Abstract
Leptospirosis is an environmentally transmitted zoonotic disease caused by pathogenic Leptospira spp. that affects poor communities worldwide. In urban slums, leptospirosis is associated with deficient sanitary infrastructure. Yet, the role of sewerage in the reduction of the environmental contamination with pathogenic Leptospira has not been explored. Here, we conducted a survey of the pathogen in soils surrounding open and closed sewer sections in six urban slums in Brazil. We found that soils surrounding conventionally closed sewers (governmental interventions) were 3 times less likely to contain pathogenic Leptospira (inverse OR 3.44, 95% CI = 1.66–8.33; p < 0.001) and contained a 6 times lower load of the pathogen (0.82 log10 units difference, p < 0.01) when compared to their open counterparts. However, no differences were observed in community-closed sewers (poor-quality closings performed by the slum dwellers). Human fecal markers (BacHum) were positively associated with pathogenic Leptospira even in closed sewers, and rat presence was not predictive of the presence of the pathogen in soils, suggesting that site-specific rodent control may not be sufficient to reduce the environmental contamination with Leptospira. Overall, our results indicate that sewerage expansion to urban slums may help reduce the environmental contamination with the pathogen and therefore reduce the risk of human leptospirosis.
Keywords: leptospirosis, sewer, public health, environment, fecal pollution
Graphical Abstract
INTRODUCTION
Leptospirosis is a neglected zoonotic disease that affects urban and rural communities worldwide1 with an estimated annual burden of over 1 million cases and approximately 60 000 deaths.2 Its clinical manifestations range from asymptomatic or a mild flu-like illness to severe disease such as Weil’s disease and pulmonary hemorrhagic syndrome for which fatality rates are higher than 10% and 50%, respectively.3,4 Leptospirosis is caused by pathogenic spirochetes from the genus Leptospira. Pathogenic Leptospira thrive in the kidneys of a wide variety of animals, some of which are chronic carriers, and are released with the urine into the environment at high concentrations5,6 where they can survive for an extended time.7,8 Human infection occurs through contact with previously contaminated water and soil or by exposure of cuts and abraded skin with animal urine, making leptospirosis an environmentally transmitted disease.1
Leptospirosis has historically been an occupational disease related to livestock raising, mining, rice farming, and other agricultural activities,9 but in the last 30 years it has emerged as an epidemic in urban communities surrounding cities in developing countries.10–13 In these neglected settings, poverty, deficient housing, and trash accumulation create the ecological conditions for the proliferation of rodents, particularly Rattus norvegicus, which are the primary reservoirs of pathogenic Leptospira in urban environments.14,15 Extreme weather events and seasonal periods of heavy rainfall increase the presence of the pathogen in the environment16,17 and the likelihood of human exposure to contaminated water, soil, and mud due to inadequate sewer and storm drainage infrastructure.18,19 Indeed, cross-sectional and prospective epidemiological studies have identified open sewers and drainage as risk factors for Leptospira infection in urban slums.19–22 As the population living in urban slums is predicted to reach 2 billion by 2025,23 the burden of leptospirosis is only expected to increase.18 There is, therefore, an urgent need to develop control measures for leptospirosis in resource-poor urban settings.
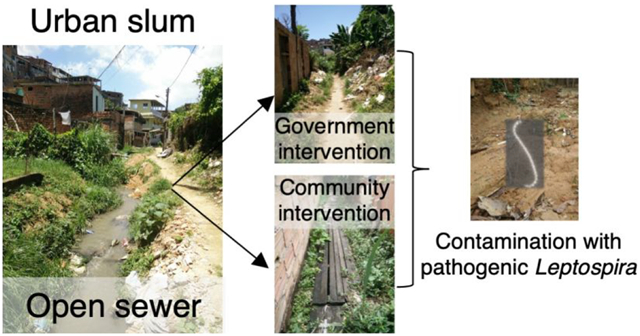
Sanitary interventions to close open sewers are an alternative to reduce exposure to environmental sources of Leptospira16,24 given the lack of efficacious vaccines for human use1,25 and the limited success of rodent control strategies due to regrowth after extermination.26,27 Sewerage construction is widely recognized to reduce the incidence of viral, bacterial, and parasitic diseases.28–30 However, its effect on the reduction of pathogenic Leptospira contamination has not been examined. Here, we aimed to determine the effect of sewerage in the environmental contamination with pathogenic Leptospira in urban slums. To this end, we performed a cross-sectional study in soils surrounding open and closed sewer sections in six Brazilian urban slums. The evaluation of the effect of sewerage in preventing environmental contamination with the pathogen is critical to inform public health interventions aimed to reduce the burden of leptospirosis in these neglected urban communities.
MATERIALS AND METHODS
Study Sites.
We conducted this study in six sites located in five urban slum settlements (favelas) in the periphery of the city of Salvador (Brazil). The incidence of severe leptospirosis in urban slums in Salvador is 19.8 cases per 100 000 inhabitants.20 The communities studied were Pau da Lima (sites 1 and 6), Sete de Abril (site 2), Campinas de Pirajá(site 3), Tancredo Neves (site 4), and Nova Constituinte (site 5) (Figure 1A). All of these slums have similar characteristics of poverty, overcrowding, marginalization, poor-quality housing, and lack of reliable sanitation infrastructure than other slum settlements in Brazil and other developing countries.23,31 Specifically, the deficient sanitation system results in untreated sewage and stormwater drainage flowing through open sewers across these communities. In each community, we selected one site containing a sewer with contiguous open and closed sections (Figure 1B and 1D). The selected sites were similar regarding the presence of impervious surfaces, exposed soil and mud, and the presence of vegetation and trash. Closed sections were classified as conventional or community based depending on the type of closing. Conventional closings (sites 1–4) were built by the local government sewage company by digging trenches and placing sewer mains to which every house drain was connected (Figure 1D). Conventional closings isolated the sewer and prevented sewage from contaminating the surrounding environment. Community closings (sites 5 and 6) had been performed informally by the local dwellers and consisted of wood planks or concrete boards placed on top of the open sewer (Figure 1E). Community interventions prevented major spills from the sewer but did not avoid leaking or major overflowing during rainfall events. In all sites, the open section was located downstream of the closed section.
