Abstract
Air quality impacts from wildfires are poorly understood, particularly indoors. As frequencies increase, it is important to optimize methodologies to understand and reduce chemical exposures from wildfires. Public health recommendations use air quality estimates from outdoor stationary air monitors, discounting indoor air conditions, and do not consider chemicals in the vapor phase, known to elicit adverse effects. We investigated vapor-phase polycyclic aromatic hydrocarbons (PAHs) in indoor and outdoor air before, during, and after wildfires using a community-engaged research approach. Paired passive air samplers were deployed at 15 locations across four states. Twelve unique PAHs were detected only in outdoor air during wildfires, highlighting a PAH exposure mixture for future study. Heavy-molecular-weight (HMW) outdoor PAH concentrations and average Air Quality Index (AQI) values were positively correlated (p < 0.001). Indoor PAH concentrations were higher in 77% of samples across all sampling events. Even during wildfires, 58% of sampled locations still had higher indoor PAH air concentrations. When AQI values exceeded 140 (unhealthy for sensitive groups), outdoor PAH concentrations became similar to or higher than indoors. Cancer and noncancer inhalation risk estimates from vapor-phase PAHs were higher indoors than outdoors, regardless of the wildfire impact. Consideration of indoor air quality and vapor-phase PAHs could inform public health recommendations regarding wildfires.
Keywords: passive sampling, vapor-phase PAHs, public health, community-engaged research, wildfire PAH exposure mixture
Short abstract
PAH outdoor air concentrations only exceeded indoor when the Air Quality Index exceeded 140, and 12 unique PAHs were detected during wildfires.
Introduction
Wildfire frequencies are increasing in some regions under a warming climate.1 In 2017, the Western United States (U.S.) had the second worst wildfire season on record, resulting in over 10 million acres burned.2 2020 was the most active wildfire year on record for the Western U.S. Over six million acres burned in California, Oregon, and Washington. Six of the 20 largest California wildfires occurred,3 and nearly 4,000 more homes were lost in Oregon than in the previous 5 years combined.4,5 Many cities across these three states saw historically poor air quality across multiple days.6,7
While the number of wildfires each year has been fairly consistent, the number of large wildfires (>1,000 acres) has increased by seven fires per year8 and the total acreage burned and the average size has increased.9,10 Since 1970, the wildfire season in the Western U.S. is 105 days longer, and high wildfire potential days are predicted to increase by 6–34 days by 2050.11 As impacts increase, it is important to understand and reduce chemical exposures from wildfires.
Wildfire smoke is a complex mixture with knowledge gaps regarding its composition and health impact.12 One class of concern is polycyclic aromatic hydrocarbons (PAHs), a widespread organic contaminant arising from both natural and anthropogenic sources with links to cancers, developmental and neurological issues, respiratory problems, and suppressed immune functions.13−15 In general, low-molecular-weight (LMW) PAHs (two or three rings) tend to be more acutely toxic, while high-molecular-weight (HMW) PAHs (4-ring and above) are usually more carcinogenic.16,17
Current wildfire public health messaging is based on risks from particulate matter and U.S.-regulated chemicals.18 However, PAHs are semivolatile chemicals and are present in both the vapor phase and bound to some types of particulate matter.19 Previous studies have shown that vapor-phase PAHs can account for up to 86% of the cancer risk from total inhalation exposure (vapor phase and particulate matter).20−23 Understanding the distributions of vapor-phase PAHs is important for more comprehensive public health recommendations.
Wildfire public health messaging also does not account for indoor air. In a study by Messier et al.,24 passive samplers were deployed indoors and outdoors at six urban sites in Eugene, Oregon, across 7 days during the 2018 wildfire season. Results showed that indoor PAH air concentrations were consistently equal to or greater than outdoor concentrations, even during periods of mild smoke impact (AQI 0–100). Messier et al.24 also found that only outdoor PAH air concentrations were associated with satellite-based wildfire smoke density. This study builds on the approach by Messier et al.24 to better understand the fate of, and potential human exposure to, vapor-phase PAHs from wildfires in indoor and outdoor air. Study objectives were to (1) compare PAH air concentrations before, during, and after wildfire sampling events; (2) compare indoor versus outdoor air concentrations; (3) assess the influence of wildfire characteristics (such as wildfire size and smoke density) and participant behaviors (woodstove use, ventilation) on indoor and outdoor PAH concentrations; and (4) explore the influence of wildfires on cancer and noncancer inhalation risks.
Materials and Methods
Sampling Locations
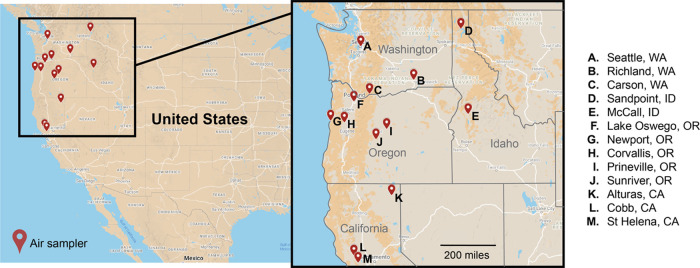
This study was performed from August to November in 2018, 2019, and 2020. Over these 3 years, passive sampling took place at 15 locations in the Western U.S. across Washington, Oregon, California, and Idaho (Figure 1). Passive sampling devices (PSDs) nonselectively sequester organic compounds in a biomimetic manner.25 Their ease of implementation and low maintenance make passive samplers an ideal approach for community-engaged research (CEnR). Through CEnR, researchers can work with trained community members across a large geographical area to rapidly deploy samplers.
Figure 1.
Sampling locations across four Western U.S. states.
Participants (18 or older) were selected using a convenience sampling design based on residence in high-risk areas for wildfires in the Western U.S. Prospective participants were contacted by the study coordinator and informed of the purpose of the study, duration, and the activities for which they would be responsible.
Participants received a kit containing the PSDs, an instruction packet, a survey, and a prepaid return label. At all locations, one passive air sampler was placed inside the home and a second sampler was placed outside the property. At one site, samplers were deployed in triplicate to assess field variability, and a field trip blank was included to assess potential contamination during the transportation process. PSDs were deployed for 3–4 weeks and then mailed back to the Food Safety and Environmental Stewardship Lab at Oregon State University.
Given the duration of the study, participant retention was important. An initial 13 locations were identified in 2018. Over time, some participants had a lack of interest (n = 3), were unavailable during some data collections (n = 2), or moved out of the state (n = 2). Where possible, new participants were recruited to fill spots, resulting in 15 unique locations across 13 cities. Overall, participant retention across the 3 years of the study was 62%. Locations were only included in the final data set if there were at least two different sampling events captured. Twelve of the 15 locations were sampled during wildfires.
Sample Preparation
One meter passive sampling strips were constructed from additive-free low-density polyethylene (LDPE) (Burntwood Plastics Ltd.) and cleaned with hexanes, as previously described.26,27 Three performance reference compounds, fluorene-d10, pyrene-d10, and benzo[b]fluoranthene-d12, were added to the LDPE prior to deployment to allow for calculations of in situ uptake rates. The average of three blank-infused LDPE strips was used to determine t = 0 performance reference compound concentrations (Table S1). The analyte sampling rate was derived from performance reference compound loss.
At all locations, T-shaped air cages28,29 containing five LDPE strips were placed in a room inside and outside the home. Indoor samplers were placed between the floor and the adult breathing-zone height (1–2 meters). Outdoor air samplers were placed approximately 1 meter above the soil. Temperature loggers were placed inside all air cages for the duration of the deployment period.
