Abstract
Aims
Vasovagal syncope (VVS) is a common clinical condition that lacks effective medical therapies despite being associated with significant morbidity. Current guidelines suggest that midodrine, a prodrug for an α1-adrenergic receptor agonist, might suppress VVS but supporting studies have utilized heterogeneous methods and yielded inconsistent results. To evaluate the efficacy of midodrine to prevent syncope in patients with recurrent VVS by conducting a systematic review and meta-analysis of published studies.
Methods and results
Relevant randomized controlled trials were identified from the MEDLINE, Embase, CENTRAL, and CINAHL databases without language restriction from inception to June 2021. All studies were conducted in clinical syncope populations and compared the benefit of midodrine vs. placebo or non-pharmacological standard care. Weighted relative risks (RRs) were estimated using random effects meta-analysis techniques. Seven studies (n = 315) met inclusion criteria. Patients were 33 ± 17 years of age and 31% male. Midodrine was found to substantially reduce the likelihood of positive head-up-tilt (HUT) test outcomes [RR = 0.37 (0.23–0.59), P < 0.001]. In contrast, the pooled results of single- and double-blind clinical trials (I2 = 54%) suggested a more modest benefit from midodrine for the prevention of clinical syncope [RR = 0.51 (0.33–0.79), P = 0.003]. The two rigorous double-blind, randomized, placebo-controlled clinical trials included 179 VVS patients with minimal between-study heterogeneity (I2 = 0%) and reported a risk reduction with midodrine [RR = 0.71 (0.53–0.95), P = 0.02].
Conclusions
Midodrine is effective in preventing syncope induced by HUT testing and less, but still significant, RR reduction in randomized, double-blinded clinical trials.
Keywords: Vasovagal syncope, Randomized clinical trials, Meta-analysis, Systematic review, Midodrine
What’s new?
Midodrine is effective in preventing syncope induced by head-up-tilt testing [relative risk (RR) = 0.37 (0.23–0.59), P < 0.001].
Midodrine significantly prevents vasovagal syncope in randomized, double-blinded clinical trials [RR = 0.71 (0.53–0.95), P = 0.02].
Introduction
Syncope is a common clinical condition with a lifetime cumulative incidence of at least 35% and a high rate of recurrence following initial presentation.1 Nearly 60% of all syncope cases are attributable to vasovagal syncope (VVS),2,3 which is due to hypotension often caused by sympathetic withdrawal. Although syncope is generally benign, recurrent VVS is associated with frequent injuries,4,5 psychological morbidities, and impaired patient quality of life.6
To date, there remain few effective medical therapies for the treatment of recurrent VVS current consensus guidelines provide weak recommendations for the use of beta-blockers, fludrocortisone, and midodrine for the management of recurrent VVS based on modest data supporting their effectiveness in selected patient populations.2,3,7 This systematic review focuses on midodrine and provides an update on the systematic review performed by Izcovich et al.8 in 2014, including the results of a recent, positive, randomized clinical trial.9 Our review stresses studies with adequate blinding and clinical outcomes.
Midodrine is a selective α1-adrenergic receptor agonist pro-drug that is thought to enhance peripheral vascular tone and reduce venous pooling, thereby preventing syncope.3 However, prior studies assessing the benefit of midodrine for syncope prevention have used heterogeneous methods in clinically disparate populations, providing inconsistent results and lower levels of evidence.9–15 Therefore, the aim of this study was to evaluate the effectiveness of midodrine for the prevention of syncope in patients with recurrent VVS through a comprehensive systematic review and meta-analysis of published studies, with special consideration of the effect of study design on apparent drug benefit.
Methods
The protocol for this systematic review was registered in PROSPERO: CRD42019132720. This protocol includes details of the search strategy, criteria for study selection, statistical methodology, and risk of bias assessments. This study was exempt from Institutional Review Board approval.
Data sources and search strategy
Multiple electronic databases were searched without language restriction from database inception to 20 July 2021, and the results of the second Prevention of Syncope Trial were added after it was published on 3 August 2021.9 These included the Medical Literature Analysis and Retrieval System Online (MEDLINE), Excerpta Medica Database (EMBASE), Cochrane Central Register of Controlled Trials (CENTRAL), and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases. The Medical Subject Headings (MeSH) terms and keywords included in the searches were related to ‘vasovagal syncope’ and ‘midodrine hydrochloride’. Database-specific search terms and results are listed in Supplementary material online, Table S1. Additional searches of Google Scholar, ClinicalTrials.gov, and screening of references from relevant articles were performed to identify relevant grey literature.
