Abstract
Producing polymers from renewable resources via more sustainable approaches has become increasingly important. Herein we present the polymerization of monomers obtained from biobased renewable resources, employing an environmentally friendly photoinduced iron-catalyzed atom transfer radical polymerization (ATRP) in low-toxicity solvents. We demonstrate that renewable monomers can be successfully polymerized into sustainable polymers with controlled molecular weights and narrow molar mass distributions (Đ as low as 1.17). This is in contrast to reversible addition–fragmentation chain-transfer (RAFT) polymerization, arguably the most commonly employed method to polymerize biobased monomers, which led to poorer molecular weight control and higher dispersities for these specific monomers (Đs ∼ 1.4). The versatility of our approach was further highlighted by the temporal control demonstrated through intermittent “on/off” cycles, controlled polymerizations of a variety of monomers and chain lengths, oxygen-tolerance, and high end-group fidelity exemplified by the synthesis of block copolymers. This work highlights photoinduced iron-catalyzed ATRP as a powerful tool for the synthesis of renewable polymers.
Directing polymerization methodologies toward more sustainable pathways is of paramount and ever-increasing importance.1,2 However, a plethora of factors should be considered to improve the feasibility of a sustainable polymerization methodology.3 To begin with, the most important factor is the origin of monomers to be polymerized. Currently, the vast majority of monomers used for the synthesis of polymers are based on fossil fuel feedstock. Recently, in search for more sustainable alternatives, the synthesis of polymers from renewable resources has attracted significant attention, showing great promise in counterbalancing the use of fossil fuel feedstock.4 Indeed, biomass-derived materials have been employed as an alternative and renewable resource for the synthesis of monomers.5−8 For example, lignocellulose has gained great popularity as an inexpensive renewable waste product which can be produced in high abundance.4,9 In particular, lignin can provide multiple phenol derivative building blocks, the secondary alcohol of which can be easily modified under mild conditions to offer polymerizable building blocks.10 Another promising family of renewable resources that can be used for monomer production is terpenes, which can be extracted from plants, providing interesting biological properties.11,12
The synthesis of polymers from renewable resources is important not only from a sustainability viewpoint, but also because it leads to the production of novel polymeric materials with unique properties.7,13−16 To maximize access over the range of polymeric materials that can be attained, reversible deactivation radical polymerization (RDRP) has recently been employed to polymerize a range of renewable monomers.9,10 For the majority of cases, reversible addition–fragmentation chain-transfer (RAFT) polymerization has been employed for the polymerization of biomass-based monomers, as it is one of the most versatile RDRP techniques.14,17−23 However, RAFT polymerization of some renewable methacrylate monomers often leads to relatively broad molar mass distributions (Đs ∼ 1.3–1.7).9,20 In parallel, copper-mediated atom transfer radical polymerization (ATRP) has also been employed in polymerization of renewable monomers, but to a relatively lesser extent.24−32 It is noted that both polymerization methods typically employ toxic components/solvents that prevent the development of a more sustainable polymerization procedure.33 Arguably, one of the most environmentally friendly RDRP methodologies is iron (Fe) ATRP.34−36 Fe is one of the most abundant metals on earth and is inexpensive, nontoxic, and biocompatible.37 The possibility to utilize light (rather than heat) as an external stimulus to mediate Fe ATRP is also advantageous from a sustainability point of view.3 In a similar fashion to conventional RAFT and copper ATRP, highly toxic solvents such as acetonitrile, anisole, or trifluoroethanol are often required to conduct a successful Fe ATRP.38,39 In this work, we aim to develop a greener and efficient approach, which will satisfy as many of the 12 principles of green chemistry as possible,3 to polymerize renewable monomers by employing the environmentally friendly photoinduced iron-catalyzed ATRP in low-toxicity solvents while maintaining narrow molar mass distributions for all the synthesized sustainable polymers.
