Abstract
Impaired exercise capacity is the key symptom of heart failure (HF) and is associated with reduced quality of life and higher mortality rates. Unfortunately, current therapies, although generally lifesaving, have only small or marginal effects on exercise capacity. Specific strategies to alleviate exercise intolerance may improve quality of life, while possibly improving prognosis as well. There is overwhelming evidence that physical exercise improves performance in cardiac and skeletal muscles in health and disease. Unravelling the mechanistic underpinnings of exercise‐induced improvements in muscle function could provide targets that will allow us to boost exercise performance in HF. With the current review we discuss: (i) recently discovered signalling pathways that govern physiological muscle growth as well as mitochondrial quality control mechanisms that underlie metabolic adaptations to exercise; (ii) the mechanistic underpinnings of exercise intolerance in HF and the benefits of exercise in HF patients on molecular, functional and prognostic levels; and (iii) potential molecular therapeutics to improve exercise performance in HF. We propose that novel molecular therapies to boost adaptive muscle growth and mitochondrial quality control in HF should always be combined with some form of exercise training.
Keywords: Heart failure, Exercise intolerance, Exercise training, Cardiac and skeletal muscle, Mitochondrial adaptation, Physiological muscle hypertrophy
Introduction: the unmet need to alleviate exercise intolerance in heart failure patients
Exercise intolerance is the main clinical symptom of heart failure (HF) and a key factor contributing to the reductions in quality of life of those affected. 1 , 2 , 3 , 4 , 5 , 6 Exercise intolerance – defined as an inability to perform physical activity – is accompanied by symptoms such as dyspnoea on exertion and fatigue, and affects HF patients with preserved and reduced ejection fraction (HFpEF and HFrEF, respectively) to the same extent. 4 While exercise intolerance in HF is primarily caused by diminutions in circulatory performance, the mechanisms are far more complex and also involve functional and structural abnormalities in cardiac and skeletal muscle as well as respiratory dysfunction and deconditioning. 4
Contemporary HF therapies are designed to reduce hospitalizations and mortality. While many HF drugs that reduce mortality also influence exercise capacity, the magnitude of improvement is modest and variable. 4 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Accordingly, exercise tolerance and activity levels of HF patients remain low. 5 , 16 , 17 , 18
On the other hand, evidence is emerging to suggest that exercise training can decrease morbidity and mortality in HF patients. 2 , 5 , 16 , 19 , 20 This was recently confirmed in the REHAB‐HF trial in which an exercise intervention improved physical function. 20 Exercise induces specific structural and molecular changes in HF patients that cause sustained improvements in exercise performance. Mechanistic insights into these pathways may uncover nodal points for therapeutic interventions to improve exercise performance in HF. With the current review, we provide an overview of exercise‐induced changes that may benefit HF patients, focusing on physiological signalling pathways and mitochondrial adaptations in cardiac and skeletal muscle.
Physiological adaptation in cardiac and skeletal muscle in response to exercise
Signalling pathways that govern physiological cardiac growth
In 1801, Corvisart was the first to hypothesize that an increase in cardiac mass was the result of increases in cardiac workload. 21 This hypothesis was confirmed by Kuelbs et al. who demonstrated that exercise in dogs resulted in an increase in cardiac mass, which was absent in sedentary controls. 22 Many years later, a variety of experimental designs using multiple species – ranging from voluntary to forced exercise – have uncovered a panel of growth factors, intracellular signalling pathways and transcriptional responses that distinguish pathological from physiological cardiac growth. 23 , 24 , 25 , 26 , 27 , 28 , 29 A critical event in the development of physiological hypertrophy is the release of the specific physiological peptide growth factors, including but not restricted to insulin‐like growth factor 1 (IGF1). 23 , 29 Binding of IGF1 to its surface receptor on cardiomyocytes activates the IGF1‐phosphoinositide 3 kinase‐protein kinase B (IGF1‐PI3K‐Akt) pathway that governs a plethora of adaptive changes (summarized in Figure 1A ). 23 , 29 , 30
Figure 1.
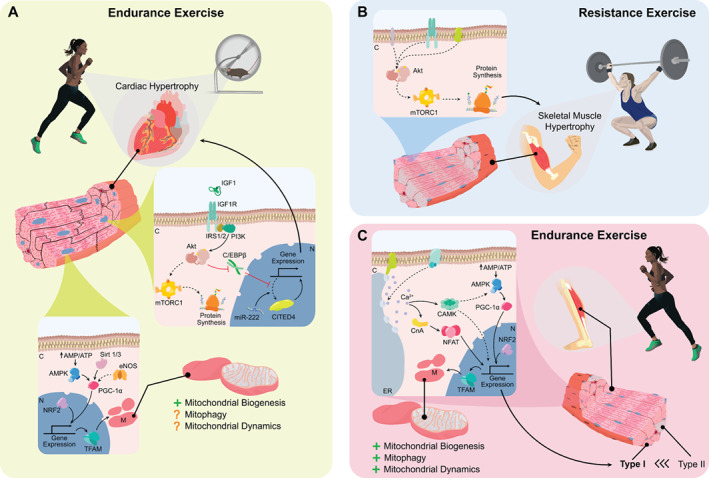
Physiological adaptation in cardiac and skeletal muscle. (A) The adaptive effects of endurance exercise on cardiac muscle are governed by special signal transduction pathways and mitochondrial quality control. Upper panel: exercise stimulates binding of insulin‐like growth factor 1 (IGF1) to a specific transmembrane tyrosine kinase membrane receptor (IGF1R), causing a conformational change that recruits and phosphorylates insulin receptor substrates 1 and 2 (IRS1/2). In turn, the activation of IRS1/2 phosphorylates phosphoinositide 3 kinase (PI3K) and further downstream activation of protein kinase B (Akt). The diverse effects of Akt activation include for example activation of endothelial nitric oxide synthase (eNOS), activation and/or inhibition of sirtuins, inhibition of glycogen synthase kinase 3β as well as inhibition of forkhead box protein O3 (FOXO3). Most importantly, however, activation of Akt subsequently (i) promotes protein synthesis through activation of mammalian target of rapamycin complex 1 (mTORC1), its downstream activation of ribosomal protein S6 kinase 1 (S6K1) and inhibition of eIF4E‐binding protein 1 (4EBP1) and (ii) by inhibiting the transcriptional repressor CCAAT/enhancer binding protein‐β (C/EBPβ) to activate a specific physiological growth programme downstream of the transcription factor CBP/p300‐interacting trans activator 4 (CITED4). Lower panel: exercise also enhances mitochondrial performance through enhanced mitochondrial biogenesis, and potentially also through increased mitochondrial clearance (mitophagy) and mitochondrial morphological changes (mitochondrial dynamics). Exercise stimulates mitochondrial biogenesis through activation of AMP‐activated protein kinase (AMPK) and upregulation of sirtuin 1/3 (SIRT1/3) and eNOS. These factors in turn promote the activity of the transcription factor peroxisome‐proliferator‐activated‐receptor gamma coactivator 1‐alpha (PGC‐1α) and its downstream factors nuclear respiratory factor 2 (NRF2) and mitochondrial transcription factor A (tFAM). NRF2 and tFAM are both essential for the generation of new mitochondrial proteins. (B) Resistance exercise induces skeletal muscle hypertrophy mediated through Akt, which activates mTORC1 leading to the synthesis of new proteins and muscle growth. (C) In skeletal muscle, endurance exercise causes an increase in mitochondrial biogenesis, mitophagy and mitochondrial dynamics. Mitochondrial biogenesis is regulated by activation of AMPK, PGC‐1α and downstream NRF2 as well as tFAM. An additional effect of exercise in skeletal muscle fibres includes a shift toward a more oxidative composition through calcineurin (CnA) mediated activation of nuclear factor of activated T‐cells (NFAT) and Ca2+/calmodulin‐dependent protein kinase (CaMK), or through modulation of AMPK and PGC‐1α. Full lines indicate direct effects, dashed lines indicate indirect effects. AMP, adenine nucleotide monophosphate; ATP, adenine nucleotide triphosphate; C, cytoplasm; Ca2+, calcium; ER, endoplasmic reticulum; M, mitochondria; miR‐222, microRNA; N, nucleus.