LDPE samples were transported in sealed polytetrafluoroethylene bags at ambient temperatures, and once at the laboratory, they were stored at −20 °C.30−32 Following established methods, samples were cleaned using isopropanol to remove particulate matter and moisture, extracted in n-hexane, quantitatively transferred, and then concentrated, as previously described.27−29,33 Prior to extraction, surrogates (naphthalene-d8, acenaphthylene-d8, phenanthrene-d10, fluoranthene-d10, chrysene-d12, benzo[a]pyrene-d12, and benzo[ghi]perylene-d12) were spiked onto samples for the calculation of surrogate recovery. Sample extracts were stored at −20 °C. Solvents were Optima grade (Fisher Scientific, Pittsburg, PA) or equivalent. Deuterated surrogates were from CDN Isotopes (Pointe-Claire, Quebec Canada).
QC Samples
A standard containing all target analytes was used to verify the continuing calibration during analysis. Laboratory quality control (QC) samples including construction, lab processing, cleaning, reagent, and instrument blanks as well as sample duplicates and overspikes were used to ensure data quality (Table S2).
GC-MS/MS
Analysis for 65 PAHs was performed with an Agilent, a 7890A gas chromatograph (GC), a 7000C triple quadrupole mass spectrometer (MS/MS), and a select PAH column (30 m × 250 μm × 0.15 μm).34 At least a 3-point (3–7 points) calibration was employed with correlations ≥0.99.34 The PAH physicochemical properties and instrument parameters are detailed in Tables S3 and S4. GC-MS/MS data was analyzed using the MassHunter Quantitative Analysis v.B.06.00 SP1 build 6.0.388.1 (Agilent Corp. Wilmington, DE) software. The Agilent GC-MS/MS method conducts automatic surrogate correction to account for any losses during sample processing in the laboratory. The average extraction surrogate recovery was 87% (59–109%). Perylene-d12 was used as the instrumental internal standard. Nine of the 65 PAHs were not detected in any samples and were excluded from further analysis.
Calculations
Time-weighted average concentrations for the air vapor phase were determined using an empirical uptake model and in situ sampling rates derived from performance reference compounds, as described by Huckins et al.25,35 The sampler–air partitioning coefficient is temperature-corrected using the average temperature during deployment. Detailed equations are provided in SI Page S5.
The quantitative human health risk assessment was used to estimate the excess lifetime cancer risk and noncancer hazard from inhalation exposure to vapor-phase PAH mixtures in indoor and outdoor air across all sampling events36−38 (SI Page S5 and Table S5). Risk assessment values calculated in this study account for risk to vapor-phase PAHs only. Additionally, reference values to estimate the inhalation cancer risk were available for only 20 of the 65 PAHs in our analytical method.38−43 Just four reference concentration (RfC) values, used for noncancer hazard calculations, are published for PAHs.38,42 Values for other PAHs are not available due to lack of or insufficient data. Additionally, most of the published RfC values are for 2-ring PAHs (naphthalene, 1-methylnaphthalene, 2 methylnaphthalene, and benzo[a]pyrene) and do not reflect risk from higher-molecular-weight PAHs. Therefore, the risk assessments provided in this study merely represent a fraction of the entire PAH mixture.
Wildfire Characteristics and Participant Behaviors
At each location, the U.S. Environmental Protection Agency (EPA) Air Quality Index (AQI) values for particulate matter (based on PM2.5 and PM10 combined) were obtained for each day of sampling using the closest available EPA monitor.44 The average distance of participant locations to AQI monitors was 15 miles (2–54 miles). AQI values are sectioned into six different categories: 0–50 “good”, 51–100 “moderate”, 101–150 “unhealthy for sensitive groups”, 151–200 “unhealthy”, 201–300 “very unhealthy”, and 301–500 “hazardous”.45 Wildfire smoke density was obtained for each day of sampling using the National Oceanic and Atmospheric Administration satellite-based model for wildfire smoke called the Hazard Mapping System (HMS).46 During wildfire impact, information for all wildfires near sampling locations was collected. AQI values, distance from AQI monitor, smoke density, and wildfire information are provided in Tables S6–S12.
Following each sampling event, participants were asked to complete a survey. The survey format used in 2018 consisted of basic questions related to the location of the air samplers, the opening of windows, the use of air conditioning, and participants’ interest in receiving results from their residence. The basic survey was expanded in 2019 and 2020 and transitioned online (Qualtrics XM Survey Software). Specifically, questions were included to identify potential PAH inhalation exposure sources other than wildfires, such as any use of or exposure to the following: air fresheners, candles/incense, use of a fryer, broiler or charcoal grill, wood-fired heating sources, smoker status (cigarette, e-cigarette, cigar), and type of kitchen stove (gas, electric). The 2019 and 2020 survey activities were conducted under the Institutional Review Board approval from Oregon State University (protocol # IRB-2019–0312). Survey compliance for basic survey questions was 79% across all 3 years. Survey compliance for expanded survey questions in 2019 and 2020 was 55%. Survey results are provided in Table S13.
Following each round of sampling, individual data sets were returned to study participants. The reports included a comparison of different PAH concentrations indoors and outdoors for each location, overall study conclusions, common sources of PAHs, and ways to reduce exposure. The 2020 reports were further expanded to include results from each sampling year to demonstrate trends in air quality across years and to evaluate air quality based on wildfire status.
Statistical Analysis and Regression Modeling
Samples were binned into one of three categories: before wildfire (10–12 months since any wildfire activity), during wildfire, and after wildfire (about 1 month after wildfire) based on wildfire activity during deployment. All statistical analyses were performed using R 3.5.2 and JMP Pro 14.0.1 statistical software. Statistical analyses were only completed for compounds that were above detection limits during at least one sampling event. Analytes below method quantification limits were assigned 1/2 method detection limits (Tables S14–S16). The relative standard deviation was calculated based on n = 3 replicates24,26,47−50 at Newport, Oregon (Table S17).
The mean PAH concentrations in air before wildfire versus wildfire, and wildfire versus after wildfire, were compared at each location using a one-sided t-test. Data was analyzed by grouping PAHs by ring size to assess trends based on the PAH physicochemical and potential toxicological properties. Two- and 3-ring PAHs are defined as low molecular weight (LMW), while 4-ring and above are defined as high molecular weight (HMW). Statistical analysis of 5-ring and 6-ring PAH air concentrations during wildfire compared to that of no wildfire or after wildfire was not completed for seven locations due to nondetect frequency or only n = 1 PAH was detected. The mean PAH concentrations in indoor and outdoor air at each sampling event were compared using a one-sided t-test. For all statistical analyses, concentrations were log-transformed, and a Bonferroni procedure was used to adjust the significance level and reduce the probability of type I errors.
A simple linear regression model was used to explore the influence of AQI values, NOAA smoke density, wildfire information, and participant survey responses on indoor and outdoor PAH air concentrations during wildfires. PAH air concentrations were first summed by ring size for each sample, grouped by indoor or outdoor, and then tested as the response variable. A Holm–Bonferroni procedure was used to adjust the significance level and reduce the probability of type I errors.
Results and Discussion
Impact of Wildfires on Indoor and Outdoor PAH Air Concentrations
We found no differences before, during, or after wildfires when evaluating the sum of all PAHs. However, LMW PAHs were detected at much higher concentrations (3–6 times higher), which dominated the analysis and drowned out the HMW PAHs. As a result, we conducted additional analyses separating out LMW and HMW PAHs.
We found 12 HMW PAHs in outdoor samples during wildfire sampling that were not detected outdoors before or after wildfires (Table 1). To the best of our knowledge, only 4 have been previously reported51−53 and 8 of the 12 PAHs have not previously been detected in air during wildfires. These 12 PAHs were never detected in indoor samples.
