Abstract
Aims
There is limited information on what clinical factors are associated with the development of pericardial effusion after leadless pacemaker implantation. We sought to determine predictors of and to develop a risk score for pericardial effusion in patients undergoing Micra leadless pacemaker implantation attempt.
Methods and results
Patients (n = 2817) undergoing implant attempt from the Micra global trials were analysed. Characteristics were compared between patients with and without pericardial effusion (including cardiac perforation and tamponade). A risk score for pericardial effusion was developed from 18 pre-procedural clinical variables using lasso logistic regression. Internal validation and future prediction performance were estimated using bootstrap resampling. The scoring system was also externally validated using data from the Micra Acute Performance European and Middle East (MAP EMEA) registry. There were 32 patients with a pericardial effusion [1.1%, 95% confidence interval (CI): 0.8–1.6%]. Following lasso logistic regression, 11 of 18 variables remained in the model from which point values were assigned. The C-index was 0.79 (95% CI: 0.71–0.88). Patient risk score profile ranged from −4 (lowest risk) to 5 (highest risk) with 71.8% patients considered low risk (risk score ≤0), 16.6% considered medium risk (risk score = 1), and 11.7% considered high risk (risk score ≥2) for effusion. The median C-index following bootstrap validation was 0.73 (interquartile range: 0.70–0.75). The C-index based on 9 pericardial effusions from the 928 patients in the MAP EMEA registry was 0.68 (95% CI: 0.52–0.83). The pericardial effusion rate increased significantly with additional Micra deployments in medium-risk (P = 0.034) and high-risk (P < 0.001) patients.
Conclusion
The overall rate of pericardial effusion following Micra implantation attempt is 1.1% and has decreased over time. The risk of pericardial effusion after Micra implant attempt can be predicted using pre-procedural clinical characteristics with reasonable discrimination.
Clinical trial registration
The Micra Post-Approval Registry (ClinicalTrials.gov identifier: NCT02536118), Micra Continued Access Study (ClinicalTrials.gov identifier: NCT02488681), and Micra Transcatheter Pacing Study (ClinicalTrials.gov identifier: NCT02004873).
Keywords: Leadless pacing, Pericardial effusion, Perforation
What’s new?
The overall rate of pericardial effusion after Micra implantation attempt is low (1.1%) and risk factors for effusion are similar to those previously reported for transvenous pacemakers.
The risk of pericardial effusion after a Micra implant attempt can be predicted using routinely obtained clinical characteristics with reasonable discrimination.
Importantly, repeated attempts at Micra deployment appear to be associated with increased risk of pericardial effusion particularly in patients with elevated risk at baseline.
The proposed risk score model may be helpful in guiding greater care with implantation technique that not only focuses on septal implants but also one that minimizes the number of deployments in moderate- to high-risk candidates.
Introduction
Leadless pacing is now an established alternative to traditional transvenous pacemakers, especially in patients who only require brief periods of pacing or only require ventricular pacing.1–3 Leadless pacing also avoids complications associated with subclavian or axillary lead access and pocket formation, including pneumothorax, acute pacemaker pocket infection, and chronic lead and pocket complications. Moreover, the Micra leadless pacemaker has been associated with lower rates of complications, particularly lead and pocket related complications, when compared with traditional single-chamber ventricular pacing.1,4 The device has advantages in patients who have limited vascular access options and appears to be associated with a lower risk of device infection.4–7 However, like all pacemakers, the Micra leadless pacemaker does carry a risk of infrequent but known complications.1,3
One of the complications that can occur during Micra implantation is myocardial perforation and the development of pericardial effusion. While low, the rates of pericardial effusion may be higher than those observed with traditional pacemaker implant procedures.3,8–11 The complication remains a concern, especially since leadless pacemaker candidates are often older and have higher rates of comorbid illness and frailty. There is limited information on the risk factors associated with an increased risk of pericardial effusion after Micra implantation. Therefore, the goal of this analysis was to determine predictors of pericardial effusion in patients undergoing attempts at Micra pacemaker implantation and develop a simple risk score that physicians may use to assess a patient’s risk for pericardial effusion prior to implant.
Methods
Study design and oversight
In order to better characterize the incidence, predictors, and outcomes of pericardial effusion following Micra leadless pacemaker implantation, we included patients with an attempted Micra implant in the Micra Investigational Device Exemption (IDE) study, Micra Continued Access (CA) study, and Micra Post-Approval Registry (PAR) in this analysis. The design and results of the Micra IDE and Micra PAR have been previously reported.1,3 The Micra IDE study enrolled 726 patients between December 2013 and May 2015 and was used to obtain regulatory approval of the Micra Transcatheter Pacing System (TPS) (Medtronic, Mounds View, MN, USA). The Micra CA study enrolled 276 patients between June 2015 and May 2016 allowing patients continued access to the Micra system during regulatory review in the USA. The Micra PAR study was designed to evaluate the short- and long-term safety and performance of the Micra system in real-world clinical practice following commercial release. The Micra PAR enrolled 1815 patients between July 2015 and March 2018 with a 9-year follow-up ongoing.
All three studies were prospective, nonrandomized, and enrolled patients that met class I or II guideline recommendations for ventricular pacing with no co-morbidity restrictions with the exception that patients with an existing pacemaker or implantable cardioverter-defibrillator were excluded from the Micra IDE study. All three studies were sponsored by Medtronic and the protocols for each study were approved by the ethics committee at each centre. All patients provided written informed consent. Adverse events, including all cardiac perforations and pericardial effusions, were adjudicated by a clinical events committee, comprised of independent physicians, for their relationship to the Micra system and implant procedure. However, the decision to perform a pre- or post-implant echocardiogram was left to the implanting clinician. Pericardial effusion severity was classified by their most severe therapeutic consequence; specifically, those resulting in death, surgery, pericardial drainage, and those resolving without surgery or pericardial drain.12
Objective
The objective of this analysis was to characterize the rate of pericardial effusion (including cardiac perforation and tamponade) and outcomes in patients undergoing Micra implantation attempt. Another aim of this analysis was to develop a model to predict the risk of pericardial effusion based on readily available and simple to obtain patient characteristics. Pericardial effusions were defined as the occurrence of any pericardial effusion reported as an adverse event during the study (including perforation or tamponade) regardless of severity or outcome.