It has been well established that the activation of the IGF1‐PI3K‐Akt pathway is required for physiological cardiac hypertrophy. 31 , 32 , 33 , 34 , 35 , 36 , 37 For instance, cardiomyocyte‐specific overexpression of the IGF1 receptor (IGF1R) results in spontaneous physiological hypertrophy as well as a more profound increase in cardiac mass in response to swimming. 37 Conversely, cardiac hypertrophy is attenuated after repetitive swimming exercises in cardiac‐specific IGF1R‐knock‐out mice. 36 Pharmacological inhibition of PI3K‐Akt attenuates cardiac hypertrophy, 31 whereas cardiac hypertrophy in response to swimming is blocked in Akt‐knock‐out mice. 35 Further evidence for the critical importance of Akt has been established with a model using the Akt‐specific PH domain leucine‐rich repeat protein phosphatase (PHLPP1). In PHLPP1‐knock‐out mice, Akt phosphorylation is increased and physiological cardiac hypertrophy in response to swimming is augmented. 32
The downstream effects of Akt that govern physiological cardiac growth are diverse, 19 , 23 , 29 , 38 , 39 but two stand out as most clearly associated with exercise‐induced cardiac growth. 23 , 39 First, the mammalian target of rapamycin complex 1 (mTORC1) is phosphorylated by Akt, which subsequently leads to downstream activation of ribosomal protein S6 kinase 1 and inhibition of eIF4E‐binding protein 1 leading to protein synthesis and in turn cardiac hypertrophy. 23 , 29 , 39 Second, the IGF‐1‐PI3K‐Akt signalling cascade was more recently also shown to regulate CCAAT/enhancer binding protein‐β (C/EBPβ), and CBP/p300‐interacting trans activator 4 (CITED4). 19 , 23 , 29 , 39 Akt phosphorylation downregulates cardiac C/EBPβ, which de‐represses the transcription factors serum response factor (SRF) and CITED4 and subsequently enables transcription of genes encoding for proteins that are critical for physiological cardiac hypertrophy. 19 , 40 , 41 , 42 , 43 , 44 Interestingly, in addition to hypertrophic growth pathways, CITED4 also appears to regulate ultrastructural changes and cardiomyocyte elongation. 19 , 40 , 42 , 43 , 45 The role of CITED4 has been studied in a CITED4‐knock‐out mouse model, which was subjected to swimming and transaortic constriction. 44 Physiological hypertrophy was attenuated, while pathological hypertrophy was exacerbated in CITED4‐knock‐out mice, indicating that CITED4 enables physiological growth while also preventing maladaptive responses. Interestingly, CITED4‐mediated protection is at least partially regulated by mTORC1. 44 Other potential factors include microRNA‐222 (miR‐222) and miR30d. 19 , 23 , 40 , 44 , 46 , 47
Exercise‐induced hypertrophy in skeletal muscle
While resistance exercise training induces robust hypertrophy in skeletal muscle – through mTORC1 – the hypertrophic response to endurance exercise is negligible. Instead, skeletal muscle increases the number of nuclei and satellite cells, adapts capillary and mitochondrial networks, and switches its muscle fibre type composition 48 , 49 , 50 , 51 (Figure 1B ). Fast twitch (type II), glycolytic muscle fibres are favourable in anaerobic and resistance exercise, whereas slow twitch (type I), oxidative muscle fibres are preferable in aerobic endurance exercise as they contain more mitochondria. 48 Skeletal muscle adapts to endurance exercise by switching towards more oxidative isoforms, primarily controlled by calcineurin signalling. 48 , 49 , 50 , 51 , 52 Reprogramming towards oxidative isoforms is also associated with adaptive changes in mitochondrial quality control and function 48 , 53 (Figure 1C ).
Mitochondrial adaptations to exercise in cardiac muscle
Because exercise increases the demand for energy, mitochondrial numbers must increase as well 54 , 55 , 56 , 57 , 58 (Figure 1A ). Mitochondrial biogenesis refers to the generation of new mitochondria from existing organelles through the process of self‐replication, 54 , 56 and represents the principal mechanism responsible for exercise‐induced increases in mitochondrial content. 59 Mitochondrial proteins are encoded by both mitochondrial and nuclear DNA, which requires accurate transcriptional regulation and protein import. 54 , 56 , 57 , 60 Several signalling pathways activate biogenesis including sirtuin 1 and 3 (SIRT1/3), AMP‐activated protein kinase (AMPK) and endothelial nitric oxide synthase (eNOS). 56 , 61 , 62 , 63 These pathways all converge in the downstream activation of the master regulator peroxisome‐proliferator‐activated‐receptor gamma coactivator 1‐alpha (PGC‐1α). 54 , 56 , 57 , 64 Subsequently, PGC‐1α activates nuclear respiratory factors 1–2 (NRF1‐2), which promote transcription of nuclear encoded mitochondrial proteins but also activate mitochondrial transcription factor A (tFAM) which promotes transcription of mitochondrial DNA. 54 , 56 , 57 , 64 , 65 The majority of the evidence indicates that exercise induces mitochondrial biogenesis. 54 , 66 , 67 , 68 , 69 , 70 Most studies have demonstrated that mitochondrial quantity increases after voluntary exercise in mice. 71 eNOS appears to be critically involved in this process, since mitochondrial biogenesis was significantly reduced in eNOS‐knock‐out mice after swimming. 72 Conversely, other animal studies have suggested that exercise‐induced mitochondrial biogenesis is more prominent in skeletal muscle than in the heart. 73 , 74 The latter may suggest that mitochondrial biogenesis is more important for the early adaptation to exercise than the later stages.
Mitophagy is the opposite of mitochondrial biogenesis as it refers to the removal of damaged or dysfunctional mitochondria. 56 The canonical mitophagy pathway is driven by the loss of mitochondrial membrane potential which fosters mitochondrial accumulation of PTEN‐induced kinase 1 (PINK1), 56 , 57 , 75 and subsequent cytoplasmic‐to‐mitochondrial translocation of the E3 ubiquitin ligase Parkin. The Parkin‐mediated ubiquination of mitochondrial proteins that ensues, targets mitochondria for engulfment by autophagosome. 56 , 57 , 75 The balance between mitochondrial biogenesis and mitophagy is tightly regulated through PGC‐1α by Parkin‐interacting substrate (PARIS), which prevents a mismatch in generation and degradation of mitochondria. 56 , 57 , 75 Theoretically, exercise‐induced biogenesis would be accompanied by similar increases in mitophagy, 56 to remove old and damaged mitochondria and to make room for the newly produced ones. 76 One research group describes several studies suggesting that mitophagy was enhanced and associated with the beneficial effects of exercise. 68 However, others reported that mitophagy was suppressed rather than stimulated. 77 , 78 These discrepancies are at least in part explained by the short‐lived mitophagy process in combination with the paucity of suitable models to study mitophagy in vivo.
Mitochondrial dynamics is a subtler quality control mechanism that controls the shape and size of the mitochondrial population through mitochondrial fission and fusion events. 79 Dynamic interactions between mitochondria and changes in the outer mitochondrial membrane allow for continuous exchange of mitochondrial content and changes the size and shape of these organelles. 79 , 80 , 81 Fission of a mitochondrion results in multiple, smaller, mitochondria, a process which is controlled by the dynamin‐1‐like protein (DRP1), mitochondrial fission 1 protein (Fis1) and mitochondrial fission factor (MFF). 80 , 81 Fission and the generation of fragmented mitochondria is required for mitosis, programmed cell death and mitophagy to occur. 80 , 81 On the other hand, fusion causes mitochondria to fuse into larger and longer mitochondria. 80 , 81 The functional purpose is therefore also different; fusion allows for mixing of mitochondrial content, improvement of mitochondrial function, and precedes mitochondrial biogenesis. 80 , 81 Critical regulators of the fusion process are mitofusin 1 and 2 (Mfn1‐2) and optical atrophy protein 1 (OPA1). 80 , 81 Physiological exercise appears to activate fission and fusion processes simultaneously. Coronado et al. 77 demonstrated that increased fission is essential for the cardiac adaptation to exercise. Other authors did not detect major changes in fission in aged rats but did detect an increase in exercise‐induced fusion activity. 82 Yoo et al. 78 did not observe changes in mitochondrial fission and fusion after acute exercise, despite detecting enhanced cardiac mitochondrial function. Taken together, relatively little and inconsistent data are available related to the role of mitochondrial dynamics in response to exercise and require further exploration.
