Abstract
Introduction
Recent evidence suggested that vitamin D deficiency was associated with Hashimoto's thyroiditis (HT) pathogenesis and thyroid hypofunction. This study aimed to investigate whether vitamin D supplementation would be effective in the prevention and progression of hypothyroidism in patients with HT.
Methods
PubMed, Embase and the Cochrane library were searched for randomized controlled trials (RCTs) and prospective cohort studies published from inception to August 2021.
Results
A total of 7 cohorts of patients from six clinical trials with 258 patients with HT were included. Significant difference was found (WMD = 19.00, 95% CI: 12.43, 25.58, p < 0.001; I 2 = 90.0%, p heterogeneity < 0.001) between the vitamin D group and control group in serum 25‐hydroxyvitamin D level. And the combined results indicated vitamin D supplementation significantly reduced the level of thyroid peroxidase antibodies (TPO‐Ab) compared to the control group (WMD = −158.18, 95% CI: −301.92, −14.45, p = 0.031; I 2 = 68.8%, p heterogeneity = 0.007). Whereas no significant differences were found on the levels of thyroid‐stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4) compared to the control group (p > 0.05).
What is New and Conclusion
Our study demonstrated that vitamin D treatment might significantly increase the serum 25(OH)D levels and produce changes in TPO‐Ab titres.
No significant association was found between serum vitamin D treatment and the levels of TG‐Ab, TSH, FT3 and FT4, suggesting that vitamin D is not associated with the function of the thyroid in patients with HT.
Keywords: hashimoto's thyroiditis, meta‐analysis, thyroid, vitamin D
1. WHAT IS KNOWN
Hashimoto's thyroiditis (HT), also called chronic lymphocytic thyroiditis, is the most prevalent organ‐specific autoimmune disorder as well as the most common cause of thyroid hypofunction, with an incidence of 0.3–1.5 cases per 1000 people in developed countries. 1 , 2 This autoimmune event is believed to be initiated by the activation of thyroid antigen‐specific auxiliary T lymphocytes and patients may present with various thyroid function states but most of them eventually evolve into hypothyroidism. 3 The main purpose of HT treatment is the control of hypothyroidism, including oral administration of a synthetic hormone to achieve normal circulating thyrotropin levels. But the effectiveness of the current treatments such as glucocorticoids, levothyroxine and specific diet for the management of HT has been questioned in recent years. 4 , 5 , 6 , 7
Vitamin D is a steroid molecule whose primary function is to control bone metabolism and calcium and phosphorus homeostasis. Recent evidence suggested that vitamin D is also associated with non‐skeletal roles including in autoimmune diseases, infectious diseases, metabolic syndrome, cardiovascular diseases and cancers. 8 , 9 , 10 A recent published meta‐analysis analysed the effects of vitamin D 11 on autoantibodies in patients with autoimmune thyroiditis (AIT). Based on the combined results of six randomized controlled trials, they concluded that vitamin D treatment might be a good choice for AIT based on vitamin D, especially for those with vitamin D deficiency, which is common worldwide. 12 Low levels of vitamin D were found to be associated with HT, and the association between vitamin D deficiency, HT pathogenesis and thyroid hypofunction has been demonstrated in several studies. 13 , 14 , 15 Given the low cost and the minimal side effects of oral vitamin D supplementation, the treatment of vitamin D supplementation in patients with HT may be recommended.
1.1. Objective
In light of the current information, vitamin D might be effective in the treatment of hypothyroidism in patients with HT. Therefore, this study aimed to review the association between vitamin D treatment in patients with HT by assessing patients’ serum circulating 25‐hydroxyvitamin D level to evaluate whether a change occurs in the course of disease.
2. METHODS
2.1. Ethical statement
We developed the framework of the current systematic review and meta‐analysis according to the recommendations issued by the Cochrane Collaboration for the purpose of ensuring the methodological quality because we did not register formal protocol. 16 We did not impose ethical approval and patients’ informed consent because all essential data in the current systematic review and meta‐analysis were extracted from published studies.
2.2. Literature search
This meta‐analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 17 The relevant clinical trials were searched based on the PICO process. 18 A systematic search was performed from PubMed, Embase and the Cochrane library for available studies published up to August 2021, using the MeSH term ‘Hashimoto Disease’ and ‘Vitamin D’, as well as relevant keywords. For studies that have not been completed and published but had previously published their design and protocol with their registration number in ClinicalTrials.gov, we manually searched them to ensure whether the results were posted or not.