Statistical methods
Summary statistics were obtained and reported using mean ± standard deviation for continuous variables and frequencies and percentages for categorical variables. Exact binomial confidence intervals (CIs) were used to compute 95% CIs for pericardial effusion rates by study and overall.
A multivariable risk prediction model was developed and internally validated in accordance with the TRIPOD statement.13 Based upon clinical factors associated with complications in traditional pacemaker candidates, we identified a candidate set of 18 prognostic variables (Table 1) to include as candidate variables in the multivariable model. Patients missing values for >10 candidate variables were excluded from the model. Logistic regression models were used to assess the univariate association between each candidate prognostic variable and effusion status. To facilitate the construction of a simple scoring system, body mass index (BMI) was dichotomized (<20 vs. ≥20 kg/m2) and age was categorized (<75, 75 to <85, and >85) based on graphical exploration of the data prior to model fitting and BMI <20 kg/m2 being associated with physical frailty in the elderly.14 Due to the presence of missing data, 20 imputed datasets were constructed using multivariate imputation by chained equations.15 Variable selection and parameter estimation were accomplished using weighted lasso logistic regression utilizing the combined imputed datasets.16 Lasso is a regression and variable selection technique that selects variables maximizing prediction accuracy while penalizing overfitting rather than performing variable selection based on traditional measures of statistical significance.17
Table 1.
Univariate association with pericardial effusion
| Characteristic | Effusion (N = 32) | No effusion (N = 2785) | Odds ratio (95% CI) | P-value |
|---|---|---|---|---|
| Body mass index | 25.7 ± 6.0 (32) | 27.8 ± 5.7 (2741) | 0.92 (0.86–1.00) | 0.038 |
| BMI <20 kg/m2 | 15.6% (5/32) | 4.3% (117/2741) | 4.15 (1.57–10.98) | 0.004 |
| Age (years) | 78.4 ± 13.6 (32) | 75.7 ± 12.7 (2782) | 1.02 (0.99–1.06) | 0.229 |
| Age ≥75 | 65.6% (21/32) | 65.0% (1809/2782) | 1.03 (0.49–2.14) | 0.944 |
| Age ≥85 | 46.9% (15/32) | 23.2% (646/2782) | 2.92 (1.45–5.87) | 0.003 |
| Female | 62.5% (20/32) | 39.6% (1103/2783) | 2.54 (1.24–5.21) | 0.011 |
| Cardiomyopathy | 15.6% (5/32) | 15.3% (427/2783) | 1.02 (0.39–2.67) | 0.965 |
| Congestive heart failure | 28.1% (9/32) | 15.6% (433/2783) | 2.12 (0.98–4.62) | 0.058 |
| Coronary artery disease | 15.6% (5/32) | 25.2% (702/2783) | 0.55 (0.21–1.43) | 0.220 |
| Myocardial infarction | 15.6% (5/32) | 8.8% (246/2783) | 1.91 (0.73–5.00) | 0.188 |
| Hypertension | 68.8% (22/32) | 69.8% (1942/2783) | 0.95 (0.45–2.02) | 0.900 |
| Pulmonary hypertension | 12.5% (4/32) | 7.9% (220/2783) | 1.66 (0.58–4.79) | 0.345 |
| Coronary artery intervention | 18.8% (6/32) | 14.8% (411/2783) | 1.33 (0.55–3.26) | 0.529 |
| COPD | 28.1% (9/32) | 11.2% (313/2783) | 3.09 (1.42–6.73) | 0.005 |
| Diabetes | 28.1% (9/32) | 27.5% (764/2783) | 1.03 (0.48–2.25) | 0.932 |
| Preclusion for transvenous pacing | 18.8% (6/32) | 19.4% (540/2782) | 0.96 (0.39–2.34) | 0.925 |
| AF history | 50.0% (16/32) | 72.9% (2028/2783) | 0.37 (0.19–0.75) | 0.006 |
| Prior cardiac surgery | 12.5% (4/32) | 24.8% (690/2783) | 0.43 (0.15–1.24) | 0.119 |
| On OAC | 67.7% (21/31) | 73.5% (2036/2769) | 0.76 (0.35–1.61) | 0.469 |
| Renal dysfunction (any) | 25.0% (8/32) | 21.5% (597/2783) | 1.22 (0.55–2.73) | 0.627 |
| Renal dysfunction (dialysis required) | 12.5% (4/32) | 7.0% (195/2783) | 1.90 (0.66–5.46) | 0.236 |
AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; OAC, oral anticoagulation.
To develop a practical risk score, variables selected by the lasso model were assigned points by increasing (or decreasing) β coefficients up or down to the next higher positive or lower negative integer, respectively. A risk score was then computed for each patient, and the population was divided into three categories: low risk relative to the entire analysis cohort (risk score ≤0), medium risk relative to the entire analysis cohort (risk score = 1), or high risk relative to the entire analysis cohort (risk score ≥2) for pericardial effusion. Discrimination ability for both the lasso regression model and risk score was assessed using the C-index. Model calibration was assessed by regressing the observed effusion rate on predicted pericardial effusion rate for each risk status (low, medium, or high) and weighting by the proportion of patients within each risk status.
Due to the small number of pericardial effusion events across all datasets, internal validation and future prediction performance of the modelling process were assessed using 1000 bootstrap samples.18 Specifically, for each bootstrap iteration, the entire model development process was repeated (missing data imputation, variable selection and estimation, and risk score development) and the model developed was used to predict the probability of effusion for patients both in and out of the bootstrap sample (i.e. those observations not selected in the bootstrap sample). Future prediction performance was assessed for each sample using the C-index and the calibration slope using Efron’s 0.632 estimator.18 Similarly, CIs for the final model coefficients as well as variable selection probability were generated using the bootstrap samples.