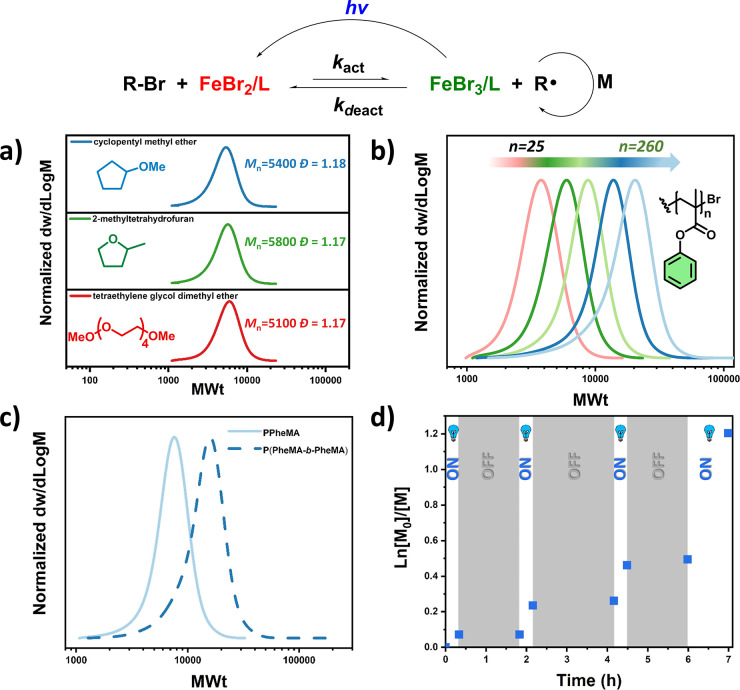
We first synthesized six different methacrylic monomers through the esterification of lignin derivatives (phenol, p-cresol, guaiacol, vanillin, syringol) and a thyme-derived terpene compound (thymol) using methacrylic anhydride, which is less toxic than methacryloyl chloride, the more frequently employed compound in this type of reaction (Scheme 1, Scheme S1, Spectra S1–S6)). 2-Methyltetrahydrofuran was used as the solvent for the methacrylation, a “greener” alternative when compared with more toxic and commonly employed organic solvents, such as dichloromethane.40 The monomers were purified thoroughly in order to avoid any contamination with the initial alcohols, which in some cases may be hazardous. After the successful synthesis of the renewable monomers, we sought to perform polymerizations via photoinduced iron-catalyzed ATRP. Phenyl methacrylate (PheMA) was used as the model monomer, tetraethylene glycol dimethyl ether (TEGDME) as the model low-toxicity solvent, FeBr3 as the metal source, tetrabutylammonium bromide (TBABr) as the ligand, and methyl α-bromophenylacetate (MBPA) as the initiator (Scheme S2). Initial experiments were conducted with a ratio of [MBPA]/[FeBr3]/[TBABr] = 1:0.1:0.1 and a targeted degree of polymerization (DP) of 50. All experiments were performed under blue light LED irradiation (48 W, λ = 465 nm (±5 nm)) in a homemade box (Figure S1). Under the aforementioned conditions, well-defined PPheMA could be obtained within 90 min with good control over the molecular weight and low dispersity as determined by size-exclusion chromatography (SEC; Mn = 5100, Đ = 1.17, Figure 1a, Table S1, entry 1). It can therefore be concluded that TEGDME does not decrease the catalyst’s activity (by ligation to Fe)41,42 and as such is an excellent solvent choice for the photoinduced iron-catalyzed ATRP of renewable monomers. The possibility to utilize alternative low-toxicity and green solvents was also investigated using 2-methyltetrahydrofuran and cyclopentyl methyl ether.40 Under otherwise identical conditions, both solvents fully solubilized the catalyst, resulting in PPheMA with very similar control over the molecular weight and dispersity as in the case of TEGDME (Figure 1a, Table S1, entries 2 and 3). Although the remaining experiments were conducted in TEGDME, the other two solvents were proven equally efficient to mediate a successful photoinduced iron-catalyzed ATRP, thus, suggesting no competing solvent–catalyst complexation.
Scheme 1. Synthesis of Sustainable Biomass-Derived Monomers and Their Corresponding Polymers.
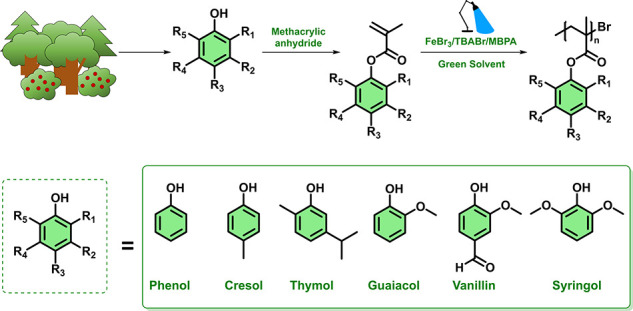
Figure 1.