Mitochondrial adaptations to exercise in skeletal muscle
The metabolic demand of skeletal muscle can increase by 100‐fold during maximal exercise, 58 making skeletal muscle highly dependent on mitochondrial function as well 66 , 83 , 84 , 85 (Figure 1C ). While the absolute mitochondrial content of skeletal muscle is low compared to the heart, 83 it contains large mitochondrial networks – the mitochondrial reticulum – which allows for unparalleled plasticity. 66 , 83 , 85 , 86 Studies indicate that mitochondrial biogenesis is activated by both acute and chronic exercise. 66 , 83 , 85 , 87 , 88 For instance, mitochondrial biogenesis is enhanced in human muscles after low‐volume high‐intensity interval training, 89 as well as after chronic endurance exercise. 90 The majority of evidence also suggests that acute and chronic exercise promotes Parkin‐dependent and Parkin‐independent mitophagy pathways. 83 , 84 , 85 , 87 , 88 , 91 , 92 Mitochondrial fission is crucial for exercise‐induced mitochondrial adaptation, 66 , 85 and Fis1‐knock‐out mice develop swollen mitochondria with reduced cristae density in the gastrocnemius muscle and have reduced exercise capacity when subjected to exhaustive exercise. 66 , 83 , 84 , 85 , 86 , 87 , 88 , 93 Similarly, DRP1‐deficient mice also display deficient exercise capacity and a maladaptive response to endurance exercise. 94 The evidence regarding fusion responses to exercise remain limited. 66 , 84 , 85 , 95
Taken together, the beneficial effects of exercise in cardiac and skeletal muscle are mediated by specific signal transduction pathways, which initiate muscle growth. In addition, mitochondrial performance is increased through orchestrated changes in mitochondrial biogenesis, mitophagy and potentially mitochondrial dynamics as well.
Exercise in heart failure
Pathophysiology of exercise intolerance in heart failure and the benefits of exercise
Exercise intolerance has a multifactorial origin, 4 , 96 and can be attributed to maladaptive central and peripheral factors. However, exercise itself can also exert beneficial effects in the setting of HF (Figure 2).
Figure 2.
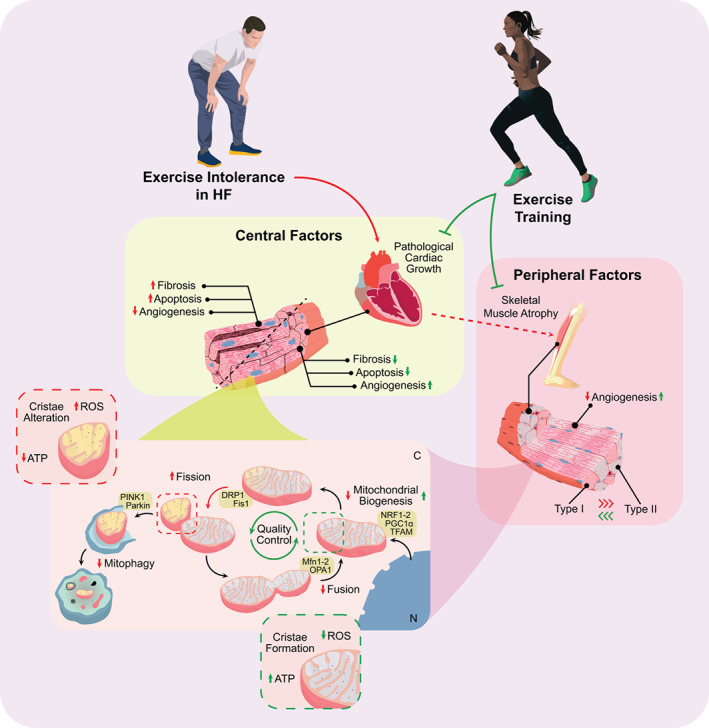
Mechanistic underpinnings of exercise intolerance in heart failure (HF) and the adaptive effects of exercise. Exercise intolerance can develop due to central and/or peripheral factors, which often include pathological cardiac remodelling and mitochondrial dysfunction. These processes can be attenuated by performing exercise, which causes adaptive effects in a disease setting in both cardiac and skeletal muscle. The mechanisms involved include growth signalling as well as mitochondrial quality control. Colours have been used structurally: red indicates effects in HF, green indicates effects of exercise training. Full lines indicate direct effects, dashed lines indicate indirect effects. ATP, adenine nucleotide triphosphate; C, cytoplasm; DRP1, dynamin‐1‐like protein; Fis1, mitochondrial fission 1 protein; Mfn1‐2, mitofusin 1 and 2; N, nucleus; NRF1‐2, nuclear respiratory factors 1 and 2; OPA1, optical atrophy protein 1; PGC‐1α, peroxisome‐proliferator‐activated‐receptor gamma coactivator 1‐alpha; PINK1, PTEN‐induced kinase 1; ROS, reactive oxygen species; tFAM, mitochondrial transcription factor A.
Central pathways underlying exercise intolerance in heart failure
Centrally, cardiac and pulmonary reserves are deteriorated in HF and lead to symptoms such as fatigue and dyspnoea during exercise. 4 , 97 HF is associated with maladaptive or pathological cardiac hypertrophy that develops in response to pathological stimuli such as hypertension or myocardial infarction. 30 , 98 , 99 , 100 , 101 , 102 Pathological hypertrophy, while initially compensatory, eventually fails to sustain cardiac function and often deteriorates into HF. 30 , 98 , 99 , 100 , 101 , 102 Molecularly, gene expression is shifted towards the foetal gene expression programme 98 , 99 , 100 , 101 , 102 and metabolically, substrate utilization is shifted from fatty acids towards increased glucose oxidation as a compensatory response. 98 , 99 , 100 , 101 , 102 , 103 Contrary to physiological hypertrophy; fibrosis, apoptosis and necrosis also occur in response to pathological stimuli. 98 , 99 , 100 , 101 , 102 Together this results in the structural and functional impairments of cardiac muscle that ultimately culminate in the HF syndrome. 29 , 104
In addition to structural defects, bioenergetic insufficiency is also thought to contribute to cardiac dysfunction and exercise intolerance in HF. 104 , 105 Cardiac mitochondrial dysfunction can be attributed to many factors, including abnormal mitochondrial ultrastructure, improper mitochondrial dynamics and functional impairments related to enhanced reactive oxygen species (ROS) and decreased ATP production. 104 Mitochondrial fission is increased, but Parkin‐dependent mitophagy is dysregulated, resulting in increased numbers of fragmented mitochondria that are not efficiently removed through mitophagy. 104 , 105 Fusion and biogenesis are downregulated, further compromising mitochondrial quality. 104 , 105 Together, both pathological cardiac remodelling and mitochondrial dysfunction may result in unmet cardiac energy demands at rest and subsequently in response to exercise (Figure 2 ).
Peripheral factors underlying exercise intolerance in heart failure
Peripherally, HF has been associated with several defects in the structure and function of skeletal muscle including but not limited to atrophy, an unfavourable switch to glycolytic muscle fibre types and general mitochondrial dysfunction. 4 , 104 , 106 , 107 , 108 Furthermore, adaptive capacity of skeletal muscle is also blunted in patients with HFrEF and HFpEF. 97 In contrast to the heart, skeletal muscle mass deteriorates in HF leading to muscular atrophy rather than hypertrophy. Bioenergetics are also impaired in skeletal muscle, which can be attributed to reductions in oxidative muscle fibres (type I) and in mitochondrial volume and density 4 , 97 , 104 , 106 , 107 , 108 , 109 (Figure 2 ).
The mechanisms causing reduced mitochondrial content and function remain relatively unexplored. Molina et al. 110 detected reductions in mitochondrial content in skeletal muscle of older HFpEF patients, associated with diminished citrate synthase activity and lower Mfn2 levels, which were all correlated with parameters for exercise intolerance. This suggests that reduced mitochondrial fusion also contributes to skeletal muscle wasting in HF. Tsuda et al. 111 discovered increased mitochondrial protein acetylation, associated with dysregulated fatty acid oxidation and decreased exercise capacity in a murine HF model. Hence, post‐translational modifications may play a role in the deterioration of skeletal muscle function.
Benefits of exercise in central and peripheral factors underlying exercise intolerance in heart failure: evidence from animal studies
Interestingly, exercise training appears to be beneficial for cardiac function in rodent models of HF. 112 , 113 , 114 , 115 For example, Campos et al. 113 also demonstrated that 8 weeks of moderate intensity running training in rats post‐myocardial infarction (MI) HF, improved left ventricular (LV) function, associated with improvements in mitochondrial oxidative capacity. Similarly, 4 weeks of treadmill exercise improved LV function and reduced cardiac fibrosis in rats post‐MI. Mechanistically, mitochondrial biogenesis is clearly upregulated by exercise, suggesting that improvements in mitochondrial function could underlie the salutary effects. 112 Swimming training has been shown to have similar effects, accompanied by reduced fibrosis and apoptosis, improved mitochondrial dynamics and reduced ROS production. 115 Interestingly, endurance training prior to ischaemia/reperfusion improved mitochondrial dynamics, reduced infarct sizes and diminished pathological cardiac remodelling, suggesting that exercise has cardioprotective properties as well. 114 Taken together, exercise training exerts beneficial effects in the HF setting, both related to pathological cardiac remodelling and mitochondrial quality control (Figure 2 ).