As an external assessment of future discrimination performance of the risk score, the C-index was estimated based on outcomes from the Micra Acute Performance European and Middle East (MAP EMEA) registry.
The association between the number of Micra deployments required during the implant procedure and pericardial effusion rate was assessed using the Jonckheere–Terpstra test within patients with low, medium, and high predicted baseline risk.
All statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, USA) or using the mice,15 glmnet,19 and ROCR,20 packages in R v4.0.2.21
Results
Incidence and outcomes of pericardial effusion
Among 2817 patients who underwent Micra implant attempt, there were 32 patients with a symptomatic pericardial effusion identified from adverse event reports for an overall rate of 1.1% (95% CI: 0.8–1.6%). The pericardial effusion rate ranged from 0.8% in the Micra PAR to 1.8% in the Micra IDE study (Figure 1). Of the 32 pericardial effusions, 2 resulted in death (1 following surgery), 5 required surgical intervention, 15 required pericardiocentesis, and 10 resolved without the need for pericardial drainage or surgery (Table 2). Of the 26 patients requiring surgical intervention or pericardiocentesis, 3 died within 30 days of their procedure (1 died on the day of implant following surgical intervention, 1 died 16 days post-implant due to septic shock resulting from an infected gallbladder, and 1 died 11 days post-procedure from respiratory failure).
Figure 1.
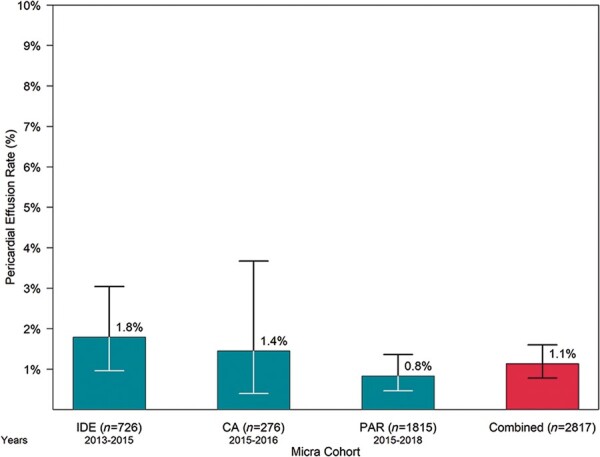
Pericardial effusion rate by Micra study cohort. Error bars represent 95% confidence intervals. Years on the x-axis indicate the years in which implants occurred during each study. CA, Continued Access; IDE, Investigational Device Exemption; PAR, Post-Approval Registry.
Table 2.
Patients with pericardial effusion (n = 32)
| Patient | Study | Day diag noseda | Outcome/ actions | Deploy ments | Age | BMI | Sex | CHF | CAD | MI | COPD | AF | Prior cardiac surgery | Dialysis | Risk score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PAS | 0 | Death | 2 | 65 | 17.6 | Female | − | − | − | − | − | − | + | 3 |
| 2 | PAS | 0 | Death | 1 | 76 | 35.9 | Female | − | − | − | − | − | − | + | 1 |
| 3 | IDE | 0 | Surgery | 3–5 | 74 | 27.9 | Female | − | − | − | − | + | − | − | 1 |
| 4 | IDE | 0 | Surgery | 3–5 | 91 | 20.7 | Female | − | − | − | − | − | − | − | 2 |
| 5 | CA | 0 | Surgery | NR | 69 | 26.6 | Female | − | − | − | − | − | − | − | 1 |
| 6 | CA | 0 | Surgery | 3–5 | 79 | 24.8 | Male | − | − | − | − | − | + | − | −2 |
| 7 | PAS | 0 | Surgery | 3–5 | 86 | 27.3 | Female | − | − | − | − | + | − | − | 1 |
| 8 | IDE | 0 | Pericardiocentesis | 1 | 85 | 25.2 | Female | + | − | − | + | + | − | − | 5 |
| 9 | IDE | 0 | Pericardiocentesis | 2 | 88 | 23.5 | Male | + | + | + | + | + | − | − | 3 |
| 10 | IDE | 0 | Pericardiocentesis | 3–5 | 88 | 26.9 | Male | − | + | − | − | − | + | − | −1 |
| 11 | IDE | 0 | Pericardiocentesis | ND | 84 | 22.8 | Female | + | + | + | + | + | − | − | 3 |
| 12 | IDE | 1 | Pericardiocentesis | >5 | 90 | 30.9 | Female | − | − | − | + | − | − | − | 4 |
| 13 | IDE | 0 | Pericardiocentesis | 1 | 83 | 24.8 | Female | − | − | − | − | − | + | − | −1 |
| 14 | IDE | 0 | Pericardiocentesis | >5 | 86 | 18.3 | Male | + | + | + | + | + | − | + | 5 |
| 15 | CA | 0 | Pericardiocentesis | 1 | 88 | 23.4 | Male | − | − | − | − | − | − | − | 1 |
| 16 | PAS | 0 | Pericardiocentesis | 2 | 87 | 28.2 | Female | − | − | − | − | + | − | − | 1 |
| 17 | PAS | 0 | Pericardiocentesis | 2 | 97 | 25.9 | Female | + | − | − | − | + | − | − | 2 |
| 18 | PAS | 0 | Pericardiocentesis | 2 | 65 | 32.2 | Male | + | − | + | − | − | − | − | 2 |
| 19 | PAS | 0 | Pericardiocentesis | >5 | 89 | 23.9 | Male | − | − | − | − | + | − | − | 0 |
| 20 | PAS | 0 | Pericardiocentesis | 1 | 69 | 49.5 | Female | − | − | − | − | − | − | − | 1 |
| 21 | PAS | 0 | Pericardiocentesis | 1 | 69 | 23.1 | Male | − | − | − | − | − | − | − | −1 |
| 22 | PAS | 0 | Pericardiocentesis | 3–5 | 88 | 30.1 | Female | + | − | − | − | + | − | − | 3 |
| 23 | IDE | 0 | Observation | 2 | 67 | 28.6 | Female | − | − | + | − | + | − | − | 1 |
| 24 | IDE | 16 | Observation | 3–5 | 85 | 22.1 | Male | − | − | − | − | − | − | − | 1 |
| 25 | IDE | 0 | Observation | 1 | 77 | 28.0 | Female | − | − | − | − | + | − | − | −1 |
| 26 | IDE | 36 | Observation | 1 | 64 | 18.4 | Female | − | − | − | − | − | − | − | 2 |
| 27 | CA | 5 | Observation | >5 | 77 | 27.8 | Male | + | − | − | + | + | − | − | 1 |
| 28 | PAS | 1 | Observation | 3–5 | 72 | 20.0 | Female | − | − | − | + | + | − | − | 3 |
| 29 | PAS | 0 | Observation | 1 | 90 | 25.0 | Male | − | − | − | − | + | − | + | 1 |
| 30 | PAS | 0 | Observation | 2 | 72 | 22.4 | Female | − | − | − | + | − | − | − | 3 |
| 31 | PAS | 0 | Observation | 3–5 | 23 | 21.5 | Female | − | − | − | − | − | − | − | 1 |
| 32 | PAS | 1 | Observation | 3–5 | 85 | 19.7 | Male | + | + | − | + | − | + | − | 3 |
Observation means the event resolved without the need for surgical intervention or pericardial drain.
