Abstract
The ability to bind, or link, different aspects of an experience in memory undergoes protracted development across childhood. Most studies of memory binding development have assessed extraobject binding between an object and some external element such as another object, whereas little work has examined the development of intraobject binding, such as between shape and color features within the same object. In this work, we investigate the development of intra- and extraobject memory binding in five-year-olds, eight-year-olds, and young adults with a memory interference paradigm. Between two experiments, we manipulate whether stimuli are presented as coherent objects (Experiment 1: n5-year-olds = 32, 19 males, 13 females; n8-year-olds = 30, 15 males, 15 females; nadults = 30, 15 males, 15 females), requiring intraobject binding between shape and color features, or as spatially separated features (Experiment 2: n5-year-olds = 24, 16 males, 8 females; n8-year-olds = 41, 19 males, 22 females; nadults = 31, 13 males, 18 females), requiring extraobject binding. To estimate the contributions of different binding structures to performance, we present a novel computational model that mathematically instantiates the memory binding, forgetting, and retrieval processes we hypothesize to underlie performance on the task. The results provide evidence of substantial developmental improvements in both intraobject and extraobject binding of shape and color features between 5 and 8 years of age, as well as stronger intraobject compared with extraobject binding of features in all age groups. These findings provide key insights into memory binding across early development.
Keywords: computational model, memory binding, memory development, memory interference
Declarative memory undergoes substantial development during childhood (Bauer, 2008; Ofen et al., 2007; Picard et al., 2012). A key component of this developmental change is the ability to bind, or link, different elements of an experience (Brockmole & Logie, 2013; Lee et al., 2016; Lorsbach & Reimer, 2005; Raj & Bell, 2010; Yim et al., 2013). Binding may occur between features within an object, such as its shape and color, or between an object and a separate element, such as its spatial location or another object. We refer to these processes as intraobject and extraobject binding, respectively. In addition to these relatively simple forms of binding, complex or configural binding between more than two elements is also possible, such as between two objects and the context in which they are presented (Humphreys et al., 1989; Sutherland & Rudy, 1989; Yonelinas, 2013). Complex binding is believed to separate highly overlapping memories, thus preventing or attenuating mutual interference among these memories (Darby & Sloutsky, 2015; McClelland et al., 1995; Shanks et al., 1998).
In the current work, we present two experiments, along with a novel computational model, investigating the development of intraobject, extraobject, and complex binding in five-year-olds, eight-year-olds, and adults with a variant of a memory interference paradigm (Darby & Sloutsky, 2015). We begin by discussing these forms of binding across development.
Intraobject and Extraobject and Memory Binding Across Development
Prior work with adults has suggested that intraobject and extraobject memory binding may differ in their attentional demands and underlying neural substrates. Some work has investigated the attentional resources required for intraobject and extraobject binding by manipulating whether features such as shape and color are presented within the same object or are perceptually separated in some way. In one study (van Geldorp et al., 2015), adults were presented with a visual working memory task and were asked to remember pairings of shapes and colors that were either parts of the same object or were spatially separated as transparent shapes alongside color blobs. Adults were less accurate at remembering shape-color associations when features were separated than when they were presented in the same object (for similar findings, see Asch et al., 1960; Walker & Cuthbert, 1998), suggesting that extraobject binding is more difficult than intraobject binding. The authors also manipulated attentional load and found that a concurrent task affected memory performance more when the shapes and colors were spatially separated, suggesting that extraobject binding is more attentionally demanding. Other studies adopting similar stimulus manipulations (Ecker et al., 2007, Ecker et al., 2013) have found evidence that intraobject binding, but not extraobject binding, may be automatic in both working memory and long-term memory (but see Hanna & Remington, 1996; Treisman & Gelade, 1980; Wheeler & Treisman, 2002; for evidence that some attention may be necessary for intraobject binding to occur).
In addition, intra- and extraobject binding may be supported by dissociable neural mechanisms. A great deal of work has suggested that extraobject binding relies on the hippocampus (Davachi, 2006; Giovanello et al., 2004; Hannula & Ranganath, 2008; Lee et al., 2020), whereas intraobject binding may begin in early perceptual areas and involve the perirhinal cortex (Staresina & Davachi, 2008; Zimmer & Ecker, 2010). These differences in attentional demands and neural pathways suggest the possibility that intraobject and extraobject binding may have dissociable properties in early development.
Prior work on memory development has focused primarily on extraobject binding. For example, some studies have demonstrated that the ability to bind an object to a scene improves between 4 and 6 years of age (Lloyd et al., 2009; Sluzenski et al., 2006). Other work has found that binding an object to external elements such as the time the object was presented, its spatial location, or another object does not reach adult-like performance until between 9.5 and 11 years of age (Lee et al., 2016) and that developmental changes in these forms of extraobject binding are related to longitudinal structural changes in the hippocampus (Lee et al., 2020).
By contrast, we are unaware of any studies directly investigating intraobject binding or how it compares to extraobject binding in early development. Although little work has examined these issues, some studies have suggested that memory binding processes may be affected by instructions or strategies that encourage participants to bind features as part of the same item, a process referred to as unitization. One way that unitization can occur is through preexisting semantic associations. For example, associating words that are commonly presented as a compound (e.g., PIN-WHEEL) are more easily associated than unrelated words (e.g., PIN-CLOUD; Giovanello et al., 2006). It has also been suggested that unitization can occur through explicit strategies. For example, participants might be shown a grayscale image or line drawing of an object surrounded by a colored frame and be asked to imagine the object in that color (Staresina & Davachi, 2010). Previous studies have found that training children to use unitization strategies can improve their memory performance. For example, Robey and Riggins (2018) trained 6- and 8-year-olds to imagine a story explaining why objects (depicted as line drawings) would be the color of a surrounding border and found that this strategy improved memory in both age groups relative to a strategy that did not encourage unitization. Given that memory may be improved by strategically treating different elements as part of the same item, and that intraobject binding may be less difficult and attention-demanding than extraobject binding, as discussed above, a reasonable hypothesis is that children will show evidence of stronger intraobject compared with extraobject memory binding. Furthermore, it is possible that intraobject binding may develop earlier and may show relatively little change during childhood.
Memory Binding Complexity
In addition to the distinction between intraobject and extraobject content, memory binding structures can differ in complexity. Whereas binding between two elements, such as between a shape and color or an object and its background, is relatively simple, it is possible to form more complex binding structures, such as between the shape, color, and size of an object, or between two objects along with the context in which they appear together. To form complex binding structures, the representations of multiple entities should be combined into a configural, or conjunctive, representation (O’Reilly & Rudy, 2001; Sutherland & Rudy, 1989). For example, two objects could be jointly associated with context, such as another object or the space where the two objects appeared together. As we discuss below, these more complex binding structures may help protect information from interference from other, overlapping memories (see Darby & Sloutsky, 2015, for evidence and related arguments).
Prior work has suggested that complex memory binding may develop relatively slowly. One study (Yim et al., 2013) used a cued recall design to measure memory binding in 4-year-olds, 7-year-olds, and adults, as well as a multinomial processing tree (MPT) model to estimate to what extent participants in each age group formed different binding structures. Specifically, the model estimated the contributions of item-experiment, item-item, item-context, and complex item-item-context binding structures to memory performance. The results suggested that the simpler binding structures, such as between two items, reach maturity by 7 years of age, whereas the item-context and complex item-item-context binding structures continue to increase after 7 years of age. However, because this work made use of a cued recall paradigm, it remains unclear how complex binding supporting recognition memory develops (see Yim et al., 2018 for evidence of complex binding in recognition memory in adults). In addition, this work considered the development of only extraobject binding, without considering the development of intraobject binding.
Memory Interference and Binding
Memory binding processes may be related to interference effects between similar memories (Darby & Sloutsky, 2015; Hedden & Park, 2003; McClelland et al., 1995; Yim et al., 2013). Before considering this hypothesis, we provide an overview of interference effects and how they may differ across development.
A long history of research suggests that memories are often more difficult to retrieve due to interference from other memories (see Anderson & Neely, 1996; Wixted, 2004; for reviews). Proactive interference occurs when new learning is more difficult as a result of memory for previously learned information, and retroactive interference occurs when retaining what was learned in the past is more difficult due to subsequent learning. Interference effects are often studied with paradigms in which participants learn to associate pairs of items (e.g., words or images) in one phase, and then learn to associate different combinations of the same items in a second phase. Less robust learning of the new combinations in the second phase reflects proactive interference, and reduced memory accuracy for the original combinations of items after the second phase reflects retroactive interference.
Although most work on interference has been performed with adults, developmental work has found evidence that not only are 4- to 7-year-old children susceptible to interference (Benear et al., 2021), they may be more susceptible than adults to both proactive (Yim et al., 2013) and retroactive (Darby & Sloutsky, 2015) interference. There are different mechanisms that could modulate interference and explain this pattern of developmental change.
Some research has suggested that interference may be modulated by inhibiting competitors at retrieval (Anderson, 2003; Anderson et al., 1994; Hulbert & Anderson, 2020). For example, proactive interference could be resolved by temporarily inhibiting previously learned information that competes for retrieval of the newly learned information. Inhibiting this information would presumably make it more difficult to retrieve, producing retroactive interference until an additional process releases the inhibition. Children could be more susceptible to retroactive interference owing to stronger inhibition, although this would imply that children should be less susceptible to proactive interference, which has not been reported in the literature (see Yim et al., 2013, for evidence of proactive interference in children). Relatedly, initially learned information could be permanently unlearned, which would facilitate new learning of similar information but would necessarily impair memory for the first-learned information. Unlearning has sometimes been rejected in theories of memory (Slamecka, 1966), although some recent computational work has explored the possibility of unlearning processes in the context of memory aging (Darby & Sederberg, 2022).