Skeletal muscle responses to exercise in HF are also generally adaptive and beneficial. 116 , 117 , 118 , 119 Cai et al. 116 demonstrated that 4 weeks of resistance and endurance training alleviated oxidative stress, protein breakdown and myocyte atrophy to the same extent. The beneficial effects of these exercise regimens were associated with activation of growth factors IGF1 and neuregulin‐1. Eight weeks of voluntary wheel running in a model of genetic heart disease, also showed that exercise reduced oxidative stress and structural damage to skeletal muscle. 117 Bacurau et al. 118 demonstrated the protective role of endurance exercise in HF mice, in which activation of the IGF1‐Akt–mTOR pathway prevented skeletal muscle atrophy. On the other hand, Moreira et al. 119 demonstrated that mitochondrial citrate synthase activity was enhanced and tFAM expression was restored by endurance exercise, indicating that mitochondrial biogenesis remained intact. Hence, these studies show that skeletal muscle atrophy in HF can be overcome by exercise, possibly through beneficial effects on skeletal muscle mitochondria (Figure 2 ; Box 1).
Box 1. Exercise in a bottle?
The future promise to replace physical exercise by a drug or supplement can be questioned. Can we promise that we can replace physical exercise by a tablet or bottle? Taking into account the paradigm shift toward iron supplementation and ketone administration, exercise in a bottle is what we ultimately thrive for. Whether this is a realistic gesture remains unanswered. Considering that physical exercise does much more than activating a specific pathway or growth hormone: the cyclic stretch, the increase blood flow, the vasodilation: it is hard to conceive that this is all to come out of one bottle. Tackling all the aspects of exercise is non‐realistic, unfortunately, this is something many physician‐scientists do hope for. Most probably opportunities lie more within therapeutic interventions in supplement to exercise training, rather than mimicking exercise itself.
A novel method to quantify exercise intolerance in heart failure
In humans, the golden standard for assessing exercise capacity entails the cardiopulmonary exercise test which is widely available. 120 , 121 However, peripheral aspects of exercise intolerance can be studied in more detail using magnetic resonance spectroscopy (MRS) with an in‐magnet ergometer. 122 , 123 , 124 With the use of 31 phosphorous (31P) MRS, in vivo skeletal muscle metabolism can be brought to light. 122 , 125 Measurements of phosphocreatine (PCr), inorganic phosphate (Pi), ATP and phosphomonoester resonances during exercise and post‐exercise recovery allow for the determination of cellular muscle bioenergetics in the larger skeletal muscles. 122 Additionally, oxidative muscle fibres can be visualized, and mitochondrial content can be estimated. 126 Changes in metabolite measurements such as the Pi/PCr ratio during incremental exercise correlate well with the degree of exercise intolerance in HF patients. 127 , 128 Furthermore, measurement of PCr resynthesis during recovery is considered to be a reliable estimate of mitochondrial functioning in skeletal muscle, because PCr resynthesis is predominantly dependent on oxidative mitochondrial ATP synthesis. 124 For instance, this technique was applied by van der Ent et al. 128 who have investigated forearm skeletal muscle metabolism in chronic HF patients and provided evidence for decreased mitochondrial oxidative capacity, resulting in a decreased exercise adaptive capacity in HF patients. Weiss et al. 96 provided the evidence that both HFrEF and HFpEF patients exhibited normal basal skeletal muscle metabolism, but in response to exercise, skeletal muscle metabolism was disrupted. This was characterized by rapid decrements in phosphates and low oxidative capacity (Box 2).
Box 2. A novel golden standard to determine exercise intolerance?
Many clinical trials utilize the 6‐minute walk test and/or Kansas City Cardiomyopathy Questionnaire to determine exercise capacity. In clinical practice, cardiopulmonary exercise testing is however golden standard. For research purposes this often is too laborious and costly but would provide more insight into specific parameters of i.e., peak oxygen uptake, which are more accurate and of prognostic value for cardiovascular outcome. Recent studies using a 31 phosphorous surface coil in Magnetic Resonance Spectroscopy with an in‐magnet ergometer, have shown to provide detailed functional status of peripheral skeletal muscles. This technique offers more specific insight into mechanistic deficits in heart failure patients with exercise intolerance. Yet its feasibility and cost‐effectiveness should be considered. Perhaps, the development of a patient‐specific panel or flow chart of tests could provide an individualized status of exercise tolerance, which could serve as a more accurate golden standard in both a research and clinical setting.
Possibilities to improve exercise intolerance in heart failure
Benefits of endurance exercise in heart failure: evidence from human studies
Clinical data also suggest that exercise is beneficial for HF patients. In fact, multiple studies including randomized controlled clinical trials such as the HF‐ACTION trial, systematic reviews and meta‐analyses have shown that exercise is safe and improves symptoms in HF patients, both HFrEF and HFpEF patients. 5 , 6 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 Exercise training is therefore recommended in HF patients. 5 , 16 Unfortunately, exercise is initially accompanied by increases in discomfort and many HF patients are apprehensive or physically challenged by non‐cardiac comorbidities. Long‐term adherence to such regimens is therefore low. 16 , 18 , 145 Accordingly, nutriceutical or pharmacological strategies to boost exercise performance in HF patients could be key. Based on the evidence presented in this review, we believe that there are multiple targets and strategies that should be explored to improve exercise performance in HF (summarized in Figure 3 ).
Figure 3.
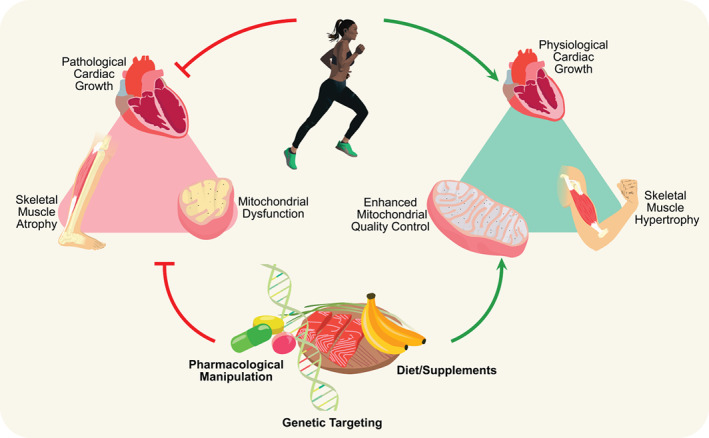
Exercise as a molecular therapy for heart failure‐associated exercise intolerance. The adaptive effects of exercise in both health and disease may provide therapeutic targets to improve exercise intolerance in heart failure. The underlying mechanistic pathways involve stimulation of physiological cardiac growth, skeletal muscle hypertrophy and enhanced mitochondrial quality control. This is accompanied by inhibition of pathological cardiac growth, skeletal muscle atrophy and mitochondrial dysfunction. These exercise‐induced effects can be enhanced by targeting these pathways with pharmacological manipulation, diets/supplements and/or genetic targeting.
Targeting growth in cardiac and skeletal muscle
There are clear distinctions in the molecular growth responses between physiological and pathological growth in cardiac and skeletal muscle. One might therefore argue that it would be beneficial to activate physiological pathways in HF patients, i.e. to superimpose physiological growth responses in a pathological setting. A potential focus should be the activation of exercise‐induced signalling pathways, such as targeting factors in the Akt‐C/EBPß‐CITED4 pathway. Considering that this pathway signals through miRs, RNA‐based therapeutics may offer opportunities to promote physiological growth in HF. 19 , 23 , 44 , 146 , 147 Another example, of a potential target to regulate physiological growth is A‐kinase interacting protein 1 (AKIP1). 148 , 149 , 150 Studies with neonatal rat ventricular cardiomyocytes demonstrated an induction of cardiomyocyte hypertrophy by overexpression of AKIP1. 149 This hypertrophic response was associated with activation of Akt signalling without enhanced expression of pathological gene markers, 149 suggesting a role for AKIP1 in physiological cardiomyocyte hypertrophy. Activation of such signalling pathways could lead to beneficial reprogramming and could improve exercise performance. In skeletal muscle, it is also of importance to stimulate physiological growth, but most specifically, novel targets should be developed to overcome skeletal muscle atrophy. The knowledge regarding resistance and endurance training is increasing, and this provides improvements in skeletal muscle mass, potentially through mechanisms involving Akt and mTORC1. 118
Boosting mitochondrial performance in cardiac and skeletal muscle
Current literature describes several cardioprotective roles of exercise due to underlying beneficial mitochondrial mechanisms, suggesting potential targets to improve exercise tolerance in HF. 151 , 152 , 153 Stimulation of these mitochondrial adaptive responses including mitochondrial biogenesis, mitophagy and mitochondrial dynamics could potentially also improve exercise capacity in HF patients. It is however important to consider that cardiac dysfunction may not be restored solely by improving mitochondrial performance. 154 In skeletal muscle, improvements in mitochondrial quality control could enhance the adaptive response to stress and block or reverse the unfavourable muscle fibre switching. Recently, our department uncovered an important role of the erythropoietin receptor in skeletal muscle, a novel target which is critical for mitochondrial biogenesis in skeletal muscle and the response to physiological exercise. 155 Potentially, stimulating skeletal muscle erythropoietin signalling could benefit for patients with mitochondrial myopathies, skeletal muscle fatigue, or atrophy.
