and a shorter sleep duration (difference 0.5 h, 95% CI 0.2–0.7) compared to controls.
Conclusion
Remote monitoring via wearables in pediatric obesity has the potential to objectively measure the disease burden in the home-setting. The novel endpoints demonstrate significant differences in PA level, HR, BP, and sleep duration between patients and controls. Future studies are needed to determine the capacity of the novel digital endpoints to detect effect of interventions.
Keywords: Pediatric obesity, Home-monitoring, Smartwatch, Physical activity, Cardiovascular parameters
Introduction
Childhood obesity is a chronic disease with an increasing prevalence worldwide [1]. The disease is associated with a wide spectrum of adverse outcomes, including cardiovascular and metabolic complications, musculoskeletal problems, and psychosocial consequences [2]. Treatment, follow-up, and clinical research in pediatric obesity is challenging. Logistical problems such as travel distance and scheduling conflicts account for 44% of non-return to pediatric weight management programs, while another important factor is patient and family expectation of the program [3]. In the outpatient clinic, patients' and parents' recall of physical activity (PA) and food intake is frequently subjective and suboptimal. Reliable blood pressure (BP) measurements are important in the follow-up of pediatric obesity [4], but BP measurements during outpatient visits can be distorted by white coat hypertension [5]. The need for improved monitoring and treatment of pediatric obesity has become increasingly relevant in the past year, and recent studies have suggested that the COVID-19 pandemic caused a significant weight gain among children [6, 7]. All these examples indicate that there is a need for objective measurements obtained in a home-setting that can monitor disease activity, and this could be provided via remote monitoring with digital biomarkers [8].
Previous studies already assessed remote monitoring of daily step count in pediatric obesity and reported that they are less physically active compared to healthy children [9]. However, wearable devices can also capture other digital biomarkers, such as heart rate (HR) and various sleep-related endpoints (e.g., sleep duration and sleep depth) [10]. Capturing PA, HR, BP, and sleep simultaneously via wearable technology has not previously been reported in pediatric obesity. Remote monitoring with this selection of biomarkers may lead to early detection of common childhood obesity-related complications such as exercise-related complications, cardiovascular alterations, or obesity-associated sleep disorders [2]. Furthermore, home-measured HR and BP provides real-life data resulting in a better indication of cardiovascular risk status. These digital endpoints could be utilized in clinical care and clinical trials to evaluate the effect of lifestyle interventions in the home-setting and may contribute to a reduction of the burden to visit outpatient clinics. Clinical validation of novel digital endpoints must be performed in the target population before integration in clinical care or clinical trials [11]. Important clinical validation criteria for the candidate endpoints in this validation process are the tolerability and the ability to detect a significant difference between patients and controls, and these two validation criteria are the focus of this study. The aim of this study was to determine the clinical potential of novel digital endpoints derived from PA, HR, sleep, and BP gathered via a home-monitoring platform in pediatric obesity.
Materials and Methods
Location and Ethics
This study was conducted at the Haga Teaching Hospital, Juliana Children's Hospital (The Hague, The Netherlands) and at the Centre for Human Drug Research (Leiden, The Netherlands). The study protocol was approved by the Medical Ethics Committee Zuid West Holland (The Hague, The Netherlands), and was conducted according to the Dutch Act on Medical Research Involving Human Subjects (WMO) and Good Clinical Practice Guideline. Written informed consent was obtained from all parents. Verbal consent was obtained from children aged younger than 12 years and written consent was obtained from children aged 12 years and older. The trial was registered at the Dutch Trial Registry (NTR, Trial NL7611, registered March 18, 2019).
Subjects and Study Design
During this prospective observational case-control study, 28 patients, aged between 6 and 16 years old, were recruited via the outpatient clinic between November 2018 and February 2020. Patients diagnosed with obesity grade 1, 2, or 3 were included [12]. Children diagnosed with a chronic condition, other than obesity, that might impair PA levels were excluded. Data from 128 healthy controls, children aged between 6 and 16 years, were collected via a separate study [8]. The control group had a similar age and sex distribution as the patient group. Before the start of the study, an informative session was planned for education on the study devices for both the patient group and the control group. Afterward, patients were monitored in the home-setting during 28 consecutive days and used a smartphone that connected to other study devices. Patients were expected to wear a Steel HR smartwatch (Withings, Issy-les-Molineux, France) 24 h per day, which measured PA in step count and several sleep-related parameters via a built-in accelerometer and registered HR through a photoplethysmography sensor every 10 min. Data were directly uploaded to the server via the CHDR MORE application. Daily BP measurements were performed by a single-sized, wireless, upper arm cuff and oscillometric determination of pressure (Withings BPM) each evening at approximately the same clock time. Patients were instructed not to physically exert themselves just before the measurement. Weekly weight assessments were conducted with Withings Body + Scales. A daily questionnaire was completed on the smartphone regarding the daily screen time of the patient.
Baseline Characteristics and Environmental Data
At the start of the study, baseline characteristics were collected, and the Children's Somatization Inventory (CSI) questionnaire and Pediatric Quality of Life Inventory (PedsQL) 4.0 questionnaire were completed [13, 14]. The calculated body mass index standard deviation (SD) score was adjusted for age and sex at baseline. Data regarding the degree of urbanization of the child's city of residence were obtained via the Dutch Central Office of Statistics. An area with an address density of >2,500 addresses/km2 was considered as an extremely urbanized area. Weather data during the study period were collected from the Royal Dutch Meteorological Institute (KNMI) at the weather station located in Hoek van Holland.