Another possibility is that interference is modulated by memory binding processes. Specifically, complex binding may help reduce interference effects by decreasing the similarity, and hence competition, between memories. This idea may be illustrated with the recognition memory interference paradigm of Darby and Sloutsky (2015). In this paradigm, participants learned to associate combinations of objects with cartoon characters across three phases. In Phase 1, participants learned that AB → X (i.e., objects A and B were associated with character X), CD → X, EF → Y, and GH → Y. In Phase 2, participants learned that AC → Y, BD → Y, IJ → X and KL → X. Two of the associative triplets in each phase were overlapping, in that the same objects (i.e., A, B, C, and D) were recombined and associated with different characters across phases, whereas the other triplets were unique in that the objects were different across phases. In Phase 3, participants were again presented with the initial set of overlapping triplets learned in Phase 1, and faced a conflict: A, B, C, and D had each been associated with both X and Y. Simply binding individual objects to a character in each phase, then, would be expected to produce interference, as each item would be bound to both characters. By contrast, specific pairs of objects were only associated with a single character across phases of the task, such that complex binding of the two objects within each pair along with the character could allow for high performance without interference.
With this paradigm, Darby and Sloutsky (2015) found that 5-year-olds exhibited substantially more retroactive interference than adults in Phase 3, as measured by a greater drop in accuracy for overlapping relative to unique triplets. Because children experienced greater interference, the authors inferred that children likely formed simple binding structures, whereas adults likely formed more complex binding structures. However, this work did not formally characterize the formation of memory binding structures, or developmental differences therein.
Importantly, participants in the Darby and Sloutsky (2015) paradigm learned to predict a character from pairs of objects, and memory for these pairings presumably relied to a large extent on extraobject binding. As discussed above, however, intraobject binding may typically be less attention-demanding and more accurate (Asch et al., 1960; Ecker et al., 2007, 2013; van Geldorp et al., 2015; Walker & Cuthbert, 1998), suggesting that features within the same object may be bound more easily with each other and with other elements in a complex binding structure, potentially reducing interference effects. Prior work has demonstrated that retroactive interference can affect intraobject binding in working memory paradigms (Allen et al., 2006; Logie et al., 2009; Ueno et al., 2011), but we are unaware of work that has directly addressed potential differences in interference between intraobject and extraobject memory binding.
The Present Work
In the present work, we examine intraobject, extraobject, and complex memory binding with a variant of the Darby and Sloutsky (2015) interference paradigm. In this variant, characters are associated not with pairs of objects, but with pairs of shapes and colors. In Experiment 1, the shapes and colors are presented within the same object, requiring intraobject binding, whereas in Experiment 2 the features are spatially separated, requiring extraobject binding. In both experiments, the shapes, colors, and characters are recombined across different phases, creating the potential for interference.
We tested 5-year-olds, 8-year-olds, and adults in both experiments to examine developmental differences in binding and interference. These age groups were chosen because prior work has found evidence of decreases in interference effects between 4- to 5-year-old children and adults (Darby & Sloutsky, 2015; Yim et al., 2013), as well as continued development of complex binding beyond 7 years of age (Yim et al., 2013).
To formally hypothesize how information was learned and retrieved in this task, we developed a novel computational model. The model parameters estimated three kinds of binding: (a) simple binding between shape and color features, (b) simple binding between each separate feature and a cartoon character, and (c) more complex binding between a conjunction of the shape and color features and the character. In addition, the model included a mechanism by which previously learned associations could be forgotten if they conflicted with current learning.
We hypothesized that extraobject binding of spatially separated shapes and colors would be weaker than intraobject binding of these features, and, as a result, memory interference would be greater when extraobject binding is required. Additionally, we expected to find greater developmental differences in extraobject compared with intraobject binding. We begin by examining the development of intraobject binding in experiment 1.
Experiment 1: The Development of Intraobject Binding
The purpose of this experiment was to assess the development of intraobject memory binding in relation to interference effects. Given evidence from prior work that intraobject binding may be less attention-demanding and more accurate compared with extraobject binding (Ecker et al., 2007, 2013; van Geldorp et al., 2015), we expected that developmental differences in binding of intraobject features would be minor. We also expected that recombining the shapes and colors in different phases of the experiment would produce relatively small interference effects.
Method
Participants
Forty-eight 5-year-olds (Mage = 5.08 years, SDage = .19, rangeage = 4.74 – 5.51; 23 females, 25 males), 35 eight-year-olds (Mage = 8.50 years, SDage = .28, rangeage = 8.01–8.99; 18 females, 17 males), and 30 adults (15 females, 15 males) participated in this experiment. The approximate sample sizes were chosen to be comparable to those of a previous study demonstrating developmental differences in interference effects using a similar paradigm (Darby & Sloutsky, 2015). See the Results and Discussion section, below, for a power analysis. Children were tested in local preschools and elementary schools located primarily in middle class neighborhoods of Columbus, Ohio. They were recruited on the basis of returned permission slips and received stickers for participating. Adults were recruited from introductory psychology classes and received partial course credit. This project was approved by The Ohio State University Institutional Review Board Protocol # 2004B042, “Comprehensive protocol for cognitive development research.”
Stimuli
Participants were presented with objects that varied by shape and color. There were eight shapes (e.g., square, circle, triangle) and eight colors (e.g., red, green, blue) that were combined in different ways into objects over the course of the experiment. The combinations of shapes and colors were randomized for every participant. In addition to objects, participants were presented with two cartoon characters (Winnie the Pooh and Mickey Mouse), which were associated with the objects as described below.
Procedure
The experiment was presented with OpenSesame software (Mathôt et al., 2012), on computer screens with a resolution of 1920 × 1080. Children were tested individually in a quiet room in their preschool or elementary school and made responses on a touchscreen. Adults were tested in groups of up to four participants in the lab with standard screens and made responses on a keyboard.
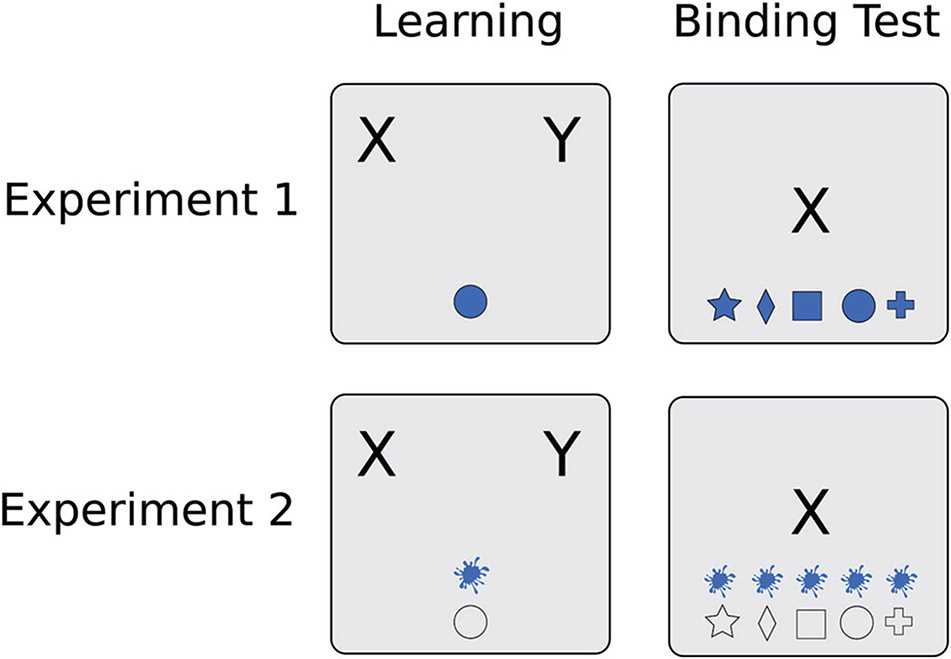
Participants first completed a Learning phase for one set of contingencies (i.e., Set A), in which they learned, with feedback, to associate four objects with one of two cartoon characters across multiple trials (each object was always associated with a single character). Next, participants completed a Learning test phase for Set A without feedback. After this phase, a one-minute break was provided, during which children received a sticker and adults were asked to sit quietly. These Learning and Learning test phase procedures were then repeated for Set B, which included four new contingencies, including two Overlapping contingencies, in which shapes and colors previously seen in Set A were recombined to make new objects that were associated with a different character, as well as two Unique contingencies, involving objects with new shapes and colors (see Figure 1C). Learning and testing of Set B associations may be assessed to measure proactive interference, which we infer from reduced accuracy in Set B compared with Set A, particularly for Overlapping contingencies. After these phases a second break was given. Set A was then revisited with a second Learning test to measure retroactive interference, or reduced memory for overlapping Set A contingencies. Finally, a Binding test phase was administered, which further tested memory for the contingencies learned in both Sets A and B in an interleaved manner. See Figure 1A for an illustration of the sequence of task phases. Details on the procedure of each phase type are provided below.
Figure 1. Design and Stimulus Overview, Experiment 1.
Note. Panel A illustrates the progression of task phases, and Panel B presents sample trials for the Learning, Learning test, and Binding test phases. Panel C shows the stimulus structure of the experiment, with example contingencies shown for Overlapping and Unique objects in Sets A and B. Panel D shows the structure of choices for two examples of the Binding Test, in which Mickey Mouse (X) and the color blue (top), or Winnie the Pooh (Y) and the cross shape (bottom), are provided as cues. See the text for details. Note that participants were shown illustrations of Mickey Mouse and Winnie the Pooh in place of the letters X and Y. C = Color, S = Shape, X = Mickey Mouse, Y = Winnie the Pooh.
Learning Phases.
