Abstract
Aims
Catheter ablation is superior to escalated antiarrhythmic drugs among patients with ventricular tachycardia (VT) and prior myocardial infarction (MI). However, it is uncertain whether clinical VT characteristics, should influence choice of therapy. The purpose of this study was to evaluate whether presentation with electrical storm and the clinical VT cycle length predicted response to ablation vs. escalated antiarrhythmic therapy.
Methods and results
All patients enrolled in the Ventricular Tachycardia Ablation vs. Escalated Antiarrhythmic Drug Therapy in Ischaemic Heart Disease (VANISH) trial were included. The association between VT cycle length and presentation with electrical storm and the primary outcome of death, subsequent VT storm or appropriate ICD shock was evaluated. Among the study population of 259 patients, escalated antiarrhythmic drug therapy had worse outcomes for those presenting with a VT cycle length >400 ms [<150 b.p.m., 89/259, hazard ratio (HR) 1.7 (1.02–3.13)]. This effect was more pronounced among those taking amiodarone at baseline [HR of 2.22 (1.19–4.16)]. Presentation with VT storm (32/259) did not affect the primary outcome between groups. However, those presenting with VT storm on amiodarone had a trend towards worse outcomes with escalated antiarrhythmic therapy [HR 4.31 (0.55–33.93)].
Conclusion
The VT cycle length can influence response to either ablation or escalated drug therapy in patients with VT and prior MI. Those with slow VT had improved outcomes with ablation. Patients presenting with electrical storm demonstrated similar outcomes to the overall trial population, with a trend to benefit of catheter ablation, particularly in those on amiodarone.
Keywords: Ventricular tachycardia, Catheter ablation, Antiarrhythmic therapy, VANISH trial
What’s new?
In patients presenting with ventricular tachycardia and prior myocardial infarction, despite antiarrhythmic drugs, those presenting with slower VT (especially <150 b.p.m.) have better outcomes with catheter ablation than escalating drug therapy.
Catheter ablation may be superior to escalating antiarrhythmic drug therapy in those patients presenting with ventricular tachycardia storm despite amiodarone.
Introduction
The Ventricular Tachycardia Ablation vs. Escalated Antiarrhythmic Drug Therapy in Ischaemic Heart Disease (VANISH) trial compared catheter ablation with escalated antiarrhythmic drug therapy in patients with a previous myocardial infarction (MI) and ventricular tachycardia (VT) despite antiarrhythmic therapy.1 The overall study demonstrated the superiority of catheter ablation, though outcomes were significantly influenced by whether patients had failed amiodarone vs. another antiarrhythmic drug, such as sotalol, at enrolment.
While the overall VANISH trial compared the efficacy of each treatment across the whole population, it did not specifically examine whether there were patient-level VT characteristics that might predict better outcomes with either therapy. Characteristics of the qualifying clinical VT may be important in determining which patients may be better suited to catheter ablation vs. antiarrhythmic drug therapy. Among patients with prior MI, clinical characteristics, such as ejection fraction and comorbidities, have been predictive of outcomes with either catheter ablation or antiarrhythmic therapy only in some studies.1–5 Presentation with electrical storm is a marker of higher risk of poor outcomes and observational studies have suggested potentially less efficacy of antiarrhythmics and better outcomes with catheter ablation in this population.6–8 Equally, however, those presenting with electrical storm may be more unstable clinically and medical therapy may be preferentially chosen by the treating health care practitioners.
The physiology of the VT circuit within the myocardial scar is reflected in the cycle length (heart rate during VT), the clinical presentation (incessant or frequently recurring VT suggests an electrically stable arrhythmia circuit), and the response to therapy [such as termination with anti-tachycardia pacing (ATP)]. Thus, the VT characteristics may be useful to help clinicians and patients refine clinical decision-making, and choose the most efficacious therapy.
The purpose of this study was to evaluate whether the (i) clinical VT cycle length and (ii) clinical presentation with electrical storm predicted response to catheter ablation vs. escalated antiarrhythmic therapy.
