Abstract
Septic arthritis of the hand is a serious disease that often results in dysfunction of the joint or even the need to perform amputation of the finger. They rank second in the frequency of occurrence after lesions of the knee joint. Many points concerning the etiology, the timing of the development of cartilage destruction and the development of osteomyelitis, approaches to surgical treatment, the duration of antibiotic therapy, and the start of rehabilitation measures remain the subject of numerous discussions. Based on a search in the PubMed, Web of Science and Google Scholar databases down to 1990-2021, publications on septic arthritis of the hand were found and analyzed. The following inclusion criteria were used in our review: (1) Septic arthritis of the hand; (2) Published in a peer review journal; (3) Written in English; and (4) Full text version available. Studies were excluded if they met any of the following criteria: (1) Letters; (2) Articles published in abstract form only; and (3) Cadaveric studies. Septic arthritis of the hand was characterized by the most frequent damage to the joints of the index and middle fingers (> 50% of cases). Up to 90% of cases, the infection enters the joint as a result of penetrating trauma, animal bites, etc. Staphylococcus aureus became the most frequently isolated microorganism (30%-55%), and its polyantibiotic-resistant form Methicillin-resistant Staphylococcus aureus was found, according to various sources, from 0% to 73% among all isolated Staphylococcus aureus. In arthritis, Pasteurella multocida (6%-11%) is often isolated as a result of animal bites. Articular cartilage destruction in the experiment developed within 24-48 h after infection. In clinical studies, the development of osteomyelitis was noted when treatment was delayed by more than 10 d. X-ray data during the first two weeks were uninformative. Priority of surgical treatment of septic arthritis. Drainage and surgical treatment, and with the development of osteomyelitis, the implementation of arthrodesis. Antibacterial therapy for 2-4 wk and early start of rehabilitation measures. Timely surgical treatment in combination with antibiotic therapy and rehabilitation makes it possible to obtain a positive result in the treatment of septic arthritis of the hand.
Keywords: Septic arthritis, Hand, Staphylococcus aureus, Metacarpophalangeal joint, Interphalangeal joint, Rehabilitation
Core Tip: Septic arthritis of the hand often occurs as a result of penetrating wounds. The most common causative agent is Staphylococcus aureus, often characterized by high antibiotic resistance. Delayed treatment of septic arthritis can lead to cartilage destruction and osteomyelitis. To achieve a positive result in the treatment of septic arthritis of the hand, timely surgery, adequate antibiotic therapy and early rehabilitation are necessary.
INTRODUCTION
The hand is a unique anatomical formation of the human body, that determines its authenticity and individuality. Injuries and diseases of the hand can have the most tragic consequences, depriving the victims of the opportunity to continue their professional activities, causing irreparable cosmetic damage, and often causing disability[1,2]. Among the infectious pathologies of the hand, septic arthritis of the metacarpophalangeal and interphalangeal joints is characterized by particular severity. Delayed or inadequate treatment of these diseases can lead to loss of joint function or even to the need for finger amputation[3,4]. In addition, persistent infection, impaired function, and chronic pain after septic arthritis may warrant arthrodesis or amputation in 50%-75% of patients[1].
PREVALENCE AND CAUSES
The incidence of purulent arthritis of the hand joints is characterized by significant regional differences and ranges from 2 to 12 cases per 100000 people per year[5]. They rank second in prevalence (15%-20%) among purulent arthritis of other locations in adults after lesions of the knee joint[3]. However, according to other data, they are much less common than 5% among septic arthritis patients[6]. Among all hand infections, the proportion of bone and joint lesions ranges from 5% to 18%[3].
The joints of the index and middle fingers are most often affected (more than 50% of cases). Information about the involvement in the inflammatory process of the distal (DIP), proximal (PIP) interphalangeal joints and metacarpophalangeal (MCP) joints is contradictory according to various authors. Some of them indicate a higher frequency of DIP lesions, while others, on the contrary, indicate a more frequent involvement of PIP or MCP in the inflammatory process[2,6,7]. At the same time, it should be noted that the judgments of most authors are based on little clinical material.
As a rule, (85%-90% of all cases) the infection entered the joint directly as a result of tissue damage as a result of a domestic or industrial injury, animal or human bites, or medical manipulations[2]. Cat bites are significantly more likely than dog bites to cause infectious complications. This is due to differences in anatomy and bite mechanism. Dog bites are characterized by a crushing and tearing mechanism associated with naturally blunt dog teeth. The sharp teeth of cats, piercing tissues, leave behind a bacterial trail like an injection needle. This mechanism is characterized by slight tissue damage, contributing to the retention of bacteria in the deep layers, which leads to severe infectious complications[8]. Quite typical is the development of purulent arthritis of the metacarpophalangeal joint in traumatic injury, called clenched fist injury. It is dangerous not only because of tissue contamination with pathogenic microflora from the oral cavity, but also due to possible damage to the extensor tendon, MCP capsule, and metacarpal head[9]. It is possible to spread the infection to the joint with the development of septic arthritis from the surrounding soft tissues with felon, and pyogenic flexor tenosynovitis[10].