Study selection
Studies were selected based on the following inclusion criteria: (i) study designs comprised parallel-group or crossover RCTs comparing oral midodrine hydrochloride against a control intervention, whether placebo or non-pharmacological standard care; (ii) patients with recurrent VVS; (iii) outcomes reported included the occurrence of syncope or a predefined syncope surrogate in all patients within the given testing or follow-up period. Inclusion was not limited by blinding procedure, follow-up duration, or patient age at the time of enrolment. The study selection process is shown in Figure 1.
Figure 1.
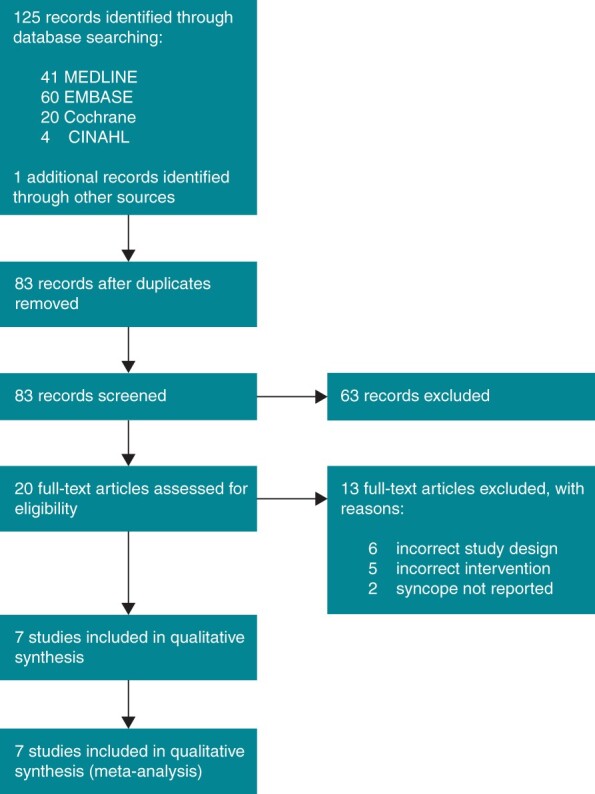
PRISMA flow diagram for systematic literature review and study selection.
Quality of evidence assessments of included studies
The risk of bias of each study was evaluated using the Cochrane Collaboration’s tool for assessing bias in randomized trials.16 The quality of evidence for each outcome was graded using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework.
Outcomes and subgroup analysis
The primary outcome was syncope, defined as a brief and complete loss of consciousness. In studies where the presence of syncope was recorded during both head-up-tilt (HUT) testing and clinical follow-up (two of seven studies),14,15 both event rates were extracted and considered independently during data synthesis. Analysis subgroups were defined based on blinding procedure (open label vs. double-blind).
Statistical analysis
Continuous variables are presented as means with standard deviation, while categorical variables are expressed as percentages. Baseline variables were compared using t test for means and z test for proportions. All tests were two-tailed, and a P-value of <0.05 was considered significant. Meta-analysis was performed using the ‘metafor’ package in R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria). The risks of syncope were expressed as relative risk (RR) ratios with 95% confidence intervals (CIs). Weighted pooled RRs were calculated using random-effects models. Heterogeneity was estimated according to the I2 statistic; values <25% were considered to represent low heterogeneity, 25–50% moderate heterogeneity, and >75% high heterogeneity.17 Where there were more than two independent studies in an analysis group, 95% CIs for I2 values were calculated using the test-based method proposed by Higgins et al.
Results
Study selection and characteristics of included studies
Among 125 unique citations identified by the search strategy, 20 full-text articles were assessed for eligibility and seven studies met inclusion criteria (Figure 1). Of the 13 studies that were excluded, six were not of the specified study design, five did not administer the correct active intervention, and two did not report the outcomes of interest.
