ABSTRACT
Transmitted by ticks, the bacterium Borrelia burgdorferi sensu lato is the causative agent of Lyme disease (LD), the most common vector-borne disease in the Northern hemisphere. No effective vaccines are currently available. B. burgdorferi sensu lato produces the CspZ protein that binds to the complement inhibitor, factor H (FH), promoting evasion of the host complement system. We previously showed that while vaccination with CspZ did not protect mice from B. burgdorferi infection, mice can be protected after immunization with CspZ-Y207A/Y211A (CspZ-YA), a CspZ mutant protein without FH-binding activity. To further study the mechanism of this protection, herein we evaluated both poly- and monoclonal antibodies recognizing CspZ FH-binding or non-FH-binding sites. We found that the anti-CspZ antibodies that recognize the FH-binding sites (i.e., block FH-binding activity) eliminate B. burgdorferi sensu lato in vitro more efficiently than those that bind to the non-FH-binding sites, and passive inoculation with anti-FH-binding site antibodies eradicated B. burgdorferi sensu lato in vivo. Antibodies against non-FH-binding sites did not have the same effect. These results emphasize the importance of CspZ FH-binding sites in triggering a protective antibody response against B. burgdorferi sensu lato in future LD vaccines.
KEYWORDS: Lyme disease, CspZ, Borrelia, factor H-binding sites, antibodies
INTRODUCTION
Lyme disease (LD) is caused by the spirochete Borrelia burgdorferi sensu lato (also known as Borreliella burgdorferi sensu lato or Lyme borreliae) transmitted by the bite of infected Ixodes ticks. LD is the most common vector-borne disease in the Northern hemisphere, with an estimated 476,000 people diagnosed annually in the United States alone (1, 2). Among all spirochete species in the B. burgdorferi sensu lato species complex, B. burgdorferi sensu stricto (hereafter B. burgdorferi) and Borrelia afzelii are the major causative species of LD in North America and Eurasia, respectively (2). Following transmission by an infected tick, Lyme borreliae spread from the bite site to various tissues, leading to severe systemic manifestations such as arthritis, carditis, and neuroborreliosis. A human LD vaccine (LYMErix) targeting a spirochete protein, OspA, was once available (but withdrawn from market later), and a newer vaccine consisting of the modified OspA is under clinical trial (3–7). However, this protein is not produced after Lyme borreliae invade humans, leading to the difficulty in maintaining effective titers of antibodies without constant boosters. Such challenges trigger the need of identifying the new target that is produced in hosts during LD infection as the candidate of LD vaccines.
To survive in a host, Lyme borreliae need to evade multiple host immune responses while establishing infection and throughout dissemination to distal tissues (8, 9). Part of the immune response in the bloodstream is a complement system, which is composed of numerous serum proteins activated through three pathways that recombine to form different protein complexes to ultimately kill pathogens (10–12). Complement regulators are produced to inhibit this cascade to avoid damage to host cells (10, 13). For example, factor H (FH) and FH-like protein 1 (FHL-1, a truncated form of FH) bind to the C3b in C3 and C5 convertases, leading to the degradation of C3b and inactivating all downstream processes (14). Lyme borreliae produce multiple outer surface proteins that recruit these complement regulators to its surface and promote the degradation of complement proteins upon binding, ultimately facilitating serum resistance and bloodstream survival of the spirochetes (15–21). These spirochete proteins include five distinct FH-binding proteins (collectively known as complement regulator-acquiring surface proteins [CRASPs]), including CspZ (CRASP-2) (22, 23). The cspZ gene is expressed only when spirochetes reside in vertebrate hosts but not in ticks (24), reflecting the induction of this gene in spirochetes under host environmental cues (e.g., blood and dialysis membrane chambers) (25–27). Additionally, when studied under blood treatment to overcome low expression during in vitro culturing, a cspZ-deficient B. burgdorferi mutant colonizes mouse tissues at reduced levels compared to the wild-type strain (27). These results suggest a role of CspZ to promote efficient spirochete dissemination.
Moreover, cspZ is highly conserved among Lyme borreliae strains (>80% sequence identity) and carried in all B. burgdorferi and B. afzelii strains isolated from human patients with systemic and more severe manifestations (e.g., arthritis and neuroborreliosis) (28–30). In addition, all LD patients in an anecdotal study develop antibodies that recognize CspZ (29). These observations raise the possibility of targeting CspZ as a human LD vaccine antigen. However, we and others reported that vaccination with CspZ does not protect mice from B. burgdorferi infection (29, 31–33). Rather, immunization with CspZ-Y207A/Y211A (CspZ-YA), a mutant CspZ protein that does not bind to FH, prevents spirochete colonization and LD-associated manifestations in mice (32, 33). These findings lead to the question, “Do antibodies that recognize the FH-binding sites of CspZ confer Lyme borreliae clearance?” In this study, we separated the antibodies that selectively recognize CspZ FH-binding or non-FH-binding sites. We then examined each of these antibodies for their ability to eradicate Lyme borreliae and prevent spirochete colonization. The resulting data ultimately facilitated the understanding of the protective epitopes and disease-preventing mechanisms of the CspZ-YA vaccine.
RESULTS
CspZ-YA immunization triggered IgGs that recognize both FH- and non-FH-binding sites of CspZ.
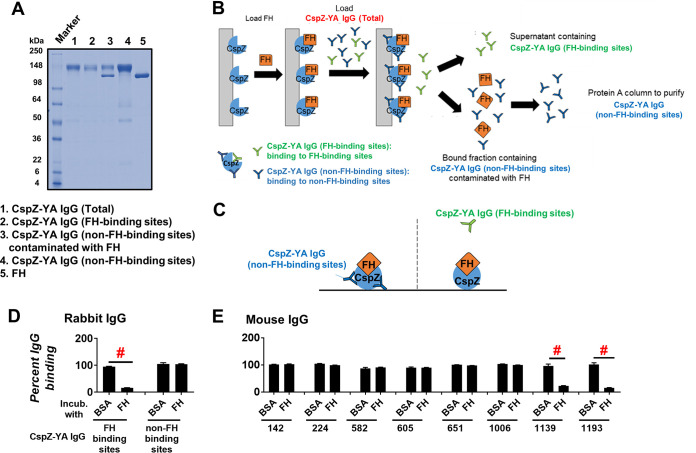
To obtain antibodies that recognize CspZ-YA, serum generated from rabbit immunized with this recombinant protein was purified using a CspZ-immobilized resin to capture all anti-CspZ-YA IgGs (CspZ-YA IgG [total]) (lane 1 in Fig. 1A). From this pool, we separated the IgGs that recognize the FH-binding site (CspZ-YA IgG [FH-binding sites]) from those that recognize non-FH-binding sites of CspZ (CspZ-YA IgG [non-FH-binding sites]) (Fig. 1B). After applying CspZ-YA IgGs (total) to a resin functionalized with a CspZ-FH complex, we collected the unbound fraction containing CspZ-YA IgGs (FH-binding sites) (lane 2 in Fig. 1A and B). The bound proteins consisting mainly of CspZ-YA IgG (non-FH-binding sites) were then eluted (lane 3 in Fig. 1A and B). This fraction also contained another protein with a molecular weight of approximately 120 kDa (lane 3 in Fig. 1A), likely FH (lane 5 Fig. 1A). The bound fraction was subsequently applied to a Protein A column to remove the FH, resulting in purified CspZ-YA IgGs (non-FH-binding sites) (lane 4 in Fig. 1A and B).
FIG 1.
