Abstract
The primary plasma cell leukemia (pPCL) is a rare but aggressive variant of multiple myeloma (MM). Few studies have focused on the differences in the causes of death between pPCL and MM. This study aimed to compare and evaluate the causes of death of patients with pPCL and MM.
The data were collected from the Surveillance Epidemiology, and End Results (SEER) database. The demographic characteristics, survival, and causes of death in pPCL and MM patients were evaluated and compared. The competing risk regression model was performed to predict the cause of death.
Between 1975 and 2009, the overall mortality rate was 96.13% and 88.71% for pPCL and MM, and the median survival was 9 and 26 months, respectively. In pPCL, leukemia caused 45.05% of the deaths, followed by myeloma (38.83%). In MM, myeloma was the leading cause of death, accounting for 74.89% of the deaths. Older age at diagnosis was a risk factor for dying of leukemia in pPCL patients (HR = 1.49, 95% CI: 1.16–1.91), while older age at death was associated with reduced risk (HR = 0.67, 95% CI: 0.52–0.86). Although the survival of pPCL patients increased with time periods of diagnosis since 1975 to 2009, the risk of dying of leukemia increased with the periods. For MM, most of the demographic characteristics were found to have independently predicting influence on the cause of death.
Patients with pPCL and MM had distinct causes of death. Leukemia was the leading and the most serious cause of death in pPCL patients. The demographic factors could not predict the causes of death in pPCL. More large-scale and multi-center studies are needed to evaluate the effect of novel agents in pPCL patients, especially for patients who have progressed to leukemia.
Keywords: causes of death, multiple myeloma, plasma cell leukemia, prognosis, SEER program
1. Introduction
Plasma cell leukemia (PCL) is a rare but highly malignant type of plasma cell dyscrasia (PCD), with typical features of increased monoclonal plasma cells in peripheral blood.[1–3] There are 2 types of PCL: primary PCL (pPCL) and secondary PCL (sPCL). As a distinct clinical and biological entity, pPCL is present as de novo, without an antecedent multiple myeloma (MM), with sPCL as a leukemic transformation of end-stage MM.[4–6] pPCL represents approximately 60% of PCL patients, and its crude incidence in Europe is 0.04–0.05/100.000 persons per year.[5,6] The prognosis of pPCL is dismal, whereby the median survival is approximately 4–24 months. Novel agents, such as lenalidomide, bortezomib, and thalidomide, have proven to be effective in MM in recent years, and have also been introduced for use in pPCL; however, a significant improvement in survival has not been observed in pPCL.[2,7] A recent study showed that the 4-year overall survival rate was still between 28% and 31% in pPCL patients despite undergoing hematopoietic cell transplantation (HCT).[4]
Based on Kyle diagnostic criteria in 1974, pPCL is considered to be a rare variant of MM, operationally defined by more than 20% circulating plasma cells and/or plasma cell count in peripheral blood of over 2 × 109/L and without previous MM.[8] However, a survival rate similar to that of pPCL has been identified in MM patients whose circulating plasma cells have not yet reached this standard in some studies.[9,10] This suggests the current definition may be too stringent, resulting in an underestimation of the incidence of pPCL.[5,10] So, in some studies, a lower diagnostic threshold (i.e., >5% circulating plasma cell and/or >0.5 × 109/L peripheral blood clonal plasma cells) has been proposed.[11–13]
Compared to MM, different clinical and biological features have been reported in pPCL, as well as an elevated genomic instability and increased number of genetic aberrations.[7,14–16] Previous studies have shown pPCL has a higher incidence of chromosomal deletions, amplification, and translocation, compared to MM.[7] A recent study has shown there is a significant difference of transcriptome between pPCL and MM, and the biological differences between pPCL and MM may be caused by the mRNA processing pathway.[17] Besides, the methylation involved in cell adhesion and migration is also one of the characteristics that may distinguish pPCL from MM.[18]
Given the rarity of pPCL, its underlying pathophysiologic mechanisms remain incompletely understood.[7,19] Several studies have conducted population-based analysis of the Surveillance, Epidemiology, and End Results (SEER) database to evaluate the survival rates of pPCL. However, there is no study focused on comparing the causes of death between pPCL and MM. We suppose that the different biological mechanisms and distinct clinical and laboratory features would induce various causes of death. In this study, we conducted a descriptive analysis to evaluate and compare the distribution of the causes of death in pPCL and MM patients, based on SEER database. The present study may provide clues to distinguishing pPCL and MM, and also provide supportive cure and prevent poor prognosis in clinical practice.
2. Methods
2.1. Data source
Detailed patient information was obtained from the SEER database. The SEER database consists of data on the incidence of cancer from 18 cancer registries around the United States, covering 34.6% of the population in the USA (https://seer.cancer.gov). The data include tumor and demographic characteristics of the patients, as well as causes of death. SEER database 1975–2016 (SEER 18), which was based on the November 2018 submission, was used for this study. Data were collected through the SEER*Stat v8.3.6 (https://seer.cancer.gov/seerstat/).
2.2. Patient selection
The data of patients, who were diagnosed with pPCL (International Classification of Diseases for Oncology, ICD-O-3 code 9733/3: Plasma cell leukemia) between 1975 and 2009, were download from SEER database. The year of 2009 was set because PCL had been included in the same category with MM (ICD-O-3 code 9732/3: Plasma cell myeloma) and stopped to be coded separately by the SEER cancer registry since 2010. In an attempt to ensure that only pPCL patients were selected in this research, we limited the patients to those with PCL as the first and only primary cancer diagnosis. Similarly, the patients with MM as the only primary cancer diagnosis during the same period were also included in the present study. Patients included in this study were restricted to those who had diagnostic verification of their tumor based on microscopic confirmation, tissue histology, or cytology. We excluded patients who were diagnosed at death or autopsy, patients with no follow-up or 0 months of the survival time, and those without the cause of death recorded.
2.3. Study variables
The demographic characteristics of pPCL and MM patients were abstracted; these included age at diagnosis, sex, race, marital status, survival status (dead or alive), the cause of death, and survival time. The race included the White race, the Black race, and other race subgroups. The marital status was divided into married and other, and the latter included divorced, widowed, and separated status in marriage. Besides, since the types of therapy not included in the SEER database, the patients were stratified into 4 time periods (1975-, 1996-, 2001-, and 2006–2009) based on the year of diagnosis to reflect the effect of different treatment modalities, according to a previous study.[20] Briefly, the autologous stem cell transplantation (ASCT) had been used as a standard treatment modality since 1995, and novel agents started to be used as of 2000. Moreover, the novel agents were available for use as first-line therapy from 2006 onward.
All the patients reported between 1975 and 2009 in the SEER database were included in this study, and the target event was the death by any cause. The survival time was the time interval since the diagnosis to death, or to the end of the follow-up in 2009 for those still alive. Subjects who were alive at the end of the follow-up were considered as censored for the target event. The causes of death were divided into 4 subgroups according to the major causes of death, which were defined by SEER Causes of Death Recode (https://seer.cancer.gov/codrecode/); based on the ICD-10, this included leukemia, myeloma, other cancers, and noncancer diseases. Leukemia included lymphocytic leukemia, myeloid and monocytic leukemia, and other types of leukemia. Other cancers included any other malignant cancers except leukemia or myeloma, and noncancer covered any cause of death from noncancer diseases. Since the death of any other cause was hindered by one cause of death, the 4 causes of death were considered as competing endpoint events.