Table 1. Twelve PAHs Were Only Detected during Wildfires in Outdoor Samplesa.
| PAH | ring number | number of detections | average AQI of samples |
|---|---|---|---|
| dibenzo[e,l]pyrene | 6-ring | n = 6 | 90, 116, 142, 184, 189, 220 |
| 6-methylchrysene | 4-ring | n = 4 | 135, 184, 189, 220 |
| 7,12-dimethylbenz[a]anthracene | 5-ring | n = 4 | 135, 184, 189, 220 |
| anthanthrene | 6-ring | n = 4 | 116, 142, 189, 220 |
| 5-methylchrysene | 4-ring | n = 3 | 135, 189, 220 |
| benzo[a]chrysene | 5-ring | n = 2 | 189, 220 |
| naphtho[2,3-a]pyrene | 6-ring | n = 2 | 189, 220 |
| naphtho[2,3-e]pyrene | 6-ring | n = 2 | 189, 220 |
| naphtho[1,2-b]fluoranthene | 6-ring | n = 2 | 189, 220 |
| coronene | 7-ring | n = 2 | 189, 220 |
| perylene | 5-ring | n = 1 | 220 |
| dibenzo[a,l]pyrene | 6-ring | n = 1 | 220 |
Eight of the PAHs (bold) have not been previously reported in air during wildfires.
HMW PAHs, in particular, are an important consideration for exposure due to their potential for increased persistence, bioaccumulation, and toxicity54 compared to LMW PAHs. Our results suggest that a common high-molecular-weight wildfire PAH exposure mixture could be prioritized for future toxicology and epidemiology studies.55,56
Before, during, and after wildfire, PAH comparisons and statistical analysis are presented in Figure S3 and Table 2, respectively. The average indoor (Figure S3a) and outdoor (Figure S3b) vapor-phase LMW PAH air concentrations were three times higher during wildfires than before or after but were not statistically significant (Table 2). Our results are consistent with previous studies on indoor air quality, where the influence of the infiltrating outdoor air was not significant for LMW PAHs.57,58
Table 2. One-Sided Paired t-Test Comparing Indoor and Outdoor PAH Air Concentrations before, during, and after Wildfires by Ring Sizea.
| LMW PAHs |
HMW PAHs |
||||||
|---|---|---|---|---|---|---|---|
| location | 2-ring | 3-ring | 4-ring | 5-ring | 6-ring | wildfire average AQI | |
| before wildfires indoorb | Alturas, CA | 0.99 | 0.90 | 0.10 | 0.10 | 0.10 | 70 |
| Newport, OR | 0.34 | 0.99 | 0.83 | N/A (n = 0) | N/A (n = 0) | 90 | |
| St. Helena, CA | <0.00010b | 0.0069b | 0.00020b | N/A (n = 0) | N/A (n = 0) | 93 | |
| Sandpoint, ID | 0.24 | 0.58 | 0.57 | N/A (n = 0) | N/A (n = 0) | 116 | |
| Richland, WA | 0.0088 | 0.25 | <0.00010b | 0.012 | N/A (n = 1) | 135 | |
| Lake Oswego, OR | 0.019 | 0.22 | 0.014 | 0.038 | N/A (n = 1) | 189 | |
| after wildfires indoorc | Carson, WA | <0.00010c | 0.88 | 0.16 | N/A (n = 0) | N/A (n = 0) | 21 |
| McCall, ID | 0.36 | 0.26 | 0.044 | N/A (n = 0) | N/A (n = 0) | 73 | |
| Prineville, OR | 0.59 | 0.92 | 0.090 | N/A (n = 1) | N/A (n = 0) | 95 | |
| Sandpoint, ID | 0.096 | 0.24 | 0.74 | N/A (n = 0) | N/A (n = 0) | 116 | |
| Richland, WA | 0.018 | 0.54 | <0.0001c | 0.012 | N/A (n = 1) | 135 | |
| Seattle, WA 2 | 0.85 | 0.91 | 0.011 | N/A (n = 0) | N/A (n = 1) | 142 | |
| Sunriver, OR | 0.14 | 0.98 | 0.0050c | N/A (n = 1) | N/A (n = 0) | 184 | |
| Lake Oswego, OR | 0.085 | 0.0030c | 0.00030c | 0.015 | N/A (n = 1) | 189 | |
| Corvallis, OR 2 | 0.10 | 0.93 | 0.0086 | 0.032 | 0.041 | 220 | |
| before wildfires outdoorb | Alturas, CA | 0.92 | 0.95 | 1.0 | 1.0 | 1.0 | 70 |
| Newport, OR | 0.79 | 0.16 | 0.00050b | N/A (n = 0) | N/A (n = 1) | 90 | |
| St. Helena, CA | 0.033 | 0.025 | <0.00010b | 0.016 | N/A (n = 1) | 93 | |
| Sandpoint, ID | 0.50 | 0.053 | <0.00010b | 0.0032b | 0.0049b | 116 | |
| Richland, WA | 0.061 | 0.033 | <0.00010b | 0.0002b | N/A (n = 1) | 135 | |
| Lake Oswego, OR | 0.14 | 0.0054b | <0.00010b | <0.00010b | <0.00010b | 189 | |
| after wildfires outdoorc | Carson, WA | 0.99 | 0.80 | 0.99 | 0.99 | N/A (n = 0) | 21 |
| McCall, ID | 0.00020c | 0.012 | <0.00010c | N/A (n = 0) | N/A (n = 0) | 73 | |
| Prineville, OR | 0.0099 | 0.051 | <0.00010c | 0.00080c | 0.044 | 95 | |
| Sandpoint, ID | 0.94 | 0.016 | <0.00010c | 0.00010c | <0.00010c | 116 | |
| Richland, WA | 0.99 | 0.011 | <0.00010c | 0.00030c | N/A (n = 1) | 135 | |
| Seattle, WA 2 | <0.0001c | 0.0020c | <0.00010c | 0.016 | 0.049 | 142 | |
| Sunriver, OR | 0.19 | 0.92 | 1.0 | 1.0 | 0.97 | 184 | |
| Lake Oswego, OR | 0.48 | 0.011 | <0.00010c | <0.00010c | <0.00010c | 189 | |
| Corvallis, OR 2 | 0.61 | 0.00020c | <0.00010c | <0.00010c | <0.00010c | 220 | |
Significant p-values indicate that vapor-phase PAH air concentrations during wildfires are significantly greater than before or after. α values are Bonferroni-corrected for each sampling event.
Significant when probability >t at α′ = 0.0083.
Significant when probability >t at α′ = 0.0056.
The average vapor-phase HMW PAH air concentrations were six times higher indoors during wildfires than before or after. However, due to nondetect frequency or only n = 1 PAH being detected, HMW air concentrations were not statistically significant at most locations (Table 2). Our results are supported by previous studies identifying outdoor air as a contributor to indoor HMW PAHs.57,58 Specifically, indoor air concentrations of 4-ring PAHs were statistically higher during wildfires at four locations (Table 2). Few 5- and 6-ring PAHs were detected in indoor air. The average outdoor air concentrations of vapor-phase HMW PAHs were 86 times higher during wildfires than before or after and were statistically significant at most locations (Table 2). Concentration increases from our study are slightly higher than other studies,51,52 though these previous studies have only analyzed for up to 23 PAHs. In our study, we analyzed for up to 65 individual PAHs.