Demonstration of a sustainable polymerization methodology via photoinduced iron-catalyzed ATRPof biobased monomers. (a) SEC traces of PPheMA synthesized in three low-toxicity/green solvents, (b) demonstration of temporal control during the polymerization of PheMA, (c) SEC traces of PPheMA with different degrees of polymerization, and (d) SEC traces showing the chain extension of PPheMA.
Next, we were interested in whether an “on/off” temporal control is possible during the polymerization of these renewable monomers. To assess this possibility, we monitored the growth of PPheMA chains during alternating periods of light and dark, using 1H NMR to calculate the monomer conversion. Under the previously established conditions ([PheMA]/[MBPA]/[FeBr3]/[TBABr] = 50:1:0.1:0.1), negligible polymerization was observed during the dark periods (<3%, Table S2), whereas a clear increase in monomer conversion was observed when the reaction was exposed to visible light irradiation (Figure 1b). The small percentage of polymerization noticeable during the dark periods was attributed to the relatively high catalyst loading and is in agreement with previous reports.38,43 To fully eliminate the conversion during the “off” cycles, a further decrease of the catalyst concentration is recommended, albeit at the expense of higher dispersity polymers.44
To explore the potential of our technique to control the polymerization of higher molecular weight polymers, a range of DPs were targeted. Good control and low dispersities were observed regardless of the initial chain length targeted (Figure 1c, Table S3). Considering the recent interest in oxygen-tolerant polymerizations,45−47 we also attempted our polymerizations in the absence of any external deoxygenation by simply minimizing the reaction vessel’s headspace to reduce the amount of the initially present oxygen. Pleasingly, a well-controlled polymerization took place, thus further simplifying the polymerization procedure (Table S4). Next, we wanted to investigate whether our method allows the low-volume synthesis of polymers as this may be of high interest to applications that require the use of low reaction scales (i.e., biological studies, bioconjugations, etc.). For this purpose, we conducted the polymerization of PheMA in reaction volumes of 50 and 100 μL (25 and 50 mg of monomer, respectively) and observed good control over the polymerization (Figure S2), thus highlighting the versatility of our method. Finally, we wanted to investigate the end-group fidelity achieved through our methodology. To assess this, a PPheMA macroinitiator (Mn = 6500, Đ = 1.17) was chain-extended, furnishing higher molecular weight polymers while maintaining low dispersity (Mn = 12300, Đ = 1.24, Figure 1d, Table S5).
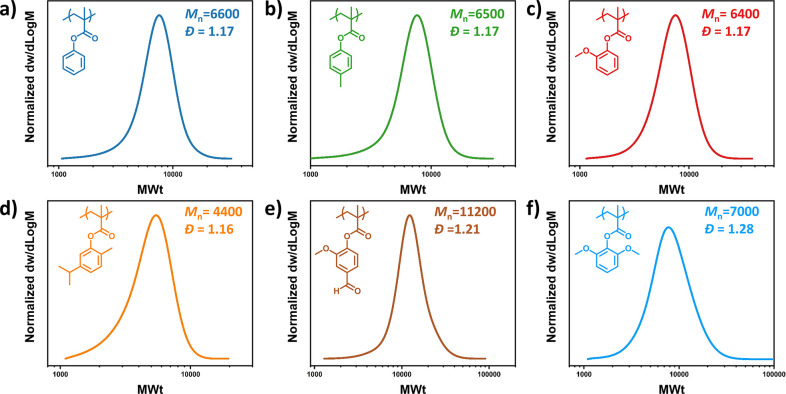
Upon establishing optimized conditions, we then successfully polymerized a range of renewable monomers, as indicated in Scheme 1. It is noted that for the liquid monomers, including cresol methacrylate (CreMA), guaiacol methacrylate (GuMA), and thymol methacrylate (ThyMA), a ratio of monomer to solvent 1:1 was employed. Instead, the polymerization of solid monomers (i.e., vanillin methacrylate (VaMA) and syringol methacrylate(SyrMA)) required an increased solvent loading to fully dissolve the initial monomer (1.5 equiv with respect to monomer). With these modifications, all monomers were efficiently polymerized, yielding controlled molecular weights and low dispersities (Figure 2 and Table S6). An additional block copolymer was also targeted, consisting of PPGuMA (Mn = 6500, Đ = 1.17, Figure 2c, and Table S7, entry 1) as the first block. In the presence of 10% of catalyst, PGuMA was chain-extended with PheMA resulting in a diblock with a higher dispersity (Đ = 1.35) (Figure S3a and Table S7, entry 2). However, further lowering of the diblock’s dispersity was possible by doubling the catalyst concentration (Đ = 1.24; Figure S3b and Table S7, entry 3).48
Figure 2.
