Abstract
Purpose of Review
Blood pressure (BP) exhibits strong diurnal variations that have been shown to be important for normal physiology and health. In this review, we highlight recent advances in both basic and clinic research on how the circadian clock affects these BP rhythms.
Recent Findings
Tissue-specific and inducible knockout rodent models have provided novel ways to dissect how circadian clocks regulate BP rhythms and demonstrated that loss of these rhythms is associated with the development of disease. The use of circadian-specific research protocols has translated findings from rodent models to humans, providing insight into circadian control of BP, as well as how sleep, activity, and other factors influence diurnal BP rhythms.
Summary
Circadian mechanisms play an important role in the regulation of diurnal BP rhythms. Future research needs to extend these findings to clinical populations and determine the extent to which circadian factors may play a role in the development of novel treatment approaches to the management of hypertension.
Keywords: Circadian rhythm, Blood pressure, Translational research
Introduction
Maintenance of normal blood pressure (BP) is important for health and longevity. Clinical guidelines highlight the importance of maintaining BP within normal levels due to the strong association between strictly controlled BP and decreased risk of major cardiovascular events and death [1–3]. The increased use of 24-h ambulatory BP monitoring has highlighted the importance of time-of-day-dependent changes in BP. BP follows a diurnal rhythm where a 10–20% decrease in pressure, or dip, occurs during the inactive or sleep period. Loss of this diurnal variation, known as “non-dipping,” is associated with metabolic disorders, and progression of chronic kidney disease, and can contribute to the onset and development of cardiovascular disease [4].
On a molecular level, the highly conserved circadian molecular clock keeps time through a series of transcriptional-translational feedback loops (Fig. 1, adapted from the UC San Diego BioClock Studio). Aryl hydrocarbon receptor nuclear translocator-like protein 1 (Arntl), also known as Bmal1, heterodimerizes with Circadian Locomotor Output Cycles Kaput (Clock) to promote the transcription of the repressor genes, Period (Per homologs Per1, Per2, and Per3), and Cryptochrome (Cry, homologs Cry1 and Cry2). Once translated, Per and Cry form a heterodimer that stops the transcription of Bmal1 and Clock. As Per and Cry are degraded, Bmal1 and Clock transcription begins again. The individual components of the molecular clock also bind and promote the transcription of other clock-controlled genes. It is interesting to note that every molecular clock gene whole-body knockout (KO) mouse has a BP phenotype, suggesting that the molecular clock is important for both BP levels and rhythms in mice.