All included studies were randomized controlled trials, with two studies performed in paediatric populations14,15 and five performed in adult populations.9–13 Across all trials, a total of 319 patients were included. Study sizes ranged from 12 to 134. Overall, patients were 33 ± 17 years of age and were primarily female (69%). Patients had a minimum of two spontaneous syncopal episodes in the year preceding study enrolment. Of the seven studies selected, two reported the occurrence of HUT-induced syncope (n = 28),10,12 three reported clinical syncope recurrence with continued intervention within a given follow-up period (n = 217),9,11,13 and two reported both HUT-induced and clinical syncope outcomes (n = 70).14,15
Individual characteristics of each study, including design, reported outcomes, and midodrine dosing regimens are summarized in Table 1 and further detailed in Supplementary material online, Table S2. Briefly, Kaufmann et al.12 and Ward et al.10 were double-blind, placebo-controlled, HUT-based crossover trials performed in adult populations. Kaufmann et al.12 included 12 patients (17% male) with a mean age of 42 ± 4 years, more than two syncopal episodes in the year preceding enrolment (range: 2 to >15), and reproducible syncope on drug-free HUT. Patients were randomized to receive a single 5 mg dose of midodrine or matching placebo 1 h prior to passive 60° HUT for up to 40 min, with a single washout day between active study days.12 Midodrine was found to significantly improve orthostatic tolerance with no indication of supine hypertension [RR = 0.25 (0.07, 0.94)].12
Table 1.
Characteristics of included studies
| Study | Study design | Interventions | Sample size | Age in years (mean ± SD) | Previous syncopal episodes | Outcome | Follow-up duration | ||
|---|---|---|---|---|---|---|---|---|---|
| Midodrine | Control | Midodrine | Control | ||||||
| Kaufmann 2002 | Double-blind crossover RCT | 5 mg PO single dose | Matching placebo | 12 | 12 | 42 ± 4 | Range 2 to > 15 | HUT-induced syncope | 3 days |
| Ward 1998 | Double-blind crossover RCT | 5 mg tid PO | Matching placebo | 16 | 16 | 56 ± 18 | Range 2 to 8 | HUT-induced syncope | 2 months |
| Liu 2009 | Open label RCT | 1.25–2.5 mg bid PO | Conventional therapy | 15 | 33 | 11 ± 3 | Not reported | HUT-induced and clinical syncope | 9 months |
| Qingyou 2006 | Open label RCT | 1.25–2.5 mg bid PO | Conventional therapy | 12 | 10 | 12 ± 3 | Range 3 to 40 | HUT-induced syncope or severe presyncopea | 10 months |
| Perez-Lugones 2001 | Open label RCT | 5–15 mg tid PO | Conventional therapy | 31 | 30 | 42 ± 17 | Range 9 to 41 | Clinical Syncope | 12 months |
| Romme 2011 | Double-blind crossover RCT | 5 mg bid PO | Matching placebo | 23 | 23 | 31 ± 12 | IQR 20 to 90 | Clinical syncope | 6 months |
| Sheldon 2021 | Double-blind RCT | 2.5–10 mg tid PO | Matching placebo | 66 | 67 | 36 ± 13 | Midodrine IQR 10 to 100 Placebo IQR 11 to 250 | Clinical syncope | 12 months |
HUT, head-up-tilt; IQR, interquartile range; RCT, randomized controlled trial.
Symptoms of severe pre-syncope accompanied by hypotension or bradycardia.
In contrast, Ward et al.10 included 16 patients (31% male) with a mean age of 56 ± 18 years, a median of four syncopal events in the month preceding enrolment (range: 2–8), and reproducible syncope on HUT with nitroglycerine. Patients were randomized to receive 5 mg of midodrine tid or matching placebo for 1 month prior to 70° HUT with sublingual nitroglycerine, and a 7-day washout phase between treatment periods.10 In this study, midodrine significantly reduced the risk of HUT-induced syncope [RR = 0.43 (0.22, 0.83)].10
Liu et al.14 and Qingyou et al.15 were both open-label, parallel-group trials performed in paediatric populations that reported the occurrence of syncope during both HUT testing and extended clinical follow-up. Liu et al.14 included 33 patients in the control group and 15 patients in the midodrine group (44% male overall) with a mean age of 11 ± 3 years and unexplained recurrent syncope. Patients assigned to the control group received conventional non-pharmacological therapy, while the midodrine group was administered an initial dose of 1.25 mg bid, titrated up to 2.5 mg bid given treatment efficacy within the first 2 weeks of the 9 ± 2 month follow-up period.14 The HUT-induced syncope with treatment was assessed at 4 weeks using an unspecified HUT test protocol.14 Midodrine was not found to reduce the risk of HUT-induced syncope at 4 weeks [RR = 0.39 (0.13, 1.13)], but did significantly reduce the risk of clinical syncope [RR = 0.24 (0.06, 0.92)].14
Qingyou et al.15 enrolled a similar patient demographic, with 13 patients in the control group and 13 patients in the midodrine group (38% male overall) with a mean age of 12 ± 3 years, unexplained recurrent syncope, a positive response to HUT testing, and a lifetime mean of 4 ± 8 syncopal episodes. Patients in the control group received conventional non-pharmacological therapy, while the midodrine group was administered an initial dose of 1.25 mg bid, titrated up to 2.5 mg bid given treatment efficacy on HUT 1 week after study commencement.15 Patients requiring a higher dosage of midodrine were subject to repeat HUT testing at 2 weeks using the same unspecified HUT test protocol as in the first test.15 All study participants continued treatment and were followed-up for a mean of 10 ± 8 months.15 Qingyou et al.15 found that midodrine significantly reduced the risk of both HUT-induced syncope [RR = 0.31 (0.11, 0.87)] and clinical syncope [RR = 0.28 (0.08, 0.98)].