Methods
The details of the VANISH trial have been previously published.1 In brief, patients were eligible if they had a prior MI, an ICD in place and had recurrent, monomorphic VT at a rate of <250 b.p.m., despite first-line antiarrhythmic drug therapy. From 2009 to 2014, 259 patients were enrolled at 22 centres across Canada, Europe, the USA, and Australia. The trial was approved by the Institutional Committee on Human Research at each of the enrolling sites and all subjects provided informed consent. The data underlying this article will be shared on reasonable request to the corresponding author.
Eligible patients were randomized, using a prospective open blinded endpoint design, to escalated drug therapy or catheter ablation in a 1 : 1 ratio, stratified by the use of amiodarone or other antiarrhythmic drugs (predominantly sotalol) at baseline. Escalated antiarrhythmic therapy followed a pre-specified protocol consisting of switching to amiodarone, increasing the dose of amiodarone or adding mexiletine to high-dose amiodarone, based on the antiarrhythmic drugs being taken at baseline. ICD programming was standardized across all patients and outcome assessment was blinded. Patients were initially continued on their baseline antiarrhythmic drugs after their ablation procedure, per protocol.
Patients
This study included all subjects enrolled in the VANISH trial. The clinical VT cycle length was determined from the qualifying VT episodes and was classified (approximating tertiles) into: (i) >400 ms (<150 b.p.m.), (ii) 400–321 ms (150–187 b.p.m.), and (iii) 320–240 ms (188–250 b.p.m.). Patients with missing VT cycle length data were excluded. In cases of multiple presenting VT cycle lengths, the most commonly observed cycle length was used.
The clinical VT presentation was the qualifying VT episode used for enrolment, prior to randomization/initiation of study therapy. The majority of patients in the VANISH trial presented with ICD shocks or VT storm, with relatively few presenting with VT below detection or VT treated with ATP alone. Therefore, in this analysis, patients were classified dichotomously by the presence or absence of electrical storm.
Outcome
The primary outcome was a composite of death at any time or, after a 30-day treatment period (to allow implementation of treatment), VT storm (≥3 documented episodes of VT within 24 h) or appropriate ICD shock. In secondary analyses, the effects of clinical VT presentation and cycle length on the primary outcome were determined for those receiving amiodarone and those receiving other antiarrhythmic agents at baseline. Randomization was stratified by this pre-specified classification within the original VANISH trial.
Statistical analysis
In the primary analyses, the effects of the clinical VT cycle length and electrical storm on the primary composite were determined for the overall trial population. Baseline demographic information were summarized using descriptive statistics. Categorical data were presented as frequencies and percentages. Continuous data were presented as medians with inter-quartile ranges or as means ± standard deviations depending on the distribution of data. Survival rates were summarized using Kaplan–Meier product-limit estimates. Given the small size of the comparison groups, unadjusted hazard ratios (HRs) and 95% confidence intervals were calculated. All analyses were performed in SAS Version 9.4 (the SAS Institute, Cary, NC, USA). The authors had access to the primary data for the trial.
Results
The VANISH trial enrolled 259 patients, of which 127 were randomized to escalated antiarrhythmic therapy and 132 were randomized to catheter ablation. The median follow-up was 23.4 months (interquartile range, 14.7–40.4). In the overall trial population, the mean age was 68.6 years (±8.1) and 93.0% (241/259) were male. Amiodarone was the baseline antiarrhythmic in 169/259 (65.2%), whereas 90/259 (34.7%) were taking other antiarrhythmics, predominantly sotalol.
Clinical ventricular tachycardia cycle length
The demographic characteristics, according to clinical VT cycle length, are presented in Table 1. In summary, 34.4% (89/259) patients had a presenting VT cycle length of >400 ms, 31.6% (82/259) had a VT cycle length of 321–400 ms, 30.1% (78/259) had a VT cycle length of 320–240 ms, and 3.9% (10/259) had missing VT cycle length. The groups had similar baseline characteristics apart from a difference in baseline chronic kidney disease and a higher rate of amiodarone use at baseline in the patients with VT cycle length >400 ms (82% vs. 59.8% and 53.8%, respectively, P < 0.001).
Table 1.