Analysis
Compliance
An important validation criterion of the fit-for-purpose validation is the tolerability of the novel endpoints [11]. This criterion was assessed by determining the compliance for each measurement type. The compliance was calculated for each participant individually by dividing the amount of completed measurements by the amount of expected measurements. When the weight assessment deviated more than 2 days from the protocolized time point, this assessment was counted as not completed. The watch wear time between 6:00 a.m. and 10:00 p.m. was calculated to include as a covariate for the analysis of PA data. Calculation of the watch wear time was based on hourly data of PA and HR. When there was no registration of both HR and PA in a particular hour, it was concluded that the watch was not worn by the subject.
Candidate Endpoints
Multiple candidate endpoints based on PA, HR, sleep, and BP were defined prior to the analysis. The selected biomarkers needed to be able to be captured non-invasively, digitally, and objectively in the home-setting. First, the proposed PA-derived candidate biomarkers consisted of the daily PA, average PA per hour of the day, and PA during the most active hour per day (peak PA). HR data between 6:00 a.m. and 10:00 p.m. were summarized as average daytime HR, and HR data between 0:00 a.m. and 5:00 a.m. were summarized as average nighttime HR. In addition, the average HR per hour of the day was calculated. Moreover, systolic BP and diastolic BP were considered as candidate biomarkers. Lastly, sleep-related candidate endpoints consisted of the average sleep duration, sleep depth (average proportion light sleep), and the amount of wake-ups per night.
Statistical Analysis of the Candidate Endpoints
The ability of the candidate endpoints to detect a significant difference between patients and controls, another validation criterion of the validation process, was also examined [11]. Days with watch wear time <50% between 6:00 a.m. and 10:00 p.m. were excluded from the analysis. Differences between the two groups were assessed for each candidate endpoint via linear mixed effect models with condition (healthy or obesity) as fixed effect and subject as random effect. Residual plots were inspected, and logarithmic and square root transformations were applied in the case of heteroscedasticity. The following parameters were tested as additional fixed effect in a model when expected to explain additional variance: age, sex, watch wear time, day of the week, type of day (school day/weekend/holiday), degree of urbanization, rain duration, temperature, sunshine duration, and step count [8]. Polynomial regression with 3 degrees of freedom was utilized when exploratory plots suggested a nonlinear relationship. Inclusion of a covariate or factor and determining the best model fit were based on Akaike's information criterion, Bayesian information criterion, and likelihood ratio tests. Interactions between included covariates and factors were tested and considered to be included in the model if the interaction was biologically plausible. Estimated marginal means were calculated for both study groups and plotted with a 95% confidence interval (CI). For all estimated means, fixed effects were held constant to their population average. Models including watch wear time were visualized with a watch wear time of 100%. A p value less than 0.05 was considered statistically significant. Within the patient group, correlations between body mass index, SD score, quality of life, and daily PA were assessed by calculating Pearson's correlation coefficient or Spearman's correlation coefficient.
Software
Promasys® 7.3 (Anju Software, Fort Lauderdale, TX, USA) was used for data management of the baseline characteristics. Statistical analysis was performed with R version 3.6.2 with utilization of the lme4, emmeans, and ggeffects packages [15, 16, 17, 18].
Results
Baseline Characteristics
Baseline characteristics of the children with obesity (n = 28) and of the healthy children (n = 128) are presented in Table 1. The mean age was 11 years and 46% of the participants was male in both study groups. The mean body mass index of patients was 30.9 (body mass index SD score 3.6) versus 18.0 of controls (body mass index SD score 0.3). The average quality of life score measured by the PedsQL 4.0 questionnaire was 78.6 out of 100 in the patient group versus 90.7 out of 100 in the control group.
Table 1.
Baseline characteristics
| Children with obesity (n = 28) | Healthy children (n = 128) [8] | |
|---|---|---|
| Age, years, mean (SD) | 11.6 (3.1) | 11.1 (3.1) |
| Sex, male, n (%) | 13 (46) | 59 (46) |
| Weight, kg, mean (SD) | 77.7 (24.6) | 42.7 (15.7) |
| Height, cm, mean (SD) | 156.3 (15.5) | 151.3 (17.7) |
| Body mass index, mean (SD) | 30.9 (5.2) | 18.0 (3.1) |
| Body mass index SD score, mean (SD) | 3.6 (0.4) | 0.3 (1.2) |
| PedsQL score, mean (SD) | 78.6 (14.2) | 90.7 (7.4) |
| CSI score, mean (SD) | 12.3 (11.4) | − |
| Obesity grade, n (%) | ||
| Grade 1 | 10 (36) | − |
| Grade 2 | 10 (36) | |
| Grade 3 | 8 (29) | |
| Plays sports, n (%) | 18 (64) | 117 (91) |
| Ethnicity, n (%) | ||
| Caucasian | 20 (71) | 122 (95) |
| Asian/Hindi | 3 (11) | 2 (2) |
| Other/Mixed | 5 (18) | 4 (3) |
| Extremely urbanized area, n (%) | 24 (86) | 97 (76) |
Compliance
The median compliance of each measurement type is listed in Table 2. The median overall compliance was 74% (interquartile range 55–85%). The median wear time of the smartwatch was 22.0 h per day. The lowest median compliance was observed for the daily questionnaire (55%), while the highest median compliance was observed for two smartwatch-related measurements, HR (100%), and step count (100%). Two patients did not complete any BP measurements and 2 patients did not wear the smartwatch at night.
Table 2.
