Abstract
Aim
To elucidate the relationship between serum selenium levels and the risk of mortality and new‐onset heart failure (HF) in the general adult population.
Methods and results
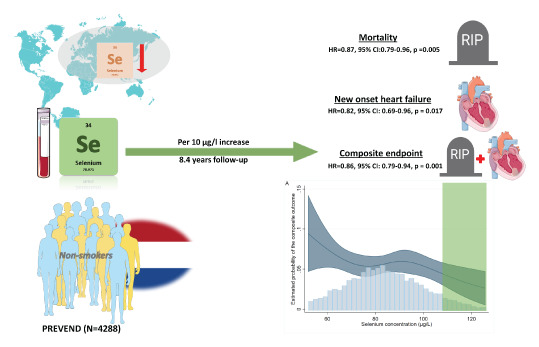
Selenium was measured in a Dutch cohort and a retrospective analysis of prospectively assessed data was performed. Main outcome measures were all‐cause mortality and incidence of new‐onset HF separately, and combined as a composite endpoint. Serum selenium was measured in 5973 subjects and mean selenium concentration was 84.6 (±19.5) µg/L. Mean age was 53.6 (±12.1) years and 3103 subjects (52%) were female. Median follow‐up was 8.4 years. Selenium levels associated positively with female sex, higher total cholesterol and glucose concentrations, and associated negatively with incidence of anaemia, iron deficiency, current smoking, increased C‐reactive protein levels, and higher body mass index. Univariate analysis on all subjects showed no association of continuous selenium concentrations, per 10 µg/L increase, with the composite endpoint (hazard ratio [HR] 0.96, 95% confidence interval [CI] 0.87–1.06, p = 0.407). However, significant interaction with smoking status was observed. In non‐smoking subjects (n = 4288), continuous selenium concentrations were independently associated with reduced mortality risk (HR 0.87, 95% CI 0.79–0.96, p = 0.005), lower risk of new‐onset HF (HR 0.82, 95% CI 0.69–0.96, p = 0.017), as well as reduced risk of the composite endpoint (HR 0.86, 95% CI 0.79–0.94, p = 0.001). In smoking subjects, no associations were found.
Conclusion
Serum selenium was independently associated with multiple indicators of the metabolic syndrome. In addition, high selenium levels were independently associated with reduced mortality and new‐onset HF in non‐smokers. Well‐powered interventional studies are necessary to evaluate the potential benefit of repleting selenium, especially in non‐smoking subjects.
Keywords: Selenium, Malnutrition, Heart failure, Mortality
Low selenium levels are not uncommon in several parts worldwide. We measured selenium concentrations in 5973 subjects of the PREVEND cohort, a general population prospective cohort of the city of Groningen. Of them, 4288 subjects were non‐smokers and after a follow‐up of 8.4 years, high selenium concentrations were associated with lower mortality as well as lower incidence of new‐onset heart failure in these non‐smokers. Non‐smoking subjects with selenium concentrations of >110 µg/L had lower risk for these endpoints as they were close to the levels needed for optimal expression for selenoproteins (which is 123 µg/L). CI, confidence interval; HR, hazard ratio.
Introduction
Malnutrition is a global health problem. Roughly 800 million people worldwide are suffering from different forms of malnutrition. 1 This is not only limited to undernourishment, but it also includes specific micronutrient deficiencies caused by an imbalanced diet in the developing as well as the developed countries. 1 Dietary habits might play essential roles in the development and prevention of the nutrition‐related chronic non‐communicable diseases (e.g. diabetes, heart failure [HF] and the metabolic syndrome). 2 However, current treatment options consist mainly of medication, which mostly targets selective pathological pathways and does not correct underlying causes. 2 For instance, suboptimal levels of minerals and trace elements (e.g. iron, iodine and selenium) may contribute to the development and progression of HF. 3 , 4 It has been shown that up to 50% of patients with HF suffer from some form of malnutrition, including micronutrient insufficiencies. 3 , 4
Selenium is an essential micronutrient that is required for proper enzymatic functioning of 25 selenoproteins. These selenoproteins have biological functions in redox hemostasis, thyroid hormone metabolism, and inflammation as well as for the respiratory capacity of mitochondria in the myocardium. 5 , 6 Dietary uptake of selenium is dependent upon selenium content and speciation in the soil. 6 Nuts (and Brazil nuts in particular), red meat, seafood and grains are rich sources of selenium. 6 Extreme selenium deficiency is associated with Keshan disease, an endemic dilated cardiomyopathy, which has been observed mainly in areas with very low levels of selenium in the soil. 5 , 6 This cardiomyopathy has been reversed by applying population‐based selenium supplementation. 7 Moreover, suboptimal selenium levels are not uncommon as several parts of Europe and Asia contain low soil selenium levels. 6 , 8 Nevertheless, there is yet no consensus on the definition of selenium deficiency in the general population. 6
Considering the involvement of selenium in various physiological processes, selenium might have particular relevance to public health and for patients with non‐communicable diseases. Selenium might be related to mortality risk, although the epidemiological prospective evidence regarding this association is inconsistent. 9 , 10 , 11 , 12 , 13 With respect to HF, suboptimal selenium levels have been thought to accelerate the progression of HF, but evidence from prospective studies in a general population on the incidence of new‐onset HF is still lacking. 6
In this study, we aim to elucidate the association of serum selenium levels with mortality and the development of HF, in a developed country with moderate selenium levels. For this purpose, we will utilize data from a large, well‐characterized, prospective, general population cohort.
