Abstract
As gene therapy enters mainstream medicine, it is more important than ever to have a grasp of exactly how to leverage it for maximum benefit. Development of new targeting strategies and tools make treating patients with genetic diseases possible. Many Mendelian disorders amenable to gene replacement or correction. These often affect post-mitotic tissues, meaning a single stably expressing therapy can be applied.
Recent years have seen the development of a large number of novel viral vectors for delivering specific therapies. These new vectors – predominately recombinant adeno-associated virus (AAV) variants – target nervous tissues with differing efficiencies. This review gives an overview of current gene therapies in in the brain, ear, and eye, and describes the optimal approaches, depending on cell type and transgene. Overall, this work aims to serve as a primer for gene therapy in central nervous and sensory systems.
Keywords: Gene therapy, AAV, retina, inner ear, brain
Introduction to gene therapy
Gene therapy has become a realistic strategy for treating diseases previously thought uncurable. For the past number of years, AAV vectors have led the field for direct in vivo gene therapy. With AAV-based medicines Luxturna and Zolgensma commercially available, and many more gene therapeutics under trial, treatment of inherited disease with viral vector-based therapy has become a clinical reality[1]. In general, AAV vectors safely transduce central nervous and sensory tissues.
The breadth and scope of gene therapy research has expanded exponentially over the past few years. This review offers a brief overview on techniques to deliver gene therapies to nervous and sensory systems in the body. In particular, we focus on the state-of-the-art in AAV delivery for the central nervous system (CNS), as well as sensory and non-sensory cell types of the inner ear and eye. Overall, this should serve as an introductory guide for those navigating the increasingly complex landscape of gene therapy in the brain and sensory organs.
AAV-mediated gene delivery in the nervous system
Gene therapy is, in simple terms, the act of delivering transgenes to rescue or correct a disease phenotype in a patient’s cells. This approach can be defined further into: a) gene addition – supplying a functional copy of a gene to a tissue or organ that may lack it; b) gene silencing – inhibiting a dominant-acting mutation through the use of RNA interference or CRISPR-based editing; c) repair – using a base editing or homology-driven repair approach to correct a mutation within the context of the genome. While there are many strategies for delivering gene therapies to target tissues, AAV has become the gold standard viral vector due to its safety, efficacy, and broad tropism for a number of tissues and organs. AAV is a small (~25nm) single-stranded DNA virus, that consists of a short genome (4.7kb) enclosed by a protein capsid. The viral genome consists of genes that direct vector replication (rep gene) and capsid structural protein (cap gene) production. Although beyond the scope of this review, in recent years, more genes have been discovered, aap and maap[2,3], which are the subject of intense study. Importantly, AAV cannot replicate independently, and requires a simultaneous infection by adenovirus (or other helper viruses, such as herpes simplex virus) to replicate[4]. AAVs used for gene therapy are devoid of rep and cap genes, instead provided by producer cells in trans. This adds to the vector’s safety profile, as well as opening up critical packaging capacity for the transgene of interest. As seen in Box 1, recombinant AAV vectors (referred to hence as AAV) only retain the inverted terminal repeats (ITRs; see Glossary) from the wild-type virus. ITRs are the only part of the wild-type AAV genome (wtAAV) required for functional packaging and transduction. Transduction is itself a multi-step process; a schematic of this is shown in Box 1.
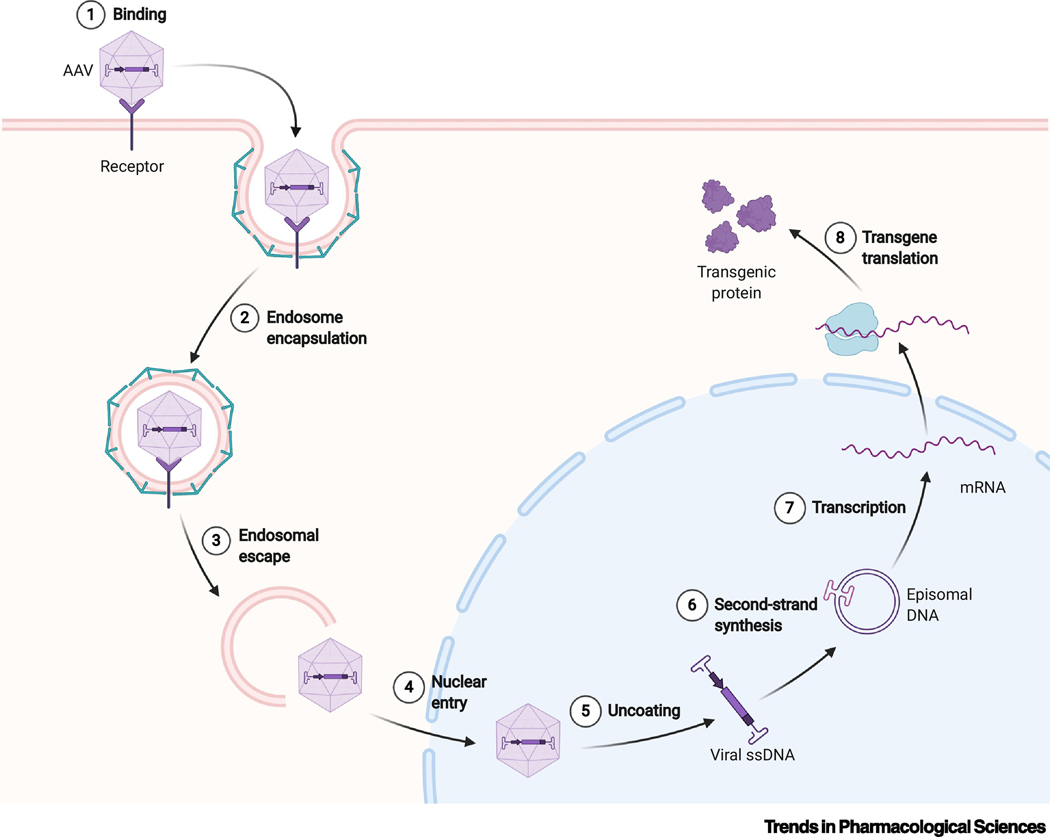
Box 1. Mechanism of AAV internalization and transduction.
There are multiple steps to AAV transduction, which are laid out sequentially in Box 1, Figure I. Transduction begins with capsid-cell adhesion (1), which is mediated by a large number of cell surface glycans, receptors and other moieties, including heparin sulfate proteoglycans (AAV2, 3b, 6), sialic acids (AAV1, 4–6), and galactose (AAV9; reviewed in [100]). As well as serotype-specific receptors, there are newly discovered “general” receptors that the bulk of AAV serotypes rely on, including AAVR and GPR108[101,102].
Following binding, AAV is internalized into an endosome (2), likely mediated by the formation of clathrin-coated pits[103]. As the endosome degrades (3), the capsid is released and enters the nucleus (4). The capsid’s ssDNA is then released (5). The final step before gene expression is second strand synthesis (6), where the single-stranded AAV genome is filled in by endogenous DNA polymerases. These DNA sequences will subsequently circularize and form stable concatemers within the nucleus[104]. A small percentage of genomes also integrate into the host genome. Finally, mRNA transcription (7) and translation into protein (8) can occur.
Box 1 Figure I:
Schematic of AAV transduction.
One of the key advantages for using AAV in gene therapy is its versatility; there are dozens of naturally occurring serotypes, still being discovered as of this year, each varying in transduction properties[5]. These properties can be further modified through use of promoters and enhancer elements to transcriptionally regulate transgene expression, such as the synapsin promoter for tailored neural expression[6]. There are many other examples of tissue specific promoters, and other genetic tools being used to direct and restrict transgene expression which fall outside the scope of this review. However, recent years have seen a massive push to modify and improve AAV capsids infection of tissue types via rational design, capsid shuffling, and directed evolution, summarized in Box 2. The result: serotypes and variants have been discovered or derived that can target almost any cell type in the body. This review seeks to cover useful serotypes and capsids for transduction of the brain, retina and inner ear.