Figure 1.

Distribution of sampling sites in the study area, and typology of sewer closing. (A) Map of the city of Salvador (Brazil) with the locations of the six urban slum communities where soil collection was performed: Pau da Lima (sites 1 and 6), Sete de Abril (site 2), Campinas de Pirajá (site 3), Tancredo Neves (site 4), and Nova Constituinte (site 5). (B) Open sewer section in site 1. (C) Soil sampling points (red dots) in the soil at an open sewer section. (D) Conventionally closed sewer section in site 2. (E) Community closed sewer section in site 5. (F) Soil sampling points (red dots) in the soil at the closed sewer section.
Sampling Design and Sample Collection.
At each site, closed and open contiguous areas containing exposed soil within a 12 m distance to the main sewer were demarcated, georeferenced, and entered in a GIS database. Polygons of 150–220 m2 were drawn in each closed and open area separated by approximately 20 m, and 24 collection points were randomly selected using a packing density of 0.4 with corresponding minimum distances between collection points for each area (Figure 1C and 1F). Because of size constraints, only 16 collection points were selected in site 6. In total, 272 collection points were selected, 136 in open and 136 in closed sewer areas.
All samples were collected on December 1, 2018, during the typically less rainy season in Salvador (Brazilian Institute of Meteorology). During the collection period, no big rainfall events (>20 mm/day) were recorded in the meteorological stations close to the selected urban slums. All collections were performed a full day after the last recorded rainfall. Samples in open and closed sections at each site were collected the same day between 10 a.m. and 12 p.m.
Each collection point, an area of ~400 cm2, was cleared from surface rocks and vegetation debris, and ~25 g of subsurface soil was collected at a depth of approximately 5 cm, stored in aseptic containers, transported to the laboratory, and processed within 4 h of collection as described previously with minor modifications.32 Soil samples were mixed with a sterile spatula for 5 min to homogenize, and a subsample of 5 g was transferred to a 50 mL sterile polypropylene tube. Then, 40 mL of sterile double-distilled water was added to each 5 g sample and shaken with a horizontal vortex adaptor at maximum speed for 2 min. Samples were centrifuged at 100 rcf for 5 min; the supernatant (approximately 45 mL) was recovered and centrifuged at 12 000 rcf for 20 min at room temperature. The supernatants were discarded, and the pellets were frozen at −20 °C. In addition to the soil samples, two paired 40 mL sewage samples were collected in sterile 50 mL polypropylene tubes at the end of the closed and open sections in each site. Sewage samples were homogenized by inversion, and a 40 mL aliquot was centrifuged at 15 000 rcf for 20 min at 4 °C. The supernatant was discarded, and the pellet was recovered and frozen at −20 °C.16
Quantification of Pathogenic Leptospira and Human Fecal Markers.
DNA was extracted from the frozen pellets within 3 days after processing using a DNA Easy PowerSoil kit (Qiagen) in batches of 20 samples and stored at −80 °C. An extraction blank (sterile double-distilled water) was included with each batch to control for cross-contamination.
Pathogenic Leptospira was quantified using a TaqMan assay targeting the lipL32 gene as described previously.32 This qPCR assay has been shown to specifically detect species from the subclade P1 in complex environmental samples.16,32 To determine the levels of human fecal contamination, we used the BacHum TaqMan qPCR assay.33,34 Calibration curves based on genomic DNA from L. interrogans serovar Copenhageni strain Fiocruz L1–130 were included in each qPCR plate with the concentration of the standard ranging from 2 × 102 to 2 × 109 GEq/mL. Samples were run in duplicate and included nontemplate controls in each plate row to control contamination. qPCR inhibition was monitored using a an Internal Amplification Control (IAC) plasmid in singleplex reactions as described previously16 for lipL32 and testing at least two sample dilutions for BacHum. For more details on the cycling parameters, primer, probe, and bovine serum albumin (BSA) concentrations, calibration curves, and tests for inhibition, see the Supporting Information.
Rat Activity Monitoring.
To evaluate the rat presence in the sampling sites during soil collection, we used a track plate method that had previously showed high correlation with rat infestation measures and trapping of rats to population exhaustion approaches.35,36 Forty-eight track plates were placed in each of the demarcated areas described above on the day of soil collection. Plates were randomly distributed within each polygon with a packing density of 0.4 with corresponding minimum distances between them (1.33 ± 0.73 m). Each site contained 96 track plates (48 in the surroundings of the open section of the sewer and 48 in the closed section) for a total of 576 plates. Track plates were evaluated daily over the course of 2 days for evidence of rat activity through the identification of footprints, scrapes, and tail slides and scored using a binary variable (presence/absence of rat marks on a plate) and a continuous variable (the intensity of marks on plates).35 In addition, environmental rodent surveys were carried out at each sampling site by looking for variables associated with rodent infestation and water or harborage sources for rodents: pavement, soil, mud vegetation, trash, food, water, building material, rubble, other animals, and rat feces.37
Data Treatment.
Samples were considered positive when both qPCR replicates showed amplification up to a CT of 40. Samples with a single positive reaction were submitted to an additional qPCR run in duplicate. If in this second run the sample was amplified in either of the replicates, it was considered positive. The genomic equivalents (GEq) per reaction in all positive qPCR replicates were averaged, normalized by the amount of wet soil or water processed, and log10 transformed to obtain concentrations in log10 GEq/g or mL. For the purpose of statistical analysis, soil samples with concentrations below the limit of detection were considered to have a concentration equivalent to the limit of detection of the lipl32 qPCR assay in soil samples (2 GC/g).32
Statistical Analysis.