Materials and methods
Patient population
Data from the second visit of the Prevention of REnal and Vascular End‐stage Disease (PREVEND) study have been utilized. This is a prospective observational Dutch cohort study based on the general population of the city of Groningen, in the North of the Netherlands. Detailed description of the study can be found elsewhere. 14 In short, the inhabitants of the city (n = 85 421) were invited to participate in the study by filling in a postal questionnaire and by providing an early morning urine sample. Following the exclusion of pregnant women and patients with type 1 diabetes mellitus, a total of 6000 participants with urine albumin excretion (UAE) ≥10 mg/L as well as a random selected control group with UAE <10 mg/L (n = 2592) have been enrolled in the study. Fasting venous blood samples (i.e. after an overnight fasting period) were drawn and stored at −80°C until analysis. Our analysis is a retrospective analysis of prospectively assessed data. Selenium has been measured in 5973 subjects (i.e. the available samples from the second visit), of which 3743 subjects with UAE ≥10 mg/L and 2204 <10 mg/L. Selenium has been measured using a validated inductively coupled plasma mass spectrometry method as described before. 4 The study was approved by the Medical Ethics Committee of the University Medical Center Groningen and was performed in accordance with the Declaration of Helsinki.
Definitions
Anaemia was defined according to the World Health Organization definition (haemoglobin level <12 g/dl in women and <13 g/dl in men). Iron deficiency was defined as ferritin levels <30 µg/L. The diagnosis of type 2 diabetes mellitus was determined if one or more of the following criteria were met: fasting blood glucose >7.0 mmol/L, non‐fasting blood glucose >11.1 mmol/L, use of antidiabetic, or self‐report of a physician diagnosis. Smoking status was defined as current smokers versus no current smokers. Kidney function was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation.
All‐cause mortality and incidence of heart failure
Data on all‐cause mortality were received from the Dutch Central Bureau for Statistics. Hospital charts of PREVEND participants were checked in the two main hospitals in the city of Groningen. The files were checked for the presence of HF at baseline (by checking signs, symptoms and objective evidence of HF) and for new‐onset HF based on the guidelines of the European Society of Cardiology. 15 In addition, the Dutch national registry of hospital discharge diagnoses (PRISMANT, Utrecht, the Netherlands) was checked for any cardiovascular hospitalization. Cases who were identified as suspected HF were examined by an endpoint adjudication committee consisting of seven independent experts in HF. In order to avoid selection bias, two different experts received anonymised clinical charts, hospitalization and physician office records and validated each case. In case of disagreement, a joint decision was made within the committee. Subjects' files were accessed after a permission given by the local Ethics Committees of both hospitals. Further details about the evaluation process have been published elsewhere. 14
Statistical analysis
All analyses were performed using Stata version 16.0 and R version 4.0.3. As the current cohort enrolls subjects from the general population, subjects at either end of the continuous spectrum of selenium concentrations were considered as either high (>85 percentile) or low (<15 percentile) in selenium. The main differences in characteristics and events were expected to be observed in these groups, because selenium concentrations that lead to optimization of SELENOP levels (i.e. the main transporter of selenium in the circulation) and thus exceed >123 µg/L, 16 were relatively uncommon in this cohort. The data were reported as mean ± standard deviation (SD) for normally distributed variables, as median and interquartile range (IQR) for non‐normally distributed variables, and categorical variables were reported as frequencies and percentages. To compare the baseline characteristics, either one‐way analysis of variance, Kruskal–Wallis, or chi‐squared test were used, where appropriate. Furthermore, we performed a stepwise multivariable linear regression analysis to identify the independent associates of selenium concentrations. The potential associating factors included all relevant variables from Table 1 . As a sensitivity analysis, we performed multivariable minimax concave penalty‐penalized regression. Our primary outcome is the composite endpoint of all‐cause mortality and new incidence of HF, while our secondary outcome is both endpoints separated. Cox proportional hazards models were fitted to evaluate the hazards ratios (HRs) of selenium status (i.e. as defined in Table 1 and where the middle group, resembling the average majority of the population, was used as a reference group) or concentrations (i.e. selenium as continuous variable, reported as: per 10 µg/L increase) in association with these endpoints. To assess whether selenium levels were associated with new incidence of HF in particular, subjects with prevalent HF were excluded from the analysis (n = 60), and a competing‐risk regression analysis was performed where death was considered as a competing risk. Meeting of proportionality assumptions was assessed using the Grambsch–Therneau test. When <5% of the subjects were at risk, the follow‐up was truncated, which was at 9.4 years. Subjects were censored at the last date of the follow‐up or at the date when they moved to an unknown destination. Three models were developed: a univariable model, a second model with the variables selected based on the stepwise regression, and a third model that included the associates of selenium concentrations (i.e. variables of the second model) in addition to age in five categories (i.e. subjects in 30s, 40s, 50s, 60s and 70s) and systolic blood pressure. Only subjects with non‐missing measurements were included in the analyses (online supplementary Table S1 ). To visualize the association between selenium concentrations and the outcomes, we constructed restricted cubic splines with four knots based on Harrell's recommended percentiles. Since the PREVEND cohort is known to have an overrepresentation of participants with increased UAE, we applied a previously developed statistical correction factor using a weighted Cox regression model. 14 As such, the conclusions may be extended to the general population, independent of microalbuminuria. 14 Given a previously reported interaction with smoking in relation to mortality 17 and several studies that reported the effects of smoking on selenium concentrations, 18 , 19 we evaluated the potential presence of statistical interaction between selenium and smoking. We also tested for the interaction with sex because of the already established sexual dimorphism known in selenium metabolism. 20 These were planned analyses. P‐values <0.1 for the interaction term were considered to be statistically significant, while a p‐value <0.05 was considered significant for other analyses.