Much rare (up to 10%-12%) is the hematogenous route of infection penetration into the joint, and the source in most cases remains unidentified[2,4]. When trauma is not evident, the differential diagnosis should include degenerative arthritis, inflammatory arthritis, crystalline arthropathy, cellulitis and soft tissue abscess[11,12].
MICROBIOLOGY
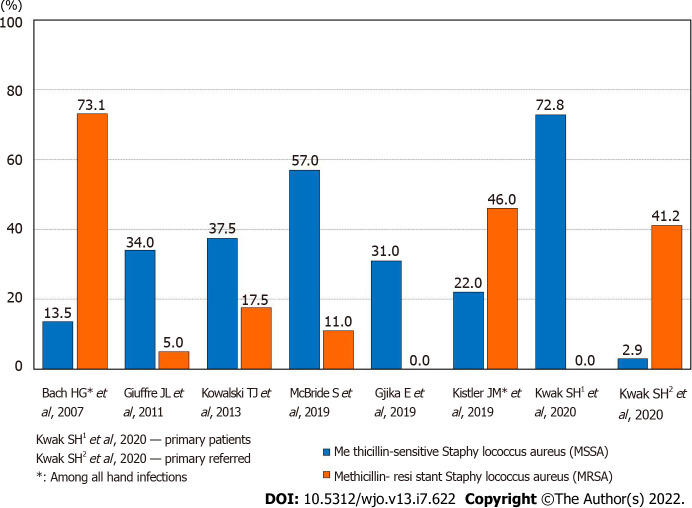
The microbiology of causative agents of septic arthritis of the hand has not been studied in sufficient detail. Data have been published that among the isolated microorganisms in septic arthritis, Staphylococcus aureus prevailed (30%-55%), and among its strains, the methicillin-resistant form was often found[7,13-15] (Figure 1). Various types of Streptococcus were also often isolated (up to 15%), including the most pathogenic Streptococcus pyogenes[16]. The level of gram-negative microorganisms remained relatively high (up to 13%)[3,16]. A feature of the microbial landscape of septic arthritis of the hand was the fairly frequent release of Pasteurella multocida (up to 6%-11%), the causative agent of zoonotic infections, which usually enter tissues with animal bites[7]. With septic arthritis of the fingers, monoinfection prevailed, although cases of isolation of associations of microorganisms (up to 5%-19%) were not uncommon[13,17]. The importance of identifying pathogens was not only in conducting scientific analysis, but also in the possibility of antibacterial therapy, taking into account the sensitivity of the isolated microflora. However, according to researchers, successful isolation of microorganisms in purulent arthritis of the hand occurred in only 50%-70% of cases[2,7,18].
Figure 1.
The frequency of isolated microorganisms of Methicillin-sensitive Staphylococcus aureus and methicillin-resistant staphylococcus aureus.
PATHOGENESIS
Pathogenic microorganisms that have penetrated into the joint produce substances that promote their adhesion and protection from humoral and cellular immunity factors. The multiplication of bacteria leads to the spread of infection. In the initial stage of the immune response of the macroorganism, a cell wall is formed, including macrophages and polymorphonuclear leukocytes. The production of proinflammatory cytokines (interleukin 1-β, interleukin-6, tumor necrosis factor-α, etc.) begins along with the activation of the complement system. If it is impossible to eliminate the infection, the immunological response continues to develop with the formation of byproducts that lead to the release of matrix proteinases and lysosomal enzymes. They, in combination with bacterial toxins, cause the degradation of host collagen. The formed articular effusion disrupts the nutrition of chondrocytes, contributing to the occurrence of cartilage destruction[19-22]. Experimental studies have shown that cartilage surface erosion, degeneration and necrosis of chondrocytes appear starting from 24 h after intra-articular injection of bacteria[23,24]. An in vivo study showed the death of chondrocytes within 48 h of interaction with pathogenic microflora (S. aureus, E. coli)[23,25,26]. Clinical studies indicate that delaying treatment by more than 10 days leads to the development of osteomyelitis[4,27,28]. Other authors note even later periods of osteomyelitis − 1 mo or more after injury[3,29]. Undoubtedly, timely treatment is the most important factor preventing the occurrence of osteochondral destruction in purulent arthritis, which was proven by a study in which, with an average treatment delay of 5.4 d, osteomyelitis was not detected in any case[2]. Similar data are given by M. Sinha et al[10] (2006), who observed 26 patients with hand arthritis. The maximum treatment delay was up to 6 days. At the same time, cartilage destruction was not detected in any case.