SEC traces of renewable polymers synthesized via photoinduced iron-catalyzed ATRP: (a) PPheMA, (b) PCreMA, (c) PGuMA, (d) PThyMA, (e) PVaMA, and (f) PSyrMA.
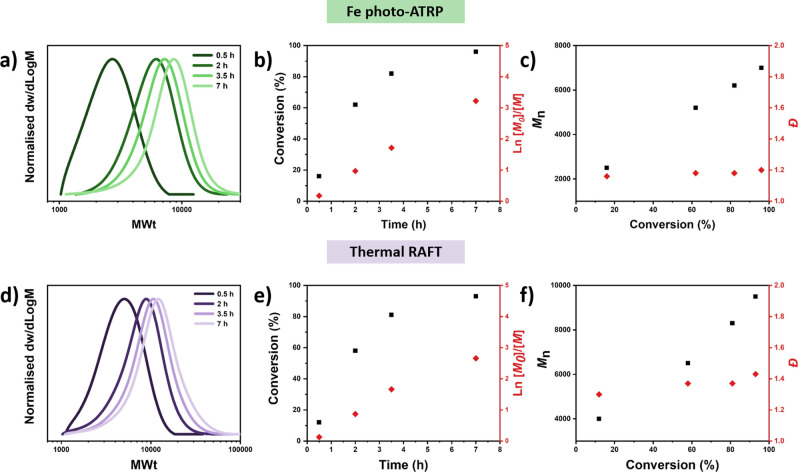
Intrigued by the excellent control over the dispersities attained through our photoinduced iron-catalyzed ATRP, we were interested in a direct comparison with conventional thermal RAFT polymerization, which is the most commonly employed method to polymerize such renewable monomers.9 By replicating the experiments in the presence of 2-cyano-2-propyl benzodithioate and 10% of AIBN, PheMA was effectively polymerized by RAFT polymerization albeit the final dispersity was as high as 1.43 (Table S8, entry 4). Similar results were also obtained for the polymerization of other monomers such as SyrMA, ThyMA, and VaMA (Figure S4). Detailed kinetic analysis was conducted to compare photoinduced iron-catalyzed ATRP with thermal RAFT polymerization under otherwise identical conditions (same monomer/targeted DP/solvent). Although both systems displayed features of a controlled polymerization such as a linear increase of ln[M0]/[M] over time (Figure 3b,e) and comparable reaction rates, some important differences were also observed. The first observation was a clear discrepancy between theoretical and experimental molecular weights. For instance, in the case of photoinduced iron-catalyzed ATRP an Mn of 5200 was obtained by SEC at 62% of conversion which is in close agreement with the theoretical Mn (5200). Instead, when RAFT polymerization was employed, a higher experimental Mn (MSEC = 6500) was observed at a similar conversion (i.e., 60%). We hypothesized that this discrepancy could be explained by the incomplete consumption of the RAFT agent.49 Indeed, the UV-SEC detector confirmed that the RAFT agent was not fully consumed, even at higher monomer conversions (>90%, Figure S5), which verified our original hypothesis. This discovery may also be associated with our second observation in that polymers synthesized by RAFT polymerization showed significantly higher dispersity values (1.37 and 1.43 at ∼60 and 90% conversion, respectively, as opposed to 1.18 and 1.20 for Fe-ATRP; Figure 3a,c,e,f and Tables S8 and S9). The higher dispersities observed by RAFT polymerization are attributed to the slow consumption of the RAFT agent as a result of less-efficient fragmentation and the potential hybrid behavior RAFT may have.50 Instead, the lower Đs obtained via photoinduced iron-catalyzed ATRP indicate a faster and more complete initiator consumption.51 To further understand our data, we conducted two additional control experiments. First, we polymerized PheMA using photoinduced electron-energy transfer (PET) RAFT instead of thermal RAFT. Under otherwise identical conditions, PET RAFT gave rise to a similarly high dispersity (Đ = 1.34, Figure S6), thus further supporting insufficient fragmentation with the selected RAFT agent. Second, to examine whether the polymerization temperature can affect the polymerization control, we conducted in parallel a polymerization of PheMA with thermal RAFT polymerization at 70 °C, and also iron-catalyzed photoinduced ATRP at the same temperature. The results show the superiority of iron-catalyzed photoinduced ATRP over RAFT in the polymerization of PheMA (Đ = 1.2 vs 1.4, Figure S7), suggesting that temperature is not the main factor behind the relatively lesser control in the RAFT polymerization of these monomers.