Box 2. New methods for new vector development.
One of the most exciting developments to take place in gene therapy has been the development of novel AAV capsid variants to expand the range and utility of the vector for gene therapy. There are three primary methods for new capsid development: capsid shuffling, library-based directed evolution, and rational design. Capsid shuffling involves blending of multiple serotypes; capsid genes of various AAVs are partially digested and then recombined by annealing of complementary regions and PCR amplification, producing variants with a mix of properties. An in vitro or in vivo selection can then be used to isolate the new variants that are superior. One of the first of these produced, AAV-DJ, was a fusion of AAV1, 2, 8 and 9, and has been recently modified with an additional peptide and used to target the inner ear[52,105]. Library evolution is additive; randomized peptides (usually 6–10 amino acids in length) are inserted into the capsid protein itself. The insertion points used are in so-called “safe harbors”, areas on the capsid surface that stick out and are permissive to editing and insertion with minimal interference to capsid assembly. By far the most common insertion point is in variable region VIII of the capsid, in the spike of the 3-fold axis – in AAV9, for example, this point is at amino acid 588/589 of the VP1 protein. Capsids with these random insertions are then subjected to rounds of selection, similar to a capsid shuffling approach, until a small number of highly successful candidates can be isolated. Several outputs of this method have been transformative in gene therapy in recent years, with some – such as AAV2–7m8, PHP.B, and AAV-F – discussed in the text. Finally, rational design involves making informed (rather than random) changes to the capsid. One of the earliest of these involved mutating tyrosine residues on the surface of capsids, as it was observed that those residues are critical to AAV2-ubiquitination and degradation; this led to enhanced expression in the retina[106,107]. Larger changes are also possible. Several attempts have been made at deriving “ancestral” serotype variants through lineage mapping of dozens of wtAAVs. There have been several attempts at this[108–110], but none more widely tested than Anc80. Indeed, as seen in this review, Anc80 has seen success in multiple systems, including the primate retina and mouse inner ear. As the field learns more about AAV itself, this knowledge can hopefully be leveraged to further a rationally designed approach.
AAV in the brain and CNS – systemic delivery
Targeting the brain effectively with any gene delivery system, including AAV, is a difficult process. In mammals – and in particular in humans – direct intraparenchymal injection does not lead to complete distribution throughout the brain. And, although the brain is highly vascular, the blood-brain barrier (BBB) effectively blocks many serotypes from effectively entering via the bloodstream after intravenous delivery. While other methods such as intrathecal administration can deliver AAV to brain, systemic delivery has great potential to broadly transduce the CNS, due to the high density of capillaries in the brain. AAV9 was the first serotype to show significant brain transduction after intravenous (IV) administration[7,8]. AAVrh8 and AAVrh10 have both subsequently been shown to be as effective as AAV9 in some studies, with rh10 also demonstrating expression in mouse and marmoset spinal cord after IV delivery[9,10]. That said, AAV9 remains the most widely adopted serotype for systemic CNS delivery, with successes such as the clinically-approved Zolgensma and trials for other diseases such as mucopolysaccharidosis[11] (clinicaltrials.gov NCT03315182).
In the last few years, however, a number of library-based, directed evolution strategies have been developed (Box 2) with a focus on more effectively targeting the brain through IV administration. A notable example of success of this approach came from Deverman et al., who used a library system called CREATE to generate PHP.B, an AAV9 variant capable of widespread transduction of the brain of C57/BL6 mice after IV delivery[12]. CREATE is a system in which genomes from capsid variants that successfully reach the nucleus could be recovered by PCR when injected into a Cre-expressing mouse, due to the existence of loxP sites in the viral genome. PHP.B has been used in a number of different studies, and is discussed later in this review, particularly with reference to the inner ear[12–15].
Interestingly, PHP.B is able to drive efficient CNS transduction in certain mouse lines, but not in others (such as BALB/c), nor in non-human primates (NHPs). It was discovered by several labs that this was due to a reliance by PHP.B on the Ly6a receptor[16–18], which is not expressed in those strains. Further, a Ly6a homolog is missing entirely in primates, including humans[19]. A number of mutant variants of PHP.B have been created (for example, PHP.eB) that give greater expression than the original in rodents, while maintaining Ly6a specificity[13,17]. Recently, our group described a transgene-expression based library platform called iTransduce[20]. The iTransduce system selects only library variants that mediate vector-encoded Cre protein expression by triggering fluorescent protein expression in a floxed-STOP mouse model. This ensures that selected variants are functional at the most downstream event of transduction: transgene expression. We used the iTransduce library to select an AAV variant, AAV-F, which shows similar high-efficiency transgene expression in the murine brain to PHP.B after IV delivery, as well as potent spinal cord transduction after intrathecal delivery. Further, AAV-F does not appear to be reliant on Ly6a, as the PHP.B family is, being able to effectively transduce BALB/c brains after systemic delivery[20]. However, it remains to be seen whether the transduction pattern with AAV-F observed in mice will translate to primates and therefore be considered a clinical candidate – for now it remains a promising tool for high efficiency genetic modification of the murine CNS.
AAV in the Brain and CNS - Non-systemic delivery
While an intravenous approach to AAV delivery can enable transduction of the brain, there are a number of risks and challenges associated with systemic AAV delivery, two key issues being off-target expression of the transgene and heightened immune response, discussed in Box 3. There is a potential concern of AAV-mediated genotoxicity in the liver, which is undergoing rigorous debate in the field and continues to be monitored in clinical trials[21]. There is also a logistical challenge in manufacturing the larger amount of vector required for systemic dosing, which translates into higher cost.
Box 3. Immune implications of AAV gene therapy.
AAV has been used in many clinical trials without serious adverse events, and patient immune responses are generally mild. Some therapies have seen over 15 years of expression in the primate CNS[111]. However, AAV does provoke an immune response from innate and adaptive immune cells. Many people are exposed to wtAAV in life, resulting in robust neutralizing antibodies to many serotypes. This ranges from 40% for AAVs 6, 8 and 9 up to 70% for AAV1 and 2[112]. Notably, anti-AAV antibodies in NHPs can show strong cross-reactivity, which is likely seen in humans as well[113]. In practice, this means that patients in clinical trials are usually pre-screened for anti-AAV antibodies. For some strategies (e.g. in the eye, or in dividing tissues), readministration may be necessary. Luxturna was shown to be safely tolerated and effective when readministered to a second eye[95]. However, in a trial treating Leber Hereditary Optic Neuropathy, the single patient receiving contralateral administration subsequently lost visual acuity in both eyes, attributed to an immune reaction caused by intravitreal injection[65].
Besides an antibody response, capsid-specific cytotoxic T-lymphocytes (CTLs) can diminish AAV-mediated gene expression. CTL-mediated destruction of transduced hepatocytes is suspected in loss of transgene expression in a hemophilia B trial[114], which was not predicted by animal models and may necessitate redosing. This response can at least partially be controlled through the use of high dose glucocorticosteroids, which reduce T-cell expansion[115]. The innate immune response to AAV is less well understood. It’s become apparent that unmethylated CpG motifs in AAV transgene cassettes can provoke inflammation[116]; the complement cascade has also been demonstrated to induce inflammation and neutralizing antibody induction[117].
The brain, eye and ear are immune-privileged and so the immune response from direct injection can usually be managed with transient immunosuppression[115]. However, systemic delivery requires large quantities of vector to be administered, which may cause toxicity. Although AAV is usually very safe, tragically, in recent months three trial patients died following an AAV8 injection targeting X-linked myotubular myopathy (NCT03199469). All three patients were injected at a very high dose of 3×1014 vg/kg, and all three reported liver dysfunction, gastrointestinal bleeding, and possibly lethal sepsis. If such extremely high doses are required to achieve significant transduction (e.g., in the brain), a more local approach may be advisable. Furthermore, developing immunomodulatory strategies in combination with more efficient capsids may deliver sufficient gene transfer while mitigating immune effects.
