Abstract
Aims
We aimed to characterize the substrate of T-wave inversion (TWI) using cardiac magnetic resonance (CMR) and the association between diffuse fibrosis and ventricular arrhythmias (VA) in patients with mitral valve prolapse (MVP).
Methods and results
TWI was defined as negative T-wave ≥0.1 mV in ≥2 adjacent ECG leads. Diffuse myocardial fibrosis was assessed by T1 relaxation time and extracellular volume (ECV) fraction by T1-mapping CMR. We included 162 patients with MVP (58% females, age 50 ± 16 years), of which 16 (10%) patients had severe VA (aborted cardiac arrest or sustained ventricular tachycardia). TWI was found in 34 (21%) patients. Risk of severe VA increased with increasing number of ECG leads displaying TWI [OR 1.91, 95% CI (1.04–3.52), P = 0.04]. The number of ECG leads displaying TWI increased with increasing lateral ECV (26 ± 3% for TWI 0-1leads, 28 ± 4% for TWI 2leads, 29 ± 5% for TWI ≥3leads, P = 0.04). Patients with VA (sustained and non-sustained ventricular tachycardia) had increased lateral T1 (P = 0.004), also in the absence of late gadolinium enhancement (LGE) (P = 0.008).
Conclusions
Greater number of ECG leads with TWI reflected a higher arrhythmic risk and higher degree of lateral diffuse fibrosis by CMR. Lateral diffuse fibrosis was associated with VA, also in the absence of LGE. These results suggest that TWI may reflect diffuse myocardial fibrosis associated with VA in patients with MVP. T1-mapping CMR may help risk stratification for VA.
Keywords: Electrocardiography, Mitral valve prolapse, Ventricular arrhythmias, Diffuse myocardial fibrosis, T1-mapping cardiac magnetic resonance
Graphical Abstract
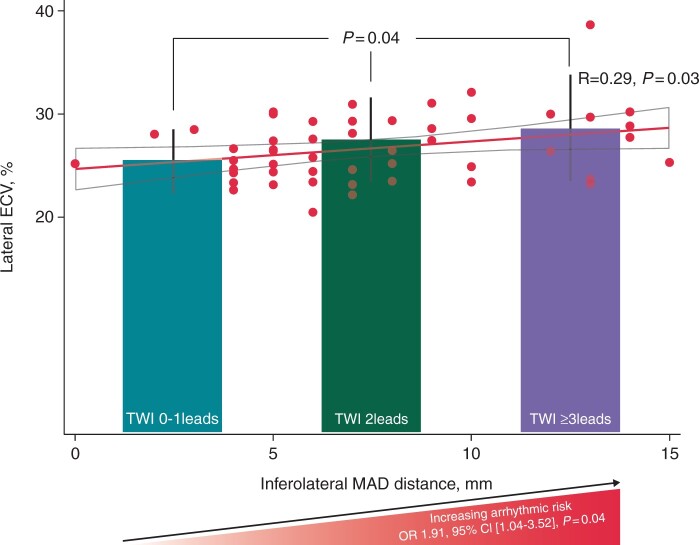
Graphical Abstract.
Higher number of ECG leads with TWI indicating higher arrhythmic risk and higher degree of lateral diffuse myocardial fibrosis Bar charts show values of lateral ECV by T1-mapping CMR in patients with no TWI or with TWI in 1 lead (left bar), in patients with TWI in 2 ECG leads (middle bar) and in patients with TWI in ≥3 ECG leads. P value was obtained by one-way ANOVA test with Bonferroni correction. The scatter plot graph shows the correlation between lateral ECV by T1-mapping CMR and inferolateral MAD distance. Inferolateral MAD distance increased with higher lateral ECV values. Correlation coefficient R and P values were calculated by Pearson correlation test. The risk of severe VA increased with the number of ECG leads with TWI. Odds ratios, 95% CI and P value for risk of severe VA were calculated by logistic regression analysis. CMR = cardiac magnetic resonance, ECG = electrocardiographic, ECV = extracellular volume, MAD = mitral annulus disjunction, TWI = T-wave inversion VA = ventricular arrhythmias.
What’s new?
T-wave inversion on ECG reflects lateral diffuse myocardial fibrosis by T1-mapping cardiac magnetic resonance potentially predisposing to ventricular arrhythmias in patients with mitral valve prolapse.
Assessment of diffuse myocardial fibrosis by T1-mapping cardiac magnetic resonance can be used for arrhythmic risk stratification in patients with mitral valve prolapse.
Introduction
Sudden cardiac death is a rare but devastating complication in patients with mitral valve prolapse (MVP). Given the high prevalence of MVP in the general population,1 identifying patients at risk of arrhythmic death is of uttermost importance.