Table 1.
Baseline characteristics
| Total | Selenium | p‐value | |||
|---|---|---|---|---|---|
| <15% | 15%–85% | >85% | |||
| No. patients | 5973 | 895 | 4182 | 896 | |
| Selenium concentration (µg/L) | 84.6 (19.5) | 57.7 (6.9) | 83.6 (9.6) | 116.1 (18.4) | <0.001 |
| Selenium range | 13.7–66.1 | 66.1–102.4 | 102.4–367.7 | ||
| Demographics | |||||
| Age (years) | 53.6 (12.1) | 53.4 (12.4) | 53.4 (12.2) | 54.7 (11.7) | 0.010 |
| Female sex | 3103 (52.0%) | 461 (51.5%) | 2148 (51.4%) | 494 (55.1%) | 0.12 |
| Body mass index (kg/m2) | 26.7 (4.3) | 26.8 (4.4) | 26.6 (4.3) | 26.7 (4.3) | 0.36 |
| Systolic blood pressure (mmHg) | 126.0 (19.0) | 126.1 (18.4) | 125.8 (19.0) | 126.7 (19.5) | 0.42 |
| Current smoker | 1630 (27.5%) | 300 (33.8%) | 1157 (27.9%) | 173 (19.5%) | <0.001 |
| History of myocardial infarction | 386 (6.6%) | 74 (8.4%) | 254 (6.2%) | 58 (6.5%) | 0.054 |
| History of diabetes | 364 (6.2%) | 50 (5.7%) | 244 (5.9%) | 70 (8.0%) | 0.062 |
| History of CVA | 71 (1.2%) | 11 (1.3%) | 53 (1.3%) | 7 (0.8%) | 0.49 |
| Use of antihypertensive medication | 937 (15.8%) | 141 (15.8%) | 648 (15.6%) | 148 (16.7%) | 0.71 |
| Laboratory values | |||||
| Glucose (mmol/L) | 5.0 (1.2) | 5.0 (1.1) | 5.0 (1.2) | 5.1 (1.3) | 0.020 |
| Cholesterol (mmol/L) | 5.4 (1.1) | 5.3 (1.1) | 5.4 (1.0) | 5.7 (1.1) | <0.001 |
| eGFR (ml/min/1.73 m2) | 91.8 (17.2) | 91.1 (18.2) | 92.1 (17.1) | 90.7 (16.5) | 0.042 |
| Kidney function <60 ml/min/1.73 m2 | 247 (4.4%) | 44 (5.4%) | 164 (4.1%) | 39 (4.5%) | 0.27 |
| Urine albumin excretion (mg/24 h) | 8.1 (5.9–13.5) | 8.2 (6.1–13.2) | 8.0 (5.9–13.4) | 8.3 (6.0–13.9) | 0.21 |
| Haemoglobin (mmol/L) | 8.5 (0.8) | 8.5 (0.8) | 8.5 (0.7) | 8.5 (0.7) | 0.48 |
| Anaemia | 564 (9.5%) | 111 (12.5%) | 401 (9.6%) | 52 (5.8%) | <0.001 |
| MCV (fL) | 90.5 (4.6) | 90.3 (5.0) | 90.5 (4.6) | 90.8 (4.4) | 0.061 |
| Haematocrit (v/v) | 0.4 (0.0) | 0.4 (0.0) | 0.4 (0.0) | 0.4 (0.0) | 0.29 |
| Serum iron (µmol/L) | 15.8 (5.6) | 15.2 (5.8) | 15.9 (5.6) | 16.3 (5.6) | <0.001 |
| Transferrin (g/L) | 2.6 (0.4) | 2.6 (0.4) | 2.6 (0.4) | 2.6 (0.4) | 0.014 |
| Ferritin (µg/L) | 96.0 (47.0–172.0) | 86.0 (41.0–165.0) | 97.0 (47.0–171.0) | 104.0 (55.0–187.0) | <0.001 |
| Iron deficiency | 856 (14.8%) | 158 (18.2%) | 604 (14.9%) | 94 (10.9%) | <0.001 |
| hs‐CRP (mg/L) | 1.4 (0.6–3.0) | 1.6 (0.7–3.5) | 1.3 (0.6–3.1) | 1.2 (0.6–2.5) | <0.001 |
CVA, cerebrovascular accident; eGFR, estimated glomerular filtration rate; hs‐CRP, high‐sensitivity C‐reactive protein; MCV, mean corpuscular volume.
Results
Baseline characteristics
Selenium was measured in 5973 subjects with a mean age of 53.6 (12.1) years. Fifty‐two percent of total participants were female and mean selenium level was 84.6 (19.5) µg/L. The upper and lower 15% extremes included 896 and 895 subjects, respectively. Compared to subjects in the lower extreme, participants with high selenium status were likely to be older (54.7 [11.7] years vs. 53.4 [12.4], p = 0.010), have lower levels of C‐reactive protein (CRP) (1.2 [0.6–2.5] mg/L vs. 1.6 [0.7–3.5] mg/L, p < 0.001), but higher levels of glucose (5.1 [1.3] mmol/L vs. 5.0 [1.1] mmol/L, p < 0.001), and total cholesterol (5.7 (1.1) mmol/L vs. 5.3 [1.1] mmol/L, p < 0.001). In addition, patients with higher selenium levels had slightly lower kidney function (90.7 [16.5] mL/min vs. 91.1 [18.2] mL/min), but were less likely to have anemia (n = 52 [5.8%] vs. n = 111 [12.5%]) or iron deficiency (n = 94 [10.9%] vs. n = 158 [18.2%]) (Table 1 ).