We used Fischer’s exact test to compare rat activity between open and closed sewer sections and the environmental variables between sites.
We built mixed generalized linear models (GLMMs) with binomial and gamma error structure to investigate the probability of the presence (binomial) and concentration of pathogenic Leptospira in soil (continuous in log10) and their association with sewer status (open/closed) and type of closing (open, conventional, or community based).38 We also included other covariates such as distance to the sewer, soil moisture, rat activity (presence/absence of rat marks and number of rat marks) and human fecal markers (BacHum), and a randomization factor for the sampling site.39,40 The modeling approach was carried out in two stages. First, we built univariate models between all of the variables and added an interaction structure between them to understand how the presence and concentration of Leptospira in soil varied. Variables with a p value below 0.1 in univariate analyses were included in the multivariate analyses subsequently performed. Various multivariate statistical models were generated, and the model with the lowest AIC (Akaike’s Information Criterion) and ΔAIC < 2 was selected as the best model using the dredge function of the R MuMIn package.41,42 We estimated the odds ratios (ORs) associated with the probability of Leptospira presence in soil and the rates (β coefficients) for the Leptospira concentration model in soil. The analyzes were performed in R 3.3.1,43 and we applied a significance level of p < 0.05.
RESULTS
Presence of Leptospira DNA in Soil Samples.
We collected a total of 272 soil samples and 24 sewage samples in the six sites studied and tested them for the presence of pathogenic Leptospira DNA. Overall, 68 soil samples (25.0%) were positive for Leptospira DNA with more samples positive in soils surrounding the open sewer sections (31.6%, 95% CI = 24.4–39.9%) than in their closed counterparts (18.4%, 95% CI = 12.7–26.8%) (Table 1). Among the 68 positive samples, the geometric mean concentration and count range of Leptospira DNA was 3.3 [2.00−1.62 × 103] GEq/g and 4.2 [2.0–52.6] GEq/g in open and closed sections, respectively (Table 1, Figure 1 and Supplemental Figure 1). The highest proportion of positive samples was detected in the open section of site 1 with 54.2% (13/24), whereas the lowest was found in the closed section of site 2 with 0.0% (0/24) (Table 1). Interestingly, while we observed a relative reduction in the percentage of positive samples in most conventionally closed sites (sites 1–3) compared to open sites, no reduction was observed in community closed sites (sites 5 and 6).
Table 1.
Occurrence and Concentration of Pathogenic Leptospira in Soils Surrounding Open and Closed Sewers in the Six Brazilian Urban Slumsa
| site and type of closing | pathogenic Leptospira positivity rate (n and %) | pathogenic Leptospira concentration (mean log10 and SD) | ||
|---|---|---|---|---|
| open sewer | closed sewer | open sewer | closed sewer | |
| conventional | ||||
| site 1: Pau da Lima 1 | 13 (54.2%) | 2 (8.3%) | 1.04 (0.70) | 0.57 (1.19) |
| site 2: Sete de Abril | 3 (12.5%) | 0 (0.0%) | 0.61 (0.82) | 0.00 (0.00) |
| site 3: Campinas de Pirajá | 8 (33.3%) | 3 (12.5%) | −0.08 (0.74) | 0.54 (0.04) |
| site 4: Tancredo Neves | 4 (16.7%) | 5 (21.7%) | 1.04 (1.99) | 0.48 (0.30) |
| total conventional | 28 (29.1%) | 10 (10.5%) | 0.67 (1.05) | 0.51 (0.44) |
| community | ||||
| site 5: Nova Constituinte | 11 (45.8%) | 11 (45.8%) | 0.20 (0.62) | 0.70 (0.56) |
| site 6: Pau da Lima 2 | 4 (26.7%) | 4 (25.0%) | 0.35 (0.45) | 0.67 (0.72) |
| total community | 15 (38.4%) | 15 (37.5%) | 0.24 (0.57) | 0.69 (0.57) |
| overall | 43 (31.9%) | 25 (18.5%) | 0.52 (0.93) | 0.62 (0.52) |
Closed sewers are classified based on the type of closing: conventional or community based. Twenty-four samples were collected in open and closed areas from sites 1–5. Sixteen samples were collected in open and closed areas from site 6.
Presence and Concentration of Human Fecal Pollution Markers.
We detected the human fecal pollution markers (BacHum) in 56.3% (153 of 272) of the soil samples collected (Supplemental Table 1). The presence of the marker was slightly higher in open than in closed areas of the sewers (58.8% [50.4–66.8%] and 53.3% [45.0–61.4%], respectively). The highest proportion of positive samples occurred in the open sections of sites 1 and 6 (91.7% and 100%, respectively), whereas the lowest was found in the open and closed sections of site 4 (8.3% and 16.7%, respectively). Among the 153 positive samples, the geometric mean concentrations and count range of BacHum was 3.04 × 103 [21.4−2.41 × 107] GEq/g and 1.26 × 103 [66.4−8.06 × 103] GEq/g in the open and closed sections, respectively (Supplemental Table 2).
Presence of Rats.
We observed a higher presence and activity of rats as measured by tracking boards in the closed sections (12.2% and 29.2%; p = 0.0139) when compared to the open sections of the sewers (9.3% and 18.1%; p < 0.001). Tracking plates placed within the area of the closed section of the sewer had higher rat presence (12.2% (±8.3) vs 93% (±9.8); p = 0.0139) and higher percent rat activity (29.2% (±6.9) vs 18.1% (±19.2); p < 0.001) than the tracking plates placed near the open section of the sewer. We did not find significant differences between the open/closed status of the sewer and the number of animals (p = 0.4484), number of rat holes (p = 1.0000), pavement (p = 0.4902), soil (p = 0.1138), mud (p = 0.5271), vegetation (p = 1.0000), trash (p = 1.0000), food (p = 0.5271), water (p = 1.000), building material (p = 0.4902), rubble (p = 0.5271), and rat feces (p = 1.000).