2.4. Ethical approval
All procedures performed in this study were in accordance with the 1964 Helsinki Declaration and its later amendments. No ethics approval was declared because the SEER is a publicly available database.
2.5. Statistical analysis
Frequency and percentage were used to describe the categorical variables of the demographic characteristics and median with range were provided for continuous variables. The difference of demographics among patients was analyzed by using Pearson chi-square test and the Kruskal-Wallis test. The pairwise multiple comparisons were performed by using Nemenyi test. The Kaplan-Meier method was adopted to create survival curves, and the comparison of the survival curves was performed by the Log-Rank test. To determine the independently predicting influence of various risk factors on the causes of death, a Fine and Gray multivariate competing risk model was performed. R software version 3.6.2 (The R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org) was adopted for all statistical analyses. All tests were 2-tailed and P < .05 represented a statistically significant difference.
3. Results
3.1. Demographics in pPCL and MM patients
In the present study, 284 pPCL patients and 50261 MM patients were included. Table 1 shows the demographics of the patients. For pPCL, median age at diagnosis was 62 years. Over 83% of the patients were between 40 and 80 years old. Among pPCL patients, 72.54% were White. There were 273 patient deaths during 1975–2009, and the overall mortality rate was 96.13%. For MM patients, the median of age at diagnosis was 68 years. Similar to pPCL, 81.78% were diagnosed between 40 and 80 years of age, and 75.91% were White. The overall mortality rate of MM was 88.71%, and 44588 among 50261 patients died during 1975 to 2009. The comparison of pPCL and MM showed that the pPCL patients were younger than MM when they were diagnosed (P < .0001) and pPCL patients had a higher mortality rate higher than MM (P < .0001). Other statistically significant differences included the distribution of age at diagnosis, gender, and time periods of diagnosis between pPCL and MM (Table 1).
Table 1.
Demographics of pPCL and MM patients.
| Characteristic | pPCL (n = 284) | MM (n = 50,261) | P value |
|---|---|---|---|
| Age at diagnosis, median (range) | 62.00 (19.00, 91.00) | 68.00 (9.00, 103.00) | <.0001 |
| Age group at diagnosis (%) | <.0001 | ||
| <40 years | 11 (3.87) | 833 (1.66) | |
| 40–59 years | 110 (38.73) | 13,148 (26.16) | |
| 60–79 years | 128 (45.07) | 27,955 (55.62) | |
| ≥80 years | 35 (12.32) | 8325 (16.56) | |
| Sex (%) | .0458 | ||
| Male | 131 (46.13) | 26,257 (52.24) | |
| Female | 153 (53.87) | 24,004 (47.76) | |
| Marital status (%) | .2359 | ||
| Married | 174 (61.27) | 29,043 (57.78) | |
| Other | 110 (38.73) | 21,218 (42.22) | |
| Race (%) | .1104 | ||
| White | 206 (72.54) | 38,151 (75.91) | |
| Black | 65 (22.89) | 9194 (18.29) | |
| Other | 13 (4.58) | 2916 (5.80) | |
| Time periods of diagnosis (%) | .0160 | ||
| 1975–1995 | 74 (26.06) | 16,332 (32.49) | |
| 1996–2000 | 39 (13.73) | 7706 (15.33) | |
| 2001–2005 | 81 (28.52) | 13,975 (27.80) | |
| 2006–2009 | 90 (31.69) | 12,248 (24.37) | |
| Survival status (%) | <.0001 | ||
| Alive | 11 (3.87) | 5673 (11.29) | |
| Dead | 273 (96.13) | 44,588 (88.71) |
MM = multiple myeloma, pPCL = primary plasma cell leukemia.
3.2. Demographics of deaths from pPCL and MM
The distributions of the deaths from all causes among pPCL and MM patients were analyzed and are shown in Table 2. For pPCL patients, the median age at death and the median survival months were 63 years and 9 months, respectively. Nearly 56.05% of the deaths from pPCL happened in the 12 months after the diagnosis. The leading cause of death was leukemia, followed by myeloma, noncancer diseases, and other cancers; the specific mortality of leukemia was 45.05% (Table 2). For MM, the median age at death and median survival were 72-years and 26 months, respectively. Only 29.13% of the deaths occurred within the first years after diagnosis. The leading cause of death was myeloma, which comprised 74.89% of all the MM deaths, followed by noncancer diseases, and both other cancers and leukemia accounted for 2.32% (Table 2). Comparing the distribution of pPCL and MM, statistical differences were identified in all the variables, except for gender, marital status, and race (P = .0629, P = .0752, and P = .1494, respectively). More details of the distribution of deaths from all causes for pPCL and MM are shown in Table 3.
Table 2.
Demographics of the deaths among pPCL and MM patients.
| Characteristic | pPCL (N = 273) | MM (N = 44,588) | P Value |
|---|---|---|---|
| Age at death, median (range) | 63.00 (19.00, 92.00) | 72.00 (12.00, 104.00) | <.0001 |
| Survival months, median (range) | 9.00 (1.00, 141.00) | 26.00 (1.00, 457.00) | <.0001 |
| Interval of survival months,n(%) | .0005 | ||
| <6 months | 104 (38.10) | 7831 (17.56) | |
| 6 months | 49 (17.95) | 5158 (11.57) | |
| 12 months | 54 (19.78) | 7745 (17.37) | |
| 24 months | 26 (9.52) | 5913 (13.26) | |
| 36 months | 13 (4.76) | 4506 (10.11) | |
| ≥48 months | 27 (9.89) | 13,435 (30.13) | |
| Age group at diagnosis (%) | <.0001 | ||
| <40 years | 11 (4.03) | 566 (1.27) | |
| 40–59 years | 104 (38.10) | 10,195 (22.86) | |
| 60–79 years | 123 (45.05) | 25,630 (57.48) | |
| ≥80 years | 35 (12.82) | 8197 (18.38) | |
| Sex (%) | .0629 | ||
| Male | 127 (46.52) | 23,339 (52.34) | |
| Female | 146 (53.48) | 21,249 (47.66) | |
| Marital status (%) | .0752 | ||
| Married | 170 (62.27) | 25,381 (56.92) | |
| Other | 103 (37.73) | 19,207 (43.08) | |
| Race (%) | .1494 | ||
| White | 200 (73.26) | 34,137 (76.56) | |
| Black | 61 (22.34) | 8020 (17.99) | |
| Other | 12 (4.40) | 2431 (5.45) | |
| Time periods of diagnosis (%) | .0001 | ||
| 1975–1995 | 74 (27.11) | 16,171 (36.27) | |
| 1996–2000 | 37 (13.55) | 7288 (16.35) | |
| 2001–2005 | 81 (29.67) | 12,221 (27.41) | |
| 2006–2009 | 81 (29.67) | 8908 (19.98) | |
| Causes of death (%) | <.0001 | ||
| Leukemia | 123 (45.05) | 322 (0.72) | |
| Myeloma | 106 (38.83) | 33,391 (74.89) | |
| Other cancers | 7 (2.56) | 713 (1.60) | |
| Noncancer diseases | 37 (13.55) | 10,162 (22.79) |
MM = multiple myeloma, pPCL = primary plasma cell leukemia.