Fig. 1.
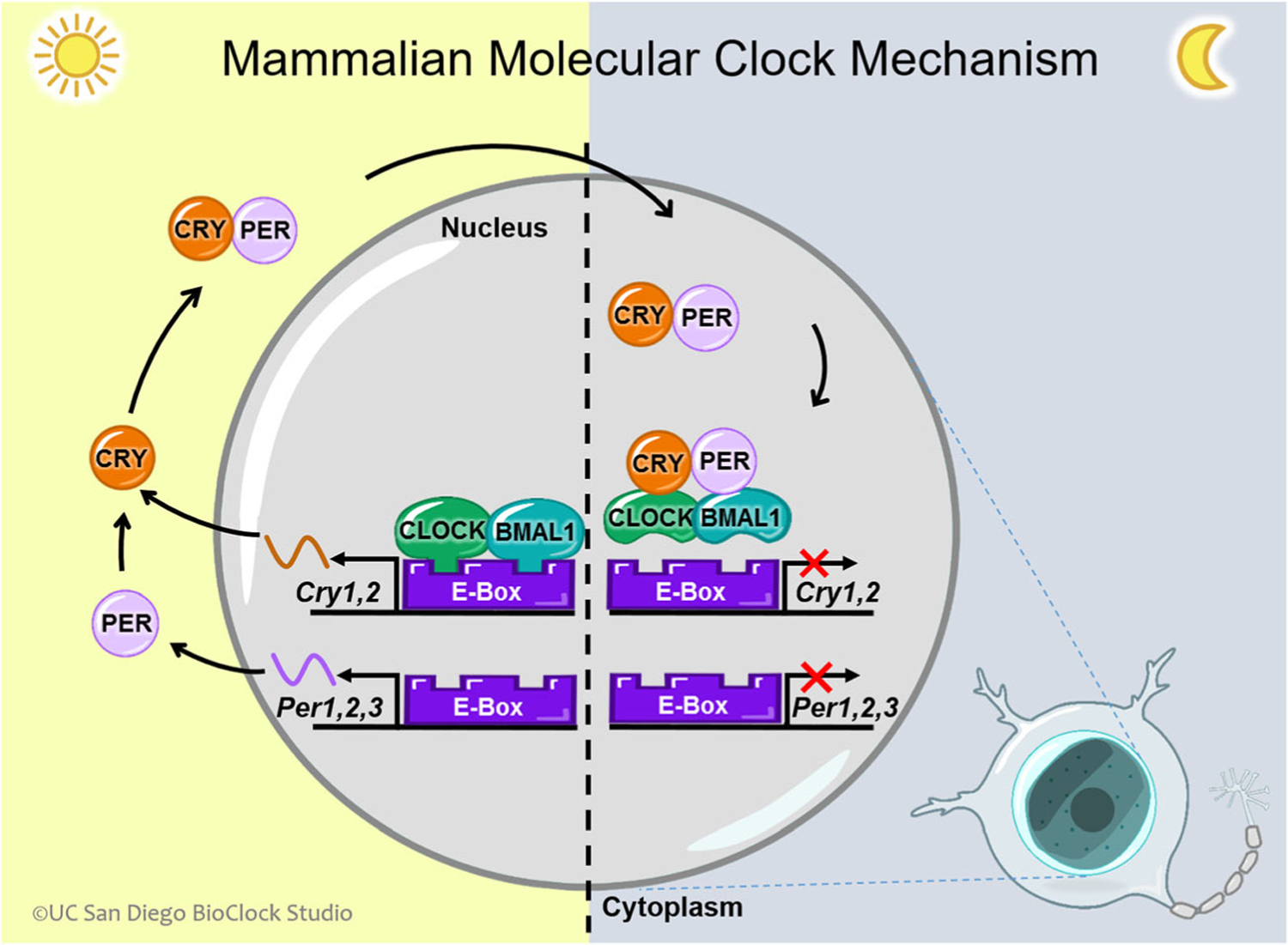
Circadian transcription-translation feedback loop, CLOCK, and BMAL1 are transcription factors that form a heterodimer and bind to E-boxes in the nucleus to promote the expression of the Per and Cry genes during the day. Per and Cry act in the negative limb of the mammalian clock translational transcriptional feedback loop (TTFL). The Per and Cry genes are transcribed to produce mRNA in the nucleus, which then travels to the cytoplasm to be translated into proteins. Forming a heterodimer with one another, PER and CRY form a complex and return to the nucleus to inhibit their own expression by binding to and inactivating CLOCK:BMAL1. Figure adapted from UC San Diego BioClock Studio
The molecular circadian clock has been found to be entrained by intrinsic (e.g., the central molecular clock) and extrinsic factors (e.g., light, exercise, and meal timing). The central clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus, serves as the master pacemaker for the body. Environmental light signals to the SCN via the retinal hypothalamic tract and the SCN is thought to set the “master time” for the rest of the body. Witte et al. established that ablation of the SCN results in loss of the diurnal variation in BP, demonstrating that the SCN is crucial to the daily rhythmic variation of BP [5]. While the SCN is the master clock of the body, the components of the molecular clock are expressed nearly ubiquitously throughout organs and tissues. These peripheral clocks are thought to integrate signals from the SCN and other Zeitgeber (translated literally as “time-givers” from German), such as exercise or nutrient availability, to keep time throughout the body (Fig. 2).
Fig. 2.
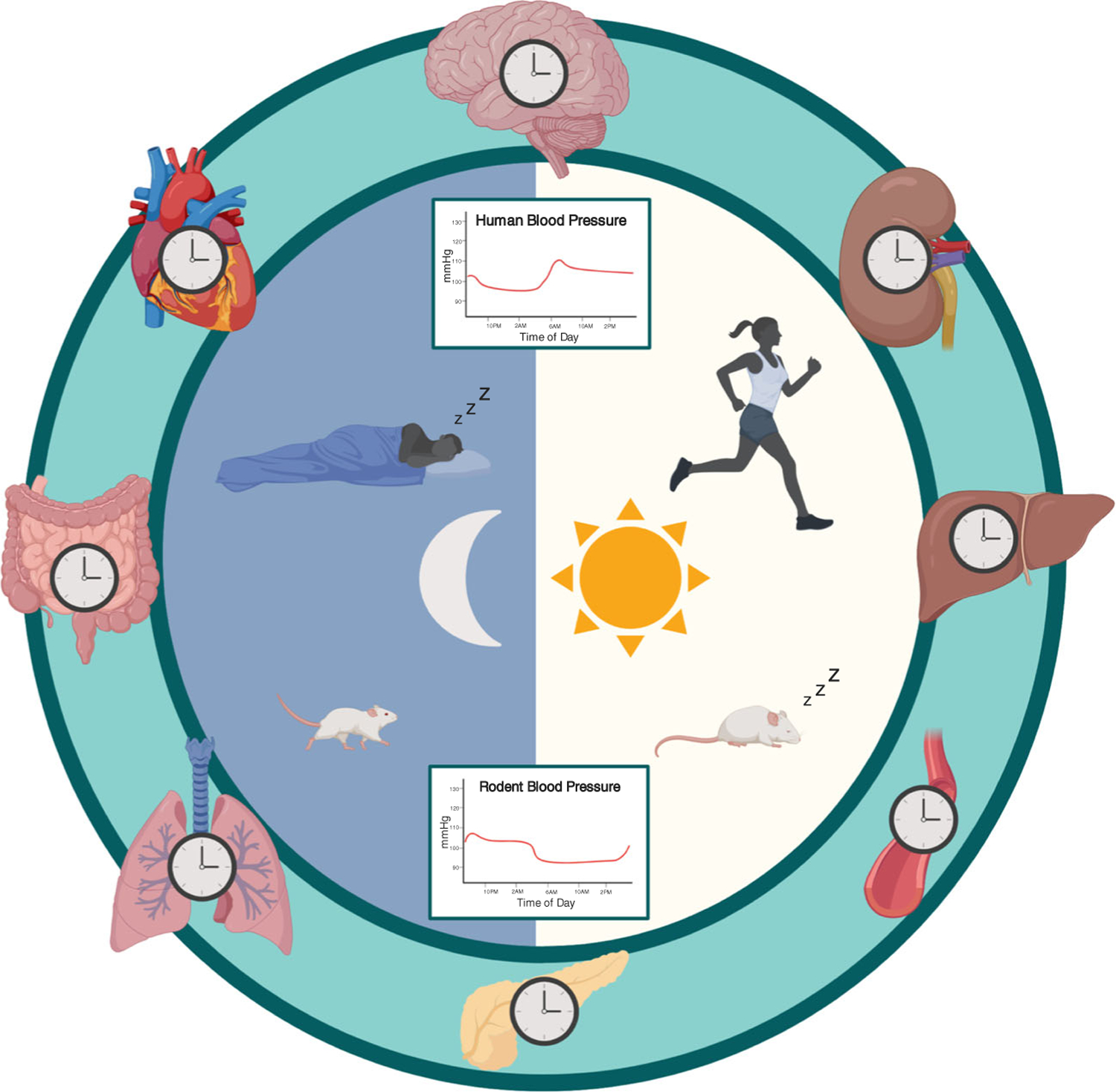
Circadian entrainment and blood pressure control, central and peripheral molecular clocks in rodents and humans keep time to coordinate processes, function, and behavior. In turn, these outputs can affect diurnal blood pressure variations, producing a rhythm that is higher during the active/wake period and lower in the inactive/sleep period
This review will highlight recent advances in basic and clinical research focused on the regulation of BP levels and diurnal rhythms by the circadian clock. Particular attention is paid to the use of tissue-specific KO rodent models, which are useful in determining the effects of peripheral circadian clocks on BP regulation. Next, this review will discuss translational circadian research and detail the utilization of specific protocols designed to determine the role of circadian BP regulation in humans. Finally, the authors discuss the potential impact of circadian rhythm sleep-wake disorders on BP levels and rhythms and future clinical and research directions.