Sewage Samples.
Pathogenic Leptospira was present in 17 of 24 of the sewage samples collected with a geometric mean and count range of 124 [20–1545] GEq/mL. In all collection sites (at the end of the closed and the open sections), at least one of the two paired samples collected was positive, indicating that sewage is a potential source of pathogenic Leptospira to soils surrounding sewers.
Predictors of Leptospira DNA Presence and Concentration.
The univariate models found significant associations between the presence and the concentration of pathogenic Leptospira in soil with the status of the sewer (open/closed), type of closing, distance to other nearby open sewers, rat activity, and concentration of fecal human markers (BacHum) (Supplemental Tables 2 and 3). However, only two covariates remained significant in the multivariate final models: type of sewer closing and presence of BacHum fecal pollution markers (Table 2 and Supplemental Table 4). First, soil samples collected in areas surrounding sewers closed by the local government were more than 3 times less likely (inverse OR 3.44, 95% CI = 1.66–8.33) to contain pathogenic Leptospira than soils collected in open areas overall. In contrast, the presence of pathogenic Leptospira was not significantly different in soils surrounding community closed sewers than that in those adjacent to open sewers. Similarly, the logistic model using Leptospira concentration as the outcome indicated that soils surrounding conventionally closed sewers contained a lower load of pathogenic Leptospira (0.82 log10 units less or approximately 6 times less). Furthermore, the logistic model showed that BacHum markers were significantly associated with the presence of pathogenic Leptospira. For every log10 unit increase in BacHum concentration, the chances of finding a positive Leptospira sample increased by 15%. Likewise, the concentration of pathogenic Leptospira in positive samples was higher in those samples that also contained BacHum markers (Table 2). Notably, none of the other variables included in the model (rat presence and activity, soil moisture, distance to open or closed sewer, and proximity to other open sewers) were found to be significantly associated with pathogenic Leptospira in the multivariate models. In summary, our model revealed that the type of closing and BacHum markers were predictors of the presence and concentration of pathogenic Leptospira in soil.
Table 2.
Final Multivariate Logistic and Linear Mixed Models on the Probability of Finding a Positive Sample and log10 Concentration for Leptospira DNAa
| predictors | logistic model for probability | model for concentration | ||
|---|---|---|---|---|
| OR | CI | estimates (β) | CI | |
| intercept | 0.40*** | 0.21 to 0.70 | −2.6*** | −3.07 to −2.16 |
| type of closing | ||||
| no closing (ref.) | ||||
| conventional | 0.29*** | 0.12 to 0.60 | −0.82** | −1.33 to −0.30 |
| community | 1.09 | 0.46 to 2.55 | 0.19 | −0.54 to 0.93 |
| fecal human markers | ||||
| concentration fecal human markers (log10 GE/mL) | 1.15** | 1.04 to 1.26 | 0.11** | 0.04 to 0.18 |
(**) p = 0.001 (***) p = 0.0001.
DISCUSSION
In this study, we compared the presence and concentration of pathogenic Leptospira in soils surrounding open and closed sewer sections in six Brazil urban slums. We found that pathogenic Leptospira occurred in both areas but was more prevalent in soils adjacent to open sewer sections, although the concentration was generally low. More importantly, our results show that soils in conventionally closed sewers have a reduced presence of the pathogen when compared to their open counterparts. However, no significant differences were observed when comparing open and community-closed sewers. These results have important implications for future public health and sewerage development in urban slums.
The soil contamination with pathogenic Leptospira was lower in soils adjacent to conventionally closed sewers than that in open sections, but no reduction was observed in community-based closings (Table 1 and Figure 2). Conventional sewer closings are implemented by the local government and completely canalize sewage, isolating it from the surrounding environment and preventing spills and overflow during heavy rainfall events. Since sewage is a recognized source of Leptospira as evidenced by this and previous studies,16,24,44 its canalization may eliminate spillage contamination in the soil. However, the imperfect closure of community-based interventions that consist of suboptimal closings made with poor-quality construction materials such as wood boards or concrete panels might still allow sewage to contaminate adjacent soils. Moreover, despite the reduction observed in conventionally closed sections, the pathogen could still be detected in 3 of the 4 sites sampled. This suggests that the presence of pathogenic Leptospira contamination in these soils may not have its origin exclusively in the adjacent open sewers.
Figure 2.

Distribution of pathogenic Leptospira based on lipl32 qPCR in soils surrounding open and closed sewers. (A) Occurrence by the type of sewer closing (mean percentage and standard error). (B) Overall concentration of pathogenic Leptospira by the type of sewer closing (median and interquartile ranges). Open sewers are denoted in gray and closed sewers in green.
We identified human fecal markers (BacHum) as a predictor of the presence and concentration of pathogenic Leptospira in soils. A previous study in streams from Hawaii also found a positive correlation of pathogenic Leptospira concentrations and fecal pollution markers (Bacteroidales and Clostridium perfringens).45 Interestingly, we observed a correlation between BacHum and pathogenic Leptospira in not only open but also closed sewer sections. This indicates that besides the adjacent sewers, there are other sources of fecal pollution and pathogenic Leptospira. Previous studies hypothesized that intense rainfall events may mobilize pathogenic Leptospira and human pollution markers occurring in soils in higher elevated areas and transport them with the storm runoff to lower areas,32,46,47 where sewers are located. Notably, the construction of conventional sewers does not canalize stormwater, and thus, runoff may still contribute to the contamination observed in conventionally closed sewer sections. Therefore, the association of pathogenic Leptospira and human fecal markers is likely a combination of the effect of sewer proximity and storm runoff.