Independent associates of selenium levels
The stepwise analysis resulted in eight clinical associates of serum selenium concentrations in this population. Current smoking, iron deficiency, anemia, body mass index (BMI) and CRP were negatively associated with selenium concentrations, while female sex, cholesterol levels and glucose levels had a positive association with selenium concentrations (Table 2 ). All of these independently associated variables were also selected when using an alternative penalized regression analysis (online supplementary Table S2 ).
Table 2.
Associates of selenium levels
| Selected associates | Coefficient | p‐value | 95% CI |
|---|---|---|---|
| Sex | 1.65 | 0.004 | 0.51 to 2.78 |
| Glucose (mmol/L) | 0.94 | <0.001 | 0.45 to 1.43 |
| Anaemia (yes/no) | −3.63 | <0.001 | −5.58 to −1.68 |
| Body mass index (kg/m2) | −0.18 | 0.011 | −0.31 to −0.04 |
| Cholesterol (mmol/L) | 2.02 | <0.001 | 1.50 to 2.54 |
| Current smoker (yes/no) | −4.11 | <0.001 | −5.34 to −2.89 |
| Iron deficiency (yes/no) | −3.03 | <0.001 | −4.69 to −1.38 |
| hs‐CRP (mg/L) | −0.26 | <0.001 | −0.37 to −0.15 |
CI, confidence interval; hs‐CRP, high‐sensitivity C‐reactive protein.
For sex variable, female was defined as 1.
Composite endpoint
During a median follow‐up period of 8.4 (IQR 7.8–8.9) years, 529 subjects either died (n = 381) or developed HF (n = 194). Of those who developed HF, 46 (24%) subjects died during the follow‐up. In almost all univariate and multivariate models, there is a non‐significant tendency to a lower HR of the composite endpoint in subjects with higher selenium levels. An exception to this is the association between the composite endpoint and continuous selenium levels after adjustment with the aforementioned associates of selenium (i.e. model b) (HR 0.92, 95% confidence interval [CI] 0.86–1.00, p = 0.043) (Table 3 ). In the univariate model, there was a significant interaction with sex (p = 0.004) as well as with smoking (p < 0.001). However, only the interaction with smoking remained statistically significant after multivariate adjustment (Table 3 ). The population subjects were stratified based on their smoking status (online supplementary Table S3 ). For the non‐smoker population (n = 4288), continuous selenium concentrations significantly associated with the composite endpoint in the univariable model (HR 0.90, 95% CI 0.83–0.97, p = 0.007) as well as after adjusting for the associates of selenium (i.e. model b) (HR 0.86, 95% CI 0.79–0.94, p = 0.001). Similarly, compared to the reference group (between 15%–85% of the population), high selenium status (upper 15% of the population) was significantly associated with reduced risk after adjusting for the associates of selenium (i.e. model b) (HR 0.53, 95% CI 0.33–0.87, p = 0.013) (Table 3 ). These associations remained significant after adjusting for age and systolic blood pressure, next to the associates of selenium (online supplementary Table S4 ).
Table 3.
Cox regression models for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure
| Model total events | Total (n = 529) | Interaction with smoking | Only non‐smokers (n = 359) | Only smokers (n = 165) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | |||
| Composite outcome | |||||||||||
| Model a | |||||||||||
| Continuous selenium | 0.407 | 0.96 | 0.87–1.06 | <0.001 | 0.007 | 0.90 | 0.83–0.97 | <0.001 | 1.13 | 1.07–1.18 | |
| Lowest 15% | 0.055 | 1.38 | 0.99–1.93 | 0.171 | 1.33 | 0.88–2.01 | 0.462 | 1.25 | 0.69–2.27 | ||
| Highest 15% | 0.230 | 0.80 | 0.55–1.15 | 0.073 | 0.67 | 0.44–1.04 | 0.304 | 1.43 | 0.72–2.84 | ||
| Model b | |||||||||||
| Continuous selenium | 0.043 | 0.92 | 0.86–1.00 | 0.004 | 0.001 | 0.86 | 0.79–0.94 | 0.135 | 1.11 | 0.97–1.27 | |
| Lowest 15% | 0.192 | 1.29 | 0.88–1.88 | 0.159 | 1.39 | 0.88–2.22 | 0.746 | 1.11 | 0.58–2.12 | ||
| Highest 15% | 0.056 | 0.67 | 0.44–1.01 | 0.013 | 0.53 | 0.33–0.87 | 0.734 | 1.15 | 0.52–2.51 | ||
| Total (n = 381) | Interaction with smoking | Only non‐smokers (n = 260) | Only smokers (n = 118) | ||||||||
| p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | |||
| All‐cause mortality | |||||||||||
| Model a | |||||||||||
| Continuous selenium | 0.807 | 0.99 | 0.88–1.11 | <0.001 | 0.019 | 0.90 | 0.82–0.98 | <0.001 | 1.15 | 1.09–1.21 | |
| Lowest 15% | 0.106 | 1.39 | 0.93–2.07 | 0.230 | 1.35 | 0.83–2.18 | 0.424 | 1.35 | 0.65–2.81 | ||