Perez-Lugones et al.13 was an open-label, parallel-group trial that included 30 patients (33% male) in the control group and 31 patients (35% male) in the midodrine group with a mean age of 43 ± 17 years and a lifetime mean of 23 syncopal episodes (range: 9–41). Patients in the control group received conventional non-pharmacological therapy, whereas those in the midodrine group were administered an initial dose of 5 mg tid, titrated up to 15 mg tid given treatment efficacy within the first 3 weeks of the 6 month follow-up period.13 In this study, midodrine was found to significantly reduce the risk of syncope [RR = 0.32 (0.15, 0.70)].13
Finally, Romme et al.11 and Sheldon et al.9 were double-blind, placebo-controlled, clinical trials performed in adult populations. Romme et al.11 was a crossover trial that included 23 patients (17% male) with a mean age of 31 ± 12 years and a lifetime median of 35 syncopal episodes (IQR: 70). Patients were randomized to receive 5 mg of midodrine bid or matching placebo and were followed for 3 months on each study intervention, with 1 week of washout between treatment periods.11 Midodrine was not found to significantly reduce the risk of clinical syncope [RR = 0.73 (0.44, 1.23)].11 Conversely, Sheldon et al.9 was a parallel-group trial that included 67 patients (25% male) randomized to placebo and 66 patients (29% male) randomized to midodrine with a mean age of 36 ± 13 years and a median of six syncopal episodes in the year preceding enrolment (IQR: 3, 20). Patients randomized to the midodrine group were administered an initial dose of 5 mg tid, titrated up to 10 mg tid or down to 2.5 mg tid within the first 2 weeks of treatment, as tolerated, and subsequently followed for 1 year.9 Midodrine significantly reduced likelihood of clinical syncope [RR = 0.69 (0.49, 0.97)].9
Quality of evidence assessments
Using the Cochrane Collaboration’s risk of bias tool, all three open-label studies were determined to have unclear risks of selection bias and high risks of performance bias due to unspecified randomization methods and lack of blinding, respectively (Figure 2).13–15 Liu et al.14 also had a high risk of detection bias due to a combination of its open-label design and lack of clearly stated criteria for appraisal of the primary outcome. All four double-blind trials,9–12 regardless of syncope endpoint, had low risks of bias across the six domains identify by the Cochrane tool.
Figure 2.