Figure 3.
Polymerization kinetics of PheMA utilizing (a–c) photoinduced iron-catalyzed ATRP and (d–f) conventional thermal RAFT polymerization.
In summary, we report a more sustainable RDRP methodology, whereby we leveraged photoinduced iron-catalyzed ATRP to polymerize a variety of renewable monomers in low-toxicity solvents. Low Đs were obtained for all cases and detailed kinetics confirmed a controlled polymerization. The high end-group fidelity of the polymers was demonstrated via chain extensions and block copolymers. Additionally, good temporal control over the polymerization could be achieved through light/dark iterations. Importantly, the polymerization reactions can be performed without any deoxygenation, simplifying the polymerization procedure. Finally, we demonstrate that photoinduced iron-catalyzed ATRP provides better control over the polymerization of the specific family of renewable methacrylate monomers than conventional RAFT polymerization, thus highlighting the superiority of our approach.
Acknowledgments
We thank Dr. Nghia P. Truong for the fruitful discussions on RAFT polymerization.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmacrolett.2c00302.
General information, experimental procedures, 1H NMR spectra, and SEC traces (PDF)
A.A. gratefully acknowledges ETH Zurich for financial support. K.P. thanks the Onassis Foundation, as this scientific paper was supported by the Onassis Foundation-Scholarship ID: FZQ051-1/2020-2021. H.S.W. acknowledges the award of the Swiss Government Excellence Scholarship (ESKAS No. 2020.0324). This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (DEPO: Grant No. 949219).
The authors declare no competing financial interest.
Supplementary Material
References
- Epps T. H. III; Korley L. T.; Yan T.; Beers K. L.; Burt T. M. Sustainability of Synthetic Plastics: Considerations in Materials Life-Cycle Management. JACS Au 2022, 2 (1), 3–11. 10.1021/jacsau.1c00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkatzidis K.; Wang H. S.; Truong N. P.; Anastasaki A. Recent developments and future challenges in controlled radical polymerization: a 2020 update. Chem. 2020, 6 (7), 1575–1588. 10.1016/j.chempr.2020.06.014. [DOI] [Google Scholar]
- Dworakowska S.; Lorandi F.; Gorczyński A.; Matyjaszewski K. Toward Green Atom Transfer Radical Polymerization: Current Status and Future Challenges. Adv. Sci. 2022, 2106076. 10.1002/advs.202106076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dea R. M.; Willie J. A.; Epps T. H. III 100th anniversary of macromolecular science viewpoint: polymers from lignocellulosic biomass. Current challenges and future opportunities. ACS Macro Lett. 2020, 9 (4), 476–493. 10.1021/acsmacrolett.0c00024. [DOI] [PubMed] [Google Scholar]
- Meier M. A.; Metzger J. O.; Schubert U. S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36 (11), 1788–1802. 10.1039/b703294c. [DOI] [PubMed] [Google Scholar]
- Biermann U.; Bornscheuer U.; Meier M. A.; Metzger J. O.; Schäfer H. J. Oils and fats as renewable raw materials in chemistry. Angew. Chem., Int. Ed. 2011, 50 (17), 3854–3871. 10.1002/anie.201002767. [DOI] [PubMed] [Google Scholar]
- Bass G. F.; Epps T. III Recent developments towards performance-enhancing lignin-based polymers. Polym. Chem. 2021, 12, 4130–4158. 10.1039/D1PY00694K. [DOI] [Google Scholar]
- Gandini A. Polymers from renewable resources: a challenge for the future of macromolecular materials. Macromolecules 2008, 41 (24), 9491–9504. 10.1021/ma801735u. [DOI] [Google Scholar]