Prior to the first Learning phase, participants were instructed that they would be shown different objects along with their friends Winnie the Pooh and Mickey Mouse, that each object belonged to one of these friends, and that their job was to figure out whether each object belonged to “Pooh Bear” or Mickey. Therefore, while participants were informed that they needed to learn object-character associations, they were not given instructions on what specific kinds of associations they should form. For example, participants were not informed that they should try to remember the specific shape-color combinations within objects, and they were not told that some of the shapes and colors would be recombined later in the task.
On each trial of the two Learning phases, a single object was shown to the participant, centered near the bottom of the screen, along with two cartoon characters (Winnie the Pooh and Mickey Mouse), which were situated in the top two corners of the screen (see Figure 1B). The position (left or right) of the characters was randomized for each participant but remained consistent throughout the experiment. The task of each Learning trial was to determine whether the presented object belonged to “Pooh Bear” or Mickey. Child participants made responses by touching one of the two characters on the touchscreen monitor; adults responded by pressing the left or right arrow key. After each response was made, feedback was given in the following ways: (a) the correct character appeared above the object, regardless of response accuracy, (b) text appeared giving explicit feedback (e.g., “Awesome, that object belongs to Mickey!”), which was read aloud to children but not adults, (c) a smiling or frowning face was shown for correct and incorrect responses, respectively, and (d) an auditory tone was presented (a high tone for correct responses or a low tone for incorrect responses). Each Learning phase included four blocks of eight trials, for a total of 32 trials; each object was presented twice per block, for a total of eight presentations across the phase.
Learning Test Phases.
The Learning test procedure was identical to that of the Learning phases except for the following changes. First, no feedback was provided on any trial; instead, the characters remained on the screen, but no object was present for an intertrial interval of 200 ms. Additionally, only two blocks of eight trials were presented in each Learning test phase. As in the Learning phases, only one set of objects was tested in each Learning test phase.
Binding Test Phase.
The purpose of the Binding test phase was to further probe the memory binding structures learned in the experiment. On each trial, participants were shown a character (either Winnie the Pooh or Mickey Mouse) in the center of the screen, and five objects positioned along an invisible horizontal line near the bottom of the screen (see Figure 1B). Participants were told that one of the presented objects had belonged to the given character earlier in the task, and that their job was to identify that exact object. Children responded by touching the chosen object on the screen; adults responded by pressing a corresponding number key on the keyboard.
Importantly, one feature (i.e., the shape or color) was given to the participants as a cue, in that every answer choice had the same feature value (e.g., all answer choices were blue). The second feature differed among the choices, requiring memory for an association with the given feature and character; we refer to this as the tested feature. Each object from both sets was tested twice per block; shape was the tested feature on one of these trials, and color the tested feature on the other. The order of trials within each block was randomized for each participant. A total of 32 trials were presented in the phase.
On every trial, one of the object choices was correct (i.e., had been associated with the given character earlier in the task) and the remaining four were incorrect. Three of the incorrect foils always had values of the tested feature that had been part of (a) an Overlapping object (which had been presented during the task but was associated with the other character), (b) a Unique object from Set A, or (c) a Unique object from Set B. For each of these categories, the particular feature value was randomly chosen from the available options for each trial. The fourth foil always had a new feature, randomly selected on each trial from two shapes or two colors that were not shown during the Learning phases. The spatial position of the five response options (referred to as Correct, Overlapping, Unique A, Unique B, and New) was randomized for every trial. See Figure 1D for examples of answer choice arrays.
We designed the Binding test so that participants could make use of different binding structures to narrow their response options. For example, we reasoned that if participants had bound the character to values of a single feature (i.e., shapes or colors), they could exclude response options with a tested feature that had not been associated with the character (e.g., the cross and diamond shapes at the top of Figure 1D). Similarly, intraobject binding of the shape and color would allow the participant to exclude objects with shape-color combinations that had not been seen in the Learning phases (e.g., the blue cross, blue square, and blue diamond in Figure 1D). Notably, shape-color binding would allow perfect accuracy for Unique objects, but not for Overlapping objects, as the Overlapping object foil had been seen previously (e.g., the blue star in Figure 1D), but was associated with the other character. Complex binding of the shape and color within the object as well as the character, however, could be used to correctly identify the correct answer choice in all trials. To quantitatively estimate the extent to which these binding structures were formed by each participant we constructed a computational model, which is summarized in the Results section and presented in detail in the online supplemental materials.
Analyses
We conducted all analyses with hierarchical Bayesian methods, including conventional regression models and our novel computational model. Hierarchical Bayesian approaches allow estimation of model parameters for each age group, while properly accounting for variability between participants. In addition, these methods allow for estimation of posterior distributions of parameter values, which inherently provide information about uncertainty in parameter estimates, such that distributions that are more broad indicate greater uncertainty in the estimates.
In addition to examining posterior distributions of parameter values for each age group, it is possible to compare these distributions between age groups. To do so, we applied a technique to calculate the overlap between two distributions based on kernel density estimates (Pastore & Calcagnì, 2019). Briefly, the overlap metric, , calculates the area shared by two distributions, compared with the total area. For two distributions that are completely nonoverlapping, , whereas for two identical distributions, . This is a continuous measure between 0 and 1, but for ease of exposition we consider <.05 values to be very strong evidence of a difference between groups (Darby & Sederberg, 2022).
Transparency and Openness
We have reported above our sample sizes and how they were determined, as well as the data exclusion procedure. We also reported all experimental manipulations and measures. All analyses were conducted in Python (Van Rossum & Drake, 2011), and all models were implemented with the Python library RunDEMC (https://github.com/compmem/RunDEMC). The study’s design and analyses were not preregistered. The data and model code associated with this study are publicly available at https://osf.io/x9m83/.
Results and Discussion
Three 5-year-olds were excluded because of computer failures, and an additional 5-year-old was excluded for not completing the task. Because children and adults made responses in different ways (a touch screen and keyboard, respectively), we could not analyze response times, but we excluded individual trials with very fast (i.e., < 200 ms) responses from all analyses because they were likely unintentional. The percentage of excluded trials was 1.3% in 5-year-olds, .2% in 8-year-olds, and 0% in adults. After excluding individual trials, we excluded all data from participants who did not perform well in both the Learning and Learning test phases, because it would be difficult to make inferences about interference or memory binding in participants who did not understand the task or were not paying attention. Specifically, for every participant we performed two one-tailed binomial tests. The first test combined all trials from Blocks 2–4 of the Set A Learning phase with Unique trials from Blocks 2–4 of the Set B Learning phase. We excluded the first Block of these phases because we anticipated accuracy near chance when the contingencies were first presented, and we excluded Overlapping trials from Set B because we reasoned that accuracy might be lower in those trials due to proactive interference. The second statistical test combined all trials from the first Learning test of Set A with Unique trials from the Learning test of Set B. The reason for excluding Overlapping trials for Set B was again the possibility of proactive interference. We excluded 12 five-year-olds, five 8-year-olds, and zero adults whose accuracy was not above chance on both binomial tests.1 The final sample included 32 five-year-olds (Mage = 5.08 years, SDage = .20, rangeage = 4.74-5.51; 13 females, 19 males), 30 eight-year-olds (Mage = 8.54 years, SDage = .28, rangeage = 8.05–8.99; 15 females, 15 males), and 30 adults (15 females, 15 males). To examine whether these final sample sizes were appropriate, we conducted a power analysis based on the overall accuracy in the Binding test phase for 5-year-olds and 8-year-olds. Power to detect a difference between five-year-olds and eight-year-olds was 1.00 in this experiment, indicating these sample sizes were more than sufficient to detect an age effect in memory binding accuracy.
The data for this experiment are presented in Figure 2 and Table 1. To analyze the data, we conducted a series of hierarchical Bayesian linear regressions predicting mean accuracy in different task phases. These analyses are presented in full in the Hierarchical Bayesian Regression Models and Regression Model Results sections of the online supplemental materials. Overall, the results suggest strong overlap-specific interference effects in all groups. Specifically, although we found little evidence of proactive interference when learning or being tested on Set B contingencies, we found strong evidence of retroactive interference in the second Learning test for Set A (i.e., in Learning test 2A, following learning and testing of Set B), as accuracy was substantially lower for Overlapping compared with Unique contingencies. Interestingly, accuracy also tended to be lower in Learning test 2A overall. There are multiple reasons why this may have been the case: memories may have decayed across time, participants may have been fatigued at this later stage of the experiment, or there may have been some interference from other contingencies that were not specific to stimulus overlap. We also found strong evidence of interference specifically attributable to stimulus overlap in the Binding test phase, as accuracy was substantially lower for Overlapping compared with Unique contingencies. We also found evidence of lower accuracy overall for 5-year-olds than the older age groups in the Binding test phase. The results of strong interference and developmental differences were somewhat surprising given that the shape and color features were presented within objects and we expected intraobject binding to be relatively strong in all age groups.
Figure 2. Observed and Model-Generated Performance in Experiment 1.
Note. Panels A and B present the task accuracy for the Learning phases and Learning test phases, respectively, in each age group. For the Learning phases, accuracy is shown across blocks for Sets A and B. Panel C presents the proportion of each of the five response options available on every trial of the Binding test phase, for both Overlapping and Unique contingencies. Dashed lines show chance levels of accuracy in each phase. Error bars represent standard errors of the mean. Also shown in each panel is the computational model-generated performance for each phase of the experiment, as indicated by the triangle symbols.
Table 1.