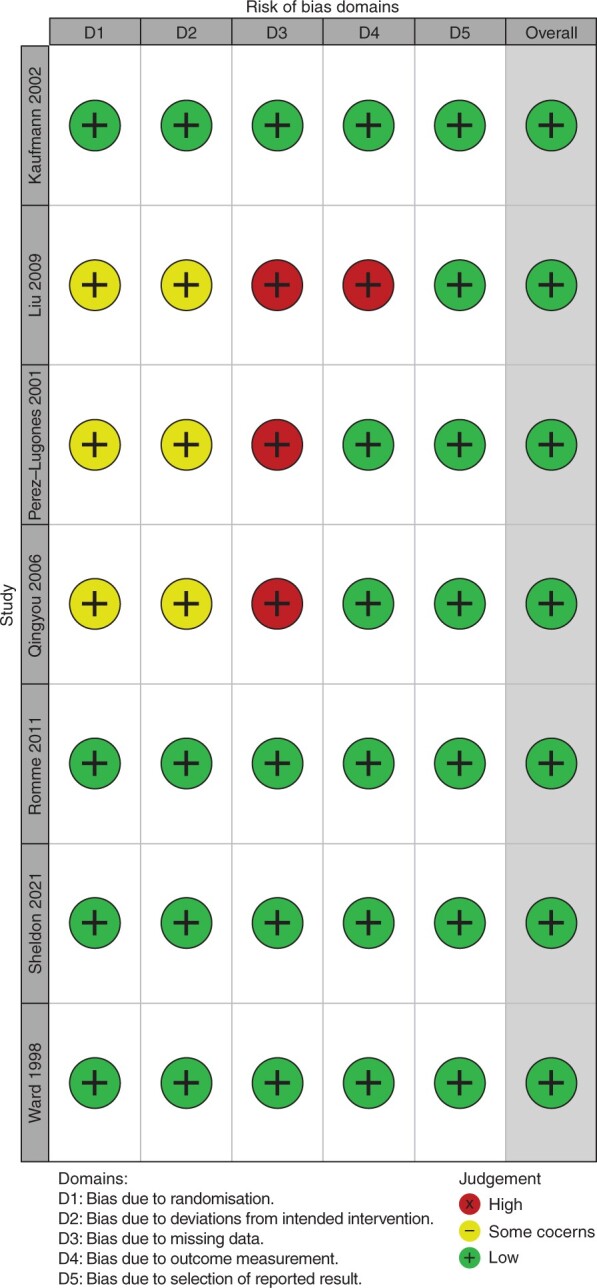
Summary of risk of bias assessments of included randomized controlled trials using the Cochrane Collaboration’s risk of bias tool (green +, low risk of bias; yellow –, unclear risk of bias).
The GRADE quality of evidence assessments for both HUT-induced syncope and clinical syncope outcomes are presented in Supplementary material online, Table S3. There was moderate- to high quality of evidence for each of the pooled subgroup outcomes.
Outcomes
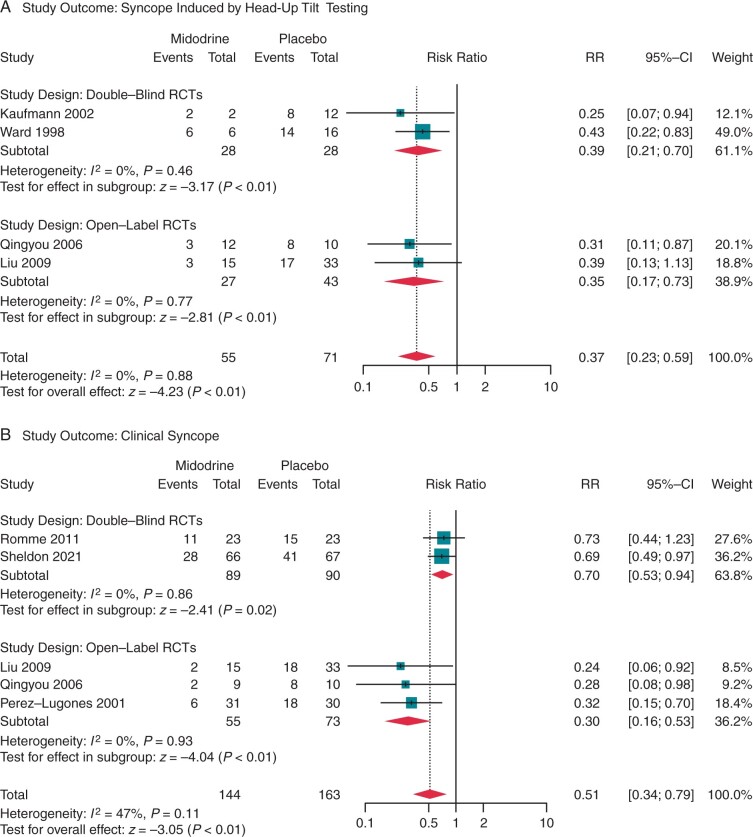
The primary outcome occurred in 210 patients (48%), consisting of 63% (147 of 234 patients) prescribed a control therapy and 32% (63 of 199 patients) that received midodrine. Unique patients were counted twice in studies that reported both HUT-induced and clinical syncope outcomes.14,15 The overall estimate of the pooled RR with midodrine vs. control was 0.47 (95% CI: 0.35–0.64, P < 0.01), with a moderate degree of heterogeneity across the seven studies [I2 = 31% (0–65%), Cochran’s Q P = 0.17]. In a subgroup analysis stratified by type of syncope outcome, heterogeneity was reduced among HUT-induced syncope studies [I2 = 0% (0–68%), P = 0.88; Figure 3A], but remained moderate among clinical syncope studies [I2 = 47% (0–73%), P = 0.11; Figure 3B].