2.3. Eligibility criteria
The eligibility criteria were as follows: (1) population: patients diagnosed with HT; (2) interventions: treated by vitamin D; (3) control: placebo or matched individualized therapy; (4) study type: any prospective studies or RCTs published in scientific peer‐reviewed journals; (5) outcome: serum 25‐hydroxyvitamin D level after treatment; and (6) language was limited to English. No ethical consent was required because this study was performed based on previous data.
2.4. Data extraction
Two investigators (Hui Jiang and Xiao‐luo Chen) independently extracted the following items using the pre‐designed data extraction sheet: basic characteristics of the study including first author, year of publication, country, and type of study design, patients’ characteristics including sample size, mean age and gender, and clinical characteristics of study including vitamin D status at inclusion, serum thyrotropin levels at inclusion, treatment in both groups, dose of treatment, follow‐up period. Serum 25‐hydroxyvitamin D level after treatment was included as primary outcome. If an included study was designed to have more than two groups, then the methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions were used to divide the individual study into two unique RCTs or combine groups to create a single pair‐wise comparison. 19 If essential information was missed from the original study, then the leading author was contacted for additional information. Any inconsistencies in data extraction were solved based on the consensus principle.
2.5. Outcomes
The primary outcome was the serum 25‐hydroxyvitamin D level after treatments. The secondary outcomes were the levels of thyroid peroxidase antibodies (TPO‐Ab), thyroglobulin antibodies (TG‐Ab), thyroid‐stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4) after treatments.
2.6. Quality of the evidence
The level of evidence of all included studies was assessed independently by two authors (Hui Jiang and Xiao‐luo Chen) using the RoB‐2 criteria or MINORS (Methodological Index for Non‐Randomized Studies) scoring system. 20 , 21 Discrepancies in the assessment were resolved through discussion until a consensus was reached.
2.7. Statistical analysis
All analyses were performed using STATA SE 14.0 (StataCorp). The outcomes were presented as weighted mean differences (WMD). The effects and corresponding 95% confidence intervals (CIs) were used to compare the outcomes. For studies that did not present their results as means ±standard deviations, the results were estimated based on the reported parameters (median, standard error, IQR or 95% CI). 22 Statistical heterogeneity among studies was calculated using Cochran's Q test and the I 2 index. An I 2 > 50% and a Q test p < 0.10 indicated high heterogeneity, and the random effects model was used; otherwise, the fixed‐effects model was applied. p‐values <0.05 were considered statistically different. Sensitivity analysis was performed using the leave‐one‐out method. 20 We did not assess the potential publication bias by funnel plots and Egger's test, because the number of studies included in every meta‐analysis was fewer than ten, in which case the funnel plots and Egger's test could yield misleading results and are not recommended.
3. RESULTS
3.1. Study inclusion
Figure 1 presents the study inclusion process. A total of 416 studies were first retrieved, and 61 trials were left after removing the duplicates. Then, 182 were excluded because of the type of article or the language. From the 173 trials left, after reviewing the full texts, 56 case‐report studies, 36 retrospective designed studies, 11 in vivo studies, 29 studies with unsuitable population, 34 with inappropriate interventions and 1 with unwanted outcome were excluded. Therefore, a total of seven cohorts of patients from six studies (3 prospective cohort study and 3 RCTs) entered our analysis (Table 1). 23 , 24 , 25 , 26 , 27 , 28 At the end of our research, 258 patients with HT were included in our analyses.
FIGURE 1.
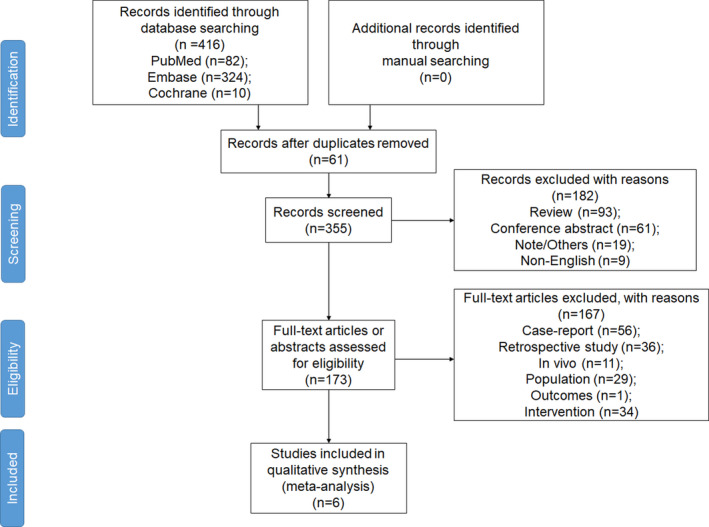
PRISMA 2009 flow diagram
TABLE 1.