Compliance of children with obesity during the study period
| Measurement | Median compliance (IQR) |
|---|---|
| Smartwatch | |
| Step count | 100% (93–100%) |
| Heart rate | 100% (93–100%) |
| Sleep | 81% (62–89%) |
| Wear time watch per day | 22.0 h (18.0–23.3 h) |
| BP | 59% (32–79%) |
| Weight | 75% (25–100%) |
| Daily questionnaire | 55% (20–79%) |
| Overall compliance | 74% (55–85%) |
Difference Patients and Controls
Physical Activity
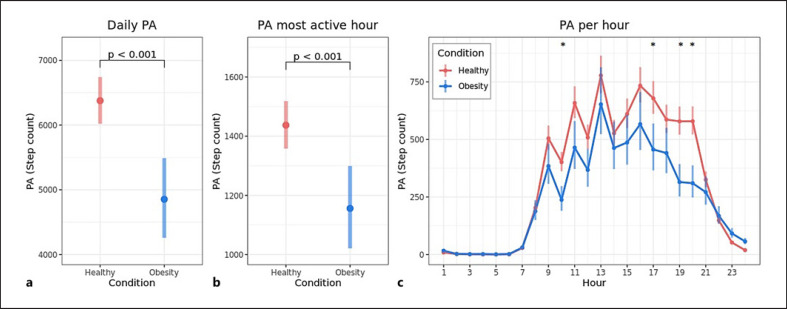
The average daily step count was 4,597 for patients versus 6,081 for controls (difference 1,485 steps, 95% CI 862–2,108, Fig. 1a). For patients, PA during the most active hour was lower compared to controls with a difference of 277 steps (1,115 steps vs. 1,392 steps, 95% CI 136–417, Fig. 1b). A separate analysis was performed to calculate the average PA per hour of the day, which was significantly lower for the patient group compared to the control group with a difference of more than 50 steps per hour at 10:00 a.m., 5:00 p.m., 7:00 p.m. and 8:00 p.m., after controlling for age and sex (Fig. 1c). Online supplementary Figure S1a and b (for all online suppl. material, see www.karger.com/doi/10.1159/000522185) displays the 10th and 90th percentile of the daily PA within a week, which were both lower for patients compared to controls with an adjusted difference of 948 steps (3,572 steps vs. 4,520 steps, 95% CI 325–1,571) and 2,257 steps (7,633 steps vs. 9,889 steps, 95% CI 1,315–3,199), respectively. An overview of all analyses with adjusted and unadjusted differences is listed in online supplementary Table S1. The relationship between daily PA and daily screen time for both study groups is visualized in online supplementary Figure S2. Daily PA decreased with an increase in screen time for both the patient group and the control group. For days with up to 2 h of screen time, patients performed less PA compared with controls (online suppl. Fig. S2).
Fig. 1.
Differences in PA between children with obesity and healthy children. Estimated marginal mean (95% CI) daily step count (a) and step count during the most active hour per day (b) for children with obesity and healthy children. Age (11 years), rain duration (1.87 h), weekday, degree of urbanization, and sex were fixed to their population average. Plots are visualized with watch wear time 100%. c Estimated marginal mean (95% CI) PA per hour during the day for children with obesity and healthy children. Age (11 years) and sex are fixed to their population average. *Indicate hours with a p value <0.05 for the difference (>50 steps per hour) after Holm's correction for multiple tests.
Heart Rate
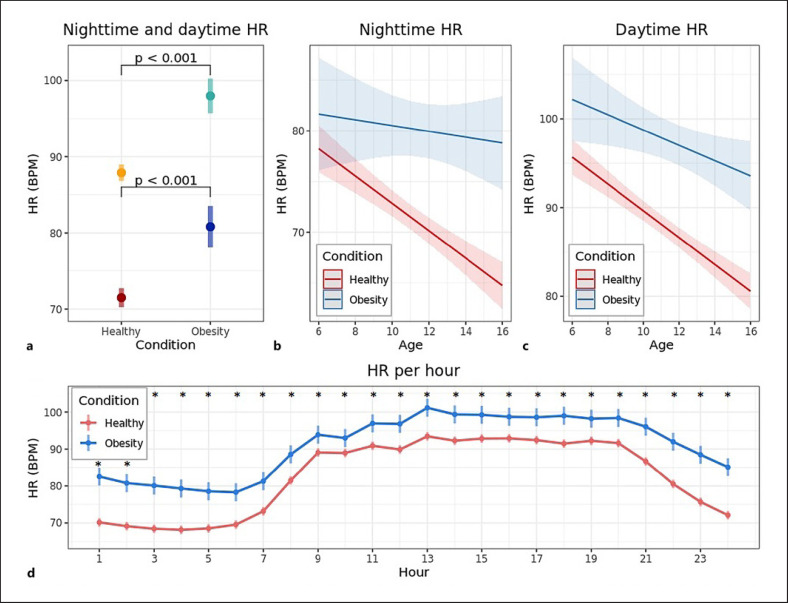
The average nighttime HR was 81 bpm for patients versus 71 bpm for controls (difference 9.3 bpm, 95% CI 6.3–12.3, Fig. 2a). In addition, the average daytime HR was also higher for patients compared to controls (98 bpm vs. 88 bpm, difference 10.1 bpm, 95% CI 7.6–12.6, Fig. 2a). The difference in average nighttime HR between patients and controls increased as a function of age by a difference of 1.1 bpm/age year (95% CI 0.1–2.0, Fig. 2b). This age-related effect was not observed for the daytime HR (Fig. 2c). A separate analysis per hour showed that patients had a significantly higher average hourly HR compared to controls for every hour of the day, after controlling for age and sex (Fig. 2d).
Fig. 2.
Differences in HR between children with obesity and healthy children. a Estimated marginal mean (95% CI) nighttime and daytime HR for children with obesity and healthy children. Light colors represent daytime HR, dark colors represent nighttime HR. Age (11 years) and sex for both nighttime and daytime HR and daily step count (7,000 steps) for daytime HR only, were fixed to their population average. b Relationship between age and nighttime HR (difference of 1.1 bpm/age year, 95% CI 0.1–2.0, p = 0.029). c Relationship between age and daytime HR (difference of 0.6 bpm/age year, 95% CI −0.2 to 1.5, p = 0.119). b–c Bold lines represent the estimated marginal means, shaded areas indicate the 95% CI of the estimated mean. d Estimated marginal mean (95% CI) HR per hour of the day for children with obesity and healthy children. Age (11 years) and sex were fixed to their population average. *Indicate hours with a p value <0.05 for the difference after Holm's correction for multiple tests.