IgGs that recognize both FH- and non-FH-binding sites of CspZ can be isolated separately from CspZ-YA-immunized rabbits and mice. (A) The integrity and purity assessment of CspZ-YA IgGs using SDS-PAGE. (B) A schematic diagram showing the purification process to retrieve CspZ-YA IgG (FH-binding sites) and CspZ-YA IgG (non-FH-binding sites). CspZ-conjugated resin was incubated with FH followed by loading CspZ-YA IgG (total). The bound and unbound fractions contain CspZ-YA IgG (non-FH-binding sites) and CspZ-YA IgG (FH-binding sites) IgG, respectively. After the bound fraction was eluted, the CspZ-YA IgG (non-FH-binding sites) was further purified using Protein A resin to remove contaminated FH. (C) A schematic diagram showing the experimental setup of panels D and E. (D and E) One microgram of CspZ was coated on ELISA plate wells, which were then incubated with human FH (500 nM), BSA (control), or PBS (control, data not shown), followed by the treatment of each of the CspZ-YA IgG samples. These IgGs include (D) CspZ-YA rabbit polyclonal IgGs that recognize the FH-binding site of CspZ (FH-binding sites) or non-FH-binding site (non-FH-binding sites) or (E) CspZ-YA mouse monoclonal IgGs (50 nM). The levels of bound CspZ-YA rabbit polyclonal and mouse monoclonal IgG were determined using HRP-conjugated goat anti-rabbit IgG (Sigma-Aldrich) and goat anti-mouse IgG (Sigma-Aldrich), respectively. Data were expressed as the percent IgG binding, derived by normalizing the levels of bound IgG from FH- or BSA-treated wells to that in PBS-treated wells. Data shown are the mean ± standard error of the mean (SEM) of the percent IgG binding from three independent experiments. # indicates the statistical significance (P < 0.05, Mann-Whitney test) of different levels of percent IgG binding between indicated groups.
To verify the ability of these fractions to recognize (or not) FH-binding sites, we incubated CspZ-coated ELISA plate wells with FH or bovine serum albumin (BSA; negative control), added CspZ-YA IgG (FH-binding sites) or CspZ-YA IgG (non-FH-binding sites) to those wells, and detected the levels of bound antibody (Fig. 1C). We found that CspZ-YA IgG (non-FH-binding sites) bound to both FH- and BSA-treated wells indistinguishably, but the levels of bound CspZ-YA IgG (FH-binding sites) were significantly reduced in FH-treated wells compared to those from BSA-treated wells (Fig. 1D). These results demonstrate the ability of CspZ-YA IgG (FH-binding sites) to recognize FH-binding sites.
We further investigated whether CspZ-YA immunization triggered the production of IgGs that recognize both FH- and non-FH-binding sites in mice. To generate enough antibodies for this effort and for subsequent experiments, we obtained CspZ-YA mouse monoclonal IgGs from eight individual mouse hybridomas (no. 142, 224, 582, 605, 651, 1006, 1139, 1193). We then assessed if these antibodies recognize (or not) CspZ FH-binding sites. We found that the binding levels of monoclonal antibodies (MAbs) no. 142, 224, 582, 605, 651, and 1006 to FH-treated wells did not significantly differ from those to BSA-treated wells (Fig. 1E). However, antibodies no. 1139 and 1193 displayed levels of binding to FH-treated wells significantly lower than those to BSA-treated wells (Fig. 1E). These results grouped the CspZ-YA mouse monoclonal antibodies into those whose CspZ-binding activity is blocked by FH and those whose CspZ-binding activity is not blocked by FH. These findings demonstrate that IgGs that recognize either the FH- or non-FH-binding sites of CspZ can be isolated from CspZ-YA-immunized mice.
The FH-binding activity of CspZ was selectively blocked by CspZ-YA IgGs that recognize FH-binding sites.
Mouse sera after CspZ-YA immunization were documented to block FH binding to CspZ (32). As the IgGs that recognize either FH- or non-FH-binding sites of CspZ can be isolated after such immunization, we sought to determine the ability of each of those IgGs to prevent FH binding to CspZ. We thus incubated CspZ-YA IgG (FH-binding sites) or CspZ-YA IgG (non-FH-binding sites) with CspZ-coated enzyme-linked immunosorbent assay (ELISA) plate wells, added FH to each of those wells, and determined the levels of bound FH (Fig. 2A). CspZ-YA IgGs before fractionation (CspZ-YA IgG [total]) and irrelevant rabbit IgGs were included as a control. As expected, CspZ-YA IgGs (total) but not irrelevant rabbit IgG antibodies inhibited FH binding to CspZ (Fig. 2B, Table S1). We found that CspZ-YA IgGs (FH-binding sites) inhibit CspZ-FH binding in a dose-dependent manner, more efficiently than CspZ-YA IgGs (total) (50% inhibitory concentration [IC50] of 4.2 nM). Conversely, CspZ-YA IgGs (non-FH-binding sites) did not inhibit the FH-binding activity of CspZ (Fig. 2B, Table S1). We also determined the capability of each of the mouse monoclonal CspZ-YA IgGs to block FH binding to CspZ. We observed that those IgGs that do not bind to the FH-binding sites of CspZ (no. 142, 224, 582, 605, 651, 1006, 1139, 1193) did not reduce FH binding to CspZ while those that bound to the FH-binding sites of CspZ (no. 1139 and 1193) could (Fig. 2C, see Table S1 for IC50). These results indicate that CspZ-YA IgGs that recognize FH-binding sites selectively prevent FH binding to CspZ.
FIG 2.
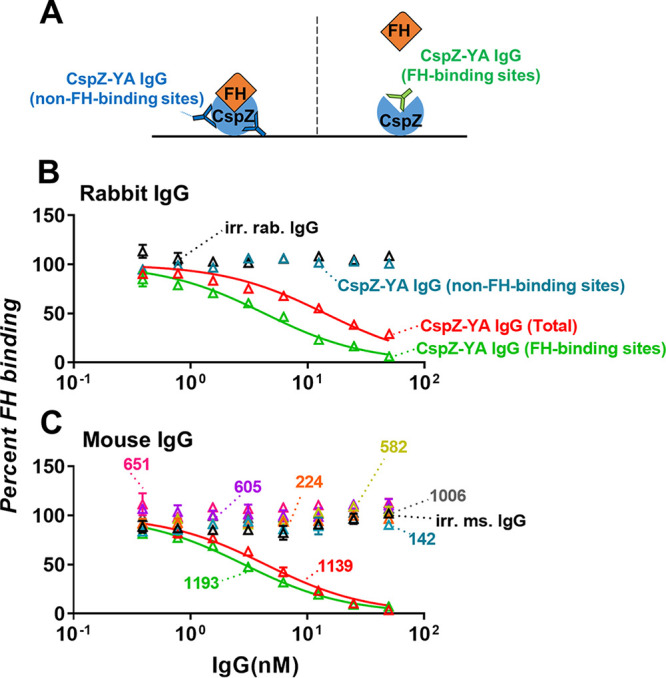
Mouse- and rabbit-derived IgGs that recognize the CspZ FH-binding site selectively block the human FH-binding activity of CspZ. (A) Experimental setup. (B and C) Each of the CspZ-YA IgGs was added into the CspZ-coated ELISA plate wells. These IgGs include (B) CspZ-YA rabbit polyclonal IgG (CspZ-YA IgG [total]), those IgGs that recognize the FH-binding site of CspZ (CspZ-YA IgG [FH-binding sites]), or non-FH-binding site (CspZ-YA IgG [non-FH-binding sites]), or (C) CspZ-YA mouse monoclonal IgGs at indicated concentrations (see Materials and Methods). The wells treated with irrelevant IgGs from rabbits (irr. rab. IgG) and mice (irr. ms. IgG) (50 nM) and PBS (data not shown) were included as a control in the same dose-dependent fashion. Each of those wells was then incubated with human FH, and the levels of bound FH were quantified using sheep anti-human FH and goat anti-sheep HRP IgG as primary and secondary antibodies, respectively. The work was performed on three independent experiments; within each experiment, samples were run in triplicate. Data are expressed as the percent human FH binding, derived by normalizing the levels of bound human FH from IgG-treated wells to those from PBS-treated wells. Data shown are the mean ± SEM of the percent human FH binding from three replicates. Shown is one representative experiment. The concentrations of the IgG to inhibit 50% of human FH bound by CspZ (IC50) were obtained from curve-fitting and extrapolation of panels B and C and shown in Table S1.
CspZ-YA IgGs that recognize CspZ FH-binding sites robustly killed Lyme borreliae in vitro.