Characteristics of the literatures
| Author, year | Country | Study design | VD status | Serum thyrotropin levels, mU/l | Intervention | Control | Sample size | Dose | Gender | Age (intervention/control), y | Follow‐up | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | ||||||||||
| Chahardoli, 2019 | Iran | RCT | Not limited | NA | VD | Placebo | 19 | 21 | 50000 IU/week | / | Female | 36.4 ± 5.2/35.9 ± 7.8 | 3 months |
| Nodehi, 2019 | Iran | RCT | Not limited | NA | VD | Placebo | 17 | 17 | 50000 IU/week | / | Female | 36.4 ± 5.2/35.9 ± 7.8 | 3 months |
| Vahabi, 2017 | Iran | RCT | Insufficiency | NA | VD | Placebo | 30 | 26 | 50000 IU/week | / | Both | 43.6 ± 1.6/44.1 ± 1.6 | 3 months |
| Krysiak, 2016 | Poland | Prospective | Normal | 0.4 to 8 | VD + Levothyrotxine | Levothyroxine | 16 | 18 | 2000 IU/d Levothyroxine: NA | NA | Female | 34 ± 7/36 ± 6 | 6 months |
| Krysiak, 2017a | Poland | Prospective | Insufficiency | 0.45 to 4.5 | VD + Simvastatin | Simvastatin | 19 | 9 | VD: 2000 IU/d Simvastatin: 40 mg/day | 40 mg/day | Female | 38 ± 7/37 ± 6 | 6 months |
| Krysiak, 2017b | Poland | Prospective | Insufficiency | 0.45 to 4.5 | VD | Simvastatin | 20 | 9 | 2000 IU/day | 40 mg/day | Female | 39 ± 5/37 ± 6 | 6 months |
| Krysiak, 2018 | Poland | Prospective | Not limited | 0.4 to 4 | VD | Selenomethionine | 20 | 17 | 4000 IU/day | 200 µg/day | Male | 35 ± 8/34 ± 7 | 6 months |
NA, not applicable; RCT, randomized control trial; VD, vitamin D.
3.2. Quality of all included studies
Among the three randomized controlled trials, 23 , 27 , 28 all three studies were graded as low risk of bias in the assessment for bias arising from the randomization process, bias due to missing outcome data and bias in selection of the reported result. Some concerns were raised in the assessment for bias due to deviations from intended interventions in one study. 27 All three studies were concerned in the assessment for bias in measurement of the outcome. Among the four prospective studies, 24 , 25 , 26 the total quality score of individual study was all more than 6. We summarized the results of appraising quality of all included studies in Online appendix A1.
3.3. Effect of vitamin D on the serum 25‐hydroxyvitamin D
All 7 cohorts of patients from six studies reported the serum 25‐hydroxyvitamin D level after treatment in both treatment and control group. Significant difference was found (WMD = 19.00, 95% CI: 12.43, 25.58, p < 0.001; I 2 = 90.0%, p heterogeneity < 0.001, Figure 2A) between the vitamin D group and control group in serum 25‐hydroxyvitamin D level. The sensitivity analyses showed that no specific study contributed to heterogeneity (Online appendix A2a).
FIGURE 2.
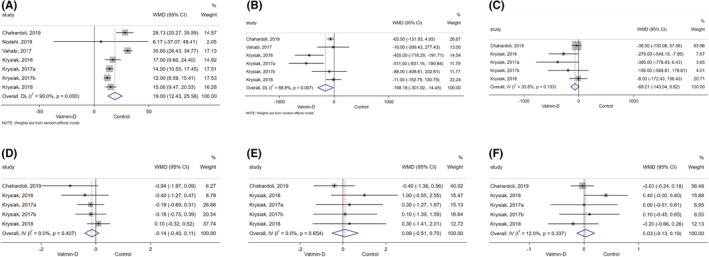
Forest plot comparing the autoimmunity markers between vitamin D group with the control group. (A) 25‐Hydroxyvitamin D Level; (B) thyroid peroxidase antibodies; (C) thyroglobulin antibodies; (D) thyroid‐stimulating hormone; (E) free thyroxine; (F) free triiodothyronine
3.4. Secondary outcomes
Six cohorts of patients from five studies reported the level of TPO‐Ab after treatment from vitamin D or control. 23 , 24 , 25 , 26 , 28 Combined results indicated vitamin D significantly reduced the level of TPO‐Ab compared to the control group (WMD = −158.18, 95% CI: −301.92, −14.45, p = 0.031; I 2 = 68.8%, p heterogeneity = 0.007, Figure 2B). The sensitivity analysis showed that no specific study contributed to heterogeneity (Online appendix A2b).