| Highest 15% | 0.353 | 0.81 | 0.53–1.26 | 0.132 | 0.67 | 0.40–1.13 | 0.296 | 1.53 | 0.69–3.40 | ||
| Model b | |||||||||||
| Continuous selenium | 0.131 | 0.93 | 0.86–1.02 | 0.023 | 0.005 | 0.87 | 0.79–0.96 | 0.256 | 1.11 | 0.93–1.33 | |
| Lowest 15% | 0.397 | 1.21 | 0.78–1.90 | 0.418 | 1.25 | 0.72–2.17 | 0.681 | 1.17 | 0.55–2.51 | ||
| Highest 15% | 0.085 | 0.66 | 0.41–1.06 | 0.033 | 0.54 | 0.31–0.95 | 0.914 | 1.05 | 0.42–2.61 | ||
| Total (n = 194) | Interaction with smoking | Only non‐smokers (n = 131) | Only smokers (n = 61) | ||||||||
| p‐value | HR | 95% CI | p‐value | HR | 95% CI | p‐value | HR | 95% CI | |||
| New‐onset heart failure | |||||||||||
| Model a | |||||||||||
| Continuous selenium | 0.097 | 0.90 | 0.80–1.02 | 0.095 | 0.102 | 0.89 | 0.77–1.02 | 0.556 | 1.03 | 0.93–1.14 | |
| Lowest 15% | 0.335 | 1.31 | 0.76–2.25 | 0.443 | 1.31 | 0.65–2.63 | 0.846 | 0.92 | 0.38–2.20 | ||
| Highest 15% | 0.276 | 0.71 | 0.38–1.32 | 0.274 | 0.68 | 0.34–1.36 | 0.871 | 0.90 | 0.24–3.30 | ||
| Model b | |||||||||||
| Continuous selenium | 0.108 | 0.90 | 0.79–1.02 | 0.015 | 0.017 | 0.82 | 0.69–0.96 | 0.252 | 1.10 | 0.94–1.29 | |
| Lowest 15% | 0.320 | 1.38 | 0.73–2.58 | 0.133 | 1.78 | 0.84–3.78 | 0.610 | 0.76 | 0.26–2.21 | ||
| Highest 15% | 0.309 | 0.69 | 0.33–1.42 | 0.187 | 0.58 | 0.26–1.30 | 0.978 | 0.98 | 0.24–3.93 | ||
Model a is a univariate model. Model b included the following variables: sex, iron deficiency, anemia, body mass index, C‐reactive protein, cholesterol, glucose, and current smoking. Lowest 15% referred to the subjects with selenium levels <15% of the total population, while highest 15% referred to selenium levels >15% of the total population. The middle group (i.e. the group with selenium levels 15%–85% of the total population) was used as reference group.
In smokers, continuous selenium variable had opposite, but non‐significant, association with the composite outcome in the multivariable model (HR 1.11, 95% CI 0.97–1.27, p = 0.135) (Table 3 ).
When stratified based on sex, continuous selenium concentrations were associated with more pronounced risk reduction in males compared to females after adjusting for the variables of model b (HR 0.89, 95% CI 0.81–0.99, p = 0.024 vs. HR 0.97, 95% CI 0.86–1.10, p = 0.661) (online supplementary Table S5 ).
All‐cause mortality and new‐onset heart failure
A total of 381 subjects died during the follow‐up period and 194 subjects developed HF. As with the composite endpoint, no significant associations were observed in the total population for the secondary endpoints. However, a significant interaction with smoking in association with all‐cause mortality and incident HF (p < 0.001 and p = 0.095, respectively) was observed (Table 3 ). Compared to the middle group, non‐smoker subjects with high selenium status had a significantly reduced mortality risk in multivariate model (HR 0.54, 95% CI 0.31–0.95, p = 0.033). Similar results were found with continuous selenium concentrations (HR 0.87, 95% CI 0.79–0.96, p = 0.005 in model b). With respect to new‐onset HF, continuous selenium concentrations were associated with lower risk of new‐onset HF (HR 0.82, 95% CI 0.69–0.96, p = 0.017 in model b) (Table 3 ). These results of both endpoints remained significant after adjusting for age and systolic blood pressure (online supplementary Table S4 ). Furthermore, restricted cubic splines showed that mainly subjects with selenium concentrations >110 µg/L had reduced risk of death or of developing HF within the duration of follow‐up. In contrast, subjects with selenium levels <75 µg/L showed to have increased risk (Figure 1 ). In stratified analyses using the categorical variable (i.e. selenium status as defined in Table 1 ), the differences between groups were not significant. However, when directly comparing the high selenium group with the low selenium group, non‐smoker subjects with a low selenium status had a significantly higher risk to develop HF in the multivariable analysis (HR 3.06, 95% CI 1.13–8.31, p = 0.028 in model b) (online supplementary Table S6 ). Similar to the composite outcome, in smoker subjects, continuous selenium concentrations associated with increased, but non‐significant, risk of all‐cause mortality (HR 1.11, 95% CI 0.93–1.33, p = 0.256 in model b) and new‐onset HF (HR 1.10, 95% CI 0.94–1.29, p = 0.252 in model b).
Figure 1.