Bmal1
Whole-body KO of the Bmal1 gene in mice typically results in a loss of diurnal BP rhythms and lower BP compared to wild-type controls [6]. Yang et al. demonstrated that loss of Bmal1 also contributes to rhythms in activity and heart rate using inducible whole-body Bmal1 KO mice. Once deleted, these conditional KO mice lose diurnal variations in BP, heart rate, and activity. Interestingly, these inducible KO mice did not show differences in life span, fertility, body weight, or blood glucose levels that are often described in other Bmal1 KO mice strains [7]. This study suggests that Bmal1 has transcriptional effects in addition to its canonical role in the molecular clock and/or a functioning circadian clock is crucial during development.
Loss of Bmal1 in perivascular adipose tissue in mice results in a unique BP phenotype that the investigators termed “super-dippers,” wherein BP is 10% lower during the light/resting phase compared to littermate controls [8••]. Vessels did not lose their ability to contract or did not have altered responses to phenylephrine or acetylcholine, suggesting the reduction in BP is not due to vessel impairment. Chang et al. observed a similar resting period phenotype in mice with KO of angiotensinogen specifically in brown adipose tissue. After identifying a Bmal1-binding site upstream of the angiotensinogen gene, they proposed that time-dependent regulation of angiotensinogen in brown adipose tissue contributes to the circadian regulation of BP.
Deletion of Bmal1 specifically in vascular smooth muscle resulted in lower BP and decreased BP amplitude, defined as the difference between peak and trough levels of BP, compared to control mice [9]. Interestingly, cardiomyocyte-specific deletion of Bmal1 did not have any effect on BP. Xie et al. suggested that this strain-specific difference is due to Bmal1 affecting time-of-day-dependent variations in smooth muscle vasoconstriction, which they demonstrated by measuring ex vivo contractile responses to serotonin and phenylephrine [9].
Ansermet et al. used podocyte-specific deletion of Bmal1 to show that the intrinsic molecular clock contributes to the circadian rhythmicity of glomerular filtration rate [10], which is crucial to the maintenance of fluid homeostasis and BP regulation. Tokonami et al. reported that deletion of Bmal1 in renin-producing cells of the kidney caused changes in urinary sodium excretion, urine volume, plasma aldosterone, and lowered BP [11]. Loss of Bmal1, whether in a whole-body, tissue-specific, or inducible knockout model, leads to a lowering of BP in rodent models.
Clock
Clock is canonical for the functioning of the molecular clock feedback loops; however, some behavioral circadian patterns (such as locomotor activity) may not require Clock [12]. Studies have found that the transcription factor Npas2 can substitute for Clock in both the SCN [13] and peripheral tissues [14]. Clock-deficient mice have lowered BP levels and altered urinary sodium excretion, and higher incidence of diabetes insipidus compared to control animals [15]. Clock-deficient mice also have decreased renal and urinary 20-hydroxyeicosatetraenoic acid, a powerful regulator of BP and blood flow [16]. Clock Δ19 KO mice, which encode a partially functioning CLOCK protein, have a blunted diurnal difference in mean arterial pressure and develop a higher prevalence of age-dependent cardiac hypertrophy and fibrosis compared to wild-type controls [17]. Together, these studies suggest Clock is important to both renal and cardiovascular function.
Period
Per1 has been shown to be highly important in the regulation of BP. Whole-body KO of Per1 results in a hypotensive phenotype with a reduced expression of the α subunit of the renal epithelial sodium channel, increased urinary sodium excretion, and elevated renal endothelin-1 [18]. Per1 also regulates the transcription of the NaCl co-transporter in the distal convoluted tubule [19], the Na+/H+ exchanger 3 and Na-glucose co-transporter in the proximal tubule [20], and plasma aldosterone [21]. Loss of Per1 in conjunction with high salt diet and mineralocorticoid treatment results in a non-dipping hypertensive phenotype in male, but not female, mice [22••, 23••]. Douma et al. indicates that Per1 coordinates sodium handling in the distal nephron to regulate 24-h BP rhythms [24]. The other Per homologs, Per2 and Per3, have been identified in mammals [25] and Per2 KO mice display decreased diurnal BP differences and decreased diastolic BP compared to controls [26]. Per3 was recently reported to be crucial to embryonic development in mice [27]; however, not much is currently known about the role of the PER3 in BP control.
Cryptochrome
As the primary transcriptional repressors, the Cry genes are critical for the proper functioning of the molecular clock. Cry1/Cry2 KO mice, or Cry-null mice, have no circadian oscillations of BP and develop salt-sensitive hypertension, likely driven by hyperaldosteronism [28]. When placed on a high salt diet, Cry-null mice display increased kidney damage, increased urinary albumin, and decreased nephrin expression [29]. In these mice, lowering BP using spironolactone did not affect renal damage, suggesting that hyperaldosteronism due to loss of Cry may be responsible for renal damage independent of BP.