Baseline characteristics according to clinical VT cycle length
| Characteristic | Overall study population (N = 259) | VT cycle length >400 ms (N = 89) | VT cycle length 400–321 ms (N = 82) | VT cycle length 320–240 ms (N = 78) | P-value for comparison across groups |
|---|---|---|---|---|---|
| Age (years) | 68.6 ± 8.1 | 70 ± 6.9 | 67.6 ± 9.3 | 68 ± 8.1 | 0.13 |
| Male sex, no. (%) | 241 (93.1%) | 78 (87.6%) | 78 (95.1%) | 75 (96.2%) | 0.082 |
| Previous PCI, no. (%) | 112 (43.2%) | 39 (43.8%) | 34 (41.5%) | 35 (44.9%) | 0.91 |
| Previous CABG, no. (%) | 118 (45.6%) | 39 (43.8%) | 33 (40.2%) | 41 (52.6%) | 0.27 |
| Diabetes, no. (%) | 77 (29.7%) | 22 (24.7%) | 24 (29.3%) | 27 (34.6%) | 0.39 |
| Hypertension, no. (%) | 180 (69.5%) | 58 (65.2%) | 54 (65.9%) | 60 (76.9%) | 0.2 |
| Chronic kidney disease, no. (%) | 49 (18.9%) | 25 (28.1%) | 9 (11%) | 13 (16.7%) | 0.014 |
| Atrial fibrillation/flutter, no. (%) | 99 (38.2%) | 37 (41.6%) | 27 (32.9%) | 32 (41%) | 0.44 |
| NYHA class, no. (%) | |||||
| I | 61 (23.6%) | 19 (21.3%) | 21 (25.6%) | 19 (24.4%) | 0.32 |
| II | 137 (52.9%) | 44 (49.4%) | 48 (58.5%) | 39 (50%) | |
| III | 61 (23.6%) | 26 (29.2%) | 13 (15.9%) | 20 (25.6%) | |
| Ejection fraction (%) | 28.8 ± 10 | 28.9 ± 9.6 | 28.1 ± 10.6 | 29.4 ± 10 | 0.82 |
| CRT defibrillator, no. (%) | 51 (19.7%) | 22 (24.7%) | 11 (13.4%) | 17 (21.8%) | 0.24 |
| Antiarrhythmic drug at qualification, no. (%) | |||||
| Amiodarone | 169 (65.3%) | 73 (82%) | 49 (59.8%) | 42 (53.8%) | 0.0002 |
| Other medication | 90 (34.7%) | 16 (18.0%) | 33 (40.2%) | 36 (46.2%) | |
| NT-proBNP (pg/mL) | 548 (274–1270) | 798 (280–1594) | 432 (243–1069) | 532 (242–1310) | 0.14 |
ATP, anti-tachycardia pacing; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; NTproBNP, N-terminal pro-hormone brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; VT, ventricular tachycardia.
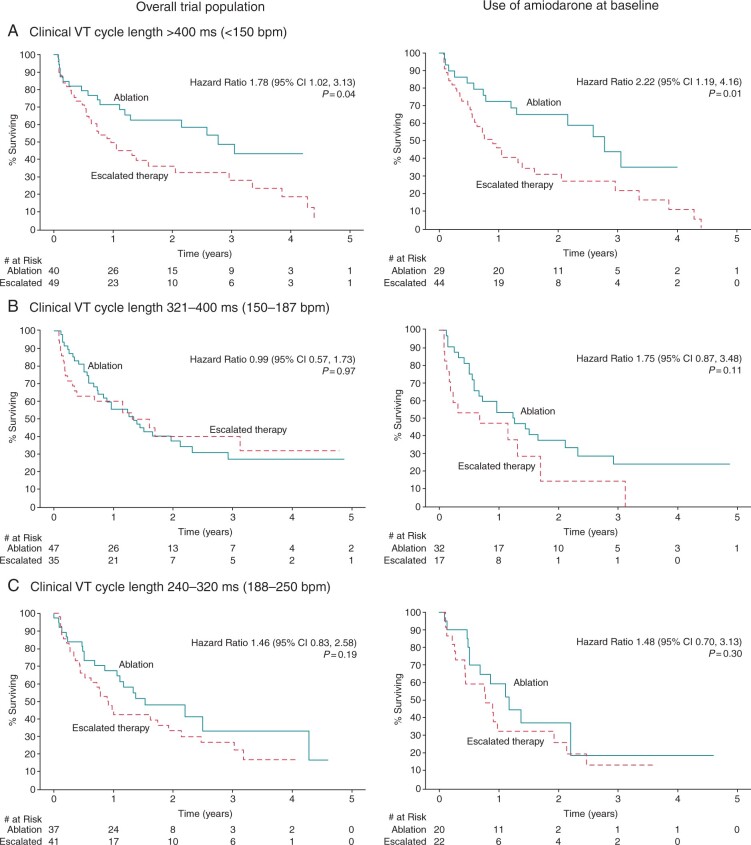
The primary composite outcomes, by clinical VT cycle length are presented in Figure 1 and Supplementary material online, Table S1. Those presenting with a VT cycle length of >400 ms had worse outcomes with escalated antiarrhythmic therapy with an unadjusted HR of 1.7 (1.02–3.13). There was no difference in the primary outcome for those with VT cycle lengths ≤400 ms. Among those taking amiodarone at baseline, those with a VT cycle length of >400 ms again had worse outcomes with escalated antiarrhythmic therapy in comparison to catheter ablation with an HR of 2.22 (1.19–4.16).
Figure 1.