The concentrations of pathogenic Leptospira in the collected soils were generally low in the six urban areas studied (mean log10 0.56 ± 0.8 and 2.00−1.62 × 103 GEq/g) (Figure 1). This finding is consistent with previous studies that reported low concentrations of the pathogen in soils and waters in high-risk environments.16,24,32,48 Besides the sewer and runoff contribution, the presence and concentration of the pathogen in soil is related to its survival and long-term persistence ability,8,49 which is affected by the soil type, composition, and physicochemical characteristics. For instance, soils rich in nutrients such as iron, manganese, copper, and nitrate have been shown to be a positive risk factor for the presence of Leptospira, just as wetter soils and basic pH can increase the survival of this pathogen.49–51 Interestingly, a soil sample in site 4 contained a particularly high concentration of pathogenic Leptospira (1.62 × 103 GEq/g), which indicates that hot spots of the pathogen occur in the urban slum environment. Yet, the highly heterogenic distribution of the pathogen in soil32 and the cross-sectional nature of our sampling strategy may have prevented the identification of these high-concentration areas and determination of their origin and temporal dynamics. Since the human infectious dose is still unknown, more studies are needed to determine the significance of these heterogenic distributions of the pathogen in human infection dynamics.
Unexpectedly, rat presence and activity were not important factors to predict the presence or concentration of the pathogen in soils. Rats are the main animal reservoir of pathogenic Leptospira in urban slums,5,52,53 and rat presence is commonly reported as a factor for leptospirosis infection.37,54 Open sewers offer an ideal ecosystem for the proliferation of rodents by providing burrowing areas, access to water, and food sources. Counterintuitively, our results suggest that the contamination of soils close to sewers is more related to the type of sewer closing than to the presence and activity of rats. Therefore, rat control strategies alone, such as rodenticide campaigns, may not be effective in reducing the presence of the pathogen in the sewer environment55,56 and should be combined with sewerage construction.
This study was limited by its cross-sectional design. Because Leptospira soil contamination may be variable over time and, specifically, around rainfall events, future prospective studies are needed to investigate the effect of sewer closing in the presence and concentration of the pathogen. In addition, the high heterogeneity of urban slum environments and diversity of community-based closings limit our ability to make wide generalizations of the effects observed in this study. Other important factors that may affect the presence, survival, and distribution of the pathogen in soil such as rainfall, stormwater hydrology, and elevation will need to be considered in future studies. Furthermore, although a higher environmental presence of pathogenic Leptospira is intuitively linked to a higher risk of infection, epidemiological studies are needed to determine how sewerage interventions and the reduction of the environmental burden of the pathogen affect the dynamics of leptospirosis infection and disease. These future studies will also need to determine whether community interventions, despite not reducing the environmental burden of Leptospira, may still decrease human infection. As community interventions are cheaper and easier to implement in neglected communities, more research is needed to understand their potential role in disease transmission.
Despite these limitations, taken together, our results suggest that conventional sewer systems may be an important but not exclusive strategy to reduce the presence and concentration of the pathogen in the environment. The closure of sewers could reduce the niches for the environmental distribution and dissemination of pathogenic Leptospira, subsequently decreasing pathogenic Leptospira exposures in these neglected communities and eventually reducing human leptospirosis. This adds to the body of evidence that sewerage reduces exposure to a wide number of human pathogens and therefore supports the expansion of sewer systems in urban slums to help decrease the burden of leptospirosis and other environmentally transmitted infectious diseases.
Supplementary Material
ACKNOWLEDGMENTS
We thank the resident associations, community leaders, and residents from Pau da Lima, Tancredo Neves, Sete de Abril, Nova Constituinte, and Campinas de Pirajá. This work was supported by the National Institutes of Health (grants F31 AI114245, U01 AI088752, and R01 TW009504), Fundação de Amparo á Pesquisa do Estado da Bahia (FAPESB/JCB0020/2016), and Wellcome Trust [218987/Z/19/Z]. M.C. was supported by a Downs International Health Student Travel Fellowship from the Yale School of Public Health. F.N.S. participated in this study under a FAPESB doctorate scholarship.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.est.1c04916
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.1c04916.
Supplementary methods for qPCR and inhibition testing, tables and figures for the presence and concentration of Leptospira; and tables for the univariate and multivariate models (PDF)
The authors declare no competing financial interest.
Contributor Information
Arnau Casanovas-Massana, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, Connecticut 06511, United States.
Fabio Neves Souza, Instituto de Saúde Coletiva, Universidade Federal da Bahia, Salvador, Bahia 40110-040, Brazil; Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil.
Melanie Curry, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, Connecticut 06511, United States.
Daiana de Oliveira, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil.
Anderson S. de Oliveira, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil
Max T. Eyre, Instituto de Saúde Coletiva, Universidade Federal da Bahia, Salvador, Bahia 40110-040, Brazil; Centre for Health Informatics, Computing, and Statistics, Lancaster University Medical School, Lancaster LA1 4YW, United Kingdom
Diogo Santiago, Instituto de Saúde Coletiva, Universidade Federal da Bahia, Salvador, Bahia 40110-040, Brazil.
Maísa Aguiar Santos, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil.
Rafael M. R. Serra, Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil
Evelyn Lopes, Instituto de Saúde Coletiva, Universidade Federal da Bahia, Salvador, Bahia 40110-040, Brazil.
Barbara IA Xavier, Instituto de Saúde Coletiva, Universidade Federal da Bahia, Salvador, Bahia 40110-040, Brazil.
Peter J. Diggle, Centre for Health Informatics, Computing, and Statistics, Lancaster University Medical School, Lancaster LA1 4YW, United Kingdom
Elsio A. Wunder, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, Connecticut 06511, United States; Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil
Mitermayer G. Reis, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, Connecticut 06511, United States; Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil; Faculdade de Medicina da Bahia, Universidade Federal da Bahia, Salvador, Bahia 40026-010, Brazil
Albert I. Ko, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, Connecticut 06511, United States; Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil.