Conclusion
Exercise intolerance is the central and most debilitating symptom in HF patients and limited therapies are available. Evidence is emerging that exercise training has beneficial effects on both cardiac and skeletal muscle, in health and disease settings. Therefore, in order to overcome exercise intolerance in HF patients, the primary advice remains exercise. However, this should be accompanied by additional therapies to alleviate initial symptoms associated with exercise in HF patients. We do propose that novel molecular therapies to boost muscle growth and mitochondrial quality control in HF should always be combined with some form of exercise training. Possibilities for such supplemental molecular therapies lie within pharmacological, genetic and/or dietary targeting of pathways associated with adaptive effects of exercise in cardiac and skeletal muscle. Hence, increased understanding of exercise‐associated growth signalling, and mitochondrial quality control mechanisms may broaden the horizon for exercise as a therapy for HF. Future studies should focus on unravelling the mechanistic underpinnings of exercise‐induced benefits in both cardiac and skeletal muscle.
Acknowledgments
Dr. Westenbrink is supported by the Netherlands Organisation for Scientific Research (NWO VENI, grant 016.176.147) and the Netherlands Heart Foundation Senior Clinical Scientist Grant (2019T064) and CVON DOUBLE DOSE (grant 2020‐8005). Dr. de Boer is supported by the Netherlands Heart Foundation (CVON DOUBLE DOSE, grant 2020‐8005, CVON SHE‐PREDICTS‐HF, grant 2017‐21, and CVON RED‐CVD, grant 2017‐11) and the European Research Council (ERC CoG 818 715, SECRETE‐HF).
Conflict of interest: The UMCG, which employs Nijholt, Sánchez‐Aguilera, Voorrips, de Boer, and Westenbrink, has received research grants and/or fees from AstraZeneca, Abbott, Boehringer Ingelheim, Cardior Pharmaceuticals Gmbh, Ionis Pharmaceuticals, Inc., Novo Nordisk, and Roche. Dr. de Boer reports having received speaker fees from Abbott, AstraZeneca, Bayer, Novartis and Roche.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al.; ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. Cattadori G, Segurini C, Picozzi A, Padeletti L, Anzà C. Exercise and heart failure: an update. ESC Heart Fail. 2018;5:222–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–18. [DOI] [PubMed] [Google Scholar]
- 4. del Buono MG, Arena R, Borlaug BA, Carbone S, Canada JM, Kirkman DL, et al. Exercise intolerance in patients with heart failure: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:2209–25. [DOI] [PubMed] [Google Scholar]
- 5. Alvarez P, Hannawi B, Guha A. Exercise and heart failure: advancing knowledge and improving care. Methodist Debakey Cardiovasc J. 2016;12:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail. 2022;24:4–131. [DOI] [PubMed] [Google Scholar]
- 7. Vitale C, Spoletini I, Rosano GM. Pharmacological interventions effective in improving exercise capacity in heart failure. Card Fail Rev 2018;4:25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maslov MY, Foianini S, Orlov MV, Januzzi JL, Lovich MA. A novel paradigm for sacubitril/valsartan: beta‐endorphin elevation as a contributor to exercise tolerance improvement in rats with preexisting heart failure induced by pressure overload. J Card Fail. 2018;24:773–82. [DOI] [PubMed] [Google Scholar]
- 9. von Haehling S, Arzt M, Doehner W, Edelmann F, Evertz R, Ebner N, et al. Improving exercise capacity and quality of life using non‐invasive heart failure treatments: evidence from clinical trials. Eur J Heart Fail. 2021;23:92–113. [DOI] [PubMed] [Google Scholar]
- 10. Karavidas A, Troganis E, Lazaros G, Balta D, Karavidas I, Polyzogopoulou E, et al. Oral sucrosomial iron improves exercise capacity and quality of life in heart failure with reduced ejection fraction and iron deficiency: a non‐randomized, open‐label, proof‐of‐concept study. Eur J Heart Fail. 2021;23:593–7. [DOI] [PubMed] [Google Scholar]
- 11. Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, et al. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yurista SR, Chong CR, Badimon JJ, Kelly DP, de Boer RA, Westenbrink BD. Therapeutic potential of ketone bodies for patients with cardiovascular disease: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2021;77:1660–9. [DOI] [PubMed] [Google Scholar]
- 13. Yurista SR, Matsuura TR, Silljé HHW, Nijholt KT, McDaid KS, Shewale SV, et al. Ketone ester treatment improves cardiac function and reduces pathologic remodeling in preclinical models of heart failure. Circ Heart Fail. 2021;14:e007684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, et al. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–68. [DOI] [PubMed] [Google Scholar]
- 15. Nielsen R, Møller N, Gormsen LC, Tolbod LP, Hansson NH, Sorensen J, et al. Cardiovascular effects of treatment with the ketone body 3‐hydroxybutyrate in chronic heart failure patients. Circulation. 2019;139:2129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13:347–57. [DOI] [PubMed] [Google Scholar]
- 17. Barbour KA, Miller NH. Adherence to exercise training in heart failure: a review. Heart Fail Rev. 2008;13:81–9. [DOI] [PubMed] [Google Scholar]
- 18. Bozkurt B, Fonarow GC, Goldberg LR, Guglin M, Josephson RA, Forman DE, et al. Cardiac rehabilitation for patients with heart failure: JACC expert panel. J Am Coll Cardiol. 2021;77:1454–69. [DOI] [PubMed] [Google Scholar]
- 19. Wei X, Liu X, Rosenzweig A. What do we know about the cardiac benefits of exercise? Trends Cardiovasc Med. 2015;25:529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kitzman DW, Whellan DJ, Duncan P, Pastva AM, Mentz RJ, Reeves GR, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Katz AM. Evolving concepts of heart failure: cooling furnace, malfunctioning pump, enlarging muscle. Part II: Hypertrophy and dilatation of the failing heart. J Card Fail. 1998;4:67–81. [DOI] [PubMed] [Google Scholar]
- 22. Hollmann W. Sports medicine in the Federal Republic of Germany. Br J Sports Med. 1989;23:142–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernardo BC, Ooi JYY, Weeks KL, Patterson NL, McMullen JR. Understanding key mechanisms of exercise‐induced cardiac protection to mitigate disease: current knowledge and emerging concepts. Physiol Rev. 2018;98:419–75. [DOI] [PubMed] [Google Scholar]
- 24. Manzanares G, Brito‐Da‐Silva G, Gandra PG. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res. 2018;52:e7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–8. [DOI] [PubMed] [Google Scholar]
- 26. Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol. 2015;5:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo S, Huang Y, Zhang Y, Huang H, Hong S, Liu T. Impacts of exercise interventions on different diseases and organ functions in mice. J Sport Health Sci. 2020;9:53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Seo DY, Kwak HB, Kim AH, Park SH, Heo JW, Kim HK, et al. Cardiac adaptation to exercise training in health and disease. Pflugers Arch. 2020;472:155–68. [DOI] [PubMed] [Google Scholar]
- 29. Nakamura M, Sadoshima J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat Rev Cardiol. 2018;15:387–407. [DOI] [PubMed] [Google Scholar]
- 30. Dorn GW. The fuzzy logic of physiological cardiac hypertrophy. Hypertension. 2007;49:962–70. [DOI] [PubMed] [Google Scholar]
- 31. Buss SJ, Riffel JH, Malekar P, Hagenmueller M, Asel C, Zhang M, et al. Chronic Akt blockade aggravates pathological hypertrophy and inhibits physiological hypertrophy. Am J Physiol Heart Circ. 2012;302:H420–30. [DOI] [PubMed] [Google Scholar]
- 32. Moc C, Taylor AE, Chesini GP, Zambrano CM, Barlow MS, Zhang X, et al. Physiological activation of Akt by PHLPP1 deletion protects against pathological hypertrophy. Cardiovasc Res. 2015;105:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ramasamy S, Velmurugan G, Rekha B, Anusha S, Shanmugha Rajan K, Shanmugarajan S, et al. Egr‐1 mediated cardiac miR‐99 family expression diverges physiological hypertrophy from pathological hypertrophy. Exp Cell Res. 2018;365:46–56. [DOI] [PubMed] [Google Scholar]