Summary of Performance (i.e., Accuracy) for Overlapping and Unique Contingencies for Each Task Phase, Age Group, and Experiment
| Experiment 1 |
Experiment 2 |
||||||
|---|---|---|---|---|---|---|---|
| Task | Age | 5 | 8 | A | 5 | 8 | A |
| Learn. A | UN | 0.84 (0.13) | 0.85 (0.10) | 0.88 (0.10) | 0.75 (0.12) | 0.84 (0.14) | 0.85 (0.11) |
| OV | 0.83 (0.12) | 0.85 (0.14) | 0.87 (0.11) | 0.77 (0.12) | 0.84 (0.13) | 0.84 (0.13) | |
| Learn. Test A1 | UN | 0.91 (0.15) | 0.95 (0.12) | 0.95 (0.11) | 0.84 (0.14) | 0.94 (0.10) | 0.91 (0.15) |
| OV | 0.92 (0.13) | 0.97 (0.07) | 0.95 (0.13) | 0.83 (0.16) | 0.91 (0.17) | 0.91 (0.15) | |
| Learn. B | UN | 0.79 (0.15) | 0.86 (0.12) | 0.88 (0.10) | 0.83 (0.13) | 0.85 (0.11) | 0.85 (0.10) |
| OV | 0.76 (0.17) | 0.81 (0.14) | 0.85 (0.12) | 0.68 (0.17) | 0.85 (0.12) | 0.82 (0.13) | |
| Learn. Test B1 | UN | 0.85 (0.17) | 0.96 (0.09) | 0.96 (0.11) | 0.88 (0.17) | 0.96 (0.09) | 0.94 (0.12) |
| OV | 0.79 (0.20) | 0.95 (0.08) | 0.94 (0.10) | 0.64 (0.24) | 0.90 (0.20) | 0.94 (0.12) | |
| Learn. Test A2 | UN | 0.81 (0.21) | 0.83 (0.25) | 0.85 (0.27) | 0.80 (0.21) | 0.76 (0.28) | 0.77 (0.25) |
| OV | 0.55 (0.28) | 0.65 (0.29) | 0.72 (0.32) | 0.51 (0.32) | 0.55 (0.33) | 0.51 (0.35) | |
| Binding Test | UN | 0.58 (0.20) | 0.83 (0.20) | 0.90 (0.13) | 0.42 (0.22) | 0.63 (0.26) | 0.54 (0.28) |
| OV | 0.29 (0.14) | 0.53 (0.20) | 0.61 (0.24) | 0.21 (0.14) | 0.37 (0.19) | 0.36 (0.21) | |
Note. Each cell shows the M proportion of correct responses (and SD). Chance accuracy is equal to 0.5 for all Learning and Learning test phases, whereas chance accuracy in the Binding test is 0.2. Learn. = Learning Phase; Learn. Test = Learning Test Phase; UN = Unique contingencies; OV = Overlapping contingencies; 5 = 5-year-olds; 8 = 8-year-olds; A = adults.
However, it is not clear from these results what associations were formed or how these binding structures may have differed between the age groups: participants may have formed simple binding structures between individual shape or color features and the character, between the shapes and colors together within objects, and potentially between both features and the characters, which would considered be a complex binding structure. It is difficult to estimate the extent to which these binding structures were formed with standard statistical models such as regression. Instead, we need a model that predicts both correct responses and various types of error responses on the basis of the underlying representation (i.e., the binding structure). To formally estimate the formation of different binding structures, and developmental differences in these processes, we created a generative computational model.
Computational Model Description
To formally characterize the binding processes that we hypothesized to underlie performance in this experiment, we developed a new computational model (for a full description of the model, see the Generative Computational Model section of the online supplemental materials). The model assumes that participants’ responses were supported by the strength of associations learned during the course of the experiment.
In the model, matrices MFF, MFC, and MFFC store associations between the shape and color features (i.e., feature-feature bindings, or FF), between each separate object feature (shape or color) and a character (i.e., feature-character bindings, or FC), and between a conjunction of both features together within the object and a character (i.e., feature-feature-character binding, or FFC), respectively. These matrices are updated on a trial level to simulate binding processes and probed by memory cues to simulate retrieval.
Each binding structure is bidirectional: for example, as a shape is associated with a character, the character is also associated with the shape (see Figure 3). Associations between different elements are increased in the three matrices on a trial level, scaled by learning rate parameters specific to each type of binding: αFF, αFC, and αFFC. The different binding structures affect the model’s performance in different ways. FF associations do not affect performance in the Learning or Learning test phases, but in the Binding test contribute to Correct responses for Unique contingencies, and to both the Correct and Overlapping response options for Overlapping contingencies. In Experiment 1, intraobject FF binding was encouraged by presenting shapes and colors within the same object, whereas in Experiment 2 the features were spatially separated to encourage extraobject binding (Figure 3, upper panel). FC associations contribute to accurate responses in the Learning and Learning test phases but can also contribute to the inaccurate response for Overlapping contingencies after Set B is introduced; these associations may also be used in the Binding test to increase the probability of choosing response options that have been associated with the given character. Finally, complex FFC associations contribute only to the Correct response for both Overlapping and Unique contingencies in all phases of the task.
Figure 3. Binding Manipulations and Computational Model.
Note. (1) Intraobject binding occurs for features within the same object, as in Experiment 1, whereas extraobject binding occurs for features not in the same object, as in Experiment 2, in which shape and color features were spatially separated. (2) FF binding (whether intraobject or extraobject) occurs in the computational model by forming bidirectional associations between separate representations of the shape and color features (indicated as vectors with different activated slots). (3) FC binding occurs by associating each of the separate shape and color features to the character (and vice versa), whereas (4) FFC binding occurs by associating a unified conjunction of the shape and color features to the character (and vice versa). Note that the conjunction of both features is represented by activating a single slot in the array, such that there is no overlap in this representation even if the individual shape or color features are shared with different objects. Importantly, the mechanisms of the computational model are exactly the same for the two experiments, such that shape and color are represented separately as well as within the conjunction in the model regardless of whether the stimuli encourage intraobject or extraobject FF binding.
In addition to strengthening these types of associations, the model is able to “forget” by weakening previously formed associations that conflict with current learning. For example, when learning Overlapping contingencies in the Learning phase for Set B, each individual object feature has been previously associated with the other character through FC binding, and the model is able to weaken those competing associations to facilitate greater accuracy, and hence reduce proactive interference, while at the same time increasing retroactive interference later when Set A is revisited in Learning test A2. The effect of forgetting, then, is similar to an inhibition process as discussed in the Introduction, although we did not include a mechanism by which forgetting could be reversed other than new learning, whereas inhibition is often considered a temporary phenomenon (Geiselman & Bagheri, 1985). As a result, the forgetting mechanism in the model could be considered an unlearning process. The extent of this forgetting process affecting all binding structures was controlled by one additional parameter, β.
These learning and forgetting processes are defined mathematically in the model. Although the full model equations are provided in the online supplemental materials, we now provide simplified equations to summarize the components of the model. Each matrix is updated on every trial:
where M is one of the three associative matrices and α is the corresponding learning rate (recall that different learning rates are estimated for FC, FF, and FFC binding: αFC, αFF αFFC). The vector representations of two elements (e.g., a shape and color) are denoted as f1 and f2. These elements are associated using an outer product, denoted by the symbol ⊗. Forgetting in the model is controlled by the parameter β, which is the same for all types of binding (FC, FF, and FFC). Forgetting occurs by downweighting the association between each element and the other elements that have been previously associated with it but are not presented on the current trial, denoted fx. For example, if in Set A blue circle and yellow star were presented, when a blue star is presented in the Learning phase for Set B, the blue-star association would be strengthened by new learning, whereas the blue-circle association would be weakened by forgetting. Note that in the full model, learning occurs bidirectionally (e.g., as blue is associated with star, star is associated with blue; see the online supplemental materials for the full model equations).
Finally, r is a single scalar value representing a trial-level novelty signal that changes from trial to trial, tracking how strongly the elements f1 and f2 have already been associated in the past: r = e−f1·(M·f2)+f2·(M·f1)). In this equation, f2 is used as a cue to retrieve an array of previously associated elements from M via a dot product, and then a second dot product “reads out” how strongly f1 in particular has been retrieved. This scalar strength value is then added to a corresponding strength value for the association in the opposite direction (i.e., how strongly f2 has been retrieved by f1). The exponential function is applied to this total strength value. If no learning has occurred for these associations previously, the strengths will be zero, resulting in r = 1, and full learning. However, as the associations become stronger, strength values become larger, and r approaches zero. The result of this mechanism is that learning is reduced for associations that have already been well-learned, which is needed to keep association strengths from growing without bound, as learning takes place over multiple trials.
To simulate decision-making, the model calculates the strength of association between the provided memory cues and possible targets on each trial. Strengths are determined by probing associative matrices with cues: s = (M · fcue) · ftarget. This equation retrieves an array of elements that have been associated with the cue (fcue) and reads out a single strength value (s) for a specific target (ftarget). These strength estimates provide the basis for calculating the probability of each possible response while allowing for competition from other possible responses that could result in interference. This competitive retrieval rule is implemented as a softmax function: . If the strengths supporting all possible choices are the same, the model will predict chance-level performance, but to the extent that a particular choice is supported by greater strength values than other choices, the model will be more likely to make the corresponding choice.
We fit the model to the observed data with four free parameters: αFC, αFF, αFFC, and β. Importantly, the model was not fit to summary statistics, such as the proportion of correct responses in a particular phase, but took into account participants’ choice for each trial in every phase of the experiment. We applied hierarchical Bayesian techniques to fit the model, allowing us to assess age differences with posterior parameter distributions. See the online supplemental materials for additional details on the model and how it was fit to data.
Computational Model Results
To assess model fit, we generated trial-level task performance given each participant’s best-fitting parameter estimates (see Figure 2 to compare observed and model-predicted performance). Despite overestimating proactive interference in the first block of Set B learning in all age groups, the model fit most patterns of performance well across all experimental phases, suggesting that it was able to capture at least some of the processes underlying task performance and how they differed between age groups.