As such, there is a role for more localized administration through direct injection, or intrathecal delivery along the spinal cord. Diseases such as Parkinson’s Disease (PD), which are characterized by dopamine deficiency and neuronal death in the brain, have seen significant clinical testing using AAVs injected directly into the putamen. AAV2s expressing aromatic L-amino acid decarboxylase (AADC), which converts L-dopa and 5-hydroxytryptophan to dopamine and serotonin, or neurturin, essential for neuron survival, finished phase II clinical trials around 2017. While treatments were tolerated well, there did not appear to be significant clinical benefits[22,23]. More recently, Voyager reported increased enzymatic activity and improved motor performance in patients receiving high dose AAV expressing AADC[24]. By directly comparing the AADC therapy trials we see that both studies used AAV2 as the vector, and an identical delivery route. However, while the NTUH trial treated each patient with 1.81×1011 vector genomes (vg) in total, the Voyager study used three escalating doses of 7.5×1011 to 4.7×1012 vg for their three cohorts. While the NTUH trial only used one dose, which was itself based on a higher dose of a previous trial[25], they observed some impact on motor development but minor increases in AADC activity in a portion of treated patients. Contrast this to the Voyager trial which observed increased AADC activity and change in UPDRS score with increasing dosage. This suggests a dosage requirement may not have been fully reached in earlier trials, accounting for their results. Other contributing factors could include mean age (Voyager, 57y; NTUH 2y), and differences in dopamine drug restrictions after treatment, but it seems likely that higher doses or more efficient capsids which transduce dopaminergic neurons and brain supporting cells will increase treatment efficacy.
Optimistically, a novel serotype called AAV2-v66 was recently demonstrated to effectively deliver transgene into the brain after intercranial injection[5]. Of particular note, the authors who described AAV2-v66 noted an apparent ability to transduce microglia of the mouse brain which has been something of a holy grail with few, low efficiency examples[26,27]. Microglia are the innate immune cells of the brain, playing both neuroprotective and destructive roles during normal and disease states[28]. The development or discovery of a vector that can target this cell type at high levels could enable a new generation of treatments for diseases such as PD and AD, by limiting microglial mediated killing of diseased neurons, or enhancing its protective functions to maintain diseased neuron function and survival. This approach could be a valuable alternative or augmentative method for treating inflammatory diseases of the brain.
Another delivery approach relies on injection into the CSF via intrathecal injection. This offers potential avenues for transduction of both brain and spinal cord. Groups have shown efficacy in transducing the CNS in small and large animals with this method using vectors such as AAV9, or our own group’s AAV-F[20,29]. PHP.B may be amenable to this method, as one report described heightened expression in macaques compared to an intravenous approach, although in this case it was not compared to AAV9[30]. A phase I clinical trial to treat Batten disease also used a lumbar administration to delivery AAV9, and is currently in a long-term follow-up phase (NCT02725580, NCT04273243). Intracerebroventricular (ICV) delivery into the ventricle of the brain allows administration of a much lower dose of vector than systemic delivery, but can cause unwanted immunological complications[29]. However, this method has also been shown to avoid neutralization by AAV antibodies in NHPs[31]. Overall, the lower dose and more restricted peripheral biodistribution (which is enhanced through slow-infusion with an osmotic pump[32]) potentially make these an attractive proposition for CNS delivery. Like in all methodologies, taking each method’s strength and weakness into account is essential.
Delivering AAV to the Inner Ear
The majority of deafness causing genes are primarily expressed in cells found within the cochlea. As such, the majority of AAV-mediated gene therapies to rescue hearing are focused on delivery to this structure. Specifically, the sound-sensing inner and outer hair cells (IHC, OHC) are the most sought-after targets for current cochlear gene therapies. The mammalian cochlea is responsible for converting sound into signals interpreted by the brain. Sensorineural hearing loss occurs when hair cells (HCs) or spiral ganglion nerves (SGN) of the cochlea are damaged or lost. Mutations within many HC-specific genes cause deafness, and delivery of AAVs to HCs has become an active focus of the gene therapy community.
There is a unique challenge in delivering AAV to the cochlea. The cochlea is a closed system, embedded in the temporal bone, and made up of an intricate set of coils and distinct features, summarized in Figure 1a. Only a tiny amount of material can be administered to the cochlea without expunging cochlear fluids, or damaging the delicate stereocilia of the hair cells. The cochlea follows a tonotopic layout, whereby HCs located at the apex are stimulated by low frequency sounds while those at the base react to high frequency sounds, making transduction of the entire length required for complete effectiveness.
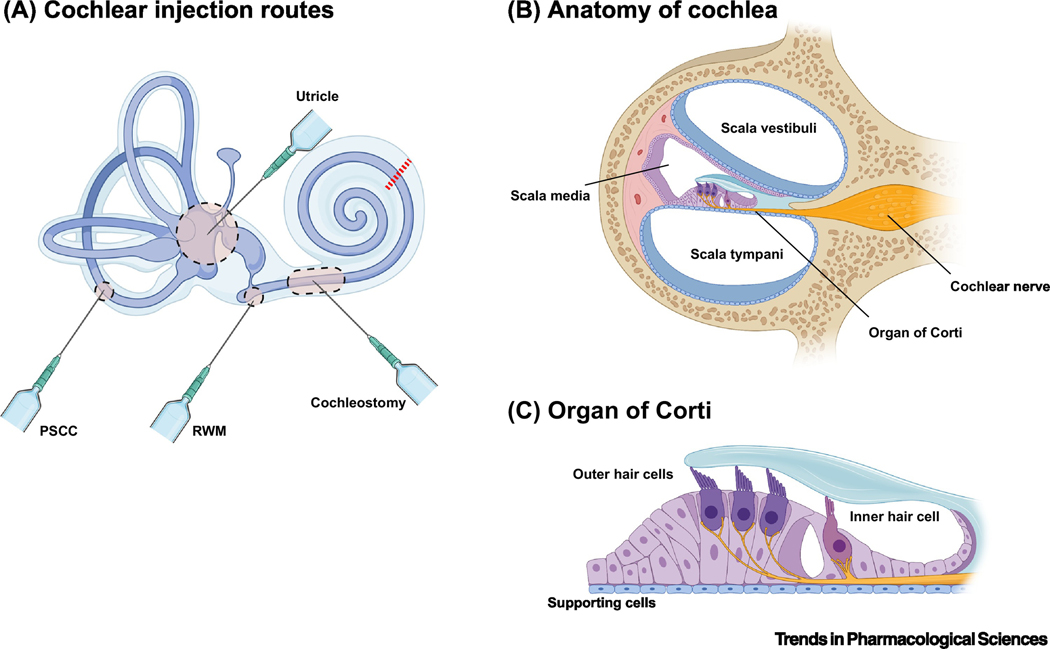
Figure 1. Cochlear Architecture.
a. Routes of injection employed in the cochlea. The round window membrane (RWM) is the most commonly used approach for neonatal mice, as well as NHPs. Cochleostomy involves penetrating the lateral wall and is used after the cochlear bone calcifies in adult subjects. Targeting the cochlea via the vestibular system is also an option. The posterior semicircular canal (PSCC) is also used for treating adult mice. More recently, injecting via the utricle has been shown to deliver high level expression in the cochlea with a number of serotypes. However, more invasive procedures like the PSCC and cochleostomy can carry a greater risk of damaging hair cells, obviating any therapeutic benefit[39,40]. b. A cross-section of the cochlea (red line in a) is shown. The scalae vestibuli and tympani are perilymph-filled vessels surrounding the organ of Corti, where the sensory apparatus resides. This perilymph must remain separate from the endolymph of the scala media; as such, any injection directed towards the organ of Corti must be minimal to limit the puncture size. c. Focus on the organ of Corti. Inner and outer hair cells are tightly nestled among a number of classes of supporting cells called Dieter, Claudius, and Hensen cells.