Federico Costa, Department of Epidemiology of Microbial Diseases, School of Public Health, Yale University, New Haven, Connecticut 06511, United States; Instituto de Saúde Coletiva, Universidade Federal da Bahia, Salvador, Bahia 40110-040, Brazil; Instituto Gonçalo Moniz, Fundação Oswaldo Cruz, Ministério da Saúde, Salvador, Bahia 40296-710, Brazil.
REFERENCES
- (1).Ko AI; Goarant C; Picardeau M Leptospira: The Dawn of the Molecular Genetics Era for an Emerging Zoonotic Pathogen. Nat. Rev. Microbiol 2009, 7 (10), 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Costa F; Hagan JE; Calcagno J; Kane M; Torgerson P; Martinez-Silveira MS; Stein C; Abela-Ridder B; Ko AI Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Neglected Trop. Dis 2015, 9 (9), No. e0003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).McBride AJA; Athanazio DA; Reis MG; Ko AI Leptospirosis. Curr. Opin. Infect. Dis 2005, 18 (5), 376–386. [DOI] [PubMed] [Google Scholar]
- (4).Gouveia EL; Metcalfe J; de Carvalho ALF; Aires TSF; Villasboas-Bisneto JC; Queirroz A; Santos AC; Salgado K; Reis MG; Ko AI Leptospirosis- Associated Severe Pulmonary Hemorrhagic Syndrome, Salvador, Brazil. Emerging Infect. Dis 2008, 14 (3), 505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Costa F; Wunder EA; De Oliveira D; Bisht V; Rodrigues G; Reis MG; Ko AI; Begon M; Childs JE Patterns in Leptospira Shedding in Norway Rats (Rattus Norvegicus) from Brazilian Slum Communities at High Risk of Disease Transmission. PLoS Neglected Trop. Dis 2015, 9 (6), No. e0003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Barragan V; Chiriboga J; Miller E; Olivas S; Birdsell D; Hepp C; Hornstra H; Schupp JM; Morales M; Gonzalez M; Reyes S; de la Cruz C; Keim P; Hartskeerl R; Trueba G; Pearson T High Leptospira Diversity in Animals and Humans Complicates the Search for Common Reservoirs of Human Disease in Rural Ecuador. PLoS Neglected Trop. Dis 2016, 10 (9), No. e0004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Casanovas-Massana A; Pedra GG; Wunder EA; Diggle PJ; Begon M; Ko AI Quantification of Leptospira Interrogans Survival in Soil and Water Microcosms. Appl. Environ. Microbiol 2018, 84 (13), No. e00507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Barragan V; Olivas S; Keim P; Pearson T Critical Knowledge Gaps in Our Understanding of Environmental Cycling and Transmission of Leptospira Spp. Appl. Environ. Microbiol 2017, 83 (19), No. e01190–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Bharti AR; Nally JE; Ricaldi JN; Matthias MA; Diaz MM; Lovett MA; Levett PN; Gilman RH; Willig MR; Gotuzzo E; Vinetz JMG Leptospirosis: A Zoonotic Disease of Global Importance. Lancet Infect. Dis 2003, 3 (12), 757–771. [DOI] [PubMed] [Google Scholar]
- (10).Karande S; Gandhi D; Kulkarni M; Bharadwaj R; Pol S; Thakare J; De A Concurrent Outbreak of Leptospirosis and Dengue in Mumbai, India, 2002. J. Trop. Pediatr 2005, 51 (3), 174–181. [DOI] [PubMed] [Google Scholar]
- (11).Kyobutungi C; Ziraba AK; Ezeh A; Yé Y The Burden of Disease Profile of Residents of Nairobi’s Slums: Results from a Demographic Surveillance System. Popul. Health Metr 2008, 6 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Matthias MA; Ricaldi JN; Cespedes M; Diaz MM; Galloway RL; Saito M; Steigerwalt AG; Patra KP; Ore CV; Gotuzzo E; Gilman RH; Levett PN; Vinetz JM Human Leptospirosis Caused by a New, Antigenically Unique Leptospira Associated with a Rattus Species Reservoir in the Peruvian Amazon. PLoS Neglected Trop. Dis 2008, 2 (4), No. e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ko AI; Galvão Reis M; Ribeiro Dourado CM; Johnson WD; Riley LW; Reis MG; Dourado CMR; Johnson WD Jr; Riley LW; Galvão Reis M; Ribeiro Dourado CM; Johnson WD; Riley LW Urban Epidemic of Severe Leptospirosis in Brazil. Lancet 1999, 354 (9181), 820–825. [DOI] [PubMed] [Google Scholar]
- (14).Panti-May JA; Carvalho-Pereira TSA; Serrano S; Pedra GG; Taylor J; Pertile AC; Minter A; Airam V; Carvalho M; Júnior NN; Rodrigues G; Reis MG; Ko AI; Childs JE; Begon M; Costa F A Two-Year Ecological Study of Norway Rats (Rattus Norvegicus) in a Brazilian Urban Slum. PLoS One 2016, 11 (3), No. e0152511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Blasdell KR; Morand S; Perera D; Firth C Association of Rodent-Borne Leptospira Spp. with Urban Environments in Malaysian Borneo. PLoS Neglected Trop. Dis 2019, 13 (2), No. e0007141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Casanovas-Massana A; Costa F; Riediger IN; Cunha M; de Oliveira D; Mota DC; Sousa E; Querino VA; Nery N; Reis MG; Wunder EA; Diggle PJ; Ko AI Spatial and Temporal Dynamics of Pathogenic Leptospira in Surface Waters from the Urban Slum Environment. Water Res. 2018, 130, 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Saito M; Miyahara S; Villanueva SYAM; Aramaki N; Ikejiri M; Kobayashi Y; Guevarra JP; Masuzawa T; Gloriani NG; Yanagihara Y; Yoshida S PCR and Culture Identification of Pathogenic Leptospira from Coastal Soil in Leyte, Philippines after a Storm Surge during Super Typhoon Haiyan (Yolanda). Appl. Environ. Microbiol 2014, 80 (22), 6926–6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lau CL; Smythe LD; Craig SB; Weinstein P Climate Change, Flooding, Urbanisation and Leptospirosis: Fuelling the Fire? Trans. R. Soc. Trop. Med. Hyg 2010, 104 (10), 631–638. [DOI] [PubMed] [Google Scholar]