CLASSIFICATION
Currently, a specialized classification of purulent arthritis of the hand has not been proposed, which would reflect important parameters such as the destruction of bone and cartilage structures, the tendons of the flexor and extensor of the finger, the presence of paraarticular wounds and fistulas. Septic arthritis of the fingers found some reflection in the classification of hand infections Brown[30] (1978). It includes such forms as: cellulitis, necrotizing fasciitis, paronychia, felon, pyogenic flexor tenosynovitis, deep space infections, septic arthritis, and osteomyelitis. The classification presents only two variants of purulent arthritis, one of which is accompanied by the development of osteomyelitis. Along with this, there is no information about the condition of the surrounding soft tissues, the patient's immune status, or clinical data. The generally accepted and recommended practice is to use the classifications developed for purulent arthritis of large joints. One of the most successful for clinical use is the classification by Tan et al[31] (1998), which includes a number of important parameters:
Joint name
Anatomic type: I: Periarticular soft-tissue infection without pyarthrosis; II: Isolated septic arthritis; III: Septic arthritis with soft-tissue extension, but no osteomyelitis; IV: Septic arthritis with contiguous osteomyelitis.
Host class: A: Normal immune system; B: Compromised system, BL: Local tissue compromise, BS: Systemic immune compromise; C: Risk associated with aggressive treatment unwarranted.
Clinical setting
(1) Less than 5 d of symptoms and nonvirulent organism; and (2) Symptoms for 5 d or more, or a virulent organism.
Clinical stage for the septic joint
Anatomic type + host class + clinical setting = stage.
DIAGNOSTICS
The diagnosis of septic arthritis of the hand is based on a set of clinical, instrumental and laboratory research methods. Among the clinical manifestations, there are symptoms such as pain, swelling, skin hyperemia, dysfunction of the joint, and signs of fluid accumulation in the joint cavity[13]. The severity of clinical symptoms may differ significantly depending on the acute or chronic course of the disease. A number of authors, extrapolating the data used in the classification of periprosthetic infections of large joints, highlight the acute or chronic course of arthritis of the hand. The acute course is associated with a duration of symptoms of < 3 wk with a hematogenous route of infection or < 4 wk with an exogenous source. In a chronic course, symptoms persist for ≥ 3 wk with a hematogenous route of infection of the joint or ≥ 4 wk with a nonhematogenous route[32].
Instrumental diagnostics may include radiography, ultrasound, computed tomography (CT) and magnetic resonance tomography (MRI). X-ray data are usually uninformative in the early stages, which can lead to diagnostic errors[1,3]. Less than 5% of acute cases of osteomyelitis of the hand are recognized on radiographs[33]. Signs characteristic of osteomyelitis, such as osteolysis (70%), osteopenia (10%), osteosclerosis (10%), periosteal reaction (10%), and sequestration (5%), appear on radiographs 2-3 wk after the onset of the disease[8]. Technetium-, gallium-, and indium-labeled white blood cell scans are helpful in identifying acute osteomyelitis before the aforementioned changes can be detected on plain radiographs[33]. Ultrasound examination makes it possible to detect intra-articular effusion, and is also useful for pointing the needle when puncturing small joints of the hand[5,34,35]. MRI is a useful diagnostic tool to visualize joint effusion, its distribution, and the destruction of soft tissues and osteochondral structures of the joint. However, MRI is expensive and usually accompanied by a significant time delay and therefore can only be used in an acute situation to a limited extent[36,37]. CT makes it possible to examine bone structures in more detail than MRI, losing the visualization of soft tissues[5,38]. Both methods are necessary in the chronic course of the inflammatory process[39]. Laboratory diagnostics consisted of conducting a microbiological and cytological study of the articular effusion. Identification of the pathogen and determination of its sensitivity to antibiotics makes it possible to conduct effective antibiotic therapy[2,10,40].
TREATMENT
Recommendations for the treatment of patients with septic arthritis of the small joints of the hand are based on data from retrospective studies and expert opinion. The choice of sanitation method depends on the nature and severity of pathological changes and may include: repeated punctures, arthroscopic drainage and open sanitation[13,41]. Puncture treatment is effective only at the earliest stages of the disease, and arthroscopic sanitation of small joints of the hand is very difficult, so open sanitation of the joint is the most frequently performed surgical intervention[4,42]. In the absence of osteochondral destruction, the concept of continuous catheter irrigation, described by Wright[43] (1943) in the treatment of tendon sheath infections. Timely continuous irrigation of the joint cavity made it possible to prevent the destruction of cartilaginous tissue that accompanies septic arthritis. The authors noted such obligatory moments during irrigation as: axial traction along with passive flexion and extension of the joint[42]. The catheter was removed after the disappearance of edema and other clinical manifestations of inflammation, usually after 2-5 d. The start of rehabilitation was critical to joint function. Another treatment option has also been described: After surgical debridement, a collagen sponge with gentamicin was installed in the joint, and immobilization was performed for a period of 4 wk[44,45].