- Hatton F. L. Recent advances in RAFT polymerization of monomers derived from renewable resources. Polym. Chem. 2020, 11 (2), 220–229. 10.1039/C9PY01128E. [DOI] [Google Scholar]
- Veith C.; Diot-Néant F.; Miller S. A.; Allais F. Synthesis and polymerization of bio-based acrylates: A review. Polym. Chem. 2020, 11 (47), 7452–7470. 10.1039/D0PY01222J. [DOI] [Google Scholar]
- Noppalit S.; Simula A.; Ballard N.; Callies X.; Asua J. M.; Billon L. Renewable Terpene Derivative as a Biosourced Elastomeric Building Block in the Design of Functional Acrylic Copolymers. Biomacromolecules 2019, 20 (6), 2241–2251. 10.1021/acs.biomac.9b00185. [DOI] [PubMed] [Google Scholar]
- Parkatzidis K.; Chatzinikolaidou M.; Koufakis E.; Kaliva M.; Farsari M.; Vamvakaki M. Multi-photon polymerization of bio-inspired, thymol-functionalized hybrid materials with biocompatible and antimicrobial activity. Polym. Chem. 2020, 11 (25), 4078–4083. 10.1039/D0PY00281J. [DOI] [Google Scholar]
- Zhan K.; Ejima H.; Yoshie N. Antioxidant and adsorption properties of bioinspired phenolic polymers: A comparative study of catechol and gallol. ACS Sustain. Chem. Eng. 2016, 4 (7), 3857–3863. 10.1021/acssuschemeng.6b00626. [DOI] [Google Scholar]
- Mahajan J. S.; O’Dea R. M.; Norris J. B.; Korley L. T.; Epps T. H. III Aromatics from lignocellulosic biomass: a platform for high-performance thermosets. ACS Sustain. Chem. Eng. 2020, 8 (40), 15072–15096. 10.1021/acssuschemeng.0c04817. [DOI] [Google Scholar]
- Upton B. M.; Kasko A. M. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 2016, 116 (4), 2275–2306. 10.1021/acs.chemrev.5b00345. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Ganewatta M. S.; Tang C. Sustainable polymers from biomass: Bridging chemistry with materials and processing. Prog. Polym. Sci. 2020, 101, 101197. 10.1016/j.progpolymsci.2019.101197. [DOI] [Google Scholar]
- Wang S.; Bassett A. W.; Wieber G. V.; Stanzione J. F. III; Epps T. H. III Effect of methoxy substituent position on thermal properties and solvent resistance of lignin-inspired poly (dimethoxyphenyl methacrylate) s. ACS Macro Lett. 2017, 6 (8), 802–807. 10.1021/acsmacrolett.7b00381. [DOI] [Google Scholar]
- Liu S.; Zhang X.; Li M.; Ren X.; Tao Y. Precision synthesis of sustainable thermoplastic elastomers from lysine-derived monomers. J. Polym. Sci., Part A: Polym. Chem. 2017, 55 (2), 349–355. 10.1002/pola.28394. [DOI] [Google Scholar]
- Holmberg A. L.; Stanzione J. F. III; Wool R. P.; Epps T. H. III A facile method for generating designer block copolymers from functionalized lignin model compounds. ACS Sustain. Chem. Eng. 2014, 2 (4), 569–573. 10.1021/sc400497a. [DOI] [Google Scholar]
- Holmberg A. L.; Reno K. H.; Nguyen N. A.; Wool R. P.; Epps T. H. III Syringyl methacrylate, a hardwood lignin-based monomer for high-T g polymeric materials. ACS Macro Lett. 2016, 5 (5), 574–578. 10.1021/acsmacrolett.6b00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmberg A. L.; Nguyen N. A.; Karavolias M. G.; Reno K. H.; Wool R. P.; Epps T. H. III Softwood lignin-based methacrylate polymers with tunable thermal and viscoelastic properties. Macromolecules 2016, 49 (4), 1286–1295. 10.1021/acs.macromol.5b02316. [DOI] [Google Scholar]
- Holmberg A. L.; Karavolias M. G.; Epps T. H. RAFT polymerization and associated reactivity ratios of methacrylate-functionalized mixed bio-oil constituents. Polym. Chem. 2015, 6 (31), 5728–5739. 10.1039/C5PY00291E. [DOI] [Google Scholar]
- Emerson J. A.; Garabedian N. T.; Burris D. L.; Furst E. M.; Epps T. H. III Exploiting feedstock diversity to tune the chemical and tribological properties of lignin-inspired polymer coatings. ACS Sustain. Chem. Eng. 2018, 6 (5), 6856–6866. 10.1021/acssuschemeng.8b00667. [DOI] [Google Scholar]