- 34. Palabiyik O, Tastekin E, Doganlar ZB, Tayfur P, Dogan A, Vardar SA. Alteration in cardiac PI3K/Akt/mTOR and ERK signaling pathways with the use of growth hormone and swimming, and the roles of miR21 and miR133. Biomed Rep. 2019;10:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, et al. Akt1 is required for physiological cardiac growth. Circulation. 2006;113:2097–104. [DOI] [PubMed] [Google Scholar]
- 36. Kim J, Wende AR, Sena S, Theobald HA, Soto J, Sloan C, et al. Insulin‐like growth factor I receptor signaling is required for exercise‐induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McMullen JR, Shioi T, Huang WY, Zhang L, Tarnavski O, Bisping E, et al. The insulin‐like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3‐kinase(p110α) pathway. J Biol Chem. 2004;279:4782–93. [DOI] [PubMed] [Google Scholar]
- 38. Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol. 2016;97:245–62. [DOI] [PubMed] [Google Scholar]
- 39. Schüttler D, Clauss S, Weckbach LT, Brunner S. Molecular mechanisms of cardiac remodeling and regeneration in physical exercise. Cell. 2019;8:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ding S, Gan T, Song M, Dai Q, Huang H, Xu Y, et al. C/EBPB‐CITED4 in exercised heart. Adv Exp Med Biol. 2017;1000:247–59. [DOI] [PubMed] [Google Scholar]
- 41. Boström P, Mann N, Wu J, Quintero PA, Plovie ER, Panáková D, et al. C/EBPβ controls exercise‐induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryall KA, Bezzerides VJ, Rosenzweig A, Saucerman JJ. Phenotypic screen quantifying differential regulation of cardiac myocyte hypertrophy identifies CITED4 regulation of myocyte elongation. J Mol Cell Cardiol. 2014;72:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bezzerides VJ, Platt C, Lerchenmüller C, Paruchuri K, Oh NL, Xiao C, et al. CITED4 induces physiologic hypertrophy and promotes functional recovery after ischemic injury. JCI Insight. 2016;1:e85904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lerchenmüller C, Rabolli CP, Yeri AS, Kitchen R, Salvador AM, Liu LX, et al. CITED4 protects against adverse remodeling in response to physiological and pathological stress. Circ Res. 2020;127:631–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Miake J, Notsu T, Higaki K, Hidaka K, Morisaki T, Yamamoto K, et al. Cited4 is related to cardiogenic induction and maintenance of proliferation capacity of embryonic stem cell‐derived cardiomyocytes during in vitro cardiogenesis. PLoS One. 2017;12:e0183225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, et al. MiR‐222 is necessary for exercise‐induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sakamoto T, Kelly DP. A case for adaptive cardiac hypertrophic remodeling is CITED. Circ Res. 2020;127:647–50. [DOI] [PubMed] [Google Scholar]
- 48. Qaisar R, Bhaskaran S, van Remmen H. Muscle fiber type diversification during exercise and regeneration. Free Radic Biol Med. 2016;98:56–67. [DOI] [PubMed] [Google Scholar]
- 49. Moghetti P, Bacchi E, Brangani C, Donà S, Negri C. Metabolic effects of exercise. Front Horm Res. 2016;47:44–57. [DOI] [PubMed] [Google Scholar]
- 50. Weihrauch M, Handschin C. Pharmacological targeting of exercise adaptations in skeletal muscle: benefits and pitfalls. Biochem Pharmacol. 2018;147:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–84. [DOI] [PubMed] [Google Scholar]
- 52. Zhang L, Zhou Y, Wu W, Hou L, Chen H, Zuo B, et al. Skeletal muscle‐specific overexpression of PGC‐1α induces fiber‐type conversion through enhanced mitochondrial respiration and fatty acid oxidation in mice and pigs. Int J Biol Sci. 2017;13:1152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mishra P, Varuzhanyan G, Pham AH, Chan DC. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 2015;22:1033–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rimbaud S, Garnier A, Ventura‐Clapier R. Mitochondrial biogenesis in cardiac pathophysiology. Pharmacol Rep. 2009;61:131–8. [DOI] [PubMed] [Google Scholar]
- 55. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:1062–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dorn GW, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stotland A, Gottlieb RA. Mitochondrial quality control: easy come, easy go. Biochim Biophys Acta. 2015;1853:2802–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Murphy RM, Watt MJ, Febbraio MA. Metabolic communication during exercise. Nat Metab. 2020;2:805–16. [DOI] [PubMed] [Google Scholar]
- 59. Holloszy JO. Regulation of mitochondrial biogenesis and GLUT4 expression by exercise. Compr Physiol. 2011;1:921–40. [DOI] [PubMed] [Google Scholar]
- 60. Quiros PM, Goyal A, Jha P, Auwerx J. Analysis of mtDNA/nDNA ratio in mice. Curr Protoc Mouse Biol. 2017;7:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Qiu Z, Wei Y, Song Q, Du B, Wang H, Chu Y, et al. The role of myocardial mitochondrial quality control in heart failure. Front Pharmacol. 2019;10:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tang BL. Sirt1 and the mitochondria. Mol Cells. 2016;39:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–9. [DOI] [PubMed] [Google Scholar]
- 64. Medeiros DM. Assessing mitochondria biogenesis. Methods. 2008;46:288–94. [DOI] [PubMed] [Google Scholar]
- 65. Viña J, Gomez‐Cabrera MC, Borras C, Froio T, Sanchis‐Gomar F, Martinez‐Bello VE, et al. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev. 2009;61:1369–74. [DOI] [PubMed] [Google Scholar]
- 66. Huertas JR, Casuso RA, Agustín PH, Cogliati S. Stay fit, stay young: mitochondria in movement: the role of exercise in the new mitochondrial paradigm. Oxid Med Cell Longev. 2019;2019:7058350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kasai S, Shimizu S, Tatara Y, Mimura J, Itoh K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules. 2020;10:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu NN, Tian H, Chen P, Wang D, Ren J, Zhang Y. Physical exercise and selective autophagy: benefit and risk on cardiovascular health. Cell. 2019;8:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Joseph AM, Pilegaard H, Litvintsev A, Leick L, Hood DA. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem. 2006;42:13–29. [DOI] [PubMed] [Google Scholar]
- 70. Roh J, Rhee J, Chaudhari V, Rosenzweig A. The role of exercise in cardiac aging: from physiology to molecular mechanisms. Circ Res. 2016;118:279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Eisele JC, Schaefer IM, Randel Nyengaard J, Post H, Liebetanz D, Brüel A, et al. Effect of voluntary exercise on number and volume of cardiomyocytes and their mitochondria in the mouse left ventricle. Basic Res Cardiol. 2008;103:12–21. [DOI] [PubMed] [Google Scholar]
- 72. Vettor R, Valerio A, Ragni M, Trevellin E, Granzotto M, Olivieri M, et al. Exercise training boosts eNOS‐dependent mitochondrial biogenesis in mouse heart: role in adaptation of glucose metabolism. Am J Physiol Endocrinol Metab. 2014;306:E519–28. [DOI] [PubMed] [Google Scholar]
- 73. Li L, Mühlfeld C, Niemann B, Pan R, Li R, Hilfiker‐Kleiner D, et al. Mitochondrial biogenesis and PGC‐1α deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol. 2011;106:1221–34. [DOI] [PubMed] [Google Scholar]
- 74. Bayod S, del Valle J, Lalanza JF, Sanchez‐Roige S, de Luxán‐Delgado B, Coto‐Montes A, et al. Long‐term physical exercise induces changes in Sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol. 2012;47:925–35. [DOI] [PubMed] [Google Scholar]
- 75. Gottlieb RA, Thomas A. Mitophagy and mitochondrial quality control mechanisms in the heart. Curr Pathobiol Rep. 2017;5:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW. Parkin‐mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science. 2015;350:aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Coronado M, Fajardo G, Nguyen K, Zhao M, Kooiker K, Jung G, et al. Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circ Res. 2018;122:282–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yoo SZ, No MH, Heo JW, Park DH, Kang JH, Kim JH, et al. Effects of acute exercise on mitochondrial function, dynamics, and mitophagy in rat cardiac and skeletal muscles. Int Neurourol J. 2019;23:S22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gottlieb RA, Gustafsson ÅB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Torrealba N, Aranguiz P, Alonso C, Rothermel BA, Lavandero S. Mitochondria in structural and functional cardiac remodeling. Adv Exp Med Biol. 2017;982:277–306. [DOI] [PubMed] [Google Scholar]