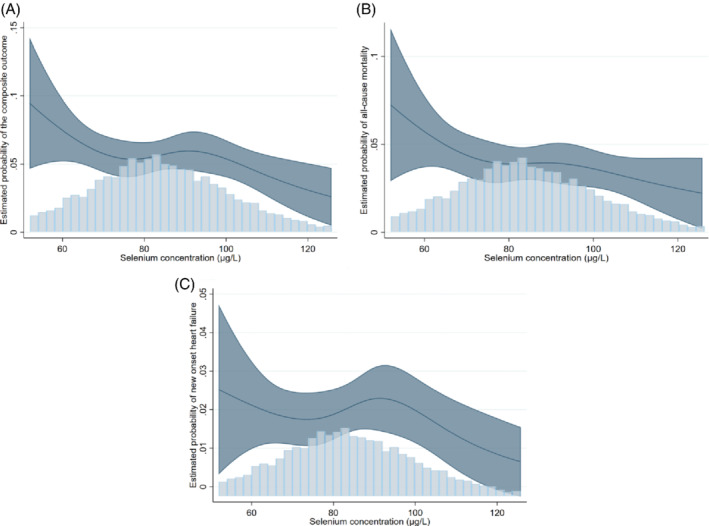
Restricted cubic splines of the association between selenium concentrations and the outcomes. (A) The association between and selenium concentration and the composite endpoint. As can be observed in the figure, subjects with selenium concentrations >110 µg/L had lower risk of mortality or new‐onset HF, while those with selenium concentrations <75 µg/L had an increased risk. Of note, 110 µg/L is the median selenium value of the group with high selenium status, while selenium concentrations <75 µg/L are considered in the range of selenium deficiency. (B) The association between and selenium concentration and all‐cause mortality. (C) The association between and selenium concentration and new onset heart failure. These association has similar trend as with the composite endpoint.
Discussion
Studying a well‐characterized cohort of the general population of the Netherlands, we report significant associations between selenium levels and components of the metabolic syndrome (i.e. high BMI, high blood glucose levels) as well as significant associations with anaemia, iron deficiency, inflammation (CRP) and current smoking. We show that high selenium levels are independently associated with a reduced risk for all‐cause mortality and new‐onset HF in non‐smokers with more pronounced risk reduction in male subjects (Figure 2). Smoking has been revealed to be a major effect‐modifier of the association of serum selenium concentrations with outcomes. These results indicate the relevance of high selenium status to public health.
Selenium in the general population
The mean serum selenium concentration in our population was 84 (19.5) µg/L, which is close to the mean of several European as well as several Asian countries, 8 but at the same time it is likely to be insufficient for optimal expression of selenoproteins. 16 The majority of the studied general populations has suboptimal selenium levels (Figure 1 ). This is in contrast to the United States, where the mean serum selenium concentrations exceed 130 µg/L. 6 , 8
Our results showed more strong associations in male but not female subjects (online supplementary Table S5 ). This could be attributed to sexual dimorphism, where selenoproteins might function differently in those subjects, although more translational studies are needed in this regard. 20 On the other hand, the potential benefits cannot be excluded in female subjects because high selenium status was significantly associated with reduced mortality in female subjects in a large American cohort. 21
Smoking
Several studies have reported significant lower serum selenium concentrations in smokers. 18 , 19 In addition, a statistical interaction between selenium and smoking was reported in relation to all‐cause mortality. 17 Moreover, a mechanistic interaction between smoking and selenium is also very likely to occur as cigarettes contain heavy metals that might have interactions with selenium. For instance, cigarette smoking increases the inhalation of arsenic, 22 an element that is known to chemically interact with selenium. 23 Arsenic affects the synthesis and the expressions of selenoproteins, and it increases the elimination of selenium via the gastrointestinal tract. 23 In addition to these antagonistic effects, synergetic toxic effects of the interaction between selenium and arsenic have been reported previously. 23 Arsenic affects selenium metabolism and may enhance the toxicity of selenium partially‐methylated forms by blocking processes related to their detoxification such as methylation. 23 These findings may explain partly the increased HRs of selenium variables in smokers (Table 3 ). The type of interaction that may occur might be influenced by the selenium status as high selenium status is associated with lower arsenic toxicity in areas with high arsenic concentrations. 24
Anaemia and iron deficiency
A higher incidence of anaemia and iron deficiency is observed in patients with low selenium levels and may indicate a general lack of a healthy and balanced diet. We previously showed that patients suffering from HF, who also are iron‐deficient, share multiple characteristics with those with selenium deficiency. 25 Selenium might have a role in the regulation of genes involved in iron metabolism 26 and concurrent deficiencies of both elements might exacerbate each other. 27 As treatment with intravenous iron has clear beneficial effects in HF, our current findings provide an extra motive to investigate the effects of selenium supplementation in HF treatment.
Glucose, cholesterol, body mass index and C‐reactive protein
Our results show that levels of glucose and total cholesterol are higher in subjects with high selenium concentrations. Patients who received selenium supplementation in a replete population had higher incidence of diabetes mellitus 28 ; however, no differences have been reported in randomized controlled trials in populations with low selenium levels. 29 Similarly, the effects of selenium on cholesterol levels are inconclusive, although a recent meta‐analysis of 11 studies has shown marginal, but significant improvement of total cholesterol levels. 30 Since our findings contrast with the results of interventional studies, it is likely that higher dietary intake may explain the increased glucose and cholesterol levels with higher selenium status in our cohort.