- (19).Hagan JE; Moraga P; Costa F; Capian N; Ribeiro GS; Wunder EA; Felzemburgh RDM; Reis RB; Nery N; Santana FS; Fraga D; dos Santos BL; Santos AC; Queiroz A; Tassinari W; Carvalho MS; Reis MG; Diggle PJ; Ko AI Spatiotemporal Determinants of Urban Leptospirosis Transmission: Four-Year Prospective Cohort Study of Slum Residents in Brazil. PLoS Neglected Trop. Dis 2016, 10 (1), No. e0004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Felzemburgh RDM; Ribeiro GS; Costa F; Reis RB; Hagan JE; Melendez AXTO; Fraga D; Santana FS; Mohr S; Dos Santos BL; Silva AQ; Santos AC; Ravines RR; Tassinari WS; Carvalho MS; Reis MG; Ko AI Prospective Study of Leptospirosis Transmission in an Urban Slum Community: Role of Poor Environment in Repeated Exposures to the Leptospira Agent. PLoS Neglected Trop. Dis 2014, 8 (5), No. e2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Reis RB; Ribeiro GS; Felzemburgh RDM; Santana FS; Mohr S; Melendez AXTO; Queiroz A; Santos AC; Ravines RR; Tassinari WS; Carvalho MS; Reis MG; Ko AI Impact of Environment and Social Gradient on Leptospira Infection in Urban Slums. PLoS Neglected Trop. Dis 2008, 2 (4), No. e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Briskin EA; Casanovas-Massana A; Ryff KR; Morales-Estrada S; Hamond C; Perez-Rodriguez NM; Benavidez KM; Weinberger DM; Castro-Arellano I; Wunder EA; Sharp TM; Rivera-Garcia B; Ko AI Seroprevalence, Risk Factors, and Rodent Reservoirs of Leptospirosis in an Urban Community of Puerto Rico, 2015. J. Infect. Dis 2019, 220, 1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Abbott J State of the World’s Cities 2012/2013 - Prosperity of Cities. Australian Planner 2015, 52, 171–173. [Google Scholar]
- (24).Ganoza CA; Matthias MA; Collins-Richards D; Brouwer K; Cunningham CB; Segura ER; Gilman RH; Gotuzzo E; Vinetz JM Determining Risk for Severe Leptospirosis by Molecular Analysis of Environmental Surface Waters for Pathogenic Leptospira. PLoS Med. 2006, 3 (8), e308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Picardeau M Virulence of the Zoonotic Agent of Leptospirosis: Still Terra Incognita? Nat. Rev. Microbiol 2017, 15 (5), 297–307. [DOI] [PubMed] [Google Scholar]
- (26).Smith L An Experimental Rat Eradication Program in an Urban Area. Public Health Rep. 1963, 78 (9), 807–811. [PMC free article] [PubMed] [Google Scholar]
- (27).de Masi E; Vilaça PJ; Razzolini MTP Evaluation on the Effectiveness of Actions for Controlling Infestation by Rodents in Campo Limpo Region, São Paulo Municipality, Brazil. Int. J. Environ. Health Res 2009, 19 (4), 291–304. [DOI] [PubMed] [Google Scholar]
- (28).Brown J; Cairncross S; Ensink JHJ Water, Sanitation, Hygiene and Enteric Infections in Children. Arch. Dis. Child 2013, 98 (8), 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Freeman MC; Garn JV; Sclar GD; Boisson S; Medlicott K; Alexander KT; Penakalapati G; Anderson D; Mahtani AG; Grimes JET; Rehfuess EA; Clasen TF The Impact of Sanitation on Infectious Disease and Nutritional Status: A Systematic Review and Meta-Analysis. Int. J. Hyg. Environ. Health 2017, 220 (6), 928–949. [DOI] [PubMed] [Google Scholar]
- (30).Norman G; Pedley S; Takkouche B Effects of Sewerage on Diarrhoea and Enteric Infections: A Systematic Review and Meta-Analysis. Lancet Infect. Dis 2010, 10 (8), 536–544. [DOI] [PubMed] [Google Scholar]
- (31).Khalil H; Santana R; de Oliveira D; Palma F; Lustosa R; Eyre MT; Carvalho-Pereira T; Reis MG; Ko AI; Diggle PJ; Alzate Lopez Y; Begon M; Costa F Poverty, Sanitation, and Leptospira Transmission Pathways in Residents from Four Brazilian Slums. PLoS Neglected Trop. Dis 2021, 15 (3), No. e0009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Schneider AG; Casanovas-Massana A; Hacker KP; Wunder EA; Begon M; Reis MG; Childs JE; Costa F; Lindow JC; Ko AI Quantification of Pathogenic Leptospira in the Soils of a Brazilian Urban Slum. PLoS Neglected Trop. Dis 2018, 12 (4), No. e0006415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Kildare BJ; Leutenegger CM; McSwain BS; Bambic DG; Rajal VB; Wuertz S 16S RRNA-Based Assays for Quantitative Detection of Universal, Human-, Cow-, and Dog-Specific Fecal Bacteroidales: A Bayesian Approach. Water Res. 2007, 41 (16), 3701–3715. [DOI] [PubMed] [Google Scholar]