If signs of infection persist, a second operation is recommended within 24-48 h after the first operation[4,5,12]. There is only fragmentary information regarding the frequency of reoperations for septic arthritis of the hand. A number of authors report that in most cases (up to 80%) patients underwent 2 or more surgical interventions. The most common of these was surgical debridement[2,16,44]. Often, this was associated with suppuration of the paraarticular soft tissues and destruction of the flexor and extensor tendons of the finger[46]. Progression of the infection, despite surgical debridement and antibiotic therapy, may require amputation[1]. Along with this, data on the treatment of septic arthritis of the hand in two hospitals in Switzerland were published, where the number of surgical interventions in 1 patient was 1. At the same time, the average delay in surgical treatment from the onset of the disease was no more than 2-3 d[47].
In cases of detection of destruction of the articular cartilage and osteomyelitis, arthrodesis is recommended to prevent the formation of painful arthrosis or contracture in a functionally disadvantageous position[6,48]. Most of the experts prefer primary arthrodesis of the interphalangeal joints, while others favor secondary arthrodesis 4−6 wk after the primary revision with immobilization with an external fixator and the introduction of a cement spacer with gentamicin[4,13,16,45]. Single reports concern the possibility of using the Masquelet technique in the treatment of septic arthritis of the interphalangeal joints[38,49]. However, given the risk of purulent complications and rejection of the bone autograft, the expediency of such an operation may be questionable.
The course of septic arthritis of the hand in patients with diabetes mellitus is especially severe[9,50]. Published data indicate that the need for arthrodesis increased by 1.7 times. The risk of finger amputation increased even more, by 2.1 times[13]. Stiffness of the interphalangeal joints after purulent arthritis in patients with diabetes mellitus developed in more than 50% of cases, despite early activation and manual therapy started 24 h after surgery[13].
Antibacterial therapy, along with surgical treatment, is an essential component in purulent arthritis of the hand. Taking into account the data obtained in the study of the microbial landscape of purulent arthritis, the main antibacterial drugs used in their treatment are: amoxicillin/clavulanate, clindamycin, levofloxacin, vancomycin, cefazolin, and ceftriaxone[51,52]. Correction of empirical antibiotic therapy is carried out taking into account the results of microbiological studies. Based on an extensive systematic review evaluating the role of antimicrobials in the treatment of bone and joint infections, it was concluded that there is no evidence that any drug is superior to others[53]. However, if the nature of antibiotic therapy in general does not cause controversy among researchers, then its duration remains the subject of numerous discussions. A frequently encountered opinion of experts indicates the need for a course of antibiotic therapy for septic arthritis of the hand lasting approximately 1 mo[16]. As a rule, both the initial parenteral and subsequent oral routes of drug administration are included here[54]. On the other hand, Gjika et al[18] (2019) conducted a prospective randomized study (154 cases), which compared the effectiveness of 2 and 4 wk of antibiotic therapy after surgical treatment of septic arthritis of the hand in adults. The conclusion made proved the absence of any advantages of a 4-wk course over a 2-wk course. According to Kowalski et al[7] (2014) found that the combination of surgical treatment of a purulent focus with parenteral (less than 1 wk) and subsequent oral (2-3 wk) administration of antibacterial drugs is optimal. To date, a combined scheme (parenterally and then orally) for the administration of antibacterial drugs in the treatment of septic arthritis of the hand is optimal, since it is designed for both inpatient and outpatient treatment of patients[18,54].