Blood Pressure
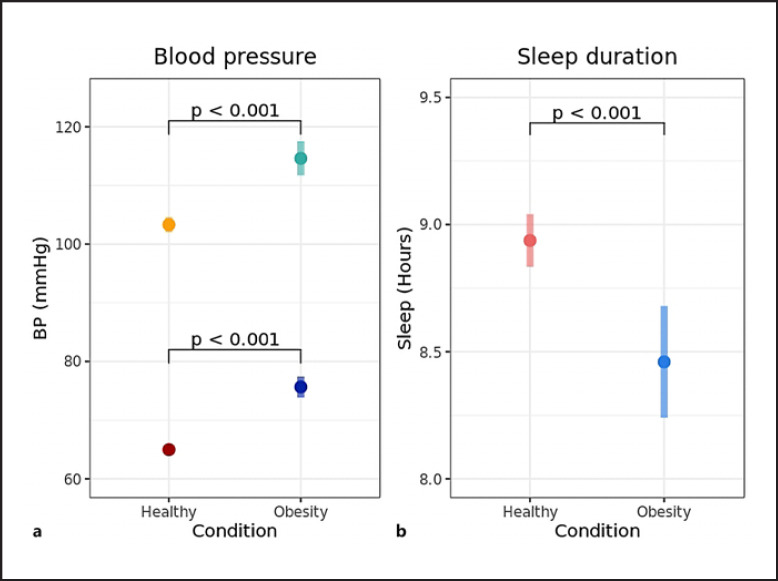
Patients had a higher systolic BP (115 mm Hg vs. 104 mm Hg, difference 11.3 mm Hg, 95% CI 8.1–14.5, Fig. 3a) as well as a higher diastolic BP (76 mm Hg vs. 65 mm Hg, difference 10.7 mm Hg, 95% CI 8.7–12.7, Fig. 3a) compared to controls. The difference in diastolic BP between patients and controls increased significantly as a function of age (difference 0.9 mm Hg/age year, 95% CI 0.3–1.5, online suppl. Fig. S3a). This age-related effect was not observed for the systolic BP (online suppl. Fig. S3b).
Fig. 3.
Difference in BP and sleep duration between children with obesity and healthy children. a Estimated marginal mean (95% CI) systolic BP and diastolic BP for children with obesity and healthy children. Light colors represent systolic BP and dark colors represent diastolic BP. Age (11 years) was fixed to the population average. b Estimated marginal mean (95% CI) sleep duration for children with obesity and healthy children. Age (11 years), sex, and type of day were fixed to their population average.
Sleep
The adjusted average sleep duration per night was shorter for patients compared to controls (8.5 h vs. 8.9 h, difference 0.5 h, 95% CI 0.2–0.7, Fig. 3b). The average proportion of light sleep per night was 52.6% for the patient group versus 56.9% for the control group (difference 4.3%, 95% CI 1.8–6.8, online suppl. Fig. S4a). There was no significant difference found in the average number of wake-ups per night between patients and controls (online suppl. Fig. S4b).
Correlations in the Patient Group
No significant correlation was found in the patient group between daily PA and body mass index SD score (Spearman's rho 0.203) as well as between daily PA and quality of life (Spearman's rho −0.05). Since a period of 28 days was too short to observe evident differences in weight, no further analyses were performed with the weight data obtained by the weekly weight assessments.
Discussion
This study evaluated digital endpoints derived from PA, HR, BP, and sleep in pediatric obesity through wearable devices at home. The novel endpoints demonstrated that children with obesity performed less PA, had a higher HR and BP, and had a shorter sleep duration compared to healthy children. These multiple digital endpoints gathered by a home-monitoring platform show potential for future use in clinical trials and clinical care because of the high tolerability and ability to differentiate patients from controls, which are prerequisites for implementation [11]. Adequate validation must be performed before implementation of the ubiquitous digital devices and applications in clinical research and ultimately in clinical practice. A previously proposed stepwise approach to fit-for-purpose validation of (digital) biomarkers was applied in this study [11].
One of the most important characteristics of digital biomarkers is the tolerability in the target population [11]. Non-compliance will lead to incomplete datasets and biased results. The smartwatch-related measurements showed the highest tolerability (median compliance 81–100%). Gathering data via a smartwatch appeared to be superior to gathering data via a questionnaire (median compliance 55%) in children with obesity. Compared to a previous study in healthy children, the overall compliance was lower for children with obesity (94% vs. 74%). This difference is predominantly caused by the difference in median compliance for the daily BP measurements (95% for healthy children vs. 59% for patients) and daily questionnaire (90% for healthy children vs. 55% for patients) [8]. Childhood obesity is associated with a less structured home environment [19]. In contrast to the smartwatch-related measurements, the daily BP measurements and questionnaire need to be planned and performed actively by the child or parents, which might be more difficult in a less structured home environment.
Another important validation criterion for biomarkers is the ability to discriminate healthy children from patients, which was assessed for all candidate biomarkers. The average daily PA was lower for patients compared to controls, which has been cited in numerous previous studies with comparable differences [9]. However, the reported differences in step count throughout the literature vary due to, inter alia, the utilization of a wide selection of pedometers and differences in age of the study populations. Accelerometry has shown to be a reliable, noninvasive, and inexpensive method to measure the amount of PA in children [20]. There is conflicting evidence whether the awareness of wearing an accelerometer is affecting the level of PA in healthy youth [21]. The majority of studies examining differences in step count between children with obesity and healthy children monitored subjects for a maximum of 8 days [9]. In this study, patients were monitored for 28 days. We did not find a decrease in PA levels comparing the first and last week in either study group. As reported by past studies, patients had a lower peak PA compared to controls [22]. We demonstrated a difference in peak PA between the two groups not only by analyzing PA levels during the most active hour per day, but also by calculating the 90th percentile of the daily PA within a week. The latter endpoint is less variable and could be a useful biomarker for long-term monitoring [8]. Based on the data presented here, interventions focused on PA after school time seem most appropriate, since the biggest differences in PA between the two groups were observed in the after-school period. The combination of PA-derived biomarkers provides a wide-ranging and objective overview of the PA level of the patient, and can be utilized to promote PA and to provide personal advice. However, by looking at the validity of PA as digital biomarker in clinical decision making, it must be considered that it is not clear if changes in PA levels are a cause or a symptom of obesity, and future studies are needed to determine the role of PA in children with obesity younger than 6 years old [9].