Figure 3.
Random-effects model analysis of the relative risk of (A) head-up-tilt-induced syncope and (B) clinical syncope in patients with recurrent vasovagal syncope treated with midodrine or control therapy.
Syncope induced by head-up-tilt testing
Two open-label14,15 and two double-blind10,12 RCTs reported the occurrence of syncope induced by HUT testing. The relative benefit of midodrine was similar among studies regardless of the blinding procedure [I2 = 0% (0–33%), P = 0.88], and showed a significantly reduced likelihood of positive HUT test outcomes with active drug [RR = 0.37 (0.23–0.59), P < 0.01; Figure 3A]. Midodrine appeared to provide a marginally higher degree of risk reduction in the two open-label RCTs [RR = 0.35 (0.17–0.73), P < 0.01] compared with the two double-blind RCTs [RR = 0.39 (0.21–0.70), P < 0.01].
Clinical syncope
Three open-label13–15 and two double-blind9,11 RCTs reported clinical syncope recurrence within a 6- to 12-month treatment period. Overall, midodrine provided significant protection against clinical syncope [RR = 0.51 (0.34–0.79), P < 0.01; Figure 3B]. The open-label studies suggested that midodrine was highly effective in the prevention of syncope provided continuous use over a follow-up duration of ≥6 months [RR = 0.30 (0.16–0.53), P < 0.01]. In contrast, pooled analysis of the rigorously performed double-blind studies (n = 179) revealed a more modest but still significant syncope risk reduction [RR = 0.70 (0.53–0.95), P = 0.02].
Adverse effects
Midodrine is a reasonably well-tolerated drug in patients without contraindications to its use. The most frequent adverse effect across all studies was supine hypertension, and to a lesser degree, nausea, skin rash, and chills. There were no reports of significant sleep disturbances.
Discussion
Principal findings
We identified seven RCTs that evaluated the efficacy of midodrine to prevent syncope induced by HUT testing and clinical syncope in patients with recurrent VVS. There is moderate- to high-quality evidence to suggest that midodrine reduces the likelihood of syncope whether induced by HUT testing or in clinical settings. The HUT study results are limited by concerns regarding the reproducibility of such tests, and their limited usefulness to predict patient response to pharmacological treatment.3,18,19 Additionally, there was significant heterogeneity among clinical syncope trials [I2 = 47% (0–73%)] owing to different degrees of study blinding. There is a strong possibility of placebo effect in syncope clinical trials,20 which was reiterated by the larger effect estimates among open-label trials [RR = 0.30 (0.16–0.53)] relative to double-blind trials [RR = 0.70 (0.53–0.94); Figure 3B]. Due to the implicit limitations of the open-labels studies and their apparent lack of external validity, it is likely that the two rigorously performed, double-blind clinical trials offer the greatest value in assessing the clinical applicability of midodrine.9,11
Potential mechanism of action
Vasovagal syncope is associated with a withdrawal of sympathetic traffic, resulting in vasodilation, a reduction in venous return and preload, and possibly a reduction in peripheral resistance. The hypotension associated with VVS may be attributed to a failure of arteriolar constriction, venoconstriction, or both. Consequently, midodrine may prevent VVS by causing both arteriolar constriction and venoconstriction, thereby increasing peripheral resistance and cardiac output, and increasing blood pressure.
Clinical considerations
To date, only midodrine has had a clinical trial with a positive primary outcome as well as a positive meta-analysis. Previously studied agents include beta-blockers, norepinephrine reuptake inhibitors, selective serotonin reuptake inhibitors, clonidine, disopyramide, fludrocortisone, verapamil, and α1-adrenergic receptor agonists. Of these, only fludrocortisone significantly reduced its primary clinical outcome,21 and that was in a secondary landmark analysis. In general, placebo-controlled RCTs have failed to show significant drug benefit over placebo in clinical settings.