Air concentrations of HMW PAH outdoors also appeared to increase with increasing smoke intensity and AQI values (Figure S3b). Five-ring PAH outdoor air concentrations were significantly higher during wildfires than before or after at seven locations (Table 2). All seven locations had average AQIs exceeding 140 during wildfire sampling (described as unhealthy for sensitive groups). Six-ring PAH air concentrations were significantly higher outdoors at three locations during wildfires than before or after (Table 2). Two of the three locations had the highest average AQI values during wildfire sampling (described as unhealthy and very unhealthy). Similar results have been observed in a previous study focused on bulk particulate matter concentrations.59
Indoor versus Outdoor PAH Air Concentrations before, during, and after Wildfires
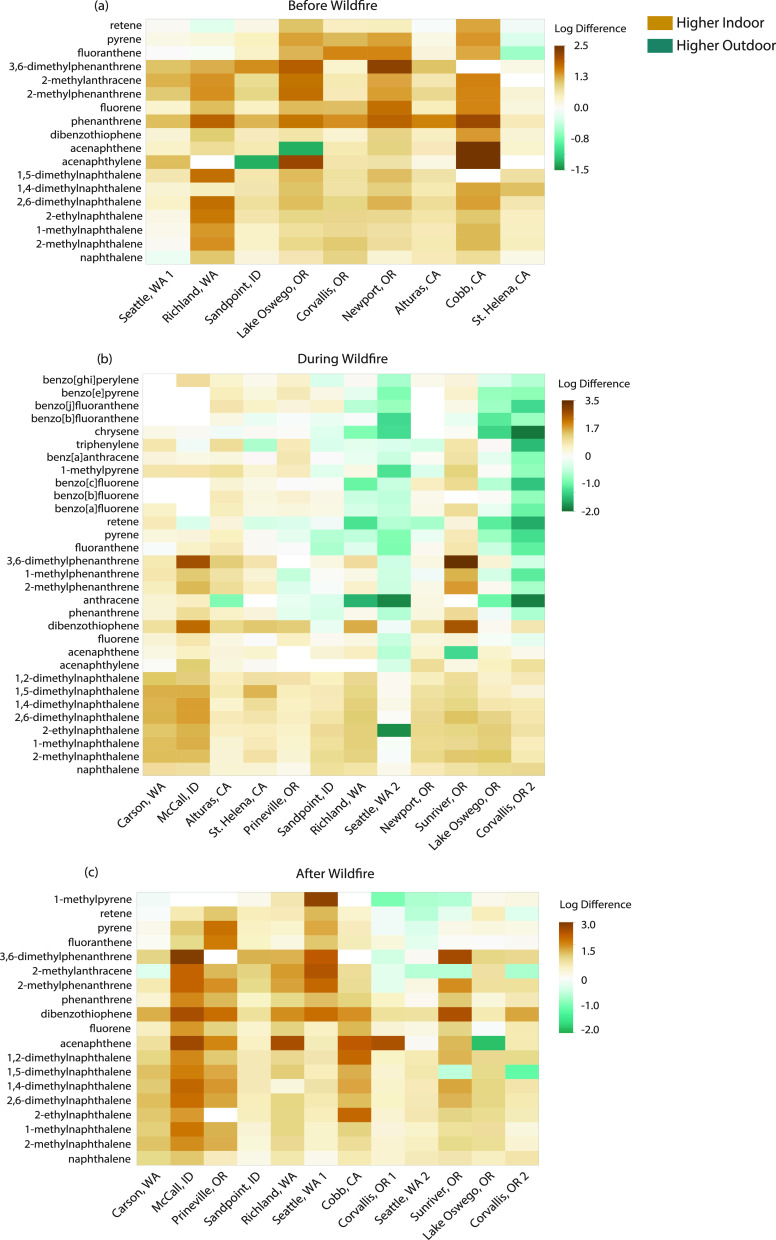
Indoor minus outdoor (indoor–outdoor) PAH air concentrations for each sampling event are presented in Figure 2.
Figure 2.
Heat map of indoor–outdoor vapor-phase PAH concentrations (a) before, (b) during, and (c) after wildfires. Air concentrations were first log-transformed and then subtracted. Only PAHs detected in at least 75% of samples are included. Shades of yellow represent higher PAH concentrations indoors, while shades of blue represent higher PAH air concentrations outdoors.
Before wildfires, indoor vapor-phase PAH air concentrations were significantly higher than outdoor concentrations at all locations except St. Helena, CA (Figure 2a and Table 3). Potential explanations for specific site differences are discussed below. A 2021 review of particulate PAH concentrations in indoor dust samples observed a similar trend, with median sum PAH indoor air concentrations being approximately 2.6 times higher than outdoor air across the globe.60 Other studies largely focused on PAHs from particulate matter indicate that indoor–outdoor ratios may differ for individual PAHs.61−63 Personal behaviors and different PAH sources used inside the home have been highlighted as potential causes of different study results.58,63,64
Table 3. One-Sided Paired t-Test Comparing Indoor–Outdoor PAH Air Concentrations before, during, and after Wildfiresa.
| location | before wildfireb | wildfirec | after wildfired | wildfire average AQI |
|---|---|---|---|---|
| Seattle, WA 1 | 0.0025* | N/A | <0.0001* | N/A |
| Cobb, CA | <0.00010* | N/A | <0.0001* | N/A |
| Corvallis, OR 1 | <0.00010* | N/A | 0.0290e | N/A |
| Carson, WA | N/A | <0.00010* | <0.00010* | 21 |
| Newport, OR | <0.00010* | <0.00010* | N/A | 70 |
| McCall, ID | N/A | <0.00010* | <0.00010* | 73 |
| Alturas, CA | <0.00010* | <0.00010* | N/A | 90 |
| St. Helena, CA | 0.043 | <0.00010* | N/A | 93 |
| Prineville, OR | N/A | <0.00010* | <0.00010* | 95 |
| Sandpoint, ID | <0.00010* | 0.473 | <0.00010* | 116 |
| Richland, WA | 0.0010* | 0.15 | <0.00010* | 135 |
| Seattle, WA 2 | N/A | 1.0 | 0.76 | 142 |
| Sunriver, OR | N/A | <0.00010* | 0.057 | 184 |
| Lake Oswego, OR | <0.00010* | 0.69 | 0.0015* | 189 |
| Corvallis, OR 2 | N/A | 1.0 | 0.18 | 220 |
Significant p-values indicate that vapor-phase PAH air concentrations indoors are significantly greater than those outdoors. α values are Bonferroni-corrected for each sampling event.
*Significant when probability <t at α′ = 0.0056.
*Significant when probability <t at α′ = 0.0042.
*Significant when probability <t at α′ = 0.0042.
Potential impact due to backyard campfire near the outdoor air sampler.
Surprisingly, during wildfires, indoor vapor-phase PAH air concentrations were still significantly higher than outdoor concentrations at locations with an average AQI of less than 115, described as good, moderate, or unhealthy for sensitive group air quality (Figure 2b and Table 3). These results are similar to those observed by Messier et al.24 However, a shift occurred at locations with an average AQI exceeding 115 (unhealthy for sensitive groups), where outdoor HMW PAH air concentrations exceeded indoor concentrations (Figure 2b). Additionally, indoor air concentrations were not significantly higher than outdoor air concentrations (Table 3) with the exception of Sunriver, OR. Potential explanations for specific site differences are discussed below. These results suggest that indoor vapor-phase PAH air quality can be worse than outdoor air quality during mild wildfire conditions.
Prior studies on particulate-bound PAHs have found different results, wherein outdoor air concentrations were consistently higher than indoor air concentrations.65−67 Since PAHs can be present in both the vapor phase and particulate phase,19 and vapor-phase PAHs can account for up to 86% of the cancer risk from total inhalation exposure (vapor phase and particulate matter),20−23 our results provide an important consideration for public health recommendations during wildfire events.
Previous studies have also focused on air infiltration indoors,65−67 and often excluded or subtracted out indoor sources of particulate matter, in attempts to only capture PAH air concentrations related to wildfire smoke infiltration.65 In this approach, PAH concentrations from outdoor air infiltration alone do not capture the total indoor air quality, where residents are being exposed to sources from inside and outside the home. Therefore, the concentrations presented in this study represent a more accurate measure of human exposure indoors during wildfires.
To evaluate the potential long-term effects of extreme wildfire smoke events, we compared indoor–outdoor vapor-phase PAH air concentrations after wildfires in 2018 and 2020. Following mild smoke impact (2018), indoor vapor-phase PAH air concentrations were significantly higher than outdoor air concentrations (Figure 2c and Table 2). In contrast, 2020 indoor air concentrations for three of the four locations (Seattle, WA, Sunriver, OR, and Corvallis, OR) were not significantly higher than outdoor air concentrations (Table 2). These results suggest that outdoor air could still be impacted by HMW vapor-phase PAHs after extreme wildfire events.