Table 3.
Distribution of causes of death for pPCL and MM patients.
| Group | Causes of death | n | % | |
|---|---|---|---|---|
| pPCL | Leukemia | 123 | 45.05 | |
| Myeloma | 106 | 38.83 | ||
| Lymphoma | 5 | 1.83 | ||
| Other cancers | Miscellaneous malignant cancer | 2 | 0.73 | |
| Diseases of heart | 13 | 4.76 | ||
| Other cause of death | 9 | 3.3 | ||
| Other infectious and parasitic diseases including HIV | 5 | 1.83 | ||
| Pneumonia and influenza | 3 | 1.1 | ||
| Cerebrovascular diseases | 2 | 0.73 | ||
| noncancers | Nephritis, nephrotic syndrome, and nephrosis | 1 | 0.37 | |
| Diabetes mellitus | 1 | 0.37 | ||
| Accidents and adverse effects | 1 | 0.37 | ||
| Hypertension without heart disease | 1 | 0.37 | ||
| Other diseases of arteries, arterioles, capillaries | 1 | 0.37 | ||
| Total | 273 | 100 | ||
| MM | Myelom | 33,391 | 74.89 | |
| Leukemia | 322 | 0.72 | ||
| Miscellaneous malignant cancer | 214 | 0.48 | ||
| Skin | 122 | 0.27 | ||
| Lymphoma | 108 | 0.24 | ||
| Respiratory system | 73 | 0.16 | ||
| Bones and joints | 69 | 0.15 | ||
| Digestive system | 56 | 0.13 | ||
| Male genital system | 19 | 0.04 | ||
| Urinary system | 14 | 0.03 | ||
| Other cancers | Brain and other nervous system | 12 | 0.03 | |
| Soft tissue including heart | 5 | 0.01 | ||
| Breast | 4 | 0.01 | ||
| Female genital system | 4 | 0.01 | ||
| Oral cavity and pharynx | 4 | 0.01 | ||
| Skin | 4 | 0.01 | ||
| Eye and orbit | 3 | 0.01 | ||
| Kaposi sarcoma | 1 | 0 | ||
| Mesothelioma | 1 | 0 | ||
| Diseases of heart | 4122 | 9.24 | ||
| Other cause of death | 1711 | 3.84 | ||
| Cerebrovascular diseases | 640 | 1.44 | ||
| Pneumonia and influenza | 622 | 1.39 | ||
| Nephritis, nephrotic syndrome and nephrosis | 580 | 1.3 | ||
| Chronic obstructive pulmonary disease and allied cond | 450 | 1.01 | ||
| Diabetes mellitus | 309 | 0.69 | ||
| Accidents and adverse effects | 272 | 0.61 | ||
| Other infectious and parasitic diseases including HIV | 264 | 0.59 | ||
| Septicemia | 240 | 0.54 | ||
| Hypertension without heart disease | 192 | 0.43 | ||
| Alzheimer | 119 | 0.27 | ||
| Noncancers | Symptoms, signs and ill-defined conditions | 119 | 0.27 | |
| In situ, benign or unknown behavior neoplasm | 104 | 0.23 | ||
| Atherosclerosis | 87 | 0.2 | ||
| Chronic liver disease and cirrhosis | 87 | 0.2 | ||
| Suicide and self-inflicted injury | 84 | 0.19 | ||
| Aortic aneurysm and dissection | 41 | 0.09 | ||
| Other diseases of arteries, arterioles, capillaries | 37 | 0.08 | ||