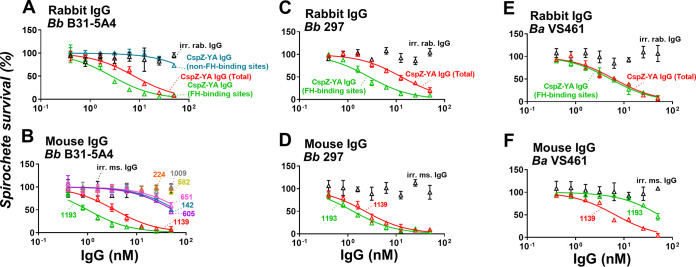
We have shown that CspZ-YA vaccination triggers borreliacidal antibodies (32), raising the possibility of IgGs that recognize FH- and/or non-FH-binding sites of CspZ to kill Lyme borreliae. We examined this possibility and found that the irrelevant rabbit IgGs do not eliminate B. burgdorferi B31-5A4, but CspZ-YA IgGs (total) killed those spirochetes in a dose-dependent manner (Fig. 3A; the 50% borreliacidal activity of each IgG [BA50] was 7.4 nM, Table S2). We found that CspZ-YA IgGs (non-FH-binding sites) did not efficiently kill B. burgdorferi B31-5A4 since the results from this IgG did not allow accurate fitting to obtain a BA50 value (Fig. 3A, Table S2). However, CspZ-YA IgGs (FH-binding sites) robustly eradicated those spirochetes, with BA50 values (BA50 = 2.56 nM) significantly lower than those of CspZ-YA IgGs (total) (Fig. 3A, Table S2). We also tested the bactericidal activity of each of the CspZ-YA mouse monoclonal IgGs in the same fashion and observed that irrelevant mouse IgG antibodies did not kill B. burgdorferi B31-5A4 (Fig. 3B). We found that the IgGs that did not recognize the FH-binding sites of CspZ could be divided into two groups based on their bactericidal activities: those IgGs that did not have any borreliacidal activities (no. 224, 582, 1009; Fig. 3B) and those that still killed bacteria but were not efficient enough to acquire accurate BA50 values (no. 142, 605, and 651; Fig. 3B, Table S2). On the contrary, both IgGs that recognized the FH-binding sites of CspZ (no. 1139 and 1193; Fig. 2C) efficiently killed spirochetes, with no. 1193 (BA50 = 1.23 nM) eradicating bacteria more efficiently than no. 1139 (BA50 = 3.45 nM) (Fig. 2C, Table S2). We further evaluated the ability of the IgGs that robustly killed B. burgdorferi B31-5A4 (CspZ-YA IgG [total], CspZ-YA IgG [FH-binding sites], no. 1139, and no. 1193) to eradicate other strains and species of Lyme borreliae, including B. burgdorferi 297 and B. afzelii VS461. Similar to the results for B. burgdorferi B31-5A4, these IgGs eradicated B. burgdorferi 297 and B. afzelii VS461 in a dose-dependent manner (Fig. 3C to E and Table S2). However, CspZ-YA IgGs (FH-binding sites) killed strain 297 more robustly than CspZ-YA IgGs (total) but showed no significantly different efficiency in killing strain VS461 compared to that of CspZ-YA IgGs (total) (Fig. 3C and E, and Table S2). In addition, no. 1139 displayed ability to eliminate strain 297 indistinguishable from that of no. 1193 but showed more robust killing of strain VS461 (Fig. 3D and F, Table S2). These results not only showed the general ability of CspZ-YA IgGs that recognize the CspZ FH-binding sites to efficiently kill Lyme borreliae but also exhibited that these antibodies varied in their efficiency against different bacterial strains and species.
FIG 3.
IgGs that recognize the CspZ FH-binding sites eliminate different Lyme borreliae species and strains more efficiently than those that bind to CspZ non-FH-binding sites. Each of the CspZ-YA IgG samples or irrelevant IgG samples from rabbits (irr. rab. IgG) or mice (irr. ms. IgG) or PBS (control, data not shown) was serially diluted as indicated and mixed with guinea pig complement and (A and B) B. burgdorferi strains B31-5A4 (Bb B31-5A4) or (C and D) 297 (Bb 297) or (E and F) B. afzelii strain VS461 (Ba VS461) (5 × 105 cells mL−1). These IgGs include (A, C, E) CspZ-YA IgG (CspZ-YA IgG [total]), those IgGs that recognize the FH-binding site of CspZ (CspZ-YA IgG [FH-binding sites]), or those that recognize the non-FH-binding site (CspZ-YA IgG [non-FH-binding sites]), or (B, D, F) indicated CspZ-YA mouse monoclonal mouse IgGs at indicated concentrations. After incubated for 24 h, surviving spirochetes were quantified from three fields of view for each sample using dark-field microscopy. The work was performed on three independent experiments. The survival percentage was derived from the proportion of IgG-treated to PBS-treated spirochetes. Data shown are the mean ± SEM of the survival percentage from three replicates. Shown is one representative experiment. The 50% borreliacidal activity of each IgG (BA50), representing the IgG concentrations that effectively killed 50% of spirochetes, was obtained and extrapolated from curve-fitting and shown in Table S2. Data shown are the mean ± SEM of the borreliacidal titers from three experiments.
Passive immunization with CspZ-YA IgGs that recognize CspZ FH-binding sites reduced the seropositivity and levels of colonization by B. burgdorferi.
Our previous efforts illustrate that passive inoculation of sera from CspZ-YA vaccination protects mice from Lyme borreliosis (32). This observation led to the hypothesis that the IgGs that recognized FH- and/or non-FH-binding sites of CspZ prevent B. burgdorferi infection. To test this hypothesis, we inoculated mice with CspZ-YA IgGs (non-FH-binding sites). Due to insufficient CspZ-YA IgGs (FH-binding sites) recovered after purification for this experiment, MAbs no. 1139 and 1193 were solely used to represent IgGs that recognize CspZ FH-binding sites. Those mice were then fed on by ticks carrying B. burgdorferi B31-5A4 at 1 day postimmunization (Fig. 4A). In addition to uninfected mice, we also included the B31-5A4-infected mice that were inoculated with CspZ-YA IgG (total), irrelevant rabbit IgG, or irrelevant mouse IgG as a control (Fig. 4A).
FIG 4.
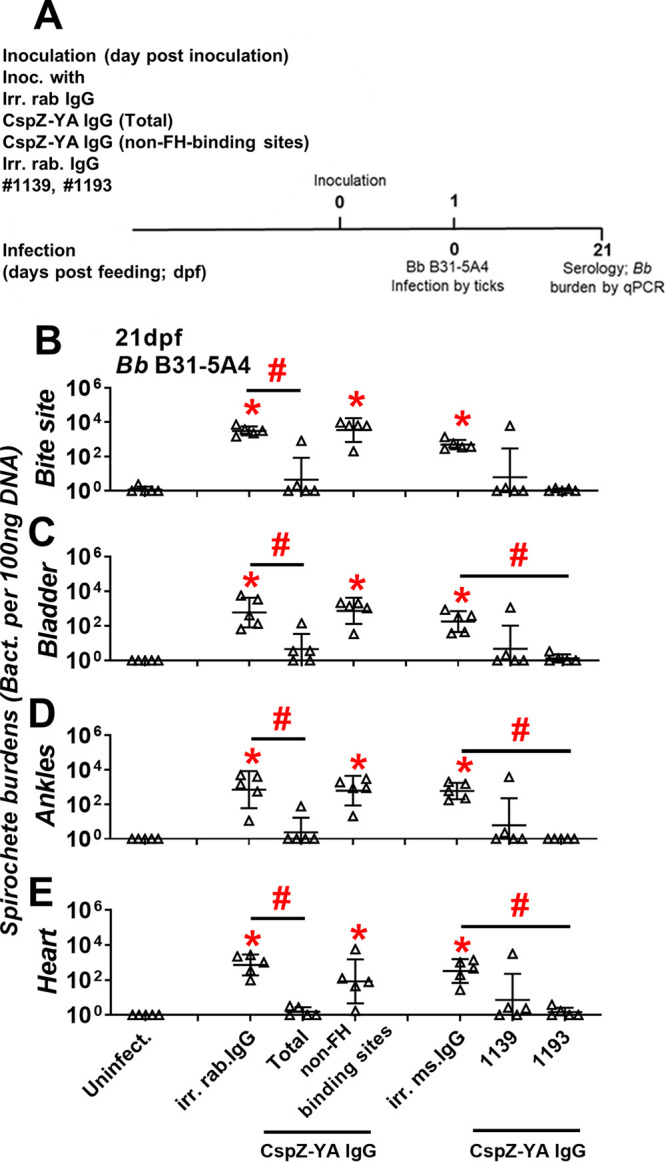
CspZ-YA IgGs that recognize CspZ FH-binding sites selectively prevent B. burgdorferi B31-5A4 infection. (A) Timeframe of the IgG inoculation and B. burgdorferi infection. (B to E) C3H/HeN mice were inoculated with irr. IgG from rabbits (irr. rab. IgG) or mice (irr. ms. IgG) or CspZ-YA IgG samples (1 mg/kg, five mice per group). These CspZ-YA IgGs include total CspZ-YA IgG (total), those IgGs that recognize non-FH-binding site (non-FH-binding sites), or mouse monoclonal IgG no. 1139 or 1193. At 24 h after IgG inoculation, these mice were fed on by I. scapularis nymphs carrying B. burgdorferi B31-5A4 (Bb B31-5A4). An additional five mice inoculated with PBS but not fed on by ticks were included as the control (Uninfect.). The tissues were collected from those mice at 21dpf. Spirochete burdens at (B) the tick feeding site (bite site), (C) bladder, (D) ankles, and (E) heart were quantitatively measured at 21 dpf, shown as the number of spirochetes per 100 ng total DNA. Data shown are the geometric mean ± geometric standard deviation of the spirochete burdens from five mice per group. Statistical significances (P < 0.05, Kruskal-Wallis test with the two-stage step-up method of Benjamini, Krieger, and Yekutieli) of differences in bacterial burdens relative to (*) uninfected mice or (#) between indicated groups of mice are presented.