The posterior distributions of hyper-parameters for each age group are shown in Figure 4A. We assess age differences for each parameter by calculating , a measure of distributional overlap described above in the Analyses section. There was no substantial evidence of any age differences in FC binding, estimated with parameter αFC , or forgetting, estimated with β ( > .17). By contrast, we found very strong evidence of weaker FF binding, estimated with parameter αFF, in 5-year-olds compared with both 8-year-olds ( = .005) and adults ( = .001). There was little evidence of a difference between the two older age groups ( = .642). These novel findings suggest strong developmental changes in intraobject binding between 5 and 8 years of age, although this ability may be adult-like by 8 years of age.
Figure 4. Computational Model Parameters, Experiments 1 and 2.
Note. Panel A presents the hyper-parameter posterior distributions for all model parameters fit to the data for Experiment 1, and Panel B shows the corresponding parameter posterior distributions for Experiment 2.
There was also strong evidence of lower values of the complex binding parameter, αFFC, in 5-year-olds compared with both 8-year-olds ( = .010) and adults ( = .002). Although the estimated parameter values tended to be higher in adults compared with eight-year-olds, this difference was not very robust ( = .429). This latter finding was somewhat surprising, because prior work using a recall task has suggested protracted development of complex binding after 7 years of age (Yim et al., 2013). Perhaps, the emerging ability to form complex bindings is more detectable in recognition tasks than in more difficult recall tasks; we return to this issue in the General Discussion. To examine whether all of the model’s mechanisms were necessary to fit the data, we performed a model comparison study in which each parameter was eliminated from the model (i.e., set to zero) while fitting the others to the data, and we found that the full model including all four parameters best fit the data even when accounting for model complexity (see the model Comparison Study section of the online supplemental materials, and see Figure S5 in the same section for how these different models predict different patterns of performance).
Overall, in this experiment we found strong evidence of memory interference effects in all age groups based on hierarchical regression models, along with developmental differences in performance in the Binding Test. Perhaps more importantly, with a novel computational model we found evidence of substantial developmental differences in intraobject binding and complex binding after 5 years of age, but not after 8 years of age. It is not clear from these results, however, how memory binding and interference were affected by presenting features within the same object. Prior work in adults suggests that extraobject binding is more attentionally demanding and is associated with less accurate associative memory performance compared with intraobject binding (Asch et al., 1960; Ecker et al., 2007, 2013; van Geldorp et al., 2015). We hypothesized, then, that spatially separating object features would disrupt binding, especially for young children, which could increase interference effects. We investigated these possibilities in Experiment 2.
Experiment 2: The Development of Extraobject Binding
Method
Participants
Forty-five 5-year-olds (Mage = 5.16 years, SDage = .23, rangeage = 4.80 −5.74; 17 females, 28 males), 43 eight-year-olds (Mage = 8.49 years, SDage = .38, rangeage = 7.74 – 8.99; 23 females, 20 males), and 34 adults (19 females, 15 males) participated in Experiment 2. See the Results and Discussion section, below, for a power analysis. Assignment to this experiment or Experiment 1 was randomized.
Stimuli and Procedure
In this experiment, the shape and color features were not presented together within the same object but were spatially separated (see Figure 5). On each trial, a transparent shape and a blob of color were positioned in vertical alignment, and the relative spatial position (top or bottom) of these features was counterbalanced for each color-shape pairing within each block of every phase. The procedure was identical to that of Experiment 1 except that the stimuli were referred to as “pairs of shapes and colors” instead of objects during instructions and performance feedback.
Figure 5. Stimulus Comparison, Experiments 1 and 2.
Note. Example stimuli are shown for a Learning phase trial (left) and Binding test trial (right) for Experiment 1 (top row) and Experiment 2 (bottom row). Note that participants were shown illustrations of Mickey Mouse and Winnie the Pooh in place of the letters X and Y. X = Mickey Mouse, Y = Winnie the Pooh.
Results and Discussion
One adult participant was excluded for failure to follow task instructions. Two 5-year-olds were excluded because of computer errors, and two additional 5-year-olds were excluded for not completing the experiment. As in Experiment 1, we excluded individual trials with response times faster than 200 ms. This resulted in excluding 1.5%, .2%, and .6% of trials in 5-year-olds, 8-year-olds, and adults, respectively. After excluding these trials, we calculated separate one-tailed binomial tests to ensure above-chance accuracy on Learning and Learning test trials in each participant, as in Experiment 1. We excluded 17 five-year-olds, two 8-year-olds, and two adults who did not pass both of these binomial tests. The final sample included 24 five-year-olds (Mage = 5.25, SDage = .22, rangeage = 4.90–5.74; 8 females, 16 males), 41 eight-year-olds (Mage = 8.49, SDage = .38, rangeage = 7.74–8.99; 22 females, 19 males), and 31 adults (18 females, 13 males). As in Experiment 1, we conducted a power analysis based on overall accuracy in the Binding test phase in 5-year-olds and 8-year-olds, and found that statistical power in this case was .98.
As in experiment 1, we analyzed performance in each phase of the task (presented in Figure 6) with hierarchical Bayesian regression models, the results of which are presented in detail in the online supplemental materials. To summarize, we found strong evidence in all age groups of retroactive interference, and of interference in the Binding Test, as well as evidence in five-year-olds of proactive interference in both the Learning and Learning test phases of the experiment. To gain insight into specific binding structures learned by participants, as well as forgetting, we fit each participant’s performance to the same computational model introduced in Experiment 1.
Figure 6. Observed and Model-Generated Performance in Experiment 2.
Note. The proportion of correct responses for the Learning and Learning test phases are presented in panels A and B, respectively. The proportions of each of the five response types in the Binding test phase are shown in Panel C. Model-generated data are shown as triangles.
Computational Model Results
As in the first experiment, we fit the model with hierarchical Bayesian techniques, and the model provided a qualitatively good fit to the data overall (see Figure 6). Figure 4B presents the posterior distributions of the free parameters of the model. Similar to experiment 1, there was little evidence of age differences in the αFC parameter ( > .47), suggesting comparable feature-character binding across age groups. There was some evidence of less forgetting, estimated by β, in 5-year-olds compared with 8-year-olds and adults ( = .143 and = .125, respectively), although these differences were not very robust.
Also, similar to Experiment 1, there was strong evidence of weaker extraobject shape-color binding, estimated by αFF, in 5-year-olds compared with both 8-year-olds ( = .002) and adults ( = .038). And (also similar to Experiment 1) there was strong evidence of weaker complex binding, estimated by αFFC, in 5-year-olds than 8-year-olds ( = .006), as well as adults ( = .024), whereas there was no evidence of greater complex binding in adults compared with 8-year-olds ( = .827).
Comparison of Performance and Model Parameters With Experiment 1
To address differences in memory binding between Experiment 1, which required only intraobject binding between the shape and color features, and Experiment 2, which required extraobject binding, we directly compared posterior distributions of the regression analyses and novel computational model. To summarize the regression results, presented in the online supplemental materials, we found only weak evidence of differences in proactive and retroactive interference effects, although there was evidence of lower performance overall in the Binding test in Experiment 2. Although we replicated previous findings of strong interference in young children (Darby & Sloutsky, 2015; Yim et al., 2013), older children and adults also exhibited evidence of substantial interference effects in both experiments.
How did the stimulus manipulation affect binding structures and forgetting, as estimated with the computational model? To investigate this, we directly compared model parameters between the two experiments. There were no strong effects of experiment on forgetting (β) or feature-character binding (αFC). By contrast, there was consistent evidence of reduced shape-color binding (αFF) in experiment 2 across age groups ( = .024, = .092, and = .002, in 5-year-olds, 8-year-olds, and adults, respectively). Interestingly, there was also evidence that complex binding between the two features and character was reduced; = .059, = .089, and = .028, in 5-year-olds, 8-year-olds, and adults, respectively.
Importantly, the modeling results suggest weaker binding between extraobject compared with intraobject features, which is in line with previous work using primarily working memory paradigms with adults (Asch et al., 1960; Ecker et al., 2007, 2013; van Geldorp et al., 2015; Walker & Cuthbert, 1998). In addition, separating features in space also decreased estimates of complex binding between conjunctions of these features and an associated cartoon character. Surprisingly, contrary to our hypotheses, we did not find that extraobject binding affected five-year-olds’ memory performance more than that of other age groups, and we did not find strong evidence of greater interference effects in Experiment 2.
General Discussion
This work investigated the development of intraobject, extraobject, and complex memory binding across three age groups: 5-year-olds, 8-year-olds, and adults. Participants learned to associate cartoon characters with different combinations of shapes and colors across phases in a variant of a memory interference paradigm (Darby & Sloutsky, 2015). The stimuli were manipulated across experiments to promote either (a) intraobject memory binding, by presenting the shapes and colors within the same object (Experiment 1), or (b) extraobject binding, by spatially separating the features (Experiment 2). We applied a novel computational model to characterize trial-by-trial learning of three types of binding structures: between the shape and color features, between individual features (shape or color) and the character, and a more complex structure between a conjunction of both features and the character. The model also included a forgetting mechanism that could down-weigh existing associations that conflicted with current learning. Removing any of these mechanisms gave rise to a worse fit to the data overall, even when accounting for model complexity, suggesting that the binding and forgetting processes instantiated in the model were each necessary, and were jointly sufficient, to provide adequate fits to the observed data (see the online supplemental materials for details). The results of the model provided evidence of stronger binding of intraobject features in Experiment 1, as well as stronger complex binding of the two features along with the associated characters, compared with when the features were spatially separated in Experiment 2. We also found strong, novel evidence in Experiment 1 of improvements in intraobject feature binding between 5 and 8 years of age, whereas by 8 years of age this type of binding was comparable to that of adults. A similar developmental pattern was seen for extraobject binding between shape and color features in Experiment 2, as well as complex binding in both experiments. In what follows, we discuss this pattern of results and its implications for memory binding, interference, and development.