Five cohorts of patients from four studies reported the level of TG‐Ab after treatment from vitamin D or control. 23 , 24 , 25 , 26 Combined results indicated vitamin D did not significantly reduce the level of TG‐Ab compared to the control group (WMD = −68.21, 95% CI: −143.04, 6.62, p = 0.074; I 2 = 35.8%, p heterogeneity = 0.183, Figure 2C). The sensitivity analysis showed that no specific study contributed to heterogeneity (Online appendix A2c).
Combined results showed vitamin D did not significantly change the levels of TSH, FT3 and FT4 compared to the control group (Figure 2D–F).
3.5. Subgroup analyses
All subgroup analyses were presented in Table 2. The level of serum 25‐hydroxyvitamin D was increased from the treatment of vitamin D regardless of the length of follow‐up, the vitamin status at initiation and the gender of patients compared to the controls group in patients with HT. The sensitivity analyses showed that no specific study contributed to heterogeneity Online appendix A2d&e&f).
TABLE 2.
Combined results of the subgroup analyses
| N | WMD (95% CI) | p (Heterogeneity) | I 2, % | p | |||
|---|---|---|---|---|---|---|---|
| 25‐hydroxyvitamin D | 7 | 19.00 (12.43, 25.58) | <0.001 | 90 | <0.001 | ||
| Follow‐up | |||||||
| 3 months | 3 | 29.89 (26.22, 33.55) | 0.482 | 0 | <0.001 | ||
| 6 months | 4 | 13.62 (11.49, 15.74) | 0.582 | 0 | <0.001 | ||
| Vitamin D status | |||||||
| Insufficient | 3 | 18.80 (8.05, 29.56) | <0.001 | 96.1 | 0.001 | ||
| Normal | 1 | 17.00 (9.60, 24.40) | . | . | <0.001 | ||
| Both | 3 | 20.25 (8.58, 31.92) | 0.023 | 73.4 | 0.001 | ||
| Gender | |||||||
| Women | 5 | 16.60 (11.43, 21.77) | 0.006 | 72 | <0.001 | ||
| Men | 1 | 15.00 (9.47, 20.53) | . | . | <0.001 | ||
| Both | 1 | 30.60 (26.43, 34.77) | . | . | <0.001 | ||
| Thyroid peroxidase antibodies | 6 | −158.18 (−301.92, −14.45) | 0.007 | 68.8 | 0.031 | ||
| Follow‐up | |||||||
| 3 months | 2 | −61.05 (−127.69, 5.59) | 0.727 | 0 | 0.073 | ||
| 6 months | 4 | −251.51 (−520.68, 17.66) | 0.003 | 78.4 | 0.067 | ||
| Vitamin D status | |||||||
| Insufficient | 3 | −201.15 (−501.79, 99.49) | 0.061 | 64.3 | 0.190 | ||
| Normal | 1 | −455.00 (−718.29, −191.71) | 0.001 | ||||
| Both | 2 | −53.58 (−115.20, 8.05) | 0.513 | 0 | 0.088 | ||
| Gender | |||||||
| Women | 4 | −260.38 (−509.17, −11.60) | 0.002 | 79.2 | 0.040 | ||
| Men | 1 | −11.00 (−152.75, 130.75) | . | . | 0.879 | ||
| Both | 1 | −16.00 (−309.42, 277.42) | . | . | 0.915 | ||
| Thyroglobulin antibodies | 5 | −68.21 (−143.04, 6.62) | 0.183 | 35.8 | 0.074 | ||
| Follow‐up | |||||||
| 3 months | 1 | −36.50 (−130.06, 57.06) | . | . | 0.445 | ||
| 6 months | 4 | −124.48 (−249.12, 0.16) | 0.171 | 40.1 | 0.050 | ||
| Vitamin D status | |||||||
| Insufficient | 2 | −285.63 (−555.97, −15.29) | 0.491 | 0 | 0.038 | ||
| Normal | 1 | −278.00 (−548.15, −7.85) | . | . | 0.044 | ||
| Both | 2 | −29.53 (−110.85, 51.79) | 0.768 | 0 | 0.477 | ||
| Gender | |||||||
| Women | 4 | −83.94 (−167.97, 0.09) | 0.134 | 46.2 | 0.050 | ||
| Men | 1 | −8.00 (−172.43, 156.43) | 0.924 | ||||
| Thyrotropin | 6 | −0.00 (−0.66, 0.66) | <0.001 | 88.9 | 0.998 | ||
| Follow‐up | |||||||
| 3 months | 2 | 0.20 (−1.92, 2.31) | <0.001 | 93.5 | 0.855 | ||
| 6 months | 4 | −0.09 (−0.36, 0.18) | 0.675 | 0 | 0.503 | ||
| Vitamin D status | |||||||
| Insufficient | 3 | 0.30 (−0.73, 1.34) | <0.001 | 93.5 | 0.569 | ||