Primary outcome, by clinical VT cycle length. The primary outcome was a composite of death at any time or VT storm (three or more documented episodes of VT within 24 h) or appropriate ICD shock after a 30-day treatment period. Kaplan–Meier survival curves for the primary outcome between escalated antiarrhythmic drug therapy and catheter ablation are shown, by presenting VT cycle length, for the overall trial population (N = 259) and for those taking amiodarone at baseline (N = 169). Log-rank P-values and unadjusted hazard ratios (escalated therapy vs. catheter ablation) are also presented. CI, confidence interval; VT, ventricular tachycardia.
Presentation with electrical storm
Within the trial population, 32/259 (12.4%) had electrical storm as their qualifying arrhythmia. Baseline patient characteristics, by electrical storm presentation are presented in Table 2. The groups were largely similar apart from baseline amiodarone use at enrolment, with those presenting with electrical storm being less likely to have been on amiodarone.
Table 2.
Baseline characteristics according to electrical storm at presentation
| Characteristic | Overall study population ((N = 259) | No electrical storm (N = 227) | Electrical storm (N = 32) | P-value for comparison across groups |
|---|---|---|---|---|
| Age (years) | 68.6 ± 8.1 | 68.4 | 70.4 ± 7.7 | 0.20 |
| Male sex, no. (%) | 241 (93.1%) | 212 (93.4%) | 29 (90.6%) | 0.56 |
| Previous PCI, no. (%) | 112 (43.2%) | 99 (43.6%) | 13 (40.6%) | 0.75 |
| Previous CABG, no. (%) | 118 (45.6%) | 102 (44.9%) | 16 (50%) | 0.59 |
| Diabetes, no. (%) | 77 (29.7%) | 68 (30.0%) | 9 (28.1%) | 0.83 |
| Hypertension, no. (%) | 180 (69.5%) | 161 (70.9%) | 19 (59.4%) | 0.18 |
| Chronic kidney disease, no. (%) | 49 (18.9%) | 43 (18.9%) | 6 (18.8%) | 0.98 |
| Atrial fibrillation/flutter, no. (%) | 99 (38.2%) | 88 (38.8%) | 11 (34.4%) | 0.63 |
| NYHA class, no. (%) | ||||
| I | 61 (23.6%) | 52 (22.9%) | 9 (28.1%) | 0.74 |
| II | 137 (52.9%) | 122 (53.7%) | 15 (46.9%) | |
| III | 61 (23.6%) | 53 (23.3%) | 8 (25%) | |
| Ejection fraction (%) | 28.8 ± 10 | 28.4 | 31.9 ± 11.1 | 0.17 |
| CRT defibrillator, no. (%) | 51 (19.7%) | 45 (19.8%) | 6 (18.8%) | 0.89 |
| Antiarrhythmic drug at qualification, no. (%) | ||||
| Amiodarone | 169 (65.3%) | 153 (67.4%) | 16 (50%) | 0.05 |
| Other medication | 90 (34.7%) | 74 (32.6%) | 16 (51.6%) | |
| NT-proBNP (pg/mL) | 548 (274–1270) | 552 (278–1199) | 437 (217–1598) | 0.28 |
ATP, anti-tachycardia pacing; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; NTproBNP, N-terminal pro-hormone brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; VT, ventricular tachycardia.