Translational Limitations of Circadian Research
Rodent models have provided a significant amount of information on how the molecular clock influences BP; however, limitations do exist. For example, direct photic input from light affects the behavior of nocturnal rodents differently when compared to humans. Preliminary evidence from Zhang et al. utilizing inactive period time-restricted feeding, where food is only available during the inactive/lights-on period of the day, has shown that the timing of food intake drastically influences diurnal BP rhythms in mice [30]. Mice and rats fed with typical ad libitum access to food consume the majority of their food during their active period, although animals can eat during their inactive period. These food intake behaviors may affect diurnal cardiovascular patterns. Interestingly, studies utilizing rabbits have shown that time of feeding can affect diurnal rhythms of BP and heart rate. In contrast to rodents, rabbits are typically presented with a fixed amount of food at the same time each day. Van den Buuse and Malpas demonstrated that changing the time of day that food was presented to the rabbits altered the observed BP and heart rate rhythms [31]. Antic et al. showed that placing rabbits on an ad libitum high-fat diet resulted in a non-dipping BP phenotype with obesity-induced hypertension [32]. These data from animal models suggest that both diet and time of feeding can influence BP and BP rhythms. While connections between diet content and BP are highly investigated in humans [33, 34], less is known about the association between time of food intake and BP. In a randomized controlled clinical trial, Sutton et al. found that limiting time of food intake to the first 6 h upon waking in men with prediabetes lowered insulin resistance and morning BP without weight loss [35]. Additional research is needed in both humans and animal models to elucidate the mechanisms controlling the BP response to diet and time of food intake.
Quadrupeds do not have the accompanying postural changes associated with sleep and wake that humans do, which complicates the translational interpretation of how the molecular clock influences BP regulation in humans compared to rodent models. Additionally, the effects of locomotor activity and rest on diurnal BP changes may be exaggerated in rodents. Sheward et al. found that bouts of locomotor activity accounted for a large portion of the variance of diurnal changes in BP and heart rate among mice that lacked a subunit of the vasoactive intestinal peptide receptor in the SCN and, thus, exhibited poorly synchronized SCN activity [36]. Van Vliet et al. reported that wild-type mice and mice with a deletion in endothelial nitric oxide synthase had changes in BP and heart rate that were temporally aligned and associated with changes in locomotor activity [37]. Thus, locomotor activity and rest should be considered an important factor in the interpretation of studies on diurnal BP in rodents.
Assessment of Circadian Rhythms in Humans
In humans, specific research protocols are required to isolate endogenous circadian rhythms, independent of the influence of potential Zeitgeber (e.g., light, meal timing, social activities, and physical activity). Unfortunately, in the scientific literature, the term “circadian” is often used loosely and physiology and behaviors that vary over 24 h are described as being under circadian control. For example, the timing of sleep is occasionally referred to as a circadian phenomenon, whereas this is not necessarily the case. Sleep/wake patterns may be misaligned with endogenous circadian rhythms that influence timing of sleep. The same holds true when describing diurnal variations in BP over 24 h. Diurnal rhythms in BP cannot be assumed to be under circadian control, as other factors (e.g., sleep, activity levels, and posture) also influence BP while exhibiting their own independent diurnal rhythms.
As described above, the SCN is believed to function as the central circadian clock and projects to a vast number of downstream structures, including the pineal gland for the regulation of melatonin. As a result, rhythms in melatonin have long been considered the primary marker for central circadian clock timing in humans. Conversely, in rodent models, melatonin rhythms are not highly utilized in research due to small pineal glands, lack of assays sensitive enough to measure variations in melatonin, and strain differences in melatonin production [38]. Some mouse strains, such as C3H and CBA, produce melatonin, while others, such as the commonly used C57BL, are melatonin-deficient. In addition, tissue-specific effects of melatonin can differ between mouse strains and may contribute to observed differences in circadian rhythms [38, 39]. Other commonly used markers of the central circadian clock include core body temperature (CBT) and cortisol. Melatonin and cortisol levels are either obtained from saliva or plasma samples. CBT is assessed using either rectal thermometers or, more recently, transmitted through ingestible pills that record CBT as they pass through the digestive system. Similar to rodent models, circadian oscillators exist in peripheral tissue in humans; however, direct examination of peripheral circadian oscillators is difficult. Recently, buccal cells have been used to examine peripheral circadian clock gene function in humans.
The constant routine (CR) protocol is used in humans to examine endogenous circadian rhythmicity in either central or peripheral clocks. In this protocol, research participants are often kept in dim light (typically < 15 lx), fed regularly timed isocaloric snacks, and forced to limit physical activity [40, 41]. The effects of sleep and posture are controlled by having the participant maintain wakefulness in a semi-recumbent position. In order to capture an entire circadian cycle, CR protocols last > 24 h with measurements of the variables of interest occurring frequently (e.g., every 30 to 60 min). Modifications in the CR protocol allow for changes in posture to urinate and/or defecate, as well as allow and adjust for sleep. Both of these modifications do introduce confounding variables and may impact variables of interest. For example, posture and sleep affect BP levels and, therefore, should be held constant when examining circadian control of BP.
Alternatively, in a forced desynchrony (FD) protocol, endogenous circadian rhythms are separated or desynchronized from sleep/wake cycles by imposing a light-dark cycle outside of the range of entrainment. However, compared with a 30-h CR protocol, FD protocols are labor intensive requiring at least 10 days when using an ultradian FD protocol and at least 20 days when using an extended-day FD protocol [40, 42]. These two types of circadian protocols are illustrated by an elegant study conducted by Shea et al., [43••] wherein 38-h CR, 196-h FD (28-h sleep/wake cycles), and 240-h FD (20-h sleep/wake cycles) protocols were used to examine circadian control of BP.