In addition to glucose, the association of low selenium levels with higher BMI adds an extra component of metabolic syndrome as a variable associated with selenium levels. This negative association has been reported in a large Chinese cohort and is supported by experimental studies. 31 In addition, the association between selenium and inflammatory markers (i.e. CRP) has been reported extensively in the literature and modulating the immune response might be one mechanism through which high selenium levels exert their benefits. 6
All‐cause mortality and new‐onset heart failure
We show that mortality risk is reduced if serum selenium concentrations are high, in a general population in a country with excellent medical care, with a broad age spectrum, and with moderate mean serum selenium levels. Our results with respect to mortality risk are consistent with previous reports from a selenium‐replete population 9 or elderly participants. 10 , 11 , 12 We observed a significant effect in non‐smokers, which is in line with the finding that selenoprotein P, the main transporter of serum selenium, was more strongly associated with mortality in non‐smokers in the Swedish general population. 19
We furthermore show that subjects with high selenium status (upper 15% of the population) had a significantly reduced risk of new incidence of HF. These results support the hypothesis that sufficient selenium levels may afford protection against the development of new‐onset HF, as observed with respect to Keshan disease. 5 , 6 , 7 To our knowledge, this is the first prospective cohort study that reports a significant association between selenium levels and new‐onset HF at a population level in areas other than endemic areas of Keshan disease. We previously showed that patients with HF and selenium deficiency have a worse prognosis and decreased exercise capacity. 4 , 25 Also, human cardiomyocytes that were exposed to low selenium levels, had reduced mitochondrial function and increased oxidative stress. 4
Taken all together, our results show a significant association between selenium levels and multiple health parameters (e.g. BMI, CRP, anaemia, smoking, etc.). Independently of these health parameters, high selenium status (mean: 116.1 µg/L) was associated with reduced risk for all‐cause mortality and incidence of HF. Although there is as yet no consensus about the definition of selenium deficiency, 6 these data provide additional evidence for the need of selenium levels that lead to plateau level of selenoprotein P (i.e. selenium levels >123 µg/L), especially as the current evidence indicates decreased mortality rate around this range.
Strengths
This is the largest European cohort study with a relatively long follow‐up period that has evaluated the association between selenium levels and the reported outcomes. This cohort includes relatively varied ages (i.e. age‐range of the participants 32–80 years) and was not limited to the elderly. In addition, the incidence of HF was evaluated strictly by a specialized HF committee. Selenium was measured using a validated inductively coupled plasma mass spectrometry method, which facilitates the comparison to other studies as well as the interpretation of the levels clinically.
Limitations
Due to the observational nature of the study, causality cannot be established. There were neither data about the dietary habits, nor about the use of supplementation. In addition, the interpretations of our findings should be limited to areas with low to moderate selenium levels as there were only 178 subjects in this cohort with optimal selenium levels (i.e. >123 µg/L).
Conclusion
In an adult population with moderate selenium levels, selenium levels were associated with several health parameters, including the incidence of anaemia, iron deficiency, current smoking, and higher BMI and CRP. Furthermore, high selenium status was independently associated with reduced risk of death and new‐onset HF in non‐smoking subjects. Populations based‐programmes and interventional well‐powered studies to evaluate the effects of increasing selenium levels in countries with moderate levels are warranted.
Supporting information
Table S1. Number of subjects with missing data of the variables of interest.
Table S2. Determinants of selenium levels using minimax concave penalty‐penalized regression.
Table S3. Baseline characteristics based on smoking status.
Table S4. Multivariate Cox regression model (model c) for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure.
Table S5. Multivariate Cox regression models for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure with stratification based on sex.
Table S6. Cox regression models for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure.
Acknowledgements
The authors thank Valentina Bracun, Martin Dokter, Sietske Zijlstra, Susanne Feringa, Jan IJmker and Jan Nijhoff for their excellent technical assistance.
Funding
This work was supported by the Dutch Research Council, through the Open Competition ENW‐KLEIN grant [OCENW.KLEIN.483 to N.B.] and the Junior Scientific Masterclass‐MD/PhD program of the UMCG [to A.A.A.]. The measurements of serum selenium in PREVEND were partly supported by Pharma Nord [to N.B.]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest: The UMCG, which employs several of the authors, has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals Gmbh, Ionis Pharmaceuticals, Inc., Novo Nordisk, Vifor and Roche. R.A.d.B. received speaker fees from Abbott, AstraZeneca, Bayer, Novartis, and Roche.
References
- 1. Webb P, Stordalen GA, Singh S, Wijesinha‐Bettoni R, Shetty P, Lartey A. Hunger and malnutrition in the 21st century. BMJ. 2018;361:k2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Afshin A, Sur PJ, Fay KA, Cornaby L, Ferrara G, Salama JS, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. 2019;393:1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKeag NA, McKinley MC, Harbinson MT, McGinty A, Neville CE, Woodside JV, et al. Dietary micronutrient intake and micronutrient status in patients with chronic stable heart failure. J Cardiovasc Nurs. 2017;32:148–55. [DOI] [PubMed] [Google Scholar]
- 4. Bomer N, Grote Beverborg N, Hoes MF, Streng KW, Vermeer M, Dokter MM, et al. Selenium and outcome in heart failure. Eur J Heart Fail. 2020;22:1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Loscalzo J. Keshan disease, selenium deficiency, and the selenoproteome. N Engl J Med. 2014;370:1756–60. [DOI] [PubMed] [Google Scholar]