- Palà M.; Woods S. E.; Hatton F. L.; Lligadas G. RDRP (Meth) acrylic Homo and Block Polymers from Lignocellulosic Sugar Derivatives. Macromol. Chem. Phys. 2022, 2200005. 10.1002/macp.202200005. [DOI] [Google Scholar]
- Bensabeh N.; Moreno A.; Roig A.; Monaghan O. R.; Ronda J. C.; Cádiz V.; Galià M.; Howdle S. M.; Lligadas G.; Percec V. Polyacrylates derived from biobased ethyl lactate solvent via SET-LRP. Biomacromolecules 2019, 20 (5), 2135–2147. 10.1021/acs.biomac.9b00435. [DOI] [PubMed] [Google Scholar]
- Bensabeh N.; Moreno A.; Roig A.; Rahimzadeh M.; Rahimi K.; Ronda J. C.; Cadiz V.; Galia M.; Percec V.; Rodriguez-Emmenegger C.; Lligadas G. Photoinduced upgrading of lactic acid-based solvents to block copolymer surfactants. ACS Sustain. Chem. Eng. 2020, 8 (2), 1276–1284. 10.1021/acssuschemeng.9b06599. [DOI] [Google Scholar]
- Bensabeh N.; Ronda J. C.; Galià M.; Cádiz V.; Lligadas G.; Percec V. SET-LRP of the hydrophobic biobased menthyl acrylate. Biomacromolecules 2018, 19 (4), 1256–1268. 10.1021/acs.biomac.8b00090. [DOI] [PubMed] [Google Scholar]
- Moreno A.; Bensabeh N.; Parve J.; Ronda J. C.; Cádiz V.; Galià M.; Vares L.; Lligadas G.; Percec V. SET-LRP of bio-and petroleum-sourced methacrylates in aqueous alcoholic mixtures. Biomacromolecules 2019, 20 (4), 1816–1827. 10.1021/acs.biomac.9b00257. [DOI] [PubMed] [Google Scholar]
- Mosnácek J.; Matyjaszewski K. Atom transfer radical polymerization of tulipalin A: A naturally renewable monomer. Macromolecules 2008, 41 (15), 5509–5511. 10.1021/ma8010813. [DOI] [Google Scholar]
- Okada S.; Matyjaszewski K. Synthesis of bio-based poly (N-phenylitaconimide) by atom transfer radical polymerization. J. Polym. Sci., Part A: Polym. Chem. 2015, 53 (6), 822–827. 10.1002/pola.27507. [DOI] [Google Scholar]
- Wang J.; Yuan L.; Wang Z.; Rahman M. A.; Huang Y.; Zhu T.; Wang R.; Cheng J.; Wang C.; Chu F.; Tang C. Photoinduced metal-free atom transfer radical polymerization of biomass-based monomers. Macromolecules 2016, 49 (20), 7709–7717. 10.1021/acs.macromol.6b01997. [DOI] [Google Scholar]
- Zain G.; Bondarev D.; Doháňošová J.; Mosnáček J. Oxygen-Tolerant Photochemically Induced Atom Transfer Radical Polymerization of the Renewable Monomer Tulipalin A. ChemPhotoChem. 2019, 3 (11), 1138–1145. 10.1002/cptc.201900151. [DOI] [Google Scholar]
- Truong N. P.; Jones G. R.; Bradford K. G.; Konkolewicz D.; Anastasaki A. A comparison of RAFT and ATRP methods for controlled radical polymerization. Nat. Rev. Chem. 2021, 5 (12), 859–869. 10.1038/s41570-021-00328-8. [DOI] [PubMed] [Google Scholar]
- Layadi A.; Kessel B.; Yan W.; Romio M.; Spencer N. D.; Zenobi-Wong M.; Matyjaszewski K.; Benetti E. M. Oxygen tolerant and cytocompatible Iron (0)-mediated ATRP enables the controlled growth of polymer brushes from mammalian cell cultures. J. Am. Chem. Soc. 2020, 142 (6), 3158–3164. 10.1021/jacs.9b12974. [DOI] [PubMed] [Google Scholar]
- Yin X.; Wu D.; Yang H.; Wang J.; Huang R.; Zheng T.; Sun Q.; Chen T.; Wang L.; Zhang T. Seawater-Boosting Surface-Initiated Atom Transfer Radical Polymerization for Functional Polymer Brush Engineering. ACS Macro Lett. 2022, 11, 693–698. 10.1021/acsmacrolett.2c00138. [DOI] [PubMed] [Google Scholar]
- Dadashi-Silab S.; Kim K.; Lorandi F.; Schild D. J.; Fantin M.; Matyjaszewski K. Effect of halogen and solvent on iron-catalyzed atom transfer radical polymerization. Polym. Chem. 2022, 13 (8), 1059–1066. 10.1039/D1PY01601F. [DOI] [Google Scholar]