Influence of Wildfire Characteristics and Participant Behaviors on Indoor and Outdoor Air PAH Concentrations
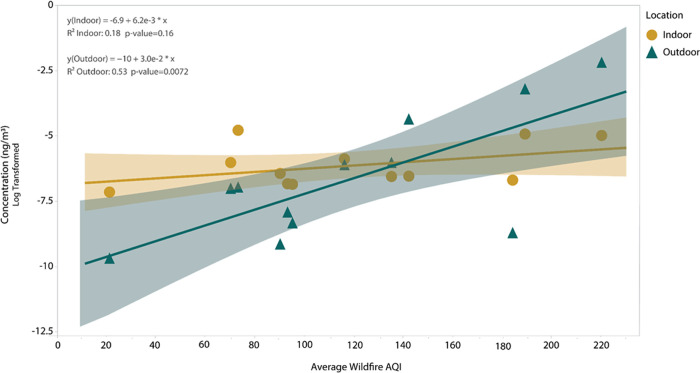
HMW PAH concentrations in indoor and outdoor air during wildfires are plotted against the average AQI in Figure 3. A significant (p < 0.010) linear relationship was found between the average AQI and outdoor air concentrations of HMW PAHs during wildfires (Figure 3) but not indoor air. Figure 3 also highlights that below an AQI of 140, indoor HMW PAH air concentrations are higher than outdoor air concentrations. Above an AQI of 140, a shift occurs, and outdoor HMW PAH air concentrations are higher than indoor air concentrations. No significant relationship was found for LMW PAH air concentrations and average AQI.
Figure 3.
Linear regression models during wildfires for sum HMW PAH air concentrations (log-transformed) and average AQI. Air concentrations for indoor (yellow circles) and outdoor (blue triangles) samples are each plotted against the average AQI at all locations. The α value is Holm–Bonferroni-corrected at α′ = 0.010.
No significant relationships were found between air concentrations and NOAA smoke density or any wildfire parameters (wildfire size, distance from wildfire, etc.). Future research should explore other parameters for their influence on PAH concentrations during wildfires.
Survey responses and comments from participants were examined to identify the potential influence of human behavior on PAH air concentrations indoors and outdoors. No significant relationships were found between indoor air and participant survey responses. A lack of significant correlations could be due to incomplete survey data, with compliance at 55% for 2019 and 2020.
Some locations, such as Alturas, CA, and Sunriver, OR, did not follow the expected trends when comparing PAH air concentrations before, during, and after wildfires. Alturas, CA, had similar PAH air concentrations indoors before and during wildfires. Sunriver, OR, had higher PAH air concentrations outdoors after wildfires than during wildfires. Survey responses indicated that the participants themselves owned a woodstove that was used when sampling before/after wildfires, and woodstoves were also being used by other residents in the area. Previous studies have noted the impact of woodstove heating as a significant source of PAH air concentrations.68,69 Therefore, woodstove use could explain higher PAH concentrations in these Alturas and Sunriver samples.
Additionally, Sunriver, OR, and St. Helena, CA, did not follow the trends seen for other locations when comparing indoor and outdoor PAH air concentrations. Sunriver, OR, had significantly higher indoor PAH air concentrations during wildfires, even though this location had an average AQI of 189. St. Helena, CA, had similar indoor and outdoor concentrations before wildfires. Survey responses did not indicate any personal behaviors that would account for higher indoor concentrations. Home age, ventilation, and other conditions could account for these differences.70,71 Another contributing factor could be the distance of the AQI monitor from the sampling location and/or prevailing wind direction of smoke in relation to the AQI monitor. The closest U.S. EPA AQI monitor was 17 miles away for Sunriver, OR, and 20 miles away for St. Helena, CA (Tables S6 and S7).
Survey responses were also examined for any potential changes in personal behaviors during each sampling event. On average, participants indicated that when sampling before wildfires, windows were opened for 10 (range: 0–44) days (Table S13). However, on average, participants only opened windows for 2 (range: 0–6) days during wildfires and 5 (range: 0–21) days after wildfires (Table S13). No changes in behavior were observed with air conditioning use. Other studies have shown the large role of personal behaviors on indoor air exposure, in addition to household characteristics.72 Future research should further explore the effects of personal and household variables on indoor PAH exposure under various wildfire conditions.
Exploratory Assessment of Wildfire Influence on Cancer and Noncancer Inhalation Risks
Cancer risk and noncancer hazard values were compared before, during, and after wildfires for indoor and outdoor PAH inhalation exposures. Indoors and outdoors, 80% of locations had a higher inhalation cancer risk and hazard quotient during wildfires than before or after (Tables S18 and S19). Indoor inhalation risk (cancer and noncancer) was three times higher during wildfires, while outdoor inhalation risk was 36 times higher. Our study results suggest that human inhalation exposure to PAHs from wildfire smoke increases both cancer and noncancer risks, regardless of indoor or outdoor location, even over a short period of exposure. Messier et al.24 did not previously observe significant changes in cancer risk with increased smoke impact but only sampled during mild wildfire conditions.
Cancer risk and noncancer hazard values were also compared for indoor versus outdoor PAH inhalation exposure during wildfires. All 12 locations had a higher risk indoors than outdoors during wildfires (Table 4). Similar results were found in Messier et al. during wildfire sampling using a toxicity equivalency approach.24 Cancer risk was largely driven by naphthalene, fluoranthene, 7,12-dimethylbenz[a]anthracene, and benzo[j]fluoranthene, while noncancer hazard was largely driven by naphthalene. A few other studies24,73−76 have compared inhalation risk to PAHs in indoor versus outdoor air. Of the studies conducted, the focus has been primarily on particulate matter and found that risk to outdoor air is higher than indoor air.73−76 However, other recent studies have found that indoor cancer risk can be higher than or similar to risk from outdoor air.77,78
Table 4. Inhalation Cancer Risk and Noncancer Hazard Indoors Compared to Outdoors during Wildfiresa.
| location | indoor cancer risk | outdoor cancer risk | indoor hazard quotient | outdoor hazard quotient | wildfire average AQI |
|---|---|---|---|---|---|
| Carson, WA | 3.0 × 10–7 | 1.2 × 10–8 | 3.5 × 10–3 | 3.4 × 10–5 | 21 |
| Newport, OR | 6.1 × 10–8 | 4.8 × 10–9 | 2.5 × 10–4 | 5.2 × 10–6 | 70 |
| McCall, ID | 4.0 × 10–7 | 1.1 × 10–8 | 2.1 × 10–3 | 2.4 × 10–5 | 73 |
| Alturas, CA | 5.2 × 10–7 | 1.5 × 10–8 | 1.6 × 10–3 | 6.2 × 10–5 | 90 |
| St. Helena, CA | 2.9 × 10–7 | 2.4 × 10–8 | 7.6 × 10–4 | 3.2 × 10–5 | 93 |
| Prineville, OR | 9.9 × 10–8 | 1.2 × 10–8 | 4.3 × 10–4 | 3.8 × 10–5 | 95 |
| Sandpoint, ID | 3.5 × 10–7 | 5.1 × 10–8 | 3.2 × 10–3 | 4.6 × 10–4 | 116 |
| Richland, WA | 3.7× 10–7 | 4.6 × 10–8 | 2.2 × 10–3 | 3.2 × 10–5 | 135 |
| Seattle, WA 2 | 1.6 × 10–7 | 1.1 × 10–7 | 7.1 × 10–4 | 6.9 × 10–5 | 142 |
| Sunriver, OR | 2.5 × 10–7 | 5.6× 10–9 | 5.9 × 10–4 | 7.9 × 10–6 | 184 |
| Lake Oswego, OR | 7.6 × 10–7 | 2.0 × 10–7 | 1.2 × 10–3 | 3.2 × 10–5 | 189 |
| Corvallis, OR 2 | 7.6 × 10–7 | 4.6 × 10–7 | 7.7 × 10–4 | 6.5 × 10–5 | 220 |
Values in bold indicate the higher risk for a particular site.