| Normal | 1 | −0.40 (−1.27, 0.47) | . | . | 0.366 | ||
| Both | 2 | −0.05 (−0.44, 0.34) | 0.066 | 70.3 | 0.808 | ||
| Gender | |||||||
| Women | 4 | −0.29 (−0.62, 0.03) | 0.594 | 0 | 0.079 | ||
| Both | 1 | 1.22 (0.89, 1.55) | <0.001 | ||||
| Men | 1 | 0.10 (−0.32, 0.52) | 0.640 | ||||
| Free thyroxine | 5 | 0.09 (−0.51, 0.70) | 0.654 | 0 | 0.760 | ||
| Follow‐up | |||||||
| 3 months | 1 | −0.40 (−1.36, 0.56) | . | . | 0.416 | ||
| 6 months | 4 | 0.43 (−0.36, 1.21) | 0.860 | 0 | 0.290 | ||
| Vitamin D status | |||||||
| Insufficient | 2 | 0.20 (−0.89, 1.28) | 0.856 | 0 | 0.723 | ||
| Normal | 1 | 1.00 (−0.55, 2.55) | . | . | 0.206 | ||
| Both | 2 | −0.23 (−1.07, 0.61) | 0.484 | 0 | 0.589 | ||
| Gender | |||||||
| Women | 4 | 0.06 (−0.59, 0.72) | 0.497 | 0 | 0.845 | ||
| Men | 1 | 0.30 (−1.41, 2.01) | . | . | 0.731 | ||
| Free triiodothyronine | 5 | 0.03 (−0.13, 0.19) | 0.337 | 12 | 0.707 | ||
| Follow‐up | |||||||
| 3 months | 1 | −0.03 (−0.24, 0.18) | 0.784 | ||||
| 6 months | 4 | 0.11 (−0.13, 0.35) | 0.280 | 21.7 | 0.378 | ||
| Vitamin D status | |||||||
| Insufficient | 2 | 0.06 (−0.35, 0.46) | 0.812 | 0 | 0.792 | ||
| Normal | 1 | 0.40 (−0.00, 0.80) | 0.052 | ||||
| Both | 2 | −0.06 (−0.25, 0.13) | 0.513 | 0 | 0.545 | ||
| Gender | |||||||
| Women | 4 | 0.06 (−0.11, 0.23) | 0.327 | 13.1 | 0.474 | ||
| Men | 1 | −0.20 (−0.66, 0.26) | 0.397 | ||||
4. DISCUSSION
The present meta‐analysis suggested that despite a significant increase in serum 25‐hydroxyvitamin D level and decrease in TPO‐Ab among patients who received vitamin D were observed, we did not find any significant effect of vitamin D treatment on TG‐Ab, TSH, FT4 and FT3.
It has been well acknowledged that vitamin D plays a significant role in modulation of the immune system. It can enhance the innate immune response while exert an inhibitory action on the adaptive immune system. 29 , 30 Our results showed that after patients with HT were treated by vitamin D supplementation, their serum 25(OH)D levels significantly increased by 19.00 (95% CI: 12.43–25.58) ng/ml compared to the patients who were treated by placebo or other thyroid replacement medication. Till now, some studies have reported an association between serum 25(OH)D levels and autoimmune thyroid disorder (AITD) or HT. 31 , 32 , 33 , 34 , 35 Kivity et al. 36 pointed out that vitamin D deficiency was linked to the presence of anti‐thyroid antibodies and abnormal thyroid functions. Their results showed a significant correlation between vitamin D deficiency and the presence of anti‐thyroid antibodies (p = 0.010), suggesting the involvement of vitamin D in the pathogenesis of AITD. Several studies demonstrated that the prevalence of vitamin D insufficiency was significantly higher in patients with HT compared to healthy controls, and the serum 25(OH)D levels in patients with HT were significantly lower than the controls. 37 Additionally, the severity of vitamin D deficiency was also correlated with the duration of HT, thyroid volume and antibody levels, suggesting a potential role of vitamin D in the development of HT and its progression to hypothyroidism. 38 Mansournia et al. 39 showed that by 5 ng/ml increases in the level of vitamin D, the risk of occurrence of HT would be decreased by 19%. The inverse association between vitamin D level and HT strongly implies that vitamin D supplementation could be effective in patients with HT.