We found that after feeding on all groups of mice to full engorgement, ticks did not have significantly different levels of bacterial burdens (Fig. S1), consistent with the finding that CspZ is not produced in ticks (24). At 21 days post tick feeding (dpf), we determined the serology of all mice. The uninfected group was seronegative, whereas all five mice inoculated with irrelevant rabbit and mouse IgG were seropositive (Fig. S2, Table 1). Only one of five mice inoculated with CspZ-YA IgGs (total) turned seropositive, a significantly lower number than that of mice inoculated with the irrelevant control (Fig. S2, Table 1). Conversely, all five mice injected with CspZ-YA IgGs (non-FH-binding sites) were seropositive, not significantly different from those injected with the rabbit IgG control (Fig. S2, Table 1). Notably, only one no. 1139-inoculated, and none of the no. 1193-inoculated, mice were seropositive (Fig. S2, Table 1). These results suggest that IgGs that recognize the FH-binding sites of CspZ reduced seroconversion of mice during infection.
TABLE 1.
Seropositivity of mice passively immunized with anti-CspZ-YA IgGs followed by the infection of B. burgdorferi
| Characteristic | Data from mice inoculated with: |
||||||
|---|---|---|---|---|---|---|---|
| PBS | Rabbit IgG |
Mouse IgG |
|||||
| Irr. rab. IgGc | CspZ-YA IgG (total) | CspZ-YA IgG (non-FH-binding sites) | Irr. ms. IgGd | 1139 | 1193 | ||
| Infected with I. scapularis nymphs carrying B. burgdorferi 5A4 | Nob | Yes | Yes | Yes | Yes | Yes | Yes |
| Positive ratea | 0/5 | 5/5e | 1/5 | 5/5e | 5/5e | 1/5 | 0/5 |
Seropositive rate, the number of mice with sera yielding the levels of IgG against C6 peptides greater than the threshold (mean ± 3-fold standard deviation of the C6 IgG levels derived from the PBS-inoculated, uninfected mice).
Uninfected mice.
Irrelevant rabbit IgG, anti-green fluorescence protein of rabbit IgG.
Irrelevant mouse IgG, anti-green fluorescence protein of mouse IgG.
Positive rates significantly greater than those of the uninfected mice determined by two-tailed Fisher test.
We further determined the levels of spirochete colonization of mouse tissues at 21 dpf. Mice inoculated with the irrelevant rabbit IgG control yielded bacterial burdens significantly higher than those of uninfected mice at the bite site, in the bladder, at the ankles, and in the heart (Fig. 4B to E). Mice inoculated with CspZ-YA IgGs (total) displayed spirochete burdens significantly lower than those of mice inoculated with the irrelevant rabbit IgG control for all tested tissues (Fig. 4B to E). Conversely, mice injected with CspZ-YA IgG (non-FH-binding sites) showed bacteria burdens that were indistinguishable from those of mice injected with the irrelevant rabbit IgG control and were significantly higher than those of uninfected mice (Fig. 4B to E). Additionally, mice inoculated with irrelevant mouse IgG control developed bacterial loads significantly greater than those of uninfected mice in all tissues (Fig. 4B to E). Except one nonprotected mouse in the no. 1139-inoculated group, all no. 1139- and no. 1193-inoculated mice yielded bacterial burdens in all tissues indistinguishable from those of uninfected mice. Moreover, the no. 1193-inoculated mice had spirochete loads significantly lower than those of the irrelevant mouse IgG-inoculated control mice in the distal tissues. These results demonstrate the ability of IgG antibodies that recognize the FH-binding sites of CspZ to significantly reduce B. burgdorferi colonization.
DISCUSSION
The antibody repertoire induced by a specific antigen comprises different populations of antibodies that recognize distinct epitopes on that antigen. Some less abundant antibody fractions are considered “immunologically subdominant” (34). When the efficacious antibody population is immunologically subdominant, host-adaptive immune responses may not efficiently eliminate pathogens and/or alleviate manifestations despite overall robust antibody titers (35). Likewise, if the protective epitopes induced by a vaccine antigen were immunologically subdominant, that vaccine may be unable to prevent infection (36). Antigen engineering can enhance the abundance of the antibodies that recognize those immunologically subdominant protective epitopes, thereby improving vaccine efficacy (34). One of the strategies for antigen engineering is to remove the structures preventing exposure of the protective epitopes (e.g., surface polysaccharides that mask the protective epitopes of some viral antigens) (37, 38).
The antigen of interest, CspZ, is upregulated immediately after spirochetes infect hosts and binds to host FH (22, 24). Therefore, any protective epitopes in/around the FH-binding site may not be fully exposed to induce sufficient bactericidal antibodies (32, 33). In fact, high titers of antibodies against CspZ are in humans and mice after exposure to Lyme borreliae, but those spirochetes still persist during infection (28, 30, 32, 33). Additionally, we previously reported null protection in mice after immunization with CspZ but full protection after vaccination with CspZ-YA, a CspZ mutant unable to bind its target ligand (28, 31–33). Thus, the protective epitopes may be immunologically subdominant, and engineering the antigen to remove the FH-binding ability may have triggered the production of these protective antibodies. In the current study, we demonstrated the ability of the antibodies that specifically recognize the CspZ FH-binding site to eradicate B. burgdorferi in vitro and in vivo, demonstrating that the protective epitopes were in/around the FH-binding site. Such an engineering of removing the ability to bind the target ligand in enhancing the levels of the protective antibodies has also been applied to other infectious agents, such as Fhbp, a Neisseria meningitidis antigen currently used as a human vaccine against meningococcal infection (39–41). However, unlike CspZ-YA, removing the FH-binding activity of Fhbp also magnified overall antibody responses (39, 42, 43). Taken together, our findings provide mechanistic insights into how such precise antigen engineering would turn immunologically subdominant epitopes to be the major region that can induce efficacious antibodies against infectious agents.
However, an unanswered question is how those antibodies that recognize the mutant FH-binding sites promote spirochete clearance to protect mice from B. burgdorferi infection. We showed here that the protection from LD in vivo is selectively mediated by the CspZ-YA IgGs that recognize CspZ FH-binding sites. Together with another finding in the current study of antibody-mediated borreliacidal abilities in vitro by the IgGs that recognize CspZ FH-binding sites, it is possible that CspZ-YA IgGs-mediated killing is conferred by the Fc regions of IgGs. As that Fc region promotes classical pathway-mediated pathogen lysis and opsonophagocytosis directly or indirectly by the binding of antibodies to leukocytes (44), these mechanisms would play a role in the bacterial clearance caused by CspZ-YA IgGs. We also found that the CspZ-YA IgGs that recognize FH-binding sites block FH binding to CspZ, which leads to the possibility that these antibodies eliminate bacteria by preventing the ability of spirochetes to evade alternative pathway-mediated killing (e.g., opsonophagocytosis and pathogen lysis) (10). Delineating the role of each of these mechanisms in promoting the protective antibodies upon CspZ-YA vaccination warrants further investigations.