Future studies should examine the risk of vapor-phase PAHs in indoor and outdoor air irrespective of wildfires to provide a more comprehensive assessment of PAH risk in both environments.
Recommendations
Results from our study suggest that current public health recommendations during wildfires, relying mostly on outdoor air quality and particulate matter, may not be sufficient for reducing human PAH exposure. Improved recommendations may highlight not only keeping smoke out of the home but also keeping indoor air clean from other vapor-phase and particulate PAH sources through increased ventilation and use of higher efficiency filters, which can remove both particulate and vapor-phase contaminants. Additionally, more protective recommendations may be needed for sensitive populations.
Due to incomplete survey responses, no significant relationships were found between indoor PAH air concentrations and other indoor PAH sources. However, our results indicate that there are ongoing indoor sources of PAHs, which contribute to high indoor concentrations. Indoor sources of vapor-phase PAHs should be explored further in a future study.
There are limitations to wildfire sampling studies using stationary monitors. Previous studies have used outdoor PAH air concentrations from public stationary monitoring systems rather than pairing an outdoor sampler at each sampling site.65−67 Distance from the monitor to the home could lead to a loss in the resolution of exposure, as seen in our study, and depending on the prevailing wind direction may not be an accurate reflection of local air quality (e.g., monitor may be upwind of smoke). However, by codeploying passive samplers at the same residential location in our study, a better representation of true indoor and outdoor PAH exposures was measured.
An additional limitation is that the PAH inhalation risk assessments provided in this study represent only a fraction of the entire PAH mixture. Without a comprehensive list of reference values for all PAHs, it is difficult to determine the true risks associated with indoor and outdoor exposures. Further research into additional PAH compounds and mixtures is needed to provide a more realistic cancer risk and hazard quotient estimate.
Acknowledgments
The authors wish to extend a special thanks to the community participants for their help in data collection and real-time observations of field conditions across 3 years of sampling. The authors also thank Michael Barton for his tremendous effort in data organization and development of participant report backs, Richard Scott, Lane Tidwell, Clarisa Caballero-Ignacio, Kaci Graber, Caoilinn Haggerty, and Jacob Del Savio for their help in gear preparation, shipping, and laboratory processing, Brianna Rivera for her help in survey question compilation, Ian Moran for his help with statistical analysis, and all of the members of Oregon State University’s Food Safety and Environmental Stewardship Program for their help in the identification of potential sampling locations.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c00619.
The Supporting Information contains additional details about sample preparation, collected metadata, analytical methodology, calculations, environmental concentrations, and data analysis (PDF)
This work was supported by the National Institute of Environmental Health Sciences (NIEHS) award numbers P42 ES016465, T32 ES007060, and P30 ES030287. This content is solely the responsibility of the authors and does not represent the official views of the NIEHS or NIH.
The authors declare no competing financial interest.
Supplementary Material
References
- Doerr S. H.; Santín C. Global trends in wildfire and its impacts: perceptions versus realities in a changing world. Philos. Trans. R. Soc., B 2016, 371, 20150345 10.1098/rstb.2015.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Interagency Coordination Center, Wildland Fire Statistics. National Interagency Coordination Fire Center, 2018.
- California Department of Forestry and Fire Protection, Top 20 Largest California Wildfires. California Department of Forestry and Fire Protection, Ed, 2021.
- Urness Z.Oregon’s 2020 wildfire season brought a new level of destruction. It could be 438 just the beginning. Statesman Journal October 30, 2020.
- Oregon Wildfire Economic Recovery Council, Recovering & Rebuilding from Oregon’s 2020 Wildfires.. Governor, O., Ed. 2021, p 32.
- Congressional Research Service, Wildfire Statistics. United States Congress, Ed. 2021, p 33.
- Childs J. W.Dangerous Wildfire Smoke Persists in California, Oregon and Washington. The Weather Channel (September 15, 2020). [Google Scholar]
- Dennison P. E.; Brewer S. C.; Arnold J. D.; Moritz M. A. Large wildfire trends in the western United States, 1984-2011. Geophys. Res. Lett. 2014, 41, 2928–2933. 10.1002/2014GL059576. [DOI] [Google Scholar]
- SciLine. Wildfire Trends in the United States. https://www.sciline.org/evidence-blog/wildfires (accessed December, 2018).
- Dennison P. E.; Brewer S. C.; Arnold J. D.; Moritz M. A. Large wildfire trends in the western United States, 1984–2011. Geophys. Res. Lett. 2014, 41, 2928–2933. 10.1002/2014GL059576. [DOI] [Google Scholar]
- Kenward A.; Sanford T.; Bronzan J.. Western Wildfires: A Fiery Future, Climate Central, 2016; p 42. [Google Scholar]
- Aguilera R.; Corringham T.; Gershunov A.; Leibel S.; Benmarhnia T. Fine Particles in Wildfire Smoke and Pediatric Respiratory Health in California. Pediatrics 2021, 147, e2020027128 10.1542/peds.2020-027128. [DOI] [PubMed] [Google Scholar]
- Margolis A. E.; Herbstman J. B.; Davis K. S.; Thomas V. K.; Tang D.; Wang Y.; Wang S.; Perera F. P.; Peterson B. S.; Rauh V. A. Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. J. Child Psychol. Psychiatry 2016, 57, 851–860. 10.1111/jcpp.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortey-Sam N.; Ikenaka Y.; Akoto O.; Nakayama S. M. M.; Asante K. A.; Baidoo E.; Obirikorang C.; Saengtienchai A.; Isoda N.; Nimako C.; Mizukawa H.; Ishizuka M. Oxidative stress and respiratory symptoms due to human exposure to polycyclic aromatic hydrocarbons (PAHs) in Kumasi, Ghana. Environ. Pollut. 2017, 228, 311–320. 10.1016/j.envpol.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Abdel-Shafy H. I.; Mansour M. S. M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. 10.1016/j.ejpe.2015.03.011. [DOI] [Google Scholar]
- Kennish M. J.Practical Handbook of Estuarine and Marine Pollution, 1st ed.; Taylor & Francis Group: Boca Raton, FL, 1997; p 544. [Google Scholar]
- Bunce N.Environmental Chemistry; Wuerz Pub Ltd.: Winnipeg, Canada, 1990; p 340. [Google Scholar]
- United States Environmental Protection Agency NAAQS Table. https://www.epa.gov/criteria-air-pollutants/naaqs-table (accessed May 25, 2020).