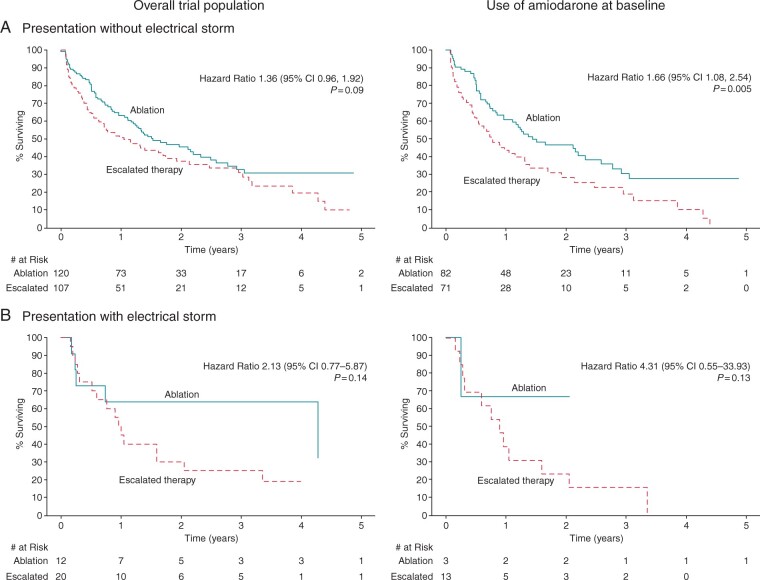
The primary composite outcome, analysed by the presence of electrical storm is presented in Figure 2 and Supplementary material online, Table S2. For the overall cohort, electrical storm did not significantly influence the occurrence of the primary outcome [HR 2.13 (0.77–5.87)]. Among those on amiodarone at baseline, those presenting with electrical storm had a trend towards worse outcomes with escalated antiarrhythmic therapy [unadjusted HR 4.31 (0.55–33.93)], though this was not statistically significant. Notably, 12/13 patients presenting with electrical storm on amiodarone, treated with escalated antiarrhythmic therapy, experienced a primary endpoint. This mirrored the findings in the overall trial population and those presenting without electrical storm [HR 1.36 (0.96–1.92)].
Figure 2.
Primary outcome, by electrical storm at presentation. The primary outcome was a composite of death at any time or VT storm (three or more documented episodes of VT within 24 h) or appropriate ICD shock after a 30-day treatment period. Kaplan–Meier survival curves for the primary outcome between escalated antiarrhythmic drug therapy vs. catheter ablation are shown, by the presence of electrical storm at presentation, for the overall trial population (N = 259) and for those taking amiodarone at baseline (N = 169). Log-rank P-values and unadjusted hazard ratios (escalated therapy vs. ablation) are also presented. AAD, antiarrhythmic drug; CI, confidence interval; VT, ventricular tachycardia.
Discussion
Although the overall VANISH trial demonstrated the superiority of catheter ablation over escalation of antiarrhythmic drug therapy, the results of this analysis can help further refine decision making for patients with a prior MI considering catheter ablation. In patients not on amiodarone at baseline, outcomes with both initiation of amiodarone and catheter ablation were similar in the VANISH trial. This study suggests that those with slow VT (cycle length >400 ms) would preferentially benefit from catheter ablation. Catheter ablation was superior to escalated antiarrhythmic drug therapy for those already on amiodarone in the overall VANISH trial and this study confirms that this benefit also applies to the higher risk population of those presenting with VT storm.