Lymphocytic infiltration of the thyroid gland correlates well with the thyroid antibodies, which are considered the most reliable prognostic factors to predict HT and the development of hypothyroidism. 40 , 41 Thyroid function tests include FT3, FT4, TSH, TPO‐Ab and TG‐Ab concentrations. In our analyses, we found a significantly lower TPO‐Ab level in patients who were treated by vitamin D compared to those who were treated by placebo or other thyroid replacement medication (WMD: −158.18, 95% CI: −301.92, −14.45, p = 0.031). Considering the significantly higher level of serum 25(OH)D in vitamin D group compared to other treatments, it is reasonable to speculate that there was a negative association between serum 25(OH)D level and TPO‐Ab in patients with HT. Comparing to the meta‐analysis 11 investigating the effect of vitamin D on AIT, they found a significant decrease of TPO‐Ab levels (three studies, random effects SMD: −0.57, 95% CI: −1.08 to −0.07, I 2 = 79.7%) in the vitamin D‐treated group as well. In addition, they also observed a significantly lower Tg‐Ab titres (four studies, random effects SMD: −0.55, 95% CI: −1.05 to −0.04, p = 0.033) in vitamin D group compared to the controls. The possible explanations for the differences are that only three studies in their analysis investigated specifically patients with HT, and only one of which was included in our analysis (the other two published in Mandarin were excluded from this study). Our result is consistent with the previous research that demonstrated a significant negative correlation between serum 25(OH)D levels and TPO‑Ab in patients with AITDs. 42 Moreover, Ucan et al. 43 observed a relatively lower 25(OH)D concentration in the HT group as compared with the control group, and a significant reduction in antibody concentration after vitamin D replacement was given. They demonstrated a statistically significant decrease in TPO‐Ab (p = 0.003) and TG‐Ab (p = 0.020) antibody concentrations after vitamin D replacement in patients with euthyroid HT. However, other studies did not find any association between serum TPO‐Ab or TG‐Ab and 25(OH)D levels in newly diagnosed HT patients. 30 , 44 , 45 As in our study, the association between TG‐Ab and 25(OH)D levels was not pronounced (p = 0.074). Though it is worth mentioning that a decrease in TPO‐Ab titres may better reflect the effect of vitamin D on thyroid autoimmunity than its action on TG‐Ab as the general measurements of TPO‐Ab often have higher sensitivity and at least equal specificity to TG‐Ab measurements in the diagnosis of autoimmune disorders of the thyroid gland. 46
Our results should be interpreted cautiously as we did not reveal any association between serum 25(OH)D and the levels of TSH, FT3 and FT4, suggesting that vitamin D supplementation is not associated with the function of the thyroid in HT. One recent retrospective study found TSH is an independent risk factor for HT, but no association was found between HT and FT3 or FT4, suggesting HT patients were more prone to subclinical hypothyroidism. 15 However, this study also revealed a potential role of 25(OH)D supplementation in regulating the thyroid hormone level based on the results of positive correlation between 25(OH)D and the level of FT3 and FT4. Moreover, Krysiak et.al suggested that vitamin D supplementation produced no effect on the post‐treatment levels of FT4 and free thyroid hormone.
The results of the present meta‐analysis must be considered in light of the study's limitation. Firstly, the estimated means ± standard deviations might potentially bias the results, but the sensitivity analysis showed a robust outcome even when the individual studies with estimated parameters were left out of the analyses. Second, some of the studies used different regimen and individualized dose of the treatment, which would probably result in heterogeneity. Of note, several studies could not be included because they did not report results specifically for patients with HT. Third, all studies have rather small sample sizes. To our knowledge, there are only a few prospective studies that investigated the effect of vitamin D supplementation on patients with HT, and the thyroid indicators in each study were reported differently. Despite only seven studies being included in this meta‐analysis in total, there were no previous meta‐analyses that analysed the effect of vitamin D supplementation in patients with HT for a given outcome. Fourth, only papers in English were included, possibly leaving out valuable results. Although the difference is statistically significant, the clinical significance should be cautiously interpreted since the patients were clustered by gender. Additional studies might be necessary to determine the exact functional impact of vitamin D treatment on HT patients. Finally, the combined estimates of our outcomes were not reported at the same duration after patients had been treated.