Several factors have been associated with an adverse outcome in patients with MVP including bileaflet MVP,2 myocardial fibrosis in the inferolateral left ventricular wall or papillary muscles detected by late gadolinium enhancement (LGE) at cardiac magnetic resonance (CMR)3 and mitral annulus disjunction (MAD), known as atrial displacement of mitral annulus from the ventricular myocardium at end-systole.4 However, risk stratification remains challenging and robust tools for prediction of severe VA are lacking in patients with MVP.
Previous studies suggested electrocardiographic (ECG) repolarization abnormalities as a characteristic of arrhythmic MVP phenotype with frequent T-wave inversion (TWI) in high-risk patients with MVP.2,3 However, the underlying morphological substrate of repolarization abnormalities in patients with MVP is not known.
Focal myocardial fibrosis reflected by LGE is the underlying substrate for re-entry arrhythmias thus predisposing to severe VA in MVP patients.4,5 However, severe VA occurs in patients without LGE suggesting the existence of additional arrhythmic substrates.4 Diffuse myocardial fibrosis reflected by a shortened post-contrast T1 time at T1-mapping CMR is associated with complex premature ventricular contractions (PVCs) in patients with MVP.6 Furthermore, diffuse fibrosis is present in chronic mitral regurgitation due to MVP and related to clinical events.7 However, whether diffuse fibrosis is a risk marker for VA beyond the presence of LGE in patients with MVP is unknown.
We aimed to describe the prevalence of TWI and to explore the association between severe VA and repolarization abnormalities in a cohort of patients with MVP. In addition, we aimed to investigate the morphological substrate of TWI using tissue characterization by CMR. We hypothesized that TWI is associated with diffuse myocardial fibrosis, forming the substrate for VA in patients with MVP.
Methods
Study population
In this multicentre cross-sectional study, patients were consecutively included from August 2015 until March 2018, at Oslo University Hospital, Rikshospitalet and Drammen Hospital, Norway and at the University Hospital Brussels, Universitair Ziekenhuis Brussel, Belgium. A subset of patients have been reported previously.4,8 Patients undergoing echocardiography at these centres were screened for MVP and MAD by experienced physicians and sonographers. Patients with confirmed MVP and MAD were invited to a comprehensive study evaluation, which included medical history recording, clinical examination, 12-lead ECG, 24 h Holter monitoring, echocardiography and CMR as previously described.4,8 Patients with history of severe VA underwent a comprehensive diagnostic work-up as clinically indicated. We excluded patients with obstructive coronary artery disease and non-mitral moderate or severe valvular disease, patients with severe VA of other plausible causes (cardiac channelopathies and cardiomyopathies) and those successfully treated by catheter ablation for VA before inclusion. All patients gave their written informed consent for participation. The study complied with The Declaration of Helsinki and was approved by the Regional Committee for Medical Research Ethics in Norway (2015/596/REKnord) and by the Ethical Committee of UZ Brussels in Belgium (2016/407). The data that support the findings of this study are available from the corresponding author upon reasonable request.
Electrocardiogram and ventricular arrhythmias
We analysed 12-lead resting ECGs for the presence and extent of inverted T-wave in inferior (II, III, and aVF), lateral (I, aVL, V5, and V6), and anterior leads (V3 and V4). We excluded ECGs recorded <5 days after an episode of severe VA and TWI secondary to bundle branch block. TWI was defined as inverted T-wave ≥0.1 mV and considered pathological when present in ≥2 adjacent ECG leads.9 We defined extended TWI as negative T-wave present in ≥3 ECG leads. We reported duration of the QRS complex and fragmentation of a narrow QRS complex defined as the presence of an additional R wave or notching in the nadir of the S wave10 and calculated QTc duration according to Bazett’s formula. All ECG analyses were performed blinded to clinical data.
The number of PVCs in 24 h by Holter monitoring was recorded as PVCs count.
We defined VA as non-sustained VT (≥3 consecutive ventricular beats >100 beats/min for <30 s) or documented history of severe VA. Severe VA were defined as history of documented sustained VT (runs of consecutive ventricular beats >100 beats/min for >30 s) or aborted cardiac arrest at inclusion.
Echocardiography
Transthoracic echocardiography was performed using a commercial cardiac ultrasound system (Vivid 7, Vivid E9 or Vivid E95; GE Vingmed Ultrasound, Horten, Norway) and images were analysed offline blinded for clinical data (EchoPac, version 202, GE Vingmed Ultrasound, Horten, Norway). We acquired standard parasternal long- and short-axis images, apical 4-, 2-, and 3-chamber views. MV regurgitation was quantified according to guidelines and graded as mild, moderate, or severe.11
Cardiac magnetic resonance
The CMR study protocol was performed using a 3-T scanner (Ingenia, Philips Healthcare, Best, the Netherlands) and images were analysed using Sectra Workstation IDS7 v18.1 (Sectra AB, Linköping, Sweden) at Oslo University Hospital, Rikshospitalet, Norway.