- Dadashi-Silab S.; Matyjaszewski K. Iron catalysts in atom transfer radical polymerization. Molecules 2020, 25 (7), 1648. 10.3390/molecules25071648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadashi-Silab S.; Matyjaszewski K. Iron-catalyzed atom transfer radical polymerization of semifluorinated methacrylates. ACS Macro Lett. 2019, 8 (9), 1110–1114. 10.1021/acsmacrolett.9b00579. [DOI] [PubMed] [Google Scholar]
- Teodorescu M.; Gaynor S. G.; Matyjaszewski K. Halide anions as ligands in iron-mediated atom transfer radical polymerization. Macromolecules 2000, 33 (7), 2335–2339. 10.1021/ma991652e. [DOI] [Google Scholar]
- Jessop P. G. Searching for green solvents. Green Chem. 2011, 13 (6), 1391–1398. 10.1039/c0gc00797h. [DOI] [Google Scholar]
- Rolland M.; Whitfield R.; Messmer D.; Parkatzidis K.; Truong N. P.; Anastasaki A. Effect of polymerization components on Oxygen-Tolerant Photo-ATRP. ACS Macro Lett. 2019, 8 (12), 1546–1551. 10.1021/acsmacrolett.9b00855. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Matyjaszewski K. ATRP of MMA in polar solvents catalyzed by FeBr2 without additional ligand. Macromolecules 2010, 43 (9), 4003–4005. 10.1021/ma1002276. [DOI] [Google Scholar]
- Dolinski N. D.; Page Z. A.; Discekici E. H.; Meis D.; Lee I. H.; Jones G. R.; Whitfield R.; Pan X.; McCarthy B. G.; Shanmugam S.; et al. What happens in the dark? Assessing the temporal control of photo-mediated controlled radical polymerizations. J. Polym. Sci., Part A: Polym. Chem. 2019, 57 (3), 268–273. 10.1002/pola.29247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield R.; Parkatzidis K.; Rolland M.; Truong N. P.; Anastasaki A. Tuning Dispersity by Photoinduced Atom Transfer Radical Polymerisation: Monomodal Distributions with ppm Copper Concentration. Angew. Chem., Int. Ed. 2019, 58 (38), 13323–13328. 10.1002/anie.201906471. [DOI] [PubMed] [Google Scholar]
- Theodorou A.; Liarou E.; Haddleton D. M.; Stavrakaki I. G.; Skordalidis P.; Whitfield R.; Anastasaki A.; Velonia K. Protein-polymer bioconjugates via a versatile oxygen tolerant photoinduced controlled radical polymerization approach. Nat. Commun. 2020, 11, 1486. 10.1038/s41467-020-15259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepaniak G.; Fu L.; Jafari H.; Kapil K.; Matyjaszewski K. Making ATRP more practical: oxygen tolerance. Acc. Chem. Res. 2021, 54 (7), 1779–1790. 10.1021/acs.accounts.1c00032. [DOI] [PubMed] [Google Scholar]
- Parkatzidis K.; Truong N. P.; Antonopoulou M. N.; Whitfield R.; Konkolewicz D.; Anastasaki A. Tailoring polymer dispersity by mixing chain transfer agents in PET-RAFT polymerization. Polym. Chem. 2020, 11 (31), 4968–4972. 10.1039/D0PY00823K. [DOI] [Google Scholar]
- Rolland M.; Truong N. P.; Whitfield R.; Anastasaki A. Tailoring polymer dispersity in photoinduced iron-catalyzed ATRP. ACS Macro Lett. 2020, 9 (4), 459–463. 10.1021/acsmacrolett.0c00121. [DOI] [PubMed] [Google Scholar]
- Whitfield R.; Parkatzidis K.; Truong N. P.; Junkers T.; Anastasaki A. Tailoring polymer dispersity by RAFT polymerization: a versatile approach. Chem. 2020, 6 (6), 1340–1352. 10.1016/j.chempr.2020.04.020. [DOI] [Google Scholar]
- Pietsch C.; Fijten M. W. M.; Lambermont-Thijs H. M. L.; Hoogenboom R.; Schubert U. S. Unexpected reactivity for the RAFT copolymerization of oligo(ethylene glycol) methacrylates. J. Polym. Sci., Part A: Polym. Chem. 2009, 47, 2811–2820. 10.1002/pola.23363. [DOI] [Google Scholar]
- Parkatzidis K.; Rolland M.; Truong N. P.; Anastasaki A. Tailoring polymer dispersity by mixing ATRP initiators. Polym. Chem. 2021, 12 (39), 5583–5588. 10.1039/D1PY01044A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.