CA, Continued Access; IDE, Investigational Device Exemption; PAR, Post-Approval Registry.
Days relative to implant procedure (Day 0 is day of implant).
AF, atrial fibrillation; CHF, congestive heart failure; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disorder; NR, not reported; ND, no deployments.
Univariate predictors of pericardial effusion
Univariate analyses among all 2817 patients demonstrated that age ≥85 years, BMI (dichotomized at< 20 kg/m2), female sex, and chronic obstructive pulmonary disease (COPD) were all positively associated with increased occurrence of pericardial effusion. In contrast, increasing BMI on a continuous basis and a history of atrial fibrillation were associated with a lower occurrence of pericardial effusion at the P < 0.05 level (Table 1).
Multivariable modelling
There were two patients without pericardial effusion who were missing >10 candidate predictor variables and excluded from the model development process. In the remaining 2815 patients, 2760 (98.0%) were missing no candidate predictor variables, while the most common missing variables were BMI and oral anticoagulation status (Supplementary material online, Figure S1). Of the 32 patients with pericardial effusion, 31 had complete data and 1 was missing oral anticoagulation status.
Following lasso logistic regression, 11 of the candidate variables remained in the model from which point values were assigned proportional to their regression coefficients (Table 3). Patient risk score profile ranged from −4 (lowest risk) to 5 (highest risk) among the 2760 patients with complete data with 1981 (71.8%) patients considered low risk (risk score ≤ 0), 457 (16.6%) considered medium risk (risk score = 1), and 322 (11.7%) considered high risk (risk score ≥ 2) for pericardial effusion (Supplementary material online, Figure S2). Predicted perforation risk was 0.4%, 1.5%, and 4.8% for low-, medium-, and high-risk patients (Figure 2). Both the lasso model and the model based on risk scores discriminated well with C-index values of 0.80 (95% CI: 0.73–0.88) and 0.79 (95% CI: 0.71–0.88), respectively (Supplementary material online, Figure S3). Model calibration slopes were 1.31 for the lasso model and 0.951 for the risk scoring system.
Table 3.
Multivariable lasso logistic regression analysis and scoring system
| Variable | Selection probabilitya | Odds ratio | β regression coefficient (95% CI)b | Pointsc |
|---|---|---|---|---|
| Age (years) | 0.984 | |||
| <75 | Reference | |||
| ≥75 to <85 | 0.77 | −0.26 (−1.44–0.0) | −1 | |
| >85 | 2.08 | 0.73 (0.0–1.49) | 1 | |
| Body mass index < 20 (kg/m2) | 0.864 | 2.39 | 0.87 (0.0–1.75) | 1 |
| Female | 0.918 | 1.70 | 0.53 (0.0–1.40) | 1 |
| Heart failure | 0.765 | 1.52 | 0.42 (0.0–1.29) | 1 |
| Coronary artery disease | 0.809 | 0.77 | −0.27 (−1.66–0.0) | −1 |
| Prior myocardial infarction | 0.731 | 1.65 | 0.50 (0.0–1.60) | 1 |
| Pulmonary hypertension | 0.596 | 1.09 | 0.08 (−0.31–1.19) | 1 |
| COPD | 0.944 | 2.83 | 1.04 (0.0–1.91) | 2 |
| Atrial fibrillation | 0.979 | 0.41 | −0.89 (−1.68 to −0.05) | −1 |
| Prior cardiac surgery | 0.761 | 0.74 | −0.31 (−1.51–0.0) | −1 |
| Dialysis | 0.664 | 1.30 | 0.26 (−0.21–1.79) | 1 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease.
Proportion of 1000 bootstrap samples in which the variable was included in the lasso logistic model.
Confidence intervals derived from the 2.5th and 97.5th percentile from the distribution of each coefficient across 1000 bootstrap samples. If the variable was not selected in the model for a particular bootstrap sample it was included as having a value of zero.
Points were assigned by increasing (or decreasing) β coefficients up or down to the next higher positive or lower negative integer, respectively.
Figure 2.