Implications for Memory Binding Development
One important finding of this work was substantial improvements in intraobject feature binding after 5 years of age, but not after 8 years of age. This finding was somewhat surprising, because prior work with adults suggests that intraobject binding may be easier and less attentionally demanding than extraobject binding. We had expected to see strong evidence of intraobject binding in all age groups, with larger developmental differences in extraobject binding. Interestingly, however, the αFF parameter estimates in Experiments 1 and 2 were higher in eight-year-olds than in five-year-olds, suggesting improvements in both intra- and extraobject feature binding between 5 and 8 years.
Another possibility, however, is that young children do not have a deficit in binding ability per se but simply did not attend to the objects sufficiently for binding to transpire in this experiment. Indeed, work in adults has suggested that some attention may be necessary to bind features together, even in the same object (Hanna & Remington, 1996; Treisman & Gelade, 1980; Wheeler & Treisman, 2002). Perhaps young children found the association between shape and color features to be less central to the task, and selectively attended to other aspects. For example, young children may have focused more on the link between individual object features and a character (e.g., “the square goes to Mickey”), which could be considered more immediately relevant to the goal of choosing the correct character. According to this explanation, children did not learn the FF associations because they simply did not attend to them. Although differences in attention may have played a role, prior work has demonstrated that preschool-aged children often demonstrate less selective attention than adults, and often show more distributed patterns of attention to many aspects of stimuli, whether they are relevant to the goals of a task or not (Darby et al., 2021; Deng & Sloutsky, 2015, 2016; Plebanek & Sloutsky, 2017). This suggests that it is unlikely that young children demonstrated more selective attention than older children and adults by ignoring the shape-color relations to focus on other aspects of the stimuli. As a result, differences in attention are less likely to explain the developmental improvements in intra- or extraobject feature binding in the current work, although future research should aim to better understand how memory and attention processes contribute to developmental changes in binding.
The pattern of robust improvements between 5- and 8-year-olds, but not between 8-year-olds and adults, was also found for complex binding between the shape and color features, as well as the associated cartoon character. This was somewhat surprising given prior work using a recall paradigm that found differences in complex binding between 7-year-olds and adults (Yim et al., 2013). One reason why evidence for protracted development of complex binding transpired in the previous research using recall tasks, but not in the current research employing recognition tasks, could be task difficulty. If this is the case, then emerging complex binding abilities in eight-year-olds would be more apparent in simpler (i.e., recognition) than in more complex (i.e., recall) tasks. Future work is needed to better understand how memory binding processes could depend on task characteristics.
Although examination of the group-level posterior distributions allowed us to make inferences about developmental changes in latent processes, it is unclear what the relative contributions of the different processes of the model might be to developmental differences in performance. To address this, we performed a simulation study (presented in the online supplemental materials) in which we used the model to generate data for different age groups and assessed the impact of different parameters on developmental differences by holding parameters constant between five-year-olds and the older age groups, one parameter at a time. We found that simulated age differences were most affected by replacing 5-year-olds’ values of the αFF parameter with values from the older age groups. This suggests that the biggest driver of developmental change in these experiments (at least in the Binding test phase) may have been binding between the shape and color features, regardless of whether this binding occurred for intraobject or extraobject features. In the simulations, we also found evidence that complex binding (controlled with parameter αFFC) likely had an impact on developmental changes as well, whereas αFC and the forgetting parameter β had little impact.
This work suggests the possibility of substantial developmental change between 5 and 8 years of age in intraobject memory binding. Future work should examine neural changes supporting the development of intraobject binding. Past work has suggested that the perirhinal cortex is an important contributor to intraobject binding (Staresina & Davachi, 2008; Zimmer & Ecker, 2010). Few studies have addressed the development of this region, but some recent work suggests potential memory-related dissociations between children ages 4–10 and adults in perirhinal cortex activity (Benear et al., 2020). A number of studies have demonstrated developmental changes in the hippocampus (Callow et al., 2020; Daugherty et al., 2016, Daugherty et al., 2017; DeMaster et al., 2014; Tang et al., 2020), which is often associated with relational or extraobject binding (Davachi, 2006; Giovanello et al., 2004; Hannula & Ranganath, 2008; Lee et al., 2020; Staresina & Davachi, 2008), and it would be useful to better understand how developmental changes in the hippocampus and perirhinal cortex relate to intraobject and extraobject binding.
In addition to the important developmental findings, we found evidence of substantial differences between intraobject and extraobject memory binding by comparing αFF parameter estimates between the two experiments. Binding between shape and color features was stronger in all age groups when the features were presented within objects in Experiment 1. This is consistent with prior work suggesting that intraobject binding is more accurate and less attention-demanding than extraobject binding in young and older adults (Asch et al., 1960; Ecker et al., 2007, 2013; van Geldorp et al., 2015; Walker & Cuthbert, 1998), and suggests that this is likely also true early in development. The computational modeling results also suggest a novel finding of stronger complex binding of the shape and color features along with the associated cartoon character, estimated by parameter αFFC, when the features were presented within the same object. One reason for this difference between experiments may be that presenting the shape and color within the same object made it easier to form a conjunction between these features, which could be associated with the character, as was implemented in our computational model. Importantly, the evidence of stronger FF binding as well as complex binding when the shapes and colors were presented in the same object was consistent across age groups, strongly suggesting differences in binding strength for intraobject compared with extraobject features across development.
Between the two experiments, we manipulated the stimuli to encourage intraobject or extraobject binding. However, other work has suggested that unitization strategies can also impact memory binding. For example, Robey and Riggins (2018) trained children to devise stories explaining why an object, presented as a line drawing, would have a particular color, presented as a colored frame. In that study, shapes and colors were spatially separated, as in Experiment 2 of the current work, but children were trained to treat them as features of the same object. This unitization training improved children’s associative memory relative to training that did not encourage unitization. An interesting avenue for future work would be to examine whether unitization training with spatially separated features would result in similar memory binding as we found with perceptually unitized objects in Experiment 1.
Implications for Interference and Forgetting
Contrary to our expectation, we did not find strong evidence of differences in interference between Experiment 1, which required intraobject binding, and Experiment 2, which required extraobject binding. One contributing factor to this may have been the presence of strong interference effects, especially retroactive interference, in Experiment 1, decreasing the likelihood of detecting greater interference in Experiment 2 without greater power. Relatedly, we found strong evidence of retroactive interference in all three age groups, without substantial differences between children and adults, in both experiments. Prior work (Darby & Sloutsky, 2015) found evidence of strong interference in 5-year-olds but relatively little interference in adults. One reason why interference was so strong in the current experiments, even in adults, could be that the stimuli were quite sparse, consisting of simple shapes and colors, whereas past work (Darby & Sloutsky, 2015) made use of perhaps more interesting and semantically meaningful stimuli, including different kinds of animals, clothing, and vehicles. Prior work has demonstrated improved memory for more meaningful over more abstract or unfamiliar stimuli (Asp et al., 2021; Shing et al., 2008), suggesting the possibility that the more abstract stimuli used in the current study may have contributed to stronger interference effects, although future work is needed to examine this possibility more closely.
Although we have focused on memory binding as a modulator of interference, inhibitory or forgetting mechanisms may also play a role (Anderson, 2003; Hulbert & Anderson, 2020). According to this account, previously learned information is inhibited when learning new information, to avoid proactive interference, which can cause retroactive interference when the initial information needs to be retrieved again later. Relatedly, an unlearning process affecting competing information could relieve proactive interference but cause permanent forgetting. In the current work, our computational model included a forgetting component that allowed previously learned information to be forgotten in a way analogous to these accounts, particularly unlearning. Overall, we found evidence that this mechanism improved the fit of the model (see the model comparison study in the online supplemental materials), suggesting that inhibition or unlearning may have played a role in this experiment, but we did not find strong evidence of developmental differences in this process.
Although forgetting may have played a role in these experiments, we caution that the mechanism used in our model was quite simple, with only one free parameter controlling forgetting of all forms of binding, which certainly put this process at a disadvantage compared with new learning in terms of explaining developmental and experiment-specific differences. In addition, our forgetting mechanism differed from inhibition in that forgetting was permanent in our model, making this process more similar to unlearning than inhibition, which is often thought to be a transient process in which inhibition is employed when it is needed for learning or retrieval, and then released (Geiselman & Bagheri, 1985). Overall, we emphasize that the current work was designed to examine memory binding processes, and we do not claim that this work provides a strong test of inhibition-based or unlearning-based accounts of interference or developmental change. We encourage future work to examine these issues more thoroughly.
Other Model Frameworks
Other studies have applied computational modeling to investigate memory binding and its development. Yim et al. (2013) presented an MPT model to estimate the contributions of different kinds of memory binding to cued recall performance, including item-experiment, item-item, item-context, and complex item-item-context binding. Although they are similar in that they both address different kinds of binding structures, the currently presented model differs from the MPT model in key ways. First, the MPT model only addressed performance on the memory test and did not attempt to account for learning. By contrast, the model presented here is able to account for trial-level learning and performance in all phases of the task, not just testing. In addition, the MPT model only estimated contributions of different binding structures to performance and did not consider potential effects of forgetting on learning and testing, as does the current model. Finally, the work by Yim and colleagues addressed only extraobject forms of binding, whereas here we used our model to account for both intraobject and extraobject binding.