Multiple candidate biomarkers based on HR were examined in this study. HR was registered through a photoplethysmography sensor, which has shown an acceptable validity in adults and has demonstrated to be accurate in measuring HR in children undergoing elective surgery [23, 24]. Patients had a higher average nighttime HR compared to controls, with similar absolute differences compared to previous research with other methods of HR monitoring [25]. Additionally, children with obesity had a higher daytime HR compared to controls, which also has been reported in the past [26, 27]. Analysis of HR per hour clearly displayed the difference in daily HR pattern for patients compared to controls. The higher HR in the patient group can be explained by sympathetic nervous system overactivation [26, 28, 29], caused by dysregulation of the release of multiple adipokines (inter alia; leptin, free fatty acids, TNF-α, IL-6, adiponectin) and baroreflex dysfunction [30, 31]. In this study, the difference in average nighttime HR between patients and controls increased as a function of age (while no correlation between body mass index SD score and age was found). This is a novel observation, possibly explained by the fact that in healthy children a progressive increase in cardiac parasympathetic activity relative to sympathetic activity occurs with an increase in age, while for children with obesity this process is disrupted [32]. This age-related effect was not observed for the average daytime HR, most likely due to the higher proportion of unexplained variability in this data. Weight loss is associated with a decrease in HR, which may suggest that the lower parasympathetic activity is reversible [33, 34]. It has been reported that a higher resting HR leads to a higher risk of cardiovascular disease and (non-)cardiovascular death in adults and is associated with dyslipidemia in children [35, 36, 37, 38]. Consequently, nighttime HR might be an attractive surrogate biomarker to assess the risk for cardiovascular disease in children with obesity.
Systolic BP and diastolic BP were also proposed as candidate endpoints, and both were elevated in patients compared to controls. The differences in BP reported here are slightly larger compared to the differences mentioned in previous research, but are within the ranges reported in the literature [4]. This relatively large difference between patients and controls could be explained by our study population, which consists of a high percentage of children diagnosed with grade 2 and grade 3 obesity (65%) compared with other studies. This might have led to a high proportion of patients at risk for cardiovascular problems in our cohort, since an increase in body mass index is associated with an increase in BP [39]. Another explanation is the BP cuff used in this study, which was identical for healthy children and children with obesity. This could have resulted in an overestimation of the BP in patients due to a bigger arm circumference, though the observed differences in BP appear too large to be entirely attributed to the utilization of the single-sized cuff [40]. Moreover, the Withings device has been validated in accordance with the ESH International Protocol Revision 2010 [41]. In the future, new BP meters compatible with multiple cuff sizes must be utilized. The pathophysiological mechanism of hypertension in children with obesity is multifactorial and complex. Suggested contributing factors are increased sympathetic nervous system activation, dysfunction of the endocrine system, disturbed sodium hemostasis, and vascular damage [42]. The difference in diastolic BP, but not systolic BP, between patients and controls increased as a function of age. Presently, a pathophysiologic explanation for this observation is lacking, and more research regarding hypertension subtypes in children with obesity may elucidate the underlying mechanism [42, 43]. Childhood hypertension has multiple adverse consequences, such as an increased carotid intima-media thickness and left ventricular hypertrophy [44, 45], both precursors to adverse cardiovascular outcomes in adulthood [46, 47]. Literature regarding the reversibility of the adverse cardiovascular effects of childhood obesity states that lifestyle interventions improve early markers of atherosclerosis and reduce the BP [48]. Hence, the combination of HR registration and BP measurements appear to be a valid option to monitor the cardiovascular status of the patient non-invasively, and might contribute to a reduction of the burden to visit outpatient clinics for the follow-up of these cardiovascular parameters.
Multiple sleep parameters were tested as candidate endpoints. Patients had a significantly shorter sleep duration than healthy children, an observation supported by previous studies [10]. Data regarding sleep quality and sleep efficiency in children with obesity compared with healthy children are inconsistent partly due to different measurement methods and definitions [10]. Since sleep parameters were measured via accelerometry, the lower proportion of light sleep for children with obesity compared to the healthy children could be caused by less movement at night due to the habitus of the patients. Also, it must be taken into account that when interpreting accelerometry-derived sleep measurements, accelerometry has shown to be less accurate compared to polysomnography [49]. On the other hand, sleep registration via accelerometry is less invasive and can be performed multiple nights in an outpatient setting, in contrast to polysomnography which is limited to an inpatient setting. Furthermore, accelerometry-derived sleep recording has shown to be more reliable than sleep registration via maternal sleep reports and avoids the recall bias related to sleep diaries [50]. In the future, with further improvement of the underlying algorithms, accelerometer-derived parameters might be useful to detect sleep-related breathing disorders non-invasively [51, 52].