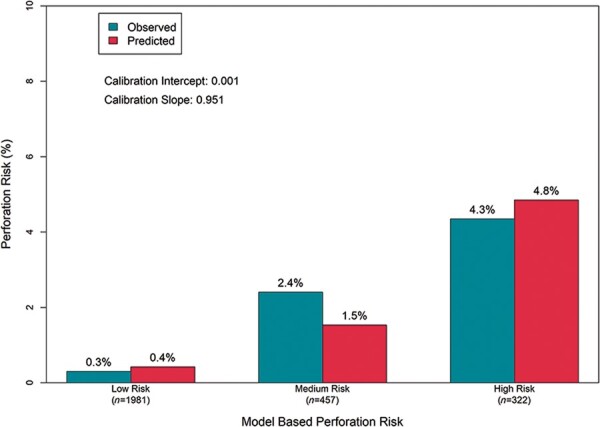
In-sample model calibration. Predicted perforation risk for low-, medium-, and high-risk patients.
Internal model validation and assessment of future prediction performance
Following bootstrap validation, the median C-index was 0.73 [interquartile range (IQR): 0.71–0.76] for the lasso logistic model and 0.73 (IQR: 0.70–0.76) for the scoring system (Supplementary material online, Figure S4). Median calibration slope was 0.79 (IQR: 0.64–0.96) for the lasso logistic model and 1.02 (IQR: 0.87–1.19) for the scoring system (Supplementary material online, Figure S5).
External assessment of discrimination performance
A total of nine pericardial effusion events (0.97%) were observed in 928 patients undergoing implant in the MAP EMEA registry. None of the nine events required surgical intervention, while five required pericardiocentesis of which one died of multiple organ failure 11 days post-procedure. Despite differences in some characteristics used to construct the risk scoring system, the distribution of risk score values was similar between the Micra IDE, CA, and PAR patients used to develop the risk scoring system and patients in the MAP EMEA cohort (Supplementary material online, Table S1). The C-index based on the scoring system was 0.68 (95% CI: 0.52–0.83) in this data source.
Relationship between number of Micra deployments and pericardial effusion rate
Of the 2815 patients, there were 2638 (including 30 of the 32 patients with pericardial effusion) with complete data that were known to have the device deployed during the implant procedure at least once. For patients with a low risk for pericardial effusion (risk score ≤ 0), there was no association between the number of Micra deployments and observed rate of pericardial effusion (Figure 3A). However, the observed rate of pericardial effusion increased significantly for those patients at medium (risk score = 1; P = 0.034; Figure 3B) and high baseline risk for effusion (risk score ≥ 2; P < 0.001; Figure 3C).
Figure 3.
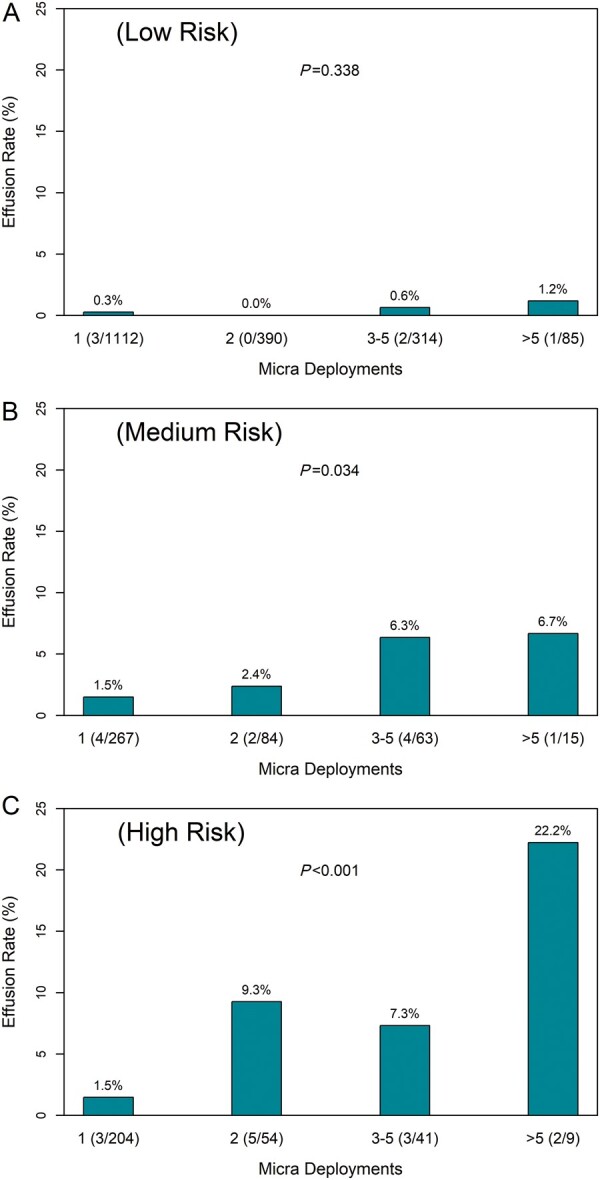
Pericardial effusion rate vs. number of Micra deployments for patients at low-risk score ≤ 0 (A), medium-risk score = 1 (B), and high-risk score ≥2 (C) for pericardial effusion. Numbers in parentheses under each bar are the number of pericardial effusions (numerator) and number of patients in category (denominator).
Discussion
In this analysis of >2800 patients undergoing Micra leadless pacemaker implant attempt, there are several clinically relevant and important findings. First, the rate of pericardial effusion following Micra implantation attempt was 1.1% overall and appears to have decreased over time in subsequent clinical studies. Second, risk factors for pericardial effusion are similar to those observed for traditional pacemaker implantation including increasing age, BMI <20, female sex, heart failure, prior myocardial infarction, COPD, absence of prior cardiothoracic surgery, and dialysis. Third, a predictive model with these clinical factors discriminated risk well (C-index 0.80) with reasonable predicted future discrimination performance based on bootstrap resampling (C-index 0.73) and an external data source (C-index 0.68) despite a small number of pericardial effusion events (n = 9) reported in the MAP EMEA registry. Finally, repeated attempts at deployment appear to be associated with increased risk of pericardial effusion in patients with elevated risk at baseline.