- (34).Van De Werfhorst LC; Sercu B; Holden PA Comparison of the Host Specificities of Two Bacteroidales Quantitative PCR Assays Used for Tracking Human Fecal Contamination. Appl. Environ. Microbiol 2011, 77 (17), 6258–6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Hacker KP; Minter A; Begon M; Diggle PJ; Serrano S; Reis MG; Childs JE; Ko AI; Costa F A Comparative Assessment of Track Plates to Quantify Fine Scale Variations in the Relative Abundance of Norway Rats in Urban Slums. Urban Ecosyst. 2016, 19 (2), 561–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Eyre MT; Carvalho-Pereira TSA; Souza FN; Khalil H; Hacker KP; Serrano S; Taylor JP; Reis MG; Ko AI; Begon M; Diggle PJ; Costa F; Giorgi E A Multivariate Geostatistical Framework for Combining Multiple Indices of Abundance for Disease Vectors and Reservoirs: A Case Study of Rattiness in a Low-Income Urban Brazilian Community. J. R. Soc., Interface 2020, 17 (170), 20200398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Costa F; Ribeiro GS; Felzemburgh RDM; Santos N; Reis RB; Santos AC; Fraga DBM; Araujo WN; Santana C; Childs JE; Reis MG; Ko AI Influence of Household Rat Infestation on Leptospira Transmission in the Urban Slum Environment. PLoS Neglected Trop. Dis 2014, 8 (12), No. e3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Zuur AF; Ieno EN; Walker NJ; Saveliev AA; Smith GM GLMM and GAMM. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, 2009; pp 323–341. [Google Scholar]
- (39).Zuur AF; Ieno EN; Walker N; Smith AA; Saveliev GM Mixed Effects Models and Extensions in Ecology with R; Springer: New York, 2009. [Google Scholar]
- (40).McCulloch CE; Neuhaus JM Misspecifying the Shape of a Random Effects Distribution: Why Getting It Wrong May Not Matter. Stat. Sci 2011, 26 (3), 388–402. [Google Scholar]
- (41).Burnham KP; Anderson DR Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, 2007. [Google Scholar]
- (42).Bozdogan H Model Selection and Akaike’s Information Criterion (AIC): The General Theory and Its Analytical Extensions. Psychometrika 1987, 52 (3), 345–370. [Google Scholar]
- (43).R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2014. [Google Scholar]
- (44).Muñoz-Zanzi C; Mason MR; Encina C; Astroza A; Romero A Leptospira Contamination in Household and Environmental Water in Rural Communities in Southern Chile. Int. J. Environ. Res. Public Health 2014, 11, 6666–6680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Viau EJ; Boehm AB Quantitative PCR-Based Detection of Pathogenic Leptospira in Hawai’ian Coastal Streams. J. Water Health 2011, 9 (4), 637–646. [DOI] [PubMed] [Google Scholar]
- (46).de Oliveira D; Airam Querino V; Sara Lee Y; Cunha M; Nery N; Wessels Perelo L; Rossi Alva JC; Ko AI; Reis MG; Casanovas-Massana A; Costa F Relationship between Physicochemical Characteristics and Pathogenic Leptospira in Urban Slum Waters. Trop. Med. Infect. Dis 2020, 5 (3), 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Bierque E; Thibeaux R; Girault D; Soupé-Gilbert M-E; Goarant C A Systematic Review of Leptospira in Water and Soil Environments. PLoS One 2020, 15 (1), No. e0227055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Thibeaux R; Geroult S; Benezech C; Chabaud S; Soupé-Gilbert M-E; Girault D; Bierque E; Goarant C Seeking the Environmental Source of Leptospirosis Reveals Durable Bacterial Viability in River Soils. PLoS Neglected Trop. Dis 2017, 11 (2), No. e0005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Casanovas-Massana A; Pedra GG; Wunder EA; Diggle PJ; Begon M; Ko AI Quantification of Leptospira Interrogans Survival in Soil and Water Microcosms. Appl. Environ. Microbiol 2018, 84 (13), e00507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Lall C; Vinod Kumar K; Raj RV; Vedhagiri K; Sunish IP; Vijayachari P Correlation between Physicochemical Properties of Soil and Presence of Leptospira. Ecohealth 2018, 15 (3), 670–675. [DOI] [PubMed] [Google Scholar]
- (51).Cucchi K; Liu R; Collender PA; Cheng Q; Li C; Hoover CM; Chang HH; Liang S; Yang C; Remais JV Hydroclimatic Drivers of Highly Seasonal Leptospirosis Incidence Suggest Prominent Soil Reservoir of Pathogenic Leptospira Spp. In Rural Western China. PLoS Neglected Trop. Dis 2019, 13 (12), No. e0007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Desvars-Larrive A; Smith S; Munimanda G; Bourhy P; Waigner T; Odom M; Gliga DS; Walzer C Prevalence and Risk Factors of Leptospira Infection in Urban Brown Rats (Rattus Norvegicus), Vienna, Austria. Urban Ecosyst. 2020, 23 (4), 775–784. [Google Scholar]
- (53).Boey K; Shiokawa K; Rajeev S Leptospira Infection in Rats: A Literature Review of Global Prevalence and Distribution. PLoS Neglected Trop. Dis 2019, 13, e0007499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Pellizzaro M; Martins CM; Yamakawa AC; Ferraz D. da C.; Morikawa VM; Ferreira F; Santos AP; dos Biondo AW; Langoni H Molecular Detection of Leptospira Spp. in Rats as Early Spatial Predictor for Human Disease in an Endemic Urban Area. PLoS One 2019, 14 (5), No. e0216830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Murray MH; Fidino M; Fyffe R; Byers KA; Pettengill JB; Sondgeroth KS; Killion H; Magle SB; Rios MJ; Ortinau N; Santymire RM City Sanitation and Socioeconomics Predict Rat Zoonotic Infection across Diverse Neighbourhoods. Zoonoses Public Health 2020, 67 (6), 673–683. [DOI] [PubMed] [Google Scholar]
- (56).Minter A; Diggle PJ; Costa F; Childs J; Ko AI; Begon M A Model for Leptospire Dynamics and Control in the Norway Rat (Rattus Norvegicus) the Reservoir Host in Urban Slum Environments. Epidemics 2018, 25, 26–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