| Stomach and duodenal ulcers | 37 | 0.08 | ||
| Congenital anomalies | 29 | 0.07 | ||
| Homicide and legal intervention | 8 | 0.02 | ||
| Tuberculosis | 6 | 0.01 | ||
| Total | 44,588 | 100 | ||
MM = multiple myeloma, pPCL = primary plasma cell leukemia.
3.3. Survival of pPCL and MM patients
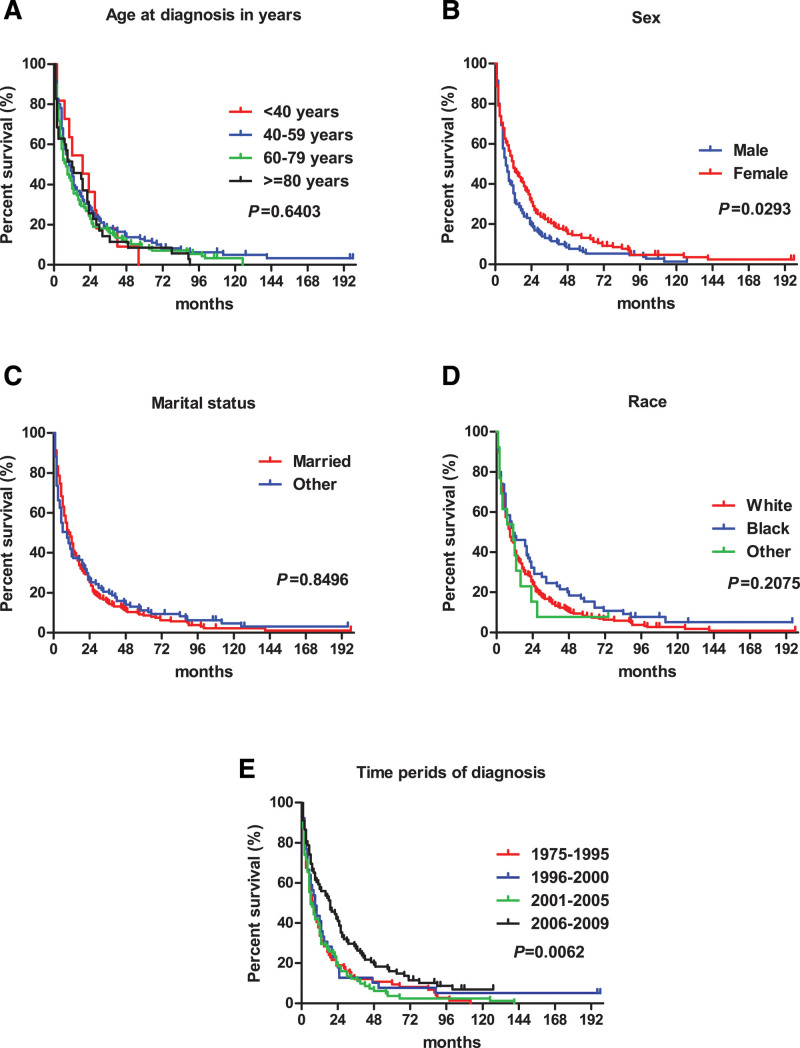
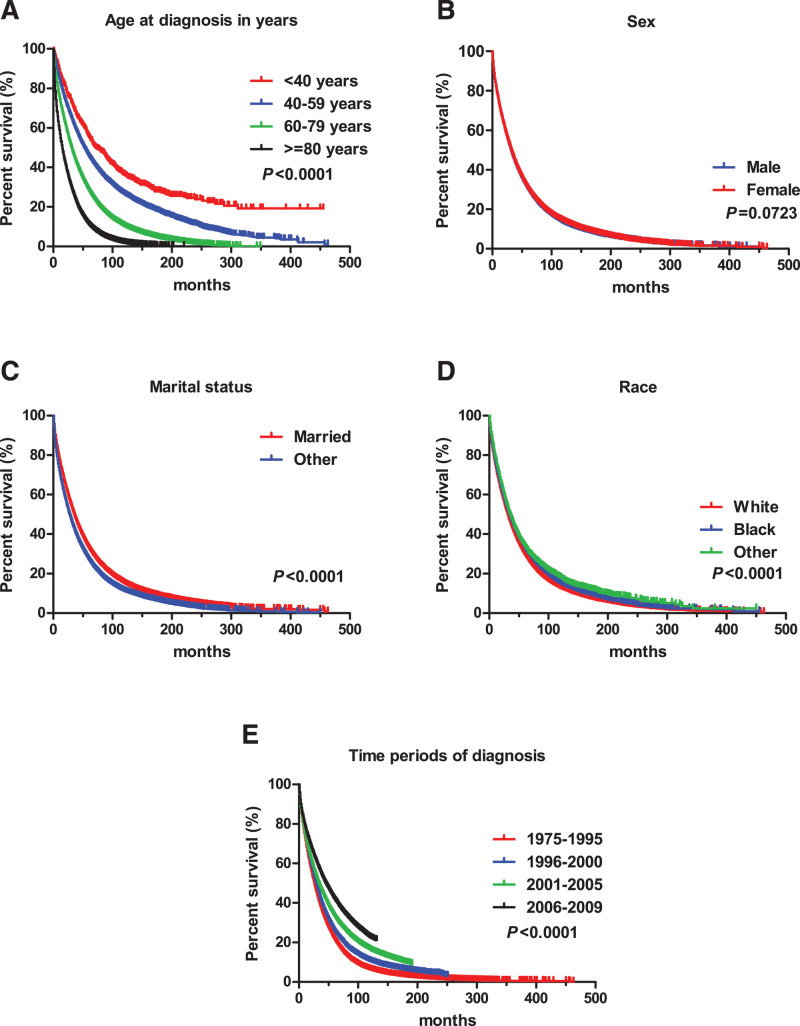
The survival curve shows the age at diagnosis, marital status, and race had no effect on the survival time of pPCL patients (Fig. 1A, C, and D). The median survival time of male pPCL patients was 7 months, which was shorter than 12 months for females (P = .0293) (Fig. 1B). Median survival for pPCL patients who were diagnosed during 2006–2009 was longer than 1975–1995, 1996–2000, and 2001–2005, with the values of 19, 7.5, 9, and 6 months, respectively (Fig. 1E). For MM, the median survival time decreased with increasing age at diagnosis (Fig. 2A). Different races and marital status had different median survival times (P < .0001) (Fig. 2C, D). The median survival time of MM patients was 26, 30, 34, and 45 months for 1975–1995, 1996–2000, 2001–2005, and 2006–2009 periods, respectively (P < .0001) (Fig. 2E).
Figure 1.
Survival curve for pPCL patients.
Figure 2.
Survival curve for MM patients.
3.4. Causes of death for pPCL
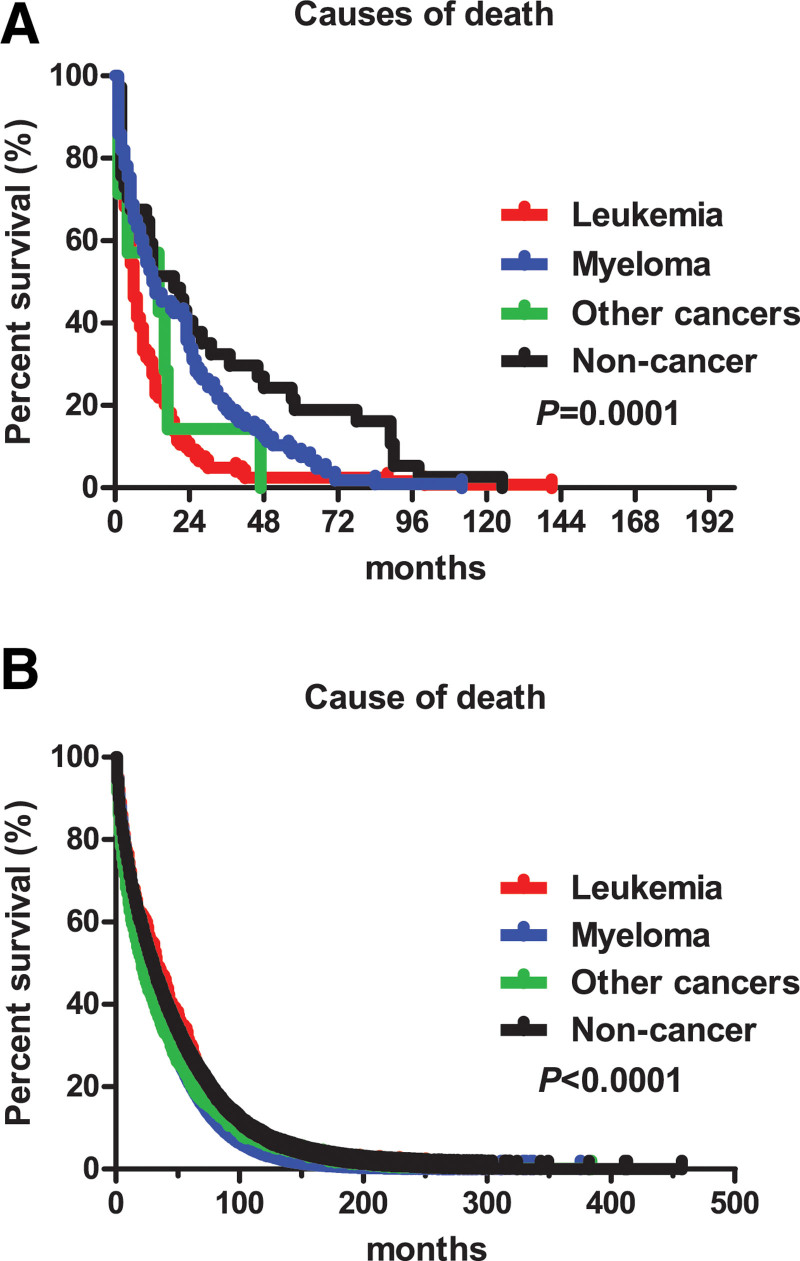
As shown in Table 4, no significant difference was identified for the distribution of the age at diagnosis, marital status, race, and time periods of diagnosis among the 4 causes for pPCL deaths. The patients who died from myeloma or noncancer diseases had a higher age at death than those who died from leukemia and other cancers (P = .0232). Leukemia caused 45.53% of the deaths within the first 6 months after diagnosis. The results also showed that a proportion of the patients dying from leukemia increased by the time periods of diagnosis. To analyze the difference among the periods of diagnosis, we also calculated the mortality rate of dying from leukemia. The specific mortality rates for dying from leukemia were 43.24% (32/74), 28.21% (11/39), 35.80% (29/81), and 37.78% (34/90) for each period. Similarly, the mortality rates for dying from myeloma were 35.14% (26/74), 51.28% (20/39), 55.56% (45/81), and 35.56% (32/90). The median survival of leukemia, myeloma, other cancers and noncancer diseases was 6, 12.5, 14, and 19 months, respectively (P = .0001) (Fig. 3A).