- 6. Al‐Mubarak AA, van der Meer P, Bomer N. Selenium, selenoproteins, and heart failure: current knowledge and future perspective. Curr Heart Fail Rep. 2021;18:122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou H, Wang T, Li Q, Li D. Prevention of Keshan disease by selenium supplementation: a systematic review and meta‐analysis. Biol Trace Elem Res. 2018;186:98–105. [DOI] [PubMed] [Google Scholar]
- 8. Stoffaneller R, Morse NL. A review of dietary selenium intake and selenium status in Europe and the Middle East. Nutrients. 2015;7:1494–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Walston J, Xue Q, Semba RD, Ferrucci L, Cappola AR, Ricks M, et al. Serum antioxidants, inflammation, and total mortality in older women. Am J Epidemiol. 2006;163:18–26. [DOI] [PubMed] [Google Scholar]
- 10. Giovannini S, Onder G, Lattanzio F, Bustacchini S, Di Stefano G, Moresi R, et al. Selenium concentrations and mortality among community‐dwelling older adults: results from ilSIRENTE study. J Nutr Health Aging. 2018;22:608–12. [DOI] [PubMed] [Google Scholar]
- 11. Lauretani F, Semba RD, Bandinelli S, Ray AL, Ruggiero C, Cherubini A, et al. Low plasma selenium concentrations and mortality among older community‐dwelling adults: the InCHIANTI study. Aging Clin Exp Res. 2008;20:153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akbaraly NT, Arnaud J, Hininger‐Favier I, Gourlet V, Roussel AM, Berr C. Selenium and mortality in the elderly: results from the EVA study. Clin Chem. 2005;51:2117–23. [DOI] [PubMed] [Google Scholar]
- 13. Wei WQ, Abnet CC, Qiao YL, Dawsey SM, Dong ZW, Sun XD, et al. Prospective study of serum selenium concentrations and esophageal and gastric cardia cancer, heart disease, stroke, and total death. Am J Clin Nutr. 2004;79:80–5. [DOI] [PubMed] [Google Scholar]
- 14. Brouwers FP, de Boer RA, van der Harst P, Voors AA, Gansevoort RT, Bakker SJ, et al. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community‐based cohort: 11‐year follow‐up of PREVEND. Eur Heart J. 2013;34:1424–31. [DOI] [PubMed] [Google Scholar]
- 15. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–847. [DOI] [PubMed] [Google Scholar]
- 16. Hurst R, Armah CN, Dainty JR, Hart DJ, Teucher B, Goldson AJ, et al. Establishing optimal selenium status: results of a randomized, double‐blind, placebo‐controlled trial. Am J Clin Nutr. 2010;91:923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bleys J, Navas‐Acien A, Guallar E. Serum selenium levels and all‐cause, cancer, and cardiovascular mortality among US adults. Arch Intern Med. 2008;168:404–10. [DOI] [PubMed] [Google Scholar]
- 18. Ellingsen DG, Thomassen Y, Rustad P, Molander P, Aaseth J. The time‐trend and the relation between smoking and circulating selenium concentrations in Norway. J Trace Elem Med Biol. 2009;23:107–15. [DOI] [PubMed] [Google Scholar]
- 19. Schomburg L, Orho‐Melander M, Struck J, Bergmann A, Melander O. Selenoprotein‐P deficiency predicts cardiovascular disease and death. Nutrients. 2019;11:1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schomburg L, Riese C, Renko K, Schweizer U. Effect of age on sexually dimorphic selenoprotein expression in mice. Biol Chem. 2007;388:1035–41. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Lo K, Shen G, Feng YQ, Huang YQ. Gender difference in the association of serum selenium with all‐cause and cardiovascular mortality. Postgrad Med. 2020;132:148–55. [DOI] [PubMed] [Google Scholar]
- 22. Pappas RS, Fresquez MR, Martone N, Watson CH. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J Anal Toxicol. 2014;38:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali W, Zhang H, Junaid M, Mao K, Xu N, Chang C, et al. Insights into the mechanisms of arsenic‐selenium interactions and the associated toxicity in plants, animals, and humans: a critical review. Crit Rev Environ Sci Technol. 2021;51:704–50. [Google Scholar]
- 24. Smits JE, Krohn RM, Akhtar E, Hore SK, Yunus M, Vandenberg A, et al. Food as medicine: selenium enriched lentils offer relief against chronic arsenic poisoning in Bangladesh. Environ Res. 2019;176:108561. [DOI] [PubMed] [Google Scholar]
- 25. Al‐Mubarak AA, Grote Beverborg N, Anker SD, Samani NJ, Dickstein K, Filippatos G, et al. A clinical tool to predict low serum selenium in patients with worsening heart failure. Nutrients. 2020;12:2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christensen MJ, Olsen CA, Hansen DV, Ballif BC. Selenium regulates expression in rat liver of genes for proteins involved in iron metabolism. Biol Trace Elem Res. 2000;74:55–70. [DOI] [PubMed] [Google Scholar]
- 27. Pighetti GM, Eskew ML, Reddy CC, Sordillo LM. Selenium and vitamin E deficiency impair transferrin receptor internalization but not IL‐2, IL‐2 receptor, or transferrin receptor expression. J Leukoc Biol. 1998;63:131–7. [DOI] [PubMed] [Google Scholar]
- 28. Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;2013:CD009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohler LN, Foote J, Kelley CP, Florea A, Shelly C, Chow HH, et al. Selenium and type 2 diabetes: systematic review. Nutrients. 2018;10:1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hasani M, Djalalinia S, Sharifi F, Varmaghani M, Zarei M, Abdar ME, et al. Effect of selenium supplementation on lipid profile: a systematic review and meta‐analysis. Horm Metab Res. 2018;50:715–27. [DOI] [PubMed] [Google Scholar]
- 31. Wang Y, Gao X, Pedram P, Shahidi M, Du J, Yi Y, et al. Significant beneficial association of high dietary selenium intake with reduced body fat in the CODING study. Nutrients. 2016;8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Number of subjects with missing data of the variables of interest.
Table S2. Determinants of selenium levels using minimax concave penalty‐penalized regression.
Table S3. Baseline characteristics based on smoking status.
Table S4. Multivariate Cox regression model (model c) for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure.
Table S5. Multivariate Cox regression models for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure with stratification based on sex.
Table S6. Cox regression models for the prediction of the combined outcome, all‐cause mortality and competing risk regression models for the prediction of new‐onset heart failure.