5. WHAT IS NEW AND CONCLUSIONS
Our study demonstrated that vitamin D might significantly increase the serum 25(OH)D levels and produce changes in TPO‐Ab titres. But no significant association was found between serum vitamin D supplementation and the levels of TG‐Ab, TSH, FT3 and FT4, suggesting that vitamin D is not associated with the function of the thyroid in patients with HT. Well‐designed RCTs with sufficient sample sizes investigating the effect of vitamin D on thyroid function are still warranted.
CONFLICT OF INTEREST
No conflicts of interest have been declared.
PATIENT CONSENT STATEMENT
This study did not contain any patients and patient consent is not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
Jiang H, Chen X, Qian X, Shao S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto's thyroiditis—A meta‐analysis of randomized controlled trials. J Clin Pharm Ther.2022;47:767–775. doi: 10.1111/jcpt.13605
Funding information
This work was supported by National Natural Science Foundation of China (grant number 81771848). The funding only gave financial support
DATA AVAILABILITY STATEMENT
All data generated or analysed during this study are included in this published article and its supplementary information files.
REFERENCES
- 1. Merrill SJ, Minucci SB. Thyroid autoimmunity: an interplay of factors. Vitam Horm. 2018;106:129‐145. [DOI] [PubMed] [Google Scholar]
- 2. Ralli M, Angeletti D, Fiore M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev. 2020;19:102649. [DOI] [PubMed] [Google Scholar]
- 3. Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391‐397. [DOI] [PubMed] [Google Scholar]
- 4. Wiersinga WM. Thyroid hormone replacement therapy. Horm Res. 2001;56(Suppl 1):74‐81. [DOI] [PubMed] [Google Scholar]
- 5. Topliss DJ. Clinical update in aspects of the management of autoimmune thyroid diseases. Endocrinol Metab (Seoul). 2016;31:493‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Watanabe T, Maruyama M, Ito T, et al. Clinical features of a new disease concept, IgG4‐related thyroiditis. Scand J Rheumatol. 2013;42:325‐330. [DOI] [PubMed] [Google Scholar]
- 7. Abbott RD, Sadowski A, Alt AG. Efficacy of the autoimmune protocol diet as part of a multi‐disciplinary, supported lifestyle intervention for Hashimoto's thyroiditis. Cureus. 2019;11:e4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Makariou S, Liberopoulos EN, Elisaf M, Challa A. Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med. 2011;22:355‐362. [DOI] [PubMed] [Google Scholar]
- 9. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266‐281. [DOI] [PubMed] [Google Scholar]
- 10. Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9:941‐955. [DOI] [PubMed] [Google Scholar]
- 11. Wang S, Wu Y, Zuo Z, Zhao Y, Wang K. The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a systematic review and a meta‐analysis. Endocrine. 2018;59:499‐505. [DOI] [PubMed] [Google Scholar]
- 12. Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080s‐1086s. [DOI] [PubMed] [Google Scholar]
- 13. Liontiris MI, Mazokopakis EE. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients.Points that need more investigation. Hell J Nucl Med. 2017;20:51‐56. [DOI] [PubMed] [Google Scholar]
- 14. Roehlen N, Doering C, Hansmann M‐L, et al. Vitamin D, FOXO3a, and Sirtuin1 in Hashimoto's thyroiditis and differentiated thyroid cancer. Front Endocrinol (Lausanne). 2018;9:527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chao G, Zhu Y, Fang L. Correlation between Hashimoto's thyroiditis‐related thyroid hormone levels and 25‐hydroxyvitamin D. Front Endocrinol (Lausanne). 2020;11:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019). 2019; www.training.cochrane.org/handbook
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aslam S, Emmanuel P. Formulating a researchable question: a critical step for facilitating good clinical research. Indian J Sex Transm Dis AIDS. 2010;31:47‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.handbookcochrane.org
- 20. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712‐716. [DOI] [PubMed] [Google Scholar]
- 22. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chahardoli R, Saboor‐Yaraghi AA, Amouzegar A, Khalili D, Vakili AZ, Azizi F. Can supplementation with vitamin D modify thyroid autoantibodies (Anti‐TPO Ab, Anti‐Tg Ab) and thyroid profile (T3, T4, TSH) in Hashimoto's thyroiditis? A double blind, randomized clinical trial. Horm Metab Res. 2019;51:296‐301. [DOI] [PubMed] [Google Scholar]