We found that the CspZ-YA IgGs that recognize FH-binding sites efficiently eliminate spirochetes across different strains and species of Lyme borreliae, but the extent of killing varied among the strains. This result is consistent with the fact that the sequences on the FH-binding interface of CspZ are highly similar among the variants of different strains within the same Lyme borreliae species (>98% identity) but moderately variable among the strains from different spirochete species (~80% identity) (29, 32). Moreover, CspZ-YA vaccination not only stops spirochete colonization at tissues but also prevents the development of LD-associated manifestations (i.e., arthritis) (30, 32). Although Lyme borreliae dissemination is a prerequisite for the onset of these manifestations, the severity of such disease symptoms has been shown to not necessarily be linked to the spirochete burdens in tissues (45). Therefore, the results from this study showing the absence of the spirochetes in the tissues from mice inoculated with CspZ-YA IgGs that recognize FH-binding sites may not fully address the mechanisms of manifestation prevention by CspZ-YA vaccines. Despite that, our finding does not preclude the possibility of the antibodies that recognize CspZ FH-binding sites killing spirochetes at the initial infection sites and blocking spirochetes from disseminating to distal tissues. In this study, the insufficient polyclonal CspZ-YA IgG (FH-binding sites) from CspZ-YA-immunized rabbits (~25 μg isolated from six rabbits) justifies the use of no. 1139 and 1193 for the in vivo work. However, both no. 1139 and 1193 may account for only part of the antibody population in the CspZ-YA IgG (FH-binding sites). The fact of the protectivity provided by no. 1139 and 1193 does not rule out the possibility that the uncovered antibody population in CspZ-YA IgG (FH-binding sites) has different phenotypes from no. 1139 and 1193, which is worth further investigation. Further, preexposure prophylaxis (PrEP) is a commonly studied strategy for LD prevention. In fact, in addition to vaccines, a yearly administered monoclonal antibody against OspA, a protein that is required for tick-to-host transmission of spirochetes, is currently in clinical trials (46, 47). Our finding of the bactericidal effect by the antibodies recognizing the CspZ FH-binding site illustrates that identifying monoclonal antibodies that bind these epitopes constitutes a promising option for a prophylactic agent against LD. Collectively, this study identified that the protective epitopes of CspZ-YA vaccines are proximal to the FH-binding site, which further elucidates the potential preventive mechanisms of this vaccine candidate. Such findings would eventually inform the strategy of precision antigen engineering in developing vaccines and monoclonal antibody-based prophylaxis against LD and other infectious diseases.
MATERIALS AND METHODS
Ethics statement.
All mouse experiments were performed in strict accordance with all provisions of the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and the PHS Policy on Humane Care and Use of Laboratory Animals. The protocol (docket number 19–451) was approved by the Institutional Animal Care and Use Agency of Wadsworth Center, New York State Department of Health. All efforts were made to minimize animal suffering.
Mouse, ticks, and bacterial strains.
Three-week-old female C3H/HeN mice were purchased from Charles River (Wilmington, MA, USA). Although such an age of the mice has not reached sexual maturity, the underdevelopment of immune system in this age of mice would allow such mice to be more susceptible to Lyme borreliae infection, increasing the signal to noise ratio of the readout. That will also provide more stringent criteria to define the protectivity. BALB/c C3-deficient mice were from in-house breeding colonies (48), and Ixodes scapularis tick larvae were obtained from BEI Resources (Manassas, VA). Escherichia coli strain BL21(DE3) and derivatives were grown at 37°C or other appropriate temperatures in Luria-Bertani broth or agar, supplemented with kanamycin (50 μg/mL). Borrelia strains were grown at 33°C in Barbour-Stoenner-Kelly II (BSK II) complete medium (49), and these strains include B. burgdorferi strains B31-5A4 (50), 297 (31, 51), and VS461 (52) (Table S3). Cultures of B. burgdorferi strain B31-5A4 were tested with PCR to ensure a full plasmid profile before use (53, 54), whereas B. burgdorferi strain 297 and B. afzelii strain VS461 were maintained as fewer than 10 passages.
Cloning, expression, and purification of CspZ and CspZ-YA.
The DNA encoding CspZ and CspZ-YA with a C-terminal TEV cleavage site (ENLYFQG) followed by a hexahistidine tag (His tag) were codon-optimized based on E. coli codon usage preference and synthesized by GenScript (Piscataway, NJ, USA), followed by subcloning into the pET41a using NdeI/XhoI restriction sites. These plasmids were transformed into E. coli BL21(DE3). The recombinant protein expression was induced with 1 mM isopropyl-β-d-1-thiogalactopyranoside (IPTG). Once expression was confirmed, the clone with the highest expression for each construct was selected to create glycerol seed stocks.
To generate His-tagged CspZ and CspZ-YA, 9 L of basal salt medium (BSM; 5 g/L of K2HPO4, 3.5 g/L of KH2PO4, 3.5 g/L of [NH4]2HPO4, 15 g/L of glucose, 5 g/L of yeast extract [pH 7.2]) was prepared and autoclaved. Before inoculation, 9 mL of K-12 trace salts solution, 36 mL of 25% MgSO4-7H2O, 9 mL of 10% antifoam, 9 mL of 50 mg/mL kanamycin, and 90 mL of 15 g/L CaCl2·2H2O were added aseptically. Nine liters of BSM was then inoculated with a CspZ or CspZ-YA seed culture to a starting optical density at 600 nm (OD600) of 0.05. The culture was grown at 37°C until OD600 reached 0.5 to 1.0 and the induction phase was initiated. During the induction phase, the culture was induced with 0.2 mM IPTG at 22°C and pH 7.2. After 8 h of induction, fed-batch feeding (50% vol/vol glucose) was optionally added at 2 mL/L/h. Thirty percent dissolved oxygen (DO) was maintained throughout fermentation. The cell paste was harvested after 19-h induction by centrifugation and stored at −80°C until purification. To further purify His-tagged CspZ or CspZ-YA, the cell paste was first thawed and resuspended in 50 mM phosphate buffer with 500 mM NaCl and 20 mM imidazole at pH 7.4 at a ratio of 20 mL buffer per gram and homogenized 3 times on ice at 15,000 lb/in2 with an EmulsiFlex-C3 high-pressure homogenizer. The lysed cells were centrifuged, and only the supernatant with the soluble proteins was then further filtered with 0.45 μm filters and loaded onto two connecting 5 mL HisTrap IMAC FF columns (GE Healthcare) which were preequilibrated with 50 mM phosphate buffer with 500 mM NaCl and 20 mM imidazole at pH 7.4. The columns were washed with 10 column volumes (CVs) of 50 mM phosphate buffer with 500 mM NaCl and 20 mM imidazole at pH 7.4 followed by eluting with a linear gradient of 20 mM to 500 mM imidazole over 20 CVs. The purified His-tagged CspZ or CspZ-YA was dialyzed against phosphate-buffered saline (PBS) or Tris-buffered saline (TBS) and verified with the integrity and purity greater than 90% using SDS-PAGE (Fig. S3) before storage at −80°C.
Generation of CspZ-YA mouse monoclonal and rabbit polyclonal antibodies.
Purified His-tagged CspZ-YA was provided to Genemed Synthesis, Inc. (San Antonio, TX) to generate CspZ-YA IgG (total). The IgGs in the immunized rabbits containing CspZ-YA IgGs and naive rabbit IgGs were purified using Protein A chromatography and formulated in 1× PBS with 10% BSA (pH 7.4). To retrieve the CspZ-YA (total), we first covalently conjugated CspZ onto AminoLink plus coupling resin (ThermoFisher Scientific, Waltham, MA) based on the manufacturer’s instructions. The 5-mL IgGs from CspZ-YA-immunized rabbits were subsequently mixed to 100 μL CspZ-conjugated resin slurry (resin to PBST buffer ratio as 1:1) for 4 h to capture CspZ-YA IgG (total). The resin was then washed twice with 1× PBST buffer (1× PBS with 0.05% Tween 20). Finally, the captured CspZ-YA IgG (total) was eluted with 0.65 mL of glycine buffer (0.1 M glycine HCl at pH 2.5) and dialyzed against 1× PBST buffer.