Midodrine is well-tolerated in most patients, but there is a risk of developing supine hypertension due to the drug’s vasoactive effects. Accordingly, midodrine should not be taken within 4–5 h of sleep, but its short half-life warrants frequent dosing during daytime hours. Additionally, while studies mainly included young females of reproductive age, midodrine should be used with caution in older adults who are more likely to have contraindications to its use such as hypertension, heart failure, liver disease, and diseases prone to cause urinary retention. Use in pregnancy must be avoided due to concerns that it might cause hypertension. It should be prescribed in conjunction with appropriate contraception in females of childbearing potential.
Romme et al.11 noted that only 28 out of 67 patients eligible for midodrine treatment actually received pharmacological crossover therapy. The primary reasons for not starting medication were that patients were either content with the partial reduction in syncopal recurrences or feared medication side effects.11 These findings support the notion that midodrine treatment should be attempted in motivated patients whose symptom severity merits it, despite ongoing non-pharmacological therapies.
Study limitations
There are noteworthy limitations. There was a lack of dose–response data, each of the studies administered a different range of drug, and the pooled analyses included doses of midodrine that ranged from 1.25 mg to 15 mg with varying interdose intervals. Even in studies that featured dose-ranging to an effective yet tolerable level, the data were not reported for each dose.
The patient populations were heterogeneous. The systematic review inclusion criteria required patients to have experienced a minimum of two syncopal episodes in the year preceding enrolment, and most participants reported higher rates of syncope recurrence. This may limit external validity when considering the treatment of many VVS patients with infrequent syncope. Similarly, two of the seven trials were performed in paediatric populations. However, despite inherent differences between paediatric and adult populations, independent trial results were pooled as age demographics introduced minimal heterogeneity (I2 = 0; Figure 3).
The studies generally lasted no longer than 1 year, so long-term benefits (and risks) are unknown. Finally, the literature suggests that there is significant potential for placebo effect in syncope clinical trials, and the results of all four open-label clinical studies introduced the possibility of significant expectation bias. Similarly, studies that reported HUT outcomes are limited by concerns regarding the reproducibility of such tests and their reliability in predicting patient response to therapy in the clinical setting. Thus, while several studies have attempted to examine the efficacy of midodrine for the prevention of syncope, only two robust double-blind clinical trials (n = 179) offer insight into the true effect and utility of this drug in practice.
Conclusions
Midodrine significantly prevents VVS in controlled settings, but its efficacy is much more modest in clinical settings. Even so, midodrine is the only medical therapy for recurrent VVS that is successful with this level of evidence. The large difference in estimates of effectiveness is mirrored by the degree of blinding and limitation of HUT testing in predicting the true patient response to pharmacological therapies.
Supplementary material
Supplementary material is available at Europace online.
Funding
Support was received from the Vanderbilt Institute for Clinical and Translational Research (VICTR), which is funded by the National Institutes of Health (grant no. 5UL1TR002243, Bethesda, MD, USA).
Conflict of interest: S.R.R. is a consultant to Lundbeck LLC and Theravance Biopharma related to neurogenic orthostatic hypotension; Honoraria from Academy for Continued Healthcare Learning for developing CME slides kits on neurogenic orthostatic hypotension; DMSB Chair for a Phase 2 study of an irritable bowel syndrome medication for Arena Pharmaceuticals with compensation. Past-president of the American Autonomic Society without financial compensation. No disclosures related to atrial fibrillation or amiodarone. L.Y.L. and R.S.S. declared none.
Data availability
All data and analytic software are publicly available.
Supplementary Material
Contributor Information
Lucy Y Lei, Department of Cardiac Sciences, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAA02 HRIC Building, 3280 Hospital Drive NW, Calgary, AB T2N 4Z6, Canada.
Satish R Raj, Department of Cardiac Sciences, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAA02 HRIC Building, 3280 Hospital Drive NW, Calgary, AB T2N 4Z6, Canada.
Robert S Sheldon, Department of Cardiac Sciences, Libin Cardiovascular Institute, Cumming School of Medicine, University of Calgary, GAA02 HRIC Building, 3280 Hospital Drive NW, Calgary, AB T2N 4Z6, Canada.