- 81. Adaniya SM, O‐Uchi J, Cypress MW, Kusakari Y, Jhun BS. Posttranslational modifications of mitochondrial fission and fusion proteins in cardiac physiology and pathophysiology. Am J Physiol Cell Physiol. 2019;16:C583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. No MH, Heo JW, Yoo SZ, Kim CJ, Park DH, Kang JH, et al. Effects of aging and exercise training on mitochondrial function and apoptosis in the rat heart. Pflugers Arch. 2020;472:179–93. [DOI] [PubMed] [Google Scholar]
- 83. Hood DA, Tryon LD, Carter HN, Kim Y, Chen CCW. Unravelling the mechanisms regulating muscle mitochondrial biogenesis. Biochem J. 2016;473:2295–314. [DOI] [PubMed] [Google Scholar]
- 84. Kim Y, Triolo M, Hood DA. Impact of aging and exercise on mitochondrial quality control in skeletal muscle. Oxid Med Cell Longev. 2017;2017:3165396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016;30:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523:617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Russell AP, Foletta VC, Snow RJ, Wadley GD. Skeletal muscle mitochondria: a major player in exercise, health and disease. Biochim Biophys Acta. 2014;1840:1276–84. [DOI] [PubMed] [Google Scholar]
- 88. Memme JM, Erlich AT, Phukan G, Hood DA. Exercise and mitochondrial health. J Physiol. 2021;599:803–17. [DOI] [PubMed] [Google Scholar]
- 89. Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low‐volume high‐intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. J Physiol. 2010;588:1011–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Caffin F, Prola A, Piquereau J, Novotova M, David DJ, Garnier A, et al. Altered skeletal muscle mitochondrial biogenesis but improved endurance capacity in trained OPA1‐deficient mice. J Physiol. 2013;591:6017–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Drake JC, Laker RC, Wilson RJ, Zhang M, Yan Z. Exercise‐induced mitophagy in skeletal muscle occurs in the absence of stabilization of Pink1 on mitochondria. Cell Cycle. 2019;18:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Laker RC, Drake JC, Wilson RJ, Lira VA, Lewellen BM, Ryall KA, et al. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise‐induced mitophagy. Nat Commun. 2017;8:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang Z, Sliter DA, Bleck CKE, Ding S. Fis1 deficiencies differentially affect mitochondrial quality in skeletal muscle. Mitochondrion. 2019;49:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Moore TM, Zhou Z, Cohn W, Norheim F, Lin AJ, Kalajian N, et al. The impact of exercise on mitochondrial dynamics and the role of Drp1 in exercise performance and training adaptations in skeletal muscle. Mol Metab. 2019;21:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Trewin AJ, Berry BJ, Wojtovich AP. Exercise and mitochondrial dynamics: keeping in shape with ROS and AMPK. Antioxidants (Basel). 2018;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Weiss K, Schär M, Panjrath GS, Zhang Y, Sharma K, Bottomley PA, et al. Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circ Heart Fail. 2017;10:e004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985). 2015;119:739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123:107–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Rohini A, Agrawal N, Koyani CN, Singh R. Molecular targets and regulators of cardiac hypertrophy. Pharmacol Res. 2010;61:269–80. [DOI] [PubMed] [Google Scholar]
- 100. Bernardo BC, Ooi JYY, McMullen JR. The yin and yang of adaptive and maladaptive processes in heart failure. Drug Discov Today Ther Strateg. 2012;9:e163–72. [Google Scholar]
- 101. Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. [DOI] [PubMed] [Google Scholar]
- 102. Tham YK, Bernardo BC, Ooi JYY, Weeks KL, McMullen JR. Pathophysiology of cardiac hypertrophy and heart failure: signaling pathways and novel therapeutic targets. Arch Toxicol. 2015;89:1401–38. [DOI] [PubMed] [Google Scholar]
- 103. Tran DH, Wang ZV. Glucose metabolism in cardiac hypertrophy and heart failure. J Am Heart Assoc. 2019;8:e012673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sabbah HN. Targeting the mitochondria in heart failure: a translational perspective. JACC Basic Transl Sci. 2020;5:88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vásquez‐Trincado C, García‐Carvajal I, Pennanen C, Parra V, Hill JA, Rothermel BA, et al. Mitochondrial dynamics, mitophagy and cardiovascular disease. J Physiol. 2016;594:509–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Keller‐Ross ML, Larson M, Johnson BD. Skeletal muscle fatigability in heart failure. Front Physiol. 2019;10:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kumar AA, Kelly DP, Chirinos JA. Mitochondrial dysfunction in heart failure with preserved ejection fraction. Circulation. 2019;139:1435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tucker WJ, Haykowsky MJ, Seo Y, Stehling E, Forman DE. Impaired exercise tolerance in heart failure: role of skeletal muscle morphology and function. Curr Heart Fail Rep. 2018;15:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Taylor DJ. Clinical utility of muscle MR spectroscopy. Sem Musculoskelet Radiol. 2000;4:481–502. [DOI] [PubMed] [Google Scholar]
- 110. Molina AJA, Bharadwaj MS, van Horn C, Nicklas BJ, Lyles MF, Eggebeen J, et al. Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC Heart Fail. 2016;4:636–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tsuda M, Fukushima A, Matsumoto J, Takada S, Kakutani N, Nambu H, et al. Protein acetylation in skeletal muscle mitochondria is involved in impaired fatty acid oxidation and exercise intolerance in heart failure. J Cachexia Sarcopenia Muscle. 2018;9:844–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Jia D, Hou L, Lv Y, Xi L, Tian Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC‐1α/PI3K/Akt signaling. J Cell Physiol. 2019;234:23705–18. [DOI] [PubMed] [Google Scholar]
- 113. Campos JC, Queliconi BB, Dourado PMM, Cunha TF, Zambelli VO, Bechara LRG, et al. Exercise training restores cardiac protein quality control in heart failure. PLoS One. 2012;7:e52764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ghahremani R, Damirchi A, Salehi I, Komaki A, Esposito F. Mitochondrial dynamics as an underlying mechanism involved in aerobic exercise training‐induced cardioprotection against ischemia‐reperfusion injury. Life Sci. 2018;213:102–8. [DOI] [PubMed] [Google Scholar]
- 115. Zhao D, Sun Y, Tan Y, Zhang Z, Hou Z, Gao C, et al. Short‐duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxid Med Cell Longev. 2018;2018:4079041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cai M, Wang Q, Liu Z, Jia D, Feng R, Tian Z. Effects of different types of exercise on skeletal muscle atrophy, antioxidant capacity and growth factors expression following myocardial infarction. Life Sci. 2018;213:40–9. [DOI] [PubMed] [Google Scholar]
- 117. Bardi E, Majerczak J, Zoladz JA, Tyrankiewicz U, Skorka T, Chlopicki S, et al. Voluntary physical activity counteracts chronic heart failure progression affecting both cardiac function and skeletal muscle in the transgenic Tgαq*44 mouse model. Physiol Rep. 2019;7:e14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bacurau AVN, Jannig PR, de Moraes WM, Cunha TF, Medeiros A, Barberi L, et al. Akt/mTOR pathway contributes to skeletal muscle anti‐atrophic effect of aerobic exercise training in heart failure mice. Int J Cardiol. 2016;214:137–47. [DOI] [PubMed] [Google Scholar]
- 119. Moreira JBN, Bechara LRG, Bozi LHM, Jannig PR, Monteiro AWA, Dourado PM, et al. High‐versus moderate‐intensity aerobic exercise training effects on skeletal muscle of infarcted rats. J Appl Physiol. 2013;114:1029–41. [DOI] [PubMed] [Google Scholar]
- 120. Tran D. Cardiopulmonary exercise testing. Methods Mol Biol. 2018;1735:285–95. [DOI] [PubMed] [Google Scholar]
- 121. Degache F, Duché P, Mckeever KH, Jones S, Tillin T, Williams S, et al. Assessment of exercise capacity and oxygen consumption using a 6 min stepper test in older adults. Front Physiol. 2017;8:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jeneson JAL, Schmitz JPJ, Hilbers PAJ, Nicolay K. An MR‐compatible bicycle ergometer for in‐magnet whole‐body human exercise testing. Magn Reson Med. 2010;63:257–61. [DOI] [PubMed] [Google Scholar]