The model we presented here is quite simple and abstract. Many more complex models have been put forward to explain memory binding processes in the brain (see Feldman, 2013, for a review). For example, dynamic field theory models have been used to bind visual objects and spatial locations (Bhat et al., 2021; Schneegans et al., 2016). In these models, populations or “fields” of neurons code for different features, such as location or color of an object, and different fields of neurons can be combined to code for associations between features. This idea has been recently extended in the Word-Object Learning via Visual Exploration in Space (WOLVES) model to account for learning and attentional dynamics supporting cross-situational word learning and how they change across development (Bhat et al., 2021). This powerful model integrates dynamic field accounts of word-object associative memory, working memory, and visual attention. In the current model, we did not attempt to link binding directly to the brain or visual attention, instead estimating how associative memory strengths supporting different decisions grow across time and experimental trials. Although there are many differences between WOLVES and the model presented here, one interesting difference is that WOLVES assumes that forgetting takes place due to associations that decay as a function of time. By contrast, in the current model, associations that conflict with current learning are downweighed, irrespective of time. An interesting prospect for future work would be to compare more interference-based forgetting mechanisms such as we implemented in the current model with time-based mechanisms of memory decay.
Limitations
This work is not without limitations. One limitation is that we did not attempt to specifically manipulate or otherwise account for participants’ strategies in the task. It is possible that older children and adults intentionally implemented strategies that were not shared by the youngest age group. For example, older participants may have been more likely to use language to help unitize the items (e.g., “the blue circle belongs to Mickey”), whereas 5-year-olds may have relied more on visual perception of the stimuli. Future work could address this possibility by training participants to use specific strategies (e.g., as in Robey & Riggins, 2018).
In addition, although we did not find evidence of differences in performance or latent binding mechanisms between eight-year-olds and adults, it is possible that our sample sizes were not sufficiently large to detect differences between these age groups. Other work has found protracted improvement on memory binding tasks well beyond 8 years of age (Lee et al., 2016), so the lack of differences between eight-year-olds and adults in the current work should be interpreted with caution. We also note that because the two child age groups and adults responded in different ways (via a touch screen and a keyboard, respectively), we did not analyze response times for this study, although given more comparable methods response times may have provided a more sensitive index of differences between eight-year-olds and adults compared with the responses themselves.
Finally, we have presented the results of one computational model, but there are likely other models that could fit these data as well or better than the current model. An interesting avenue for future work would be to implement other models with different encoding and retrieval mechanisms, as well as models that account for other kinds of data that we did not consider with the current study, such as response times, eye movements, or neural responses.
Conclusions
This work examined the development of intraobject and extraobject memory binding structures of differing complexity in 5-year-olds, 8-year-olds, and adults with an interference design and a novel computational model. We found evidence of stronger binding for features within the same object than when spatially separated in all three age groups, extending prior work in adults (Asch et al., 1960; Ecker et al., 2007, 2013; van Geldorp et al., 2015; Walker & Cuthbert, 1998). We also found novel evidence of substantially weaker intraobject binding in five-year-olds than the older age groups, which suggests that this seemingly simple form of memory binding develops a great deal during childhood, similar to extraobject memory binding (Lee et al., 2016; Raj & Bell, 2010; Sluzenski et al., 2006). Importantly, many of the insights gained in this study would not have been possible with standard statistical analyses, demonstrating the usefulness of computational modeling in developmental science. Overall, this work improves our understanding of memory binding and development.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01HD078545 and P01HD080679 to Vladimir M. Sloutsky. The authors have no conflicts of interest to disclose.
Footnotes
The data and model code that support the findings of this study are available at https://osf.io/x9m83/ (Darby et al., 2022). This study was not preregistered. A portion of this work was presented in a presentation at the 2019 Biennial Meeting of the Society for Research in Child Development.
Although we excluded a relatively high number of participants for low performance in this Experiment and Experiment 2, particularly 5-year-olds, for both experiments the patterns of results are very similar when no performance-based criteria are implemented.
Supplemental materials: https://doi.org/10.1037/dev0001355.supp
References
- Allen RJ, Baddeley AD, & Hitch GJ (2006). Is the binding of visual features in working memory resource-demanding? Journal of Experimental Psychology: General, 135(2), 298–313. 10.1037/0096-3445.135.2.298 [DOI] [PubMed] [Google Scholar]
- Anderson MC (2003). Rethinking interference theory: Executive control and the mechanisms of forgetting. Journal of Memory and Language, 49(4), 415–445. 10.1016/j.jml.2003.08.006 [DOI] [Google Scholar]
- Anderson MC, & Neely JH (1996). Interference and inhibition in memory retrieval. In Ligon Bjork E & Bjork RA (Eds.), Memory (pp. 237–313). Elsevier. 10.1016/B978-012102570-0/50010-0 [DOI] [Google Scholar]
- Anderson MC, Bjork RA, & Bjork EL (1994). Remembering can cause forgetting: Retrieval dynamics in long-term memory. Journal of Experimental Psychology: Learning, Memory, and Cognition, 20(5), 1063–1087. 10.1037/0278-7393.20.5.1063 [DOI] [PubMed] [Google Scholar]
- Asch SE, Ceraso J, & Heimer W (1960). Perceptual conditions of association. Psychological Monographs, 74(3), 1–48. 10.1037/h0093755 [DOI] [Google Scholar]
- Asp IE, Störmer VS, & Brady TF (2021). Greater visual working memory capacity for visually matched stimuli when they are perceived as meaningful. Journal of Cognitive Neuroscience, 33(5), 902–918. 10.1162/jocn_a_01693 [DOI] [PubMed] [Google Scholar]
- Bauer PJ (2008). Toward a neuro-developmental account of the development of declarative memory. Developmental Psychobiology, 50(1), 19–31. 10.1002/dev.20265 [DOI] [PubMed] [Google Scholar]
- Benear SL, Horwath EA, Cowan E, Camacho MC, Ngo C, Newcombe NS, Olson IR, Perlman SB, & Murty VP, Murty VP (2020). Children show adult-like hippocampal pattern similarity for familiar but not novel events. PsyArXiv. 10.31234/osf.io/3d8tv [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benear SL, Ngo CT, Olson IR, & Newcombe NS (2021). Understanding relational binding in early childhood: Interacting effects of overlap and delay. Journal of Experimental Child Psychology, 208, 105152. 10.1016/j.jecp.2021.105152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat AA, Spencer JP, & Samuelson LK (2021). Word-Object Learning via Visual Exploration in Space (WOLVES): A neural process model of cross-situational word learning. Psychological Review. Advance online publication. 10.1037/rev0000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockmole JR, & Logie RH (2013). Age-related change in visual working memory: A study of 55,753 participants aged 8-75. Frontiers in Psychology, 4, 12. 10.3389/fpsyg.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burling JM, & Yoshida H (2017). Highlighting in early childhood: Learning biases through attentional shifting. Cognitive Science, 41 (Suppl. 1), 96–119. 10.1111/cogs.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callow DD, Canada KL, & Riggins T (2020). Microstructural integrity of the hippocampus during childhood: Relations with age and source memory. Frontiers in Psychology, 11, 568953. 10.3389/fpsyg.2020.568953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby KP, & Sederberg PB (2022). Transparency, replicability, and discovery in cognitive aging research: A computational modeling approach. Psychology and Aging, 37(1), 10–29. 10.1037/pag0000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby KP, & Sloutsky VM (2015). The cost of learning: Interference effects in memory development. Journal of Experimental Psychology: General, 144(2), 410–431. 10.1037/xge0000051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby KP, Deng SW, Walther DB, & Sloutsky VM (2021). The development of attention to objects and scenes: From object-biased to unbiased. Child Development, 92(3), 1173–1186. 10.1111/cdev.13469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby KP, Sederberg PB, & Sloutsky V (2022, February 3). Data and code for intra-object and extra-object memory binding across early development. osf.io/x9m83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Bender AR, Raz N, & Ofen N (2016). Age differences in hippocampal subfield volumes from childhood to late adulthood. Hippocampus, 26(2), 220–228. 10.1002/hipo.22517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Flinn R, & Ofen N (2017). Hippocampal CA3-dentate gyrus volume uniquely linked to improvement in associative memory from childhood to adulthood. NeuroImage, 153, 75–85. 10.1016/j.neuroimage.2017.03.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16(6), 693–700. 10.1016/j.conb.2006.10.012 [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, & Ghetti S (2014). Structural development of the hippocampus and episodic memory: Developmental differences along the anterior/posterior axis. Cerebral Cortex, 24(11), 3036–3045. 10.1093/cercor/bht160 [DOI] [PubMed] [Google Scholar]
- Deng WS, & Sloutsky VM (2015). The development of categorization: Effects of classification and inference training on category representation. Developmental Psychology, 51(3), 392–405. 10.1037/a0038749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WS, & Sloutsky VM (2016). Selective attention, diffused attention, and the development of categorization. Cognitive Psychology, 91, 24–62. 10.1016/j.cogpsych.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker UKH, Maybery M, & Zimmer HD (2013). Binding of intrinsic and extrinsic features in working memory. Journal of Experimental Psychology: General, 142(1), 218–234. 10.1037/a0028732 [DOI] [PubMed] [Google Scholar]