Figure 1b details the most commonly used injection routes into the cochlea. AAV dose, capsid serotype, and transcriptional regulation are thought to be more critical factors for transduction of the inner ear than delivery method[33–36], with direct comparisons often yielding similar transduction rates of HCs[15,34,35,37,38]. However, more invasive procedures like PSCC and cochleostomy carry a greater risk of damaging hair cells[39,40].Transduction of the cochlear hair cells in mice tends to occur in a gradient along the organ with IHCs and OHCs of the apex transduced in higher numbers and decreasing towards the base, regardless of methodology[38,41]. These unequal transduction profiles have stymied attempts to fully restore hearing in animal models, as treatment is unable to rescue high frequency- HCs of the base[15,42,43].
Cochlear Hair Cell Transduction by AAV
Choice of vector is critical in maximizing transduction. As discussed in Box 2, AAV capsids can be modified by designed or random mutagenesis to preferentially infect targeted cell types. There are many reports on the efficacy of different AAVs to transduce IHCs and OHCs; while dose is still the main consistent factor, both PHP.B and Anc80L65 transduce HCs better than other studied AAVs in neonatal mice. This can reach near 100% transduction in the apex of injected cochleas[15,38,44]. This efficiency is highly dependent on injected dose, with deliveries under 109 vg per cochlea having drastically reduced transduction rates[36,38,45]. Transduction rates of HCs fall steeply with dose, following a sigmoidal curve in transgene expression with viral copies delivered[38]. Anc80L65 poorly transduces supporting cells in mice, and PHP.B with variable efficacy[14,15,38], but PHP.B has been shown to faithfully transduce spiral ganglion neurons in C57/B6 mice and non-human primates[12,46]. Both vectors transduce murine OHCs as well. Despite their high efficacy, both PHP.B and Anc80L65 transduction efficiencies decrease along the tonotopic axis of the cochlea in mice. Notably, however, our own group has shown that PHP.B is capable of broad transduction in non-human primates. At doses greater than 3×1011vg, we have demonstrated complete transduction of IHCs and OHCs in cynomolgus macaques, along with broad expression in cell types such as SGNs and supporting cells[14,15]. With this in mind it is still unknown why certain treatments fail to restore basal HC functionality, even when viral titers are high enough to transduce the entirety of the organ of Corti. Conversely, there are some studies showing a reversal in tonotopic transduction with higher transduction of basal HCs than apical HCs with PHP.B, AAV3, and AAV8[36,40]. The few reported observations of this make it unclear why this occurs.
Following Anc80 and PHP.B, there are AAVs which intermediately transduce murine IHCs. AAV 2, 3, 5, 8, DJ, and AAV2.7m8 have all been shown to transduce IHCs at rates varying from 20–80% making them less reliable than PHP.B or Anc80[35,36,38–40,42,44,45,47]. On the other hand, AAVs 1, 6, and rh43 consistently transduce IHCs very poorly[34,35,42,48]. One major drawback of these AAVs is the transduction of OHCs in neonates. While Anc80 and PHP.B can reliably transduce OHCs, the other serotypes offer inconsistent or undetectable transduction. Consistent through all these reports, is the use of broadly expressing CMV and CBA promoters to drive transgene expression within the cochlea[40,49].
AAV Transduction of Cochlear Supporting Cells
Targeting the supporting cells of the organ of Corti in mice is reported much less throughout the literature. This is interesting given that the most common congenital deafness is caused by mutations in the GJB2 gene, which is expressed throughout the supporting cells of the organ of Corti, but not in sensory hair cells. Only a few studies have delivered Gjb2 to conditional knockout mice with AAV. While they observed rescue of cochlear architecture, there was no rescue of hearing[47,49,50]. Two studies used AAV1, taking advantage of its inefficiency to transduce hair cells, while the other used bovine AAV. Aside from these studies there are sparse reports on transducing supporting cells in vivo[35,51]. A recent report detailed the engineering of AAV-ie – an AAV-DJ capsid containing the PHP.eB peptide insertion – demonstrated efficient transduction of supporting cells when delivering 1010 vg/cochlea. However, AAV-ie also shows high efficiency transduction of IHCs[52]. Several publications demonstrate transduction of supporting cells using AAV1, 2, and bovine AAV in cochlear explants with CBA promoters[50,53], but little else. This highlights the fact that directed transduction of supporting cells represents a blind spot for gene therapy research in the ear. Unlike the sensory cells of the inner ear, there is a lack of good marker proteins for co-localization in supporting cells to observe AAV transduction. This could be addressed through intrepid transgenic mice and/or development of better antibodies for supporting cell specific proteins. Another avenue would be to use in situ RNA labelling of supporting cell-specific mRNAs[54–56]; this has been used to successfully label supporting cells, but is significantly more involved than an antibody-based approach. Targeting supporting cells is also complicated by the fact that they may divide[56], albeit slowly, which may dilute episomal AAV DNA over time.
Despite its relative novelty, remarkable progress has been made in recent years in auditory gene therapy, including our own demonstration of efficient transduction of NHP inner ear with an AAV vector[14,15]. That said, there is ample space for the development of new AAVs to transduce specific cell types, develop novel surgical methods, or restrain expression with transcriptional regulatory sequences in their vectors. The window of therapeutic intervention for the different deafness causing genes is an important factor to consider, as some cause collapse of cochlear architecture too quickly to intervene in mice. As the perinatal period in mice corresponds to in utero development in humans, this may limit the ability to intervene in some human diseases. That said, some effort is being made to transduce the inner ear in utero in mice, with one recent publication highlighting in the effectiveness of Anc80 in this regard[57].
Delivering AAV to the retina
The retina is a highly accessible organ for AAV delivery. It is composed of several densely packed and highly organized layers of cells, but unlike the brain or inner ear it lacks many of the physical barriers that prevent direct access. Mutations in over 270 genes are implicated in inherited retinal degeneration[58], with the vast majority of these mutations affecting rod and cone photoreceptors (Figure 2a). Cell types such as photoreceptors and the retinal pigment epithelium (RPE) are effectively transduced by several standard AAV serotypes, such as AAV2, AAV5, AAV8 and AAVrh10[59–62]. AAV9 has also been shown to have efficacy in cone photoreceptors in NHPs[63].
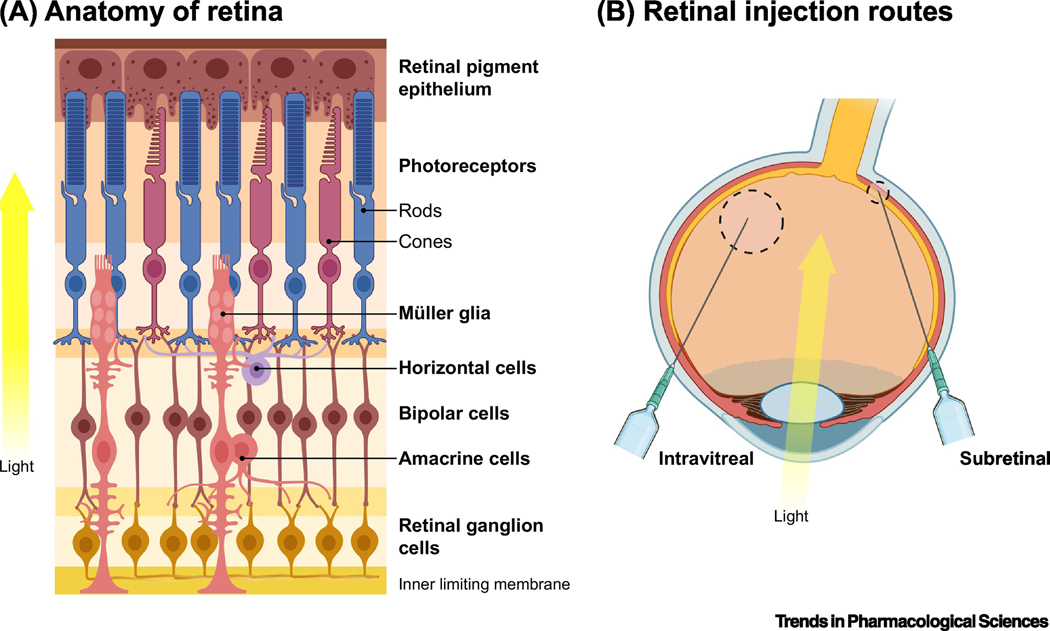
Figure 2. Retinal architecture.