Circadian Control of Blood Pressure in Humans
BP varies over 24 h, decreasing by approximately 10–20% during sleep, increasing just prior to waking, and peaking shortly after waking [44]. BP that does not decrease by at least 10% from wakefulness to sleep is termed “non-dipping,” and is associated with increased risk for cardiovascular morbidity and mortality [45–48]. An extreme form of non-dipping, termed “reverse dipping,” occurs when BP during sleep is higher than that during wakefulness. The mechanisms underlying a non-dipping BP pattern are not fully understood, although autonomic dysregulation [49, 50], alterations in the HPA system [51], renin-angiotensin-aldosterone system [52], and kidney function/sodium handling [53–55] have all been implicated [56, 57]. Recently, a population-based study reported that mid-sleep time, a proxy for chronotype and possibly underlying circadian rhythmicity, was associated with non-dipping SBP. Specifically, an earlier mid-sleep time (earlier bedtime and waketime) was associated with a higher prevalence of non-dipping SBP [58]. The physiological underpinnings of a non-dipping BP pattern may also be mediated, in part, by the presence of sleep disorders such as obstructive sleep apnea (OSA). Untreated OSA has been associated with increased sympathetic activation that may, in turn, result in higher BP levels during both sleep and wakefulness [59–61]. Recent evidence suggests that awake and asleep systolic BP levels, but not the decline in awake-to-asleep systolic BP, are associated with increased prevalence of left ventricular hypertrophy [62••]. This lack of association is likely due to a floor effect, wherein individuals’ awake-to-asleep systolic BP may not be able to decline by 10% when the awake systolic BP is already low. Therefore, absolute BP levels during sleep (nocturnal hypertension) may be more strongly associated with adverse outcomes compared with the awake-to-asleep decline in BP (non-dipping BP).
Only a part of the diurnal variation in BP described above can be attributed to circadian control. BP variation across 24 h is also affected by sleep/wake cycles, posture, activity levels, and exercise. Therefore, the extent to which circadian factors are implicated in BP levels over 24 h is not entirely clear. In a sample of 6 normotensive, healthy, non-smoking, non-medicated males undergoing a 24-h CR protocol, an endogenous circadian rhythm in heart rate but not BP was observed [63]. The authors concluded that this absence of circadian rhythm in BP, combined with previous studies that allowed for sleep and observed diurnal variations in BP, suggests that the sleep/wake cycle and not circadian factors govern diurnal variation in BP. However, in a sample of 28 normotensive, non-obese, and healthy adults (16 men and 12 women), a robust circadian rhythm in BP was observed, wherein endogenous circadian control of BP peaked in the evening at approximately 9 pm and reached its nadir in the early morning [43••]. The peak-to-trough amplitudes were 3–6 mmHg for systolic BP and 2–3 mmHg for diastolic BP. Furthermore, the rhythm in BP did not appear to be related to circadian rhythms in cortisol, catecholamines, cardiac vagal tone, heart rate, or urine flow, as there were significant phase differences between BP and these other variables. The authors concluded that an endogenous circadian rhythm in BP exists with a peak in the evening, making it unlikely that BP rhythms contribute to the morning peak in adverse cardiovascular events. In both of these studies, circadian control of BP was examined in young, healthy individuals. It is unknown whether circadian control of BP is altered in other populations, including individuals at higher risk for hypertension (e.g., African Americans) and/or who experience circadian misalignment (e.g., social jet lag or shift work).
In addition to these studies employing CR and FD protocols, repeated dosing of melatonin, a hormone involved in the regulation of the sleep/wake cycle, has been shown to reduce systolic and diastolic BP, as well as improve nocturnal systolic and diastolic BP dipping in patients with hypertension. The observed improvements in sleep and BP occurred independently of each other and were attributed to restoration of circadian function [64].
Circadian Rhythm Sleep-Wake Disorders and Impact on Blood Pressure
In both the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), and the International Classification of Sleep Disorders, Third Edition (ICSD-3), circadian rhythm sleep-wake disorders are defined by a persistent or recurrent disruption in the sleep-wake pattern that is primarily due to (1) an alteration of the circadian system (intrinsic/endogenous disorders) or (2) a misalignment between the endogenous circadian rhythm and the sleep-wake schedule required by an individual’s physical environment or social or professional schedule (extrinsic/exogenous disorders) [65, 66]. Furthermore, this disruption typically leads to symptoms of insomnia or excessive sleepiness, as well as distress or impairment in function. Circadian misalignment, when the endogenous circadian system does not match 24-h environmental/behavioral cycles, has been shown to raise 24-h BP and circulating inflammatory markers in chronic shift workers [67]. Another study found that shift work was associated with greater disruption in the circadian period (e.g., time from peak-to-peak of BP; assumed to be close to 24 h) of SBP and DBP [68]. Although these authors concluded that health outcomes resulting from misalignment in circadian rhythms in BP are unknown, shift work has long been associated with an increased risk of cardiometabolic diseases such as type 2 diabetes and cardiovascular disease [69]. The mechanisms underlying the association between chronic circadian misalignment and cardiometabolic pathophysiology are undergoing investigation; however, evidence suggests that melatonin may mediate this association [69]. While shift work can be considered an extreme example of circadian misalignment, circadian phase disorders (i.e., advanced and delayed sleep-wake phase disorders) may result in subtler misalignments between the central circadian clock and one’s sleep-wake rhythm. In delayed sleep-wake phase disorder, one’s central circadian clock may be phase delayed (later melatonin onset and later minimum core body temperature) in comparison with sleep-wake rhythms imposed by school or work schedules. However, it is unclear how circadian phase disorders may impact BP rhythms. It is possible that a delayed sleep-wake phase disorder would be accompanied by a delayed dip in BP. Lastly, irregular sleep-wake rhythm, non-24-h sleep-wake rhythm, or social jet lag disorders may also impact BP rhythms across a 24-h period. In the above cases, misalignment as a consequence of not following one’s endogenous circadian rhythm may be more harmful than following the endogenous rhythm. Indeed, it has been reported that circadian misalignment increases BP in non-shift workers [67, 70].
Conclusions
Recent evidence in both rodents and humans demonstrates clear circadian control of diurnal BP rhythms. In rodents, both central and peripheral circadian clocks influence BP rhythms. Although less research has been conducted in humans, at least one elegant study has demonstrated a robust circadian rhythm in BP, wherein endogenous circadian control of BP peaked in the evening and reached its nadir in the early morning. This BP rhythm was independent of circadian rhythms in cortisol, catecholamines, cardiac vagal tone, heart rate, or urine flow. Despite these studies, the extent to which circadian mechanisms impact BP levels and rhythms, including pathology (e.g., nocturnal hypertension and non-dipping BP) and among clinical populations, remains unclear.