We performed balanced steady-state free precession cine sequences in six left ventricular long axis image planes separated by 30° and in consecutive short axis image planes of 8 mm slice thickness as previously described.4 The CMR study protocol included modified Look-Locker inversion recovery sequences in standard long axis and three short axis image planes both prior to (native) and 10 min after intravenous injection of 0.20 mmol/kg gadoterate meglutamine (Dotarem™, Guerbet, Villepinte, France).
We measured native and post-contrast T1 relaxation time on midventricular short axis slices by placing the region of interest in the interventricular septum and in the lateral left ventricular wall and a circular region of interest in the left ventricular blood pool. We calculated myocardial extracellular volume (ECV) as the ratio between myocardial and blood pool T1 relaxivity change multiplied by a factor equal to 1-haematocrit.12 All T1-mapping CMR studies were performed on the same scanner.
We defined MVP as atrial displacement of any part of the mitral leaflets of ≥2 mm from the line connecting annular hinge points at end-systole.11 We inspected the entire mitral annulus circumference for the presence of MAD of ≥1 mm by measuring MAD distance from the left atrium-MV leaflet junction to the top of the left ventricular myocardium at end-systole. Greatest MAD assessed from 90° to 240° was reported as inferolateral MAD distance.
We assessed the presence of LGE on sequential post-contrast short axis slices of the left ventricle from the atrioventricular valve plane to the apex and we measured left ventricular volumes and ejection fraction using standard techniques.13
In patients not eligible for our CMR study protocol, previously obtained clinical CMR examinations were retrospectively analysed using Sectra Workstation IDS7 v18.1 [Sectra AB, Linköping, Sweden (in Oslo)] or Circle [Circle Cardiovascular Imaging, Calgary, Alberta, Canada (in Brussels] for the presence of LGE at basal left ventricular wall and papillary muscles.
Statistics
Continuous variables were presented as mean±standard deviation (SD) or median [inter-quartile range (IQR)] and compared using Student’s t-test, one-way ANOVA, or Mann–Whitney U non-parametric test, as appropriate. Categorical variables were presented as frequencies (%) and compared using χ2 test or Fisher’s exact test as appropriate. Association between indices of diffuse myocardial fibrosis and electrical and imaging parameters was assessed using univariate linear regression and Pearson correlation analysis. Odds ratios and 95% confidence interval (CI) were calculated for VA, LGE and TWI using univariate and multivariate logistic regression analysis. Statistical analysis was performed using Stata/SE 16.0 (StataCorp LLC, Texas); P values were two-sided and values <0.05 were considered significant. P values for CMR parameters were adjusted for centre cluster confounding.
Results
We included 162 patients with MVP (58% women, age 50 ± 16 years) (Table 1). Sixteen (10%) patients had documented severe VA at inclusion (13 with aborted cardiac arrest and 3 with sustained VT) and 18 (11%) patients were implanted with a cardiac device (15 patients with an implantable cardiac defibrillator and 3 patients with a pacemaker).
Table 1.
Characteristics of 162 patients with MVP dichotomized according to the presence of TWI in ≥3 ECG leads (n = 20) and TWI in <3 ECG leads (n = 142)
| Total (n = 162) | TWI < 3ECG leads (n = 142) | TWI ≥ 3ECG leads (n = 20) | P value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years±SD | 50 ± 16 | 50 ± 16 | 45 ± 15 | 0.20 |
| Female, n (%) | 93 (57) | 78 (55) | 15 (75) | 0.10 |
| Syncope, n (%) | 29 (18) | 26 (18) | 3 (15) | 1.00 |
| Palpitations, n (%) | 116 (72) | 100 (70) | 16 (80) | 0.60 |
| Arrhythmias | ||||
| VA, n (%) | 66 (41) | 54 (38) | 12 (60) | 0.06 |
| Severe VA, n (%) | 16 (10) | 11 (8) | 5 (25) | 0.02 |
| Electrocardiogram | ||||
| QRS duration, ms±SD | 95 ± 13 | 95 ± 14 | 90 ± 8 | 0.12 |
| QRS fragmentation, n (%) | 23 (14) | 22 (15) | 1 (5) | 0.31 |
| QTc, ms±SD | 412 ± 37 | 409 ± 37 | 429 ± 31 | 0.02 |
| 24 h Holter monitoring | ||||
| PVCs count,an±SD | 2.34 ± 1.09 | 2.29 ± 1.08 | 2.71 ± 1.17 | 0.16 |
| Cardiac magnetic resonanceb | ||||
| Bileaflet MVP, n (%) | 43 (27) | 34 (24) | 9 (45) | 0.02 |
| Inferolateral MAD distance | 7 ± 3 | 7 ± 3 | 10 ± 3 | 0.005 |
| LVEDVi, mL/m2±SD | 82 ± 24 | 81 ± 22 | 89 ± 33 | 0.26 |
| LVESVi, mL/m2±SD | 35 ± 12 | 34 ± 12 | 40 ± 17 | 0.12 |
| LV EF, %±SD | 58 ± 7 | 58 ± 7 | 55 ± 7 | 0.18 |
| LGE, n (%) | 54 (33) | 46 (32) | 8 (40) | 0.48 |
| Basal LV wall LGE, n (%) | 34 (21) | 30 (21) | 4 (20) | 1.00 |
| Papillary muscles LGE, n (%) | 21 (13) | 17 (12) | 4 (20) | 0.30 |
| Average T1 time, ms±SD | 1267 ± 43 | 1262 ± 43 | 1290 ± 42 | 0.09 |
| Septal T1 time, mc±SD | 1273 ± 47 | 1268 ± 46 | 1298 ± 46 | 0.08 |
| Lateral T1 time, ms±SD | 1257 ± 45 | 1253 ± 44 | 1280 ± 42 | 0.09 |
| Average ECV, %±SD | 27 ± 3 | 26 ± 3 | 29 ± 4 | 0.01 |
| Septal ECV, %±SD | 27 ± 3 | 26 ± 3 | 29 ± 4 | 0.02 |
| Lateral ECV, %±SD | 27 ± 3 | 26 ± 3 | 29 ± 5 | 0.03 |
Continuous variables are presented as mean (SD) and categorical variables as frequencies (%). P values are calculated by Student’s t-test, χ2, or Fisher’s exact test as appropriate. Bold values denote statistical significance.