- Ecker UKH, Zimmer HD, & Groh-Bordin C (2007). Color and context: An ERP study on intrinsic and extrinsic feature binding in episodic memory. Memory & Cognition, 35(6), 1483–1501. 10.3758/BF03193618 [DOI] [PubMed] [Google Scholar]
- Feldman J (2013). The neural binding problem(s). Cognitive Neurodynamics, 7(1), 1–11. 10.1007/s11571-012-9219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiselman RE, & Bagheri B (1985). Repetition effects in directed forgetting: Evidence for retrieval inhibition. Memory & Cognition, 13(1), 57–62. 10.3758/BF03198444 [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Keane MM, & Verfaellie M (2006). The contribution of familiarity to associative memory in amnesia. Neuropsychologia, 44(10), 1859–1865. 10.1016/j.neuropsychologia.2006.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, & Verfaellie M (2004). A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus, 14(1), 5–8. 10.1002/hipo.10182 [DOI] [PubMed] [Google Scholar]
- Hanna A, & Remington R (1996). The representation of color and form in long-term memory. Memory & Cognition, 24(3), 322–330. 10.3758/BF03213296 [DOI] [PubMed] [Google Scholar]
- Hannula DE, & Ranganath C (2008). Medial temporal lobe activity predicts successful relational memory binding. The Journal of Neuroscience, 28(1), 116–124. 10.1523/JNEUROSCI.3086-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, & Park DC (2003). Contributions of source and inhibitory mechanisms to age-related retroactive interference in verbal working memory. Journal of Experimental Psychology: General, 132(1), 93–112. 10.1037/0096-3445.132.1.93 [DOI] [PubMed] [Google Scholar]
- Hulbert JC, & Anderson MC (2020). Does retrieving a memory insulate it against memory inhibition? A retroactive interference study. Memory, 28(3), 293–308. 10.1080/09658211.2019.1710216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys MS, Bain JD, & Pike R (1989). Different ways to cue a coherent memory system: A theory for episodic, semantic, and procedural tasks. Psychological Review, 96(2), 208–233. 10.1037/0033-295X.96.2.208 [DOI] [Google Scholar]
- Lee JK, Fandakova Y, Johnson EG, Cohen NJ, Bunge SA, & Ghetti S (2020). Changes in anterior and posterior hippocampus differentially predict item-space, item-time, and item-item memory improvement. Developmental Cognitive Neuroscience, 41, 100741. 10.1016/j.dcn.2019.100741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Wendelken C, Bunge SA, & Ghetti S (2016). A Time and Place for Everything: Developmental Differences in the Building Blocks of Episodic Memory. Child Development, 87(1), 194–210. 10.1111/cdev.12447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd ME, Doydum AO, & Newcombe NS (2009). Memory binding in early childhood: Evidence for a retrieval deficit. Child Development, 80(5), 1321–1328. 10.1111/j.1467-8624.2009.01353.x [DOI] [PubMed] [Google Scholar]
- Logie RH, Brockmole JR, & Vandenbroucke ARE (2009). Bound feature combinations in visual short-term memory are fragile but influence long-term learning. Visual Cognition, 17(1-2), 160–179. 10.1080/13506280802228411 [DOI] [Google Scholar]
- Lorsbach TC, & Reimer JF (2005). Feature binding in children and young adults. The Journal of Genetic Psychology, 166(3), 313–327. 10.3200/GNTP.166.3.313-328 [DOI] [PubMed] [Google Scholar]
- Mathôt S, Schreij D, & Theeuwes J (2012). OpenSesame: An open-source, graphical experiment builder for the social sciences. Behavior Research Methods, 44(2), 314–324. 10.3758/s13428-011-0168-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, & O'Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102(3), 419–457. 10.1037/0033-295X.102.3.419 [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, & Rudy JW (2001). Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychological Review, 108(2), 311–345. 10.1037/0033-295X.108.2.311 [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, & Gabrieli JDE (2007). Development of the declarative memory system in the human brain. Nature Neuroscience, 10(9), 1198–1205. 10.1038/nn1950 [DOI] [PubMed] [Google Scholar]
- Pastore M, & Calcagnì A (2019). Measuring distribution similarities between samples: A distribution-free overlapping index. Frontiers in Psychology, 10, 1089. 10.3389/fpsyg.2019.01089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard L, Cousin S, Guillery-Girard B, Eustache F, & Piolino P (2012). How do the different components of episodic memory develop? Role of executive functions and short-term feature-binding abilities. Child Development, 83(3), 1037–1050. 10.1111/j.1467-8624.2012.01736.x [DOI] [PubMed] [Google Scholar]
- Plebanek DJ, & Sloutsky VM (2017). Costs of selective attention: When children notice what adults miss. Psychological Science, 28(6), 723–732. 10.1177/0956797617693005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj V, & Bell MA (2010). Cognitive processes supporting episodic memory formation in childhood: The role of source memory, binding, and executive functioning. Developmental Review, 30(4), 384–402. 10.1016/j.dr.2011.02.001 [DOI] [Google Scholar]
- Robey A, & Riggins T (2018). Increasing relational memory in childhood with unitization strategies. Memory & Cognition, 46(1), 100–111. 10.3758/s13421-017-0748-6 [DOI] [PubMed] [Google Scholar]
- Schneegans S, Spencer JP, & Schöner G (2016). Integrating “what” and “where”: Visual working memory for objects in a scene. In Spencer JP & Schöner G (Eds.), Dynamic thinking: A primer on dynamic field theory (pp. 197–226). Oxford University Press. [Google Scholar]
- Sederberg PB, Howard MW, & Kahana MJ (2008). A context-based theory of recency and contiguity in free recall. Psychological Review, 115(4), 893–912. 10.1037/a0013396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanks DR, Darby RJ, & Charles D (1998). Resistance to interference in human associative learning: Evidence of configural processing. Journal of Experimental Psychology: Animal Behavior Processes, 24(2), 136–150. 10.1037/0097-7403.24.2.136 [DOI] [PubMed] [Google Scholar]
- Shing YL, Werkle-Bergner M, Li S-C, & Lindenberger U (2008). Associative and strategic components of episodic memory: A life-span dissociation. Journal of Experimental Psychology: General, 137(3), 495–513. 10.1037/0096-3445.137.3.495 [DOI] [PubMed] [Google Scholar]
- Slamecka NJ (1966). Differentiation versus unlearning of verbal associations. Journal of Experimental Psychology, 71(6), 822–828. 10.1037/h0023223 [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, & Kovacs SL (2006). Binding, relational memory, and recall of naturalistic events: A developmental perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32(1), 89–100. 10.1037/0278-7393.32.1.89 [DOI] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2008). Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience, 20(8), 1478–1489. 10.1162/jocn.2008.20104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, & Davachi L (2010). Object unitization and associative memory formation are supported by distinct brain regions. The Journal of Neuroscience, 30(29), 9890–9897. 10.1523/JNEUROSCI.0826-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland RJ, & Rudy JW (1989). Configural association theory: The role of the hippocampal formation in learning, memory, and amnesia. Psychobiology, 17(2), 129–144. 10.3758/BF03337828 [DOI] [Google Scholar]
- Tang L, Pruitt PJ, Yu Q, Homayouni R, Daugherty AM, Damoiseaux JS, & Ofen N (2020). Differential functional connectivity in anterior and posterior hippocampus supporting the development of memory formation. Frontiers in Human Neuroscience, 14, 204. 10.3389/fnhum.2020.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman AM, & Gelade G (1980). A feature-integration theory of attention. Cognitive Psychology, 12(1), 97–136. 10.1016/0010-0285(80)90005-5 [DOI] [PubMed] [Google Scholar]
- Turner BM, & Sederberg PB (2012). Approximate Bayesian computation with differential evolution. Journal of Mathematical Psychology, 56(5), 375–385. 10.1016/j.jmp.2012.06.004 [DOI] [Google Scholar]
- Turner BM, & Van Zandt T (2014). Hierarchical approximate Bayesian computation. Psychometrika, 79(2), 185–209. 10.1007/s11336-013-9381-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM, Sederberg PB, Brown SD, & Steyvers M (2013). A method for efficiently sampling from distributions with correlated dimensions. Psychological Methods, 18(3), 368–384. 10.1037/a0032222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Allen RJ, Baddeley AD, Hitch GJ, & Saito S (2011). Disruption of visual feature binding in working memory. Memory & Cognition, 39(1), 12–23. 10.3758/s13421-010-0013-8 [DOI] [PubMed] [Google Scholar]
- van Geldorp B, Parra MA, & Kessels RPC (2015). Cognitive and neuropsychological underpinnings of relational and conjunctive working memory binding across age. Memory, 23(8), 1112–1122. 10.1080/09658211.2014.953959 [DOI] [PubMed] [Google Scholar]
- Van Rossum G, & Drake FL Jr. (2011). The Python language reference manual. Network Theory. [Google Scholar]
- Walker P, & Cuthbert L (1998). Remembering visual feature conjunctions: Visual memory for shape-colour associations is object-based. Visual Cognition, 5(4), 409–455. 10.1080/713756794 [DOI] [Google Scholar]
- Weigard AS, Sathian K, & Hampstead BM (2020). Model-based assessment and neural correlates of spatial memory deficits in mild cognitive impairment. Neuropsychologia, 136, 107251. 10.1016/j.neuropsychologia.2019.107251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, & Treisman AM (2002). Binding in short-term visual memory. Journal of Experimental Psychology: General, 131(1), 48–64. 10.1037/0096-3445.131.1.48 [DOI] [PubMed] [Google Scholar]
- Wixted JT (2004). The psychology and neuroscience of forgetting. Annual Review of Psychology, 55, 235–269. 10.1146/annurev.psych.55.090902.141555 [DOI] [PubMed] [Google Scholar]
- Yim H, Dennis SJ, & Sloutsky VM (2013). The development of episodic memory: Items, contexts, and relations. Psychological Science, 24(11), 2163–2172. 10.1177/0956797613487385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim H, Osth AF, Sloutsky VM, & Dennis SJ (2018). Evidence for the use of three-way binding structures in associative and source recognition. Journal of Memory and Language, 100, 89–97. 10.1016/j.jml.2018.02.002 [DOI] [Google Scholar]
- Yonelinas AP (2013). The hippocampus supports high-resolution binding in the service of perception, working memory and long-term memory. Behavioural Brain Research, 254, 34–44. 10.1016/j.bbr.2013.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer HD, & Ecker UKH (2010). Remembering perceptual features unequally bound in object and episodic tokens: Neural mechanisms and their electrophysiological correlates. Neuroscience and Biobehavioral Reviews, 34(7), 1066–1079. 10.1016/j.neubiorev.2010.01.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.