There is a relative paucity of literature evaluating the impact of VT cycle length at presentation and outcomes with catheter ablation in comparison to antiarrhythmic drugs. There is a large body of experiential literature in which patients with slower VTs after MI were preferentially selected for catheter ablation, in large part because they were more haemodynamically stable, permitting activation and entrainment mapping.9,10 In two large, multicentre observational studies of irrigated catheter ablation for VT, shorter inducible VT cycle lengths (faster VTs) at the time of ablation were associated with a higher rate of failure of catheter ablation.3,11 Paradoxically, amiodarone may have less efficacy in treating VTs with longer cycle lengths.12 This may be intuitive; slower VTs (longer cycle lengths) may be mediated by more stable circuits with a wider excitable gap, limiting the ability of a drug such as amiodarone to change the effective refractory period or conduction velocity sufficiently to prevent the VT. Conversely, faster VTs may have more rapid conduction times and may be both less likely to be haemodynamically stable enough to map and easier to miss within ventricular scar using a substrate ablation approach.13 The lack of gradient of benefit of catheter ablation with respect to VT cycle length in those not on amiodarone at baseline may reflect the fact that most patients received sotalol therapy. Sotalol predominantly affects the refractory period of the tissue but does not affect conduction velocity.14 Thus, lack of response to sotalol may not be directly related to VT circuit characteristics that may subsequently dictate a favourable response to catheter ablation (such as a long isthmus with slow conduction velocity).
Electrical storm portends a poor prognosis in patients with VT, regardless of the aetiology,15 and aggressive therapy with antiarrhythmic drugs and/or catheter ablation is almost always warranted.16 Because of the poor prognosis, there has always been interest in catheter ablation for the acute treatment of VT storm. Multiple smaller series17–20 and a recent large, multicentre collaboration8 have shown reasonable efficacy and outcomes among patients undergoing catheter ablation for electrical storm. However, most of these studies have not included a robust control group, particularly with antiarrhythmic therapy. Though the current analysis was limited by sample size, the data certainly demonstrate poor outcomes in patients presenting with electrical storm already on amiodarone, who received enhanced antiarrhythmic therapy. Overall, the electrical storm population in VANISH had similar outcomes to the overall trial population with a strong trend to benefit of catheter ablation despite the higher baseline risk.
Limitations
This was a subgroup analysis of the VANISH trial and thus, although the treatment groups were randomized, between-subgroup comparisons are not, nor was randomization stratified by these subgroups. However, the baseline characteristics among the different clinical VT presentations and cycle lengths were remarkably similar, with the exception of the influence of baseline amiodarone on VT cycle length.
The main VANISH trial results were dominated by the superiority of catheter ablation in the stratum of patients with VT despite amiodarone at baseline. It is therefore possible that the current findings simply reflect this overall effect. However, VT cycle length appeared to show a ‘dose–response’ effect with the superiority of catheter ablation increasing as the VT cycle length increased.
Another significant limitation of this analysis is the relatively small size of the subgroups analysed, which limits statistical power to detect potentially important differences between groups. It also precluded adjustment for some of the baseline differences between groups and precluded sex and race-based analyses. Randomized trials of catheter ablation have been relatively small and difficult to complete.21 Nonetheless, this study takes advantage of rigorous trial design including blinded outcome adjudication and a strict ICD programming protocol.
Conclusion
The clinical VT cycle length of the VT can influence the response to either catheter ablation or escalated drug therapy in patients with monomorphic VT in the setting of a prior MI. Patients presenting with slow VT on amiodarone have comparatively better outcomes with catheter ablation. Patients presenting with electrical storm demonstrated similar outcomes to the overall trial population, with a trend to benefit of catheter ablation, particularly in those on amiodarone at baseline.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Contributor Information
Marc W Deyell, Division of Cardiology, Department of Medicine, Heart Rhythm Services and Centre for Cardiovascular Innovation, University of British Columbia, St. Paul’s Hospital, #200–1033 Davie Street, Vancouver, British Columbia V6E 1M7, Canada.
Steve Doucette, Department of Medicine, QEII Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada.