a. Anatomy of the retina. The retina is made up of seven main types (and over sixty subtypes) of cells. Rods and cones within the retina receive light input; this signal is transferred to retinal ganglion cells (RGCs) via bipolar cells. The signal is decomposed and processed by inhibitory horizontal and amacrine cells, before undergoing further processing by RGCs and transmittance to the brain via the optic nerve. Müller glia support retinal neurons through homeostatic maintenance and cycling of neurotransmitter levels[118]. Glia also help form the inner limiting membrane (ILM), a barrier that separates the retina from the vitreous. b. Routes of administration to the retina. Intravitreal (IVit) and subretinal (SR) injections are the primary routes for drugs being delivered to the retina. IVit involves direct delivery to the vitreous humor of the eye – a well tolerated procedure millions of patients receive monthly for diseases such as age-related macular degeneration. While safe and routine to perform, the ILM prevents passage of most AAV serotypes beyond RGCs[119]. Subretinal injection delivers to the space between the retinal pigment epithelium (RPE) and photoreceptors. It is the most effective method for transducing these cell types. However, this more invasive surgical method involves isolated detachment of the retina through blebbing, which – particularly in vulnerable, diseased retinas – can lead to damage. Further, AAV injections into the subretinal space of larger animals result in only a small area of the retina immediately surrounding the bleb being transduced.
As stated in Figure 2b, IVit injection is inefficient at targeting cells outside of the ganglion cell layer (GCL). AAV2 has been used in an IVit injection route targeting the GCL in multiple clinical trials[64,65]. One key factor with IVit delivery is the increased inflammation and immune infiltration after administration[66,67]; indeed, one study in a human patient showed a lack of effect when vector was administered to the second eye 12 months later, which may indicate an immune response stymying contralateral delivery[65]. SR injection is more widely used as, again, most forms of blindness affect photoreceptors and the RPE. AAV2-based vectors are being tested in patients with retinitis pigmentosa, RPE65 Leber congenital amaurosis, Choroideremia, and Achromatopsia (reviewed in [68]). Other serotypes such as AAV5 and AAV8 have been extensively tested in large animals[62,63,69,70], and are now testing in patients for diseases such as X-linked retinitis pigmentosa and age-related macular degeneration ([71]; NCT04671433, NCT03116113, NCT04704921, NCT04514653). However, it has been shown that SR delivery in degenerated retinas can present a risk, as the surgery may worsen an already fragile state[72]. More recently new targeting strategies have been developed to overcome this. This includes subILM delivery, where AAV is delivered between the ILM and GCL (Figure 2a;[73]); “pararetinal” delivery where AAV is delivered IVit as close to the retina as possible[74]; and physically peeling back or disrupting the ILM temporarily before vector delivery[75,76]. Of course, it must be noted that these techniques are new and not widely adopted – especially in large animals or patients – and so carry a greater inherent risk at present. As such, another approach to improving delivery is modifying the delivery vehicle itself.
Improving AAV Transduction in the Retina
The glut of new AAV serotypes made available in recent years has, like the ear, made it possible to test newly developed vectors for retinal tropism, as well as repurpose those designed for another organ. Anc80L65 has shown an ability to transduce a wide variety of cell types, including the retina. Anc80L65 was shown to provide high-level expression in the outer retinas of mice and primates when administered subretinally[77]. PHP.B can effectively transduce murine retinas, possibly more effectively than conventional serotypes[15]. A recently discovered serotype, AAV44.9, has shown efficacy in NHPs, with much greater spread observed after subretinal injection – a critical factor in transduction in larger animals[78]. Overcoming limited spread of AAV vectors following subretinal delivery is a key barrier to the success of retinal gene therapy.
Efforts have been undertaken to engineer AAV vectors specifically for the eye. Some groups have success with mutating tyrosine residues on the surface of the AAV2 capsid to improve transduction[79,80]. This is hypothesized to prevent phosphorylation by receptor tyrosine kinases and subsequent degradation, and has been shown to be adaptable to other serotypes[81]. Two variants, AAV2–7m8 and AAV8-BP2, have been designed to target bipolar cells of the retina (developed using library screens), in order to deliver optogenetic therapies to render these cells light-sensitive in cases where photoreceptors have already died off[82,83]. Of note, 7m8 was selected for an ability to transduce these cells via intravitreal administration in mice. It was subsequently shown that these vectors could efficiently transduce NHP retina as well[84], although 7m8 was unable to recapitulate this expression intravitreally, transducing only cones in the parafoveal region of the retina. This may relate to differences in makeup or thickness of the ILM in primates, as well as the comparatively far larger volume of the primate vitreous. That said, more recent work with 7m8 has shown that, with a cone-specific promoter, central foveal expression is possible[85], which may indicate the capsid can in fact traverse the ILM. This is critical, as the majority of human vision is capitulated by cones in the fovea, despite making up less than 1% of total photoreceptors. A study recently developed a large number of “minipromoters” to direct expression in the retina to specific cell types[86]. This is particularly relevant to intravitreal delivery to photoreceptors, as the risk of off-target expression by this method is higher. The retina is so tightly organized that the development of novel, more efficient vectors will likely lead to off-target expression. As such, expanding the array of viable promoters for use in AAV is extremely important, both for the eye and for gene therapy as a whole.
Concluding remarks
There are challenges when it comes to designing a strategy for a novel gene therapy. The choice of transgene or CRISPR-based guide RNA, the optimal promoter, and the delivery method are all critical factors influencing the success of the intervention – as is choosing the best delivery vector. The wide array of AAV serotypes provides significant flexibility to gene therapy researchers.
The role of the adaptive and innate immune system’s response to AAV vectors will become much more relevant as vector delivery shifts toward clinical application. While the tissue types discussed in this review each possess varying degrees of immune privilege, inflammatory events are still possible. Controlling the immune response to AAV may represent the next frontier for the field (Outstanding Questions). This is particularly true with respect to intravenous delivery, as discussed in Box 3. One strategy being taken to reduce the immune burden is by evolving variants that retain their transduction properties while “detargeting” from tissues such as liver[87,88]. In animal models, liver expression of AAV-transgenes may lead to tolerance developing for the transgene product, through induction of regulatory T-cells in the liver; this can prevent the development of anti-drug antibodies[89]. However, this may require liver-specific gene expression to occur, and it remains unclear whether this occurs in humans as it does in models[90].
Other methods can be applied to AAVs such as incorporating miRNA targeting sequences to restrict transgene expression, as done with AAV9 and some oncolytic vectors[91,92], or labelling capsids with antibody fragments to more specifically infect certain cells[93]. The eye is insulated from much of the immune system, and re-administration of therapies to both animals and humans has been well tolerated[94–96]. However, as discussed previously, intravitreal delivery of AAV may provoke a stronger immune reaction[65].
The effect of AAV on recruitment of the immune system to the cochlea is also poorly studied. Immune cells reside in the cochlea and respond to pathogens, often causing disease formation following microbial or viral insult[51]. However, unlike other organs which have multiple reports detailing AAV-induced adaptive immunity[97], the cochlea is notably absent. Much better research is available on the infiltration after ototoxic drug or noise damage occurs, sterile inflammation[98]. AAV delivery to primate cochlea has already been shown to elicit an infiltration of immune cells[48,52], although a recent study by our group showed only minimal immune infiltration following high-dose AAV administration in NHPs[14]. Still, questions remain on how the cochlear immune environment will interact with AAV, especially as researchers begin testing therapeutic candidates in NHPs and, indeed, patients.