To isolate the CspZ-YA IgG (FH-binding sites) and CspZ-YA IgG (non-FH-binding sites) from CspZ-YA IgG (total), 100 μL of CspZ-conjugated resin slurry was mixed with excess factor H (Complement Technology, Tyler, TX, USA) for 2 h, allowing the formation of CspZ-FH. This step is essential to block the FH-binding site on CspZ. After washing off the excess FH using 1× PBST buffer, that CspZ-FH resin was mixed with the CspZ-YA IgG (total) for 90 min. After incubation and the centrifugation of the resin, the unbound fraction containing CspZ-YA IgG (FH-binding sites) was collected from the supernatant. The resulting resin was then washed with 1× PBST, and the bound fraction containing CspZ-YA IgG (non-FH-binding sites) was eluted using 0.1 M glycine HCl at pH 2.5. However, FH was found to be eluted with CspZ-YA IgG (non-FH-binding sites); thus, to remove that FH, the eluted proteins were further dialyzed against 1× PBST, followed by Protein A affinity purification to capture the CspZ-YA IgG (non-FH-binding sites). The purified CspZ-YA IgG (non-FH-binding sites) was eventually eluted from Protein A resin using the glycine buffer and dialyzed against 1× PBST.
The medium samples from each of the eight clones of mouse hybridoma cells producing CspZ-YA mouse monoclonal antibodies were generated by Protein and Monoclonal Antibody Production Core at Baylor College of Medicine. Each of these medium samples was then applied to NA protein G spin columns to purify the CspZ-YA mouse monoclonal IgG (ThermoFisher Scientific) and then quantitated as described in the vendor’s manual.
ELISAs.
To verify the ability of rabbit polyclonal and mouse monoclonal CspZ-YA IgGs to recognize FH-binding sites of CspZ, we compared the levels of each of these IgGs in binding to FH-saturated CspZ with those in binding to CspZ proteins treated with BSA (control). One microgram of histidine-tagged CspZ was coated on ELISA plate wells, followed by being blocked with 5% BSA in PBS buffer. Those wells were subsequently treated with human FH (500 nM, ComTech, Tyler, TX), BSA (control; 500 nM, Sigma-Aldrich, St. Louis, MO), or PBS (control), followed by incubation with each of the tested rabbit polyclonal and mouse monoclonal CspZ-YA IgGs (50 nM). Horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG (Sigma-Aldrich) and goat anti-rabbit IgG (Sigma-Aldrich) were used to detect the binding of mouse monoclonal and rabbit polyclonal CspZ-YA IgGs, respectively. Tetramethylbenzidine solution (ThermoFisher) was added to each well and incubated for 5 min, and then the reaction was stopped with hydrosulfuric acid. Plates were read at 405 nm using a Tecan Sunrise microplate reader (Tecan, Morrisville, NC). The resulting absorption values were normalized to those from PBS-treated wells to obtain the percentage of CspZ-YA IgG binding.
To determine the ability of rabbit polyclonal and mouse monoclonal CspZ-YA IgGs to prevent FH binding to CspZ, the ELISA was performed as described previously with modifications (32). Each ELISA microtiter well was coated with 1 μg of histidine-tagged CspZ. After being blocked with 5% BSA in PBS buffer, the wells were incubated with PBS (control), serially diluted irrelevant mouse IgG (anti-green fluorescence protein of mouse IgG; ThermoFisher) or irrelevant rabbit IgG (anti-green fluorescence protein of rabbit IgG; ThermoFisher), or each of the mouse monoclonal or rabbit polyclonal CspZ-YA IgGs (0.4 nM, 0.8 nM, 1.6 nM, 3.125 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM) followed by being mixed with 500 nM human FH. Sheep anti-human FH (1:200×; ThermoFisher) and then goat anti-sheep HRP (1:2,000×; ThermoFisher) were added, and the levels of FH binding were detected by ELISA as mentioned above. Data were expressed as the proportion of FH binding from serum-treated to PBS-treated wells. The 50% inhibitory concentration (IC50) (Table S1), representing the IgG concentration that blocks 50% of FH binding, was calculated using dose-response stimulation fitting in GraphPad Prism 5.04.
The seropositivity of the mice after infection with B. burgdorferi was determined by detecting the presence or absence of the antibodies that recognize C6 peptides, which has been commonly used for human Lyme disease diagnosis (55). Fifty microliters of serially diluted mouse serum (1:100×, 1:300×, 1:900×) from 21 dpf was added to microtiter wells coated with C6 peptides (Genemed Synthesis, Inc.) (55). Total IgG was detected using HRP-conjugated goat anti-mouse IgG (1:20,000×; Bethyl, Montgomery, TX, USA). After the incubation with antibodies for 1 h, tetramethyl benzidine solution (ThermoFisher) was added, and the absorbance was detected at 620 nm for 10 cycles of 60-s kinetic intervals with 10-s shaking duration using Tecan Sunrise Microplate reader as described above. For each serum sample, the maximum slope of optical density per minute of all the dilutions was multiplied by the respective dilution factor, and the greatest value was used as representative of antibody titers (arbitrary unit [AU]). The seropositive mice were defined as the mice with the serum samples yielding a value greater than the threshold, the mean plus 3-fold standard deviation of the IgG values derived from the uninfected mice.
Borreliacidal assays.
The ability of CspZ-YA mouse monoclonal and rabbit polyclonal IgGs to eradicate spirochetes was determined as described previously with modifications (32). Briefly, irrelevant mouse or rabbit IgGs, or each of these CspZ-YA mouse monoclonal or rabbit polyclonal antibodies, were serially diluted to the indicated concentrations (0.4 nM, 0.8 nM, 1.6 nM, 3.125 nM, 6.25 nM, 12.5 nM, 25 nM, 50 nM). The diluted IgGs were then mixed with complement-preserved guinea pig serum (Sigma-Aldrich; final concentration 5%). Note that this concentration of guinea pig serum has been verified to not kill spirochetes in the absence of CspZ-YA antibodies (data not shown). The PBS treatment was included as a control. After incubation with the strains B31-5A4, 297, or VS461, the mixture was incubated at 33°C for 24 h. Surviving spirochetes were quantified by directly counting the motile spirochetes using dark-field microscopy and expressed as the proportion of IgG-treated to PBS-treated Lyme borreliae. The 50% borreliacidal titer is shown in Table S2, representing the serum dilution rate that kills 50% of spirochetes, which was calculated using dose-response stimulation fitting in GraphPad Prism 5.04.
Generation of infected ticks.
Generating infected I. scapularis ticks has been described previously (56). BALB/c C3-deficient mice were infected intradermally with 105 of the strains B31-5A4 (27, 48). Ear tissues were collected via ear punch, and bacterial gDNA was purified for detection with quantitative PCR (qPCR) to confirm infection (see “IgG inoculation, B. burgdorferi infection, and quantification of spirochete burdens”). Approximately 100 to 200 uninfected larvae were then allowed to feed to repletion on the infected mice as described previously (56). The engorged larvae were collected and allowed to molt into nymphs in a desiccator at room temperature with 95% relative humidity and light-dark control (light to dark, 16:8 h).
IgG inoculation, B. burgdorferi infection, and quantification of spirochete burdens.
Three-week-old female C3H/HeN mice were subcutaneously inoculated with irrelevant mouse or rabbit IgG or each of the tested CspZ-YA mouse monoclonal or rabbit polyclonal IgGs (1 mg/kg). Five mice per group were used in this study. This number was justified by the power analysis. Using the means ± standard deviation from this study (10 ± 10 for the uninfected control group, 55 ± 10 for the infection group), a power analysis for attaining a statistically significant difference (P < 0.05; a one-way analysis of variance [ANOVA]) between the negative-control group and other groups with 95% probability requires a minimum of 5 animals per group (57). This value is consistent with numbers that we and others have used in the past for similar studies (32, 58). At 24 h after inoculation, five nymphs carrying B. burgdorferi strain B31-5A4 were allowed to feed to repletion on each mouse, and a subset of nymphs was collected pre- and post-feeding as described previously (32, 48). Mice were sacrificed at 21 dpf to collect the biting site of skin, knees, heart, and bladder. DNA was purified from these nymphs, and spirochetes were quantified as described with modifications (32). DNA was purified using EZ-10 spin column animal genomic DNA mini-prep kit (Bio Basic, Inc., Markham, Ontario, CA). Spirochete burdens were quantified based on the amplification of recA from B. burgdorferi strain B31-5A4 using the primers (BBRecAfp [5′-GTGGATCTATTGTATTAGATGAGGCTCTCG-3′] and BBRecArp [5′-GCCAAAGTTCTGCAACATTAACACCTAAAG-3′]) with qPCR using an Applied Biosystems 7500 real-time PCR system (ThermoFisher) in conjunction with PowerUp SYBR green master mix (ThermoFisher) as described previously (33, 59). The number of recA copies was calculated by establishing a quantification cycle (Cq) standard curve of a known number of recA genes extracted from strain B31-5A4, and burdens were reported as the number of recA copies per tick or normalized to 100 ng of total DNA and reported as the number of recA copies per 100 ng of total DNA.