This study has several limitations. The sample size was limited to 28 patients. Nevertheless, important baseline characteristics (age, sex and obesity grade) were well distributed in the patient group. The median compliance for the smartwatch-related measurements was high in this study. However, further studies are needed to examine the long-term compliance, which is very important in monitoring treatment effects. If the long-term compliance is sufficient, home-monitoring via wearables could reduce outpatient visits. Moreover, a disadvantage of gathering data in a home-setting is missing data due to non-compliance. Although the amount of missing data was low and therefore unlikely to impact the overall results, watch wear time was included as a covariate in the PA models. Moreover, when appraising PA-related endpoints, it must be considered that PA has been measured in step count and that activities like cycling and swimming were not registered. Finally, the child's eating behavior, which also plays a crucial role in treating childhood obesity, has not been examined in this study. Creating an objective overview of the child's dietary intake remains an enormous challenge. Monitoring food intake via daily food records is associated with a high subject burden and misreporting [53, 54]. Developing an application that is able to scan food might be a solution for these problems [55]. Strengths of this study consisted of the utilization of a structured validation process of the candidate endpoints, the inclusion of a large control group, with a similar distribution of age and sex compared to the patient group, and the relatively long-term monitoring period of 28 days. Additionally, the linear mixed effect models utilized for the analysis of the candidate endpoints can handle small sample sizes, missing data points, and unequal groups. Hence, a matched group-design was not needed. The defined endpoints based on PA, HR, BP, and sleep could be utilized to promote and track PA, to assess the risk for cardiovascular disease and to detect sleep-related alterations of childhood obesity. In the future, the new biomarkers can be utilized in clinical care and clinical trials to capture changes in condition either through interventions (e.g., for comparing different lifestyle interventions in the home-setting) or as a result of condition progression.
Conclusion
Remote-monitoring via wearable technology has the potential to objectively measure the disease-burden in the home-setting in pediatric obesity. The digital biomarkers based on PA, HR, BP, and sleep have a high tolerability. Furthermore, the novel endpoints demonstrate critical differences in PA level, multiple cardiovascular parameters, and sleep duration between children with obesity and healthy children, in line with previous studies gathering the endpoints via conventional clinic-based methods. Future studies are needed to determine the capacity of these novel digital endpoints to detect the effect of interventions.
Statement of Ethics
This study protocol was reviewed and approved by the Medical Ethics Committee Zuid West Holland (The Hague, The Netherlands), approval number 18-077. Written informed consent was obtained from all parents. Verbal consent was obtained from children aged less than 12 years and written consent was obtained from children aged 12 years and older. Trial registration: The trial was registered at the Dutch Trial Registry (NTR, Trial NL7611).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors received no specific funding for this work.
Author Contributions
J.M.K. analyzed the data and wrote the manuscript. E.C.A.M.H., D.C.M.K., and Y.B. recruited patients and reviewed the final manuscript. L.F. supported data analysis and reviewed the final manuscript. F.E.S., A.F.C., and G.J.A.D. conceptualized and supervised the study conduct and reviewed the final manuscript. M.D.K. conceptualized the study, recruited patients, supervised data analysis, and co-wrote the manuscript.
Data Availability Statement
All data presented in this manuscript are available from the corresponding author upon reasonable request.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors thank Ahnjili Zhuparris for help with data aggregation, as well as the participants and their parents for their enthusiasm during the study.
References
- 1.World Health Organization [Internet]. Obesity and overweight [cited 2021 Jan 11] Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017 Feb;92((2)):251–65. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Barlow SE, Ohlemeyer CL. Parent reasons for nonreturn to a pediatric weight management program. Clin Pediatr. 2006 May;45((4)):355–60. doi: 10.1177/000992280604500408. [DOI] [PubMed] [Google Scholar]
- 4.Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. 2012 Sep 25;345:e4759. doi: 10.1136/bmj.e4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krmar RT. White-coat hypertension from a paediatric perspective. Acta Paediatr. 2019 Jan;108((1)):44–9. doi: 10.1111/apa.14416. [DOI] [PubMed] [Google Scholar]
- 6.Woolford SJ, Sidell M, Li X, Else V, Young DR, Resnicow K, et al. Changes in body mass index among children and adolescents during the COVID-19 pandemic. JAMA. 2021 Oct 12;326((14)):1434–6. doi: 10.1001/jama.2021.15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lange SJ, Kompaniyets L, Freedman DS, Kraus EM, Porter R, Blanck HM, et al. Longitudinal trends in body mass index before and during the COVID-19 pandemic among persons aged 2–19 years: United States, 2018–2020. MMWR Morb Mortal Wkly Rep. 2021 Sep 17;70((37)):1278–83. doi: 10.15585/mmwr.mm7037a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruizinga MD, Heide NV, Moll A, Zhuparris A, Yavuz Y, Kam ML, et al. Towards remote monitoring in pediatric care and clinical trials-tolerability, repeatability and reference values of candidate digital endpoints derived from physical activity, heart rate and sleep in healthy children. PLoS One. 2021;16((1)):e0244877. doi: 10.1371/journal.pone.0244877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miguel-Berges ML, Reilly JJ, Moreno Aznar LA, Jiménez-Pavón D. Associations between pedometer-determined physical activity and adiposity in children and adolescents: systematic review. Clin J Sport Med. 2018 Jan;28((1)):64–75. doi: 10.1097/JSM.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 10.Morrissey B, Taveras E, Allender S, Strugnell C. Sleep and obesity among children: a systematic review of multiple sleep dimensions. Pediatr Obes. 2020 Apr;15((4)):e12619. doi: 10.1111/ijpo.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruizinga MD, Stuurman FE, Exadaktylos V, Doll RJ, Stephenson DT, Groeneveld GJ, et al. Development of novel, value-based, digital endpoints for clinical trials: a structured approach toward fit-for-purpose validation. Pharmacol Rev. 2020 Oct;72((4)):899–909. doi: 10.1124/pr.120.000028. [DOI] [PubMed] [Google Scholar]
- 12.Van Binsbergen JJ, Langens FNM, Dapper ALM, Van Halteren MM, Glijsteen R, Cleyndert GA, et al. Obesity guideline [cited 2021 Jan 11] Available from: https://richtlijnen.nhg.org/standaarden/obesitas#volledige-tekst-kinderen.other.