In addition to any direct influence of circadian rhythms on BP, it is possible that circadian factors may moderate the impact of other factors on BP. Aging, for example, has been shown to affect the daily oscillation of both CLOCK and BMAL1 in the murine brain [71]. Aging also affects the rhythms of behavior, body temperatures, and hormone release in humans. Therefore, circadian rhythms may play an indirect role in the regulation of BP in the context of specific populations (e.g., older adults or African Americans). Evidence suggests that disruption of circadian rhythms may be an early-warning sign of neurodegenerative diseases, such as Alzheimer’s and Huntington’s diseases [72]. Additionally, circadian rhythms may play an important role in moderating the association between diet (e.g., timing of sodium intake) and BP regulation.
Circadian rhythm research using rodent models has limitations; however, the insight into how the highly conserved molecular circadian clock affects BP in these models is invaluable. Understanding the physiology of how the highly conserved molecular clock regulates BP allows scientists to investigate how the circadian clock may influence the etiology and pathophysiology of disease. Research on circadian control of BP in humans typically enrolls small samples of healthy, young adults, limiting its generalizability to diverse populations and/or clinical settings. However, this foundational knowledge presents physicians and scientists with the possibility of timing treatments and interventions for their greatest possible efficacy. As personalized medicine grows more realistic, the fields of chronopharmacology, chrononutrition, and chronoactivity [73] allow for patients, scientists, and physicians to work together to optimize health and slow disease progression by accounting for inherent timing cues. Additionally, including larger and more diverse samples may allow for the examination of race and sex differences in circadian rhythms in order to better address differences and disparities in hypertension and hypertension-related outcomes.
Acknowledgments
This work was supported by grants 15SFRN2390002 and 19CDA34660139 from the American Heart Association to SJT and the National Institute of Health T32 DK007545 to MKR.
Footnotes
This article is part of the Topical Collection on Sleep and Hypertension
Compliance with Ethical Standards
Conflict of Interest The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Ko MJ, Jo AJ, Park CM, Kim HJ, Kim YJ, Park DW. Level of blood pressure control and cardiovascular events: SPRINT criteria versus the 2014 hypertension recommendations. J Am Coll Cardiol 2016;67(24):2821–31. [DOI] [PubMed] [Google Scholar]
- 2.Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373(22):2103–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71(6):e13–e115. [DOI] [PubMed] [Google Scholar]
- 4.de la Sierra A, Segura J, Gorostidi M, Banegas JR, de la Cruz JJ, Ruilope LM. Diurnal blood pressure variation, risk categories and antihypertensive treatment. Hypertens Res 2010;33(8):767–71. [DOI] [PubMed] [Google Scholar]
- 5.Witte K, Schnecko A, Buijs RM, van der Vliet J, Scalbert E, Delagrange P, et al. Effects of Scn lesions on orcadian blood pressure rhythm in normotensive and transgenic hypertensive rats. Chronobiol Int 1998;15(2):135–45. [DOI] [PubMed] [Google Scholar]
- 6.Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci U S A 2007;104(9):3450–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang G, Chen L, Grant GR, Paschos G, Song WL, Musiek ES, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med 2016;8(324): 324ra16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.••. Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, et al. Bmal1 in perivascular adipose tissue regulates resting-phase blood pressure through transcriptional regulation of angiotensinogen. Circulation 2018;138(1):67–79 This study used tissue-specific deletion of Bmal1 from the perivascular adipose tissue to identify the time-dependent regulation of angiotensinogen on inactive period blood pressure.
- 9.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, et al. Smoothmuscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest 2015;125(1):324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansermet C, Centeno G, Nikolaeva S, Maillard MP, Pradervand S, Firsov D. The intrinsic circadian clock in podocytes controls glomerular filtration rate. Sci Rep 2019;9(1):16089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokonami N, Mordasini D, Pradervand S, Centeno G, Jouffe C, Maillard M, et al. Local renal circadian clocks control fluidelectrolyte homeostasis and BP. J Am Soc Nephrol 2014;25(7): 1430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debruyne JP, Noton E, Lambert CM, Maywood ES, Weaver DR, Reppert SM. A clock shock: mouse CLOCK is not required for circadian oscillator function. Neuron 2006;50(3):465–77. [DOI] [PubMed] [Google Scholar]
- 13.DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci 2007;10(5):543–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landgraf D, Wang LL, Diemer T, Welsh DK. NPAS2 compensates for loss of CLOCK in peripheral circadian oscillators. PLoS Genet 2016;12(2):e1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zuber AM, Centeno G, Pradervand S, Nikolaeva S, Maquelin L, Cardinaux L, et al. Molecular clock is involved in predictive circadian adjustment of renal function. Proc Natl Acad Sci U S A 2009;106(38):16523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolaeva S, Pradervand S, Centeno G, Zavadova V, Tokonami N, Maillard M, et al. The circadian clock modulates renal sodium handling. J Am Soc Nephrol 2012;23(6):1019–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alibhai FJ, LaMarre J, Reitz CJ, Tsimakouridze EV, Kroetsch JT, Bolz SS, et al. Disrupting the key circadian regulator CLOCK leads to age-dependent cardiovascular disease. J Mol Cell Cardiol 2017;105:24–37. [DOI] [PubMed] [Google Scholar]
- 18.Stow LR, Richards J, Cheng KY, Lynch IJ, Jeffers LA, Greenlee MM, et al. The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 2012;59(6):1151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards J, Ko B, All S, Cheng KY, Hoover RS, Gumz ML. A role for the circadian clock protein Per1 in the regulation of the NaCl cotransporter (NCC) and the with-no-lysine kinase (WNK) cascade in mouse distal convoluted tubule cells. J Biol Chem 2014;289(17): 11791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solocinski K, Richards J, All S, Cheng KY, Khundmiri SJ, Gumz ML. Transcriptional regulation of NHE3 and SGLT1 by the circadian clock protein Per1 in proximal tubule cells. Am J Physiol Ren Physiol 2015;309(11):F933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards J, Cheng KY, All S, Skopis G, Jeffers L, Lynch IJ, et al. A role for the circadian clock protein Per1 in the regulation of aldosterone levels and renal Na+ retention. Am J Physiol Ren Physiol 2013;305(12):F1697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.••. Solocinski K, Holzworth M, Wen X, Cheng KY, Lynch IJ, Cain BD, et al. Desoxycorticosterone pivalate-salt treatment leads to non-dipping hypertension in Per1 knockout mice. Acta Physiol (Oxford) 2017;220(1):72–82 This study utilized a high salt/mineralocorticoid model of hypertension in Per1 knockout mice to recapitulate non-dipping hypertension.