Using data from the Truven Health MarketScan, Cantillon et al.22 found that patients undergoing leadless pacemaker implantation in clinical practice had a higher rate of pericardial effusion at 1.53% compared with 0.35% in those undergoing single-chamber transvenous pacemaker implantation. There are several potential explanations for the higher rate of observed pericardial effusions in the Cantillon study, including but not limited to true intrinsic higher rates of effusion, higher rates of effusion due to the learning curve, or residual confounding in sicker leadless pacemaker patients. The most significant finding in this analysis of the Micra IDE trial, CA trial, and PAR (n = 1815) is that the incidence of pericardial effusion following attempted Micra leadless pacemaker implantation was low at 1.1%. Evaluation of reports of pericardial effusion in MAUDE suggested that pericardial injury due to leadless pacemakers may have greater morbidity and mortality.23 In our analysis, 2 of the 32 pericardial effusions resulted in death, which is similar to mortality following tamponade due to transvenous pacemaker implantation (6.8%)24 and cardiac implanted electronic device procedures (4%).25
The risk of pericardial effusion, regardless of severity, complicating Micra implantation has decreased over time from a rate of 1.8% in the initial investigational device exemption study to 0.8% in the Micra PAR.1,3 The decreased rate of pericardial effusion is likely due to several improvements in procedural technique, including targeting implantation to the septum (as opposed to the right ventricular apex) and the use of contrast injection to confirm device location prior to deployment. Thus, while pericardial effusion is an important complication that implanting physicians must be prepared for, it is infrequent and continues to become less frequent over time.
We found that the risk factors for pericardial effusion are similar to those observed for traditional pacemaker implantation including increasing age, BMI <20, female sex, heart failure, prior myocardial infarction, COPD, absence of prior cardiothoracic surgery, and dialysis.26–28 In this cohort, the presence of COPD and low BMI exhibited particularly strong association with the risk of effusion. Similar to prior observations in lead extraction and catheter ablation, prior cardiothoracic surgery was protective against perforation as prior scar formation and obliteration of the pericardial space is protective against pericardial bleeding.29,30 The apparent lower risk observed with atrial fibrillation may reflect the increased risk associated with other reasons patients get a single-chamber VVI pacemaker, including limited vascular access. The risk of pericardial effusion increased with the number of risk factors such that the predicted pericardial effusion risk was 0.4%, 1.5%, and 4.8% for low-, medium-, and high-risk patients. While many of these risk factors are common in patients who may be preferred candidates for leadless pacemakers (e.g. limited vascular access options, high infection risk, etc.), estimation of risk can be useful for pre-procedural planning, risk counselling, and device selection for patients eligible for transvenous pacing therapy. A predictive model with these pre-procedural risk factors discriminated risk well with a C-index of 0.80. The risk model is designed to help estimate risk. It may be helpful in guiding greater care with implantation technique that not only focuses on septal implants but also one that minimizes the number of deployments in moderate- to high-risk candidates. The overall risk of pericardial effusion in the high-risk group was notable with an estimated risk of 4.8%. Careful pre-procedural counselling and caution is required when implanting leadless pacemakers in these patients. However, each patient and implant decision needs to be evaluated on a case-by-case basis for several reasons. First, patients at higher risk for complications may also have the most compelling indications for leadless pacing, including lack of vascular access or lack of candidacy for a transvenous device. Moreover, the models have reasonable but not perfect discrimination. Thus, while caution is required when discussing and approaching leadless pacing in these high-risk patients, it should not be considered prohibitory. This is especially true since the Micra leadless pacemaker has been associated with lower rates of overall complications compared with transvenous pacing.1,3
We also evaluated procedural characteristics associated with pericardial effusion. As repeated engagement of the delivery catheter/cup and engagement of the four nitinol tines could increase the risk of myocardial perforation, we examined the association between the number of Micra device deployments and the occurrence of pericardial effusion. Interestingly, we found among patients with no risk factors for pericardial effusion (risk score ≤ 0), there was no evidence for an association between the number of Micra deployments and pericardial effusion. However, in patients with moderate to high (≥1 risk factors), there was a clear association between the number of deployments and the risk of effusion. Notably, in moderate- to high-risk patients, five or more deployments were associated with a risk of effusion in excess of 5%. These data suggest that implanting physicians should exercise greater caution in situations where moderate- to high-risk patients require multiple deployments, particularly when the tines appear well-engaged and with repeatedly elevated capture thresholds >2 V.31
Limitations
There are several limitations that should be kept in mind when considering the results from this study. First, as with any observational analysis, the modelling may be subject to confounding. Second, we were unable to evaluate the location of attempted deployments and the risk of perforation. However, in our attempt to build a predictive model, we intentionally focused on clinical information available to clinicians when they are making the decision about what type of pacemaker to implant (as opposed to intraoperative data). While our analysis included a large number of patients from across three different clinical studies, the results may not be generalizable to populations with characteristics significantly different from this cohort. Moreover, consecutive patients may not have been enrolled at the registry sites. Echocardiograms were performed at the discretion of the treating physicians and were not protocol-driven or systematic. Finally, given the small number of perforations we were unable to divide the data into a training and validation set, though the scoring system provided adequate discrimination in an external dataset with a small number of perforations.
Conclusion
The rate of pericardial effusion following Micra implantation attempt is 1.1%, similar to that observed in transvenous pacemaker implantation. The risk factors for pericardial effusion are also similar to those observed for traditional pacemaker implantation including increasing age, BMI <20, female sex, heart failure, prior myocardial infarction, COPD, absence of prior cardiothoracic surgery, and haemodialysis. The risk of pericardial effusion after a Micra implant attempt can be predicted using routinely obtained clinical characteristics with reasonable discrimination. Importantly, repeated attempts at Micra deployment appear to be associated with increased risk of pericardial effusion particularly in patients with elevated risk at baseline.
Supplementary material
Supplementary material is available at Europace online.
Funding
The Micra Post-Approval Registry, Micra Continued Access Study, and Micra Transcatheter Pacing Study were funded by Medtronic, Inc. J.P.P. was supported by the National Heart, Lung and Blood Institute (R01HL128595).