REHABILITATION
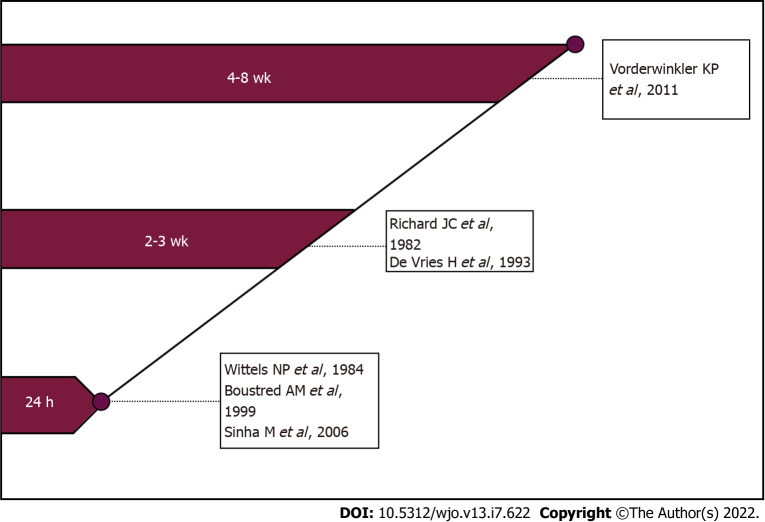
It is known that one of the negative consequences of septic arthritis of the hand is stiffness of the joints[16]. In this regard, the question of the timing of the start of rehabilitation of patients who underwent surgery for septic arthritis of the metacarpophalangeal or interphalangeal joint remains important and controversial[5,7,42]. The duration of postoperative immobilization depends on the presence of articular cartilage destruction and is the subject of numerous discussions. The concept of supporters of early rehabilitation is that it contributes to the restoration of range of motion after inflammation. It is believed that "ideal" rehabilitation should begin 24 h after surgery[10]. Along with these, there is an opinion that it is advisable to apply a splint for several days or external fixation devices for 2-4 wk[4,44] (Figure 2).
Figure 2.
Review of beginning postoperative mobilisation of patients with the hand or wrist septic arthritis.
The most important component in evaluating the results of treatment of septic arthritis of the hand is not only the elimination of the infection but also the restoration of the function of the affected joint, as well as the hand as a whole. Among the currently existing questionnaires and scales, Disabilities of the Arm, Shoulder, and Hand (DASH) and total active motion (TAM) are the most widely used in assessing hand function. Originally published in 1996 in the American Journal of Industrial Medicine, the DASH was a collaborative initiative between the American Academy of Orthopedic Surgeons, the Council of Musculoskeletal Specialty Societies, and the Institute for Work and Health. This outcome measure was designed to be a standardized assessment of the impact on function of a variety of musculoskeletal diseases and injuries in the upper extremities. The DASH is a 30-item self-report questionnaire in which the response options are presented on 5-point Likert scales. Scores range from 0 (no disability) to 100 (most severe disability). This score was designed to be useful in patients with any musculoskeletal disorder of the upper limb[55]. However, this questionnaire is characterized by a significant degree of subjectivity and reflects the function of the hand as a whole to a greater extent than a specific joint or finger.
TAM is described by the American Society for Surgery of the Hand as the sum of active MCP, PIP and DIP arc of motion in degrees of an individual digit. This calculation can then be compared to the TAM of the contralateral hand[56]. The TAM scale is objective and with high accuracy makes it possible to assess the degree of dysfunction of a particular finger. Complementing each other, DASH and TAM make it possible to obtain maximum information characterizing the functional result of the treatment of septic arthritis.
CONCLUSION
Thus, septic arthritis of the hand is a serious disease that can lead to the destruction of articular cartilage and the development of osteomyelitis, which, in turn, leads to loss of joint function or even the need for amputation of the finger. Even isolated septic arthritis is often accompanied by joint stiffness, which negatively affects the function of the hand as a whole. In the treatment of this disease, timely surgical treatment is of decisive importance, which, along with antibacterial therapy and a complex of rehabilitation measures, makes it possible to achieve a positive result.
Footnotes
Conflict-of-interest statement: All the authors declare no conflict of interests for this article.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 27, 2022
First decision: June 16, 2022
Article in press: July 8, 2022
Specialty type: Surgery
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chisthi MM, India; Jahantigh J, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
Contributor Information
Konstantin V Lipatov, Department of General Surgery, Institute of Clinical Medicine named after N.V. Sklifosovsky, Sechenov First Moscow State Medical University (Sechenov University), Moscow 119021, Russia. lipatov_k_v@staff.sechenov.ru.
Arthur Asatryan, Wound and Wound Infection Surgery, State Budgetary Institution “City Clinical Hospital named after S.S. Yudin of Moscow Healthcare Department”, Moscow 115446, Russia.
George Melkonyan, Department of General Surgery, Physician of The Hospital for War Veterans No 3, Moscow 129336, Russia.
Aleksandr D Kazantcev, Department of General Surgery, Institute of Clinical Medicine named after N.V. Sklifosovsky, Sechenov First Moscow State Medical University (Sechenov University), Moscow 119021, Russia.
Ekaterina I Solov’eva, Department of General Surgery, I.M. Sechenov First Moscow State Medical University, Moscow 119048, Russia.
Urii E Cherkasov, Department of General Surgery, I.M. Sechenov First Moscow State Medical University, Moscow 119048, Russia.