- 13.Walker LS, Beck JE, Garber J, Lambert W. Children's somatization inventory: psychometric properties of the revised form (CSI-24) J Pediatr Psychol. 2009 May;34((4)):430–40. doi: 10.1093/jpepsy/jsn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001 Aug;39((8)):800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team [Internet]. R: the R project for statistical computing [cited 2021 Jan 11] Available from: https://www.r-project.org/
- 16.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models Usinglme4. J Stat Soft. 2015;67((1)) [Google Scholar]
- 17.Lenth R [Internet]. Emmeans: estimated marginal means, aka least-squares means [cited 2021 Jan 11] Available from: https://cran.r-project.org/web/packages/emmeans/index.html.
- 18.Lüdecke D. Ggeffects: tidy data frames of marginal effects from regression models. J Open Source Softw. 2018;3((26)):772. [Google Scholar]
- 19.Bates CR, Buscemi J, Nicholson LM, Cory M, Jagpal A, Bohnert AM. Links between the organization of the family home environment and child obesity: a systematic review. Obes Rev. 2018 Mar 8;19((5)):716–27. doi: 10.1111/obr.12662. [DOI] [PubMed] [Google Scholar]
- 20.Reilly JJ, Penpraze V, Hislop J, Davies G, Grant S, Paton JY. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch Dis Child. 2008 Jul;93((7)):614–9. doi: 10.1136/adc.2007.133272. [DOI] [PubMed] [Google Scholar]
- 21.Clemes SA, Biddle SJ. The use of pedometers for monitoring physical activity in children and adolescents: measurement considerations. J Phys Act Health. 2013 Feb;10((2)):249–62. doi: 10.1123/jpah.10.2.249. [DOI] [PubMed] [Google Scholar]
- 22.Elmesmari R, Martin A, Reilly JJ, Paton JY. Comparison of accelerometer measured levels of physical activity and sedentary time between obese and non-obese children and adolescents: a systematic review. BMC Pediatr. 2018 Mar 9;18((1)):106. doi: 10.1186/s12887-018-1031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Weaver RG, Armstrong B, Burkart S, Zhang S, Beets MW. Validity of wrist-worn photoplethysmography devices to measure heart rate: a systematic review and meta-analysis. J Sports Sci. 2020 Sep;38((17)):2021–34. doi: 10.1080/02640414.2020.1767348. [DOI] [PubMed] [Google Scholar]
- 24.Pelizzo G, Guddo A, Puglisi A, De Silvestri A, Comparato C, Valenza M, et al. Accuracy of a wrist-worn heart rate sensing device during elective pediatric surgical procedures. Children. 2018 Mar 8;5((3)):E38. doi: 10.3390/children5030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Archbold KH, Johnson NL, Goodwin JL, Rosen CL, Quan SF. Normative heart rate parameters during sleep for children aged 6 to 11 years. J Clin Sleep Med. 2010 Feb 15;6((1)):47–50. [PMC free article] [PubMed] [Google Scholar]
- 26.Sorof JM, Poffenbarger T, Franco K, Bernard L, Portman RJ. Isolated systolic hypertension, obesity, and hyperkinetic hemodynamic states in children. J Pediatr. 2002 Jun;140((6)):660–6. doi: 10.1067/mpd.2002.125228. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes RA, Freitas Júnior IF, Codogno JS, Christofaro DG, Monteiro HL, Roberto Lopes DM. Resting heart rate is associated with blood pressure in male children and adolescents. J Pediatr. 2011 Apr;158((4)):634–7. doi: 10.1016/j.jpeds.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Rossi RC, Vanderlei LC, Gonçalves AC, Vanderlei FM, Bernardo AF, Yamada KM, et al. Impact of obesity on autonomic modulation, heart rate and blood pressure in obese young people. Auton Neurosci. 2015 Dec;193:138–41. doi: 10.1016/j.autneu.2015.07.424. [DOI] [PubMed] [Google Scholar]
- 29.Rodríguez-Colón SM, Bixler EO, Li X, Vgontzas AN, Liao D. Obesity is associated with impaired cardiac autonomic modulation in children. Int J Pediatr Obes. 2011 Apr;6((2)):128–34. doi: 10.3109/17477166.2010.490265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith MM, Minson CT. Obesity and adipokines: effects on sympathetic overactivity. J Physiol. 2012 Apr 15;590((8)):1787–801. doi: 10.1113/jphysiol.2011.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Silva AA, do Carmo J, Dubinion J, Hall JE. The role of the sympathetic nervous system in obesity-related hypertension. Curr Hypertens Rep. 2009 Jun;11((3)):206–11. doi: 10.1007/s11906-009-0036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eyre EL, Duncan MJ, Birch SL, Fisher JP. The influence of age and weight status on cardiac autonomic control in healthy children: a review. Auton Neurosci. 2014 Dec;186:8–21. doi: 10.1016/j.autneu.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Pidlich J, Pfeffel F, Zwiauer K, Schneider B, Schmidinger H. The effect of weight reduction on the surface electrocardiogram: a prospective trial in obese children and adolescents. Int J Obes Relat Metab Disord. 1997 Nov;21((11)):1018–23. doi: 10.1038/sj.ijo.0800220. [DOI] [PubMed] [Google Scholar]
- 34.Arone LJ, Mackintosh R, Rosenbaum M, Leibel RL, Hirsch J. Autonomic nervous system activity in weight gain and weight loss. Am J Physiol. 1995 Jul;269((1 Pt 2)):R222–5. doi: 10.1152/ajpregu.1995.269.1.R222. [DOI] [PubMed] [Google Scholar]