- 123. Sedivy P, Dezortova M, Rydlo J, Drobny M, Krššák M, Valkovič L, et al. MR compatible ergometers for dynamic 31P MRS. J Appl Biomed. 2019;17:91–8. [DOI] [PubMed] [Google Scholar]
- 124. Meyerspeer M, Boesch C, Cameron D, Dezortová M, Forbes SC, Heerschap A, et al. 31P magnetic resonance spectroscopy in skeletal muscle: experts' consensus recommendations. NMR Biomed. 2020;34:e4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Šedivý P, Christina Kipfelsberger M, Dezortová M, Krššák M, Drobný M, Chmelík M, et al. Dynamic 31P MR spectroscopy of plantar flexion: influence of ergometer design, magnetic field strength (3 and 7 T), and RF‐coil design. Med Phys. 2015;42:1678–89. [DOI] [PubMed] [Google Scholar]
- 126. Valkovič L, Chmelík M, Krššák M. In‐vivo 31P‐MRS of skeletal muscle and liver: a way for non‐invasive assessment of their metabolism. Anal Biochem. 2017;529:193–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Melenovsky V, Hlavata K, Sedivy P, Dezortova M, Borlaug BA, Petrak J, et al. Skeletal muscle abnormalities and iron deficiency in chronic heart failure an exercise 31P magnetic resonance spectroscopy study of calf muscle. Circ Heart Fail. 2018;11:e004800. [DOI] [PubMed] [Google Scholar]
- 128. van der Ent M, Jeneson JA, Remme WJ, Berger R, Ciampricotti R, Visser F. A non‐invasive selective assessment of type I fibre mitochondrial function using 31P NMR spectroscopy. Evidence for impaired oxidative phosphorylation rate in skeletal muscle in patients with chronic heart failure. Eur Heart J. 1998;19:124–31. [DOI] [PubMed] [Google Scholar]
- 129. Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al.; HF‐ACTION Investigators . Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Ambrosy AP, Cerbin LP, DeVore AD, Greene SJ, Kraus WE, O'Connor CM, et al. Aerobic exercise training and general health status in ambulatory heart failure patients with a reduced ejection fraction – findings from the Heart Failure and A Controlled Trial Investigating Outcomes of Exercise Training (HF‐ACTION) trial. Am Heart J. 2017;186:130–8. [DOI] [PubMed] [Google Scholar]
- 131. van der Meer S, Zwerink M, van Brussel M, van der Valk P, Wajon E, van der Palen J. Effect of outpatient exercise training programmes in patients with chronic heart failure: a systematic review. Eur J Prev Cardiol. 2012;19:795–803. [DOI] [PubMed] [Google Scholar]
- 132. Acanfora D, Scicchitano P, Casucci G, Lanzillo B, Capuano N, Furgi G, et al. Exercise training effects on elderly and middle‐age patients with chronic heart failure after acute decompensation: a randomized, controlled trial. Int J Cardiol. 2016;225:313–23. [DOI] [PubMed] [Google Scholar]
- 133. Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10‐year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol. 2012;60:1521–8. [DOI] [PubMed] [Google Scholar]
- 134. Chan E, Giallauria F, Vigorito C, Smart NA. Exercise training in heart failure patients with preserved ejection fraction: a systematic review and meta‐analysis. Monaldi Arch Chest Dis. 2016;86:759. [DOI] [PubMed] [Google Scholar]
- 135. Gielen S, Laughlin MH, O'Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis. 2015;57:347–55. [DOI] [PubMed] [Google Scholar]
- 136. Morris JH, Chen L. Exercise training and heart failure: a review of the literature. Card Fail Rev. 2019;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Mediano MFF, Leifer ES, Cooper LS, Keteyian SJ, Kraus WE, Mentz RJ, et al. Influence of baseline physical activity level on exercise training response and clinical outcomes in heart failure: the HF‐ACTION trial. JACC Heart Fail. 2018;6:1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al.; HF‐ACTION Investigators . Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. O'Donnell J, Smith‐Byrne K, Velardo C, Conrad N, Salimi‐Khorshidi G, Doherty A, et al. Self‐reported and objectively measured physical activity in people with and without chronic heart failure: UK Biobank analysis. Open Heart. 2020;7:e001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Taylor RS, Long L, Mordi IR, Madsen MT, Davies EJ, Dalal H, et al. Exercise‐based rehabilitation for heart failure: Cochrane systematic review, meta‐analysis, and trial sequential analysis. JACC Heart Fail. 2019;7:691–705. [DOI] [PubMed] [Google Scholar]
- 141. Taylor RS, Walker S, Smart NA, Piepoli MF, Warren FC, Ciani O, et al. Impact of exercise rehabilitation on exercise capacity and quality‐of‐life in heart failure: individual participant meta‐analysis. J Am Coll Cardiol. 2019;73:1430–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta‐analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hieda M, Sarma S, Hearon CM, Macnamara JP, Dias KA, Samels M, et al. One‐year committed exercise training reverses abnormal left ventricular myocardial stiffness in patients with stage B heart failure with preserved ejection fraction. Circulation. 2021;144:934–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Alonso WW, Kupzyk KA, Norman JF, Lundgren SW, Fisher A, Lindsy ML, et al. The HEART Camp exercise intervention improves exercise adherence, physical function, and patient‐reported outcomes in adults with preserved ejection fraction heart failure. J Card Fail. 2021. 10.1016/j.cardfail.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cooper LB, Mentz RJ, Sun JL, Schulte PJ, Fleg JL, Cooper LS, et al. Psychosocial factors, exercise adherence, and outcomes in heart failure patients: insights from Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF‐ACTION). Circ Heart Fail. 2015;8:1044–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Yang L, Li Y, Wang X, Mu X, Qin D, Huang W, et al. Overexpression of miR‐223 tips the balance of pro‐ and anti‐hypertrophic signaling cascades toward physiologic cardiac hypertrophy. J Biol Chem. 2016;291:15700–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Gevaert AB, Witvrouwen I, van Craenenbroeck AH, van Laere SJ, Boen JRA, van de Heyning CM, et al.; OptimEx‐Clin Study Group . miR‐181c level predicts response to exercise training in patients with heart failure and preserved ejection fraction: an analysis of the OptimEx‐Clin trial. Eur J Prev Cardiol. 2021;28:1722–33. [DOI] [PubMed] [Google Scholar]
- 148. Lu B, Yu H, Zwartbol M, Ruifrok WP, van Gilst WH, de Boer RA, et al. Identification of hypertrophy‐ and heart failure‐associated genes by combining in vitro and in vivo models. Physiol Genomics. 2012;44:443–54. [DOI] [PubMed] [Google Scholar]
- 149. Yu H, Tigchelaar W, Lu B, van Gilst WH, de Boer RA, Westenbrink BD, et al. AKIP1, a cardiac hypertrophy induced protein that stimulates cardiomyocyte growth via the Akt pathway. Int J Mol Sci. 2013;14:21378–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Booij HG, Yu H, de Boer RA, van de Kolk CWA, van de Sluis B, van Deursen JM, et al. Overexpression of a kinase interacting protein 1 attenuates myocardial ischaemia/reperfusion injury but does not influence heart failure development. Cardiovasc Res. 2016;111:217–26. [DOI] [PubMed] [Google Scholar]
- 151. Lee Y, Min K, Talbert EE, Kavazis AN, Smuder AJ, Willis WT, et al. Exercise protects cardiac mitochondria against ischemia‐reperfusion injury. Med Sci Sports Exerc. 2012;44:397–405. [DOI] [PubMed] [Google Scholar]
- 152. Jiang HK, Wang YH, Sun L, He X, Zhao M, Feng ZH, et al. Aerobic interval training attenuates mitochondrial dysfunction in rats post‐myocardial infarction: roles of mitochondrial network dynamics. Int J Mol Sci. 2014;15:5304–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Campos JC, Queliconi BB, Bozi LHM, Bechara LRG, Dourado PMM, Andres AM, et al. Exercise reestablishes autophagic flux and mitochondrial quality control in heart failure. Autophagy. 2017;13:1304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. McCoy J, Bates M, Eggett C, Siervo M, Cassidy S, Newman J, et al. Pathophysiology of exercise intolerance in chronic diseases: the role of diminished cardiac performance in mitochondrial and heart failure patients. Open Heart. 2017;4:e000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Nijholt KT, Meems LMG, Ruifrok WPT, Maass AH, Yurista SR, Pavez‐Giani MG, et al. The erythropoietin receptor expressed in skeletal muscle is essential for mitochondrial biogenesis and physiological exercise. Pflügers Arch. 2021;473:1301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