- 23.••. Douma LG, Solocinski K, Holzworth MR, Crislip GR, Masten SH, Miller AH, et al. Female C57BL/6J mice lacking the circadian clock protein PER1 are protected from nondipping hypertension. Am J Phys Regul Integr Comp Phys 2019;316(1):R50–8 This study postulates that sex may alter the non-dipping response to high salt/mineralocorticoid treatment in Per1 knockout mice.
- 24.Douma LG, Holzworth MR, Solocinski K, Masten SH, Miller AH, Cheng KY, et al. Renal Na-handling defect associated with PER1-dependent nondipping hypertension in male mice. Am J Physiol Ren Physiol 2018;314(6):F1138–F44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zylka MJ, Shearman LP, Weaver DR, Reppert SM. Three period homologs in mammals: differential light reponses in the suprachiasmatic circadian clock and oscillating transcripts outside of the brain. Neuron 1998;20(6):1103–10. [DOI] [PubMed] [Google Scholar]
- 26.Vukolic A, Antic V, Van Vliet BN, Yang Z, Albrecht U, Montani J-P. Role of mutation of the circadian clock gene Per2 in cardiovascular circadian rhythms. Am J Phys Regul Integr Comp Phys 2010;298(3):R627–34. [DOI] [PubMed] [Google Scholar]
- 27.Noda M, Iwamoto I, Tabata H, Yamagata T, Ito H, Nagata KI. Role of Per3, a circadian clock gene, in embryonic development of mouse cerebral cortex. Sci Rep 2019;9(1):5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamura H, Doi M, Yamaguchi Y, Fustin JM. Hypertension due to loss of clock: novel insight from the molecular analysis of Cry1/Cry2-deleted mice. Curr Hypertens Rep 2011;13(2):103–8. [DOI] [PubMed] [Google Scholar]
- 29.Nugrahaningsih DA, Emoto N, Vignon-Zellweger N, Purnomo E, Yagi K, Nakayama K, et al. Chronic hyperaldosteronism in cryptochrome-null mice induces high-salt- and blood pressure-independent kidney damage in mice. Hypertens Res 2014;37(3): 202–9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D, Jin C, Speed JS, Pollock DM. Evidence that food intake controls diurnal blood pressure rhythm in mice. FASEB J 2019;33(1_supplement):533.12. [Google Scholar]
- 31.van den Buuse M, Malpas SC. 24-hour recordings of blood pressure, heart rate and behavioral activity in rabbits by radio-telemetry: effects of feeding and hypertension. Physiol Behav 1997;62(1):83–9. [DOI] [PubMed] [Google Scholar]
- 32.Antic V, Van Vliet BN, Montani J-P. Loss of nocturnal dipping of blood pressure and heart rate in obesity-induced hypertension in rabbits. Auton Neurosci: Basic & Clinical 2001;90:152–7. [DOI] [PubMed] [Google Scholar]
- 33.Whelton PK. Sodium, potassium, blood pressure, and cardiovascular disease in humans. Curr Hypertens Rep 2014;16(8):465. [DOI] [PubMed] [Google Scholar]
- 34.Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial1–3. Am J Clin Nutr 2016;103(2):341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab 2018;27(6):1212–21.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheward WJ, Naylor E, Knowles-Barley S, Armstrong JD, Brooker GA, Seckl JR, et al. Circadian control of mouse heart rate and blood pressure by the suprachiasmatic nuclei: behavioral effects are more significant than direct outputs. PLoS One 2010;5(3):e9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. J Physiol 2003;549(Pt 1):313–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kennaway DJ, Voultsios A, Varcoe TJ, Moyer RW. Melatonin in mice: rhythms, response to light, adrenergic stimulation and metabolism. Am J Phys Regul Integr Comp Phys 2002;282:R358–R65. [DOI] [PubMed] [Google Scholar]
- 39.Stehle JH, von Gall C, Korf HW. Organisation of the circadian system in melatonin-proficient C3H and melatonin-deficient C57BL mice: a comparative investigation. Cell Tissue Res 2002;309(1):173–82. [DOI] [PubMed] [Google Scholar]
- 40.Klerman EB, Dijk DJ, Kronauer RE, Czeisler CA. Simulations of light effects on the human circadian pacemaker: implications for assessment of intrinsic period. Am J Phys 1996;270(1 Pt 2): R271–82. [DOI] [PubMed] [Google Scholar]
- 41.Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythm 2002;17(1):4–13. [DOI] [PubMed] [Google Scholar]
- 42.Stack N, Barker D, Carskadon M, Diniz BC. A model-based approach to optimizing ultradian forced desynchrony protocols for human circadian research. J Biol Rhythm 2017;32(5):485–98. [DOI] [PubMed] [Google Scholar]
- 43.••. Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res 2011;108(8):980–4 This study used three different protocols to identify circadian control of blood pressure in humans.