Table 4.
Characteristics of different causes of death for pPCL patients.
| Characteristics | Causes of death | P value | |||
|---|---|---|---|---|---|
| Leukemia (n = 123) | Myeloma (n = 106) | Other cancers (n = 7) | noncancer diseases (n = 37) | ||
| Age at diagnosis, median (range) | 62.00 (19.00, 91.00) | 63.50 (30.00, 91.00) | 60.00 (43.00, 90.00) | 66.00 (28.00, 89.00) | .0739 |
| Age at death, median (range) | 62.00 (19.00, 91.00) | 65.50 (30.00, 92.00) | 61.00 (45.00, 90.00) | 67.00 (29.00, 92.00) | .0232 |
| Interval of survival months, n (%) | .0004 | ||||
| <6 months | 56 (45.53) | 33 (31.13) | 3 (42.86) | 12 (32.43) | |
| 6 months | 29 (2.36) | 17 (16.04) | 0 (0.00) | 3 (8.11) | |
| 12 months | 26 (21.14) | 18 (16.98) | 3 (42.86) | 7 (18.92) | |
| 24 months | 6 (4.88) | 17 (16.04) | 0 (0.00) | 3 (8.11) | |
| 36 months | 3 (2.44) | 7 (6.60) | 1 (14.29) | 2 (5.41) | |
| ≥48 months | 3 (2.44) | 14 (13.21) | 0 (0.00) | 10 (27.03) | |
| Age group at diagnosis (%) | .1398 | ||||
| <40 years | 5 (4.07) | 5 (4.72) | 0 (0.00) | 1 (2.70) | |
| 40–59 years | 52 (42.28) | 40 (37.74) | 3 (42.86) | 9 (24.32) | |
| 60–79 years | 57 (46.34) | 46 (43.40) | 2 (28.57) | 18 (48.65) | |
| ≥80 years | 9 (7.32) | 15 (14.15) | 2 (28.57) | 9 (24.32) | |
| Sex (%) | <.0001 | ||||
| Male | 60 (48.78) | 62 (58.49) | 3 (42.86) | 21 (56.76) | |
| Female | 63 (51.22) | 44 (41.51) | 4 (57.14) | 16 (43.24) | |
| Marital status (%) | .0990 | ||||
| Married | 81 (65.85) | 66 (62.26) | 6 (85.71) | 17 (45.95) | |
| Other | 42 (34.15) | 40 (37.74) | 1 (14.29) | 20 (54.05) | |
| Race (%) | .1346 | ||||
| White | 92 (74.80) | 77 (72.64) | 5 (71.43) | 26 (70.27) | |
| Black | 26 (21.14) | 26 (24.53) | 0 (0.00) | 9 (24.32) | |
| Other | 5 (4.07) | 3 (2.83) | 2 (28.57) | 2 (5.41) | |
| Time periods of diagnosis (%) | .0854 | ||||
| 1975–1995 | 32 (30.19) | 26 (21.14) | 2 (28.57) | 14 (37.84) | |
| 1996–2000 | 11 (10.38) | 20 (16.26) | 2 (28.57) | 4 (10.81) | |
| 2001–2005 | 29 (27.36) | 45 (36.59) | 2 (28.57) | 5 (13.51) | |
| 2006–2009 | 34 (32.08) | 32 (26.02) | 1 (14.29) | 14 (37.84) | |
pPCL = primary plasma cell leukemia.
Figure 3.
Survival curve of different causes of death. A: For pPCL; B: for MM.
3.5. Causes of death for MM
The results in Table 5 show a significant difference for all variables among different death causes. The patients who died from leukemia had the least median age at both diagnosis and death, compared to the other causes of death (P < .0001). For each cause of death, the largest proportion of the deaths happened after 48 months of the diagnosis. The specific mortality rates of each time period of diagnosis for myeloma were 75.73% (12,368/16,332), 70.76% (5453/7706), 64.47% (9009/13,975) and 53.57% (6561/12,248), respectively, and decreased with the time periods of diagnosis, as well as in the other 3 causes of death. The median survival of leukemia, myeloma, other cancers and noncancer diseases was 33, 26, 21, and 29 months, respectively (P < .0001) (Fig. 3B).
Table 5.
Characteristics of different causes of death for MM patients.
| Characteristics | Causes of death | P value | |||
|---|---|---|---|---|---|
| Myeloma (n = 33391) | Leukemia (n = 322) | Other cancer (n = 713) | noncancer diseases (n = 10162) | ||
| Age at diagnosis, median (range) | 68.00 (17.00, 103.00) | 65.00 (31.00, 93.00) | 69.00 (9.00, 96.00) | 73.00 (23.00, 103.00) | <.0001 |
| Age at death, median (range) | 71.00 (18.00, 104.00) | 69.00 (36.00, 96.00) | 72.00 (12.00, 96.00) | 76.00 (25.00, 103.00 | <.0001 |
| Interval of survival months, n (%) | .0005 | ||||
| <6 months | 5754 (17.23) | 54 (16.77) | 174 (24.4) | 1849 (18.2) | |
| 6 months | 3946 (11.82) | 35 (10.87) | 92 (12.9) | 1085 (10.68) | |
| 12 months | 6031 (18.06) | 42 (13.04) | 114 (15.99) | 1558 (15.33) | |
| 24 months | 4613 (13.82) | 39 (12.11) | 73 (10.24) | 1188 (11.69) | |
| 36 months | 3480 (10.42) | 31 (9.63) | 62 (8.7) | 933 (9.18) | |
| ≥48 months | 9567 (28.65) | 121 (37.58) | 198 (27.77) | 3549 (34.92) | |
| Age group at diagnosis (%) | <.0001 | ||||
| <40 years | 477 (1.43) | 5 (1.55) | 11 (1.54) | 73 (0.72) | |
| 40–59 years | 8307 (24.88) | 109 (33.85) | 165 (23.14) | 1614 (15.88) | |
| 60–79 years | 19,226 (57.58) | 180 (55.90) | 392 (54.98) | 5832 (57.39) | |
| ≥80 years | 5381 (16.12) | 28 (8.70) | 145 (20.34) | 2643 (26.01) | |
| Sex (%) | <.0001 | ||||
| Male | 16,098 (48.21) | 155 (48.14) | 320 (44.88) | 4676 (46.01) | |
| Female | 17,293 (51.79) | 167 (51.86) | 393 (55.12) | 5486 (53.99) | |
| Marital status (%) | <.0001 | ||||
| Married | 19,627 (58.78) | 212 (65.84) | 376 (52.73) | 5166 (50.84) | |
| Other | 13,764 (41.22) | 110 (34.16) | 337 (47.27) | 4996 (49.16) | |
| Race (%) | <.0001 | ||||
| White | 25,980 (77.81) | 238 (73.91) | 535 (75.04) | 7384 (72.66) | |
| Black | 5634 (16.87) | 65 (20.19) | 148 (20.76) | 2173 (21.38) | |
| Other | 1777 (5.32) | 19 (5.90) | 30 (4.21) | 605 (5.95) | |
| Time periods of diagnosis (%) | <.0001 | ||||
| 1975–1995 | 12,368 (37.04) | 142 (44.1) | 217 (30.43) | 3444 (33.89) | |
| 1996–2000 | 5453 (16.33) | 48 (14.91) | 117 (16.41) | 1670 (16.43) | |
| 2001–2005 | 9009 (26.98) | 74 (22.98) | 225 (31.56) | 2913 (28.67) | |
| 2006–2009 | 6561 (19.65) | 58 (18.01) | 154 (21.60) | 2135 (21.01) | |
MM = multiple myeloma.