- Maharaj Kumari K.; Lakhani A.. PAHs in Gas and Particulate Phases: Measurement and Control. In Environmental Contaminants: Measurement, Modelling and Control; Gupta T.; Agarwal A. K.; Agarwal R. A.; Labhsetwar N. K., Eds.; Springer: Singapore: Singapore, 2018; pp 43–75. [Google Scholar]
- Ramírez N.; Cuadras A.; Rovira E.; Marce R. M.; Borrull F. Risk assessment related to atmospheric polycyclic aromatic hydrocarbons in gas and particle phases near industrial sites. Environ. Health Perspect. 2011, 119, 1110–1116. 10.1289/ehp.1002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samburova V.; Zielinska B.; Khlystov A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity?. Toxics 2017, 5, 17 10.3390/toxics5030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. N.; Tao S.; Dou H.; Zhang T. W.; Zhang X. L.; Dawson R. Exposure of traffic police to Polycyclic aromatic hydrocarbons in Beijing, China. Chemosphere 2007, 66, 1922–1928. 10.1016/j.chemosphere.2006.07.076. [DOI] [PubMed] [Google Scholar]
- Tsai P.-J.; Shieh H.-Y.; Lee W.-J.; Lai S.-O. Characterization of PAHs in the atmosphere of carbon black manufacturing workplaces. J. Hazard. Mater. 2002, 91, 25–42. 10.1016/S0304-3894(01)00384-3. [DOI] [PubMed] [Google Scholar]
- Messier K. P.; Tidwell L. G.; Ghetu C. C.; Rohlman D.; Scott R. P.; Bramer L. M.; Dixon H. M.; Waters K. M.; Anderson K. A. Indoor versus Outdoor Air Quality during Wildfires. Environ. Sci. Technol. Lett. 2019, 6, 696–701. 10.1021/acs.estlett.9b00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins J. N.; Petty J. D.; Booij K.. Monitors of the Organic Chemicals in the Environment. In Monitors of the Organic Chemicals in the Environment; Springer: New York, NY, 2006; p 223. [Google Scholar]
- Anderson K. A.; Sethajintanin D.; Sower G.; Quarles L. Field Trial and Modeling of Uptake Rates of In Situ Lipid-Free Polyethylene Membrane Passive Sampler. Environ. Sci. Technol. 2008, 42, 4486–4493. 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- Donald C. E.; Anderson K. A. Assessing soil-air partitioning of PAHs and PCBs with a new fugacity passive sampler. Sci. Total Environ. 2017, 596–597, 293–302. 10.1016/j.scitotenv.2017.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell L. G.; Allan S. E.; O’Connell S. G.; Hobbie K. A.; Smith B. W.; Anderson K. A. PAH and OPAH Flux during the Deepwater Horizon Incident. Environ. Sci. Technol. 2016, 50, 7489–7497. 10.1021/acs.est.6b02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell L. G.; Blair Paulik L.; Anderson K. A. Air-water exchange of PAHs and OPAHs at a superfund mega-site. Sci. Total Environ. 2017, 603–604, 676–686. 10.1016/j.scitotenv.2017.01.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald C. E.; Elie M. R.; Smith B. W.; Hoffman P. D.; Anderson K. A. Transport stability of pesticides and PAHs sequestered in polyethylene passive sampling devices. Environ. Sci. Pollut. Res. 2016, 23, 12392–12399. 10.1007/s11356-016-6453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty J. D.; Huckins J. N.; Martin D. B.; Adornato T. G. Use of semipermeable membrane devices (SPMDS) to determine bioavailable organochlorine pesticide residues in streams receiving irrigation drainwater. Chemosphere 1995, 30, 1891–1903. 10.1016/0045-6535(95)00070-O. [DOI] [Google Scholar]
- Allan S. E.; Smith B. W.; Anderson K. A. Impact of the Deepwater Horizon Oil Spill on Bioavailable Polycyclic Aromatic Hydrocarbons in Gulf of Mexico Coastal Waters. Environ. Sci. Technol. 2012, 46, 2033–2039. 10.1021/es202942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik L. B.; Donald C. E.; Smith B. W.; Tidwell L. G.; Hobbie K. A.; Kincl L.; Haynes E. N.; Anderson K. A. Emissions of Polycyclic Aromatic Hydrocarbons from Natural Gas Extraction into Air. Environ. Sci. Technol. 2016, 50, 7921–7929. 10.1021/acs.est.6b02762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. A.; Szelewski M. J.; Wilson G.; Quimby B. D.; Hoffman P. D. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J. Chromatogr. A 2015, 1419, 89–98. 10.1016/j.chroma.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins J. N. P.; J D; Booij K.. Monitors of the Organic Chemicals in ihe Environment; Springer: New York, NY, 2006; p 223. [Google Scholar]
- United States Environmental Protection Agency, Risk Assessment Guidance for Superfund Office of Superfund Remediation and Technology Innovation, Ed.; Vol. I: Human Health Evaluation Manual, 2009.
- Klepeis N. E.; Nelson W. C.; Ott W. R.; Robinson J. P.; Tsang A. M.; Switzer P.; Behar J. V.; Hern S. C.; Engelmann W. H. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J. Exposure Sci. Environ. Epidemiol. 2001, 11, 231–252. 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency, Human Health Toxicity Values in Superfund Risk Assessments Memorandum, Office of Superfund Remediation and Technology Innovation, Ed, 2003.
- United States Environmental Protection Agency, Integrated Risk Information System Assessments. Office of Research and Development, 2017.
- United States Environmental Protection Agency, Provisional Peer-Reviewed Toxicity Values (PPRTVs) Assessments. Office of Research and Development, 2013.
- California Environmental Protection Agency, Toxic Air Contaminant List with Staff Reports/Executive Summaries. California Office of Environmental Health Hazard Assessment, 2008.
- United States Environmental Protection Agency, CompTox Chemicals Dashboard. Office of Research and Development.
- Oregon Department of Environmental Quality and Oregon Health Authority, Cleaner Air Oregon Toxicity Refererence Values. State of Oregon, 2018.
- AirNow Interactive Map of Air Quality. https://gispub.epa.gov/airnow/ (accessed December, 2018, December, 2019, December, 2020).
- AirNow Air Quality Index (AQI) Basics. https://www.airnow.gov/aqi/aqi-basics/ (accessed December, 2019).
- McNamara D.; Stephens G.; Ruminski M.; Kasheta T. In The Hazard Mapping System (HMS) - NOAA’S Multi-Densor Gire and Dmoke Fetection Program Using Rnvironmental Satellites; Conference on Satellite Meteorology and Oceanography, 2004.
- Matzke M. M.; Allan S. E.; Anderson K. A.; Waters K. M. An approach for calculating a confidence interval from a single aquatic sample for monitoring hydrophobic organic contaminants. Environ. Toxicol. Chem. 2012, 31, 2888–2892. 10.1002/etc.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sower G. J.; Anderson K. A. Spatial and Temporal Variation of Freely Dissolved Polycyclic Aromatic Hydrocarbons in an Urban River Undergoing Superfund Remediation. Environ. Sci. Technol. 2008, 42, 9065–9071. 10.1021/es801286z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald C. E.; Scott R. P.; Wilson G.; Hoffman P. D.; Anderson K. A. Artificial turf: chemical flux and development of silicone wristband partitioning coefficients. Air Qual, Atmos. Health 2019, 12, 597–611. 10.1007/s11869-019-00680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik L. B.; Hobbie K. A.; Rohlman D.; Smith B. W.; Scott R. P.; Kincl L.; Haynes E. N.; Anderson K. A. Environmental and individual PAH exposures near rural natural gas extraction. Environ. Pollut. 2018, 241, 397–405. 10.1016/j.envpol.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth G. R.; Aklilu Y.-a.; Landis M. S.; Hsu Y.-M. Impacts of a large boreal wildfire on ground level atmospheric concentrations of PAHs, VOCs and ozone. Atmos. Environ. 2018, 178, 19–30. 10.1016/j.atmosenv.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.; Tao S.; Li B.; Lang C.; Cao J.; Coveney R. M. Emission and outflow of polycyclic aromatic hydrocarbons from wildfires in China. Atmos. Environ. 2008, 42, 6828–6835. 10.1016/j.atmosenv.2008.05.033. [DOI] [Google Scholar]