Development of novel techniques like CREATE and iTransduce to refine library selection has increased the library of AAV vectors available to researchers. Meanwhile, CRISPR/Cas technologies have continued to expand over the last three years making it possible to edit almost any individual base-pair at any region of the genome regardless of PAM sequence[99]. Additionally, with CRISPR technologies more often developed with the end goal of being utilized for gene therapy, the advancement of techniques to deliver dual-AAVs or transgenes previously thought to be too large within AAVs increases the potential for AAV mediated genetic manipulation. While explosive in its growth, the field of gene therapy is still developing and will require dedicated research to understand the best ways to deliver, monitor, and improve, the vectors, transgenes, and outcomes we seek.
Outstanding questions.
What host factors restrict AAV transgene expression in cells of interest in the retina, cochlea, and CNS?
What are the receptors that can facilitate blood-brain barrier penetration after systemic AAV injection?
What immunomodulatory strategies can make systemic AAV delivery and redosing more safely tolerated and efficacious?
Will targeting/detargeting approaches enable precise, single-tissue transduction by AAV vectors?
Will development of more efficient capsids reduce the vector load and subsequent toxicity observed in systemic based gene therapy which now use doses up to 3e14 gc/kg?
Highlights.
AAV has become the pre-eminent vector for gene therapy, and is particularly well-suited to delivering to the nervous system.
There are an increasingly large range of AAV serotypes and variants, with varying tropisms and functions.
Choosing the right vector for the right tissue type is important, and will be a prime determinant in how effective a gene therapy is.
Acknowledgements
All figures were created using BioRender.com. Due to the concise length and limited scope of this review, there are many studies we were unable to reference or discuss, especially with respect to the fundamental work that has gone into AAV biology and capsid engineering development over the past 20 years. We sincerely apologize to the many scientists and body of research we were unable to include.
Glossary of terms
- Inner/Outer hair cells
Specialized ciliated cells responsible for hearing. Inner hair cells (IHCs) are each innervated by several spiral ganglion neurons, which relay stimuli to the brain. The cilia of IHCs form organized bundles which allow detection of vibration through the basilar membrane in response to sound. Outer hair cells (OHCs) amplify low input sound locally throughout the cochlea, using a motor protein called prestin
- Intracerebroventricular injection (ICV)
Injection into the cerebrospinal fluid (CSF) through cerebral ventricles of the brain itself
- Intrathecal delivery
Administration of an agent into the cerebrospinal fluid (CSF) via the subarachnoid lumbar space of the spinal cord, or through the cisterna magna, usually via lumbar puncture or cisterna magna
- ITR
Inverted terminal repeats; hairpin structures of repetitive DNA that direct packaging into the vector capsid, involved also in integration, replication, and gene expression
- Scala media, tympani, vestibuli
Fluid-filled ducts that surround the cochlea. The scalae tympani and vestibuli transmit sound to the membranes surrounding the organ of Corti and contain perilymph. The organ of Corti is housed in the scala media, which contains endolymph
- Transduction
Successful transgene expression in a cell by a viral vector
- Vector genomes (vg)
The viral vector nucleic acid, packaged within a capsid. Used as a measurement of a vector’s presence. The concentration (titer) of a virus preparation is often expressed as vg/ml as measured by ddPCR or qPCR. Dosing of AAV vectors is based on vg for local delivery and vg/kg of body weight for systemic dosing
Footnotes
Disclaimer Statement
The authors declare the following competing interests: CAM has financial interests in Chameleon Biosciences, Inc. and Sphere Gene Therapeutics, companies developing enveloped adeno-associated virus (AAV) vector platform technologies. CAM also has a financial interest in Claritas Bio, a company developing gene therapies to treat hearing disorders. CAM’s interests were reviewed and are managed by the Massachusetts General Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keeler AM and Flotte TR (2019) Recombinant Adeno-Associated Virus Gene Therapy in Light of Luxturna (and Zolgensma and Glybera): Where Are We, and How Did We Get Here? Annu. Rev. Virol. 6, 601–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sonntag F et al. (2010) A viral assembly factor promotes AAV2 capsid formation in the nucleolus. Proc. Natl. Acad. Sci. U. S. A. 107, 10220–10225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogden PJ et al. (2019) Comprehensive AAV capsid fitness landscape reveals a viral gene and enables machine-guided design. Science (80-. ). 366, 1139–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meier AF et al. The interplay between adeno-associated virus and its helper viruses., Viruses. (2020), 12(6), 662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsu H-L et al. (2020) Structural characterization of a novel human adeno-associated virus capsid with neurotropic properties. Nat. Commun. 11, 3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson KL et al. (2016) Better targeting, better efficiency for wide-scale neuronal transduction with the synapsin promoter and AAV-PHP.B. Front. Mol. Neurosci. 9, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duque S. et al. (2009) Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 17, 1187–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foust KD et al. (2009) Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 27, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang B. et al. (2014), Global CNS transduction of adult mice by intravenously delivered rAAVrh.8 and rAAVrh.10 and nonhuman primates by rAAVrh.10., in Molecular Therapy, pp. 22(7):1299–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoshino Y. et al. (2019) The adeno-associated virus rh10 vector is an effective gene transfer system for chronic spinal cord injury. Sci. Rep. 9, 9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowes LP et al. (2019) Impact of Age and Motor Function in a Phase 1/2A Study of Infants With SMA Type 1 Receiving Single-Dose Gene Replacement Therapy. Pediatr. Neurol. 98, 39–45 [DOI] [PubMed] [Google Scholar]
- 12.Deverman BE et al. (2016) Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat. Biotechnol. 34, 204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan KY et al. (2017) Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat. Neurosci. 20, 1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanchenko MV et al. (2020) Preclinical testing of AAV9-PHP.B for transgene expression in the non-human primate cochlea. Hear. Res. 394, 107930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.György B. et al. (2019) Gene Transfer with AAV9-PHP.B Rescues Hearing in a Mouse Model of Usher Syndrome 3A and Transduces Hair Cells in a Non-human Primate. Mol. Ther. - Methods Clin. Dev. 20, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hordeaux J. et al. (2019) The GPI-Linked Protein LY6A Drives AAV-PHP.B Transport across the Blood-Brain Barrier. Mol. Ther. 27, 912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Q. et al. (2019) Delivering genes across the blood-brain barrier: LY6A, a novel cellular receptor for AAV-PHP.B capsids. PLoS One 14(11), e0225206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista AR et al. (2020) Ly6a Differential Expression in Blood-Brain Barrier Is Responsible for Strain Specific Central Nervous System Transduction Profile of AAV-PHP.B. Hum. Gene Ther. 31(1–2), 90–102 [DOI] [PubMed] [Google Scholar]
- 19.Loughner CL et al. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes., Human genomics. (2016), 10, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanlon KS et al. (2019) Selection of an Efficient AAV Vector for Robust CNS Transgene Expression. Mol. Ther. - Methods Clin. Dev. 15, 320–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davé UP and Cornetta K. (2021) AAV Joins the Rank of Genotoxic Vectors. Mol. Ther. 29, 418–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chien YH et al. (2017) Efficacy and safety of AAV2 gene therapy in children with aromatic L-amino acid decarboxylase deficiency: an open-label, phase 1/2 trial. Lancet Child Adolesc. Heal. 1, 265–273 [DOI] [PubMed] [Google Scholar]
- 23.Warren Olanow C. et al. (2015) Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: A double-blind, randomized, controlled trial. Ann. Neurol. 78, 248–257 [DOI] [PubMed] [Google Scholar]