Statistical analyses.
Significant differences were determined with a Mann-Whitney test (between two groups) (60), Kruskal-Wallis test with the two-stage step-up method of Benjamini, Krieger, and Yekutieli (61) (more than two groups), or two-tailed Fisher test (for seropositivity in Table S3) (62) using GraphPad Prism 5.04. A P value of <0.05 was used to determine significance.
ACKNOWLEDGMENTS
We thank John Leong for providing B. burgdorferi strains B31-5A4 and 297, as well as B. afzelii strain VS461. We also thank Nicholas Mantis, Klemen Strle, and ChingLin Hsieh for valuable advice, and the Wadsworth Animal Core for assistance with animal care. We appreciate Dean Edwards, Yingmin Zhu, Kurt Christensen, and Karen Moberg from Baylor College of Medicine Protein and Monoclonal Antibody Production Core for the consultation and generation of the CspZ-YA monoclonal antibody. This work was supported by NIH grant R21AI144891 (Y.L.C., A.L.M., T.A.N., M.E.B., W.H.C., Y.P.L., R.T.K., and Z.L.) and NCI‐CA125123 (supporting the generation of CspZ-YA monoclonal antibody in Baylor College of Medicine Protein and Monoclonal Antibody Production Core). The funders had no role in study design, data collection, interpretation, or the decision to submit the work for publication. We have no conflict of interest to declare.
Footnotes
Supplemental material is available online only.
Contributor Information
Wen-Hsiang Chen, Email: Wen-Hsiang.Chen@bcm.edu.
Yi-Pin Lin, Email: Yi-Pin.Lin@health.ny.gov.
De’Broski R. Herbert, University of Pennsylvania
REFERENCES
- 1.Kugeler KJ, Schwartz AM, Delorey MJ, Mead PS, Hinckley AF. 2021. Estimating the frequency of Lyme disease diagnoses, United States, 2010–2018. Emerg Infect Dis 27:616–619. 10.3201/eid2702.202731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, Li X, Mead PS. 2016. Lyme borreliosis. Nat Rev Dis Primers 2:16090. 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomes-Solecki M, Arnaboldi PM, Backenson PB, Benach JL, Cooper CL, Dattwyler RJ, Diuk-Wasser M, Fikrig E, Hovius JW, Laegreid W, Lundberg U, Marconi RT, Marques AR, Molloy P, Narasimhan S, Pal U, Pedra JHF, Plotkin S, Rock DL, Rosa P, Telford SR, Tsao J, Yang XF, Schutzer SE. 2019. Protective immunity and new vaccines for Lyme disease. Clin Infect Dis 70:1768–1773. 10.1093/cid/ciz872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Embers ME, Narasimhan S. 2013. Vaccination against Lyme disease: past, present, and future. Front Cell Infect Microbiol 3:6. 10.3389/fcimb.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuijt TJ, Hovius JW, van der Poll T, van Dam AP, Fikrig E. 2011. Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol 27:40–47. 10.1016/j.pt.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Shen AK, Mead PS, Beard CB. 2011. The Lyme disease vaccine–a public health perspective. Clin Infect Dis 52 Suppl 3:s247–s252. 10.1093/cid/ciq115. [DOI] [PubMed] [Google Scholar]
- 7.Steere AC, Drouin EE, Glickstein LJ. 2011. Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis. Clin Infect Dis 52 Suppl 3:s259–s265. 10.1093/cid/ciq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurokawa C, Lynn GE, Pedra JHF, Pal U, Narasimhan S, Fikrig E. 2020. Interactions between Borrelia burgdorferi and ticks. Nat Rev Microbiol 18:587–600. 10.1038/s41579-020-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reis ES, Mastellos DC, Hajishengallis G, Lambris JD. 2019. New insights into the immune functions of complement. Nat Rev Immunol 19:503–516. 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zipfel PF, Hallstrom T, Riesbeck K. 2013. Human complement control and complement evasion by pathogenic microbes–tipping the balance. Mol Immunol 56:152–160. 10.1016/j.molimm.2013.05.222. [DOI] [PubMed] [Google Scholar]
- 12.Blom AM. 2017. The role of complement inhibitors beyond controlling inflammation. J Intern Med 282:116–128. 10.1111/joim.12606. [DOI] [PubMed] [Google Scholar]
- 13.Ricklin D, Reis ES, Lambris JD. 2016. Complement in disease: a defence system turning offensive. Nat Rev Nephrol 12:383–401. 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meri T, Amdahl H, Lehtinen MJ, Hyvarinen S, McDowell JV, Bhattacharjee A, Meri S, Marconi R, Goldman A, Jokiranta TS. 2013. Microbes bind complement inhibitor factor H via a common site. PLoS Pathog 9:e1003308. 10.1371/journal.ppat.1003308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YP, Diuk-Wasser MA, Stevenson B, Kraiczy P. 2020. Complement evasion contributes to Lyme borreliae-host associations. Trends Parasitol 36:634–645. 10.1016/j.pt.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skare JT, Garcia BL. 2020. Complement evasion by Lyme disease spirochetes. Trends Microbiol 28:889–899. 10.1016/j.tim.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulipati V, Meri S, Panelius J. 2020. Complement evasion strategies of Borrelia burgdorferi sensu lato. FEBS Lett 594:2645–2656. 10.1002/1873-3468.13894. [DOI] [PubMed] [Google Scholar]
- 18.Blom AM, Hallstrom T, Riesbeck K. 2009. Complement evasion strategies of pathogens—acquisition of inhibitors and beyond. Mol Immunol 46:2808–2817. 10.1016/j.molimm.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 19.Miajlovic H, Smith SG. 2014. Bacterial self-defence: how Escherichia coli evades serum killing. FEMS Microbiol Lett 354:1–9. 10.1111/1574-6968.12419. [DOI] [PubMed] [Google Scholar]
- 20.Guerry P, Poly F, Riddle M, Maue AC, Chen YH, Monteiro MA. 2012. Campylobacter polysaccharide capsules: virulence and vaccines. Front Cell Infect Microbiol 2:7. 10.3389/fcimb.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zipfel PF, Skerka C. 2009. Complement regulators and inhibitory proteins. Nat Rev Immunol 9:729–740. 10.1038/nri2620. [DOI] [PubMed] [Google Scholar]
- 22.Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, Miller JC, Stevenson B, Wallich R, Zipfel PF, Kraiczy P. 2006. Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol 61:1220–1236. 10.1111/j.1365-2958.2006.05318.x. [DOI] [PubMed] [Google Scholar]
- 23.Lin YP, Frye AM, Nowak TA, Kraiczy P. 2020. New insights into CRASP-mediated complement evasion in the Lyme disease enzootic cycle. Front Cell Infect Microbiol 10:1. 10.3389/fcimb.2020.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, Kraiczy P, Stevenson B. 2007. Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete's mammal-tick infection cycle. Infect Immun 75:4227–4236. 10.1128/IAI.00604-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tokarz R, Anderton JM, Katona LI, Benach JL. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun 72:5419–5432. 10.1128/IAI.72.9.5419-5432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brooks CS, Hefty PS, Jolliff SE, Akins DR. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun 71:3371–3383. 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcinkiewicz AL, Dupuis AP, 2nd, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, Kramer LD, Lin YP. 2019. Blood treatment of Lyme borreliae demonstrates the mechanism of CspZ-mediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol 21:e12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers EA, Marconi RT. 2007. Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun 75:5272–5281. 10.1128/IAI.00850-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers EA, Abdunnur SV, McDowell JV, Marconi RT. 2009. Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun 77:4396–4405. 10.1128/IAI.00393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraiczy P, Seling A, Brissette CA, Rossmann E, Hunfeld KP, Bykowski T, Burns LH, Troese MJ, Cooley AE, Miller JC, Brade V, Wallich R, Casjens S, Stevenson B. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 (CspZ) as a serological marker of human Lyme disease. Clin Vaccine Immunol 15:484–491. 10.1128/CVI.00415-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, Kenedy MR, Anderson JF, Akins DR, Pal U. 2008. Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One 3:3010e. 10.1371/journal.pone.0003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcinkiewicz AL, Lieknina I, Yang X, Lederman PL, Hart TM, Yates J, Chen WH, Bottazzi ME, Mantis NJ, Kraiczy P, Pal U, Tars K, Lin YP. 2020. The factor H-binding site of CspZ as a protective target against multistrain, tick-transmitted Lyme disease. Infect Immun 88:e00956-19. 10.1128/IAI.00956-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcinkiewicz AL, Lieknina I, Kotelovica S, Yang X, Kraiczy P, Pal U, Lin YP, Tars K. 2018. Eliminating factor H-binding activity of Borrelia burgdorferi CspZ combined with virus-like particle conjugation enhances its efficacy as a Lyme disease vaccine. Front Immunol 9:181. 10.3389/fimmu.2018.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caradonna TM, Schmidt AG. 2021. Protein engineering strategies for rational immunogen design. NPJ Vaccines 6:154. 10.1038/s41541-021-00417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angeletti D, Gibbs JS, Angel M, Kosik I, Hickman HD, Frank GM, Das SR, Wheatley AK, Prabhakaran M, Leggat DJ, McDermott AB, Yewdell JW. 2017. Defining B cell immunodominance to viruses. Nat Immunol 18:456–463. 10.1038/ni.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman MO, Angeletti D, Yewdell JW. 2018. Antibody immunodominance: the key to understanding influenza virus antigenic drift. Viral Immunol 31:142–149. 10.1089/vim.2017.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubrovskaya V, Tran K, Ozorowski G, Guenaga J, Wilson R, Bale S, Cottrell CA, Turner HL, Seabright G, O'Dell S, Torres JL, Yang L, Feng Y, Leaman DP, Vazquez Bernat N, Liban T, Louder M, McKee K, Bailer RT, Movsesyan A, Doria-Rose NA, Pancera M, Karlsson Hedestam GB, Zwick MB, Crispin M, Mascola JR, Ward AB, Wyatt RT. 2019. Vaccination with glycan-modified HIV NFL envelope trimer-liposomes elicits broadly neutralizing antibodies to multiple sites of vulnerability. Immunity 51:915–929.e7. 10.1016/j.immuni.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu WC, Jan JT, Huang YJ, Chen TH, Wu SC. 2016. Unmasking stem-specific neutralizing epitopes by abolishing N-linked glycosylation sites of influenza virus hemagglutinin proteins for vaccine design. J Virol 90:8496–8508. 10.1128/JVI.00880-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beernink PT, Shaughnessy J, Braga EM, Liu Q, Rice PA, Ram S, Granoff DM. 2011. A meningococcal factor H binding protein mutant that eliminates factor H binding enhances protective antibody responses to vaccination. J Immunol 186:3606–3614. 10.4049/jimmunol.1003470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granoff DM, Costa I, Konar M, Giuntini S, Van Rompay KK, Beernink PT. 2015. Binding of complement factor H (FH) decreases protective anti-FH binding protein antibody responses of infant Rhesus macaques immunized with a meningococcal serogroup B vaccine. J Infect Dis 212:784–792. 10.1093/infdis/jiv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Granoff DM, Giuntini S, Gowans FA, Lujan E, Sharkey K, Beernink PT. 2016. Enhanced protective antibody to a mutant meningococcal factor H-binding protein with low-factor H binding. JCI Insight 1:e88907. 10.1172/jci.insight.88907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giuntini S, Beernink PT, Granoff DM. 2015. Effect of complement Factor H on anti-FHbp serum bactericidal antibody responses of infant rhesus macaques boosted with a licensed meningococcal serogroup B vaccine. Vaccine 33:7168–7175. 10.1016/j.vaccine.2015.10.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossi R, Granoff DM, Beernink PT. 2013. Meningococcal factor H-binding protein vaccines with decreased binding to human complement factor H have enhanced immunogenicity in human factor H transgenic mice. Vaccine 31:5451–5457. 10.1016/j.vaccine.2013.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park HI, Yoon HW, Jung ST. 2016. The highly evolvable antibody Fc domain. Trends Biotechnol 34:895–908. 10.1016/j.tibtech.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. 2011. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or lyme arthritis. Arthritis Rheum 63:2238–2247. 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Kern A, Boatright NK, Schiller ZA, Sadowski A, Ejemel M, Souders CA, Reimann KA, Hu L, Thomas WD, Jr, Klempner MS. 2016. Pre-exposure prophylaxis with OspA-specific human monoclonal antibodies protects mice against tick transmission of Lyme disease spirochetes. J Infect Dis 214:205–211. 10.1093/infdis/jiw151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anonymous. 2021. First clinical study of the safety and blood levels of a human monoclonal antibody (2217LS) against lyme disease bacteria in healthy people. https://ClinicalTrials.gov/show/NCT04863287. Celerion Inc., Lincoln, NE. [Google Scholar]
- 48.Hart T, Nguyen NTT, Nowak NA, Zhang F, Linhardt RJ, Diuk-Wasser M, Ram S, Kraiczy P, Lin YP. 2018. Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog 14:e1007106. 10.1371/journal.ppat.1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barbour AG, Burgdorfer W, Grunwaldt E, Steere AC. 1983. Antibodies of patients with Lyme disease to components of the Ixodes dammini spirochete. J Clin Invest 72:504–515. 10.1172/jci110998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elias AF, Stewart PE, Grimm D, Caimano MJ, Eggers CH, Tilly K, Bono JL, Akins DR, Radolf JD, Schwan TG, Rosa P. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect Immun 70:2139–2150. 10.1128/IAI.70.4.2139-2150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, Malawista SE. 1983. The spirochetal etiology of Lyme disease. N Engl J Med 308:733–740. 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- 52.Benoit VM, Fischer JR, Lin YP, Parveen N, Leong JM. 2011. Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect Immun 79:3501–3509. 10.1128/IAI.00163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Purser JE, Norris SJ. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc Natl Acad Sci USA 97:13865–13870. 10.1073/pnas.97.25.13865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bunikis I, Kutschan-Bunikis S, Bonde M, Bergstrom S. 2011. Multiplex PCR as a tool for validating plasmid content of Borrelia burgdorferi. J Microbiol Methods 86:243–247. 10.1016/j.mimet.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Liang FT, Bowers LC, Philipp MT. 2001. C-terminal invariable domain of VlsE is immunodominant but its antigenicity is scarcely conserved among strains of Lyme disease spirochetes. Infect Immun 69:3224–3231. 10.1128/IAI.69.5.3224-3231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kern A, Zhou CW, Jia F, Xu Q, Hu LT. 2016. Live-vaccinia virus encapsulation in pH-sensitive polymer increases safety of a reservoir-targeted Lyme disease vaccine by targeting gastrointestinal release. Vaccine 34:4507–4513. 10.1016/j.vaccine.2016.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faul F, Erdfelder E, Lang AG, Buchner A. 2007. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39:175–191. 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 58.Yang X, Coleman AS, Anguita J, Pal U. 2009. A chromosomally encoded virulence factor protects the Lyme disease pathogen against host-adaptive immunity. PLoS Pathog 5:e1000326. 10.1371/journal.ppat.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin YP, Benoit V, Yang X, Martinez-Herranz R, Pal U, Leong JM. 2014. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the Lyme disease spirochete. PLoS Pathog 10:e1004238. 10.1371/journal.ppat.1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mann HB, Whitney DR. 1947. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Statist 18:50–60. 10.1214/aoms/1177730491. [DOI] [Google Scholar]
- 61.Benjamini Y, Krieger AM, Yekutieli D. 2006. Adaptive linear step-up procedures that control the false discovery rate. Biometrika 93:491–507. 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- 62.Fisher RA. 1934. Statistical methods for research workers, 5th ed. Oliver and Boyd, Edinburgh, United Kingdom. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download iai.00062-22-s0001.pdf, PDF file, 0.8 MB (846.4KB, pdf)