References
- 1.McKay P, Formby PM, Dickens J, Gibson M. Osteomyelitis and septic arthritis of the hand and wrist. Curr Orthop Pract. 2010;21:542–550. [Google Scholar]
- 2.Meier R, Wirth T, Hahn F, Vögelin E, Sendi P. Pyogenic Arthritis of the Fingers and the Wrist: Can We Shorten Antimicrobial Treatment Duration? Open Forum Infect Dis. 2017;4:ofx058. doi: 10.1093/ofid/ofx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sendi P, Kaempfen A, Uçkay I, Meier R. Bone and joint infections of the hand. Clin Microbiol Infect. 2020;26:848–856. doi: 10.1016/j.cmi.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Chenoweth B. Septic Joints: Finger and Wrist. Hand Clin. 2020;36:331–338. doi: 10.1016/j.hcl.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Lamou HJ, Kim S, Kuchenbuch C, Thelen S, Eisenschenk A, Hakimi M. Septic Arthritis of the Hand and Wrist. Handchir Mikrochir Plast Chir. 2021;53:290–295. doi: 10.1055/a-1512-0321. [DOI] [PubMed] [Google Scholar]
- 6.Angly B, Steiger R, Zimmerli W. Septic arthritis of finger joints. Handchir Mikrochir Plast Chir. 2007;39:118–123. doi: 10.1055/s-2007-965137. [DOI] [PubMed] [Google Scholar]
- 7.Kowalski TJ, Thompson LA, Gundrum JD. Antimicrobial management of septic arthritis of the hand and wrist. Infection. 2014;42:379–384. doi: 10.1007/s15010-013-0566-0. [DOI] [PubMed] [Google Scholar]
- 8.McDonald LS, Bavaro MF, Hofmeister EP, Kroonen LT. Hand infections. J Hand Surg Am. 2011;36:1403–1412. doi: 10.1016/j.jhsa.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Osterman M, Draeger R, Stern P. Acute hand infections. J Hand Surg Am. 2014;39:1628–1635; quiz 1635. doi: 10.1016/j.jhsa.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Sinha M, Jain S, Woods DA. Septic arthritis of the small joints of the hand. J Hand Surg Br. 2006;31:665–672. doi: 10.1016/j.jhsb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Flevas DA, Syngouna S, Fandridis E, Tsiodras S, Mavrogenis AF. Infections of the hand: an overview. EFORT Open Rev. 2019;4:183–193. doi: 10.1302/2058-5241.4.180082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshy JC, Bell B. Hand Infections. J Hand Surg Am. 2019;44:46–54. doi: 10.1016/j.jhsa.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 13.Giuffre JL, Jacobson NA, Rizzo M, Shin AY. Pyarthrosis of the small joints of the hand resulting in arthrodesis or amputation. J Hand Surg Am. 2011;36:1273–1281. doi: 10.1016/j.jhsa.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 14.Bach HG, Steffin B, Chhadia AM, Kovachevich R, Gonzalez MH. Community-associated methicillin-resistant Staphylococcus aureus hand infections in an urban setting. J Hand Surg Am. 2007;32:380–383. doi: 10.1016/j.jhsa.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Kistler JM, Thoder JJ, Ilyas AM. MRSA Incidence and Antibiotic Trends in Urban Hand Infections: A 10-Year Longitudinal Study. Hand (N Y) 2019;14:449–454. doi: 10.1177/1558944717750921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride S, Mowbray J, Caughey W, Wong E, Luey C, Siddiqui A, Alexander Z, Playle V, Askelund T, Hopkins C, Quek N, Ross K, Orec R, Mistry D, Coomarasamy C, Holland D. Epidemiology, Management, and Outcomes of Large and Small Native Joint Septic Arthritis in Adults. Clin Infect Dis. 2020;70:271–279. doi: 10.1093/cid/ciz265. [DOI] [PubMed] [Google Scholar]
- 17.Houshian S, Seyedipour S, Wedderkopp N. Epidemiology of bacterial hand infections. Int J Infect Dis. 2006;10:315–319. doi: 10.1016/j.ijid.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Gjika E, Beaulieu JY, Vakalopoulos K, Gauthier M, Bouvet C, Gonzalez A, Morello V, Steiger C, Hirsiger S, Lipsky BA, Uçkay I. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomised, non-inferiority trial. Ann Rheum Dis. 2019;78:1114–1121. doi: 10.1136/annrheumdis-2019-215116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanangat S, Postlethwaite A, Hasty K, Kang A, Smeltzer M, Appling W, Schaberg D. Induction of multiple matrix metalloproteinases in human dermal and synovial fibroblasts by Staphylococcus aureus: implications in the pathogenesis of septic arthritis and other soft tissue infections. Arthritis Res Ther. 2006;8:R176. doi: 10.1186/ar2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair R, Schweizer ML, Singh N. Septic Arthritis and Prosthetic Joint Infections in Older Adults. Infect Dis Clin North Am. 2017;31:715–729. doi: 10.1016/j.idc.