Conflict of interest: J.P.P. receives grants for clinical research from Abbott, American Heart Association, Association for the Advancement of Medical Instrumentation, Bayer, Boston Scientific, and Philips and serves as a consultant to Abbott, Allergan, ARCA Biopharma, Biotronik, BMS, Boston Scientific, ElectroPhysiology Frontiers, LivaNova, Medtronic, Milestone, Sanofi, Philips, and Up-to-Date. R.C. has received consulting support and honoraria from Medtronic, Boston Scientific, Phillips, and Biotronik. J.S. has received consultant and/or speaker fees from Abbott, Amgen, Astra-Zeneca, Bayer, Berlin-Chemie, Biosense Webster, Biotronik, Boehringer-Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Medscape, Medtronic, Merck/MSD, Novartis, Roche Diagnostics, Pfizer, and WebMD. He reports ownership of CorXL. He has received grant support through his institution from Abbott, Bayer Healthcare, Biosense Webster, Biotronik, Boston Scientific, Daiichi Sankyo, and Medtronic. M.F.E.-C. has received consultant fees from Medtronic, Boston Scientific, and Biotronik. D.R. has received consultant/advisor fees from Medtronic. P.R.R. has received honoraria from Boston Scientific and Medtronic. K.S. has received speaking fees from Medtronic and Abbott Japan. C.S. has been a member of the advisory board for Biotronik and Medtronic and has been a member of the Speakers Bureau for Abbott, Biotronik, Boston Scientific, and Medtronic. C.G. serves as a consultant for Medtronic. L.C. has received fees services from Abbott, Biosense Webster, Pfizer, Biotronik, and Medtronic and had received fellowship support from Biotronik, Boston Scientific, and Medtronic. C.R.E. has received research funding from Medtronic, Atricure, Thoratec, and Boston Scientific and consulting fees from Medtronic, Sentre Heart, Spectranetics, Biosense Webster, Boston Scientific, and Atricure. K.S. is an employee and shareholder of Medtronic, Inc. D.H.F. is an employee and shareholder of Medtronic, Inc. L.M. has served as a consultant and received support for research and fellowship programs from Abbott, Biosense Medical, Biotronik, Boston Scientific, and Medtronic and is a shareholder of ADAS 3D. All other authors report no conflicts of interest.
Data availability
Reasonable requests will be considered by the authors.
Supplementary Material
Contributor Information
Jonathan P Piccini, Electrophysiology Section, Duke Clinical Research Institute, Duke University Medical Center, PO Box 17969, Durham, NC 27710, USA.
Ryan Cunnane, University of Michigan, Ann Arbor, MI, USA.
Jan Steffel, Department of Cardiology, University Heart Center Zurich, Zurich, Switzerland.
Mikhael F El-Chami, Emory University Hospital, Atlanta, GA, USA.
Dwight Reynolds, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA.
Paul R Roberts, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Kyoko Soejima, Kyorin University Hospital, Tokyo, Japan.
Clemens Steinwender, Kepler University Hospital, Linz, Austria; Paracelsus Medical University Salzburg, Salzburg, Austria.
Christophe Garweg, University Hospitals Leuven, Leuven, Belgium.
Larry Chinitz, NYU Langone Medical Center, New York, NY, USA.
Christopher R Ellis, Vanderbilt University Medical Center, Vanderbilt Heart and Vascular Institute, Nashville, TN, USA.
Kurt Stromberg, Medtronic, Inc., Mounds View, MN, USA.
Dedra H Fagan, Medtronic, Inc., Mounds View, MN, USA.
Lluis Mont, Institut Clinic Cardiovascular (ICCV), Hospital Clinic, Universitat de Barcelona, Institut per la Recera Biomèdica IDIBAPS, Catalonia, Barcelona, Spain; Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain.
References
- 1. Reynolds D, Duray GZ, Omar R, Soejima K, Neuzil P, Zhang Set al. A leadless intracardiac transcatheter pacing system. N Engl J Med 2016;374:533–41. [DOI] [PubMed] [Google Scholar]
- 2. Piccini JP, Stromberg K, Jackson KP, Kowal RC, Duray GZ, El-Chami MF, et al. ; Micra Transcatheter Pacing Study Group . Patient selection, pacing indications, and subsequent outcomes with de novo leadless single-chamber VVI pacing. Europace 2019;21:1686–93. [DOI] [PubMed] [Google Scholar]
- 3. El-Chami MF, Al-Samadi F, Clementy N, Garweg C, Martinez-Sande JL, Piccini JPet al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: a comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018;15:1800–7. [DOI] [PubMed] [Google Scholar]
- 4. Garg A, Koneru JN, Fagan DH, Stromberg K, Padala SK, El-Chami MFet al. Morbidity and mortality in patients precluded for transvenous pacemaker implantation: experience with a leadless pacemaker. Heart Rhythm 2020;17:2056–63. [DOI] [PubMed] [Google Scholar]
- 5. El-Chami MF, Clementy N, Garweg C, Omar R, Duray GZ, Gornick CCet al. Leadless pacemaker implantation in hemodialysis patients: experience with the micra transcatheter pacemaker. JACC Clin Electrophysiol 2019;5:162–70. [DOI] [PubMed] [Google Scholar]
- 6. El-Chami MF, Johansen JB, Zaidi A, Faerestrand S, Reynolds D, Garcia-Seara Jet al. Leadless pacemaker implant in patients with pre-existing infections: results from the Micra postapproval registry. J Cardiovasc Electrophysiol 2019;30:569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Chami MF, Soejima K, Piccini JP, Reynolds D, Ritter P, Okabe Tet al. Incidence and outcomes of systemic infections in patients with leadless pacemakers: data from the Micra IDE study. Pacing Clin Electrophysiol 2019;42:1105–10. [DOI] [PubMed] [Google Scholar]