BSA, body surface area; ECG, electrocardiogram; ECV, extracellular volume; EF, ejection fraction; LV, left ventricular; LVEDVi, LV end-diastolic volume indexed to BSA; LVESVi, LV end-systolic volume indexed to BSA; LGE, late gadolinium enhancement; MAD, mitral annulus disjunction; MVP, mitral valve prolapse; TWI, T-wave inversion; PVCs, premature ventricular contractions; VA, ventricular arrhythmias.
Log base 10 transformation of the PVCs count was performed to assure normal distribution.
CMR was available in 120 patients and LGE in 113 patients, T1-mapping CMR sequences were available in 56 patients.
T-wave inversion and severe ventricular arrhythmias
We found 34 (21%) patients with TWI ≥2 leads of which 20 (59%) patients had extended TWI (≥3 leads), while the remaining had TWI in 1 lead or no TWI.
Extended TWI was associated with severe VA (P = 0.02) (Table 1) and the risk of severe VA increased with the number of leads with TWI [OR 1.91, 95% CI (1.04–3.52), P = 0.04] (Graphical Abstract). Extended TWI was also associated with longer duration of QTc (Table 1). Other ECG abnormalities were not associated with higher arrhythmic risk including duration of the QRS complex (91 ± 12 vs. 95 ± 13 ms, P = 0.28), prevalence of QRS fragmentation [3/16 with VA (19%) vs. 20/146 without VA (14%), P = 0.70] and QTc duration (416 ± 28 vs. 411 ± 37 ms, P = 0.63).
T-wave inversion and myocardial fibrosis
A CMR study was available in 120 (74%) patients. CMR was performed prospectively at inclusion in 114 (70%) patients, while in the remaining 6 (4%) patients, we used a previous CMR study performed on clinical indication. CMR with LGE was available in 113 (70%) patients and 56 (35%) patients underwent study protocol CMR with T1-mapping sequences.
Of the 113 patients with LGE CMR, 54 (48%) patients had fibrosis by LGE (34 in the left ventricular inferolateral wall and 37 in the papillary muscles). LGE was not associated with extended TWI (≥3 leads) (Table 1). Furthermore, odds of LGE presence did not increase with the number of leads with TWI [OR 1.07, 95% CI (0.80–1.45), P = 0.62] nor when adjusted for centre cluster confounding [multivariate OR 1.41, 95% CI (0.80–2.48), P = 0.24].
Lateral ECV values were higher in patients with extended TWI (≥3 leads) (Table 1), also when adjusted for age and sex [lateral ECV: multivariate OR 1.32, 95% CI (1.00–1.74), P = 0.048]. Lateral ECV increased with the number of ECG leads displaying TWI (Graphical Abstract).
Myocardial fibrosis and ventricular arrhythmias
Among the 113 patients with CMR LGE protocol, 50 (44%) patients had VA and among the 56 patients with TI-mapping, 15 (28%) patients had VA.