- Masclet P.; Cachier H.; Liousse C.; Wortham H. Emissions of Polycyclic aromatic hydrocarbons by savanna fires. J. Atmos. Chem. 1995, 22, 41–54. 10.1007/BF00708180. [DOI] [Google Scholar]
- Andersson J. T.; Achten C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl Aromat. Compd. 2015, 35, 330–354. 10.1080/10406638.2014.991042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon H. M.; Armstrong G.; Barton M.; Bergmann A. J.; Bondy M.; Halbleib M. L.; Hamilton W.; Haynes E.; Herbstman J.; Hoffman P.; Jepson P.; Kile M. L.; Kincl L.; Laurienti P. J.; North P.; Paulik L. B.; Petrosino J.; Points G. L.; Poutasse C. M.; Rohlman D.; Scott R. P.; Smith B.; Tidwell L. G.; Walker C.; Waters K. M.; Anderson K. A. Discovery of common chemical exposures across three continents using silicone wristbands. R. Soc. Open Sci. 2019, 6, 181836 10.1098/rsos.181836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J. M.; Gennings C.; Hauser R.; Webster T. F. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health?. Environ. Health Perspect. 2016, 124, A6–A9. 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A.; Schoonover T. M.; Zou Q.; Norlock F.; Conroy L. M.; Scheff P. A.; Wadden R. A. Polycyclic aromatic hydrocarbons in residential air of ten Chicago area homes: Concentrations and influencing factors. Atmos. Environ. 2005, 39, 3491–3501. 10.1016/j.atmosenv.2005.02.029. [DOI] [Google Scholar]
- Hyunok Choi R. H.; Komulainen H.; Saborit J. M. D.. WHO Guidelines for Indoor Air Quality: Selected Pollutants, Organization, G. W. H., Ed., 2010. [PubMed] [Google Scholar]
- Wu J.; M Winer A.; J Delfino R. Exposure assessment of particulate matter air pollution before, during, and after the 2003 Southern California wildfires. Atmos. Environ. 2006, 40, 3333–3348. 10.1016/j.atmosenv.2006.01.056. [DOI] [Google Scholar]
- Wang M.; Jia S.; Lee S. H.; Chow A.; Fang M. Polycyclic aromatic hydrocarbons (PAHs) in indoor environments are still imposing carcinogenic risk. J. Hazard. Mater. 2021, 409, 124531 10.1016/j.jhazmat.2020.124531. [DOI] [PubMed] [Google Scholar]
- Ohura T.; Amagai T.; Sugiyama T.; Fusaya M.; Matsushita H. Characteristics of particle matter and associated polycyclic aromatic hydrocarbons in indoor and outdoor air in two cities in Shizuoka, Japan. Atmos. Environ. 2004, 38, 2045–2054. 10.1016/j.atmosenv.2004.01.038. [DOI] [Google Scholar]
- Naumova Y. Y.; Eisenreich S. J.; Turpin B. J.; Weisel C. P.; Morandi M. T.; Colome S. D.; Totten L. A.; Stock T. H.; Winer A. M.; Alimokhtari S.; Kwon J.; Shendell D.; Jones J.; Maberti S.; Wall S. J. Polycyclic Aromatic Hydrocarbons in the Indoor and Outdoor Air of Three Cities in the U.S. Environ. Sci. Technol. 2002, 36, 2552–2559. 10.1021/es015727h. [DOI] [PubMed] [Google Scholar]
- Halsall C. J.; Maher B. A.; Karloukovski V. V.; Shah P.; Watkins S. J. A novel approach to investigating indoor/outdoor pollution links: Combined magnetic and PAH measurements. Atmospheric Environment 2008, 42, 8902–8909. 10.1016/j.atmosenv.2008.09.001. [DOI] [Google Scholar]
- Ohura T.; Amagai T.; Fusaya M.; Matsushita H. Polycyclic Aromatic Hydrocarbons in Indoor and Outdoor Environments and Factors Affecting Their Concentrations. Environ. Sci. Technol. 2004, 38, 77–83. 10.1021/es030512o. [DOI] [PubMed] [Google Scholar]
- Xiang J.; Huang C.-H.; Shirai J.; Liu Y.; Carmona N.; Zuidema C.; Austin E.; Gould T.; Larson T.; Seto E. Field measurements of PM2.5 infiltration factor and portable air cleaner effectiveness during wildfire episodes in US residences. Sci. Total Environ. 2021, 773, 145642 10.1016/j.scitotenv.2021.145642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May N. W.; Dixon C.; Jaffe D. A. Impact of Wildfire Smoke Events on Indoor Air Quality and Evaluation of a Low-cost Filtration Method. Aerosol Air Qual. Res. 2021, 10.4209/aaqr.210046. [DOI] [Google Scholar]
- Liang Y.; Sengupta D.; Campmier M. J.; Apte J.; Lunderberg D.; Goldstein A. Wildfire Smoke Impacts on Indoor Air Quality Assessed Using Crowdsourced Data in California. Proc. Natl. Acad. Sci. U.S.A. 2021, 118, e2106478118 10.1073/pnas.2106478118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daisey J. M.; Spengler J. D.; Kaarakka P. A comparison of the organic chemical composition of indoor aerosols during woodburning aero non-woodburning periods. Environ. Int. 1989, 15, 435–442. 10.1016/0160-4120(89)90059-7. [DOI] [Google Scholar]
- Schauer J. J.; Kleeman M. J.; Cass G. R.; Simoneit B. R. T. Measurement of Emissions from Air Pollution Sources. 3. C1–C29 Organic Compounds from Fireplace Combustion of Wood. Environ. Sci. Technol. 2001, 35, 1716–1728. 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency Introduction to Indoor Air Quality (accessed July 10, 2020).
- Shrestha P. M.; Humphrey J. L.; Carlton E. J.; Adgate J. L.; Barton K. E.; Root E. D.; Miller S. L. Impact of Outdoor Air Pollution on Indoor Air Quality in Low-Income Homes during Wildfire Seasons. Int. J. Environ. Res. Public Health 2019, 16, 3535. 10.3390/ijerph16193535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardoulakis S.; Giagloglou E.; Steinle S.; Davis A.; Sleeuwenhoek A.; Galea K. S.; Dixon K.; Crawford J. O. Indoor Exposure to Selected Air Pollutants in the Home Environment: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 8972. 10.3390/ijerph17238972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Saborit J. M.; Stark C.; Harrison R. M. Carcinogenic potential, levels and sources of polycyclic aromatic hydrocarbon mixtures in indoor and outdoor environments and their implications for air quality standards. Environ. Int. 2011, 37, 383–392. 10.1016/j.envint.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Jung K. H.; Yan B.; Chillrud S. N.; Perera F. P.; Whyatt R.; Camann D.; Kinney P. L.; Miller R. L. Assessment of Benzo(a)pyrene-equivalent Carcinogenicity and Mutagenicity of Residential Indoor versus Outdoor Polycyclic Aromatic Hydrocarbons Exposing Young Children in New York City. Int. J. Environ. Res. Public Health 2010, 7, 1889–1900. 10.3390/ijerph7051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Wang W.; Yang L.; Chen X.; Gao Y.; Jiang P.; Zhang J.; Yu H. PM2.5-Bound PAHs in Indoor and Outdoor of Hotels in Urban and Suburban of Jinan, China: Concentrations, Sources, and Health Risk Impacts. Aerosol Air Qual. Res. 2017, 17, 2463–2473. 10.4209/aaqr.2017.08.0286. [DOI] [Google Scholar]
- Zhang L.; Yang Z.; Liu J.; Zeng H.; Fang B.; Xu H.; Wang Q. Indoor/outdoor relationships, signatures, sources, and carcinogenic risk assessment of polycyclic aromatic hydrocarbons-enriched PM2.5 in an emerging port of northern China. Environ. Geochem. Health 2021, 43, 3067–3081. 10.1007/s10653-021-00819-z. [DOI] [PubMed] [Google Scholar]
- Hu Y.-J.; Bao L.-J.; Huang C.-L.; Li S.-M.; Liu P.; Zeng E. Y. Assessment of airborne polycyclic aromatic hydrocarbons in a megacity of South China: Spatiotemporal variability, indoor-outdoor interplay and potential human health risk. Environ. Pollut. 2018, 238, 431–439. 10.1016/j.envpol.2018.03.040. [DOI] [PubMed] [Google Scholar]
- Wang J.; Guinot B.; Dong Z.; Li X.; Xu H.; Xiao S.; Ho S. S. H.; Liu S.; Cao J. PM2.5-Bound Polycyclic Aromatic Hydrocarbons (PAHs), Oxygenated-PAHs and Phthalate Esters (PAEs) inside and outside Middle School Classrooms in Xi’an, China: Concentration, Characteristics and Health Risk Assessment. Aerosol Air Qual. Res. 2017, 17, 1811–1824. 10.4209/aaqr.2017.03.0109. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.