3.6. Competing risk regression model
Table 6 provides details about the multivariable competing risk regression model of the causes of death for pPCL deaths. Since the nonconvergence model caused by the small size in pPCL, we merged the 2 causes of Other cancer and noncancer disease into one group (other causes). The results showed most of the demographic variables had no independently predicting influence on the causes of death of pPCL. Older age at the time of diagnosis had an increased risk of death from leukemia (HR = 1.49, 95%CI: 1.16, 1.91). Older age at death had a reduced risk of death from leukemia (HR = 0.67, 95%CI: 0.52, 0.86). Compared to the 1975–1995 periods, patients diagnosed during 1996–2000 and 2001–2005 had a higher risk of death from leukemia (HR = 1.94, 95%CI: 1.06, 3.55; HR = 2.12, 95%CI: 1.28, 3.53). Diagnosis during 2001–2005 was a protective factor for death from other causes. Similar to pPCL, older age at diagnosis showed a higher risk of death for all causes in MM, except leukemia, and a younger at death had a higher risk of death from leukemia, myeloma, and other cancers (Table 7). With regard to the risk of death among races, whites had a higher risk of death from myeloma and lower risk of death of noncancer diseases (HR = 1.14, 95%CI: 1.11, 1.18; HR = 0.71, 95%CI: 0.68, 0.75). Male subjects were more likely to die from other cancers and noncancer diseases (HR = 1.21, 95%CI: 1.03, 1.41; HR = 1.28, 95%CI: 1.22, 1.33), but not myeloma (HR = 0.96, 95%CI: 0.93, 0.98). Time periods of diagnosis was a protective factor for leukemia and myeloma death, but not for other cancers and noncancer diseases.
Table 6.
Competing risk regression model for pPCL patients.
| Variables | Causes of death | |||||
|---|---|---|---|---|---|---|
| Leukemia | Myeloma | Other causes | ||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age at diagnosis | 1.49 (1.16, 1.91) | .0020 | 1.05 (1.00, 1.11) | .0673 | 0.99 (0.94, 1.05) | .7369 |
| Age at death | 0.67 (0.52, 0.86) | .0016 | 0.95 (0.91, 1.00) | .0731 | 1.04 (0.98, 1.10) | .2389 |
| Race | ||||||
| Black | Reference | Reference | Reference | |||
| White | 1.08 (0.69, 1.69) | .7383 | 0.97 (0.63, 1.50) | .8882 | 1.04 (0.47, 2.30) | .9161 |
| Other | 0.77 (0.31, 1.95) | .5812 | 0.50 (0.14, 1.73) | .2715 | 3.39 (0.89, 12.93) | .0737 |
| Sex | ||||||
| Female | Reference | Reference | Reference | |||
| Male | 1.13 (0.77,1.66) | .5215 | 0.75 (0.51, 1.12) | .1612 | 1.14 (0.55, 2.40) | .7228 |
| Marital status | ||||||
| Married | Reference | Reference | Reference | |||
| Other | 0.861 (0.57, 1.30) | .4728 | 0.96 (0.64, 1.44) | .8506 | 1.45 (0.74, 2.87) | .2816 |
| Time periods of diagnosis | ||||||
| 1975–1995 | Reference | Reference | Reference | |||
| 1996–2000 | 1.94 (1.06, 3.55) | .0326 | 0.52 (0.26, 1.04) | .0633 | 0.69 (0.25, 1.92) | .4802 |
| 2001–2005 | 2.12 (1.28, 3.53) | .0037 | 0.75 (0.44, 1.26) | .2713 | 0.36 (0.15, 0.87) | .0228 |
| 2006–2009 | 1.48 (0.84, 2.60) | .1738 | 0.82 (0.51, 1.33) | .4254 | 0.64 (0.29, 1.39) | .2557 |
pPCL = primary plasma cell leukemia.
Table 7.
Competing risk regression model for MM patients.
| Variables | Causes of death | |||||||
|---|---|---|---|---|---|---|---|---|
| Leukemia | Myeloma | Other cancers | Noncancer diseases | |||||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | |
| Age at diagnosis | 1.01 (1.00, 1.02) | .1562 | 1.06 (1.05, 1.06) | <.0001 | 1.03 (1.02, 1.04) | <.0001 | 1.04 (1.03, 1.04) | <.0001 |
| Age at death | 0.97 (0.96, 0.98) | <.0001 | 0.94 (0.94, 0.95) | <.0001 | 0.97 (0.96, 0.98) | <.0001 | 1.00 (1.00, 1.00) | .4717 |
| Race | ||||||||
| Black | Reference | Reference | Reference | Reference | ||||
| White | 0.86 (0.65, 1.14) | .2928 | 1.14 (1.11, 1.18) | <.0001 | 0.87 (0.72, 1.04) | .1289 | 0.71 (0.68, 0.75) | <.0001 |
| Other | 0.92 (0.55, 1.53) | .7382 | 0.98 (0.92, 1.03) | .4031 | 0.65 (0.44, 0.96) | .0307 | 0.83 (0.76, 0.91) | .0001 |
| Sex | ||||||||
| Female | Reference | Reference | Reference | Reference | ||||
| Male | 0.88 (0.70, 1.12) | .3010 | 0.96 (0.93, 0.98) | .0003 | 1.21 (1.03, 1.41) | .0175 | 1.28 (1.22, 1.33) | <.0001 |
| Marital status | ||||||||
| Married | Reference | Reference | Reference | Reference | ||||
| Other | 0.72 (0.57, 0.93) | .0102 | 0.95 (0.93, 0.98) | .0001 | 1.22 (1.04, 1.43) | .0146 | 1.24 (1.19, 1.30) | <.0001 |
| Time periods of diagnosis | ||||||||
| 1975–1995 | Reference | Reference | Reference | Reference | ||||
| 1996–2000 | 0.74 (0.53, 1.02) | .0664 | 0.89 (0.86, 0.92) | <.0001 | 1.15 (0.92, 1.44) | .2271 | 1.01 (0.95, 1.07) | .7891 |
| 2001–2005 | 0.62 (0.47, 0.83) | .0010 | 0.77 (0.75, 0.79) | <.0001 | 1.24 (1.03, 1.49) | .0241 | 0.99 (0.94, 1.04) | .7211 |
| 2006–2009 | 0.58 (0.42, 0.78) | .0004 | 0.58 (0.56, 0.60) | <.0001 | 0.99 (0.81, 1.21) | .9131 | 0.85 (0.80, 0.90) | <.0001 |
MM = multiple myeloma.