- 24.Christine CW et al. (2019) Magnetic resonance imaging–guided phase 1 trial of putaminal AADC gene therapy for Parkinson’s disease. Ann. Neurol. 85, 704–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mittermeyer G. et al. (2011) Long-term evaluation of a phase 1 study of AADC gene therapy for parkinson’s disease. Hum. Gene Ther. 23, 377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosario AM et al. (2016) Microglia-specific targeting by novel capsid-modified AAV6 vectors. Mol. Ther. - Methods Clin. Dev. 3, 16026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y. et al. (2015) Adenoassociated virus serotype 9-mediated gene therapy for X-linked adrenoleukodystrophy. Mol. Ther. 23, 824–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao N. et al. Microglia-targeting nanotherapeutics for neurodegenerative diseases., APL Bioengineering. (2020), 4, 030902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinderer C. et al. (2018) Evaluation of Intrathecal Routes of Administration for Adeno-Associated Viral Vectors in Large Animals. Hum. Gene Ther. 29, 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liguore WA et al. (2019) AAV-PHP.B Administration Results in a Differential Pattern of CNS Biodistribution in Non-human Primates Compared with Mice. Mol. Ther. 27, 2018–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray SJ et al. (2013) Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther. 20, 450–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Y. et al. (2019) Intrathecal Adeno-Associated Viral Vector-Mediated Gene Delivery for Adrenomyeloneuropathy. Hum. Gene Ther. 30, 544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akil O. et al. (2015) Surgical method for virally mediated gene delivery to the mouse inner ear through the round window membrane. J. Vis. Exp. 97, 52187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y. et al. (2005) Specific and efficient transduction of cochlear inner hair cells with recombinant adeno-associated virus type 3 vector. Mol. Ther. 12, 725–733 [DOI] [PubMed] [Google Scholar]
- 35.Tao Y. et al. (2018) Delivery of Adeno-Associated Virus Vectors in Adult Mammalian Inner-Ear Cell Subtypes Without Auditory Dysfunction. Hum. Gene Ther. 29, 492–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim MA et al. (2019) Targeted Gene Delivery into the Mammalian Inner Ear Using Synthetic Serotypes of Adeno-Associated Virus Vectors. Mol. Ther. - Methods Clin. Dev. 13, 197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akil O. et al. (2019) Dual AAV-mediated gene therapy restores hearing in a DFNB9 mouse model. Proc. Natl. Acad. Sci. U. S. A. 116, 4496–4501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J. et al. (2020) Efficient viral transduction in mouse inner ear hair cells with utricle injection and AAV9-PHP.B. Hear. Res. 394, 107882 [DOI] [PubMed] [Google Scholar]
- 39.Suzuki J. et al. (2017) Cochlear gene therapy with ancestral AAV in adult mice: Complete transduction of inner hair cells without cochlear dysfunction. Sci. Rep. 7, 45524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chien WW et al. (2015) Cochlear gene transfer mediated by adeno-associated virus: Comparison of two surgical approaches. Laryngoscope 125, 2557–2564 [DOI] [PubMed] [Google Scholar]
- 41.Isgrig K. et al. (2019) AAV2.7m8 is a powerful viral vector for inner ear gene therapy. Nat. Commun. 10, 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.György B. et al. (2017) Rescue of Hearing by Gene Delivery to Inner-Ear Hair Cells Using Exosome-Associated AAV. Mol. Ther. 25, 379–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.György B. et al. (2019) Allele-specific gene editing prevents deafness in a model of dominant progressive hearing loss. Nat. Med. 25, 1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gu X. et al. (2019) Transduction of adeno-associated virus vectors targeting hair cells and supporting cells in the neonatal mouse cochlea. Front. Cell. Neurosci. 13, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh WH et al. (2020) In vivo base editing restores sensory transduction and transiently improves auditory function in a mouse model of recessive deafness. Sci. Transl. Med. 12, 546, eaay9101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hordeaux J. et al. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice., Molecular Therapy. (2018), 26, 664–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iizuka T. et al. (2015) Perinatal GJB2 gene transfer rescues hearing in a mouse model of hereditary deafness. Hum. Mol. Genet. 24, 3651–3661 [DOI] [PubMed] [Google Scholar]
- 48.Shu Y. et al. (2016) Identification of Adeno-Associated Viral Vectors That Target Neonatal and Adult Mammalian Inner Ear Cell Subtypes. Hum. Gene Ther. 27, 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Q. et al. (2014) Virally expressed connexin26 restores gap junction function in the cochlea of conditional Gjb2 knockout mice. Gene Ther. 21, 71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crispino G. et al. (2011) BAAV mediated GJB2 gene transfer restores gap junction coupling in cochlear organotypic cultures from deaf Cx26Sox10Cre mice. PLoS One 6, e23279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu X. et al. Screened AAV variants permit efficient transduction access to supporting cells and hair cells., Cell Discovery. (2019), 5, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan F. et al. (2019) AAV-ie enables safe and efficient gene transfer to inner ear cells. Nat. Commun. 10, 3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stone IM et al. (2005) Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol. Ther. 11, 843–848 [DOI] [PubMed] [Google Scholar]
- 54.Kersigo J. et al. (2018) A RNAscope whole mount approach that can be combined with immunofluorescence to quantify differential distribution of mRNA. Cell Tissue Res. 374, 351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu KS et al. Development of the Mouse and Human Cochlea at Single Cell Resolution., bioRxiv. (2019) [Google Scholar]
- 56.Hoa M. et al. (2020) Characterizing Adult Cochlear Supporting Cell Transcriptional Diversity Using Single-Cell RNA-Seq: Validation in the Adult Mouse and Translational Implications for the Adult Human Cochlea. Front. Mol. Neurosci. 13, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu CJ et al. (2020) Efficient in Utero Gene Transfer to the Mammalian Inner Ears by the Synthetic Adeno-Associated Viral Vector Anc80L65. Mol. Ther. - Methods Clin. Dev. 18, 493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whelan L. et al. (2020) Findings from a genotyping study of over 1000 people with inherited retinal disorders in Ireland. Genes (Basel). 11, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watanabe S. et al. (2013) Tropisms of AAV for Subretinal Delivery to the Neonatal Mouse Retina and Its Application for In Vivo Rescue of Developmental Photoreceptor Disorders. PLoS One 8, e54146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palfi A. et al. (2012) Efficacy of Codelivery of Dual AAV2/5 Vectors in the Murine Retina and Hippocampus. Hum. Gene Ther. 23, 847–858 [DOI] [PubMed] [Google Scholar]
- 61.Palfi A. et al. (2015) Efficient gene delivery to photoreceptors using AAV2/rh10 and rescue of the Rho(−/−) mouse. Mol. Ther. Methods Clin. Dev. 2, 15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenberghe LH et al. (2011) Dosage Thresholds for AAV2 and AAV8 Photoreceptor Gene Therapy in Monkey. Sci. Transl. Med. 3, 88ra54–88ra54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vandenberghe LH et al. (2013) AAV9 Targets Cone Photoreceptors in the Nonhuman Primate Retina. PLoS One 8, e54363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guy J. et al. (2017) Gene Therapy for Leber Hereditary Optic Neuropathy Low- and Medium-Dose Visual Results. Ophthalmology 124, 1621–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang S. et al. (2016) Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine 10, 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timmers AM et al. (2020) Ocular Inflammatory Response to Intravitreal Injection of Adeno-Associated Virus Vector: Relative Contribution of Genome and Capsid. Hum. Gene Ther. 31(1–2), 80–89 [DOI] [PubMed] [Google Scholar]
- 67.Bouquet C. et al. (2019) Immune Response and Intraocular Inflammation in Patients with Leber Hereditary Optic Neuropathy Treated with Intravitreal Injection of Recombinant Adeno-Associated Virus 2 Carrying the ND4 Gene: A Secondary Analysis of a Phase 1/2 Clinical Trial. JAMA Ophthalmol. 137, 399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ong T. et al. Adeno-Associated Viral Gene Therapy for Inherited Retinal Disease., Pharmaceutical Research. (2019), 36, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boye SE et al. (2012) The human rhodopsin kinase promoter in an AAV5 vector confers rod- and cone-specific expression in the primate retina. Hum. Gene Ther. 23, 1101–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ye GJ et al. (2017) Safety and Efficacy of AAV5 Vectors Expressing Human or Canine CNGB3 in CNGB3-Mutant Dogs. Hum. Gene Ther. Clin. Dev. 28, 197–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cehajic-Kapetanovic J. et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR., Nature Medicine. (2020), 26, 354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobson SG et al. (2012) Gene therapy for leber congenital amaurosis caused by RPE65 mutations: Safety and efficacy in 15 children and adults followed up to 3 years. Arch. Ophthalmol. 130, 9–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gamlin PD et al. (2019) SubILM injection of AAV for gene delivery to the retina. In Methods in Molecular Biology pp. 1950, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng Y. et al. (2020) “Para-retinal” Vector Administration into the Deep Vitreous Enhances Retinal Transgene Expression. Mol. Ther. - Methods Clin. Dev. 18, 422–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Teo KYC et al. (2018) Surgical removal of internal limiting membrane and layering of AAV vector on the retina under air enhances gene transfection in a nonhuman primate. Investig. Ophthalmol. Vis. Sci. 59, 3574–3583 [DOI] [PubMed] [Google Scholar]