- 35.Cooney MT, Vartiainen E, Laatikainen T, Juolevi A, Dudina A, Graham IM. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010 Apr;159((4)):612–9.e3. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Kannel WB, Kannel C, Paffenbarger RS, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987 Jun;113((6)):1489–94. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- 37.Jensen MT, Suadicani P, Hein HO, Gyntelberg F. Elevated resting heart rate, physical fitness and all-cause mortality: a 16-year follow-up in the Copenhagen Male Study. Heart. 2013 Jun;99((12)):882–7. doi: 10.1136/heartjnl-2012-303375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freitas Júnior IF, Monteiro PA, Silveira LS, Cayres SU, Antunes BM, Bastos KN, et al. Resting heart rate as a predictor of metabolic dysfunctions in obese children and adolescents. BMC Pediatr. 2012 Jan 12;12:5. doi: 10.1186/1471-2431-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong J, Guo XL, Lu ZL, Cai XN, Wang HC, Zhang JY, et al. Prevalence of overweight and obesity and their associations with blood pressure among children and adolescents in Shandong, China. BMC Public Health. 2014 Oct 17;14:1080. doi: 10.1186/1471-2458-14-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whincup PH, Cook DG, Shaper AG. Blood pressure measurement in children: the importance of cuff bladder size. J Hypertens. 1989 Oct;7((10)):845–50. doi: 10.1097/00004872-198910000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Topouchian J, Agnoletti D, Blacher J, Youssef A, Chahine MN, Ibanez I, et al. Validation of four devices: Omron M6 Comfort, Omron HEM-7420, Withings BP-800, and Polygreen KP-7670 for home blood pressure measurement according to the European Society of Hypertension International Protocol. Vasc Health Risk Manag. 2014;10:33–44. doi: 10.2147/VHRM.S53968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirix AJ, Kaspers PJ, Nauta J, Chinapaw MJ, Kist-van Holthe JE. Pathophysiology of hypertension in obese children: a systematic review. Obes Rev. 2015 Oct;16((10)):831–42. doi: 10.1111/obr.12305. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Haseler E, Chowienczyk P, Sinha MD. Haemodynamics of hypertension in children. Curr Hypertens Rep. 2020 Aug 25;22((8)):60. doi: 10.1007/s11906-020-01044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lande MB, Carson NL, Roy J, Meagher CC. Effects of childhood primary hypertension on carotid intima media thickness: a matched controlled study. Hypertension. 2006 Jul;48((1)):40–4. doi: 10.1161/01.HYP.0000227029.10536.e8. [DOI] [PubMed] [Google Scholar]
- 45.Jing L, Nevius CD, Friday CM, Suever JD, Pulenthiran A, Mejia-Spiegeler A, et al. Ambulatory systolic blood pressure and obesity are independently associated with left ventricular hypertrophic remodeling in children. J Cardiovasc Magn Reson. 2017 Nov 9;19((1)):86. doi: 10.1186/s12968-017-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bots ML, Dijk JM, Oren A, Grobbee DE. Carotid intima-media thickness, arterial stiffness and risk of cardiovascular disease: current evidence. J Hypertens. 2002 Dec;20((12)):2317–25. doi: 10.1097/00004872-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 47.Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications for regression. Prog Cardiovasc Dis. 2009 Sep–Oct;52((2)):153–67. doi: 10.1016/j.pcad.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 48.Ayer J, Charakida M, Deanfield JE, Celermajer DS. Lifetime risk: childhood obesity and cardiovascular risk. Eur Heart J. 2015 Jun 7;36((22)):1371–6. doi: 10.1093/eurheartj/ehv089. [DOI] [PubMed] [Google Scholar]
- 49.Kolla BP, Mansukhani S, Mansukhani MP. Consumer sleep tracking devices: a review of mechanisms, validity and utility. Expert Rev Med Devices. 2016 May;13((5)):497–506. doi: 10.1586/17434440.2016.1171708. [DOI] [PubMed] [Google Scholar]
- 50.Martinez SM, Greenspan LC, Butte NF, Gregorich SE, De Groat CL, Deardorff J, et al. Mother-reported sleep, accelerometer-estimated sleep and weight status in Mexican American children: sleep duration is associated with increased adiposity and risk for overweight/obese status. J Sleep Res. 2014 Jun;23((3)):326–34. doi: 10.1111/jsr.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bixler EO, Vgontzas AN, Lin HM, Liao D, Calhoun S, Vela-Bueno A, et al. Sleep disordered breathing in children in a general population sample: prevalence and risk factors. Sleep. 2009 Jun;32((6)):731–6. doi: 10.1093/sleep/32.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gruwez A, Bruyneel AV, Bruyneel M. The validity of two commercially-available sleep trackers and actigraphy for assessment of sleep parameters in obstructive sleep apnea patients. PLoS One. 2019;14((1)):e0210569. doi: 10.1371/journal.pone.0210569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poslusna K, Ruprich J, de Vries JH, Jakubikova M, van't Veer P. Misreporting of energy and micronutrient intake estimated by food records and 24 hour recalls, control and adjustment methods in practice. Br J Nutr. 2009 Jul;101(Suppl 2):S73–85. doi: 10.1017/S0007114509990602. [DOI] [PubMed] [Google Scholar]
- 54.Magarey A, Watson J, Golley RK, Burrows T, Sutherland R, McNaughton SA, et al. Assessing dietary intake in children and adolescents: considerations and recommendations for obesity research. Int J Pediatr Obes. 2011 Feb;6((1)):2–11. doi: 10.3109/17477161003728469. [DOI] [PubMed] [Google Scholar]
- 55.Martin CK, Nicklas T, Gunturk B, Correa JB, Allen HR, Champagne C. Measuring food intake with digital photography. J Hum Nutr Diet. 2014 Jan;27((Suppl 1)):72–81. doi: 10.1111/jhn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Data Availability Statement
All data presented in this manuscript are available from the corresponding author upon reasonable request.