Ratika Parkash, Department of Medicine, QEII Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada.
Isabelle Nault, Department of Medicine, Université Laval, Québec City, Québec, Canada.
Lorne Gula, Department of Medicine, Western University, London, Ontario, Canada.
Christopher Gray, Department of Medicine, QEII Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada.
Martin Gardner, Department of Medicine, QEII Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada.
Laurence D Sterns, Department of Medicine, Royal Jubilee Hospital, Victoria, British Columbia, Canada.
Jeff S Healey, Population Health Research Institute, McMaster University, Hamilton, Ontario, Canada.
Vidal Essebag, Department of Medicine, McGill University Health Centre and Hôpital Sacré-Coeur de Montréal, Montreal, Québec, Canada.
John L Sapp, Department of Medicine, QEII Health Sciences Centre and Dalhousie University, Halifax, Nova Scotia, Canada.
Funding
The VANISH trial was supported by the Canadian Institutes of Health Research (Grant 102695) with additional financial support from St. Jude Medical Inc. and Biosense-Webster Inc.
Conflict of interest: M.W.D. has received honoraria from Biosense-Webster, Abbott Medical, Boston Scientific, and Medtronic and research grants from Biosense-Webster Inc. I.N. reports honoraria from Biosense Webster. V.E. has received honoraria from Biosense-Webster, Abbott Medical, Boston Scientific, and Medtronic. J.L.S. has received honoraria and research grants from Biosense-Webster and honoraria and research grants from Abbott, as well as modest honoraria from Medtronic. S.D. and R.P., L.G., C.G., M.G.., L.D.S., and J.S.H. have no disclosures.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sapp JL, Wells GA, Parkash R, Stevenson WG, Blier L, Sarrazin JFet al. . Ventricular tachycardia ablation versus escalation of antiarrhythmic drugs. N Engl J Med 2016;375:1499–500. [DOI] [PubMed] [Google Scholar]
- 2. Schmitt C, Brachmann J, Waldecker B, Rizos I, Senges J, Kübler W.. Amiodarone in patients with recurrent sustained ventricular tachyarrhythmias: results of programmed electrical stimulation and long-term clinical outcome in chronic treatment. Am Heart J 1987;114:279–83. [DOI] [PubMed] [Google Scholar]
- 3. Stevenson WG, Wilber DJ, Natale A, Jackman WM, Marchlinski FE, Talbert Tet al. . Irrigated radiofrequency catheter ablation guided by electroanatomic mapping for recurrent ventricular tachycardia after myocardial infarction. Circulation 2008;118:2773–82. [DOI] [PubMed] [Google Scholar]
- 4. Parkash R, Nault I, Rivard L, Gula L, Essebag V, Nery Pet al. . Effect of baseline antiarrhythmic drug on outcomes with ablation in ischemic ventricular tachycardia: a VANISH Substudy (Ventricular Tachycardia Ablation Versus Escalated Antiarrhythmic Drug Therapy in Ischemic Heart Disease). Circ Arrhythm Electrophysiol 2018;11:e005663. [DOI] [PubMed] [Google Scholar]
- 5. Kuck KH, Schaumann A, Eckardt L, Willems S, Ventura R, Delacretaz Eet al. . Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. Lancet 2010;375:31–40. [DOI] [PubMed] [Google Scholar]
- 6. Kumar S, Fujii A, Kapur S, Romero J, Mehta NK, Tanigawa Set al. . Beyond the storm: comparison of clinical factors, arrhythmogenic substrate, and catheter ablation outcomes in structural heart disease patients with versus those without a history of ventricular tachycardia storm. J Cardiovasc Electrophysiol 2017;28:56–67. [DOI] [PubMed] [Google Scholar]