- 8. Gimbel JR, Bello D, Schmitt M, Merkely B, Schwitter J, Hayes DL, et al. ; Advisa MRI System Study Investigators . Randomized trial of pacemaker and lead system for safe scanning at 1.5 Tesla. Heart Rhythm 2013;10:685–91. [DOI] [PubMed] [Google Scholar]
- 9. Rickard J, Taborsky M, Bello D, Johnson WB, Ramza B, Chang Yet al. Short- and long-term electrical performance of the 5086MRI pacing lead. Heart Rhythm 2014;11:222–9. [DOI] [PubMed] [Google Scholar]
- 10. Udo EO, Zuithoff NP, van Hemel NM, de Cock CC, Hendriks T, Doevendans PAet al. Incidence and predictors of short- and long-term complications in pacemaker therapy: the FOLLOWPACE study. Heart Rhythm 2012;9:728–35. [DOI] [PubMed] [Google Scholar]
- 11. Piccini JP, El-Chami M, Wherry K, Crossley GH, Kowal RC, Stromberg Ket al. Contemporaneous comparison of outcomes among patients implanted with a leadless vs transvenous single-chamber ventricular pacemaker. JAMA Cardiol 2021;6:1187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vamos M, Erath JW, Benz AP, Bari Z, Duray GZ, Hohnloser SH.. Incidence of cardiac perforation with conventional and with leadless pacemaker systems: a systematic review and meta-analysis. J Cardiovasc Electrophysiol 2017;28:336–46. [DOI] [PubMed] [Google Scholar]
- 13. Collins GS, Reitsma JB, Altman DG, Moons KG.. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ 2015;350:g7594. [DOI] [PubMed] [Google Scholar]
- 14. Rietman ML, van der AD, van Oostrom SH, Picavet HSJ, Dolle MET, van Steeg Het al. The association between BMI and different frailty domains: a U-shaped curve? J Nutr Health Aging 2018;22:8–15. [DOI] [PubMed] [Google Scholar]
- 15. van Buuren S, Karin G-O.. Mice: multivariate imputation by chained equations in R. J Stat Soft 2011;45:1–67. [Google Scholar]
- 16. Thao LTP, Geskus R.. A comparison of model selection methods for prediction in the presence of multiply imputed data. Biom J 2019;61:343–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B 1996;58:267–88. [Google Scholar]
- 18. Hastie T, Tibshirani R, Friedman J.. The Elements of Statistical Learning: Data Mining, Inference, and Prediction. Springer Science & Business Media; 2009. [Database] [Google Scholar]
- 19. Friedman J, Hastie T, Tibshirani R.. Regularization paths for generalized linear models via coordinate descent. J Stat Softw 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]
- 20. Sing T, Sander O, Beerenwinkel N, Lengauer T.. ROCR: visualizing classifier performance in R. Bioinformatics 2005;21:3940–1. [DOI] [PubMed] [Google Scholar]
- 21. Team RC . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. [Google Scholar]
- 22. Cantillon DJ, Dukkipati SR, Ip JH, Exner DV, Niazi IK, Banker RSet al. Comparative study of acute and mid-term complications with leadless and transvenous cardiac pacemakers. Heart Rhythm 2018;15:1023–30. [DOI] [PubMed] [Google Scholar]
- 23. Hauser RG, Gornick CC, Abdelhadi RH, Tang CY, Casey SA, Sengupta JD.. Major adverse clinical events associated with implantation of a leadless intracardiac pacemaker. Heart Rhythm 2021;18:1132–9. [DOI] [PubMed] [Google Scholar]
- 24. Moazzami K, Dolmatova E, Kothari N, Mazza V, Klapholz M, Waller AH.. Trends in cardiac tamponade among recipients of permanent pacemakers in the United States: from 2008 to 2012. JACC Clin Electrophysiol 2017;3:41–6. [DOI] [PubMed] [Google Scholar]
- 25. Adamczyk M, Niedziela JT, Wasilewski J, Zembala MO, Kalarus Z, Gąsior M.. Prevalence, management and outcomes of cardiac tamponade complicating 66,812 invasive cardiac procedures: single-center clinical registry. Postepy Kardiol Interwencyjnej 2021;17:193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cano O, Andres A, Alonso P, Osca J, Sancho-Tello MJ, Olague J.. Incidence and predictors of clinically relevant cardiac perforation associated with systematic implantation of active-fixation pacing and defibrillation leads: a single-centre experience with over 3800 implanted leads. Europace 2017;19:96–102. [DOI] [PubMed] [Google Scholar]
- 27. Mahapatra S, Bybee KA, Bunch TJ, Espinosa RE, Sinak LJ, McGoon MDet al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005;2:907–11. [DOI] [PubMed] [Google Scholar]
- 28. Ohlow MA, Lauer B, Brunelli M, Geller JC.. Incidence and predictors of pericardial effusion after permanent heart rhythm device implantation: prospective evaluation of 968 consecutive patients. Circ J 2013;77:975–81. [DOI] [PubMed] [Google Scholar]
- 29. Bashir J, Lee AJ, Philippon F, Mondesert B, Krahn AD, Sadek MMet al. Predictors of perforation during lead extraction; results of the Canadian Lead ExtrAction Risk (CLEAR) Study. Heart Rhythm 2021;S1547-5271(21)02310-9. [DOI] [PubMed] [Google Scholar]
- 30. Friedman DJ, Pokorney SD, Ghanem A, Marcello S, Kalsekar I, Yadalam Set al. Predictors of cardiac perforation with catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2020;6:636–45. [DOI] [PubMed] [Google Scholar]
- 31. Piccini JP, Stromberg K, Jackson KP, Laager V, Duray GZ, El-Chami Met al. Long-term outcomes in leadless Micra transcatheter pacemakers with elevated thresholds at implantation: results from the Micra Transcatheter Pacing System Global Clinical Trial. Heart Rhythm 2017;14:685–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reasonable requests will be considered by the authors.