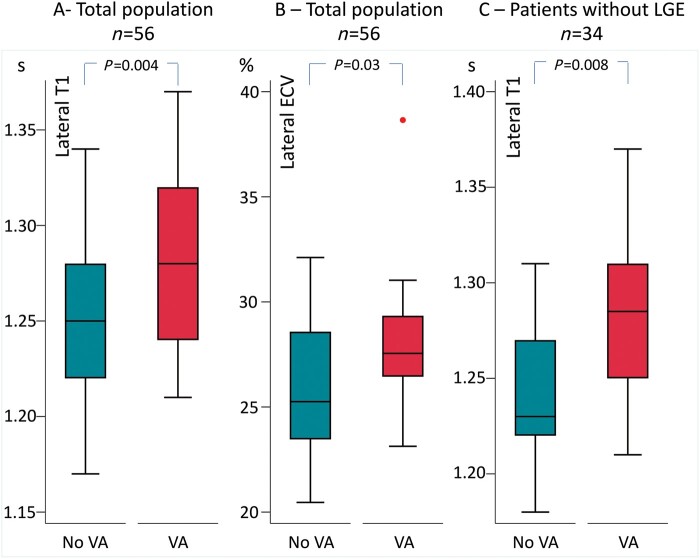
Patients with VA had more frequently papillary muscles LGE compared to those without VA (50% vs. 19%, P = 0.001). Furthermore, patients with VA had higher lateral T1 relaxation time and higher lateral ECV (Figure 1A and B; Table 2). The association between lateral T1 and VA was present even in the group of patients without LGE (Figure 1C).
Figure 1.
Lateral diffuse myocardial fibrosis by T1-mapping cardiac magnetic resonance in patients with and without VA. Boxplots representing lateral T1 and ECV values in patients with and without VA. (A) Lateral T1 in the total study population. (B) Lateral ECV in the total study population. (C) Lateral T1 in patients without LGE. P values are calculated by Student’s t-test. ECV, extracellular volume; LGE, late gadolinium enhancement; VA, ventricular arrhythmias.
Table 2.
Linear regression between diffuse fibrosis by T1-mapping cardiac magnetic resonance and clinical, electrical, and imaging parameters
| Univariate B (95% CI) Lateral T1 (ms) | P value | Univariate B (95% CI) Lateral ECV (%) | P value | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Agea (years) | 4.95 (−3.43–13.33) | 0.24 | 0.52 (−0.12–1.16) | 0.11 |
| Sex, female | 0.95 (−24.20–26.10) | 0.69 | 1.66 (−0.15–3.47) | 0.07 |
| Syncope | 39.96 (−0.88–80.79) | 0.06 | 1.36 (−1.69–4.41) | 0.38 |
| Palpitations | 14.58 (−11.81–40.96) | 0.27 | 0.96 (−1.01–2.94) | 0.33 |
| Arrhythmias | ||||
| VA | 36.68 (11.37–61.99) | 0.005 | 2.07 (0.16–3.98) | 0.03 |
| Severe VA | −25.87 (−116.61–64.87) | 0.57 | −1.29 (−7.88–5.31) | 0.70 |
| Electrocardiogram | ||||
| TWI | 27.94 (1.81–54.07) | 0.04 | 2.31 (0.42–4.19) | 0.02 |
| QRS duration (ms) | 0.49 (−0.46–1.44) | 0.30 | 0.01 (−0.07–0.08) | 0.89 |
| QRS fragmentation | −16.48 (−54.22–21.27) | 0.39 | −1.54 (−4.40–1.31) | 0.28 |
| QTc (ms) | 0.44 (0.05–0.83) | 0.03 | 0.06 (0.03–0.08) | <0.001 |
| 24 h Holter monitoring | ||||
| PVCs count,bn | 18.21 (5.51–30.94) | 0.006 | 1.17 (0.16–2.18) | 0.03 |
| Cardiac magnetic resonance | ||||
| Bileaflet MVP | 21.17 (−3.98–46.33) | 0.10 | 1.75 (−0.08–3.58) | 0.06 |
| Inferolateral MAD distance | 4.50 (1.26–7.73) | 0.007 | 0.27 (0.02–0.52) | 0.03 |
| LVEDVic (mL/m2) | 1.93 (−0.86–4.72) | 0.17 | 0.13 (−0.08–0.33) | 0.21 |
| LVEDVic (mL/m2) | 4.49 (−1.20–10.19) | 0.12 | 0.32 (−0.09–0.74) | 0.13 |
| LV EFc (%) | −4.01 (−15.42–7.39) | 0.68 | −0.45 (−1.28–0.37) | 0.28 |
| LGE | 12.10 (−13.16–37.36) | 0.34 | 1.10 (−0.75–2.95) | 0.24 |
| Basal LV wall LGE | 23.64 (−4.80–52.08) | 0.10 | 2.24 (0.18–4.29) | 0.03 |
| Papillary muscles LGE | 20.39 (−21.03–61.81) | 0.33 | 3.30 (0.37–6.23) | 0.03 |
P values are calculated by univariate linear regression analysis. Bold values denote statistical significance.
ECV, extracellular volume; EF, ejection fraction; LGE, late gadolinium enhancement; LV, left ventricular; LVEDVi, LV end-diastolic volume indexed; LVESVi, LV end-systolic volume indexed; MAD, mitral annulus disjunction; MVP, mitral valve prolapse; PM, papillary muscles; PVCs, premature ventricular contractions; TWI, T-wave inversion; VA, ventricular arrhythmias.
Per 10 years increments.
Log base 10 transformation of the PVCs count was performed to assure model linearity assumptions.