- 24. Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D on thyroid autoimmunity in levothyroxine‐treated women with Hashimoto's thyroiditis and normal vitamin D status. Exp Clin Endocrinol Diabetes. 2017;125:229‐233. [DOI] [PubMed] [Google Scholar]
- 25. Krysiak R, Szkróbka W, Okopień B. Moderate‐dose simvastatin therapy potentiates the effect of vitamin D on thyroid autoimmunity in levothyroxine‐treated women with Hashimoto's thyroiditis and vitamin D insufficiency. Pharmacol Rep. 2018;70:93‐97. [DOI] [PubMed] [Google Scholar]
- 26. Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic‐pituitary‐thyroid axis activity and thyroid function tests in men with Hashimoto's thyroiditis: a pilot study. Pharmacol Rep. 2019;71:243‐247. [DOI] [PubMed] [Google Scholar]
- 27. Nodehi M, Ajami A, Izad M, et al. Effects of vitamin D supplements on frequency of CD4+ T‐cell subsets in women with Hashimoto’s thyroiditis: a double‐blind placebo‐controlled study. Eur J Clin Nutr. 2019;73:1236‐1243. [DOI] [PubMed] [Google Scholar]
- 28. Anaraki PV, Aminorroaya A, Amini M, et al. Effect of vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto’s thyroiditis: a double‐blind randomized placebo‐controlled clinical trial. J Res Med Sci. 2017;22:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D'Aurizio F, Villalta D, Metus P, Doretto P, Tozzoli R. Is vitamin D a player or not in the pathophysiology of autoimmune thyroid diseases? Autoimmun Rev. 2015;14:363‐369. [DOI] [PubMed] [Google Scholar]
- 31. Metwalley KA, Farghaly HS, Sherief T, Hussein A. Vitamin D status in children and adolescents with autoimmune thyroiditis. J Endocrinol Invest. 2016;39:793‐797. [DOI] [PubMed] [Google Scholar]
- 32. Wang X, Zynat J, Guo Y, et al. Low serum vitamin D is associated with anti‐thyroid‐globulin antibody in female individuals. Int J Endocrinol. 2015;2015:285290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim D. Low vitamin D status is associated with hypothyroid Hashimoto's thyroiditis. Hormones (Athens). 2016;15:385‐393. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Lv S, Chen G, et al. Meta‐analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients. 2015;7:2485‐2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Muscogiuri G, Mari D, Prolo S, et al. 25 hydroxyvitamin D deficiency and its relationship to autoimmune thyroid disease in the elderly. Int J Environ Res Public Health. 2016;13:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kivity S, Agmon‐Levin N, Zisappl M, et al. Vitamin D and autoimmune thyroid diseases. Cell Mol Immunol. 2011;8:243‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamer G, Arik S, Tamer I, Coksert D. Relative vitamin D insufficiency in Hashimoto's thyroiditis. Thyroid. 2011;21:891‐896. [DOI] [PubMed] [Google Scholar]
- 38. Bozkurt NC, Karbek B, Ucan B, et al. The association between severity of vitamin D deficiency and Hashimoto's thyroiditis. Endocr Pract. 2013;19:479‐484. [DOI] [PubMed] [Google Scholar]
- 39. Mansournia N, Mansournia MA, Saeedi S, Dehghan J. The association between serum 25OHD levels and hypothyroid Hashimoto's thyroiditis. J Endocrinol Invest. 2014;37:473‐476. [DOI] [PubMed] [Google Scholar]
- 40. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet (London, England). 2017;390:1550‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab. 2005;19:1‐15. [DOI] [PubMed] [Google Scholar]
- 42. Shin DY, Kim KJ, Kim D, Hwang S, Lee EJ. Low serum vitamin D is associated with anti‐thyroid peroxidase antibody in autoimmune thyroiditis. Yonsei Med J. 2014;55:476‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ucan B, Sahin M, Sayki Arslan M, et al. Vitamin D treatment in patients with Hashimoto's thyroiditis may decrease the development of hypothyroidism. Int J Vitam Nutr Res. 2016;86:9‐17. [DOI] [PubMed] [Google Scholar]
- 44. Ma J, Wu DI, Li C, et al. Lower serum 25‐hydroxyvitamin D level is associated with 3 types of autoimmune thyroid diseases. Medicine. 2015;94:e1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Effraimidis G, Badenhoop K, Tijssen JG, Wiersinga WM. Vitamin D deficiency is not associated with early stages of thyroid autoimmunity. Eur J Endocrinol. 2012;167:43‐48. [DOI] [PubMed] [Google Scholar]
- 46. Muller AF, Drexhage HA, Berghout A. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev. 2001;22:605‐630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.