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Ross K, Mehr J, Carothers B, Greeley R, Benowitz I, McHugh L, Henry D, DiFedele L, Adler E, Naqvi S, Lifshitz E, Tan C, Montana B. Outbreak of Septic Arthritis Associated with Intra-Articular Injections at an Outpatient Practice - New Jersey, 2017. MMWR Morb Mortal Wkly Rep. 2017;66:777–779. doi: 10.15585/mmwr.mm6629a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sultana S, Dey R, Bishayi B. Dual neutralization of TNFR-2 and MMP-2 regulates the severity of S. aureus induced septic arthritis correlating alteration in the level of interferon gamma and interleukin-10 in terms of TNFR2 blocking. Immunol Res. 2018;66:97–119. doi: 10.1007/s12026-017-8979-y. [DOI] [PubMed] [Google Scholar]
- 23.Curtiss PH Jr. The pathophysiology of joint infections. Clin Orthop Relat Res. 1973:129–135. [PubMed] [Google Scholar]
- 24.Roy S, Bhawan J. Ultrastructure of articular cartilage in pyogenic arthritis. Arch Pathol. 1975;99:44–47. [PubMed] [Google Scholar]
- 25.Smith RL, Merchant TC, Schurman DJ. In vitro cartilage degradation by Escherichia coli and Staphylococcus aureus. Arthritis Rheum. 1982;25:441–446. doi: 10.1002/art.1780250413. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Wang L. Novel therapeutic interventions towards improved management of septic arthritis. BMC Musculoskelet Disord. 2021;22:530. doi: 10.1186/s12891-021-04383-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vries H, van der Werken C. Septic arthritis of the hand. Injury. 1993;24:32–34. doi: 10.1016/0020-1383(93)90079-l. [DOI] [PubMed] [Google Scholar]
- 28.Boustred AM, Singer M, Hudson DA, Bolitho GE. Septic arthritis of the metacarpophalangeal and interphalangeal joints of the hand. Ann Plast Surg. 1999;42:623–628; discussion 628. doi: 10.1097/00000637-199906000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Reilly KE, Linz JC, Stern PJ, Giza E, Wyrick JD. Osteomyelitis of the tubular bones of the hand. J Hand Surg Am. 1997;22:644–649. doi: 10.1016/S0363-5023(97)80122-0. [DOI] [PubMed] [Google Scholar]
- 30.Brown H. Hand infections. Am Fam Physician. 1978;18:79–85. [PubMed] [Google Scholar]
- 31.Tan V, Pepe MD, Esterhai JL. Sepsis of the shoulder girdle. In: Disoders of the shoulder: diagnosis and management. Edited by J. Iannotti, G.R. Williams. Philadelphia, Baltimore, New York, London, Buenos Aires, Hong Kong, Sydney, Tokyo. Lippincott Williams and Wilkins, 1998.-P.951-976. [Google Scholar]
- 32.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR Infectious Diseases Society of America. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56:e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- 33.Barbieri RA, Freeland AE. Osteomyelitis of the hand. Hand Clin. 1998;14:589–603, ix. [PubMed] [Google Scholar]
- 34.Hillman D, Rheinboldt M, Petraszko A. Sonographic imaging of hand and wrist injuries: applications in the ER setting. Emerg Radiol. 2019;26:227–240. doi: 10.1007/s10140-018-1649-0. [DOI] [PubMed] [Google Scholar]
- 35.Kim S, Baradia H, Sambasivan A. The Use of Ultrasonography in Expediting Septic Joint Identification and Treatment: A Case Report. Am J Phys Med Rehabil. 2020;99:449–451. doi: 10.1097/PHM.0000000000001284. [DOI] [PubMed] [Google Scholar]
- 36.Yu JS, Habib P. MR imaging of urgent inflammatory and infectious conditions affecting the soft tissues of the musculoskeletal system. Emerg Radiol. 2009;16:267–276. doi: 10.1007/s10140-008-0786-2. [DOI] [PubMed] [Google Scholar]
- 37.Patel DB, Emmanuel NB, Stevanovic MV, Matcuk GR Jr, Gottsegen CJ, Forrester DM, White EA. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics. 2014;34:1968–1986. doi: 10.1148/rg.347130101. [DOI] [PubMed] [Google Scholar]