Per 5-units increments.
Lateral ECV and T1 relaxation time were associated with VA in a multiple logistic regression independently of age, sex, LGE, left ventricular end-diastolic volume index and severe mitral regurgitation [lateral ECV: multivariate OR 1.37, 95% CI (1.02–1.84), P = 0.03; lateral T1: multivariate OR 1.04, 95% CI (1.01–1.07), P = 0.008].
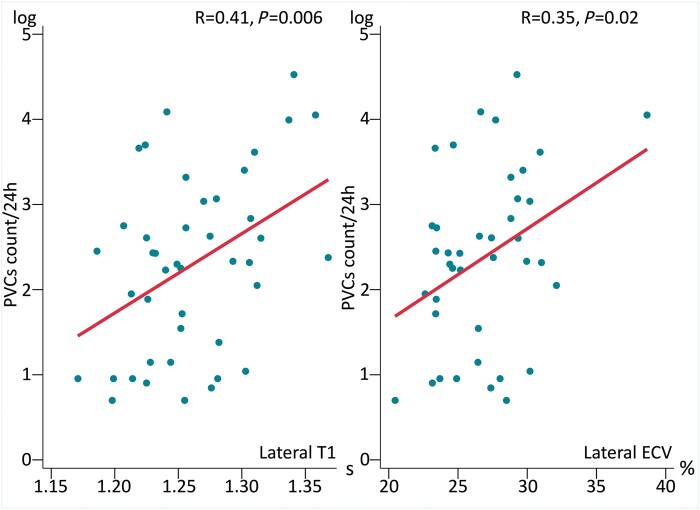
Number of PVCs per 24 h by Holter monitoring increased with lateral T1 time and lateral ECV values (Figure 2; Table 2).
Figure 2.
Correlation between PVCs count/24 h by Holter monitoring and lateral T1 and ECV values by T1-mapping cardiac magnetic resonance. Correlation plots between PVCs count/24 h as evaluated by Holter monitoring (y-axis) and lateral T1 (left panel) and ECV values (right panel) by T1-mapping cardiac magnetic resonance (x-axis). Log base 10 transformation of the PVCs count was performed to assure model linearity assumptions. Correlation coefficient R and P values are calculated by Pearson correlation analysis. ECV, extracellular volume; PVCs, premature ventricular contractions.
T-wave inversion, fibrosis and mitral valve abnormalities
Patients with extended TWI (≥3 leads) had higher prevalence of bileaflet MVP (P = 0.02) (Table 1), and had greater inferolateral MAD distance (P = 0.005) (Table 1).
Lateral ECV was marginally higher in patients with bileaflet MVP than in those without (28 ± 4% vs. 26 ± 3%, P = 0.06) (Table 2) and correlated positively with inferolateral MAD distance (P = 0.03) (Graphical Abstract).
Discussion
In this study, we confirmed the association between TWI and severe VA and added to the current knowledge by showing that arrhythmic risk increased with the extent of TWI. Interestingly, the extent of TWI correlated with the degree of diffuse myocardial fibrosis by T1-mapping CMR suggesting diffuse fibrosis to be the substrate of electrical changes. Importantly, patients with VA had more diffuse myocardial fibrosis even in the absence of LGE, suggesting diffuse myocardial fibrosis alone as an arrhythmogenic factor independently of fibrosis detected by LGE. Whether diffuse fibrosis is an early sign or concomitant with LGE detected focal fibrosis remains to be explored.
Prevalence of repolarization abnormalities and arrhythmic risk
We found TWI ≥2 leads in 21% and extended TWI in 13% of patients with MVP. The prevalence found in our study is comparable with a recent study showing TWI in 20% of cases in a general MVP population.14 Other previous studies2,3 found a prevalence of repolarization abnormalities (inverted or biphasic T-wave in inferior leads) as high as 80% when including high-risk patients with a frequent bileaflet MVP. Our study confirmed the association between extended TWI and bileaflet MVP, supporting the theory that TWI is associated with more severe mitral valve disease.
We showed that the risk of severe VA increased with greater number of ECG leads displaying TWI. Several studies have indicated T-wave changes as a characteristic of the arrhythmic MVP phenotype and its association with increased arrhythmic risk.2,3,14 Our study supported the link between TWI and severe VA and added the finding of a continuous increase in risk by more extended repolarization abnormalities.
Tissue characterization for identification of substrate of repolarization abnormalities
For the first time, we were able to link more extended TWI to lateral diffuse myocardial fibrosis by T1-mapping CMR. These findings indicate that diffuse fibrosis forms the substrate for ECG repolarization abnormalities and relate the ECG changes to possible arrhythmogenic substrates.