4. Discussion
The present study analyzed the data from SEER database to evaluate and compare the causes of death in pPCL and MM patients. The results showed the distinct characteristics of death causes among pPCL and MM. For pPCL patients, leukemia was the leading and the most serious cause of death, followed by myeloma, whereas in MM, the main cause was myeloma. Leukemia could induce earlier death than the other causes in pPCL patients. However, the MM patients, who died from leukemia, had the longest survival time than the other causes. In the competing risk regression model, the demographic characteristics had no predictive role on the death causes of pPCL.
Studies have shown pPCL is more aggressive than MM.[11,21] Despite the utilization of novel agents in recent clinical practice, including bortezomib, lenalidomide, and thalidomide, the prognosis of pPCL is still frustrating.[16,22] In a previous study, the patients with pPCL and MM who were reported in SEER database between 1973 and 2004 were included. The median overall survival for patients with pPCL was 4 months, and that for MM was 2.08 years.[23] This was similar with our results. The current study shows that the mortality was 96.13% among pPCL patients, and median survival was 9 months. The mortality rate within 6 months after the diagnosis was 36.62% (104 of 284) in pPCL patients. Death from leukemia mostly happened within 2 years after the diagnosis. By contrast, MM had a lower mortality rate of 88.71%, and a longer survival period (26 months) than pPCL. The mortality rate within 6 months after diagnosis was only 15.56%. Besides, the age at diagnosis and age at death in pPCL patients were lower than MM. The high mortality and rapid death after diagnosis suggest pPCL should be treated immediately after diagnosis, and the ideal treatments should be based on shorter treatment free intervals.[24]
Our results also showed there were different distributions of prognosis and death causes between pPCL and MM. The age at diagnosis, marital status, and race had no relationship with the prognosis of pPCL. However, only sex was found to be not associated with the prognosis of MM patient. Leukemia was the most serious cause of death among pPCL patients; 45.05% of the deaths were caused by leukemia and 45.53% of leukemia deaths happened in the first 6 months after diagnosis. Additionally, the median survival of pPCL patients who died of leukemia was 6 months, significantly shorter than the other causes of death. However, leukemia accounted for only 0.72% of deaths in MM and the median survival was 33 months. A recent study showed that, myeloma caused 71.3% of deaths in SEER database and 71.7% in the database of the Puerto Rico Central Cancer Registry, among MM patients aged more than 40 between 1987 and 2013.[25] In our study, it was similar that 74.89% of deaths in MM patients were attributed to myeloma, but it was the second ranking cause of death in pPCL, which caused only 38.83% of deaths. Interestingly, 31.13% of pPCL patients who died of myeloma occurred in the first 6 months after diagnosis, however, the proportion was only 17.23% in MM. It seemed to suggest that pPCL and MM patients had distinct characteristics of death, even though they had the same cause of death. Since the SEER database does not provide details about the types of therapy used, we divided the patients into 4 periods based on the year of diagnosis to reflect the effect of different treatment modalities. Our results showed the survival in both pPCL and MM patients increased with the year at diagnosis. This finding is similar with a prior study, in which the median overall survival based on stratification by time periods of diagnosis including 1973-, 1996-, 2001-, and 2006–2009 were 5, 6, 4, and 12 months.[20] To some extent, our results were able to detect an improvement in survival in pPCL because of the use of novel agents.
To predict the causes of death in pPCL and MM patients and adjust the bias from other demographic factors, the competing risk model was performed. The results showed that most of the demographic characteristics had no independently predicting influence for causes of death in pPCL. Only older age at diagnosis, and younger age at death were risk factors for death from leukemia in pPCL patients. Interestingly, we also found that the increasing time periods of diagnosis was the risk factors for death of leukemia compared to 1975–1995 period. This suggests that although the new treatment modalities could increase the survival of pPCL patients, the effect on those who progressed leukemia was still limited. Considering the rarity of pPCL, additional multi-center studies with larger size are needed. Instead of pPCL, nearly all demographic factors were independently predictive factors for causes of death in MM patients. We also noticed that the risk of dying from leukemia and myeloma decreased with time periods of diagnosis. To some extent, it seemed to confirm the positive effect of new treatments on MM, and also demonstrated the difference of causes of death between pPCL and MM. The different causes of death may be associated with the distinct cytogenetic and molecular features of pPCL with respect to MM.[16] The gene, such as adhesion molecule CD56 and the B cell marker CD20, show differential expression in PCL and MM.[19] Except for the differential gene expression, the differences of mutations, genetic transcriptions, and phenotypic profile between PCL and MM may also cause the diverse prognosis of these 2 malignancies,[17,26] as well as the causes of death.
This study has several limitations. The first is the lack of details concerning the treatment records in this SEER database, so we were unable to analyze the influence of the different therapies on death. Referred to the previous study, we use 4 time periods based on the year of diagnosis to represent the effect of the standard treatment modalities at different periods. The second is the small cohort of the patients; pPCL is a rare disease and accounts for 60% of PCL cases. Additionally, to include pPCL cases and reduce the bias from the complicating cancer, we set the strict inclusion criteria of including only patients with a primary cancer diagnosis in this study. This contributed to the small sample size. Finally, the details about the predicting factors are lacking in the registries of SEER program. Although we analyzed and predicted the risk for different death causes by using some demographics of the patients, almost all of the demographic factors had no independently predicting influence in pPCL. So, large-scale prospective clinical trials with more details on therapies are still needed.
5. Conclusions
This study compared and evaluated the causes of death amongst pPCL and MM patients, based on data from the SEER database. The results showed pPCL and MM had distinct causes of death. Leukemia was the leading and the most serious cause of death in pPCL patients. The demographic characteristics could not predict the causes of death. More large-scale and multi-center studies are needed to evaluate the effect of novel agents on pPCL, especially those progressed to leukemia.
Acknowledgment
We acknowledge the SEER database of NIH for the high-quality data used in this study.
Author contributions
Conceptualization: Xiaoyan Ge, Weihan Meng, Wenbo Wang, Kai Cui.