- 44.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med 2006;354(22):2368–74. [DOI] [PubMed] [Google Scholar]
- 45.Ohkubo T, Hozawa A, Nagai K, Kikuya M, Tsuji I, Ito S, et al. Prediction of stroke by ambulatory blood pressure monitoring versus screening blood pressure measurements in a general population: the Ohasama study. J Hypertens 2000;18(7):847–54. [DOI] [PubMed] [Google Scholar]
- 46.Dolan E, Stanton AV, Thom S, Caulfield M, Atkins N, McInnes G, et al. Ambulatory blood pressure monitoring predicts cardiovascular events in treated hypertensive patients–an Anglo-Scandinavian cardiac outcomes trial substudy. J Hypertens 2009;27(4):876–85. [DOI] [PubMed] [Google Scholar]
- 47.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension 2001;38(4):852–7. [DOI] [PubMed] [Google Scholar]
- 48.Ben-Dov IZ, Kark JD, Ben-Ishay D, Mekler J, Ben-Arie L, Bursztyn M. Blunted heart rate dip during sleep and all-cause mortality. Arch Intern Med 2007;167(19):2116–21. [DOI] [PubMed] [Google Scholar]
- 49.Sherwood A, Steffen PR, Blumenthal JA, Kuhn C, Hinderliter AL. Nighttime blood pressure dipping: the role of the sympathetic nervous system. Am J Hypertens 2002;15(2 Pt 1):111–8. [DOI] [PubMed] [Google Scholar]
- 50.Kario K, Shimada K, Pickering TG. Abnormal nocturnal blood pressure falls in elderly hypertension: clinical significance and determinants. J Cardiovasc Pharmacol 2003;41(Suppl 1):S61–6. [PubMed] [Google Scholar]
- 51.Zelinka T, Strauch B, Pecen L, Widimsky J Jr. Diurnal blood pressure variation in pheochromocytoma, primary aldosteronism and Cushing’s syndrome. J Hum Hypertens 2004;18(2):107–11. [DOI] [PubMed] [Google Scholar]
- 52.Hermida RC, Ayala DE, Fernandez JR, Portaluppi F, Fabbian F, Smolensky MH. Circadian rhythms in blood pressure regulation and optimization of hypertension treatment with ACE inhibitor and ARB medications. Am J Hypertens 2011;24(4):383–91. [DOI] [PubMed] [Google Scholar]
- 53.Uzu T, Ishikawa K, Fujii T, Nakamura S, Inenaga T, Kimura G. Sodium restriction shifts circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 1997;96(6):1859–62. [DOI] [PubMed] [Google Scholar]
- 54.Fujii T, Uzu T, Nishimura M, Takeji M, Kuroda S, Nakamura S, et al. Circadian rhythm of natriuresis is disturbed in nondipper type of essential hypertension. Am J Kidney Dis 1999;33(1):29–35. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int 2006;69(7):1175–80. [DOI] [PubMed] [Google Scholar]
- 56.Fabbian F, Smolensky MH, Tiseo R, Pala M, Manfredini R, Portaluppi F. Dipper and non-dipper blood pressure 24-hour patterns: circadian rhythm-dependent physiologic and pathophysiologic mechanisms. Chronobiol Int 2013;30(1–2):17–30. [DOI] [PubMed] [Google Scholar]
- 57.Sica DA. What are the influences of salt, potassium, the sympathetic nervous system, and the renin-angiotensin system on the circadian variation in blood pressure? Blood Press Monit 1999;4(Suppl 2):S9–S16. [PubMed] [Google Scholar]
- 58.Thomas SJ, Booth JN 3rd, Jaeger BC, Hubbard D, Sakhuja S, Abdalla M. Association of sleep characteristics with nocturnal hypertension and non-dipping blood pressure in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. J Am Heart Assoc 2020;70:A131 In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96(4):1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE, Somers VK. Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension 1998;32(6): 1039–43. [DOI] [PubMed] [Google Scholar]
- 61.Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation 1999;100(23):2332–5. [DOI] [PubMed] [Google Scholar]
- 62.••. Bello NA, Jaeger BC, Booth JN 3rd, Abdalla M, Anstey DE, Pugliese DN, et al. Associations of awake and asleep blood pressure and blood pressure dipping with abnormalities of cardiac structure: the Coronary Artery Risk Development in Young Adults study. J Hypertens 2019;38(1):102–10 This study suggests that high awake and high asleep SBP, but not non-dipping SBP, are associated with left ventricular hypertrophy and hypertensive end-organ damage.
- 63.Van Dongen HP, Maislin G, Kerkhof GA. Repeated assessment of the endogenous 24-hour profile of blood pressure under constant routine. Chronobiol Int 2001;18(1):85–98. [DOI] [PubMed] [Google Scholar]
- 64.Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004;43(2): 192–7. [DOI] [PubMed] [Google Scholar]
- 65.Medicine AAoS. The international classification of sleep disorders 3rd ed. Darien: American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.American Psychiatric Association. American Psychiatric Association. DSM-5 Task Force. In: Diagnostic and statistical manual of mental disorders : DSM-5, vol. xliv. 5th ed. Washington: American Psychiatric Association; 2013. p. 947. [Google Scholar]
- 67.Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A 2016;113(10):E1402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reinberg AE, Smolensky MH, Riedel M, Riedel C, Brousse E, Touitou Y. Do night and around-the-clock firefighters’ shift schedules induce deviation in tau from 24 hours of systolic and diastolic blood pressure circadian rhythms? Chronobiol Int 2017;34(8): 1158–74. [DOI] [PubMed] [Google Scholar]
- 69.Strohmaier S, Devore EE, Zhang Y, Schernhammer ES. A review of data of findings on night shift work and the development of DM and CVD events: a synthesis of the proposed molecular mechanisms. Curr Diab Rep 2018;18(12):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A 2009;106(11):4453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res 2010;1337:21–31. [DOI] [PubMed] [Google Scholar]
- 72.Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest 2017;127(2):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaix A, Panda S. Timing tweaks exercise. Nat Rev Endocrinol 2019;15(8):440–1. [DOI] [PMC free article] [PubMed] [Google Scholar]