- 7. Morawski S, Pruszkowska P, Sredniawa B, Lenarczyk R, Kalarus Z.. Long-term outcome of catheter ablation and other form of therapy for electrical storm in patients with implantable cardioverter-defibrillators. J Interv Card Electrophysiol 2017;50:227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vergara P, Tung R, Vaseghi M, Brombin C, Frankel D, Di Biase Let al. . Successful ventricular tachycardia ablation in patients with electrical storm reduces recurrences and improves survival. Heart Rhythm 2018;15:48–55. [DOI] [PubMed] [Google Scholar]
- 9. Morady F, Harvey M, Kalbfleisch SJ, el-Atassi R, Calkins H, Langberg JJ.. Radiofrequency catheter ablation of ventricular tachycardia in patients with coronary artery disease. Circulation 1993;87:363–72. [DOI] [PubMed] [Google Scholar]
- 10. Richardson AW, Josephson ME.. Ablation of ventricular tachycardia in the setting of coronary artery disease. Curr Cardiol Rep 1999;1:157–64. [DOI] [PubMed] [Google Scholar]
- 11. Calkins H, Epstein A, Packer D, Arria AM, Hummel J, Gilligan DMet al. . Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy. J Am Coll Cardiol 2000;35:1905–14. [DOI] [PubMed] [Google Scholar]
- 12. Nademanee K, Hendrickson J, Kannan R, Singh BN.. Antiarrhythmic efficacy and electrophysiologic actions of amiodarone in patients with life-threatening ventricular arrhythmias: potent suppression of spontaneously occurring tachyarrhythmias versus inconsistent abolition of induced ventricular tachycardia. Am Heart J 1982;103:950–9. [DOI] [PubMed] [Google Scholar]
- 13. Nishimura T, Upadhyay GA, Aziz ZA, Beaser AD, Shatz DY, Nayak HMet al. . Circuit determinants of ventricular tachycardia cycle length: characterization of fast and unstable human VT. Circulation 2021;143:212–26. [DOI] [PubMed] [Google Scholar]
- 14. Patterson E, Lynch JJ, Lucchesi BR.. Antiarrhythmic and antifibrillatory actions of the beta adrenergic receptor antagonist, dl-sotalol. J Pharmacol Exp Ther 1984;230:519–26. [PubMed] [Google Scholar]
- 15. Elsokkari I, Parkash R, Tang A, Wells G, Doucette S, Yetisir Eet al. . Mortality risk increases with clustered ventricular arrhythmias in patients with implantable cardioverter-defibrillators. JACC Clin Electrophysiol 2020;6:327–37. [DOI] [PubMed] [Google Scholar]
- 16. Deyell MW, AbdelWahab A, Angaran P, Essebag V, Glover B, Gula LJet al. ; Members of the Secondary Panel . 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society Position Statement on the management of ventricular tachycardia and fibrillation in patients with structural heart disease. Can J Cardiol 2020;36:822–36. [DOI] [PubMed] [Google Scholar]
- 17. Deneke T, Shin D-I, Lawo T, Bösche L, Balta O, Anders Het al. . Catheter ablation of electrical storm in a Collaborative Hospital Network. Am J Cardiol 2011;108:233–9. [DOI] [PubMed] [Google Scholar]
- 18. Izquierdo M, Ruiz-Granell R, Ferrero A, Martínez A, Sánchez-Gomez J, Bonanad Cet al. . Ablation or conservative management of electrical storm due to monomorphic ventricular tachycardia: differences in outcome. Europace 2012;14:1734–9. [DOI] [PubMed] [Google Scholar]
- 19. Kozeluhova M, Peichl P, Cihak R, Wichterle D, Vancura V, Bytesnik Jet al. . Catheter ablation of electrical storm in patients with structural heart disease. Europace 2011;13:109–13. [DOI] [PubMed] [Google Scholar]
- 20. Carbucicchio C, Santamaria M, Trevisi N, Maccabelli G, Giraldi F, Fassini Get al. . Catheter ablation for the treatment of electrical storm in patients with implantable cardioverter-defibrillators: short- and long-term outcomes in a prospective single-center study. Circulation 2008;117:462–9. [DOI] [PubMed] [Google Scholar]
- 21. Pokorney SD, Friedman DJ, Calkins H, Callans DJ, Daoud EG, Della-Bella Pet al. . Catheter ablation of ventricular tachycardia: lessons learned from past clinical trials and implications for future clinical trials. Heart Rhythm 2016;13:1748–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.