In contrast to T1-mapping CMR, we found no association between the presence of LGE and ECG repolarization abnormalities. The presence of LGE is a qualitative and observer-dependent finding based on differences in signal intensity between adjacent myocardium regions15 and is categorized in presence or absence of fibrosis. LGE might not be sensitive enough to detect small amount of fibrosis. Our findings of TWI relation to fibrosis by T1-mapping suggest that the continuum of myocardial fibrosis is more precisely characterized by T1-mapping CMR.16 Furthermore, missing CMR data in patients at highest arrhythmic risk might have impacted the association between LGE and TWI.
Substrate for arrhythmias in mitral valve prolapse
We showed that patients with MVP and VA had higher indices of diffuse myocardial fibrosis, linking diffuse fibrosis to arrhythmic risk. The arrhythmic relation to diffuse fibrosis was further supported by a positive correlation between lateral diffuse fibrosis and PVCs count in 24 h by Holter monitoring, supporting one previous study.6 This finding also suggests the lateral left ventricular wall to be predominantly affected in MVP. Our findings support the hypotheses that mechanical stretch of the basal inferolateral left ventricular wall and adjacent segments induces specific regional myocardial changes in MVP.17,18
In this study, we showed the value of assessing diffuse myocardial fibrosis for depicting arrhythmic risk also in patients without LGE.6 This finding is important by indicating that different types of fibrosis may play a role in MVP. LGE is considered to reflect replacement fibrosis while parameters assessed by T1-mapping CMR reflect interstitial fibrosis. LGE is a recognized arrhythmic marker in MVP patients,3 with inferolateral left ventricular wall and papillary muscles LGE related to highest arrhythmic risk.4,5 However, LGE has limited abilities in predicting arrhythmic risk as also shown in our previous study4 where LGE was absent in more than half of patients with MVP and severe VA thus indicating other mechanisms for the electrical instability. Detection of interstitial fibrosis in patients with MVP may further improve risk stratification.16
Myocardial fibrosis and relation to mitral valve abnormalities
The prevalence of bileaflet MVP was higher in patients with extended TWI. Our finding supports the hypothesis that stretching of the papillary muscles and of the adjacent ventricular wall by a severely degenerated valve leads to myocardial fibrosis.19 Our results further add to these theories by showing the association between bileaflet MVP and ECG abnormalities and the marginally significant relation of bileaflet MVP to CMR diffuse fibrosis.
Interestingly, we showed that a greater inferolateral MAD distance was linked to extended TWI and more lateral diffuse myocardial fibrosis. These findings suggest that greater MAD distance imposes greater mechanical stretch on the lateral ventricular wall, leading to myocardial fibrosis presented on the ECG as extended repolarization abnormalities.
Clinical implications
We propose that detection of ECG TWI could help identify patients with underlying diffuse myocardial fibrosis who might benefit from closer heart rhythm monitoring. Importantly, diffuse myocardial fibrosis in MVP patients could indicate arrhythmic risk even in the absence of LGE. Assessment of myocardial fibrosis by T1-mapping CMR may help in arrhythmic risk stratification of patients with MVP.
Limitations
This was an ambispective cross-sectional study with a prospective CMR study protocol in addition to retrospective CMR examinations and partly pre-existing data collection with inherent design-associated limitations and possible selection bias.
The limited number of severe VA could have influenced the robustness of the statistical analysis. We included MVP patients from referral centres and the prevalence of arrhythmias was therefore overestimated compared to a general population. Furthermore, the referral bias resulted in a high proportion of patients with an arrhythmic MVP phenotype and our findings may not be applicable to populations with other characteristics.
Lack of CMR examinations in survivors of severe VA already implanted with an implantable cardioverter-defibrillator and not suitable for CMR scanning may have caused underestimation of the prevalence of fibrosis in the individuals at highest risk. Only one patient with history of severe VA performed T1-mapping CMR. Therefore, we were not able to investigate a direct association between diffuse fibrosis by T1-mapping CMR and severe VA.
CMR with evaluation of the presence of LGE was not available in all and T1-mapping sequences were available only in a subgroup of patients which could have influenced the results. Patients with VA were clearly distinguished by higher native T1 values. However, small differences of native T1 values between diseased and normal myocardium might limit the clinical application of this technique.20
We cannot exclude other causes of TWI as the phenomenon of cardiac memory in the presence of frequent PVCs or transitory states responsible for dynamic TWI.
CMR protocol did not include velocity encoding necessary for regurgitation quantification and therefore the degree of mitral regurgitation was analysed using echocardiography.
Conclusions
Extended ECG TWI was associated with higher risk of severe VA and was present in 13% of patients with MVP. The extent of TWI was associated with higher degree of lateral diffuse fibrosis, suggesting diffuse fibrosis as the underlying substrate for ECG repolarization abnormalities. Lateral diffuse myocardial fibrosis indicated higher arrhythmic risk even in the absence of focal fibrosis by LGE. These results suggest that ECG TWI reflects myocardial diffuse fibrosis associated with increased arrhythmic risk in patients with MVP.
Acknowledgements
The authors have no relationship with industry.
Funding
This study was supported by the South-Eastern Norway Regional Health Authority (#2019025).