Data curation: Xiaoyan Ge, Kai Cui.
Funding acquisition: Kai Cui.
Methodology: Xiaoyan Ge, Weihan Meng, Honglin Ma, Kai Cui.
Software: Xiaoyan Ge, Honglin Ma, Siqi Zhao, Kai Cui.
Writing – original draft: Xiaoyan Ge, Kai Cui.
Writing – review & editing: Xiaoyan Ge, Weihan Meng, Wenbo Wang, Kai Cui.
Abbreviations:
- MM =
- multiple myeloma
- PCL =
- plasma cell leukemia
- pPCL =
- primary plasma cell leukemia
- SEER =
- Surveillance Epidemiology, and End Results.
How to cite this article: Ge X, Meng W, Wang W, Ma H, Zhao S, Cui K. Causes of death in primary plasma cell leukemia differ from multiple myeloma: A STROBE-compliant descriptive study based on SEER database. Medicine 2022;101:29(e29578).
Funding: This study was supported by National Natural Science Foundation of China (Grant No. 81903289), and Natural Science Foundation of Liaoning Province of China (Grant No. 2019-ZD-0611).
All authors declare that there is no conflict of interest.
Data availability statement: The datasets generated during and/or analyzed during the current study are publicly available.
Contributor Information
Xiaoyan Ge, Email: gexiaoyan@jzmu.edu.cn.
Weihan Meng, Email: 1004711616@qq.com.
Wenbo Wang, Email: 419531327@qq.com.
Honglin Ma, Email: 345446454@qq.com.
Siqi Zhao, Email: 70624447@qq.com.
References
- [1].Jelinek T, Kryukov F, Rihova L, et al. Plasma cell leukemia: from biology to treatment. Eur J Haematol. 2015;95:16–26. [DOI] [PubMed] [Google Scholar]
- [2].Musto P, Simeon V, Todoerti K, et al. Primary plasma cell leukemia: identity card 2016. Curr Treat Options Oncol. 2016;17:19. [DOI] [PubMed] [Google Scholar]
- [3].Iriuchishima H, Ozaki S, Konishi J, et al. Primary plasma cell leukemia in the era of novel agents: a multicenter study of the Japanese Society of Myeloma. Acta Haematol. 2016;135:113–21. [DOI] [PubMed] [Google Scholar]
- [4].Dhakal B, Patel S, Girnius S, et al. Hematopoietic cell transplantation utilization and outcomes for primary plasma cell leukemia in the current era. Leukemia. 2020;34:3338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Musto P, Statuto T, Valvano L, et al. An update on biology, diagnosis and treatment of primary plasma cell leukemia. Expert Rev Hematol. 2019;12:245–53. [DOI] [PubMed] [Google Scholar]
- [6].Neri A, Todoerti K, Lionetti M, et al. Primary plasma cell leukemia 2.0: advances in biology and clinical management. Expert Rev Hematol. 2016;9:1063–73. [DOI] [PubMed] [Google Scholar]
- [7].Schinke C, Boyle EM, Ashby C, et al. Genomic analysis of primary plasma cell leukemia reveals complex structural alterations and high-risk mutational patterns. Blood Cancer J. 2020;10:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kyle RA, Maldonado JE, Bayrd ED. Plasma cell leukemia. Report on 17 cases. Arch Intern Med. 1974;133:813–8. [DOI] [PubMed] [Google Scholar]
- [9].An G, Qin X, Acharya C, et al. Multiple myeloma patients with low proportion of circulating plasma cells had similar survival with primary plasma cell leukemia patients. Ann Hematol. 2015;94:257–64. [DOI] [PubMed] [Google Scholar]
- [10].Yu T, Xu Y, An G, et al. Primary plasma cell leukemia: real-world retrospective study of 46 patients from a single-center study in China. Clin Lymphoma Myeloma Leuk. 2020;20:652–9. [DOI] [PubMed] [Google Scholar]
- [11].Fernandez de Larrea C, Kyle RA, Durie BG, et al. Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia. 2013;27:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ravi P, Kumar SK, Roeker L, et al. Revised diagnostic criteria for plasma cell leukemia: results of a Mayo Clinic study with comparison of outcomes to multiple myeloma. Blood Cancer J. 2018;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yadav N, Aggarwal M, Mehta P, et al. Primary plasma cell leukemia: a retrospective study of a rare disease from tertiary cancer centre from India. Indian J Hematol Blood Transfus. 2019;35:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cazaubiel T, Leleu X, Perrot A, et al. Primary Plasma Cell Leukemia displaying t(11;14) have specific genomic, transcriptional and clinical feature. Blood. 2022;139:2666–72. [DOI] [PubMed] [Google Scholar]
- [15].Gowda L, Shah M, Badar I, et al. Primary plasma cell leukemia: autologous stem cell transplant in an era of novel induction drugs. Bone Marrow Transplant. 2019;54:1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gavriatopoulou M, Musto P, Caers J, et al. European myeloma network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias. Leukemia. 2018;32:1883–98. [DOI] [PubMed] [Google Scholar]
- [17].Rojas EA, Corchete LA, Mateos MV, et al. Transcriptome analysis reveals significant differences between primary plasma cell leukemia and multiple myeloma even when sharing a similar genetic background. Blood Cancer J. 2019;9:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Todoerti K, Calice G, Trino S, et al. Global methylation patterns in primary plasma cell leukemia. Leuk Res. 2018;73:95–102. [DOI] [PubMed] [Google Scholar]
- [19].Gundesen MT, Lund T, Moeller HEH, et al. Plasma cell leukemia: definition, presentation, and treatment. Curr Oncol Rep. 2019;21:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gonsalves WI, Rajkumar SV, Go RS, et al. Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood. 2014;124:907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tuazon SA, Holmberg LA, Nadeem O, et al. A clinical perspective on plasma cell leukemia; current status and future directions. Blood Cancer J. 2021;11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mina R, Joseph NS, Kaufman JL, et al. Survival outcomes of patients with primary plasma cell leukemia (pPCL) treated with novel agents. Cancer. 2019;125:416–23. [DOI] [PubMed] [Google Scholar]
- [23].Ramsingh G, Mehan P, Luo J, et al. Primary plasma cell leukemia: a surveillance, epidemiology, and end results database analysis between 1973 and 2004. Cancer. 2009;115:5734–9. [DOI] [PubMed] [Google Scholar]
- [24].Musto P. Progress in the treatment of primary plasma cell leukemia. J Clin Oncol. 2016;34:2082–4. [DOI] [PubMed] [Google Scholar]
- [25].Castañeda-Avila MA, Ortiz-Ortiz KJ, Torres-Cintrón CR, et al. Trends in cause of death among patients with multiple myeloma in Puerto Rico and the United States SEER population, 1987-2013. Int J Cancer. 2020;146:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bezdekova R, Jelinek T, Kralova R, et al. Necessity of flow cytometry assessment of circulating plasma cells and its connection with clinical characteristics of primary and secondary plasma cell leukaemia. Br J Haematol. 2021;195:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]