- 76.Takahashi K. et al. (2017) Improved Intravitreal AAV-Mediated Inner Retinal Gene Transduction after Surgical Internal Limiting Membrane Peeling in Cynomolgus Monkeys. Mol. Ther. 25, 296–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wassmer SJ et al. (2017) Exosome-associated AAV2 vector mediates robust gene delivery into the murine retina upon intravitreal injection. Sci. Rep. 7, 45329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boye SL et al. (2020) Novel AAV44.9-Based Vectors Display Exceptional Characteristics for Retinal Gene Therapy. Mol. Ther. 28, 1464–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petrs-Silva H. et al. (2009) High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol. Ther. 17, 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petrs-Silva H. et al. (2011) Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol. Ther. 19, 293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mowat FM et al. (2014) Tyrosine capsid-mutant AAV vectors for gene delivery to the canine retina from a subretinal or intravitreal approach. Gene Ther. 21, 96–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cronin T. et al. (2014) Efficient transduction and optogenetic stimulation of retinal bipolar cells by a synthetic adeno-associated virus capsid and promoter. EMBO Mol. Med. 6, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dalkara D. et al. (2013) In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci. Transl. Med. 5, 189ra76 [DOI] [PubMed] [Google Scholar]
- 84.Ramachandran P. et al. (2016) Evaluation of dose and safety of AAV7m8 and AAV8BP2 in the non-human primate retina. Hum. Gene Ther. 28, 154–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khabou H. et al. (2018) Noninvasive gene delivery to foveal cones for vision restoration. JCI Insight 3, e96029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jüttner J. et al. (2019) Targeting neuronal and glial cell types with synthetic promoter AAVs in mice, non-human primates and humans. Nat. Neurosci. 22, 1345–1356 [DOI] [PubMed] [Google Scholar]
- 87.Wang D. et al. (2018) A Rationally Engineered Capsid Variant of AAV9 for Systemic CNS-Directed and Peripheral Tissue-Detargeted Gene Delivery in Neonates. Mol. Ther. - Methods Clin. Dev. 9, 234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pulicherla N. et al. (2011) Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol. Ther. 19, 1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keeler GD et al. Liver induced transgene tolerance with AAV vectors., Cellular Immunology. (2019), 342, 103728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verdera HC et al. AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer., Molecular Therapy. (2020), 28, 723–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mazzacurati L. et al. (2015) Use of miRNA response sequences to block off-target replication and increase the safety of an unattenuated, glioblastoma-targeted oncolytic HSV. Mol. Ther. 23, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xie J. et al. (2012) Long-term, efficient inhibition of microRNA function in mice using rAAV vectors. Nat. Methods 9, 403–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eichhoff AM et al. (2019) Nanobody-Enhanced Targeting of AAV Gene Therapy Vectors. Mol. Ther. - Methods Clin. Dev. 15, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weed L. et al. (2019) Safety of Same-Eye Subretinal Sequential Readministration of AAV2-hRPE65v2 in Non-human Primates. Mol. Ther. - Methods Clin. Dev. 15, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bennett J. et al. (2016) Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet 388, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Annear MJ et al. (2011) Gene therapy in the second eye of RPE65-deficient dogs improves retinal function. Gene Ther. 18, 53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Martino AT and Markusic DM Immune Response Mechanisms against AAV Vectors in Animal Models., Molecular Therapy - Methods and Clinical Development. (2020), 17, 198–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wood MB and Zuo J. (2017) The contribution of immune infiltrates to ototoxicity and cochlear hair cell loss. Front. Cell. Neurosci. 11, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Walton RT et al. (2020) Unconstrained genome targeting with near-PAMless engineered CRISPR-Cas9 variants. Science (80-. ). 368, 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srivastava A. In vivo tissue-tropism of adeno-associated viral vectors., Current Opinion in Virology. (2016), 21, 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pillay S. et al. (2016) An essential receptor for adeno-associated virus infection. Nature 530, 108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dudek AM et al. (2020) GPR108 Is a Highly Conserved AAV Entry Factor. Mol. Ther. 28, 367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uhrig S. et al. (2012) Successful target cell transduction of capsid-engineered rAAV vectors requires clathrin-dependent endocytosis. Gene Ther. 19, 210–218 [DOI] [PubMed] [Google Scholar]
- 104.Yang J. et al. (1999) Concatamerization of Adeno-Associated Virus Circular Genomes Occurs through Intermolecular Recombination. J. Virol. 73, 9468–9477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grimm D. et al. (2008) In Vitro and In Vivo Gene Therapy Vector Evolution via Multispecies Interbreeding and Retargeting of Adeno-Associated Viruses. J. Virol. 82, 5887–5911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhong L. et al. (2007) A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol. Ther. 15, 1323–1330 [DOI] [PubMed] [Google Scholar]
- 107.Zhong L. et al. (2008) Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc. Natl. Acad. Sci. U. S. A 105, 7827–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zinn E. et al. (2015) In silico reconstruction of the viral evolutionary lineage yields a potent gene therapy vector. Cell Rep. 12, 1056–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith RH et al. (2016) Germline viral “fossils” guide in silico reconstruction of a mid-Cenozoic era marsupial adeno-associated virus. Sci. Rep. 6, 28965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santiago-Ortiz J. et al. (2015) AAV ancestral reconstruction library enables selection of broadly infectious viral variants. Gene Ther. 22, 934–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sehara Y. et al. (2017) Persistent Expression of Dopamine-Synthesizing Enzymes 15 Years after Gene Transfer in a Primate Model of Parkinson’s Disease. Hum. Gene Ther. Clin. Dev. 28, 74–79 [DOI] [PubMed] [Google Scholar]
- 112.Vandamme C. et al. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial., Human Gene Therapy. (2017), 28, 1061–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calcedo R. and Wilson JM (2016) AAV Natural Infection Induces Broad Cross-Neutralizing Antibody Responses to Multiple AAV Serotypes in Chimpanzees. Hum. Gene Ther. Clin. Dev. 27, 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun J. et al. (2018) An Observational Study from Long-Term AAV Re-administration in Two Hemophilia Dogs. Mol. Ther. - Methods Clin. Dev. 10, 257–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nathwani AC et al. (2014) Long-Term Safety and Efficacy of Factor IX Gene Therapy in Hemophilia B. N. Engl. J. Med. 371, 1994–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Butterfield JSS et al. (2019) TLR9-Activating CpG-B ODN but Not TLR7 Agonists Triggers Antibody Formation to Factor IX in Muscle Gene Transfer. Hum. Gene Ther. Methods 30, 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ronzitti G. et al. (2020) Human Immune Responses to Adeno-Associated Virus (AAV) Vectors. Front. Immunol. 11, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vecino E. et al. Glia-neuron interactions in the mammalian retina., Progress in Retinal and Eye Research. (2016), 51, 1–40 [DOI] [PubMed] [Google Scholar]
- 119.Dalkara D. et al. (2009) Inner limiting membrane barriers to aav-mediated retinal transduction from the vitreous. Mol. Ther. 17, 2096–2102 [DOI] [PMC free article] [PubMed] [Google Scholar]