References
- 1. Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W, Van Dijk N.. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol 2006;17:1172–6. [DOI] [PubMed] [Google Scholar]
- 2. Sheldon RS, Grubb BP, Olshansky B, Shen W-KK, Calkins H, Brignole Met al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shen W-K, Sheldon RS, Benditt DG, Cohen MI, Forman DE, Goldberger ZDet al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope. J Am Coll Cardiol 2017;70:e39–110. [DOI] [PubMed] [Google Scholar]
- 4. Jorge JG, Pournazari P, Raj SR, Maxey C, Sheldon RS.. Frequency of injuries associated with syncope in the prevention of syncope trials. Europace 2020;22:1896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jorge JG, Raj SR, Teixeira PS, Teixeira JA, Sheldon RS.. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace 2021;23:1092–9. [DOI] [PubMed] [Google Scholar]
- 6. Ng J, Sheldon RS, Ritchie D, Raj V, Raj SR.. Reduced quality of life and greater psychological distress in vasovagal syncope patients compared to healthy individuals. Pacing Clin Electrophysiol 2019;42:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brignole M, Moya A, De Lange FJ, Deharo JC, Elliott PM, Fanciulli Aet al. ; ESC Scientific Document Group . 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018;39:1883–948. [DOI] [PubMed] [Google Scholar]
- 8. Izcovich A, Gonzalez Malla C, Manzotti M, Catalano HN, Guyatt G.. Midodrine for orthostatic hypotension and recurrent reflex syncope: a systematic review. Neurology 2014;83:1170–7. [DOI] [PubMed] [Google Scholar]
- 9. Sheldon R, Faris P, Tang A, Ayala-Paredes F, Guzman J, Marquez Met al. ; POST 4 investigators . Midodrine for the prevention of vasovagal syncope. Ann Intern Med 2021;174:1349–56. [DOI] [PubMed] [Google Scholar]
- 10. Ward CR, Gray JC, Gilroy JJ, Kenny RA.. Midodrine: a role in the management of neurocardiogenic syncope. Heart 1998;79:45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Romme JJCM, Van Dijk N, Go-Schön IK, Reitsma JB, Wieling W.. Effectiveness of midodrine treatment in patients with recurrent vasovagal syncope not responding to non-pharmacological treatment (STAND-trial). Europace 2011;13:1639–47. [DOI] [PubMed] [Google Scholar]
- 12. Kaufmann H, Saadia D, Voustianiouk A.. Midodrine in neurally mediated syncope: a double-blind, randomized, crossover study. Ann Neurol 2002;52:342–5. [DOI] [PubMed] [Google Scholar]
- 13. Perez-Lugones A, Schweikert R, Pavia S, Sra J, Akhtar M, Jaeger Fet al. Usefulness of midodrine in patients with severely symptomatic neurocardiogenic syncope: a randomized control study. J Cardiovasc Electrophysiol 2001;12:935–8. [DOI] [PubMed] [Google Scholar]
- 14. Liu XY, Wang C, Wu LJ, Hu CY, Lin P, Li MXet al. Efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope [Article in Chinese]. Zhonghua Yi Xue Za Zhi 2009;89:1951–4. [PubMed] [Google Scholar]
- 15. Qingyou Z, Junbao D, Chaoshu T.. The efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. J Pediatr 2006;149:777–80. [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. ; Cochrane Bias Methods Group . The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JPT, Thompson SG, Deeks JJ, Altman DG.. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raviele A, Gasparini G, Di Pede F, Delise P, Bonso A, Piccolo E.. Usefulness of head-up tilt test in evaluating patients with syncope of unknown origin and negative electrophysiologic study. Am J Cardiol 1990;65:1322–7. [DOI] [PubMed] [Google Scholar]
- 19. Moya A, Permanyer-Miralda G, Sagrista-Sauleda J, Carne X, Rius T, Mont Let al. Limitations of head-up tilt test for evaluating the efficacy of therapeutic interventions in patients with vasovagal syncope: results of a controlled study of etilefrine versus placebo. J Am Coll Cardiol 1995;25:65–9. [DOI] [PubMed] [Google Scholar]
- 20. Pournazari P, Sahota I, Sheldon R.. High remission rates in vasovagal syncope: systematic review and meta-analysis of observational and randomized studies. JACC Clin Electrophysiol 2017;3:384–92. [DOI] [PubMed] [Google Scholar]
- 21. Sheldon R, Raj SR, Rose MS, Morillo CA, Krahn AD, Medina Eet al. ; POST 2 Investigators . Fludrocortisone for the prevention of vasovagal syncope: a randomized, placebo-controlled trial. J Am Coll Cardiol 2016;68:1–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and analytic software are publicly available.