- 38.Saito T, Noda T, Kondo H, Demiya K, Nezu S, Yokoo S, Matsuhashi M, Uehara T, Shimamura Y, Kodama M, Ozaki T. The Masquelet technique for septic arthritis of the small joint in the hands: Case reports. Trauma Case Rep. 2020;25:100268. doi: 10.1016/j.tcr.2019.100268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Unglaub F, Langer MF, Unglaub JM, Hohendorff B, Müller LP, Hahn P, Löw S, Spies CK. Joint infections of the hand. Unfallchirurg. 2016;119:943–953. doi: 10.1007/s00113-016-0261-6. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy N, Chambers ST, Nolan I, Gallagher K, Werno A, Browne M, Stamp LK. Native Joint Septic Arthritis: Epidemiology, Clinical Features, and Microbiological Causes in a New Zealand Population. J Rheumatol. 2015;42:2392–2397. doi: 10.3899/jrheum.150434. [DOI] [PubMed] [Google Scholar]
- 41.Kwak SH, Bae JY, Oh Y, Jang HS, Ahn TY, Lee SH. Primarily treated patients versus referred patients in the treatment of native septic arthritis of digits: a retrospective comparative study. BMC Musculoskelet Disord. 2020;21:780. doi: 10.1186/s12891-020-03770-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung SR, Kang YC, McGrouther DA. Techniques for Continuous Irrigation of Septic Joints of the Hand. Tech Hand Up Extrem Surg. 2019;23:133–137. doi: 10.1097/BTH.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 43.Wright DA. Tendon sheath infection. Proc R Soc Med. 1943;37:504–505. [Google Scholar]
- 44.Vorderwinkler KP, Mühldorfer M, Pillukat T, van Schoonhoven J. Treatment of bacterial infection in the interphalangeal joints of the hand. Oper Orthop Traumatol. 2011;23:192–203. doi: 10.1007/s00064-011-0024-z. [DOI] [PubMed] [Google Scholar]
- 45.Allieu Y, Chammas M, Hixson ML. External fixation for treatment of hand infections. Hand Clin. 1993;9:675–682. [PubMed] [Google Scholar]
- 46.Richard JC, Vilain R. Acute septic arthritis of the fingers. A clinical study of 87 cases. Ann Chir Main. 1982;1:214–220. doi: 10.1016/s0753-9053(82)80004-5. [DOI] [PubMed] [Google Scholar]
- 47.Rotunno T, Müller C, Heidekrueger P, Gjika E, Gauthier M, Lauper N, Beaulieu J-Y, Erba P, Christen T, Uçkay I. Outcomes of Septic Joint Arthritis of the Hand: A DualCenter Study. Clin Surg. 2019;4:2356. [Google Scholar]
- 48.Spies CK, Hohendorff B, Löw S, Müller LP, Oppermann J, Hahn P, Unglaub F. Arthrodesis of the distal interphalangeal joint using the headless compression screw. Oper Orthop Traumatol. 2017;29:374–384. doi: 10.1007/s00064-017-0507-7. [DOI] [PubMed] [Google Scholar]
- 49.Ono R, Komura S, Hirakawa A, Hirose H, Tsugita M, Masuda T, Ito Y, Akiyama H. Staged arthrodesis using the Masquelet technique for osteomyelitis of the finger with articular destruction: a report of two cases. Arch Orthop Trauma Surg. 2019;139:1025–1031. doi: 10.1007/s00402-019-03197-5. [DOI] [PubMed] [Google Scholar]
- 50.Jalil A, Barlaan PI, Fung BK, Ip JW. Hand infection in diabetic patients. Hand Surg. 2011;16:307–312. doi: 10.1142/S021881041100559X. [DOI] [PubMed] [Google Scholar]
- 51.Clerc O, Prod'hom G, Greub G, Zanetti G, Senn L. Adult native septic arthritis: a review of 10 years of experience and lessons for empirical antibiotic therapy. J Antimicrob Chemother. 2011;66:1168–1173. doi: 10.1093/jac/dkr047. [DOI] [PubMed] [Google Scholar]
- 52.Türker T, Capdarest-Arest N, Bertoch ST, Bakken EC, Hoover SE, Zou J. Hand infections: a retrospective analysis. PeerJ. 2014;2:e513. doi: 10.7717/peerj.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stengel D, Bauwens K, Sehouli J, Ekkernkamp A, Porzsolt F. Systematic review and meta-analysis of antibiotic therapy for bone and joint infections. Lancet Infect Dis. 2001;1:175–188. doi: 10.1016/S1473-3099(01)00094-9. [DOI] [PubMed] [Google Scholar]
- 54.Pinder R, Barlow G. Osteomyelitis of the hand. J Hand Surg Eur Vol. 2016;41:431–440. doi: 10.1177/1753193415612373. [DOI] [PubMed] [Google Scholar]
- 55.Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG) Am J Ind Med. 1996;29:602–608. doi: 10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 56. American Society for Surgery of the Hand. Clinical Assessment Committee Report, IL, Rosemont, 1976. [Google Scholar]