Conflict of interest: none declared.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Contributor Information
Monica Chivulescu, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, University of Oslo, 0318 Oslo, Norway.
Eivind W Aabel, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, University of Oslo, 0318 Oslo, Norway.
Erik Gjertsen, Department of Medicine, Drammen Hospital, Venstre Viken Hospital Trust, 3004 Drammen, Norway.
Einar Hopp, Division of Radiology and Nuclear Medicine, The Intervention Centre, Oslo University Hospital, Rikshospitalet, 0424 Oslo, Norway.
Esther Scheirlynck, Centrum voor Hart-en Vaatziekten, Universitair Ziekenhuis Brussel-Vrije Universiteit Brussel, 1090 Brussels, Belgium.
Bernard Cosyns, Centrum voor Hart-en Vaatziekten, Universitair Ziekenhuis Brussel-Vrije Universiteit Brussel, 1090 Brussels, Belgium.
Erik Lyseggen, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway.
Thor Edvardsen, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, University of Oslo, 0318 Oslo, Norway.
Øyvind H Lie, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, University of Oslo, 0318 Oslo, Norway.
Lars A Dejgaard, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway.
Kristina H Haugaa, Department of Cardiology, ProCardio Centre for Innovation, Oslo University Hospital, Rikshospitalet, PO Box 4950 Nydalen, 0424 Oslo, Norway; Institute of Clinical Medicine, University of Oslo, 0318 Oslo, Norway; Department of Cardiology, Karolinska University Hospital, Stockholm, Sweden; Department of Medicine, Huddinge, Karolinska Institutet, Stockholm, Sweden.
References
- 1. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DLet al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 2. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta Fet al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol 2013;62:222–30. [DOI] [PubMed] [Google Scholar]
- 3. Basso C, Perazzolo Marra M, Rizzo S, De Lazzari M, Giorgi B, Cipriani Aet al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015;132:556–66. [DOI] [PubMed] [Google Scholar]
- 4. Dejgaard LA, Skjolsvik ET, Lie OH, Ribe M, Stokke MK, Hegbom Fet al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600–9. [DOI] [PubMed] [Google Scholar]
- 5. Marra MP, Basso C, De Lazzari M, Rizzo S, Cipriani A, Giorgi Bet al. Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bui AH, Roujol S, Foppa M, Kissinger KV, Goddu B, Hauser THet al. Diffuse myocardial fibrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart 2017;103:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kitkungvan D, Yang EY, El Tallawi KC, Nagueh SF, Nabi F, Khan MA, et al. Extracellular volume in primary mitral regurgitation. JACC Cardiovasc Imaging 2021;14:1146–60. [DOI] [PubMed] [Google Scholar]
- 8. Scheirlynck E, Dejgaard LA, Skjolsvik E, Lie OH, Motoc A, Hopp Eet al. Increased levels of sST2 in patients with mitral annulus disjunction and ventricular arrhythmias. Open Heart 2019;6:e001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rautaharju PM, Surawicz B, Gettes LS, Bailey JJ, Childers R, Deal BJet al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:982–91. [DOI] [PubMed] [Google Scholar]
- 10. Pietrasik G, Zaręba W.. QRS fragmentation: diagnostic and prognostic significance. Cardiol J 2012;19:114–21. [DOI] [PubMed] [Google Scholar]
- 11. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PAet al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 12. Messroghli DR, Moon JC, Ferreira VM, Grosse-Wortmann L, He T, Kellman Pet al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson 2018;20:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E.. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson 2020;22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Essayagh B, Sabbag A, Antoine C, Benfari G, Yang LT, Maalouf Jet al. Presentation and outcome of arrhythmic mitral valve prolapse. J Am Coll Cardiol 2020;76:637–49. [DOI] [PubMed] [Google Scholar]
- 15. Karamitsos TD, Arvanitaki A, Karvounis H, Neubauer S, Ferreira VM.. Myocardial tissue characterization and fibrosis by imaging. JACC Cardiovasc Imaging 2020;13:1221–34. [DOI] [PubMed] [Google Scholar]
- 16. Schelbert EB, Messroghli DR.. State of the art: clinical applications of cardiac T1 mapping. Radiology 2016;278:658–76. [DOI] [PubMed] [Google Scholar]
- 17. Kitkungvan D, Nabi F, Kim RJ, Bonow RO, Khan MA, Xu Jet al. Myocardial fibrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol 2018;72:823–34. [DOI] [PubMed] [Google Scholar]
- 18. Han HC, Parsons SA, Curl CL, Teh AW, Raaijmakers AJA, Koshy ANet al. Systematic quantification of histologic ventricular fibrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm 2021;18:570–6. [DOI] [PubMed] [Google Scholar]
- 19. Hutchins GM, Moore GW, Skoog DK.. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535–40. [DOI] [PubMed] [Google Scholar]
- 20. Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S.. Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol 2016;71:768–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.